Abstract
Lethal mutagenesis is an antiviral approach that consists in extinguishing a virus by an excess of mutations acquired during replication in the presence of a mutagen. Here we show that favipiravir (T-705) is a potent mutagenic agent for hepatitis C virus (HCV) during its replication in human hepatoma cells. T-705 leads to an excess of G → A and C → U transitions in the mutant spectrum of preextinction HCV populations. Infectivity decreased significantly in the presence of concentrations of T-705 which are 2- to 8-fold lower than its cytotoxic concentration 50 (CC50). Passaging the virus five times in the presence of 400 μM T-705 resulted in virus extinction. Since T-705 has undergone advanced clinical trials for approval for human use, the results open a new approach based on lethal mutagenesis to treat hepatitis C virus infections. If proven effective for HCV in vivo, this new anti-HCV agent may be useful in patient groups that fail current therapeutic regimens.
Introduction
Lethal mutagenesis is an antiviral approach consisting of achieving viral extinction by an excess of mutations [1–6]. It is an application of the error threshold relationship of quasispecies theory that can be applied to finite populations of viruses in changing fitness landscapes [7]. We were interested in exploring lethal mutagenesis for the treatment of HCV infections, based on the evidence that ribavirin (1-β-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide), an important component of several anti-HCV therapies, might be exerting its antiviral action partly through lethal mutagenesis [8,9]. Effective antiviral lethal mutagenesis therapy will require additional agents that mutagenize the virus and not the cells, and provide an advantage over standard non-mutagenic inhibitors and their combinations.
Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pirazinecarboxamide) is one of several pyrazinecarboxamide derivatives that display a broad spectrum antiviral activity against RNA viruses. Work by Furuta and colleagues has documented that T-705 is active against influenza virus, and with lower potency also against poliovirus, rhinovirus and respiratory syncytial virus [10,11], and that T-1105 (3-hydroxy-2-pyrazinecarboxamide) inhibited foot-and-mouth disease virus (FMDV) replication in cell culture and in vivo [12]. T-1106, the nucleoside derivative of T-1105, inhibited replication of bovine viral diarrhea virus and HCV [13]. RNA viruses as diverse as picornaviruses, alphaviruses, flaviviruses, rhabdoviruses, orthomyxoviruses, paramyxoviruses, arenaviruses, hantaviruses and bunyaviruses are inhibited by members of this family of antiviral agents [14–28]. Moreover, T-705 potentiated the anti-influenza activity of oseltamivir [24] and the anti-arenavirus activity of ribavirin [29,30].
Present evidence suggests that these inhibitors target the viral RNA-dependent RNA polymerase (RdRp) resulting in inhibition of viral RNA synthesis [31,32]. T-705 is converted into nucleotide derivatives inside the cell, and T-705-4-ribofuranosyl-5’-triphosphate (T-705-RTP) inhibited the influenza virus polymerase in a GTP-competitive manner [11]. In replicating influenza RNA, T-705-RTP can be ambiguously recognised as G or A, and the consecutive incorporation of two T-705-RMP residues in the RNA produced chain termination [33].
The ambiguous base pairing of T-705-RTP is consistent with a dominance of G → A and C → U transitions in viral RNA that led to lethal mutagenesis of influenza virus [34]. T-705 induced also lethal mutagenesis of norovirus in cell culture and in vivo, although in this case progeny RNA acquired an excess of A → G and U → C transitions [35]. In the present study we show that T-705 is a mutagenic agent for HCV that produces an excess of G → A and C → U transitions, leading to loss of infectivity through a decrease of specific infectivity. The results reinforce the possibility of lethal mutagenesis as an alternative antiviral design to treat HCV infections.
Materials and Methods
Cells and viruses
The origin of Huh-7.5, Huh-7-Lunet, Huh-7.5 reporter cell lines and procedures for cell growth in Dulbecco’s modification of Eagle’s medium (DMEM), have been previously described [36,37]. Infected and uninfected cells were cultured at 37°C and 5% CO2. The viruses used in the experiments reported here are HCVcc [Jc1FLAG2(p7-nsGluc2A)] (a chimera of J6 and JFH-1 from genotype 2a) and GNN [GNNFLAG2(p7-nsGluc2A)] (carrying a mutation in the NS5B RNA-dependent RNA polymerase rendering it replication-defective) [38]. To control for the absence of contamination, the supernatants of mock-infected cells, which were maintained in parallel with the infected cultures, were titrated; no infectivity in the mock-infected cultures was detected in any of the experiments.
Production of viral progeny and titration of infectivity
The procedures used to prepare the initial virus stock HCV p0 and for serial infections of the human hepatoma Huh-7.5 cells have been previously described [39]. Briefly, Huh-7-Lunet cells were electroporated with 10 μg of the infectious transcript of HCVcc (Jc1 or the negative control GNN) (Gene Pulser Xcell electroporation system; Bio-Rad; 260 volts, 950 μF). Electroporated cells were then passaged every 3–4 days without allowing the cells to reach confluence; passages were continued until 30 days post-electroporation, and the cell culture supernatants were pooled. The virus was then concentrated 20 times using 10,000 MWCO spin columns (Millipore) as instructed by the manufacturer, and stored in aliquots (at -70°C). To increase virus infectivity, Huh-7.5 reporter cells were infected with concentrated virus stocks at a MOI of 0.5 TCID50/cell, and the cells were passaged to obtain the working viral stock HCV p0. The infection of Huh-7.5 cells with HCV p0 can be sustained for at least 100 serial passages [39]. For titration of HCV infectivity, serially diluted cell culture supernatants were applied to Huh-7.5 cells and 3 days post-infection the cells were washed with PBS, fixed with ice-cold methanol, and stained using anti-NS5A monoclonal antibody 9E10, as previously described [39,40].
Treatment with favipiravir (T-705)
A solution of T-705 (Atomax Chemicals Co. Ltd) was prepared at a concentration of 20 mM in H2O. It was sterilized by filtration, and stored at –70°C. Prior to use, the stock solution was diluted in DMEM to reach the desired concentration. Huh-7.5 reporter cells were pretreated with the appropriate drug concentrations (or with DMEM without drug) during 16 h prior to infection. Then, 4 x 105 Huh-7.5 reporter cells were infected (or mock infected) with 1.2 x 104 TCID50 of HCV p0; the adsorption time was 5 h, and the infection continued for 72 to 96 h in the absence or presence of T-705. For successive viral passages, 4 x 105 Huh-7.5 reporter cells were infected with 0.5 ml of the supernatant from the previous infection; the MOI ranged between 0.6 and 5 x 10−5 TCID50/cell; each MOI can be calculated from the infectivity values given for each experiment.
Toxicity assays
The CC50 of T-705 was measured by seeding 96-well plates with Huh-7.5 cells to 70% confluence and exposing the cells to a range of T-705 concentration for up to 142 h. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was added to each well at a final concentration of 500 μg/ml; 4 h later crystals were dissolved in 100 μl of DMSO and the O.D. measured at 550 nm; 50% cytotoxicity was calculated from four different determinations as previously described [38].
Inhibitory concentration
The IC50 of T-705 was calculated relative to the progeny infectivity of the untreated controls (defined as 100% infectivity), as described previously [41,42]; determinations were carried out in triplicate.
RNA extraction, cDNA synthesis, and PCR amplification for Sanger nucleotide sequencing
Intracellular viral RNA was extracted from infected cells using the Qiagen RNeasy kit according to the manufacturer’s instructions (Qiagen, Valencia, CA, USA). RT-PCR amplification was carried out using AccuScript (Agilent), as specified by the manufacturers. NS5B genomic region was amplified using the specific oligonucleotides Jc1-NS5B-F1 (5’-TGGTCTACTTGCTCCGAGGAGGAC-3’) and Jc1-NS5B-R4 (5’-AGTTAGCTATGGAGTGTACCTAG-3’). Nucleotide sequences of genomic HCV RNA were determined using the 23 ABI 3730XLS sequencer. To evaluate the complexity of mutant spectra, HCV RNA was extracted as described above and subjected to RT-PCR to amplify the NS5B-coding region as previously described [39]. Amplification products were analyzed by agarose gel electrophoresis using HindIII-digested Ф-29 DNA as molar mass standard. Negative controls (amplifications in the absence of RNA) were included in parallel to ascertain the absence of contamination by template nucleic acids. To ensure an excess of template in the RT-PCR amplifications for quasispecies analysis, and to avoid complexity biases due to redundant amplifications of the same initial RNA templates, amplifications were carried out with template preparations diluted 1:10, 1:100 and 1:1000; only when at least the 1:100 diluted template produced a visible DNA band was molecular cloning pursued using the DNA amplified from undiluted template [43]. Controls to ascertain that mutation frequencies were not affected by the basal error rate during amplification have been previously described [44].
Ultra deep sequencing
For the ultra deep sequencing (UDPS) analysis (GS-Junior platform, 454 Life Sciences-Roche), reverse transcription (RT) was performed for 60 min at 40°C using Accuscript High Fidelity Reverse Transcriptase (Agilent) with a specific oligonucleotide covering the NS5A region. The products were then subjected to a PCR using Pfu Ultra II Fusion HS DNA polymerase (Agilent); the primers were composed of a specific sequence and a universal M13 primer, either upstream or downstream of the specific sequence (S1 Table). For the PCR, 5 μl of reverse transcription product were mixed with 5 μl of 10X buffer, 0.8 mM of dNTPs, 2 ng/μl of each sense and antisense primer. The initial denaturing step was at 95°C for 1 min, and it was followed by 40 cycles of a denaturing step at 95°C for 20 seconds, annealing at 60°C for 20 seconds, extension at 72°C for 1 min, and then a final extension at 72°C for 5 min.
The PCR products were then subjected to a nested PCR using Pfu Ultra II Fusion HS DNA polymerase (Agilent). The primers were composed of a complementary universal M13 primer, upstream or downstream followed by a Roche’s Validated Multiplex Identifier (MID) with oligonucleotide A or B (supplier nomenclature) at the 5’ or 3’ end of the upstream or downstream primer, respectively. For the PCR, 5 μl DNA of the previous PCR amplification mixture was added to 5 μl of a mixture containing 0.8 mM of dNTPs, 0.4 μM of sense and antisense PCR primers. The initial denaturing step was at 95°C for 1 min, and it was followed by 15 cycles of a denaturing step at 95°C for 20 seconds, annealing at 60°C for 30 seconds, extension at 72°C for 1 min, and then a final extension at 72°C for 5 min. The PCR products were purified (QIAquick Gel Extraction Kit), quantified (Pico Green Assay), and analyzed for quality (Bioanalyzer) prior to the UDPS procedure. Negative controls (without template RNA) were run in parallel to ascertain absence of contamination with undesired templates.
Data treatment methods in ultra deep sequencing
The fasta file obtained from the 454/GS-Junior system was subjected to demultiplexing and quality filtering as previously described [45,46]. The haplotypes common to the forward and reverse strand with abundances 0.1% or higher in each strand were considered established haplotypes. The post-filter coverage of each amplicon, ranged from 4566 to 9807 reads, median 7874 and standard deviation 2075. To balance biases, the amplicons were down sampled (DS) to a common size of 4500 reads (coverage of the smallest sample), and the resulting frequencies were subjected to fringe trimming (FT), excluding haplotypes with estimated frequencies below 0.2% with 95% confidence; this procedure yielded the DSFT haplotypes [47,48].
Diversity indices were computed using the DSFT haplotypes. A set of incidence-based indices (number of haplotypes, number of mutations, and number of polymorphic sites), abundance-based indices (Shannon entropy, Gini-Simpson index, and Hill numbers of order 1,2 and infinity), functional incidence-based indices (Mfe, FAD and ^πe), and functional abundance-based indices (Mf minimum, Mfm and ^π) were calculated for each amplicon as previously described [47](S1 Fig). Standard deviations and confidence intervals were computed by a semiparametric bootstrap, where the haplotype frequencies are the parameters of a multinomial distribution. Each multinomial resample (2000 cycles of bootstrap) was then subjected to DSFT and the resulting haplotypes and frequencies were used to calculate diversity indices. The standard deviations were calculated as the standard deviation of the bootstrap values obtained for each index, and an approximate 95% confidence interval (CI) was computed as the basic bootstrap CI [49,50]. P-values were computed as the number of bootstrap value differences larger or equal than the observed difference; for this purpose, 10,000 bootstrap cycles were performed. The observed diversity differences were calculated with the full set of diversity indices using the DSFT haplotypes of the two amplicons to be compared. The null hypothesis is that favipiravir had no effect, that is all reads might belong to the same quasispecies; the alternative hypothesis is that favipiravir is mutagenic and increases quasispecies complexity. The null distribution is the pool of all haplotypes, prior to the DSFT procedure, with corresponding frequencies for sequences obtained in absence and presence of favipiravir. As most of the observed values of diversity lie far beyond the null distribution (see S2 Fig with boxplots), the bootstrap p-values are a conservative upper bound. An alternative approach was to consider the asymptotic normality of the difference of diversity values obtained in the bootstrap (S2 Fig), and to calculate a p-value from a normal distribution with mean and standard deviation as estimated by the bootstrap itself. Both sets of p-values were multitest-adjusted by the Bonferroni correction [51] to take into account that the full set of diversity indices was simultaneously tested. The new sequences derived from this study can be found as S3 Fig.
Quantification of HCV RNA
Real time quantitative RT-PCR was carried out using the Light Cycler RNA Master SYBR Green I kit (Roche), according to the manufacturer’s instructions, as previously described [52]. The 5’-UTR non-coding region of the HCV genome was amplified using as primers oligonucleotide HCV-5UTR-F2 (5’- TGAGGAACTACTGTCTTCACGCAGAAAG; sense orientation; the 5’ nucleotide corresponds to genomic residue 47), and oligonucleotide HCV-5UTR-R2 (5’- TGCTCATGGTGCACGGTCTACGAG; antisense orientation; the 5’ nucleotide corresponds to genomic residue 347). Quantification was relative to a standard curve obtained with known amounts of HCV RNA, synthesized by in vitro transcription of plasmid GNN DNA. The specificity of the reaction was monitored by determining the denaturation curve of the amplified DNAs. Negative controls (without template RNA and RNA from mock-infected cells) were run in parallel with each amplification reaction, to ascertain absence of contamination with undesired templates.
Results
Inhibition of hepatitis C virus replication in hepatoma cells by T-705
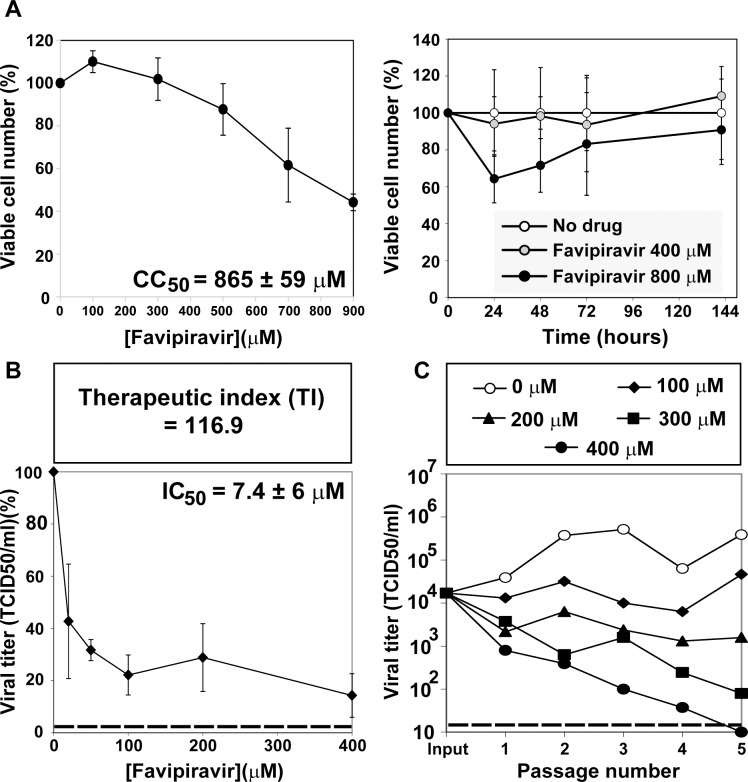
The cytotoxicity of T-705 for human hepatoma Huh-7.5 cells was quantified in experiments of exposure of different drug concentrations to the cells for a fixed time, or two drug concentrations for variable times, up to 142 h. The T-705 concentration that reduced cell viability by 50% (CC50) was 865 ± 59 μM (Fig 1A), and the T-705 concentration that produced a 50% decrease in infectious progeny production (IC50) of HCV p0 was IC50 = 7.4 ± 6 μM (Fig 1B). These values yield a therapeutic index (TI = CC50 / IC50) of 116.9. The inhibition was sustained over at least five serial passages of the virus, in a dose-dependent manner (Fig 1C). The differences in progeny production in the absence and presence of T-705 at 200 μM, 300 μM and 400 μM concentration were statistically significant over the five passages (p = 0.007 for 200 μM, p = 0.0004 for 300 μM and p <0.0001 for 400 μM; ANOVA test). No infectivity was rescued when subjecting the cell culture supernatant from passage five in the presence of 400 μM T-705 to three blind passages in the absence of drug. Thus, T-705 is a potent inhibitor of HCV during replication in Huh-7.5 cells that can lead to virus extinction.
Fig 1. Cytotoxicity for Huh-7.5 cells, and inhibition of HCV progeny production by T-705.
(A) Determinations of cytotoxic concentration 50 (CC50) and the effect of 400 μM and 800 μM T-705 on cell viability, (B) drug concentration required for 50% inhibition, or inhibitory concentration 50 (IC50); experiments were carried out in triplicate. Values and standard deviations were calculated using the program Sigma Plot. (C) Huh-7.5 reporter cells were infected with HCV p0 at a MOI of 0.03 TCID50/cell (4 x 105 Huh-7.5 cells infected with 1.2 x 104 TCID50), in the absence or presence of the T-705 concentrations indicated in the box. Infections with HCV GNN were carried out in parallel (negative control). Experimental conditions for cell growth, HCV infection, determination of cell viability, HCV infectivity, and serial virus passages are described in Materials and Methods. Discontinuous horizontal lines indicate the limit of detection.
Mutagenic activity of T-705 for hepatitis C virus
To investigate if the inhibition of HCV replication might be associated with a mutagenic activity for HCV, the mutant spectra of the virus passaged three times in the absence or presence of T-705 was analyzed, and several diversity indices were calculated [47]. Three amplicons of NS5A were analyzed by ultra-deep pyrosequencing (Table 1 and S1 and S2 Figs). All indices, except those denoted as being at entity level, increased significantly (p<0.01; bootstrap) when T-705 was present during replication, suggesting a mutagenic activity of this compound on HCV. Variation of indices at the entity level (Mfe and ˄πe) would require an increase in the number of mutations per haplotype induced by T-705. Despite T-705 increasing the number of genomes with any number of mutations, an increase in the number of mutations per haplotype was not observed.
Table 1. Ultra deep pyrosequencing analysis of HCV p0 subjected to three passages in the absence or presence of 400 μM favipiravira.
| NS5A ampliconb | ||||
|---|---|---|---|---|
| Parameter or diversity indexc | Favipiravir | A1 (6152–6454) | A2 (6446–6767) | A4 (6910–7252) |
| Number of nucleotides sequenced | - | 2,366,733 | 2,589,202 | 1,477,987 |
| + | 2,156,148 | 2,895,746 | 1,459,465 | |
| Number of haplotypesd | - | 5 (2/2/0/0) | 9 (7/0/1/0) | 27 (23/3/0/0) |
| + | 30 (26/3/0/0) | 33 (30/2/0/0) | 66 (55/8/1/1) | |
| Number of different mutations | - | 6 | 10 | 24 |
| + | 30 | 34 | 64 | |
| Number of total mutations | - | 679 | 1,318 | 1,023 |
| + | 1,788 | 2,590 | 2,234 | |
| Number of polymorphic sites | - | 6 | 9 | 24 |
| + | 29 | 33 | 64 | |
| Dominant haplotype abundance (%) | - | 92.36 | 84.90 | 78.32 |
| + | 76.01 | 72.18 | 55.14 | |
| ˄HS | - | 0.3374 | 0.6640 | 1.1820 |
| + | 1.2367 | 1.3756 | 2.5032 | |
| ˄HGS, sample-based Gini-Simpson index | - | 0.1438 | 0.2731 | 0.3836 |
| + | 0.4154 | 0.4682 | 0.6918 | |
| 1D (p), Hill numbers | - | 1.40 | 1.94 | 3.26 |
| + | 3.44 | 3.96 | 12.22 | |
| 2D (p), Hill numbers | - | 1.17 | 1.38 | 1.62 |
| + | 1.71 | 1.88 | 3.24 | |
| ∞D (p), Hill numbers | - | 1.08 | 1.18 | 1.28 |
| + | 1.32 | 1.38 | 1.81 | |
| Mfe, mutation frequency, entity level | - | 4.0 x 10−3 | 3.4 x 10−3 | 3.1 x 10−3 |
| + | 3.5 x 10−3 | 3.2 x 10−3 | 3.4 x 10−3 | |
| FAD, Functional Attribute Diversity | - | 0.16 | 0.49 | 4.33 |
| + | 6.09 | 6.75 | 29.25 | |
| ˄πe, sample nucleotide diversity, entity level | - | 7.9 x 10−3 | 6.8 x 10−3 | 6.2 x 10−3 |
| + | 7.0 x 10−3 | 6.4 x 10−3 | 6.8 x 10−3 | |
| Mf min, minimum mutation frequency | - | 2.5 x 10−6 | 3.9 x 10−6 | 1.6 x 10−5 |
| + | 1.4 x 10−5 | 1.2 x 10−5 | 4.4 x 10−5 | |
| Mf max (Mfm), maximum mutation frequency | - | 2.9 x 10−4 | 5.1 x 10−4 | 6.9 x 10−4 |
| + | 8.3 x 10−4 | 8.9 x 10−4 | 1.5 x 10−3 | |
| ˄π, sample nucleotide diversity | - | 5.5 x 10−4 | 9.8 x 10−4 | 1.3 x 10−3 |
| + | 1.6 x 10−3 | 1.7 x 10−3 | 3.0 x 10−3 | |
aThe populations analyzed correspond to passage 3 of the infections described in Fig 1.
bThe HCV genome residue numbering corresponds to the JFH-1 genome (accession number #AB047639). The number of reads on which the parameters were calculated was 4,500 for each amplicon. Procedures are described in Materials and Methods. Mutation types are summarized in Fig 3 and their position in the HCV genome and deduced amino acid substitutions are given in S2 and S3 Tables.
cDiversity indices are defined and calculated as described in [47].
dIn parenthesis the number of haplotypes with one, two, three, and four mutations is given; no haplotypes with a higher number of mutations were found.
To obtain an independent confirmation of the mutagenic activity of T-705 on HCV, the mutant spectrum of the polymerase NS5B-coding region of the same populations was analyzed by molecular cloning and Sanger sequencing. The results (Table 2) indicate a mutagenic activity of T-705, with significant increases in mutation frequencies (p<0.0001;χ2 test). Thus, T-705 is mutagenic for HCV. No infectivity was detected in the cell culture supernatant of HCV that was passaged five times in the presence of 400 μM T-705.
Table 2. Quasispecies analysis of the NS5B-coding region of hepatitis C virus population HCV p3 in the absence and presence of favipiravira.
| Parameter or diversity indexb | Favipiravir | NS5B |
|---|---|---|
| Number of nucleotides sequenced | - | 31,968 |
| + | 35,520 | |
| Number of haplotypesc | - | 9 (2/3/3/0/0/0/0/0/0) |
| + | 20 (1/0/6/5/3/1/3/0/1) | |
| Number of different mutations | - | 17 |
| + | 69 | |
| Number of total mutations | - | 17 |
| + | 71 | |
| Number of polymorphic sites | - | 17 |
| + | 69 | |
| ˄HS | - | 1.8334 |
| + | 2.9957 | |
| ˄HGS, sample-based Gini-Simpson index | - | 0.7059 |
| + | 1.0000 | |
| 1D (p), Hill numbers | - | 6.25 |
| + | 20 | |
| 2D (p), Hill numbers | - | 3.0 |
| + | 20 | |
| ∞D (p), Hill numbers | - | 1.8 |
| + | 20 | |
| Mfe, mutation frequency, entity level | - | 1.0 x 10−3 |
| + | 2.5 x 10−3 | |
| FAD, Functional Attribute Diversity | - | 0.15 |
| + | 1.51 | |
| ˄πe, sample nucleotide diversity, entity level | - | 2.1 x 10−3 |
| + | 4.0 x 10−3 | |
| Mf min, minimum mutation frequency | - | 5.3 x 10−4 |
| + | 2.0 x 10−3 | |
| Mf max (Mfm), maximum mutation frequency | - | 5.3 x 10−4 |
| + | 1.9 x 10−3 | |
| ˄π, sample nucleotide diversity | - | 1.1 x 10−3 |
| + | 4.0 x 10−3 |
aThe populations analyzed correspond to passage 3 of the infections described in Fig 1. The NS5B residues analyzed are 7667–9442. The HCV genome residue numbering corresponds to the JFH-1 genome (accession number #AB047639). Mutation types are summarized in Fig 3 and their position in the HCV genome and deduced amino acid substitutions are given in S3 Table.
bDiversity indices are defined and calculated as described in [47].
cIn parenthesis the number of haplotypes with one, two, three, four, five, six, seven, eight and nine mutations is given; no haplotypes with a higher number of mutations were found.
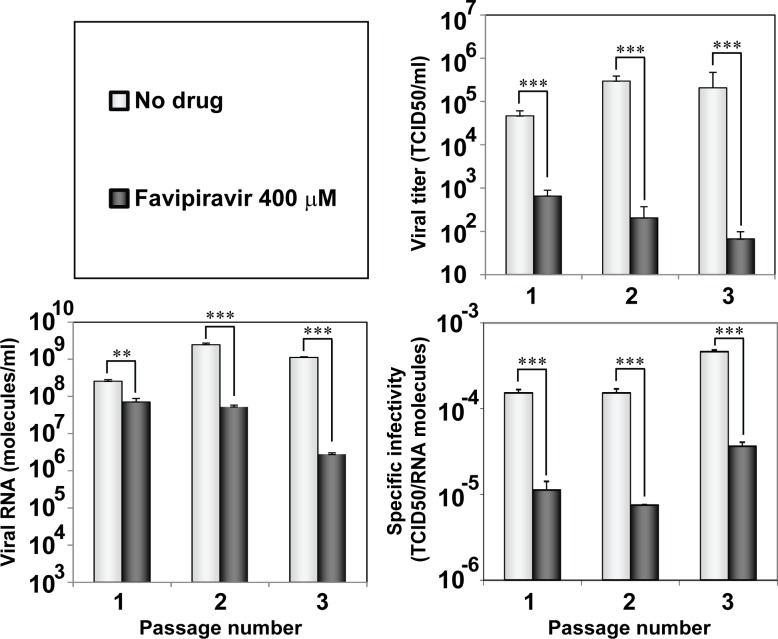
To confirm that loss of infectivity of HCV by T-705 followed a hallmark of lethal mutagenesis, the specific infectivity (the ratio between viral infectivity and the amount of genomic viral RNA) of the virus replicating at a concentration of 400 μM T-705 was calculated (Fig 2). A 13-fold to 20-fold decrease of specific infectivity occurred over the first three passages of treatment with the drug (that are those in which measurement of infectivity and viral RNA in samples of cell culture supernatant were reliable); differences were statistically significant between values in the absence and presence of the drug (p<0.0001 for passages 1 and 3, and p = 0.0001 for passage 2; t-test). In addition, treatment with T-705 did not alter the consensus genomic nucleotide sequence, again an observation made during lethal mutagenesis of viruses [53,54].
Fig 2. Effect of T-705 on the specific infectivity of HCV.
Huh-7.5 reporter cells were infected with HCV p0 at an initial MOI of 0.03 TCID50/cell, in the absence or presence of 400 μM T-705; infection with GNN was performed as negative control. The infectivity values (upper right panel) have been redrawn from those shown in Fig 1(C). Extracellular viral RNA was measured by quantitative RT-PCR (bottom letf panel). Specific infectivities (bottom right panel) were calculated by dividing the infectivity by the amount of viral RNA. Statistically significant differences are indicated by three asterisks [(p<0.001); one way analysis of variance]. The range of specific infectivities determined at passages 1, 2 and 3 was 1.3 x 10−4 to 2.2 x 10−5 TCID50/RNA molecules for T-705 100 μM, 1.1 x 10−5 to 2.7 x 10−5 for T-705 200 μM, and 8.0 x 10−5 to 3.1 x 10−6 for T-705 300 μM. Procedures are described in Materials and Methods.
Mutational bias evoked by T-705
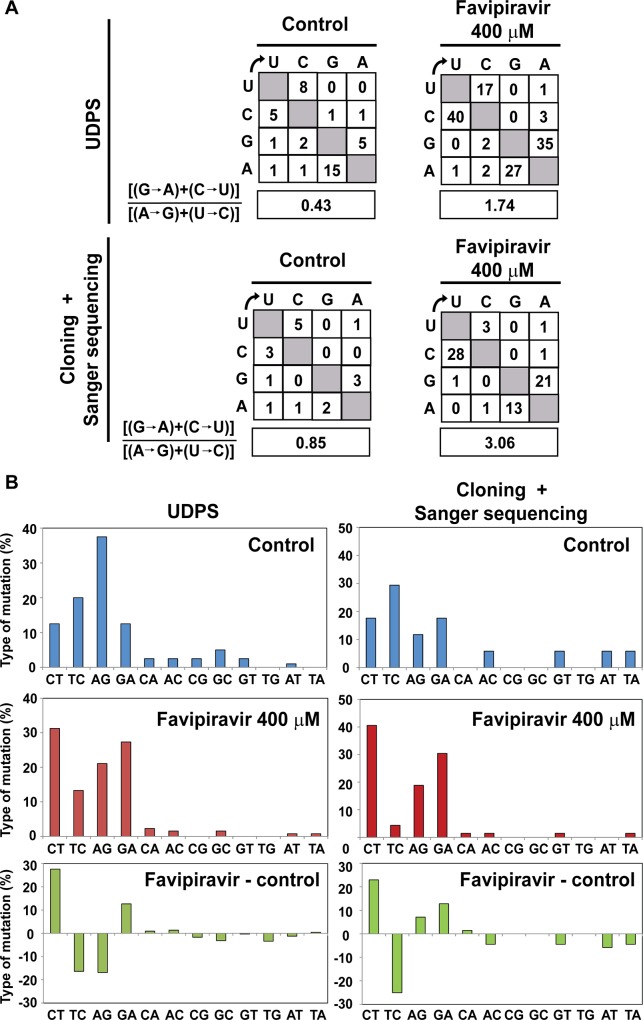
The types of the different mutations at the NS5A and NS5B regions analyzed in the populations passaged in the absence and presence of T-705 (S2, S3 and S4 Tables) indicate a predominance of C→ U and G → A transitions, with a 3.6—to 4.0- fold increase in the ratio [(G → A) + (C→ U)] / [(A → G) + (U→ C)] ratio, associated with replication in the presence of T-705 (Fig 3). Thus, T-705 is a potent mutagenic agent for HCV that produces a bias in favor of G → A and C→ U transitions preceding loss of infectivity.
Fig 3. Mutational spectrum induced by favipiravir on hepatitis C virus.
(A) Matrix of mutation types found in the NS5A-coding region of HCV p0 passaged three times in absence or presence of favipiravir (400 μM), based on haplotypes of three amplicons determined by UDPS, as detailed in Table 1. Below, matrix of mutation types found in the NS5B-coding region of the same viral populations, based on molecular cloning and Sanger sequencing, as detailed in Table 2. The box below each matrix quantifies the mutational bias, according to the transition type ratio shown on the left. (B) Percentage of mutation types considering 100% as the sum of all mutation types in the same populations and genomic regions analyzed in (A). The bottom panels indicate the difference in mutation types between the population passaged in presence and absence (control) of favipiravir. Procedures are detailed in Materials and Methods.
Discussion
In the present report we have shown that favipiravir (T-705) is a potent inhibitor of HCV replication in Huh-7.5 cells, with a therapeutic index (TI) value of 116.9 which is seven to nine times the value obtained previously for ribavirin in two independent determinations in the same virus-host system (TI = 12.8 [42]; TI = 15.6 [55]). According to the IC50 values, the inhibitory activity of T-705 for HCV is comparable to the activity exhibited against other RNA viruses [14,16,31,34,56]. The TI values for different, non-mutagenic anti-HCV agents using the same HCV p0 and Huh-7.5 cell culture system vary by orders of magnitude: 252.9, 602.4, >2000, 1.49x106 and >2x108 for telaprevir, cyclosporine A, sofosbuvir, daclatasvir and IFN-α, respectively [41,55,57]. Therapeutic efficacy may be different in vivo than in cell culture. Despite differences of values measured with HCV p0 in Huh-7.5 cells, each of the inhibitors tested has had a significant role in anti-HCV therapy.
The toxicity of T-705 for Huh-7.5 cells and the calculated CC50 value (Fig 1A) exclude that virus extinction (Fig 1C) was due to toxicity of T-705 for Huh-7.5 cells. The evidence that T-705 can act as a lethal mutagen includes also an increase in mutation frequency associated with a bias in favor of G→A and C→U transitions, a decrease of specific infectivity, and invariance of the consensus sequence. These are features typical of lethal mutagenesis, as previously established with several viruses and mutagenic nucleotide analogues (reviewed in [58,59]). The mutational bias evoked by T-705 is similar to that induced by ribavirin on HCV [42] and on FMDV [43,60,61]. The movement of viral genomic sequences towards extreme regions of sequence space is a critical deleterious event preceding extinction [60,62]. Our previous studies with FMDV have shown that a class of ribavirin- or 5-fluorouracil-resistant mutants harboring amino acid replacements in the viral polymerase or in non-structural protein 2C has as its mechanism of action to counteract the mutational bias induced by the mutagen [63,64]. The present study adds the important human pathogen HCV to a growing list of viral pathogens reported to be mutagenized by T-705 [34,35]. It is not clear whether the inhibition of HCV p0 replication by T-705 is exclusively a consequence of its mutagenic activity or T-705 has an inhibitory activity independent of its mutagenic activity, as previously documented for 5-fluorouracil acting on FMDV [65].
Despite the success of direct acting antiviral agents (DAAs) that can reach sustained response levels exceeding 90% [66–82], we have identified five reasons that justify exploration of new antiviral compounds to treat HCV infections: (i) There are patients who do not eliminate the virus with the new DAAs, in particular those infected with the so called “hard to treat” HCV genotypes such as genotype 3 HCV [83–85]. (ii) Inhibitor-escape mutants have been described for virtually every anti-HCV agent used alone or in combination, and their frequency is expected to increase with the extended use of new treatments, as judged by the pattern observed with HIV-1 during the AIDS pandemic. Selection of resistant mutants within individual patients or their increase during the epidemiological spread of the virus will require drugs with new mechanisms of action (reviewed in [58,59]). (iii) There are reports of patients who fail therapy and that in the breakthrough virus no resistance mutations to the drugs used in the treatment are detected [86–89]. One possibility to explain these clinical observations is that high fitness or a fitness-associated trait confers resistance to several anti-HCV agents [55,57]; high fitness viruses may be more sensitive to lethal mutagenesis than to standard inhibitors, a possibility that we are currently investigating. (iv) A recent report indicates that DAA-based treatments may induce tumor recurrence in about 27% of HCV-infected patients previously treated successfully of HCV-associated liver cancer [90]. If extended to other patient cohorts, the possibility of cancer recurrence may impose a limitation for the use of some DAAs. Although the recurrence mechanism is not known, tumor recurrence was not reported during the years in which patients were treated with pegylated interferon-alpha and ribavirin (pegIFN-α+Rib), the standard of care one decade ago. Although ribavirin has several mechanisms of activity [91–98], genetic and clinical evidences suggest that mutagenesis may be part ot its detrimental activity for HCV in vivo [8,9]. The possibility that lethal mutagens may extinguish HCV without the side effect of tumor recurrence is worth exploring. (v) The benefits of a treatment option depend on the HCV genotype. In the present DAA era, genotype 3 is a “hard to treat” HCV while sustained response rates of 65% to 80% were achieved after 24-week treatment with pegIFN-α+Rib (comparative efficacies for different HCV genotypes with various treatments described in [99–102], among other examples). The quasispecies dynamics of HCV [59,103–105] helps interpreting not only the existence of genotypes but also their origin and complexity. Genotypes are sets of related genomes that accumulate at some regions of sequence space due to a combination of adequate replicative and epidemiological fitness [58]. Given that antiviral efficacy is multifactorial −involving host and viral traits− it is expected that different treatments will not exhibit the same efficacy across genotypes. According to our model studies in cell culture, replicative fitness –one of the factors likely involved in genotype differentiation− is also a determinant of inhibitor efficacy [55,57]. Therefore, the available evidence suggests that if T-705 or other viral mutagens were licensed for a clinical application, it would not be possible to predict their efficacy in vivo, or their relative efficacy against the different existing HCV genotypes, as well as new genotypes likely to come. Assuming, however, that T-705 and ribavirin have a similar anti-HCV activity in the clinic, it is likely that the efficacy of T-705 would require its use in combination with other antiviral agents.
An advantage of considering T-705 as a potential anti-HCV inhibitor is that the drug has already undergone advanced clinical trials of efficacy and safety for treatment of other human viral diseases such as uncomplicated influenza in adults (US National Institutes of Health, identifier NCT02008344) and Ebola infection (JIKI trial, US National Institutes of Health, identifier NCT02662855 [106]). Thus, T-705 use for HCV treatment would be an example of drug repurposing, increasingly practiced in pharmacology to accelate testing and approval of drugs for new indications.
In summary, given the clinical evidence of still incomplete efficacy of the DAA-based treatments, of DAA-promoted hepatocarcinoma recurrence in patients previously subjected to successful tumor resection and treatment, and the continuing HCV diversification that will necessitate new treatments for optimal efficacy, favipiravir and other lethal mutagens may find a new role in anti-HCV treatment.
Supporting Information
The diversity indices are abbreviated in ordinate (Hpl, number of haplotypes; nMuts, number of different mutations; PolySites, number of polymorfic sites; Mpct; dominant haplotype abundance; Shannon, ^HS; GiniS, ^HGS, sample-based Gini-Simpson index; D1, D2, Dinf, Hill numbers; Mfe, mutation frequency, entity level; FAD, Functional Attribute Diversity; Pi.e, sample nucleotide diversity, entity level; Mf min, minimum mutation frequency; Mf.max, maximum mutation frequency; Pi, sample nucleotide diversity, and their calculation is described in reference [47] of the main text. Standard deviation interval (left column), and basic bootstrap with 95% confidence intervals (CI)(right column) are shown for each index.
(PDF)
The diversity index is given in the abscissa, with the same abbreviations used in S1 Fig. Density means the probability density of the corresponding distribution. The panels on the right indicate the boxplot of null distribution bootstraped diversity differences, with observed difference as red dot and red dash-dot line. The distance from this line to the boxplot, in terms of boxplot width, is an illustration of the low p-values obtained. A1, A2 and A4 mean amplicons 1, 2 and 4, respectively.
(PDF)
(ZIP)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We thank E. Moreno for help with the statistical analyses.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work in Madrid was supported by grants BFU-2011-23604, SAF2014-52400-R, S2013/ABI-2906 (PLATESA from Comunidad de Madrid/FEDER) and Fundación R. Areces. The work in Barcelona was funded by Instituto de Salud Carlos III, grants PI13-00456 and PI15-00829, cofinanced by the European Regional Development Fund (ERDF). CIBERehd (Centro de Investigación en Red de Enfermedades Hepáticas y Digestivas) is funded by Instituto de Salud Carlos III. C.P. is supported by the Miguel Servet program of the Instituto de Salud Carlos III (CP14/00121) cofinanced by the European Regional Development Fund (ERDF). C.M.R. is supported by a grant from the U.S. Public Health Service, National Institute of Allergy and Infectious Diseases, R01 AI099284. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Eigen M, Schuster P (1979) The hypercycle. A principle of natural self-organization Berlin: Springer. [DOI] [PubMed] [Google Scholar]
- 2.Loeb LA, Essigmann JM, Kazazi F, Zhang J, Rose KD, Mullins JI (1999) Lethal mutagenesis of HIV with mutagenic nucleoside analogs. Proc Natl Acad Sci USA 96: 1492–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graci JD, Cameron CE (2008) Therapeutically targeting RNA viruses via lethal mutagenesis. Future Virol 3: 553–566. 10.2217/17460794.3.6.553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dapp MJ, Patterson SE, Mansky LM (2013) Back to the future: revisiting HIV-1 lethal mutagenesis. Trends Microbiol 21: 56–62. 10.1016/j.tim.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holland JJ, Domingo E, de la Torre JC, Steinhauer DA (1990) Mutation frequencies at defined single codon sites in vesicular stomatitis virus and poliovirus can be increased only slightly by chemical mutagenesis. J Virol 64: 3960–3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domingo E, Schuster P (2016) Quasispecies: from theory to experimental systems. Current Topics in Microbiology and Immunology. Vol. 392. Springer. [PubMed] [Google Scholar]
- 7.Schuster P (2016) Quasispecies on fitness landscapes. In: E. Domingo and P. Schuster, eds. Quasispecies: From Theory to Experimental Systems. Curr Top Microbiol Immunol 392: 61–120. [DOI] [PubMed]
- 8.Dietz J, Schelhorn SE, Fitting D, Mihm U, Susser S, Welker MW, et al. (2013) Deep sequencing reveals mutagenic effects of ribavirin during monotherapy of hepatitis C virus genotype 1-infected patients. J Virol 87: 6172–6181. 10.1128/JVI.02778-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuevas JM, Gonzalez-Candelas F, Moya A, Sanjuan R (2009) Effect of ribavirin on the mutation rate and spectrum of hepatitis C virus in vivo. J Virol 83: 5760–5764. 10.1128/JVI.00201-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Furuta Y, Takahashi K, Fukuda Y, Kuno M, Kamiyama T, Kozaki K, et al. (2002) In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother 46: 977–981. 10.1128/AAC.46.4.977-981.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuta Y, Takahashi K, Kuno-Maekawa M, Sangawa H, Uehara S, Kozaki K, et al. (2005) Mechanism of action of T-705 against influenza virus. Antimicrob Agents Chemother 49: 981–986. 10.1128/AAC.49.3.981-986.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto K, Ohashi S, Yamazoe R, Takahashi K, Furuta Y (2006) The inhibition of FMD virus excretion from the infected pigs by an antiviral agent, T-1105. Appendix 64 Session of the Research Group of the Standing Technical Committee of the Eu FMD.
- 13.Furuta Y, Takahashi K, Maekawa M, Maegawa H, Egawa H, Terashima N (2004) T-1106, a novel pyrazine nucleoside, hepatitis C virus polymerase inhibitor. In: Abstr 44th Intersci Conf Antimicrob Agents Chemother, pp 199–200, Abstr F-487.
- 14.Gowen BB, Wong MH, Jung KH, Sanders AB, Mendenhall M, Bailey KW, et al. (2007) In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother 51: 3168–3176. 10.1128/AAC.00356-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrey JD, Taro BS, Siddharthan V, Wang H, Smee DF, Christensen AJ, et al. (2008) Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res 80: 377–379. 10.1016/j.antiviral.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furuta Y, Takahashi K, Shiraki K, Sakamoto K, Smee DF, Barnard DL, et al. (2009) T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res 82: 95–102. 10.1016/j.antiviral.2009.02.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smee DF, Hurst BL, Egawa H, Takahashi K, Kadota T, Furuta Y (2009) Intracellular metabolism of favipiravir (T-705) in uninfected and influenza A (H5N1) virus-infected cells. J Antimicrob Chemother 64: 741–746. 10.1093/jac/dkp274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julander JG, Shafer K, Smee DF, Morrey JD, Furuta Y (2009) Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother 53: 202–209. 10.1128/AAC.01074-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gowen BB, Wong MH, Jung KH, Smee DF, Morrey JD, Furuta Y (2010) Efficacy of favipiravir (T-705) and T-1106 pyrazine derivatives in phlebovirus disease models. Antiviral Res 86: 121–127. 10.1016/j.antiviral.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiso M, Takahashi K, Sakai-Tagawa Y, Shinya K, Sakabe S, Le QM, et al. (2010) T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci U S A 107: 882–887. 10.1073/pnas.0909603107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mendenhall M, Russell A, Smee DF, Hall JO, Skirpstunas R, Furuta Y, et al. (2011) Effective oral favipiravir (T-705) therapy initiated after the onset of clinical disease in a model of arenavirus hemorrhagic Fever. PLoS Negl Trop Dis 5: e1342 10.1371/journal.pntd.0001342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gowen BB, Juelich TL, Sefing EJ, Brasel T, Smith JK, Zhang L, et al. (2013) Favipiravir (T-705) inhibits Junin virus infection and reduces mortality in a guinea pig model of Argentine hemorrhagic fever. PLoS Negl Trop Dis 7: e2614 10.1371/journal.pntd.0002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Safronetz D, Falzarano D, Scott DP, Furuta Y, Feldmann H, Gowen BB (2013) Antiviral efficacy of favipiravir against two prominent etiological agents of hantavirus pulmonary syndrome. Antimicrob Agents Chemother 57: 4673–4680. 10.1128/AAC.00886-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smee DF, Tarbet EB, Furuta Y, Morrey JD, Barnard DL (2013) Synergistic combinations of favipiravir and oseltamivir against wild-type pandemic and oseltamivir-resistant influenza A virus infections in mice. Future Virol 8: 1085–1094. 10.2217/fvl.13.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caroline AL, Powell DS, Bethel LM, Oury TD, Reed DS, Hartman AL (2014) Broad spectrum antiviral activity of favipiravir (T-705): protection from highly lethal inhalational Rift Valley Fever. PLoS Negl Trop Dis 8: e2790 10.1371/journal.pntd.0002790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scharton D, Bailey KW, Vest Z, Westover JB, Kumaki Y, Van Wettere A, et al. (2014) Favipiravir (T-705) protects against peracute Rift Valley fever virus infection and reduces delayed-onset neurologic disease observed with ribavirin treatment. Antiviral Res 104: 84–92. 10.1016/j.antiviral.2014.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada K, Noguchi K, Komeno T, Furuta Y, Nishizono A (2016) Efficacy of Favipiravir (T-705) in Rabies Postexposure Prophylaxis. J Infect Dis 213: 1253–1261. 10.1093/infdis/jiv586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Safronetz D, Rosenke K, Westover JB, Martellaro C, Okumura A, Furuta Y, et al. (2015) The broad-spectrum antiviral favipiravir protects guinea pigs from lethal Lassa virus infection post-disease onset. Sci Rep 5: 14775 10.1038/srep14775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westover JB, Sefing EJ, Bailey KW, Van Wettere AJ, Jung KH, Dagley A, et al. (2016) Low-dose ribavirin potentiates the antiviral activity of favipiravir against hemorrhagic fever viruses. Antiviral Res 126: 62–68. 10.1016/j.antiviral.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oestereich L, Rieger T, Ludtke A, Ruibal P, Wurr S, Pallasch E, et al. (2016) Efficacy of Favipiravir Alone and in Combination With Ribavirin in a Lethal, Immunocompetent Mouse Model of Lassa Fever. J Infect Dis 213: 934–938. 10.1093/infdis/jiv522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 100: 446–454. 10.1016/j.antiviral.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sangawa H, Komeno T, Nishikawa H, Yoshida A, Takahashi K, Nomura N, et al. (2013) Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother 57: 5202–5208. 10.1128/AAC.00649-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Z, Smith LK, Rajwanshi VK, Kim B, Deval J (2013) The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5'-triphosphate towards influenza A virus polymerase. PLoS ONE 8: e68347 10.1371/journal.pone.0068347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baranovich T, Wong SS, Armstrong J, Marjuki H, Webby RJ, Webster RG, et al. (2013) T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 87: 3741–3751. 10.1128/JVI.02346-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arias A, Thorne L, Goodfellow I (2014) Favipiravir elicits antiviral mutagenesis during virus replication in vivo. Elife 3: e03679 10.7554/eLife.03679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blight KJ, McKeating JA, Rice CM (2002) Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol 76: 13001–13014. 10.1128/JVI.76.24.13001-13014.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones CT, Catanese MT, Law LM, Khetani SR, Syder AJ, Ploss A, et al. (2010) Real-time imaging of hepatitis C virus infection using a fluorescent cell-based reporter system. Nat Biotechnol 28: 167–171. 10.1038/nbt.1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandamme A, Witvrouw M, Pannecouque C, Balzarini J, Van Laethem K, Schmit J, et al. (2000) Evaluating Clinical Isolates for Their Phenotypic and Genotypic Resistance Against Anti-HIV Drugs; Kinchington D, Schinazi R, editors. Totowa, NJ: Humana Press Inc. [DOI] [PubMed] [Google Scholar]
- 39.Perales C, Beach NM, Gallego I, Soria ME, Quer J, Esteban JI, et al. (2013) Response of hepatitis C virus to long-term passage in the presence of alpha interferon. Multiple mutations and a common phenotype. J Virol 87: 7593–7607. 10.1128/JVI.02824-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindenbach BD, Rice CM (2003) Evasive maneuvers by hepatitis C virus. Hepatology 38: 769–771. 10.1002/hep.510380327 [DOI] [PubMed] [Google Scholar]
- 41.Perales C, Beach NM, Gallego I, Soria ME, Quer J, Esteban JI, et al. (2013) Response of hepatitis C virus to long-term passage in the presence of alpha interferon: multiple mutations and a common phenotype. J Virol 87: 7593–7607. 10.1128/JVI.02824-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortega-Prieto AM, Sheldon J, Grande-Perez A, Tejero H, Gregori J, Quer J, et al. (2013) Extinction of hepatitis C virus by ribavirin in hepatoma cells involves lethal mutagenesis. PLoS One 8: e71039 10.1371/journal.pone.0071039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Airaksinen A, Pariente N, Menendez-Arias L, Domingo E (2003) Curing of foot-and-mouth disease virus from persistently infected cells by ribavirin involves enhanced mutagenesis. Virology 311: 339–349. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez G, Bosch A, Gomez-Mariano G, Domingo E, Pinto RM (2003) Evidence for quasispecies distributions in the human hepatitis A virus genome. Virology 315: 34–42. [DOI] [PubMed] [Google Scholar]
- 45.Gregori J, Esteban JI, Cubero M, Garcia-Cehic D, Perales C, Casillas R, et al. (2013) Ultra-Deep Pyrosequencing (UDPS) Data Treatment to Study Amplicon HCV Minor Variants. PLoS ONE 8: e83361 10.1371/journal.pone.0083361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramirez C, Gregori J, Buti M, Tabernero D, Camos S, Casillas R, et al. (2013) A comparative study of ultra-deep pyrosequencing and cloning to quantitatively analyze the viral quasispecies using hepatitis B virus infection as a model. Antiviral Res 98: 273–283. 10.1016/j.antiviral.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 47.Gregori J, Perales C, Rodriguez-Frias F, Esteban JI, Quer J, Domingo E (2016) Viral quasispecies complexity measures. Virology 493: 227–237. 10.1016/j.virol.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 48.Gregori J, Salicru M, Domingo E, Sanchez A, Esteban JI, Rodriguez-Frias F, et al. (2014) Inference with viral quasispecies diversity indices: clonal and NGS approaches. Bioinformatics 30:1104–1111. [DOI] [PubMed] [Google Scholar]
- 49.Efron B, Tibshirani R (1994) An introduction to the bootstrap, Chapman & Hall/CRC, Boca Raton. [Google Scholar]
- 50.Davidson AC, Hinkley DV (1997) Bootstrap Methods and their applications, Cambridge University Press, Cambridge. [Google Scholar]
- 51.Dudoit S, van der Laan MJ (2008) Multiple testing procedures with applications to genomics Springer, New York. [Google Scholar]
- 52.Lindenbach BD (2009) Measuring HCV infectivity produced in cell culture and in vivo. Methods Mol Biol 510: 329–336. 10.1007/978-1-59745-394-3_24 [DOI] [PubMed] [Google Scholar]
- 53.Grande-Pérez A, Gómez-Mariano G, Lowenstein PR, Domingo E (2005) Mutagenesis-induced, large fitness variations with an invariant arenavirus consensus genomic nucleotide sequence. J Virol 79: 10451–10459. 10.1128/JVI.79.16.10451-10459.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.González-López C, Gómez-Mariano G, Escarmís C, Domingo E (2005) Invariant aphthovirus consensus nucleotide sequence in the transition to error catastrophe. Infection Genetics and Evolution 5: 366–374. [DOI] [PubMed] [Google Scholar]
- 55.Sheldon J, Beach NM, Moreno E, Gallego I, Pineiro D, Martinez-Salas E, et al. (2014) Increased replicative fitness can lead to decreased drug sensitivity of hepatitis C virus. J Virol 88: 12098–12111. 10.1128/JVI.01860-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rocha-Pereira J, Jochmans D, Dallmeier K, Leyssen P, Nascimento MS, Neyts J (2012) Favipiravir (T-705) inhibits in vitro norovirus replication. Biochem Biophys Res Commun 424: 777–780. 10.1016/j.bbrc.2012.07.034 [DOI] [PubMed] [Google Scholar]
- 57.Gallego I, Sheldon J, Moreno E, Gregori J, Quer J, Esteban JI, et al. (2016) Barrier-Independent, Fitness-Associated Differences in Sofosbuvir Efficacy against Hepatitis C Virus. Antimicrob Agents Chemother 60: 3786–3793. 10.1128/AAC.00581-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Domingo E (2016) Virus as Populations. Academic Press, Elsevier, Amsterdam. [Google Scholar]
- 59.Domingo E, Sheldon J, Perales C (2012) Viral quasispecies evolution. Microbiol Mol Biol Rev 76: 159–216. 10.1128/MMBR.05023-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perales C, Henry M, Domingo E, Wain-Hobson S, Vartanian JP (2011) Lethal mutagenesis of foot-and-mouth disease virus involves shifts in sequence space. J Virol 85: 12227–12240. 10.1128/JVI.00716-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sierra M, Airaksinen A, González-López C, Agudo R, Arias A, Domingo E (2007) Foot-and-mouth disease virus mutant with decreased sensitivity to ribavirin: implications for error catastrophe. J Virol 81: 2012–2024. 10.1128/JVI.01606-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grande-Pérez A, Lazaro E, Lowenstein P, Domingo E, Manrubia SC (2005) Suppression of viral infectivity through lethal defection. Proc Natl Acad Sci USA 102: 4448–4452. 10.1073/pnas.0408871102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agudo R, Ferrer-Orta C, Arias A, de la Higuera I, Perales C, Perez-Luque R, et al. (2010) A multi-step process of viral adaptation to a mutagenic nucleoside analogue by modulation of transition types leads to extinction-escape. PLoS Pathog 6: e1001072 10.1371/journal.ppat.1001072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agudo R, de la Higuera I, Arias A, Grande-Perez A, Domingo E (2016) Involvement of a joker mutation in a polymerase-independent lethal mutagenesis escape mechanism. Virology 494: 257–266. 10.1016/j.virol.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agudo R, Arias A, Pariente N, Perales C, Escarmis C, Jorge A, et al. (2008) Molecular characterization of a dual inhibitory and mutagenic activity of 5-fluorouridine triphosphate on viral RNA synthesis. Implications for lethal mutagenesis. J Mol Biol 382: 652–666. 10.1016/j.jmb.2008.07.033 [DOI] [PubMed] [Google Scholar]
- 66.Keating GM (2014) Sofosbuvir: a review of its use in patients with chronic hepatitis C. Drugs 74: 1127–1146. 10.1007/s40265-014-0247-z [DOI] [PubMed] [Google Scholar]
- 67.Koff RS (2014) Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther 39: 478–487. 10.1111/apt.12601 [DOI] [PubMed] [Google Scholar]
- 68.Gane EJ, Stedman CA, Hyland RH, Ding X, Svarovskaia E, Symonds WT, et al. (2013) Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 368: 34–44. 10.1056/NEJMoa1208953 [DOI] [PubMed] [Google Scholar]
- 69.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. (2013) Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 368: 1867–1877. 10.1056/NEJMoa1214854 [DOI] [PubMed] [Google Scholar]
- 70.Kowdley KV, Lawitz E, Crespo I, Hassanein T, Davis MN, DeMicco M, et al. (2013) Sofosbuvir with pegylated interferon alfa-2a and ribavirin for treatment-naive patients with hepatitis C genotype-1 infection (ATOMIC): an open-label, randomised, multicentre phase 2 trial. Lancet 381: 2100–2107. 10.1016/S0140-6736(13)60247-0 [DOI] [PubMed] [Google Scholar]
- 71.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, et al. (2014) Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 370: 1879–1888. 10.1056/NEJMoa1402355 [DOI] [PubMed] [Google Scholar]
- 72.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. (2013) Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 368: 1878–1887. 10.1056/NEJMoa1214853 [DOI] [PubMed] [Google Scholar]
- 73.Lawitz E, Lalezari JP, Hassanein T, Kowdley KV, Poordad FF, Sheikh AM, et al. (2013) Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 13: 401–408. 10.1016/S1473-3099(13)70033-1 [DOI] [PubMed] [Google Scholar]
- 74.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, et al. (2014) Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 370: 1483–1493. 10.1056/NEJMoa1316366 [DOI] [PubMed] [Google Scholar]
- 75.Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. (2014) Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 370: 1889–1898. 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 76.Asselah T (2014) Daclatasvir plus sofosbuvir for HCV infection: an oral combination therapy with high antiviral efficacy. J Hepatol 61: 435–438. 10.1016/j.jhep.2014.04.042 [DOI] [PubMed] [Google Scholar]
- 77.Lawitz E, Poordad FF, Pang PS, Hyland RH, Ding X, Mo H, et al. (2014) Sofosbuvir and ledipasvir fixed-dose combination with and without ribavirin in treatment-naive and previously treated patients with genotype 1 hepatitis C virus infection (LONESTAR): an open-label, randomised, phase 2 trial. Lancet 383: 515–523. 10.1016/S0140-6736(13)62121-2 [DOI] [PubMed] [Google Scholar]
- 78.Lawitz E, Sulkowski MS, Ghalib R, Rodriguez-Torres M, Younossi ZM, Corregidor A, et al. (2014) Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 384: 1756–1765. 10.1016/S0140-6736(14)61036-9 [DOI] [PubMed] [Google Scholar]
- 79.Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, et al. (2014) Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 370: 1993–2001. 10.1056/NEJMoa1316145 [DOI] [PubMed] [Google Scholar]
- 80.Asselah T, Boyer N, Saadoun D, Martinot-Peignoux M, Marcellin P (2016) Direct-acting antivirals for the treatment of hepatitis C virus infection: optimizing current IFN-free treatment and future perspectives. Liver Int 36 Suppl 1: 47–57. [DOI] [PubMed] [Google Scholar]
- 81.Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, et al. (2015) Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med 373: 2599–2607. 10.1056/NEJMoa1512610 [DOI] [PubMed] [Google Scholar]
- 82.Kohli A, Kapoor R, Sims Z, Nelson A, Sidharthan S, Lam B, et al. (2015) Ledipasvir and sofosbuvir for hepatitis C genotype 4: a proof-of-concept, single-centre, open-label phase 2a cohort study. Lancet Infect Dis 15: 1049–1054. 10.1016/S1473-3099(15)00157-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buti M, Esteban R (2015) Hepatitis C virus genotype 3: a genotype that is not 'easy-to-treat'. Expert Rev Gastroenterol Hepatol 9: 375–385. 10.1586/17474124.2015.960396 [DOI] [PubMed] [Google Scholar]
- 84.Buti M, Llaneras J, Riveiro-Barciela M, Esteban R (2015) Therapy for hepatitis C genotype 3: moving forward. J Viral Hepat 22: 683–690. 10.1111/jvh.12419 [DOI] [PubMed] [Google Scholar]
- 85.Ferenci P (2015) Treatment of hepatitis C in difficult-to-treat patients. Nat Rev Gastroenterol Hepatol 12: 284–292. 10.1038/nrgastro.2015.53 [DOI] [PubMed] [Google Scholar]
- 86.Sato M, Maekawa S, Komatsu N, Tatsumi A, Miura M, Muraoka M, et al. (2015) Deep sequencing and phylogenetic analysis of variants resistant to interferon-based protease inhibitor therapy in chronic hepatitis induced by genotype 1b hepatitis C virus. J Virol 89: 6105–6116. 10.1128/JVI.03127-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Svarovskaia ES, Dvory-Sobol H, Parkin N, Hebner C, Gontcharova V, Martin R, et al. (2014) Infrequent development of resistance in genotype 1–6 hepatitis C virus-infected subjects treated with sofosbuvir in phase 2 and 3 clinical trials. Clin Infect Dis 59: 1666–1674. 10.1093/cid/ciu697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sullivan JC, De Meyer S, Bartels DJ, Dierynck I, Zhang EZ, Spanks J, et al. (2013) Evolution of treatment-emergent resistant variants in telaprevir phase 3 clinical trials. Clin Infect Dis 57: 221–229. 10.1093/cid/cit226 [DOI] [PubMed] [Google Scholar]
- 89.Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, et al. (2015) Efficacy of sofosbuvir plus ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology 149: 1462–1470. 10.1053/j.gastro.2015.07.043 [DOI] [PubMed] [Google Scholar]
- 90.Reig M, Marino Z, Perello C, Inarrairaegui M, Ribeiro A, Lens S, et al. (2016) Unexpected early tumor recurrence in patients with hepatitis C virus -related hepatocellular carcinoma undergoing interferon-free therapy: a note of caution. J Hepatol In press. [DOI] [PubMed] [Google Scholar]
- 91.Hultgren C, Milich DR, Weiland O, Sallberg M (1998) The antiviral compound ribavirin modulates the T helper (Th) 1/Th2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol 79 (Pt 10): 2381–2391. 10.1099/0022-1317-79-10-2381 [DOI] [PubMed] [Google Scholar]
- 92.Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, et al. (1998) Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol 160: 3487–3493. [PubMed] [Google Scholar]
- 93.Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, et al. (2007) Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology 46: 1548–1563. 10.1002/hep.21853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y, Jamaluddin M, Wang S, Tian B, Garofalo RP, Casola A, et al. (2003) Ribavirin treatment up-regulates antiviral gene expression via the interferon-stimulated response element in respiratory syncytial virus-infected epithelial cells. J Virol 77: 5933–5947. 10.1128/JVI.77.10.5933-5947.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eriksson B, Helgstrand E, Johansson NG, Larsson A, Misiorny A, Noren JO, et al. (1977) Inhibition of influenza virus ribonucleic acid polymerase by ribavirin triphosphate. Antimicrob Agents Chemother 11: 946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bougie I, Bisaillon M (2003) Initial binding of the broad spectrum antiviral nucleoside ribavirin to the hepatitis C virus RNA polymerase. J Biol Chem 278: 52471–52478. 10.1074/jbc.M308917200 [DOI] [PubMed] [Google Scholar]
- 97.Streeter DG, Witkowski JT, Khare GP, Sidwell RW, Bauer RJ, Robins RK, et al. (1973) Mechanism of action of 1- -D-ribofuranosyl-1,2,4-triazole-3-carboxamide (Virazole), a new broad-spectrum antiviral agent. Proc Natl Acad Sci USA 70: 1174–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goswami BB, Borek E, Sharma OK, Fujitaki J, Smith RA (1979) The broad spectrum antiviral agent ribavirin inhibits capping of mRNA. Biochem Biophys Res Commun 89: 830–836. [DOI] [PubMed] [Google Scholar]
- 99.Hezode C, Asselah T, Reddy KR, Hassanein T, Berenguer M, Fleischer-Stepniewska K, et al. (2015) Ombitasvir plus paritaprevir plus ritonavir with or without ribavirin in treatment-naive and treatment-experienced patients with genotype 4 chronic hepatitis C virus infection (PEARL-I): a randomised, open-label trial. Lancet 385: 2502–2509. 10.1016/S0140-6736(15)60159-3 [DOI] [PubMed] [Google Scholar]
- 100.Wyles D, Pockros P, Morelli G, Younes Z, Svarovskaia E, Yang JC, et al. (2015) Ledipasvir-sofosbuvir plus ribavirin for patients with genotype 1 hepatitis C virus previously treated in clinical trials of sofosbuvir regimens. Hepatology 61: 1793–1797. 10.1002/hep.27814 [DOI] [PubMed] [Google Scholar]
- 101.Sarrazin C (2016) The importance of resistance to direct antiviral drugs in HCV infection in clinical practice. J Hepatol 64: 486–504. 10.1016/j.jhep.2015.09.011 [DOI] [PubMed] [Google Scholar]
- 102.Wyles DL, Gutierrez JA (2014) Importance of HCV genotype 1 subtypes for drug resistance and response to therapy. J Viral Hepat 21: 229–240. 10.1111/jvh.12230 [DOI] [PubMed] [Google Scholar]
- 103.Farci P, Purcell RH (2000) Clinical significance of hepatitis C virus genotypes and quasispecies. Semin Liver Dis 20: 103–126. [PubMed] [Google Scholar]
- 104.Farci P (2011) New insights into the HCV quasispecies and compartmentalization. Semin Liver Dis 31: 356–374. 10.1055/s-0031-1297925 [DOI] [PubMed] [Google Scholar]
- 105.Harouaka D, Engle RE, Wollenberg K, Diaz G, Tice AB, Zamboni F, et al. (2016) Diminished viral replication and compartmentalization of hepatitis C virus in hepatocellular carcinoma tissue. Proc Natl Acad Sci U S A 113: 1375–1380. 10.1073/pnas.1516879113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sissoko D, Laouenan C, Folkesson E, M'Lebing AB, Beavogui AH, Baize S, et al. (2016) Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med 13: e1001967 10.1371/journal.pmed.1001967 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The diversity indices are abbreviated in ordinate (Hpl, number of haplotypes; nMuts, number of different mutations; PolySites, number of polymorfic sites; Mpct; dominant haplotype abundance; Shannon, ^HS; GiniS, ^HGS, sample-based Gini-Simpson index; D1, D2, Dinf, Hill numbers; Mfe, mutation frequency, entity level; FAD, Functional Attribute Diversity; Pi.e, sample nucleotide diversity, entity level; Mf min, minimum mutation frequency; Mf.max, maximum mutation frequency; Pi, sample nucleotide diversity, and their calculation is described in reference [47] of the main text. Standard deviation interval (left column), and basic bootstrap with 95% confidence intervals (CI)(right column) are shown for each index.
(PDF)
The diversity index is given in the abscissa, with the same abbreviations used in S1 Fig. Density means the probability density of the corresponding distribution. The panels on the right indicate the boxplot of null distribution bootstraped diversity differences, with observed difference as red dot and red dash-dot line. The distance from this line to the boxplot, in terms of boxplot width, is an illustration of the low p-values obtained. A1, A2 and A4 mean amplicons 1, 2 and 4, respectively.
(PDF)
(ZIP)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.