Abstract
The horse was domesticated only 5.5 KYA, thousands of years after dogs, cattle, pigs, sheep, and goats. The horse nonetheless represents the domestic animal that most impacted human history; providing us with rapid transportation, which has considerably changed the speed and magnitude of the circulation of goods and people, as well as their cultures and diseases. By revolutionizing warfare and agriculture, horses also deeply influenced the politico-economic trajectory of human societies. Reciprocally, human activities have circled back on the recent evolution of the horse, by creating hundreds of domestic breeds through selective programs, while leading all wild populations to near extinction. Despite being tightly associated with humans, several aspects in the evolution of the domestic horse remain controversial. Here, we review recent advances in comparative genomics and paleogenomics that helped advance our understanding of the genetic foundation of domestic horses.
THE history of the domestication of the horse remains enigmatic in several aspects due to the absence of clear morphological and osteological differences between wild and early domestic individuals, but also due to the scarcity of paleontological records from some key periods, especially the one preceding the earliest evidence of domestication. This evidence is given by the ∼5500-year-old archaeological site of Botai (modern-day Kazakhstan) (Outram et al. 2009), at a considerable spatial and temporal distance from the Anatolian domestication centers for sheep and goats (Zeder et al. 2006). Unlike other ungulates, horses were not only used as a source of meat and milk, but their stamina and speed also revolutionized warfare and transportation. This also promoted cultural exchange, including the spread of Indo-European languages, religions, science, and art (Kelekna 2009; Anthony 2010). With the introduction of the horse collar and horseshoes in agriculture, the horse was increasingly used for tilling soils, incrementing farmland productivity in medieval Europe, and remains today an essential asset to the agriculture of the least-developed countries.
Human activities have conversely influenced, directly or indirectly, the evolution of horses, causing a drastic reduction of truly wild populations. After the extinction of the Tarpan horse in 1909, which populated Eastern Europe a few centuries ago, the only surviving wild relative is the endangered Przewalski’s horse. The latter was described in the Asian steppes in the 1870s, and overhunted to such an extent that it was, not >90 years later, officially declared extinct in the wild by the International Union for Conservation Nature. The Przewalski’s horse survived in captivity due to successful conservation programs, which raised a stock of 12–16 captive founders to >2000 individuals, one-quarter of which are in Mongolian and Chinese reintroduction reserves (King et al. 2015). Once “extinct in the wild,” its current conservation status has been upgraded to “endangered.” In parallel to the extinction of wild populations, human-driven management, in particular through selection, has dramatically influenced the recent history of domestic horses, developing multiple breeds with a wide range of phenotypic peculiarities. Although some horse breeds, such as the Thoroughbred racing horses, are still extremely popular, a significant part of this great diversity is currently endangered. According to the Food and Agricultural Organization of the United Nations (FAO 2015), 87 horse breeds are already extinct and among the remaining 905, almost a quarter are categorized as at risk.
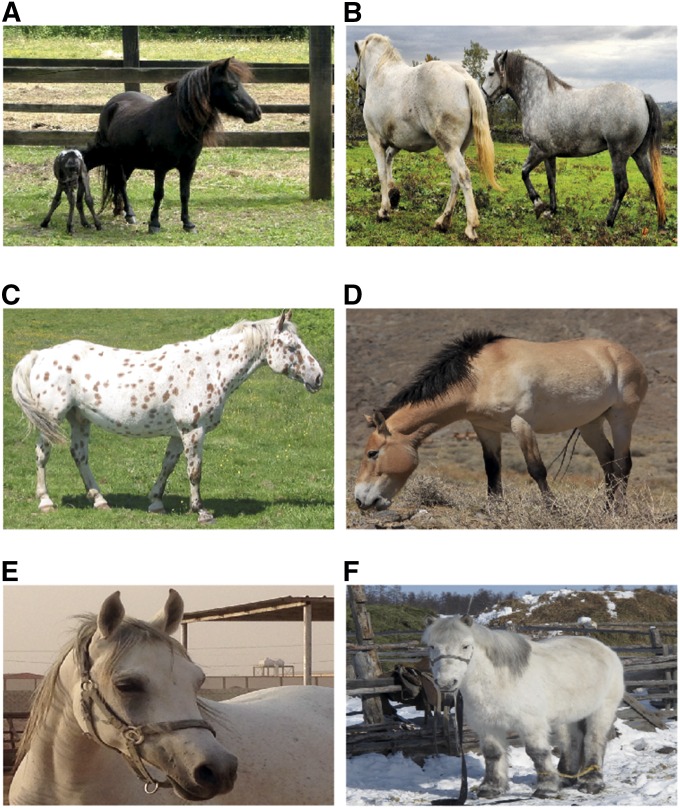
The population structure resulting from selective breeding is characterized by high interbreed and low intrabreed genetic diversity (McCue et al. 2012), and reflected by a huge array of morphological and behavioral traits (Figure 1). The height at withers, for example, extends from 70 cm in miniature Falabella horses to over 2 m in Shire and Percheron horses; an intraspecific range that is only exceeded by height variation in domestic dogs (Brooks et al. 2010). Domestic horses also exhibit striking variation in coat coloration, including the bay or bay-dun wild-type phenotypes, other basic colors like chestnut and black, as well as dilution (e.g., cream and silver), and spotting patterns (e.g., leopard complex, tobiano, and sabino) (Ludwig et al. 2009). Horse locomotion has also been recurrently selected, including their ability to perform alternate gaits, such as four-beat, lateral, or diagonal ambling. These alternate gaits come in addition to the three natural gaits (walk, trot, and gallop), and are known to increase the comfort of the rider and positively influence racing performance (Andersson et al. 2012; Promerová et al. 2014). Due to pleiotropic and/or epistatic effects, some of the traits selected in domestic breeds are, however, indirectly associated with congenital diseases (Bellone et al. 2008; McCue et al. 2008; Sandmeyer et al. 2012). These undesirable associations can be magnified by the extensive level of linkage disequilibrium (LD) that results from the low effective population size (Ne) within breeds.
Figure 1.
Diversity of breed phenotypes (size, shapes, and coat colors). (A) Falabella (image: E. H. Eckholdt, Wikimedia Commons). (B) Percheron horses (image: Carl Wycoff; Wikimedia Commons). (C) Appaloosa with LP coat (image: Jean-Pol Grandmont and Kersti Nebelsiek; Wikimedia Commons). (D) Przewalski’s horse (image: Ludovic Orlando at Seer, one of the Mongolian reintroduction reserves). (E) The Arabian horse (image: Ludovic Orlando at Riyad, Saudi Arabia). (F) Yakutian horse [image: Morgane Gibert, Centre National de la Recherche Scientifique (CNRS) UMR Anthropobiologie Moléculaire et Imagerie de Synthèse, France. Copyright Morgane Gibert-CNRS-Mountain Areas Farmer Support Organization].
Despite the loss of DNA diversity in wild relatives, many of the phenotypic traits and congenital diseases found in breeds of major economic impact have been successfully mapped into particular genomic regions (Bellone et al. 2010; Andersson et al. 2012; Makvandi-Nejad et al. 2012). This has been facilitated by the inherent structure between modern breeds, but also by recent methodological advances in horse genomics. The three major milestones in horse genomics include the relatively recent sequencing and assembly of a reference genome, which was generated from the Thoroughbred mare Twilight (Wade et al. 2009); the development of dedicated DNA-hybridization microarrays (McCue et al. 2012; Petersen et al. 2013a,b), now targeting up to 670K single nucleotide polymorphisms (SNPs) scattered across the entire genome; and, more recently, the DNA sequencing from fossil remains, which has commenced uncovering the genetic diversity present in extinct populations of wild horses and ancient domestic animals (Box 1).
Box 1. Ancient DNA: From Small Mitochondrial Fragments to Complete Nuclear Genomes.
The history of ancient DNA research is intimately linked to the equid family since the sequencing of short mitochondrial fragments from the extinct quagga zebra, which was achieved from DNA molecules preserved in the tissues of a museum specimen prepared in 1883 (Higuchi et al. 1984). After 30 years, the whole nuclear genome of this species has been characterized to an average coverage of depth of approximately eightfold, which confirmed the close genetic affinities with plains zebras from Southern Africa, while unveiling genomic loci underlying their unique genetic makeup (Jónsson et al. 2014). The development of innovative molecular methods (for a review, see Orlando et al. 2015) has clearly facilitated the transition from the characterization of short sequences to whole-genome sequencing, improving our ability (i) to extract ultrashort and damaged DNA fragments from archaeological material (Dabney et al. 2013; Gamba et al. 2016), (ii) to construct DNA libraries encompassing the whole extract complexity (Meyer et al. 2012; Gansauge and Meyer 2013), and (iii) to even preferentially enrich DNA libraries for authentic ancient templates (Carpenter et al. 2013; Fu et al. 2013; Gansauge and Meyer 2014). However, the main driver of this transition has been the advent of high-throughput DNA sequencing, which considerably increased the sensitivity and reduced both the cost and time related to the analysis of ancient DNA extracts.
Besides the quagga zebra, other members of the horse family have also attracted the attention of researchers of ancient DNA. These include the horse, but also the donkey (Kimura et al. 2011), and a range of extinct equine species such as the hydruntine European ass, which flourished in Central and Western Europe at least until the Iron Age (Orlando et al. 2006); the giant Cape zebra (Orlando et al. 2009); as well as North and South American specimens (Vilà et al. 2001; Weinstock et al. 2005; Vilstrup et al. 2013; Sarkissian et al. 2015). The horse itself currently holds the world record for the oldest genome ever sequenced, which was characterized from bone material preserved in the Yukon permafrost and dated to 560–780 KYA (Orlando et al. 2013). More recent horse genomes have been now sequenced, including two radiocarbon dated to ∼43 and 16 KYA and spanning the Upper Paleolithic (Schubert et al. 2014), one ∼5,200 years old from the Holocene (Librado et al. 2015), and a handful that lived within the last couple of centuries (Der Sarkissian et al. 2015; Librado et al. 2015). Many more are underway with the aim to reconstruct the history of genetic changes that have accompanied the emergence of the modern horse.
In addition to whole-genome sequencing, more targeted approaches have illuminated the process of horse domestication. Already 10 years ago, a method pioneered the genotyping of functionally or evolutionarily relevant nuclear markers from ancient specimens. It basically consisted in two-round multiplex PCR amplifications, whereby a range of short loci (∼50-bp long) are first coamplified within the same tube, and then separated in different tubes following a second series of individual amplifications (Römpler et al. 2006). Coupled with pyrosequencing, this method provided population-wide genotype information from ancient horses along the 5,500 years of domestication, revealing coat-color modifications as an early target of selection (Ludwig et al. 2009) and dynamic patterns of preferences in different sociocultural contexts (Ludwig et al. 2015). In the near future, we expect that this approach will be superseded by target-enrichment methods coupled with high-throughput sequencing. In horses, such approaches have been applied to only a limited number of loci (Vilstrup et al. 2013; Sarkissian et al. 2015), but recent procedures enable genotyping millions of loci genome wide (Fu et al. 2016), and even entire chromosomes (Fu et al. 2013) and genomes (Carpenter et al. 2013). This will likely facilitate the identification of the whole series of genetic modifications that have accompanied the recent evolutionary history of this family, domestication and extinction processes included.
Here, we review the recent evolution of the horse lineage, with a main focus on the specificities of its domestication process, including the recent demographic history and the genetic basis underlying the domestication and makeup of modern domestic breeds.
Predomestication Times
The equid family emerged some 55 MYA in North America, where it further diversified into several genera, including leaf browsers, grazers, and mixed feeders. It later expanded into South America and the Old World, following a complex radiation process (MacFadden 2005). Currently, the equid family is represented merely by the Equus genus, which—depending on the taxonomic classification considered—is comprised of seven to nine species, including zebras, asses, donkeys, and horses. Most of the past diversity is thus extinct, especially in the Americas, which experienced a mass extinction of large mammal species (“megafauna”) during the transition from the Late Quaternary to the Holocene, some 11.7 KYA (Burney and Flannery 2005; Faith and Surovell 2009). The underlying circumstances causing this extinction are highly debated, with the proposed drivers including a meteorite impact ∼12.9 KYA (Firestone et al. 2007), a series of anthropogenic factors (Alroy 2001; Koch and Barnosky 2006), and climate changes (Owen-Smith 1987; Guthrie 2006; Koch and Barnosky 2006).
By sequencing ancient DNA preserved in sediment cores, Haile and colleagues revealed that humans and megafaunal species, including horses, coexisted in Alaska until at least 10.5 KYA (Haile et al. 2009), some 3700 years after the last appearance of horses in the fossil record. These data challenge the ∼12.9 KYA meteorite impact as an extinction driver (Firestone et al. 2007), and imply a coexistence with humans of at least 2300 years (Rasmussen et al. 2014), disproving blitzkrieg overhunting as a credible extinction process for horses (Alroy 2001; Koch and Barnosky 2006). In line with abundance patterns in the horse fossil record (Orlando et al. 2013), further genetic analyses confirmed strong correlations between changes in the horse effective population size (Ne) and the climate and habitat availability (i.e., open grasslands) (Lorenzen et al. 2011; Orlando et al. 2013). These findings collectively support that the extinction of wild horses in northern America was mainly driven by a combination of climate and vegetation changes. This evidence, however, does not rule out the possibility of anthropogenic effects in Eurasia, where horse remains become increasingly rare in the fossil assemblages after the Last Glacial Maximum until the onset of domestication.
The beginning of the Holocene, some 11.7 KYA, indeed came with a reduction of open landscapes in Western Eurasia (Huntley and Webb 1988), and a modification in the distribution range of wild horses (Boyle 2006; Sommer et al. 2011; Bendrey 2012). Simulation-based vegetation reconstructions, integrating paleo-environmental data, showed that Iberia and the Pontic–Caspian area were grassland steppes during the mid-Holocene (Gallimore et al. 2005). Interestingly, these regions still represent hotspots of genetic diversity for horses, as measured by heterozygosity levels and allelic richness at 12 autosomal microsatellites from 24 “traditional” breeds (e.g., native from specific regions). This continuity in the spatial patterns of genetic diversity suggests that local populations of wild horses from Iberia and the Pontic–Caspian steppes could have contributed to some extent to the genetic pool of modern-day European breeds (Warmuth et al. 2011).
Gender-Biased Contributions to Domestication
Being paternally and maternally inherited, the nonrecombining region of the Y chromosome (NRY) and the mitochondrial DNA (mtDNA) directly reflect the male and female demographic histories, respectively. Although the repetitive nature of the NRY region makes its assembly challenging, the NRY and mtDNA markers have provided important insights into the domestication process for a range of livestock and domestic species (Meadows et al. 2004; Götherström et al. 2005; Natanaelsson et al. 2006; Sundqvist et al. 2006). In horses, however, the assembly for the whole NRY region is not available yet, as the reference genome was characterized from a single mare individual (Wade et al. 2009). Studies targeting specific NRY regions have shown limited variation, with haplotypes differing by one mutational step at best, which indicates that only a handful of paternal lines survived until present-day in domestic horses (Lindgren et al. 2004; Brandariz-Fontes et al. 2013; Wallner et al. 2013; Kreutzmann et al. 2014; Han et al. 2015). By contrast, analyses initially based on the mtDNA control region (D-loop) (Lister et al. 1998; Vilà et al. 2001; Jansen et al. 2002; Cieslak et al. 2010), and more recently on complete mitochondrial genomes (Lippold et al. 2011a; Achilli et al. 2012), revealed horses as the domestic animal showing one of the largest pools of mitochondrial genetic diversity.
The striking difference in paternally and maternally inherited markers reflects different effective population sizes of mares and stallions. Although this could partly result from the horse polygamous mating system, the sequence diversity found in ancient wild horses suggests that current levels of variability cannot be explained without a sex-biased contribution to the domestic stock (Lippold et al. 2011b). More specifically, the decline in NRY diversity appears to be a domestication by-product, perhaps as a result of recent breeding programs aimed at producing valuable stallions for rural regions, as (i) the only Scythian horse sampled, dating to ∼2.8 KYA, exhibits an NRY haplotype different from that found in modern individuals (Lippold et al. 2011b); and (ii) pedigree-based analyses can trace the most common patrilines back to a few, but extremely influential, stallions that lived ∼200 years ago (Wallner et al. 2013). Only the Yakutian breed is known to display substantial levels of NRY diversity (Librado et al. 2015), probably because it originated in the 13th to 15th century, following the migration of Mongolian tribes to the Sakha Republic (Russia) (Pakendorf et al. 2006; Crubézy et al. 2010). This area has been traditionally isolated from main trade routes, such as the Silk Road, which acted as gene-flow corridors, shaping patterns of population differentiation in Eurasia (Warmuth et al. 2013).
The diversity patterns found in mtDNA sequences point to a completely different demographic history for mares. Present-day mitochondrial haplogroups are almost evenly distributed worldwide and coalesce ∼93–160 KYA (Lippold et al. 2011a; Achilli et al. 2012; Schubert et al. 2014; Der Sarkissian et al. 2015; Librado et al. 2015), a time that not only predates the earliest archaeological evidence of domestication, but also the expansion of anatomically modern humans out of Africa. These studies estimated that a minimum of 17 (Achilli et al. 2012) and 46 (Lippold et al. 2011a) maternal lines successfully passed and survived into the domestic gene pool, with the latter estimate representing 73% of the mtDNA haplotypes that were already segregating prior to domestication (Lippold et al. 2011a). This proportion is higher than that inferred from partial mtDNA hypervariable sequences (∼34%) (Cieslak et al. 2010).
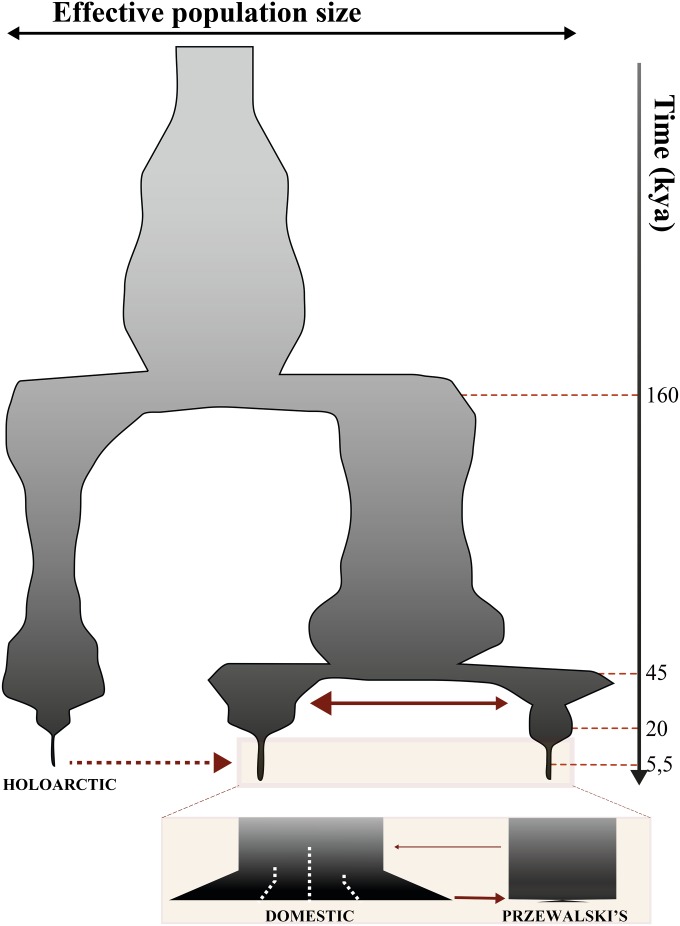
This implies that horse domestication involved a massive incorporation of maternal lines, possibly through recurrent restocking of wild mares during the spread of horse husbandry. This extensive introgression from the wild opens intriguing questions about the mechanisms maintaining phenotypic traits desirable in domestic horses. It is known, for example, that the Przewalski’s horse can hybridize with domestic horses, and produce a fully viable progeny, despite having an extra chromosome pair (2n = 66 vs. 2n = 64 in domestic horses). A recent study sequenced the genomes of 11 recent and 5 historical Przewalski’s horses, and compared them to the hitherto most extensive genome data set for domestic horses (Der Sarkissian et al. 2015). This study confirmed three phases of secondary contacts between the ancestral populations of Przewalski’s and domestic horses after their divergence ∼45 KYA (Figure 2). The first phase lasted probably until the Last Glacial Maximum and maintained these populations connected by reciprocal gene flow. The second phase involved a drastic reduction of gene flow, which consisted only of an input of the ancestral population of Przewalski’s horses into that of domestic horses. The extent of gene flow was apparently not reduced following domestication. However, an additional signature of reverse gene flow could be detected from domestic horses into Przewalski’s horses, in the beginning of the 20th century, right at the foundation of the captive stock of Przewalski’s horses (Der Sarkissian et al. 2015).
Figure 2.
Demographic model summarizing the recent evolution of the horse. Red arrows depict secondary contact events, with their size representing the magnitude of gene flow. The area of the shaded surface is proportional to the effective population size over time, as inferred with the pairwise sequentially Markov coalescent program (Li and Durbin 2011). The dashed arrow from the Holoartic to the domestic population indicates that the date for the gene-flow event represented is currently unknown. The last 18K years are zoomed in to illustrate recent evolutionary processes, such as the bottleneck experienced by the Przewalski’s horses, and the population structure and/or expansion observed since the horse domestication (∼5.5 KYA).
In addition to Przewalski’s and domestic horses, complete genome sequencing of ancient horses recently revealed the existence of a third divergent lineage corresponding to a wild population that inhabited the Holarctic region (Schubert et al. 2014; Librado et al. 2015) This population is currently known from the genomes of only three fossil specimens but survived at least until ∼5.2 KYA, a period contemporary with the early stages of the domestication process. Schubert et al. (2014) found that this population significantly contributed to the genetic makeup of domestic horses, with at least 12.9% of modern domestic genomes showing ancestry to this now-extinct lineage (Figure 2). Future work is required to delineate the geographic and temporal limits of this lineage, date the admixture event(s), identify the genomic blocks introgressed in domestic horses, and test which blocks show signatures of positive selection.
Domestication Centers and Geographic Structure
Phylogeographic studies have first exploited the patterns of mtDNA variation across space, and eventually across time, to successfully date and identify the geographic centers of domestication for a wide range of livestock species (reviewed in Bruford et al. 2003; Groeneveld et al. 2010). In horses, however, early surveys of the hypervariable control region showed a minimal correspondence between mtDNA haplogroups, geographic areas, and breed types (Vilà et al. 2001; Jansen et al. 2002; Cieslak et al. 2010). This lack of phylogeographic structure was not an artifact resulting from the limited phylogenetic information present in the hypervariable control region, since it could be further confirmed by independent studies based on complete mitochondrial genomes (Lippold et al. 2011a; Achilli et al. 2012).
The reasons for an absence of phylogeographic mitochondrial structure are manifold, and include complex interactions between long-distance dispersal rates, extensive restocking from the wild, as well as recent human management. According to mtDNA-based studies leveraging on fossil remains, the ancient patterns of isolation by distance were characterized by an almost panmitic population from the Late Pleistocene until Early Holocene and the Copper Age (Cieslak et al. 2010). Only at that time, the horse population started to exhibit a certain degree of substructure within the Eurasian steppes and the Iberian Peninsula (Cieslak et al. 2010). This ancient substructure is still faint but detectable today from autosomal microsatellites (Warmuth et al. 2011), but recent selective breeding likely contributed to erode the corresponding signature at the mtDNA level. This is indirectly reflected by the Bayesian-skyline-plot (BSP) reconstructions (Drummond et al. 2005), which show an almost constant effective population size over time, until the domestication started, and led to a continuous expansion (Lippold et al. 2011a; Achilli et al. 2012). Although this BSP profile is generally taken as the mark of demographic expansion postdomestication, it can equally well result from violating the random mating assumption made by the BSP model. Selective breeding indeed introduces reproductive islets that impede recent coalescent events, which might mimic the genealogy of an expanding population (Ho and Shapiro 2011; Heller et al. 2013).
In contrast to mtDNA, nuclear data display weak but significant patterns of isolation by distance in nonbreed horses (i.e., remote animals maintained outside of stud farms). Fitting explicit stepping-stone scenarios of horse radiation to 26 microsatellite loci genotyped on 322 nonbreed animals, Warmuth et al. (2012) found strongest support for domestication starting in the western Eurasian steppes (Warmuth et al. 2012), in agreement with the earliest archaeological evidence (Outram et al. 2009). The discovery of a second hotspot of microsatellite diversity among Iberian breeds recently revived the question of Iberia as a possible second domestication center (Warmuth et al. 2011). It has been proposed, for instance, that the mtDNA haplogroup D1, which is the most-commonly found among Iberian and North-African barb horses, reflects local domestication in the Iberian Peninsula (Jansen et al. 2002). However, the molecular analysis of 22 ancient horses failed to detect the haplogroup D1 prior to the medieval period. The limited antiquity of this haplotype within Iberia is consistent with its star-like genealogy, suggestive of an expansion in recent times, which rules out this haplogroup as a marker of a local domestication (Lira et al. 2010). Furthermore, the horse domestication that occurred in the Eurasian steppes was accompanied by an explosion of alleles involved in coat-color variation, which was not observed in Iberia until medieval times (Ludwig et al. 2009). Yet, the so-called Lusitano haplogroup C, which is currently restricted to horses native from Portugal, was found to be already present in the Neolithic and Bronze Ages (Lira et al. 2010), which leaves the possibility of a secondary domestication in Iberia open and controversial.
The Genetic Makeup of Domestic Horses
During the domestication of the horse, humans acted as strong selective forces by favoring particular traits of interest, and inadvertently through the use of animals for various tasks far outside the range of their normal behavior. This resulted in a specialization of horses for strength, aesthetics, racing performance, or endurance. Several lines of evidence suggest selection on standing genetic variation as a major component of this process.
Early Selection Targets
Both prehistoric cave paintings and ancient DNA data have corroborated that some coat-color variants, including black and bay, as well as the leopard complex spotting (LP), were already segregating in wild populations (Ludwig et al. 2009; Pruvost et al. 2011). The latter is particularly noteworthy since individuals homozygous for the LP allele show congenital stationary night blindness (Bellone et al. 2008, 2010; Sandmeyer et al. 2012). Despite its detrimental effect, the LP allele appears to have been relatively common in early domestic horses, based on its discovery in 6 out of the 10 samples from the Kirklareli–Kanligecit archaeological site (Turkish Thrace, dating 4.2–4.7 KYA). The LP allele thereafter remained at undetectable frequencies until the early Iron Age, where it was identified again in a 3300–3400-year-old specimen from the Chicha archaeological site (western Siberia). By using the Approximate Bayesian Computation framework (Beaumont et al. 2002; Csilléry et al. 2010), the authors estimated that this nonmonotonic variation of the LP frequency cannot be solely explained by genetic drift, but must have involved fluctuating selection, first favoring the LP allele during the early Bronze Age, but later counterselecting it during the middle Bronze Age (Ludwig et al. 2015). This study highlights dynamic preferences for horse traits and types in past human groups from different social and cultural contexts. In addition to the LP allele, another example of selection targeting standing variation is provided by a 1617-bp deletion on chromosome 8, which prevents the development of a wild-type dun coloration (Imsland et al. 2016). Notably, this deletion has been documented in a ∼43K-year-old horse from the Taymyr Peninsula in Central Siberia (Imsland et al. 2016). Overall, current data thus indicates that a relatively wide range of coat-color variants existed in Eurasian landscapes, long before the earliest evidence of domestication.
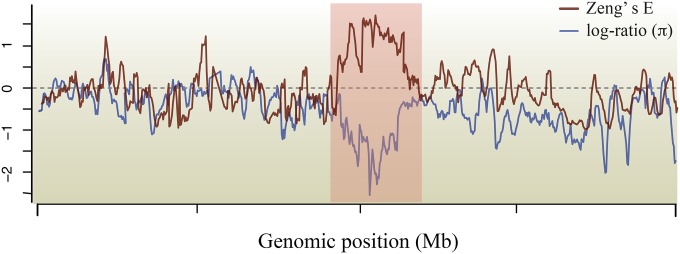
Such studies have clearly advanced our understanding of the recent horse evolution, especially with regard to their domestication. They were, however, limited in scope as they targeted only a handful of candidate loci. The whole-genome sequencing of ancient predomestic horses (Box 1) has instead allowed for genome-wide scans of positive selection (Figure 3), pinpointing the broader range of genetic changes associated with horse domestication. Schubert and colleagues implemented this approach for the first time by sequencing the complete genomes of two prehistoric horses dating back to ∼43 and 16.5 KYA, and comparing them with a range of genomes spanning a wide panel of domestic breeds (Schubert et al. 2014). This provided a set of 125 candidate genes that underwent episodes of positive selection following horse domestication, including some that were previously identified using different approaches, such as MC1R (involved in coat-coloration patterns). It also revealed a majority of novel candidates involved in the (i) cardiac and circulatory system, probably reflecting increasing energetic demands related to the extensive horse usage in transportation and locomotion; (ii) bone, limb, and face morphogenesis in relation to the morphological changes documented along the domestication history; and (iii) brain development, neuron growth, and behavior, which likely contributed to the cognitive and social changes associated with domestication.
Figure 3.
Genome-wide scan of positive selection. The shaded area showcases a genomic region that has potentially undergone a recent episode of positive selection, as the Zeng’s E statistic (Zeng et al. 2006) shows an excess of high-frequency derived variants, and the levels of nucleotide diversity (π) are lower in present-day than in a hypothetical wild ancestor (i.e., the log ratio of their respective π values is below zero and low over a genomic block of significant size).
Schubert and colleagues also estimated that the genome of domestic horses harbors an excess of variants in genomic positions that are highly constrained across mammals, and are thus likely to be deleterious. This accumulation of detrimental variants is a side effect of human-driven selective breeding. The increased variance in reproductive success, especially in stallions, yields a reduction in the effective population size, which compromises the effectiveness of natural selection to purge deleterious mutations (for a review, see Charlesworth 2009). This is in line with evidence observed from other species, such as dogs (Cruz et al. 2008; Marsden et al. 2016), rice (Lu et al. 2006), and tomatoes (Koenig et al. 2013); indicating that human preferences have not only favored certain variants during domestication, but also introduced an important genomic cost represented by an accumulation of deleterious mutations, which increased the propensity of modern domestic breeds to develop genetic disorders.
Engendering Modern Breeds
With a few notable exceptions, such as the Arabian, Mongolian, and Icelandic horses, breeds are relatively recent human constructs on an evolutionary timescale. The earliest horse studbook, that of the Thoroughbred racing horses, was created in 1791. Therefore, within the last two centuries, humans have imposed strong diverging selection among breeds. This low within-breed variability, and thus relatively high LD patterns, have made the ∼54K SNPs targeted by the EquineSNP50 BeadChip extremely useful for horse genetic research (McCue et al. 2012). More recently, the Equine Genome Diversity Consortium exploited the complete genome sequences from 20 breeds to develop an Affymetrix array covering ∼670K SNPs scattered along the horse genome. In addition to its higher SNP density, this array corrects for known limitations of the EquineSNP50 BeadChip system, such as the ascertainment bias toward Thoroughbred horses (which were overrepresented in the original SNP discovery panel) and the absence of X-chromosome markers. As the Axiom Equine Genotyping microarray (Axiom MNEC670) was made commercially available only since 2015, the EquineSNP50 BeadChip has been the most popular system in horse genomics thus far, and has been applied to address a full range of biological questions. It was, for instance, used to identify genomic blocks that are highly differentiated in particular breeds, as a proxy for understanding the genetic basis of their phenotypic peculiarities (Petersen et al. 2013a). Remarkably, the results revealed noncoding regions as breed-specific targets of selection, in accordance with selection acting both on the coding sequence of genes, and on the regulation of their expression. Putatively selected haplotypes encompassed genes previously proposed to be involved in gait (DMRT2 and DMRT3), coat color (MC1R), performance (MSTN), and size (IGF1, NCAPG, and HMGA2) (Petersen et al. 2013a).
Beyond representing an interesting list of selected candidates, the phenotypic consequences of these mutations were further demonstrated in a range of functional studies. For example, three noncoding polymorphisms around MSTN have been associated with racing capabilities in elite and common Thoroughbreds: (i) the g66493797T > C SNP at its first intron (Hill et al. 2010a,b; Tozaki et al. 2010), (ii) the insertion of a 227-bp-long short interspersed nuclear element (SINE) at its promoter (Hill et al. 2010a), and (iii) the BIEC2-417495 SNP located 692 kb upstream of MSTN and 30 kb upstream of the glutaminase gene GLS (Binns et al. 2010). In elite Thoroughbreds, homozygotes for the g66493797 C allele performed better in short (<1300 m) and fast races, heterozygotes in middle distance races (between 1301 and 1900 m), and homozygotes for the g66493797 T allele in long distance races (Hill et al. 2010a,b). A correlation between genotypes at the g66493797 locus and expression was established in untrained Thoroughbreds, with MSTN messenger RNA levels decreasing from g66493797 C/C to g66493797 C/T and g66493797 T/T; whereas in trained horses, reduced but comparable MSTN expression was observed across all genotypes (McGivney et al. 2012). Moreover, and although the precise mechanisms of transcription regulation are presently unknown, the 227-bp SINE insertion was predicted in silico to create a CpG island, as well as few novel putative transcription factor binding sites, which may play a role in regulating the MSTN expression (van den Hoven et al. 2015).
The nonsense mutation in the DMRT3 transcription factor underpins alternate gaits in horses, such as ambling and tölt (Andersson et al. 2012). This mutation, named “gait keeper,” segregates at high frequencies in breeds classified as gaited or bred for harness racing; in contrast to horses lacking this ability which show only marginal allele frequencies (Petersen et al. 2013a; Promerová et al. 2014). In Finnhorses and Icelandic horses, which can perform alternate gaits, the gait-keeper mutation was found to be beneficial only for homozygous racehorses. When used in other classical riding disciplines, such as jumping or dressage, homozygous horses obtained lower scores than heterozygous or nongait-keeper homozygous animals (Andersson et al. 2012; Kristjansson et al. 2014; Jäderkvist Fegraeus et al. 2015). Different DMRT3 genotypes thus appear to benefit different types of performance (harness racing vs. classical riding), and both gait-keeper and nongait-keeper alleles segregate at relatively similar frequencies (Jäderkvist Fegraeus et al. 2015).
Subtle molecular signals of positive selection, reflecting complex selective regimes, including context-dependent fitness and pleiotropic effects, have started to be detected. One such case is provided by the gain-of-function mutation at the glycogen-synthase GYS1 gene, which increases the accumulation of glycogen in skeletal muscles and facilitates a better restoration of glycogen postexercise (McCue et al. 2008). The GYS1 mutation is, however, also associated with the postexercise equine polysaccharide storage myopathy type 1, a disease causing a breakdown of type 2A and 2B muscle fibers (rhabdomyolysis) (McCue et al. 2008). Such deleterious effects should have purged out the GYS1 mutation from modern livestock, but it is still found at considerable frequencies in breeds of heavy horses and with long breeding histories, such as the Belgian draft horses (Baird et al. 2010; McCue et al. 2010). To explain this apparent paradox, the GYS1 mutation has been proposed to represent a “thrifty” allele, which was once beneficial in the past when conditions of regular hard work and limited nutritional input could favor genotypes leading to a more efficient storage of energy sources in the muscles. This allele would be maladaptive in modern management conditions of unlimited feeding resources and relatively limited exercise. In line with this model, high frequency, LD, and extended haplotype homozygosity patterns found in Belgian heavy draft horses in association with the GYS1 mutation are unlikely to be explained under neutrality (McCoy et al. 2014). The mutation in GYS1 might thus have undergone selection in the past, but the relaxed constraints prevailing today, including a limited population size, might ultimately lead to the disappearance of this allele in this breed (McCoy et al. 2014). It is noteworthy that in such cases of fluctuating selection, ancient DNA can help recover full time series of allele frequencies (Ludwig et al. 2015) and better characterize the dynamics of underlying selective regimes (Malaspinas et al. 2012; Schraiber et al. 2016).
Controlling for the underlying demographic process is important to accurately pinpoint the targets of natural selection. For example, Yakutian horses, which survive the most extreme winter temperatures in the Northern hemisphere, developed their striking cold adaptations in only ∼100 generations (Librado et al. 2015). The cross-comparison of the variation present in their genome and that from non-Artic horse breeds revealed that the few kilobases located upstream of translation start sites harbor the highest proportion of adaptive mutations (Librado et al. 2015). Such cis-regulatory candidates potentially drive the expression of genes participating in adaptive phenotypic features, such as hair density. Numerous genes involved in glucose metabolism indicated that sugar-related antifreezing properties and the ability to regulate seasonal thermogenic requirements might also be essential to survive the Arctic cold. Interestingly, the biological significance of some of these candidates was corroborated in woolly mammoths (Lynch et al. 2015), where parallel episodes of positive selection have been described at the BARX2 and PHIP genes, associated with hair development and insulin metabolism, respectively. Similarly, human groups from Siberia (Cardona et al. 2014) also show adaptive footprints at the PRKG1 gene, which participates in shivering by regulating blood vessel constriction. Altogether, such patterns of convergent evolution support regulatory changes as a key mechanism for driving rapid adaptive processes, mainly because these regions offer an important fraction of the segregating variation readily available for natural selection. Considering the equally short evolutionary timescale related to the formation of modern horse breeds, the role of noncoding regions in modern horse phenotypes will thus likely receive a lot of attention in the near future, especially in light of the Functional Annotation of Animal Genomes (FAANG) consortium, which aims at cataloging all the “functional” elements, protein coding or not, present in the horse genome (Andersson et al. 2015).
Conclusions
In this review, we have shown that the horse domestication process was complex and involved continuous genetic restocking from the wild, in a sex-biased manner, mostly from mares (Vilà et al. 2001; Jansen et al. 2002; Achilli et al. 2012; Warmuth et al. 2012). The absence of genetic isolation between domestic livestock and wild ancestors observed in the horse does not stand as an exception though, but is increasingly recognized in other domestic animals, such as cattle (Park et al. 2015) and pigs (Frantz et al. 2015). Understanding how distinct phenotypic features can be maintained in the presence of homogenizing gene flow thus seems to become one central question common to most, if not all, animal domestication processes. Developing comparative genomics approaches such as those aimed at identifying genomic islands of speciation (Turner et al. 2005)—the process underlying species formation and ultimately genetic isolation—will likely help solve this nascent paradox in the near future.
In addition to revealing the true extent of gene flow, in particular from yet-unrecognized and extinct wild lineages (Schubert et al. 2014), ancient DNA has also showed that the domestication process was quite dynamic and uneven through space and time, as particular human groups selected different phenotypic traits (Ludwig et al. 2015). This suggests that the history of changes that accompanied the early domestication of horses and their further transformation in the course of history will be difficult, if not impossible, to reconstruct from current patterns of genetic diversity. Indeed, the latter mostly reflect the recent history of intensive selection and extensive admixture that followed the development of breeds since the 18th century. As such, it pleads for increased ancient genome surveys of horse remains spread across the whole temporal and geographic range where the human–horse relationship developed. We expect that similar investigations will also largely advance our understanding of domestication processes when applied to other domestic animals. This work has already started in cattle (Edwards et al. 2007; Bollongino et al. 2008; Orlando 2015; Park et al. 2015; Scheu et al. 2015), dogs (Ollivier et al. 2013; Thalmann et al. 2013), swine (Edwards et al. 2007; Meiri et al. 2013; Ottoni et al. 2013; Frantz et al. 2015; Ramírez et al. 2015), and chickens (Flink et al. 2014).
Recent work based on ancient DNA has revealed that, beyond genomes, ancient genome-wide methylation and nucleosome maps can be reconstructed using patterns of DNA degradation along the genome (Gokhman et al. 2014, 2016; Orlando and Willerslev 2014; Orlando et al. 2015; Pedersen et al. 2014). Combined with current efforts of the FAANG consortium (Andersson et al. 2015), such approaches promise to reveal the true extent of past individual plasticity and how patterns of gene regulation were modified in response to new domestication/selection targets. Additionally, the recent discovery that dental calculus represents a fantastic source of ancient microbial DNA (Adler et al. 2013) and genetic traces of major components of the diet (Warinner et al. 2014), encourages the genetic investigation of dietary changes and modification of the oral microbiota that accompanied horse domestication. We anticipate that, together with ancient genome and epigenome reconstruction, these approaches will reveal the true extent of biological transformation that forged the modern horse.
Finally, as informative as the genetic analysis of fossils can be, it remains that a growing number of domestic breeds are currently endangered. It is thus important to undertake massive genetic efforts aimed at their conservation both as a unique biological heritage of traditional human activities and as an invaluable resource of variation for future selection programs.
Acknowledgments
This work is dedicated to the memory of Dr. Teri Lear, a fantastic scientist, friend and horse lover, who recently passed away. This work was supported by the Danish Council for Independent Research, Natural Sciences (Grant 4002-00152B); the Danish National Research Foundation (Grant DNRF94); Initiative d'Excellence Chaires d'attractivité, Université de Toulouse (OURASI); the International Research Group Program (Grant IRG14-08), Deanship of Scientific Research, King Saud University; a Marie-Curie Individual Fellowship (MSCA-IF-657852); a Villum Fonden Blokstipendier grant, and; a Villum Fonden research project grant (miGENEPI).
Footnotes
Communicating editor: J. Rine
Literature Cited
- Achilli A., Olivieri A., Soares P., Lancioni H., Hooshiar Kashani B., et al. , 2012. Mitochondrial genomes from modern horses reveal the major haplogroups that underwent domestication. Proc. Natl. Acad. Sci. USA 109: 2449–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler C. J., Dobney K., Weyrich L. S., Kaidonis J., Walker A. W., et al. , 2013. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 45: 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alroy J., 2001. A Multispecies Overkill Simulation of the End-Pleistocene Megafaunal Mass Extinction. Science 292: 1893–1896. [DOI] [PubMed] [Google Scholar]
- Andersson L. S., Larhammar M., Memic F., Wootz H., Schwochow D., et al. , 2012. Mutations in DMRT3 affect locomotion in horses and spinal circuit function in mice. Nature 488: 642–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L., Archibald A. L., Bottema C. D., Brauning R., Burgess S. C., et al. , 2015. Coordinated international action to accelerate genome-to-phenome with FAANG, the Functional Annotation of Animal Genomes project. Genome Biol. 16: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony D. W., 2010. The Horse, the Wheel, and Language: How Bronze-Age Riders from the Eurasian Steppes Shaped the Modern World. Princeton University Press, Princeton, NJ. [Google Scholar]
- Baird J. D., Valberg S. J., Anderson S. M., McCue M. E., Mickelson J. R., 2010. Presence of the glycogen synthase 1 (GYS1) mutation causing type 1 polysaccharide storage myopathy in continental European draught horse breeds. Vet. Rec. 167: 781–784. [DOI] [PubMed] [Google Scholar]
- Beaumont M. A., Zhang W., Balding D. J., 2002. Approximate Bayesian Computation in Population Genetics. Genetics 162: 2025–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone R. R., Brooks S. A., Sandmeyer L., Murphy B. A., Forsyth G., et al. , 2008. Differential Gene Expression of TRPM1, the Potential Cause of Congenital Stationary Night Blindness and Coat Spotting Patterns (LP) in the Appaloosa Horse (Equus caballus). Genetics 179: 1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone R. R., Forsyth G., Leeb T., Archer S., Sigurdsson S., et al. , 2010. Fine-mapping and mutation analysis of TRPM1: a candidate gene for leopard complex (LP) spotting and congenital stationary night blindness in horses. Brief. Funct. Genomics 9: 193–207. [DOI] [PubMed] [Google Scholar]
- Bendrey R., 2012. From wild horses to domestic horses: a European perspective. World Archaeol. 44: 135–157. [Google Scholar]
- Binns M. M., Boehler D. A., Lambert D. H., 2010. Identification of the myostatin locus (MSTN) as having a major effect on optimum racing distance in the Thoroughbred horse in the USA. Anim. Genet. 41: 154–158. [DOI] [PubMed] [Google Scholar]
- Bollongino R., Elsner J., Vigne J.-D., Burger J., 2008. Y-SNPs Do Not Indicate Hybridisation between European Aurochs and Domestic Cattle. PLoS One 3: e3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle, K. V., 2006 Neolithic wild game animals in Western Europe: the question of hunting, pp. 10–23 in Animals in the Neolithic of Britain and Europe, edited by D. Serjeantson and D. Field. Oxbow Books, Oxford.
- Brandariz-Fontes C., Leonard J. A., Vega-Pla J. L., Backström N., Lindgren G., et al. , 2013. Y-Chromosome Analysis in Retuertas Horses. PLoS One 8: e64985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. A., Makvandi-Nejad S., Chu E., Allen J. J., Streeter C., et al. , 2010. Morphological variation in the horse: defining complex traits of body size and shape. Anim. Genet. 41: 159–165. [DOI] [PubMed] [Google Scholar]
- Bruford M. W., Bradley D. G., Luikart G., 2003. DNA markers reveal the complexity of livestock domestication. Nat. Rev. Genet. 4: 900–910. [DOI] [PubMed] [Google Scholar]
- Burney D. A., Flannery T. F., 2005. Fifty millennia of catastrophic extinctions after human contact. Trends Ecol. Evol. 20: 395–401. [DOI] [PubMed] [Google Scholar]
- Cardona A., Pagani L., Antao T., Lawson D. J., Eichstaedt C. A., et al. , 2014. Genome-Wide Analysis of Cold Adaptation in Indigenous Siberian Populations. PLoS One 9: e98076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter M. L., Buenrostro J. D., Valdiosera C., Schroeder H., Allentoft M. E., et al. , 2013. Pulling out the 1%: Whole-Genome Capture for the Targeted Enrichment of Ancient DNA Sequencing Libraries. Am. J. Hum. Genet. 93: 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlesworth B., 2009. Effective population size and patterns of molecular evolution and variation. Nat. Rev. Genet. 10: 195–205. [DOI] [PubMed] [Google Scholar]
- Cieslak M., Pruvost M., Benecke N., Hofreiter M., Morales A., et al. , 2010. Origin and History of Mitochondrial DNA Lineages in Domestic Horses. PLoS One 5: e15311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crubézy E., Amory S., Keyser C., Bouakaze C., Bodner M., et al. , 2010. Human evolution in Siberia: from frozen bodies to ancient DNA. BMC Evol. Biol. 10: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz F., Vilà C., Webster M. T., 2008. The legacy of domestication: accumulation of deleterious mutations in the dog genome. Mol. Biol. Evol. 25: 2331–2336. [DOI] [PubMed] [Google Scholar]
- Csilléry K., Blum M. G. B., Gaggiotti O. E., François O., 2010. Approximate Bayesian Computation (ABC) in practice. Trends Ecol. Evol. 25: 410–418. [DOI] [PubMed] [Google Scholar]
- Dabney J., Knapp M., Glocke I., Gansauge M.-T., Weihmann A., et al. , 2013. Complete mitochondrial genome sequence of a Middle Pleistocene cave bear reconstructed from ultrashort DNA fragments. Proc. Natl. Acad. Sci. USA 110: 15758–15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Sarkissian, C., L. Ermini, M. Schubert, M. A. Yang, P. Librado et al, 2015 Evolutionary Genomics and Conservation of the Endangered Przewalski’s Horse. Curr. Biol. 25: 2577–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. J., Rambaut A., Shapiro B., Pybus O. G., 2005. Bayesian Coalescent Inference of Past Population Dynamics from Molecular Sequences. Mol. Biol. Evol. 22: 1185–1192. [DOI] [PubMed] [Google Scholar]
- Edwards C. J., Bollongino R., Scheu A., Chamberlain A., Tresset A., et al. , 2007. Mitochondrial DNA analysis shows a Near Eastern Neolithic origin for domestic cattle and no indication of domestication of European aurochs. Proc. R. Soc. Lond. B Biol. Sci. 274: 1377–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith J. T., Surovell T. A., 2009. Synchronous extinction of North America’s Pleistocene mammals. Proc. Natl. Acad. Sci. USA 106: 20641–20645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2015 The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture, edited by B. D. Scherf and D. Pilling. FAO Commision on Genetic Resources for Food and Agriculture Assessments, Rome. Available at: http://www.fao.org/3/a-i4787e/index.html.
- Firestone R. B., West A., Kennett J. P., Becker L., Bunch T. E., et al. , 2007. Evidence for an extraterrestrial impact 12,900 years ago that contributed to the megafaunal extinctions and the Younger Dryas cooling. Proc. Natl. Acad. Sci. USA 104: 16016–16021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flink L. G., Allen R., Barnett R., Malmström H., Peters J., et al. , 2014. Establishing the validity of domestication genes using DNA from ancient chickens. Proc. Natl. Acad. Sci. USA 111: 6184–6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz L. A. F., Schraiber J. G., Madsen O., Megens H.-J., Cagan A., et al. , 2015. Evidence of long-term gene flow and selection during domestication from analyses of Eurasian wild and domestic pig genomes. Nat. Genet. 47: 1141–1148. [DOI] [PubMed] [Google Scholar]
- Fu Q., Meyer M., Gao X., Stenzel U., Burbano H. A., et al. , 2013. DNA analysis of an early modern human from Tianyuan Cave, China. Proc. Natl. Acad. Sci. USA 110: 2223–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q., Posth C., Hajdinjak M., Petr M., Mallick S., et al. , 2016. The genetic history of Ice Age Europe. Nature 534: 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore R., Jacob R., Kutzbach J., 2005. Coupled atmosphere-ocean-vegetation simulations for modern and mid-Holocene climates: role of extratropical vegetation cover feedbacks. Clim. Dyn. 25: 755–776. [Google Scholar]
- Gamba C., Hanghøj K., Gaunitz C., Alfarhan A. H., Alquraishi S. A., et al. , 2016. Comparing the performance of three ancient DNA extraction methods for high-throughput sequencing. Mol. Ecol. Resour. 16: 459–469. [DOI] [PubMed] [Google Scholar]
- Gansauge M.-T., Meyer M., 2013. Single-stranded DNA library preparation for the sequencing of ancient or damaged DNA. Nat. Protoc. 8: 737–748. [DOI] [PubMed] [Google Scholar]
- Gansauge M.-T., and M. Meyer, 2014 Selective enrichment of damaged DNA molecules for ancient genome sequencing. Genome Res. 24: 1543–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokhman D., Lavi E., Prüfer K., Fraga M. F., Riancho J. A., et al. , 2014. Reconstructing the DNA Methylation Maps of the Neandertal and the Denisovan. Science 344: 523–527. [DOI] [PubMed] [Google Scholar]
- Gokhman D., Meshorer E., Carmel L., 2016. Epigenetics: It’s Getting Old. Past Meets Future in Paleoepigenetics. Trends Ecol. Evol. 31: 290–300. [DOI] [PubMed] [Google Scholar]
- Götherström A., Anderung C., Hellborg L., Elburg R., Smith C., et al. , 2005. Cattle domestication in the Near East was followed by hybridization with aurochs bulls in Europe. Proc. R. Soc. Lond. B Biol. Sci. 272: 2345–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld L. F., Lenstra J. A., Eding H., Toro M. A., Scherf B., et al. , 2010. Genetic diversity in farm animals—a review. Anim. Genet. 41: 6–31. [DOI] [PubMed] [Google Scholar]
- Guthrie R. D., 2006. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441: 207–209. [DOI] [PubMed] [Google Scholar]
- Haile J., Froese D. G., MacPhee R. D. E., Roberts R. G., Arnold L. J., et al. , 2009. Ancient DNA reveals late survival of mammoth and horse in interior Alaska. Proc. Natl. Acad. Sci. USA 106: 22352–22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Zhang Q., Gao K., Yue X., Zhang T., et al. , 2015. Y-Single Nucleotide Polymorphisms Diversity in Chinese Indigenous Horse. Asian-Australas. J. Anim. Sci. 28: 1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R., Chikhi L., Siegismund H. R., 2013. The Confounding Effect of Population Structure on Bayesian Skyline Plot Inferences of Demographic History. PLoS One 8: e62992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Bowman B., Freiberger M., Ryder O. A., Wilson A. C., 1984. DNA sequences from the quagga, an extinct member of the horse family. Nature 312: 282–284. [DOI] [PubMed] [Google Scholar]
- Hill E. W., McGivney B. A., Gu J., Whiston R., Machugh D. E., 2010a A genome-wide SNP-association study confirms a sequence variant (g.66493737C>T) in the equine myostatin (MSTN) gene as the most powerful predictor of optimum racing distance for Thoroughbred racehorses. BMC Genomics 11: 552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill E. W., Gu J., Eivers S. S., Fonseca R. G., McGivney B. A., et al. , 2010b A sequence polymorphism in MSTN predicts sprinting ability and racing stamina in thoroughbred horses. PLoS One 5: e8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. Y. W., Shapiro B., 2011. Skyline-plot methods for estimating demographic history from nucleotide sequences. Mol. Ecol. Resour. 11: 423–434. [DOI] [PubMed] [Google Scholar]
- van den Hoven R., Gür E., Schlamanig M., Hofer M., Onmaz A. C., et al. , 2015. Putative regulation mechanism for the MSTN gene by a CpG island generated by the SINE marker Ins227bp. BMC Vet. Res. 11: 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley, B., and T. Webb, III (Editors), 1988 Vegetation history, Kluwer Academic Publishers, Dordrecht, The Netherlands. [Google Scholar]
- Imsland F., McGowan K., Rubin C.-J., Henegar C., Sundström E., et al. , 2016. Regulatory mutations in TBX3 disrupt asymmetric hair pigmentation that underlies Dun camouflage color in horses. Nat. Genet. 48: 152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäderkvist Fegraeus K., Johansson L., Mäenpää M., Mykkänen A., Andersson L. S., et al. , 2015. Different DMRT3 Genotypes Are Best Adapted for Harness Racing and Riding in Finnhorses. J. Hered. 106: 734–740. [DOI] [PubMed] [Google Scholar]
- Jansen T., Forster P., Levine M. A., Oelke H., Hurles M., et al. , 2002. Mitochondrial DNA and the origins of the domestic horse. Proc. Natl. Acad. Sci. USA 99: 10905–10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsson H., Schubert M., Seguin-Orlando A., Ginolhac A., Petersen L., et al. , 2014. Speciation with gene flow in equids despite extensive chromosomal plasticity. Proc Natl Acad Sci USA 111: 18655–18660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekna, P., 2009 The Horse in Human History, Cambridge University Press, New York. [Google Scholar]
- King, S.R.B., L. Boyd, W. Zimmerman, and B.E. Kendall, 2015 Equus ferus ssp. przewalskii The IUCN Red List of Threatened Species 2015: e.T7961A45172099. Available at: http://dx.doi.org/10.2305/IUCN.UK.2015-2.RLTS.T7961A45172099.en.
- Kimura B., Marshall F. B., Chen S., Rosenbom S., Moehlman P. D., et al. , 2011. Ancient DNA from Nubian and Somali wild ass provides insights into donkey ancestry and domestication. Proc. R. Soc. Lond. B Biol. Sci. 278: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch P. L., Barnosky A. D., 2006. Late Quaternary Extinctions: State of the Debate. Annu. Rev. Ecol. Evol. Syst. 37: 215–250. [Google Scholar]
- Koenig D., Jiménez-Gómez J. M., Kimura S., Fulop D., Chitwood D. H., et al. , 2013. Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc. Natl. Acad. Sci. USA 110: E2655–E2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzmann N., Brem G., Wallner B., 2014. The domestic horse harbours Y-chromosomal microsatellite polymorphism only on two widely distributed male lineages. Anim. Genet. 45: 460. [DOI] [PubMed] [Google Scholar]
- Kristjansson T., Bjornsdottir S., Sigurdsson A., Andersson L. S., Lindgren G., et al. , 2014. The effect of the “Gait keeper” mutation in the DMRT3 gene on gaiting ability in Icelandic horses. J. Anim. Breed. Genet. 131: 415–425. [DOI] [PubMed] [Google Scholar]
- Li H., Durbin R., 2011. Inference of human population history from individual whole-genome sequences. Nature 475: 493–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Librado P., Sarkissian C. D., Ermini L., Schubert M., Jónsson H., et al. , 2015. Tracking the origins of Yakutian horses and the genetic basis for their fast adaptation to subarctic environments. Proc. Natl. Acad. Sci. USA 112: E6889–E6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren G., Backström N., Swinburne J., Hellborg L., Einarsson A., et al. , 2004. Limited number of patrilines in horse domestication. Nat. Genet. 36: 335–336. [DOI] [PubMed] [Google Scholar]
- Lippold S., Matzke N. J., Reissmann M., Hofreiter M., 2011a Whole mitochondrial genome sequencing of domestic horses reveals incorporation of extensive wild horse diversity during domestication. BMC Evol. Biol. 11: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippold S., Knapp M., Kuznetsova T., Leonard J. A., Benecke N., et al. , 2011b Discovery of lost diversity of paternal horse lineages using ancient DNA. Nat. Commun. 2: 450. [DOI] [PubMed] [Google Scholar]
- Lira J., Linderholm A., Olaria C., Brandström Durling M., Gilbert M. T. P., et al. , 2010. Ancient DNA reveals traces of Iberian Neolithic and Bronze Age lineages in modern Iberian horses. Mol. Ecol. 19: 64–78. [DOI] [PubMed] [Google Scholar]
- Lister A., Kadwell M., Kaagen L. M., Richards M. B., Stanley H. F., 1998. Ancient and modern DNA in a study of horse domestication. Anc. Biomol. 2: 267–280. [Google Scholar]
- Lorenzen E. D., Nogués-Bravo D., Orlando L., Weinstock J., Binladen J., et al. , 2011. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479: 359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Tang T., Tang H., Huang J., Shi S., et al. , 2006. The accumulation of deleterious mutations in rice genomes: a hypothesis on the cost of domestication. Trends Genet. TIG 22: 126–131. [DOI] [PubMed] [Google Scholar]
- Ludwig A., Pruvost M., Reissmann M., Benecke N., Brockmann G. A., et al. , 2009. Coat Color Variation at the Beginning of Horse Domestication. Science 324: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig A., Reissmann M., Benecke N., Bellone R., Sandoval-Castellanos E., et al. , 2015. Twenty-five thousand years of fluctuating selection on leopard complex spotting and congenital night blindness in horses. Phil Trans R Soc B 370: 20130386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch V. J., Bedoya-Reina O. C., Ratan A., Sulak M., Drautz-Moses D. I., et al. , 2015. Elephantid Genomes Reveal the Molecular Bases of Woolly Mammoth Adaptations to the Arctic. Cell Reports 12: 217–228. [DOI] [PubMed] [Google Scholar]
- MacFadden B. J., 2005. Fossil Horses–Evidence for Evolution. Science 307: 1728–1730. [DOI] [PubMed] [Google Scholar]
- Makvandi-Nejad, S., G. E. Hoffman, J. J. Allen, E. Chu, E. Gu et al., 2012 Four Loci Explain 83% of Size Variation in the Horse. PLoS ONE 7: e39929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspinas A.-S., Malaspinas O., Evans S. N., Slatkin M., 2012. Estimating allele age and selection coefficient from time-serial data. Genetics 192: 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden C. D., Vecchyo D. O.-D., O’Brien D. P., Taylor J. F., Ramirez O., et al. , 2016. Bottlenecks and selective sweeps during domestication have increased deleterious genetic variation in dogs. Proc. Natl. Acad. Sci. USA 113: 152–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. M., Schaefer R., Petersen J. L., Morrell P. L., Slamka M. A., et al. , 2014. Evidence of Positive Selection for a Glycogen Synthase (GYS1) Mutation in Domestic Horse Populations. J. Hered. 105: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue M. E., Valberg S. J., Miller M. B., Wade C., DiMauro S., et al. , 2008. Glycogen synthase (GYS1) mutation causes a novel skeletal muscle glycogenosis. Genomics 91: 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCue M. E., Anderson S. M., Valberg S. J., Piercy R. J., Barakzai S. Z., et al. , 2010. Estimated prevalence of the Type 1 Polysaccharide Storage Myopathy mutation in selected North American and European breeds. Anim. Genet. 41: 145–149. [DOI] [PubMed] [Google Scholar]
- McCue M. E., Bannasch D. L., Petersen J. L., Gurr J., Bailey E., et al. , 2012. A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies. PLoS Genet. 8: e1002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGivney B. A., Browne J. A., Fonseca R. G., Katz L. M., MacHugh D. E., et al. , 2012. MSTN genotypes in Thoroughbred horses influence skeletal muscle gene expression and racetrack performance. Anim. Genet. 43: 810–812. [DOI] [PubMed] [Google Scholar]
- Meadows J. R. S., Hawken R. J., Kijas J. W., 2004. Nucleotide diversity on the ovine Y chromosome. Anim. Genet. 35: 379–385. [DOI] [PubMed] [Google Scholar]
- Meiri M., Huchon D., Bar-Oz G., Boaretto E., Horwitz L. K., et al. , 2013. Ancient DNA and Population Turnover in Southern Levantine Pigs- Signature of the Sea Peoples Migration? Sci. Rep. 3: 3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M., Kircher M., Gansauge M.-T., Li H., Racimo F., et al. , 2012. A High-Coverage Genome Sequence from an Archaic Denisovan Individual. Science 338: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanaelsson C., Oskarsson M. C., Angleby H., Lundeberg J., Kirkness E., et al. , 2006. Dog Y chromosomal DNA sequence: identification, sequencing and SNP discovery. BMC Genet. 7: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollivier M., Tresset A., Hitte C., Petit C., Hughes S., et al. , 2013. Evidence of Coat Color Variation Sheds New Light on Ancient Canids. PLoS One 8: e75110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando L., 2015. The first aurochs genome reveals the breeding history of British and European cattle. Genome Biol. 16: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando L., Willerslev E., 2014. An epigenetic window into the past? Science 345: 511–512. [DOI] [PubMed] [Google Scholar]
- Orlando L., Mashkour M., Burke A., Douady C. J., Eisenmann V., et al. , 2006. Geographic distribution of an extinct equid (Equus hydruntinus: Mammalia, Equidae) revealed by morphological and genetical analyses of fossils. Mol. Ecol. 15: 2083–2093. [DOI] [PubMed] [Google Scholar]
- Orlando L., Metcalf J. L., Alberdi M. T., Telles-Antunes M., Bonjean D., et al. , 2009. Revising the recent evolutionary history of equids using ancient DNA. Proc. Natl. Acad. Sci. USA 106: 21754–21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlando L., Ginolhac A., Zhang G., Froese D., Albrechtsen A., et al. , 2013. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 499: 74–78. [DOI] [PubMed] [Google Scholar]
- Orlando L., Gilbert M. T. P., Willerslev E., 2015. Reconstructing ancient genomes and epigenomes. Nat. Rev. Genet. 16: 395–408. [DOI] [PubMed] [Google Scholar]
- Ottoni C., Flink L. G., Evin A., Geörg C., Cupere B. D., et al. , 2013. Pig Domestication and Human-Mediated Dispersal in Western Eurasia Revealed through Ancient DNA and Geometric Morphometrics. Mol. Biol. Evol. 30: 824–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outram A. K., Stear N. A., Bendrey R., Olsen S., Kasparov A., et al. , 2009. The Earliest Horse Harnessing and Milking. Science 323: 1332–1335. [DOI] [PubMed] [Google Scholar]
- Owen-Smith N., 1987. Pleistocene Extinctions: The Pivotal Role of Megaherbivores. Paleobiology 13: 351–362. [Google Scholar]
- Pakendorf B., Novgorodov I. N., Osakovskij V. L., Danilova A. P., Protod’jakonov A. P., et al. , 2006. Investigating the effects of prehistoric migrations in Siberia: genetic variation and the origins of Yakuts. Hum. Genet. 120: 334–353. [DOI] [PubMed] [Google Scholar]
- Park S. D. E., Magee D. A., McGettigan P. A., Teasdale M. D., Edwards C. J., et al. , 2015. Genome sequencing of the extinct Eurasian wild aurochs, Bos primigenius, illuminates the phylogeography and evolution of cattle. Genome Biol. 16: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen J. S., Valen E., Velazquez A. M. V., Parker B. J., Rasmussen M., et al. , 2014. Genome-wide nucleosome map and cytosine methylation levels of an ancient human genome. Genome Res. 24: 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. L., Mickelson J. R., Rendahl A. K., Valberg S. J., Andersson L. S., et al. , 2013a Genome-wide analysis reveals selection for important traits in domestic horse breeds. PLoS Genet. 9: e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen J. L., Mickelson J. R., Cothran E. G., Andersson L. S., Axelsson J., et al. , 2013b Genetic Diversity in the Modern Horse Illustrated from Genome-Wide SNP Data. PLoS One 8: e54997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promerová M., Andersson L. S., Juras R., Penedo M. C., Reissmann M. et al, 2014. Worldwide frequency distribution of the “Gait keeper” mutation in the DMRT3 gene. Anim. Genet. 45: 274–282. [DOI] [PubMed] [Google Scholar]
- Pruvost M., Bellone R., Benecke N., Sandoval-Castellanos E., Cieslak M., et al. , 2011. Genotypes of predomestic horses match phenotypes painted in Paleolithic works of cave art. Proc. Natl. Acad. Sci. USA 108: 18626–18630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez O., Burgos-Paz W., Casas E., Ballester M., Bianco E., et al. , 2015. Genome data from a sixteenth century pig illuminate modern breed relationships. Heredity 114: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen M., Anzick S. L., Waters M. R., Skoglund P., DeGiorgio M., et al. , 2014. The genome of a Late Pleistocene human from a Clovis burial site in western Montana. Nature 506: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römpler H., Dear P. H., Krause J., Meyer M., Rohland N., et al. , 2006. Multiplex amplification of ancient DNA. Nat. Protoc. 1: 720–728. [DOI] [PubMed] [Google Scholar]
- Sandmeyer L. S., Bellone R. R., Archer S., Bauer B. S., Nelson J., et al. , 2012. Congenital stationary night blindness is associated with the leopard complex in the miniature horse. Vet. Ophthalmol. 15: 18–22. [DOI] [PubMed] [Google Scholar]
- Sarkissian C. D., Vilstrup J. T., Schubert M., Seguin-Orlando A., Eme D., et al. , 2015. Mitochondrial genomes reveal the extinct Hippidion as an outgroup to all living equids. Biol. Lett. 11: 20141058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheu A., Powell A., Bollongino R., Vigne J.-D., Tresset A., et al. , 2015. The genetic prehistory of domesticated cattle from their origin to the spread across Europe. BMC Genet. 16: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraiber, J. G., S. N. Evans, and M. Slatkin, 2016 Bayesian inference of natural selection from allele frequency time series. GENETICS 23: 493–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Jónsson H., Chang D., Der Sarkissian C., Ermini L., et al. , 2014. Prehistoric genomes reveal the genetic foundation and cost of horse domestication. Proc Natl Acad Sci USA 111: E5661–E5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer R. S., Benecke N., Lõugas L., Nelle O., Schmölcke U., 2011. Holocene survival of the wild horse in Europe: a matter of open landscape? J. Quat. Sci. 26: 805–812. [Google Scholar]
- Sundqvist A.-K., Björnerfeldt S., Leonard J. A., Hailer F., Hedhammar Å., et al. , 2006. Unequal Contribution of Sexes in the Origin of Dog Breeds. Genetics 172: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thalmann O., Shapiro B., Cui P., Schuenemann V. J., Sawyer S. K., et al. , 2013. Complete Mitochondrial Genomes of Ancient Canids Suggest a European Origin of Domestic Dogs. Science 342: 871–874. [DOI] [PubMed] [Google Scholar]
- Tozaki T., Miyake T., Kakoi H., Gawahara H., Sugita S., et al. , 2010. A genome-wide association study for racing performances in Thoroughbreds clarifies a candidate region near the MSTN gene: A genome-wide scan for racing performances. Anim. Genet. 41: 28–35. [DOI] [PubMed] [Google Scholar]
- Turner T. L., Hahn M. W., Nuzhdin S. V., 2005. Genomic Islands of Speciation in Anopheles gambiae. PLoS Biol. 3: e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilà C., Leonard J. A., Götherström A., Marklund S., Sandberg K., et al. , 2001. Widespread Origins of Domestic Horse Lineages. Science 291: 474–477. [DOI] [PubMed] [Google Scholar]
- Vilstrup J. T., Seguin-Orlando A., Stiller M., Ginolhac A., Raghavan M., et al. , 2013. Mitochondrial Phylogenomics of Modern and Ancient Equids. PLoS One 8: e55950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade C. M., Giulotto E., Sigurdsson S., Zoli M., Gnerre S. et al, 2009. Genome sequence, comparative analysis, and population genetics of the domestic horse. Science 326: 865–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner B., Vogl C., Shukla P., Burgstaller J. P., Druml T., et al. , 2013. Identification of Genetic Variation on the Horse Y Chromosome and the Tracing of Male Founder Lineages in Modern Breeds. PLoS One 8: e60015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warinner C., Hendy J., Speller C., Cappellini E., Fischer R., et al. , 2014. Direct evidence of milk consumption from ancient human dental calculus. Sci. Rep. 4: 7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmuth V., Eriksson A., Bower M. A., Cañon J., Cothran G. et al, 2011. European Domestic Horses Originated in Two Holocene Refugia. PLoS One 6: e18194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmuth V., Eriksson A., Bower M. A., Barker G., Barrett E., et al. , 2012. Reconstructing the origin and spread of horse domestication in the Eurasian steppe. Proc. Natl. Acad. Sci. USA 109: 8202–8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmuth V. M., Campana M. G., Eriksson A., Bower M., Barker G., et al. , 2013. Ancient trade routes shaped the genetic structure of horses in eastern Eurasia. Mol. Ecol. 22: 5340–5351. [DOI] [PubMed] [Google Scholar]
- Weinstock J., Willerslev E., Sher A., Tong W., Ho S. Y. W., et al. , 2005. Evolution, Systematics, and Phylogeography of Pleistocene Horses in the New World: A Molecular Perspective. PLoS Biol. 3: e241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder M. A., Emshwiller E., Smith B. D., Bradley D. G., 2006. Documenting domestication: the intersection of genetics and archaeology. Trends Genet. 22: 139–155. [DOI] [PubMed] [Google Scholar]
- Zeng K., Fu Y.-X., Shi S., Wu C.-I., 2006. Statistical Tests for Detecting Positive Selection by Utilizing High-Frequency Variants. Genetics 174: 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]