Summary
Mucor fungus infection is a rare opportunistic infection, rapidly progressive and often fatal in immunocompromised patients, or in patients with chronic debilitating diseases. We report six cases of trauma patients with mucormycosis. Three had severe thermal burns, one of them with a medical history of diabetes mellitus. The other three patients suffered from severe soft tissue injuries caused by traffic accidents. In all cases there had been spontaneous exposure and contact of the wounds with soil. During hospitalization, fungi cultures and/or biopsies of all wounds were performed and all resulted positive. The patients were treated with Amphotericin B (AmB) and surgical debridement. Two of them died and the other four were fully healed and discharged. Mucormycosis should be considered in any case of aggressive skin tissue necrosis with a history of soiled wounds. We suggest that mucormycosis is treated by intravenous and local administration of AmB, extensive and repeated debridement and cautious coverage of the wound. The plastic surgeon must wait for negative swab cultures and biopsies before covering the defects with skin grafts or flaps. Reconstruction may be challenging, depending on the extent, depth, location and special indications of the affected site and the donor site availability.
Keywords: infection fongique, mucor mycose, brûlures, traumatismes
Abstract
L’infection fongique (mucor mycose) est une infection rare, opportuniste, d’aggravation rapide et d’évolution souvent fatale chez les patients immunodéprimés ou porteurs d’affections chroniques fragilisantes. Nous rapportons 6 cas de traumatismes avec mucor mycoses; 3 d’entre eux présentaient des brûlures thermiques sévères: l’un était atteint de diabète, les 3 autres patients souffraient de graves lésions des parties molles secondaires à des accidents de circulation; dans tous les cas, il s’agissait de plaies largement ouvertes et souillées; pendant l’hospitalisation les cultures ou les biopsies objectivaient la présence fongique; les patients furent traités par Amphotiricine B (AmB) et détersion chirurgicale; 2 patients moururent et les 4 autres évoluèrent vers la cicatrisation complète. La mucor mycose doit être évoquée devant tout cas de lésions cutanées profondes et nécrotiques avec notion de souillure. Nous suggérons que la mucor mycose soit traitée par administration intraveineuse et locale d’AmB avec détersion répétée, large et protection prudente de la plaie. Le chirurgien plastique doit attendre la négativité des cultures après écouvillonnage et biopsie avant de couvrir les pertes de substance par greffe ou lambeau. La reconstruction est un challenge qui dépend de l’étendue, de la profondeur, de la localisation et des particularités de la lésion ainsi que de la disponibilité de zone donneuse.
Introduction
Although fungi are common in nature, intrinsic fungal pathogenesis in healthy individuals is low. However, they can cause very aggressive infections in people with certain clinical conditions. The incidence of fungal infections has increased recently, and represents a major issue in burn infections. At the same time, there is a worldwide decrease in bacterial infections due to the fact that burn patients receive better care and more effective antibiotic coverage.1
The two main categories of fungal infections are pathogenic and opportunistic mycoses. Pathogenic mycoses are histoplasmosis, blastomycosis, coccidioidomycosis, paracoccidioidomycosis and cryptococcosis, which can cause progressive and uncontrolled infections in patients with Tcell deficiency. Candidiasis, aspergillosis and mucormycosis are classified as opportunistic mycoses. The increase in the incidence of sepsis and sepsis-related deaths coincides with the rising number of cases of sepsis caused by fungal organisms. Candida spp. is the most common cause of fungal sepsis and the fourth most common organism overall which can cause infection. Helpful microbiological and histological examinations are: arterial and venous blood cultures, dermal tissue biopsies, fungal cultures from bronchoscopy, urine cultures and retinal examination of the characteristic lesions of Candida. Candida species are frequently encountered as part of the human flora, responsible for the colonization of the burn wound. On the contrary, aspergillus and mucor are soil fungi. Opportunistic mycoses can occur only when there is phagocyte dysfunction.
Rhizopus, Absidia, Rhizomucor and Basidiobolus are the most common fungal species responsible for mucormycosis infection, and they all belong to the broader taxonomic order of Mucorales.2,3
In burn patients, normal bacterial flora is suppressed due to the prolonged period of treatment with antibiotics and this, coupled with the omnipresence of fungi in the environment, promotes fungal infection.4,5 Predisposing factors for severe fungal infections are: treatment with high doses of glycocorticoids and antibiotics, diabetes mellitus and other degenerating diseases, such as immunosuppression and malignancy.6 True fungi are not uncommon and have much greater invasive potential than expected; in patients with predisposing factors under hospitalization their invasive potential is even greater.7,8,9 Most infections are misdiagnosed due to lack of clinical awareness, similar presentation to a bacterial infection and paucity of mycology laboratories. Nonetheless, early diagnosis and treatment of these mycoses can be life-saving as the mortality rate is otherwise very high.1
Mucor infection may involve the skin, lungs or brain (rhinocerebral infection is associated with diabetes) and can cause arterial invasion with embolization, thrombosis and infarction (angio-invasive). It can also cause vascular invasion by hyphae, which leads to progressive tissue necrosis. Skin biopsies must be multiple and repeated because some tissue samples can be negative if the necrotic tissue does not contain any organisms. Positive biopsies reveal broad, non-septate hyphae with an irregular diameter. 1 The suggested therapy is systemic intravenous and topical administration of AmB (lipid formulation or deoxycholate), itraconazole, ketoconazole, voriconazole and immediate wound debridement, reducing the fungal load with early wound closure.10,10
We report six cases of burn injury and soft tissue trauma complicated by invasive cutaneous fungal infection caused by mucormycosis (Table I). All the patients were treated in our Plastic Surgery Department and Burn Unit, which is a national referral center and designated trauma service in Athens, Greece.
Table I. Our six reported cases of burn injury and soft tissue trauma that were complicated by mucormycosis.
In the 10 year period of our retrospective study (2005-2014), 477 adult patients were admitted to our Burn Unit, in accordance with the inclusion criteria of the American Burn Association11 and 3 burn patients in total were diagnosed with cutaneous mucormycosis (0.63%). In a recent retrospective study by Schaal et al., the incidence of mucormycosis infection in a French military burn center was similar (0.5%),12 unlike the results of Katz et al., who reported that the incidence of non-candida fungal infections was only 0.04% among acute burns treated in their institution, with 2 out of the 12 non-candida infected patients diagnosed with Mucor species. It should be noted that the total reference population (3300 patients) was not specified in terms of the extent and severity of burn injuries and thus epidemiology data cannot be compared with our study.13
In our study, mortality associated with mucormycosis sepsis and confirmed by post mortem histology was 33% (2 cases) and the deceased were both burn patients. The patients diagnosed with mucormycosis after severe soft tissue traumas caused in road accidents had a 100% survival rate.
For decades, the mortality rate of mucormycosis has remained ≥40% despite aggressive surgical and antifungal therapy, as reviewed by Spellberg et al.,14 and Brandt et al. described a mortality rate of 100% in patients with Mucor and Alternaria species.15 In our study, despite reviewing 10 years of cutaneous mucormycosal colonisations or infections, we observed a low incidence of these events, which limited statistical analysis.
The diagnosis of non-candida fungal infection in an immunosuppressed burn patient requires timely and effective management to reduce morbidity and mortality. Our institution’s treatment protocols for these infections include extensive surgical debridement at first suspicion of an aggressive non-candida fungal infection, alongside anti-fungal therapy. Zygomycoses, such as Mucor, are known to be particularly aggressive and typically receive radical surgical treatment to stabilize their progression. Our review illustrated a mortality rate of 20%, in the setting of early antifungal therapy and effective surgical debridement. The purpose of this study was to review and analyze the incidence, treatment and outcomes of a limited cohort of 6 burn and soft tissue trauma patients with mucormycosis fungal infections, at our state-wide burns and plastic surgery service.
Wound swabs were regularly performed in order to detect the presence of microorganisms, as part of our standard of care. In cases of clinically suspected wound infection, culture of full thickness skin biopsy was performed either directly on full thickness burns/necrotic tissue before excision, or after a swab culture positive for moulds on other sites. Other cutaneous and/or blood stream co-infections were also recorded (Table I).
In all 6 cases, the precise location of the initial accident (closed or open space, rural or urban area) was mentioned in the medical records, bypassing the limitations of other studies,12 and this helped us to develop a better understanding of the contamination mode in our patients. All deceased patients (2 cases) underwent post-mortem examination with confirmation of lethal mucormycosis sepsis.
Case Report
1st Case
A 36-year-old man was admitted to our hospital in diabetic shock and with skin-soft tissue necrosis of his right elbow. A month earlier, he had been involved in a road accident and had sustained friction burns on his right upper limb (5% TBSA). His burn wounds and abrasions were treated with oral antibiotics and dressings as an outpatient. Ten days later, he presented with a delayed onset of right elbow infection, complicated by skin and soft tissue necrosis. Despite surgical debridement and empirical alteration antimicrobial therapy, the patient sustained further skin and tissue necrosis (Fig. 1a).
Fig. 1. a) Delayed onset of right elbow infection, complicated by skin and soft tissue necrosis in a patient with untreated diabetes who had sustained friction burns one month earlier; b) Surgical debridement of the necrotic tissue led to elbow joint exposure. Tissue cultures revealed mucormycosis; c) Reconstruction with a pedicled latissimus dorsi musculocutaneous flap; d) Final result with complete wound healing, 1 month post-op.

At that point in time, diabetes mellitus was diagnosed and he was treated accordingly with insulin. He received IV antibiotics pre-operatively and underwent surgical debridement of the necrotic tissue, involving the skin, muscles, radial and ulnar nerves that led to elbow joint exposure (Fig. 1b). Intraoperative tissue samples were sent for biopsy and swab culture, which established the diagnosis of mucormycosis. AmB was immediately added to his therapy regimen, in addition to regular wound dressing changes using silver dressings. Twenty-seven days later, when infection treatment was considered to have been effective, the patient underwent the final surgical debridement and reconstruction with a pedicled latissimus dorsi musculocutaneous flap (Fig. 1c). After a month of hospitalization, he was discharged with completely healed wounds and a prescription for oral antifungal antibiotics (Fig. 1d).
2nd Case
A 38-year-old fireman was admitted to a non-urban hospital for a major burn injury sustained on duty while extinguishing a forest blaze. He had no history of diabetes mellitus or any immunosuppressive disease. The extensive burn injury was estimated to cover 50% TBSA and approximately 20% of it was full-thickness burn. Primary survey reported mild inhalation injury without indication for intubation and the patient was satisfactorily resuscitated and referred to our burn unit. 35% TBSA healed conservatively. Escharectomy and skin grafting were performed on the 15% full-thickness burns. On the 28th post-burn day, a pedicled groin flap was raised to cover a full-thickness defect on his right forearm and wrist. On the 33rd day he presented signs of severe infection with acute necrosis on his skin and subcutis involving both hand and flap. This condition indicated forearm amputation and debridement of the flap donor site. Meanwhile, the patient became septic and developed multiple organ failure. On the 39th day, he presented necrotizing infection of the abdominal wall, groin and thigh with a remaining 5% TBSA burn. The patient’s overall condition was extremely critical. He was intubated due to sepsis, in the presence of low blood pressure, extensive oedema, jaundice and impaired hepatic and renal function. At this point in time, the specimen for swab culture and incisional biopsy from the infected area revealed mucor fungus infection. The patient was treated with AmB at a daily dosage of 0.5 mg/kg body weight intravenously, taking into consideration serum creatinine levels. The patient underwent repeated surgical debridements down to the level of the femoral vessels and a broad necrotic area around the umbilicus was excised. The excised areas were locally covered with soaked AmB dressings twice a day. The necrosis rapidly spread in depth and over the wound margins. The patient’s condition gradually deteriorated and the patient succumbed on the 48th post-burn day.
3rd Case
The third patient was a 47-year-old male with 45% TBSA thermal injuries that occurred during a forest blaze, with coexisting inhalation injury. The patient prevented charring by digging a hole in the ground and covering himself with soil.
He presented to our hospital with clinical signs of severe infection and acute necrosis of the skin. The necrosis appeared in non-burned areas of his skin and muscles such as on the rectus abdominis muscle, in soft tissue and abdominal skin (Fig. 2a).
Progressively, he became septic and developed multiple organ failure. Cultures of swabs and incisional biopsies from the infected areas of the patient revealed Rhizopus Stolonifer. He was treated with AmB 0.5 mg/kg body weight per day intravenously. He also received enteral nutrition solutions.
Despite early systemic antimycotic treatment and serial surgical debridements of the infected tissues, including amputation of his right arm, his general condition gradually deteriorated and the patient succumbed (Fig. 2b).
Fig. 2. a) In a 47-year-old male with 45% TBSA, necrosis of skin and soft tissue appeared in non-burned areas, such as the abdomen. Biopsies from the infected areas revealed Rhizopus Stolonifer; b) Despite early systemic antimycotic treatment, compulsory serial surgical debridements of the infected tissues led to amputation of his right arm.
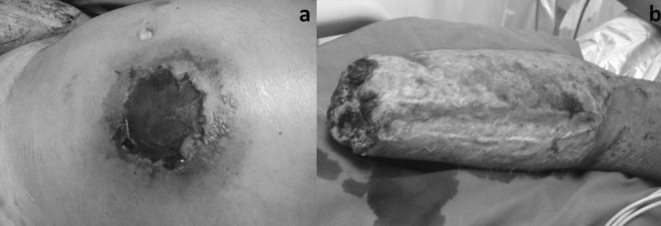
4th Case
The fourth patient was a 63-year-old male, who had been involved in a road accident as a pedestrian and suffered a degloving injury to his right thigh. He was admitted to our plastic surgery department after three days of hospitalization in an orthopaedic clinic at a peripheral hospital. He presented with high fever and bad general condition. The skin of the thigh and subcutaneous tissue was necrotised (Fig. 3a). Surgical debridement and tissue culture specimens were performed immediately (Fig. 3b). A vacuum sponge was set on the 7% TBSA deficit. The cultures revealed a fungal infection (Rhizomucor) and AmB was administered immediately. The patient’s medical record was free of diseases. After four days, his fever dropped and there was an improvement in his general condition. The first change of the vacuum device revealed a clean wound and new swab cultures were negative (Fig. 3c). On the twelfth post-op day, the patient underwent surgery again for wound coverage with split thickness skin grafts (Fig. 3d). He completely recovered after forty-two days of hospitalisation.
Fig. 3. a) 63 year-old male patient, involved in a road accident, suffered a degloving injury on his right thigh; b) Extended surgical debridement resulted in 7% TSBA deficit. Tissue cultures revealed fungal infection (Rhizomucor); c) Vacuum sponge therapy was set and promoted wound bed preparation for final reconstruction; d) Final reconstruction with STSG (split thickness skin grafts), with satisfactory wound coverage.

5th Case
The fifth patient was a 71-year-old male who had partial and full thickness burns covering 50% TBSA and inhalation injury caused by indoor flames from his fireplace. The patient was rescued by neighbours, who carried him outside and laid him down on the ground until the paramedics arrived. He was transferred to our emergency room, underwent acute intubation and was admitted to the Burn Unit. Cultures of the burn surface where positive for fungal infection (Rhizopus). We administered AmB therapy and took him to the operating theatre for an escharectomy. The patient did not have a medical history of diabetes mellitus or any other predisposing factors. One week later, the cultures were negative so we proceeded with STSG coverage of the burn surfaces. The patient made a slow but full recovery after 140 days of hospitalization
6th Case
The sixth patient was a 26-year-old male who had been involved in a motorbike collision and suffered an open thigh fracture. He required orthopaedic surgery to insert an external osteosynthesis for fracture stabilization but this was complicated by skin necrosis. We performed surgical debridement and, meanwhile, cultures and biopsies revealed a fungal infection (Rhizopus). We treated him with I.V. AmB and applied negative pressure wound therapy. After one week, biopsies and cultures were negative and the patient underwent coverage of the skin loss (5% TBSA) with STSG’s. He had had a clear medical record until the accident and eventually fully recovered.
Discussion
Infections in burn and severe soft tissue trauma patients can be caused by bacteria fungi or viruses. Candida is the most common fungal infection, frequently encountered as part of the human flora, but other spores from the environment may colonize the skin as well, such as: aspergillus, rhizopus, absidia, rhizomucor and basidiobolus. Predisposing factors of fungal infections are: a) prolonged period of treatment with antibiotics, b) immunosuppression caused by the burn or preexisting immunological conditions, c) colonization of the wound surface at the place of the accident by soil fungi or colonization in the hospital and d) diabetes mellitus.6
In five of our cases the accident had occurred in an outdoor environment. In three cases, the wounds were smeared with soil; moreover, one of these three patients had untreated diabetes mellitus. In the other three cases, the burns were extensive (50% and 45% TBSA) with inhalation co-injury. None of our patients had a history of immunosuppression, but underwent treatment for their conditions with antibiotics for a long period of time (mean duration 1 month).
Early diagnosis is very difficult to establish or even suspect in the absence of objective characteristic pathognomonic signs of this rare infection, explaining the disappointing rates of pre-mortem diagnosis (15-40%). Mortality is reported to be more than 90%. After diagnosis with swab or/and tissue cultures, alterations in topical agent wound treatment do not seem to influence the survival rate.4,16-21
Since 1928, the group of “fungi” family “Mucoracea” organisms have been referred to, in most papers, as potential pathogens in medical mucology.22 Mucormycosis of the central nervous system was reported by Gregory et al.23 in 1943 and Lecompte et al.24 in 1947. The first two cases of mucormycosis in severely burned patients were reported in 1961 by Rabin et al.25 A year earlier, in 1960, the first case of treatment of mucormycosis with AmB in a non-burned patient had been reported by Prout et al..10 In 1970, an interesting article by Munster et al. reported that IgM antibodies responded to Phycomycosis (Mucormycosis) faster than IgG antibodies.26
The severity of upper extremity fungal invasions secondary to large burns was first reported by Salisbury in 1974.27 An article published by Codish et al. in 1979 points out the importance of tissue biopsies and the use of prophylactic antibiotic agents.28 In 1981, MacMillan reviewed the course of burn wound sepsis in a group of 621 acute patients with only one case of mucormycosis.29 Two more cases were reported in 1983 by Chuntrasakul et al..30 One case successfully treated with AmB on a local fungal infection (petrol bomb burn wound) was reported in 1989 by Goldschmied-Reouven.31 In 1992, Cocanour et al. reported 2 cases with burns greater than 50% TBSA infected by mucormycosis and treated with AmB.32 Another case was reported by Kraut et al., where he performed aggressive surgical debridement and amputation, and administered parenteral AmB in 1993.33 In 1996, Fridkin et al. listed all the different epidemiologic ways of fungal infection in the nosocomial environment. However burn injuries are the traumas with the highest potential and subsequently the Burn Unit is the most common clinical ward of appearance of these infections.34
A case of a burned hand, infected with Rhizopus oryzae, was reported by Lidor et al. in 1997, wherein amputation resulted in successful treatment for the patient.35 Rhinocerebral mucormycosis in a burn patient was also reviewed by Stern in 1999. The author stresses the importance of glucose control, correction of acidosis, treatment with systemic antifungal agents and extensive surgical debridement. 36 In 2001, Tsoutsos et al. reported a case of persisting mucor fungus infection in a burn patient who had previously undergone reconstruction with a pedicled groin flap for forearm and wrist exposure, which led to multiple organ failure and death, despite aggressive treatment with I.V. AmB, repeated debridements, flap removal and amputation.37 Reconstruction of soft tissue necrosis following sino-nasal mucormycosis, using a well-vascularised galeal frontalis-pericranial flap from the mid-forehead region was presented by Lari et al. in 2002.38
Use of contaminated bandages in the Burn Unit was the reason for an outbreak of Absidia corymbifera infection in 2005, according to Christiaens et al.39 In 2006, Vega et al. reported 1 of the 27 worldwide cases of infection by Saksenaea vasiformis in an Ecuadorian adolescent patient, burned in a car accident, who was successfully treated with surgical debridement and AmB.40 In 2008, Ledgard et al. wrote an excellent review on primary cutaneus zygomycosis of burn patients, counting a mortality rate of 31% when treated with antifungal therapy and aggressive surgical debridement or amputation.41 From 2008 to 2012, nine different single cases of burn patients with mucormycosis were reported in nine articles.42-50 In 2009, Branski et al. presented the continuous battle between physicians-antibiotics and microorganisms over the treatment of burn patients facing infections.1
After diagnosis is set, immediate extensive wound debridement accompanied by early coverage of the defective areas with allografts or other temporary biological/artificial skin substitutes, when available in our armamentarium, is considered to be the appropriate treatment of mucormycosis in burn patients.
The plastic surgeon must be patient and wait for negative swab cultures and biopsies before proceeding to the final reconstruction of the defects with skin grafts or flaps. Free flaps are indicated, while pedicled flaps should be avoided due to the possibility of pathogen dissemination to the donor site, as demonstrated in the second case of this report. Haematogenous disseminations to distal sites are also possible, like in case 3 (Fig. 2b). Amputation is inevitable in some cases, but it may determine survival or prolong expectancy. In two cases we performed amputations but did not manage to intercept fatal sepsis. In cases 4 and 6, we performed thorough surgical debridement as soon as possible and proceeded to final coverage of the defects with split thickness skin, after receiving negative cultures and biopsies; the final outcomes justified our treatment strategy. In case 5, burn escharectomies were performed and AmB was administered immediately after receiving the first positive tissue culture, indicating that early diagnosis, systemic antimycotic treatment, reversal of underlying predisposing risk factors when feasible and efficient debridement prove to be the most important lifesaving factors and determine survival.
Although AmB deoxycholate has been the cornerstone of mucormycosis therapy for decades, lipid formulations of AmB (with a possible preference for CNS infections) are less nephrotoxic and can be safely administered at higher doses for a longer period of time than AmB.14
In a 2-case study of rhino-cerebral zygomycosis and an overview of 120 patients from the literature with underlying hematological or oncological disorders, described by Gleissner et al., patients seemed to benefit from liposomal amphotericin B treatment compared to AmB deoxycholate. The presented data documents improved survival (6/9 patients surviving) in cutaneous disease.51
Unfortunately, the currently proposed treatment of mucormycosis infections with lipid formulations of amphotericin has only recently become an available treatment option in Greece, and is still not available in sufficient quantities in public health institutions.
Nevertheless, Chamilos et al. quantified the benefit of early initiation of AmB antifungal therapy. They reported that if treatment was initiated within 5 days of diagnosis of mucormycosis, survival was markedly improved compared to initiation of polyene therapy at ≥6 days after diagnosis (83% vs. 49% survival). Hence, establishing an early diagnosis of mucormycosis is critical to enabling early initiation of active antifungal therapy.52
Wound infection surveillance seems to be the gold standard in order to avoid the devastating results of this rare, life-threatening infection. Regular fungal cultures and tissue biopsies should be performed when signs of fungal infection or sepsis are present. If these patients remain hospitalized for a long period of time or if they have been exposed to prolonged antibiotic therapy, underlying fungal infection should be suspected.
On the other hand, in the unlikely event of wounds complicated by mucormycosis, treatment strategies are summarized in the following algorithm: a) early initiation of intravenous antifungal therapy and local application of impregnated dressings on wound bed; b) extensive and repeated burn eschar and tissue necrosis debridement; c) final reconstruction/coverage of the healthy wound bed.
With this report, our intentions are to add value to the limited list of case study publications on this rare entity, by sharing our experience of the challenging management of patients with burns and other wounds infected by mucormycosis, in order to collaborate in the battle against fungal infection morbidity and contribute to a further reduction in mortality rate for these patients.
Conclusion
Mucormycosis is a rare, opportunistic infection in patients with burns and contaminated open wounds and should be suspected in any case of aggressive skin tissue necrosis. Treatment is challenging, with uncertain outcomes, but in any case must include the following: prompt intravenous and local administration of AmB, extensive and repeated debridement, cautious coverage of the burn wound and diminution of underlying host defects predisposing to infection. The plastic surgeon must be patient and wait for negative swab cultures and biopsies prior to reconstruction. Selection of the appropriate reconstruction option, such as pedicled or free tissue transfer, negative pressure therapy and/or skin autografts is based on the amount of soft tissue needed for coverage, the depth and the anatomical location of the deficit and other special considerations
References
- 1.Branski KL, Al-Mousawi A, Rivero H, Jeschke GM, et al, et al. Emerging Infections in Burns. Surg Inf. 2009;10(5):389–397. doi: 10.1089/sur.2009.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehrer RL, Howard DH, Sypherd PS et al. Mucormycosis. Ann Int Med. 1980;93:93–108. [Google Scholar]
- 3.Tang D, Wang W. Successful cure of an extensive burn injury complicated with mucor wound sepsis. Burns. 1998;24:72–73. doi: 10.1016/s0305-4179(97)00099-5. [DOI] [PubMed] [Google Scholar]
- 4.Bruck HM, Nash G, Stein JM et al. Studies on the occurrence and significance of yeast and fungi in the burn wound. Ann Surg. 1972;176:108–110. doi: 10.1097/00000658-197207000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker WK, Cioffi WG Jr, McManus AT et al. Fungal burn wound infection. A ten-year experience. Arch Surg. 1991;126:44–48. doi: 10.1001/archsurg.1991.01410250048008. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon WH, Bauer H. The role of predisposing factors in experimental fungus infections. Lab Invest. 1962;11:1184–1191. [Google Scholar]
- 7.Zimmerman LE. Fatal fungus infections complicating other disease. Amer J Clin Path. 1955;24:46–65. doi: 10.1093/ajcp/25.1.46. [DOI] [PubMed] [Google Scholar]
- 8.Baker RD. Pulmonary mucormycosis. Amer J Path. 1956;32:287–313. [PMC free article] [PubMed] [Google Scholar]
- 9.Straatsma BR, Zimmerman LE, Gross J et al. Phycomycosis: A clinical co-pathologic study of 51 cases. Lab Invest. 1962;11:963–985. [PubMed] [Google Scholar]
- 10.Prout RG, Goddard AR. Renal Mucormycosis - Survival after Nephrectomy and AmB Therapy. N Engl J Med. 1960;263:1246–1248. doi: 10.1056/NEJM196012152632408. [DOI] [PubMed] [Google Scholar]
- 11.Committee on Trauma American College of Surgeons & American Burn Association: Resources for Optimal Care of the Injured Patient. Guidelines for Trauma Centers Caring for Burn Patients. 2014. pp. 100–106. [Google Scholar]
- 12.Schaal JV, Leclerc T, Soler C, Donat N et al. Epidemiology of filamentous fungal infections in burned patients: A French retrospective study. Burns. 2015;41:853–863. doi: 10.1016/j.burns.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Katz T, Wasiak J, Cleland H, Padiglione A. Incidence of non-candidal fungal infections in severe burn injury: An Australian perspective. Burns. 2014;40:881–886. doi: 10.1016/j.burns.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 14.Spellberg B, Ibrahim AS. Recent Advances in the Treatment of Mucormycosis. Curr Infect Dis Rep. 2010;12:423–429. doi: 10.1007/s11908-010-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brandt ME, Warnock DW. Epidemiology, clinical manifestations, and therapy of infections caused by dematiaceous fungi. J Chemother. 2003;15 (Suppl. 2):36–47. doi: 10.1179/joc.2003.15.Supplement-2.36. [DOI] [PubMed] [Google Scholar]
- 16.Cooper BH. Introduction to clinical mycology. In: Lennette EH, Balows A, Hausler, WJ Jr., Truant JP (eds): “Manual of clinical microbiology”, 3rd ed. Washington, D.C: American Society for Microbiology; 1980. pp. 553–540. [Google Scholar]
- 17.Klein J, Watakunahem C. Hospital-acquired fungemia. Am J Med. 1979;67:51–58. doi: 10.1016/0002-9343(79)90073-1. [DOI] [PubMed] [Google Scholar]
- 18.Pruitt BA. Phycomycotic infections. Probl Gen. Surg. 1984;1:664–678. [Google Scholar]
- 19.Solomkin JS, Floh A, Simmons RL. Candida infections in surgical patients. Ann Surg. 1982;195:177–185. doi: 10.1097/00000658-198202000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gartenberg G, Bottone EJ, Keush GT, Weitzman I. Hospital-acquired mucormycosis of skin and subcutaneous tissue, epidemiology, mycology, and treatment. New Engl J Med. 1978;299:1115–1118. doi: 10.1056/NEJM197811162992007. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein E, Hoeprich PD. Problems in the diagnosis and treatment of systemic conditions. J Infect Dis. 1980;125:190–193. doi: 10.1093/infdis/125.2.190. [DOI] [PubMed] [Google Scholar]
- 22.Casteliani A. Fungi and Fungous Diseases. Chicago,: American Medical Association; 1928. pp. 132–133. [Google Scholar]
- 23.Gregory JE, Golden A, Haymaker W. Mucormycosis of the central nervous system. A report of three cases. Bull Johns Hopkins Hosp. 1943;73:405–419. [Google Scholar]
- 24.Lecompte PM, Meissner WA. Mucormycosis of the Central Nervous System Associated with Hemochromatosis: Report of a case. Am J Pathol, Jul. 1947;23(4):673–677. [PMC free article] [PubMed] [Google Scholar]
- 25.Rabin RE, Lundberg DG, Mitchell TE. Mucormycosis in severely burned patients - report of two cases with extensive destruction of the face and nasal cavity. N Engl J Med. 1961;264:1286–1289. doi: 10.1056/NEJM196106222642504. [DOI] [PubMed] [Google Scholar]
- 26.Munster AM, Hoagland HG, Pruitt BA. The effect of thermal injury on serum immunoglobulins. Jr Ann Surg. 1970;Dec 172(6)::965–969. doi: 10.1097/00000658-197012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salisbury RE, Silverstein P, Goodwin MN. Upper extremity fungal invasions secondary to large burns. Jr Plast Reconstr Surg. 1974;Dec 54(6):654–659. doi: 10.1097/00006534-197412000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Codish SD, Sheridan ID, Monaco AP. Mycotic wound infections. A new challenge of the surgeon. Arch Surg. 1979;Jul 114(7):831–835. doi: 10.1001/archsurg.1979.01370310073013. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan BG. The control of burn wound sepsis. Intensive Care Med. 1981;Jan 7(2):63–69. doi: 10.1007/BF01687262. [DOI] [PubMed] [Google Scholar]
- 30.Chuntrasakul C, Chantarakul N. Mucormycosis in severely burned patients. Report of two cases with extensive destructive lesions. J Med Assoc Thai. 1983;Feb 66(2):132–138. [PubMed] [Google Scholar]
- 31.Goldschmied-Reouven A, Shvoron A, Topaz M, Block C. Saksenaea vasiformis infection in a burn wound. J Med Vet Mycol. 1989;27(6):427–429. [PubMed] [Google Scholar]
- 32.Cocanour CS, Miller-Crotchett P, Reed RL 2nd, Johnson PC, Fischer RP. Mucormycosis in trauma patients. J Trauma. 1992;Jan 32(1):552–556. doi: 10.1097/00005373-199201000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Kraut EJ, Jordan MH, Steiner CR 3rd. Arterial occlusion and progressive gangrene caused by mucormycosis in a patient with burns. J Burn Care Rehabil. 1993;Sep-Oct 14(5):552–556. doi: 10.1097/00004630-199309000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;Oct 9(4):499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lidor C, Nunley JA. Images in clinical medicine. Mucormycosis of the hand and forearm. N Engl J Med. 197;Nov 20, 337(21):1511–1511. doi: 10.1056/NEJM199711203372105. [DOI] [PubMed] [Google Scholar]
- 36.Stern LE, Kagan RJ. Rhinocerebral mucormycosis in patients with burns: case report and review of the literature. J Burn Care Rehabil. 1999;Jul-Aug 20(4):303–306. doi: 10.1097/00004630-199907000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Tsoutsos D, Tsati E, Metaxotos N, Keramidas E et al. Extensive Burn Injury Complicated by Mucormycosis: A case report. Ann Burn Fire Disasters. 2001;v14(3):126–128. [Google Scholar]
- 38.Lari AR, Kanjoor JR, Vulvoda M, Katchy KC, Khan ZU. Orbital reconstruction following sino-nasal mucormycosis. Br J Plast Surg. 2002;Jan 55(1):72–75. doi: 10.1054/bjps.2001.3725. [DOI] [PubMed] [Google Scholar]
- 39.Christiaens G, Hayette MP, Jacquemin D, Melin P et al. An outbreak of Absidia corymbifera infection associated with bandage contamination in a burns unit. J Hosp Infect. 2005;Sep 61(1):88–88. doi: 10.1016/j.jhin.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 40.Vega W, Orellana M, Zaror L, Gené J, Guarro J. Saksenaea vasiformis infections: case report and literature review. Mycopathologia. 2006;Oct 162(4):289–294. doi: 10.1007/s11046-006-0061-6. [DOI] [PubMed] [Google Scholar]
- 41.Ledgard JP, van Hal S, Greenwood JE. Primary cutaneous zygomycosis in a burns patient: a review. J Burn Care Res. 2008;Mar-Apr 29(2):286–290. doi: 10.1097/BCR.0b013e31816673b1. [DOI] [PubMed] [Google Scholar]
- 42.Constantinides J, Misra A, Nassab R, Wilson Y. Absidia corymbifera fungal infection in burns: a case report and review of the literature. J Burn Care Res. 2008;Mar-Apr 29(2):416–419. doi: 10.1097/BCR.0b013e318166da78. [DOI] [PubMed] [Google Scholar]
- 43.Antonetti J, Killyon GW, Chang P, McCauley RL. Microvascular transfer of burned tissue for mandibular reconstruction. J Burn Care Res. 2009;May-Jun 30(3):536–539. doi: 10.1097/BCR.0b013e3181a28e5f. [DOI] [PubMed] [Google Scholar]
- 44.Lipový B, Rihová H, Hanslianová M, Kocmanová I et al. Unsuccessful therapy of combined mycotic infection in a severely burned patient: a case study. Acta Chir Plast. 2009;51(3-4):83–84. [PubMed] [Google Scholar]
- 45.Piazza RC, Thomas WL, Stawski WS, Ford RD. Mucormycosis of the face. J Burn Care Res. 2009;May-Jun 30(3):520–523. doi: 10.1097/BCR.0b013e3181a28d2f. [DOI] [PubMed] [Google Scholar]
- 46.Rapidis AD. Orbitomaxillary mucormycosis (zygomycosis) and the surgical approach to treatment: perspectives from a maxillofacial surgeon. Clin Microbiol Infect. 2009;Oct 15 Suppl 5:98–102. doi: 10.1111/j.1469-0691.2009.02989.x. [DOI] [PubMed] [Google Scholar]
- 47.Woo PC, Lau SK, Ngan AH, Tung ET et al. Lichtheimia hongkongensis sp. nov., a novel Lichtheimia spp. associated with rhinocerebral, gastrointestinal, and cutaneous mucormycosis. Diagn Microbiol Infect Dis. 2010;Mar 66(3):274–284. doi: 10.1016/j.diagmicrobio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Struck MF, Illert T, Stiller D, Steen M. Basilar artery occlusion after multifactor coagulopathy including Rhizopus oryzae infection in burns. J Burn Care Res. 2010;Nov-Dec 31(6):955–958. doi: 10.1097/BCR.0b013e3181f93912. [DOI] [PubMed] [Google Scholar]
- 49.Radowsky JS, Strawn AA, Sherwood J, Braden A, Liston W. Invasive mucormycosis and aspergillosis in a healthy 22-year-old battle casualty: case report. Surg Infect (Larchmt) 2011;Oct 12(5):397–400. doi: 10.1089/sur.2010.065. [DOI] [PubMed] [Google Scholar]
- 50.Li F, Yang HM, Chai JK, Wang HW. Burn wound mucormycosis: a case report. J Burn Care Res. 2012;Jan 33(1):e24–e25. doi: 10.1097/BCR.0b013e3182335a39. [DOI] [PubMed] [Google Scholar]
- 51.Gleissner B, Schilling A, Anagnostopolous I et al. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45:1351–1360. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 52.Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47:503–509. doi: 10.1086/590004. [DOI] [PubMed] [Google Scholar]



