Abstract
Rationale
VRP26 displays mu opioid receptor agonist and delta opioid receptor antagonist activity in vitro, a pharmacological profile purported to produce reduced tolerance, dependence, and rewarding effects. We hypothesized that VRP26 would display reduced adverse effects after chronic administration as compared with the traditional opioid analgesic fentanyl.
Objective
To explore the development of tolerance, dependence and conditioned place preference of VRP26 as compared with the traditional opioid analgesic fentanyl.
Methods
The antinociceptive effects of VRP26 and fentanyl were assessed using the mouse warm water tail withdrawal (WWTW) assay. Measurement of antinociceptive tolerance and physical dependence occurred after seven days of continuous administration of either fentanyl (0.3 mg/kg/day) or VRP26 (10 mg/kg/day); tolerance was measured by a shift in the antinociceptive dose response curve in the WWTW assay. Physical dependence was determined by observation of withdrawal symptoms after precipitated withdrawal. Rewarding effects were measured by the ability of VRP26 or fentanyl to produce conditioned place preference.
Results
Fentanyl produced significant tolerance and dependence, as well as significant conditioned place preference. VRP26 produced neither tolerance nor physical dependence, nor did it produce significant conditioned place preference.
Conclusions
These results suggest that chronic treatment with VRP26 may produce less tolerance or physical dependence than chronic treatment with clinically available mu opioid analgesics such as fentanyl. Additionally, VRP26 produces less rewarding effects than fentanyl. This desirable in vivo profile may be due to the mixed efficacy nature of VRP26 and could provide the framework for safer opioid analgesics.
Keywords: Mixed efficacy, mu opioid receptor, delta opioid receptor, tolerance, dependence, conditioned place preference, reward
Introduction
The growing consensus that the simultaneous modulation of multiple targets often generates a more desirable drug profile has opened new avenues of research in the development of therapeutic agents (Dietis et al. 2009; Morphy et al. 2004; Morphy and Rankovic 2009). This is of particular interest in the field of opioid analgesics, as the co-administration of a mu opioid receptor (MOR) agonist with a delta opioid receptor (DOR) antagonist has been proposed to produce MOR-mediated analgesia with reduced tolerance and dependence liabilities (Abdelhamid et al. 1991; Fundytus et al. 1995; Hepburn et al. 1997; Purington et al. 2009; Schiller 2009a). The development of antinociceptive tolerance often leads to dose escalation which may contribute to the prevalence of opioid misuse and abuse (Ballantyne and LaForge 2007). Additionally, individuals who are dependent on opioids may misuse or abuse opioids to prevent withdrawal (Bailey and Connor 2005; Ballantyne and LaForge 2007; Johnston et al. 2009; Ross and Peselow 2009). Opioid compounds that produce robust analgesia with limited development of tolerance and dependence would meet a significant unmet medical need by addressing these concerns.
For pharmacokinetic simplicity, it is advantageous to combine both MOR agonist and DOR antagonist pharmacophores into a single compound. As a result, ligands that interact simultaneously with both MOR and DOR have been the subject of much research and many peptide, peptide-like, and alkaloid ligands have been described; however, few have the desired profile both in vitro and in vivo (Anand et al. 2012; Anathan et al. 2004; Balboni et al. 2002; Bender et al. 2015; Healy et al. 2013; Purington et al. 2011; Salvadori et al. 1999; Schiller et al. 1999). The peptide DIPPψNH2 (Schiller et al. 1999), the bivalent ligand MDAN-21 (Lenard et al. 2007), and the multifunctional opioid alkaloid UMB425 (Healy et al. 2013) all show some improvement over morphine in vivo, but both DIPPψNH2 and UMB425 produce significant tolerance and dependence after chronic administration. Further, DIPPψNH2 does not cross the blood brain barrier (Schiller 2009b) and MDAN-21 was effective in some (Aceto et al. 2012; Daniels et al. 2005), but not all (Aceto et al. 2012), measures of antinociception. While these compounds demonstrate proof of concept that MOR agonist/DOR antagonist compounds have a more desirable profile than traditional opioid analgesics, there is still room for improvement.
We have previously described a cyclic pentapeptide, VRP26 (Figure 1), which displays low nanomolar affinity for both MOR and DOR, with selectivity relative to KOR as well as agonist activity at MOR similar to morphine and antagonism at DOR in vitro (Mosberg et al. 2014). VRP26 produces dose-dependent antinociception in the warm water tail withdrawal (WWTW) assay in mice after peripheral administration and, more significantly, does not produce acute tolerance (Mosberg et al. 2014). VRP26 is therefore a good lead for further investigation of MOR agonist/DOR antagonist compounds as novel analgesics. In this report we explore the in vivo pharmacology of VRP26 to evaluate 1) its in vivo MOR agonist and DOR antagonist effects, 2) the development of tolerance and dependence after 7d continuous infusion of the lowest, fully effective, acute doses of fentanyl (0.3 mg/kg/day) or VRP26 (10 mg/kg/day), and 3) its rewarding effects.
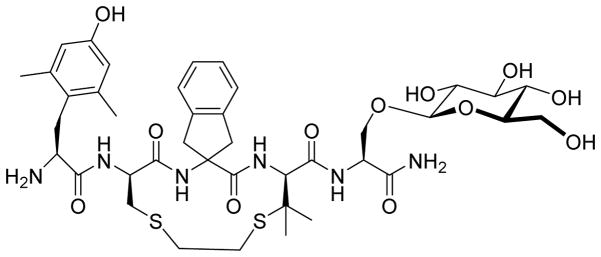
Figure 1.
Structure of MOR agonist/DOR antagonist peptide VRP26 (Dmt-c(SEtS)[DCys-Aic-DPen]-Ser(β-glucose)-NH2
MATERIALS AND METHODS
Peptide Synthesis
VRP26 (Dmt-c(SEtS)[DCys-Aci-DPen]-Ser(Glc)-NH2) was synthesized as previously described (Mosberg et al. 2014).
Drug preparation
All compounds were administered by intraperitoneal (ip) or subcutaneous (sc) injection in a volume of 10 mL/kg of body weight. Fentanyl citrate (Sigma-Aldrich, St. Louis, MO, USA), VRP26 TFA, methylnaltrexone HBr (MNTX) (Sigma-Aldrich, St. Louis, MO, USA), ketamine HCl (Henry Schein, Wixom, MI, USA) and xylazine HCl (Akorn, Decator, IL, USA) were dissolved in sterile saline (0.9% NaCl w/v), SNC80 was dissolved in 3% (v/v) 1 M HCl and brought to volume with sterile water. Naltrindole HCl (NTI) (Tocris Biosciences, Minneapolis, MN, USA) and carprofen (Zoetis Inc., Kalamazoo, MI, USA) were prepared in sterile water.
Animals
Male and female C57BL/6 wild-type mice (n= 209) or homozygous MOR knockout mice and their wild-type littermates (n = 12; Jackson Laboratory) bred in-house and weighing between 20–30g at 8–16 weeks old, were used for behavioral experiments. Mice were group-housed with free access to food and water for all experiments that did not require surgery. Mice that underwent pump implantation were single housed after surgery with free access to food and water. Experiments were conducted in the housing room, maintained on a 12h light/dark cycle with lights on at 7:00am; all experiments were conducted during the light cycle. Each mouse was used in only one experiment either for acute antinociception, antinociceptive tolerance, physical dependence, conditioned place preference, or antidepressant effects in the tail suspension test. Studies were performed in accordance with the University of Michigan Committee on the Use and Care of Animals and the Guide for the Care and Use of Laboratory Animals (National Research Council, 2011 publication).
Antinociception
Antinociceptive effects were evaluated in the WWTW assay. Withdrawal latencies were determined by briefly placing a mouse into a cylindrical plastic restrainer and immersing 2–3 cm of the tail tip into a water bath maintained at 50°C. The latency to tail withdrawal or rapidly flicking the tail back and forth was recorded with a maximum cutoff time of 20 sec to prevent tissue damage.
Acute antinociceptive effects were determined using a cumulative dosing procedure in wild-type or transgenic mice. Each mouse received an injection of saline ip and then 30 min later baseline withdrawal latencies were recorded. Following baseline determinations, increasing cumulative doses of the test compound were given ip or sc at approximate 30 min intervals. Thirty min after each injection, the tail withdrawal latency was measured as described above. Injections were spaced such that there was time for each animal to be restrained, tail withdrawal latency determined, and the next injection given, before the next animal was restrained.
To determine if the antinociceptive effects were peripherally-mediated, mice were injected with either vehicle or 10 mg/kg MNTX ip, a peripherally-restricted opioid antagonist, after baseline determination in the WWTW assay described above. This dose of MNTX has been shown to block peripheral, but not central, effects of opioids (Ramabadran 1982). Following vehicle or MNTX treatment cumulative dosing of test compound was completed as described above.
Effect of Chronic Drug Administration on Tolerance and Dependence
Osmotic pump calibration
Osmotic pumps that deliver 0.5 μL/hr for 7 days were purchased from Alzet (Cupertino, CA). To ensure that the osmotic pumps were delivering the correct volume and concentration of VRP26 drug solution, an osmotic pump was loaded with VRP26 and incubated in 5 mL of 50 mM Tris buffer (pH 7.4) for 1 week at 37°C in a sealed container. A standard curve for VRP26 was established using UV absorption at 230 nm. HPLC was performed on a Waters analytical HPLC; UV absorbance was monitored at 230 nm as described previously (Mosberg et al. 2014) and used to determine the amount of drug delivered under test conditions.
Osmotic pump implantation surgery
Osmotic pumps were loaded with drug or saline solution and incubated for 4 hours at 37°C in sterile saline to prime the pumps for solution delivery prior to implantation. For pump implantation surgery, mice were anesthetized with 100 mg/kg ketamine and 10 mg/kg xylazine ip; carprofen (5 mg/kg sc) was administered as an analgesic. Incisions were made between the scapulae and the subcutaneous space was cleared of connective tissue. Pumps were inserted and the incision closed with sutures. Following surgery, mice were single housed and wound healing was monitored for seven days.
For pump removal mice were anesthetized as described above, given carprofen, and a new incision was made between the scapulae. Pumps were removed and the incision closed with sutures.
Tolerance experimental design
Antinociceptive dose effect curves for VRP26 (n=12) or fentanyl (n=12) were established on the morning of day 1 using the WWTW assay. Each group was then divided such that 6 mice received pumps containing drug and 6 mice received pumps containing saline. The lowest, maximally-effective, acute dose of fentanyl (0.3 mg/kg) or VRP26 (10 mg/kg) in the WWTW assay was delivered by continuous infusion over a 24h period for 7d via osmotic pump (0.3 mg/kg/day fentanyl or 10 mg/kg/day VRP26). Immediately after determining the dose effect curve on day 1, mice were implanted with osmotic pumps (as described above) that were removed on the afternoon of day 7. Cumulative dose/effect curves were established for all mice on the morning of day 8. To determine agonist potency before and after chronic treatment with drug or vehicle, dose-response curves and ED50 values were calculated for each mouse. It was not expected that continuous, slow infusion of 0.3 mg/kg fentanyl over 24 h for 7 d or 10 mg/kg VRP26 over 24 h for 7 d would produce antinociceptive effects and, therefore, was not assessed.
Physical dependence experimental design
On day 1 mice were implanted with pumps containing 0.3 mg/kg/day fentanyl, 10 mg/kg/day VRP26, or saline (n=6 in each group). On the morning of day 7, mice were placed individually in clear plastic observation cages (10 in X 6 in X 8 in) without bedding and given 10 mg/kg naltrexone ip. Pumps were not removed prior to naltrexone administration to prevent spontaneous withdrawal from occurring. Mice were observed for signs of withdrawal for 15 min after naltrexone injection; behaviors were scored as follows and added together to generate a global withdrawal score: one point for the observation of each behavior (teeth chattering, paw tremor, piloerection, wet dog shakes or soft stool) and up to 3 points for jumps: 0 jumps (0 pts), 1–9 jumps (1 pt), 10–19 jumps (2 pts), greater than 20 jumps (3 pts).
Conditioned Place Preference and Locomotor Activity
Apparatus
A two-compartment place conditioning apparatus (MedAssociates, Inc. St. Albans, VT) was used for all conditioned place preference (CPP) studies. The compartmentalized box was divided into two equal size sections (8 in x 5 in x 5 in), accessed through a single, manual, guillotine door. The compartments differed in the wall color and floor texture (black walls with rod flooring vs. white walls with mesh flooring). Time spent in each chamber, number of beam breaks (used as a measure of locomotor activity), and number of entrances to each side were recorded using infrared photobeam detectors.
Conditioned place preference protocol
Experiments consisted of three phases: bias evaluation (2 days), conditioning (5 days), and testing (1 day).
Bias evaluation of CPP
Mice were placed randomly into one chamber on day 1 and the opposite chamber on day 2 and then allowed to freely explore the apparatus for 30 min. Mice that showed a greater than 70% pre-conditioning compartment bias were discarded from the study; two mice were discarded based on this criterion.
Conditioning phase of CPP
Mice were randomly assigned to receive test compound (fentanyl, VRP26, or saline; n=6 in each group) in the black or white chamber. During fentanyl and saline conditioning mice were given a saline injection (ip) and immediately placed in the saline-paired chamber for 30 min; 6 hr later mice were given an injection of fentanyl or saline (ip) and immediately placed in the drug-paired chamber for 30 min. As VRP26 has a slower onset than fentanyl (Mosberg et al. 2014), the conditioning sessions were modified: for VRP26 conditioning, mice were given a saline injection ip and returned to their home cage for 10 minutes before placement in the saline-paired chamber for 30 minutes; 6 hours later mice were given an injection of VRP26 and returned to their home cage for 10 minutes before being placed in the drug-paired chamber for 30 minutes. During all conditioning sessions, movement and activity were recorded.
Test day of CPP
Test day was always the day after the final conditioning session. Mice were randomly placed in either compartment and allowed to roam freely for 30 minutes. No injection was given on test day. Time spent in each chamber, beam breaks, and entrances to each side were recorded. CPP scores were calculated as the difference between time spent on the drug-paired side on test day and the average of time spent on the future drug-paired side on the two bias evaluations.
Tail Suspension Test (TST)
Mice were pretreated with either vehicle, 3.2 mg/kg naltrindole (NTI), 10 mg/kg VRP26, or 0.3 mg/kg fentanyl sc 30 min prior to injection of 3.2 mg/kg SNC80 or vehicle sc. Thirty minutes after SNC80 (or vehicle) injection, mice were suspended by their tail from a height of ~40 cm using tape for 6 minutes and behavior was recorded using a Sony HDR-CX220 digital camcorder. Videos were scored by observers blind to the testing conditioning and the total time mice spent immobile was summed for each animal and then averaged within each treatment group. Immobility was defined as the animal remaining motionless or making only minor, non-escape related movements.
Determination of Relative Efficacy in vitro
In vitro data for binding affinity and GTPγS binding for VRP26 and fentanyl were obtained as described in a previous report (Mosberg et al. 2014). The Ki for VRP26 and fentanyl were determined in buffer consisting of 50 mM Tris buffer, 100 mM NaCl, 5mM MgCl2, 1 mM EDTA (pH 7.4) Relative efficacy was calculated using the Ehlert equation: ½(Emaxtest/Emaxstandard)(1 + (KDstandard/EC50test)) = relative efficacy (Quock et al. 1999).
Statistics and Data Analysis
Statistical comparisons were made by either a Student’s two-tailed t test, a one way, two way repeated measures, or factorial ANOVA with Tukey’s post-hoc tests. Statistical analyses were performed using GraphPad Prism version 6.0 or SPSS version 22.
To calculate ED50 values for each mouse in the warm water tail withdrawal assay, the 50% level of maximum effect was determined from a linear regression analysis of individual latency to tail flick data, using only the linear portion of the curve and including only one dose that produced <10% of the baseline latency and one dose that produced >90% of the maximum latency. ED50 values from each mouse were then averaged across treatment group; group averages were compared using a Student’s t test. Data are presented as mean ± standard error of the mean (SEM) for each treatment group before and after chronic treatment.
RESULTS
Comparison of acute antinociceptive effect following ip vs sc administration
The antinociceptive effects of VRP26 and fentanyl were assessed using the mouse WWTW assay after both ip and sc administration (Figure 2A). VRP26 when given ip or sc produced a maximal effect at 10 mg/kg with similar ED50s (5.44 ± 0.10 mg/kg and 5.08 ± 0.23 mg/kg, respectively). Following ip and sc administration, 0.3 mg/kg fentanyl also produced similar maximal effects and had similar ED50s (0.17 ± 0.013 mg/kg and 0.20 ± 0.001 mg/kg, respectively).
Figure 2.
(A) Cumulative antinociceptive dose response curves for fentanyl ip (n=6), fentanyl sc (n=4), VRP26 ip (n=6), and VRP26 sc (n=4) in the mouse warm water tail withdrawal assay. (B) Cumulative antinociceptive dose response curves for VRP26 ip in mu knock-out mice (n=6) and their wild type littermates (n=6) in the mouse warm water tail withdrawal assay (C) Cumulative antinociceptive dose response curves for VRP26 in animals pretreated with vehicle (n=6) or 10 mg/kg methylnaltrexone (MNTX, n=6) a peripherally-restricted opioid antagonist. Data are plotted as mean +/− standard error of the mean (SEM) for all groups.
Comparison of acute antinociceptive effects in MOR knock-out mice and wild type littermates
The antinociceptive effects of VRP26 was assessed using the mouse WWTW assay in MOR knock-out mice and their wild type littermates to determine if antinociceptive effects were mediated by MOR in vivo (Figure 2B). Consistent with earlier results, VRP26 produced a maximal antinociceptive effect in wild type littermates at 10 mg/kg with and ED50 of 4.77 ± 0.32 mg/kg. In MOR knock-out mice, VRP26 failed to produce a significant increase in tail withdrawal latency up to 32 mg/kg. Baseline withdrawal latencies for MOR knock-out mice and their wild type counterparts were not significantly different, but 10 mg/kg VRP26 significantly increased withdrawal latency in wild type mice as compared with MOR knock-out littermates (t(10) = 9.161, p < 0.0001).
Evaluation of peripherally-mediated antinociceptive effects of VRP26
The antinociceptive effects of VRP26 were assessed using the mouse WWTW assay in animals pretreated with either vehicle or 10 mg/kg methylnaltrexone (MNTX) ip to determine if antinociception produced by VRP26 is peripherally-mediated in vivo (Figure 2C). This dose of MNTX was shown to block peripherally-mediated, but not centrally-mediated, opioid effects in mice (Ramabadran 1982). In both the vehicle and MNTX pretreated mice, VRP26 produced dose-dependent increases in tail withdrawal latency, with a maximal response at 10 mg/kg VRP26 ip and ED50 values that were not statistically different (4.73 ± 0.22 mg/kg for vehicle pretreated mice and 4.73 ± 0.13 mg/kg for MNTX pretreated mice). A two way ANOVA showed a significant effect dose of VRP26 (F (4, 40) = 393.6, p < 0.0001), no effect of pretreatment (vehicle vs MNTX) and no interaction between dose of VRP26 and pretreatment.
Chronic Treatment Studies
For both antinociceptive tolerance and physical dependence studies, mice were implanted with osmotic pumps sc to deliver drug continuously for 7 days. To ensure that VRP26 was stable under these conditions and eliminated from the pumps as expected, a pump loaded with VRP26 was incubated in 5 mL of 50 mM Tris buffer (pH 7.4) for seven days at 37°C. Over the course of seven days, the pump delivered >95% of the expected drug concentration as determined via analytical HPLC, indicating that the drug delivery occurred as expected and that VRP26 was stable under these conditions (data not shown).
Antinociceptive Tolerance
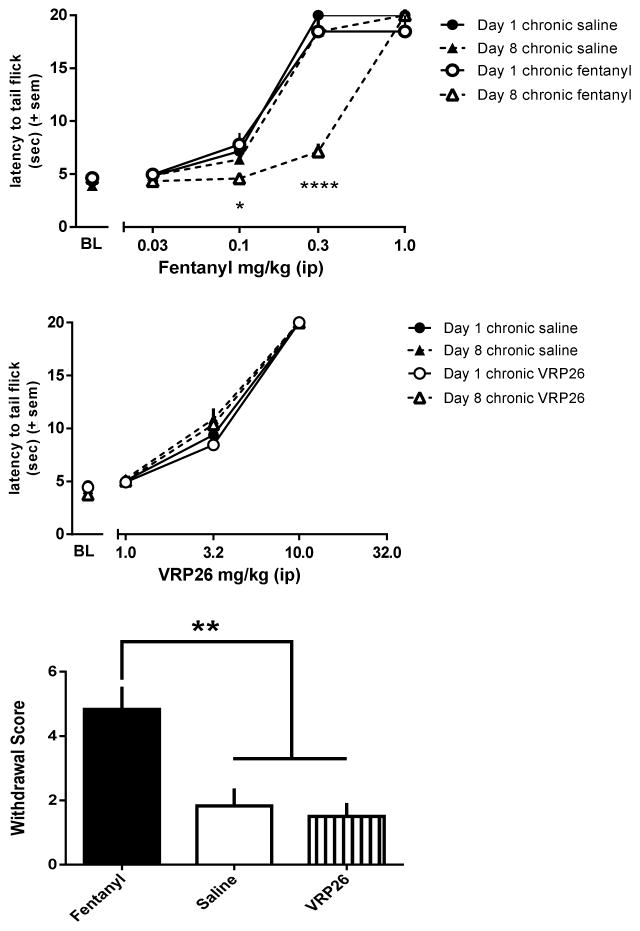
To explore the effects of chronic treatment with fentanyl, mice were treated for 7 days with either saline or 0.3 mg/kg/day fentanyl (Figure 3A). A factorial ANOVA comparing fentanyl dose effect curves before and after chronic treatment showed an interaction (fentanyl dose X day tested X chronic treatment; F(4,40) = 12.17, p < 0.0001), as well as a main effects of fentanyl dose (F(4,40) = 499.91, p < 0.0001), day (1 vs 8; F(1,10) = 21.73, p < 0.001), and chronic treatment (saline vs fentanyl, F(1,10) = 7.691, p = 0.02). Chronic fentanyl, but not chronic saline, treatment produced a three-fold rightward, parallel shift in the fentanyl dose response curve (chronic treatment X day interaction (F(1,10) = 36.36, p = 0.01)). After 7 days of continuous administration of 0.3 mg/kg/day fentanyl, the ED50 of the fentanyl dose effect curve was 3-fold larger on day 8 (0.57 ± 0.018 mg/kg) as compared with day 1 (0.18 ± 0.006 mg/kg, t(10) = 18.71, p < 0.0001). The fentanyl dose effect curves in mice treated for 7 days with saline were not different on day 1 and 8 (ED50 Day 1 0.18 ± 0.006 mg/kg vs ED50 Day 8 0.23 ± 0.04 mg/kg).
Figure 3.
Cumulative antinociceptive dose response curve for fentanyl ip in mouse warm water tail withdrawal assay before and after (A) 7 days of chronic 0.3 mg/kg/day fentanyl treatment sc (n=6) or 7 days chronic saline treatment sc (n=6) (B) 7 days of chronic 10 mg/kg/day VRP26 treatment sc (n=6) or 7 days chronic saline treatment sc (n=6). (C) Global withdrawal scores for animals treated for 7 days with either 0.3 mg/kg/day fentanyl (n=6), 10 mg/kg/day VRP26 (n=6), or saline (n=6) sc. Withdrawal was precipitated with 10 mg/kg naltrexone ip and jumps, wet dog shakes, paw tremor, soft stool, teeth chattering, and piloerection were all counted as physical symptoms of withdrawal. Data are plotted as mean +/− standard error of the mean (SEM) for all groups. * p< 0.05; **p< 0.01; **** p< 0.0001
Separate groups of mice were treated for 7 days with either saline or 10 mg/kg/day VRP26 (Figure 3B). A factorial ANOVA of the VRP26 dose effect curves before and after chronic treatment showed no interaction between factors (VRP26 dose X day tested X chronic treatment). There was a main effect of VRP26 dose (F(3,8) = 4859.98, p < 0.0001), demonstrating that VRP26 produced dose-dependent increases in antinociceptive effects, but there was no effect of day tested (before and after chronic treatment) or chronic treatment (saline vs VRP26). There was no shift in the dose response curve for VRP26 before and after chronic treatment in either saline or VRP26 treated groups (Day 1 ED50 5.35 ± 0.17 mg/kg saline treated group, 5.54 ± 0.12 VRP26 treated group; Day 8 ED50 5.07 ± 0.23 mg/kg saline treated group, 5.16 ± 0.17 VRP26 treated group).
Physical Dependence
The effects of chronic treatment on the development of physical dependence were also explored (Figure 3C). Naltrexone (10 mg/kg, ip) after chronic treatment with 0.3 mg/kg/day fentanyl precipitated significantly more withdrawal signs than after chronic saline treatment (p < 0.01) or chronic VRP26 treatment (p < 0.001), (one way ANOVA, (F(2, 15) = 10.40; p = 0.002). There were no differences in the number of withdrawal signs observed following chronic treatment with 10 mg/kg/day VRP26 or saline.
Conditioned Place Preference
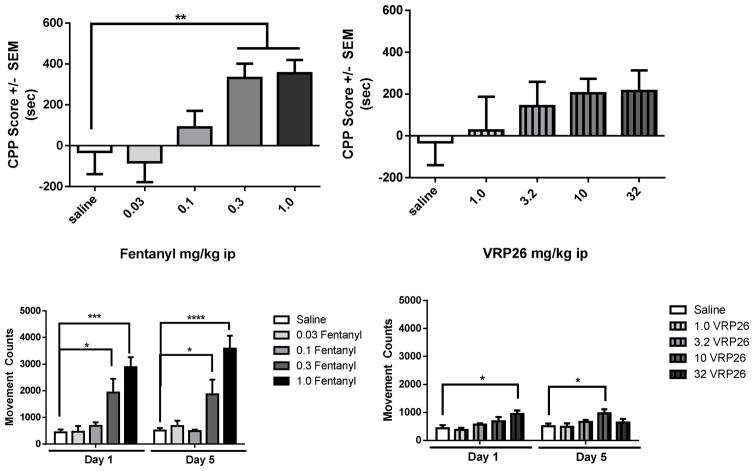
The rewarding effects of both fentanyl and VRP26 were explored using the conditioned place preference (CPP) assay (Figure 4). Fentanyl produced robust dose-dependent increases in time spent on the fentanyl-paired side of the apparatus (one way ANOVA: (F(4,36) = 5.31, p = 0.0018, Figure 4A). Mice conditioned with 0.3 mg/kg or 1.0 mg/kg fentanyl, spent more time on the drug-paired side of the apparatus than animals conditioned with saline (p < 0.01). While conditioning with VRP26 slightly increased time spent on the VRP26-paired side of the apparatus, the difference between saline conditioning and VRP26 conditioning was not statistically different at any of the doses tested (Figure 4B).
Figure 4.
Conditioned place preference (CPP) scores for animals trained for 5 days on various doses of fentanyl or VRP26 (ip). CPP scores are defined as the difference between time spent on drug paired side pre- and post- conditioning measured in seconds. (A) Animals conditioned with fentanyl showed significant place preference at doses of 0.3 and 1.0 mg/kg (ip) (p < 0.01). (B) Animals conditioned with VRP26 did not show significant place preference at any of the tested doses. Dose response curves for locomotor activity over 30 mins for (C) fentanyl and (D) VRP26 on the afternoon of Day 1 and Day 5. Data are plotted as mean +/− standard error of the mean (SEM) for all groups (n=7–11 for each group) * p< 0.05, **p< 0.01, ***p< 0.001, ****p< 0.0001
Locomotor activity was recorded during all conditioning sessions. Fentanyl produced dose-dependent increases in locomotor activity (main effect of dose: F(4,37) = 11.90, p < 0.0001), with increases in activity on both day 1 and day 5 at 0.3 mg/kg ip (p < 0.05) and 1.0 mg/kg ip (p < 0.0001) compared with saline (Figure 4C). However, there were no interactions between day and dose demonstrating that fentanyl-induced locomotor activity did not change with daily administration for 5 d. VRP26 also produced dose-dependent increases in locomotor activity on both day 1 and day 5 (Figure 4D); a factorial ANOVA of these data showed a main effect of dose (F(3, 62) = 3.71, p = 0.02), as well as an interaction of day X dose (F(3, 31) = 3.65, p = 0.02). 32 mg/kg VRP26 ip increased locomotor activity on day 1 only (p < 0.05) and 10 mg/kg VRP26 ip increased locomotor activity (p < 0.05) on day 5 only.
Antagonism of DOR
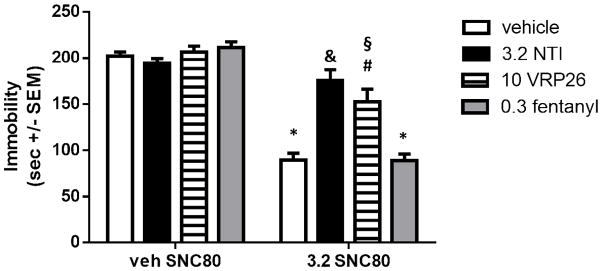
We sought to confirm that VRP26 was a centrally-active DOR antagonist by examining whether it was capable of blocking the antidepressant-like effects of a DOR agonist, SNC80, in the tail suspension test (TST; Figure 5). Animals were pretreated with vehicle, the prototypic DOR antagonist naltrindole (NTI, 3.2 mg/kg), VR26 (10 mg/kg), or fentanyl (0.3 mg/kg), then administered either 3.2 mg/kg SNC80 or vehicle. A two way ANOVA showed a significant main effect of SNC80 dose (F (1, 51) = 123.8, p < 0.0001) and pretreatment (NTI, VRP26, fentanyl, or vehicle, F (3, 51) = 8.518, p < 0.0001)), as well as an interaction of pretreatment X dose (F (3, 51) = 12.50, p < 0.0001). Mice treated with 3.2 mg/kg SNC80 (sc) alone displayed a decrease in immobility in the mouse TST as compared with vehicle treated mice (p < 0.0001). This SNC80-induced decrease in immobility was blocked by pretreatment with 3.2 mg/kg NTI sc (p < 0.0001); NTI pretreated mice had immobility scores that were not statistically different from immobility in vehicle treated mice. Pretreatment with 10 mg/kg (sc) VRP26 partially blocked SNC80-induced decreases in immobility, such that VRP26 pretreatment was significantly different from both SNC80 alone (p < 0.001) and vehicle pretreatment (p < 0.01). VRP26 alone has no effect on immobility as compared with control. Pretreatment with fentanyl had no effect on immobility scores in vehicle- or SNC80-treated mice.
Figure 5.
Animals were pretreated with either vehicle, 3.2 mg/kg naltrindole (NTI), 10 mg/kg VRP26, or 0.3 mg/kg fentanyl sc. Thirty minutes later animals were given 3.2 mg/kg SNC80 or vehicle sc. After 30 minutes, animals were suspended by their tail and their behavior was recorded for 6 minutes. Immobility was defined as the animal remaining motionless or making only minor, non-escape related movements. Data are plotted as mean +/− standard error of the mean (SEM) for all groups (n=6–10 for each group) * denotes p < 0.0001 relative to veh/veh, § denotes p < 0.01 relative to veh/veh # denotes p <0.001 relative to veh/SNC80, & denotes p<0.0001 relative to veh/SNC80.
Determination of Relative Efficacy in vitro
The relative efficacy of VRP26 and fentanyl at MOR were determined in vitro relative to the standard agonist DAMGO to examine if the lack of tolerance to chronic treatment with VRP26 is due to differences in relative efficacy. It was found that fentanyl is more efficacious (relative efficacy 0.5) than VRP26 (relative efficacy 0.28).
DISCUSSION and CONCLUSIONS
We have previously described the novel peptide MOR agonist/DOR antagonist compound, VRP26, which produced opioid receptor-mediated, dose-dependent antinociception in vivo after peripheral administration, without the development of acute tolerance (Mosberg et al. 2014). Consistent with its in vitro profile, VRP26 also demonstrates MOR agonist and DOR antagonist activity in vivo. VRP26 produces maximal antinociceptive effects in WWTW by MOR activation; these antinociceptive effects are attenuated by administration of a MOR antagonist and genetic deletion. Additionally, by blocking DORs, VRP26 attenuates the antidepressant-like effects of the DOR agonist SNC80 in the tail suspension test. Although VRP26 levels in brain tissue were not measured directly, VRP26 likely enters the central nervous system, as it attenuates the centrally-mediated antidepressant-like effects and its antinociceptive effects are not blocked by the peripherally-restricted opioid antagonist methylnaltrexone (MNTX). Together these results suggest that the antinociceptive effects of VRP26 are not mediated by peripheral mechanisms alone; however, future PK studies will examine CNS penetration of VRP26 in vivo.
In this report we evaluated the development of tolerance and physical dependence following chronic administration of VRP26. In contrast to their acute antinociceptive effects, fentanyl and VRP26 have significantly different effects following chronic administration. Continuous administration of 10 mg/kg/day VRP26 does not shift its antinociceptive dose effect curve (Figure 3B) in this warm water tail withdrawal procedure, demonstrating that, unlike fentanyl (Figure 3A), antinociceptive tolerance does not develop to VRP26 under these conditions.
While we attribute the lack of tolerance development under these conditions to be due to DOR antagonism, it is possible that differences in tolerance development between fentanyl and VRP26 could be due to differences in relative efficacy. High efficacy agonists have large receptor reserves and, therefore, are less affected by the loss of receptors on the cell surface as the result of desensitization and down-regulation (i.e., greater receptor reserve). Therefore, we evaluated the relative efficacy of VRP26 and fentanyl in vitro as compared to the standard MOR agonist DAMGO. Fentanyl is more efficacious (relative efficacy 0.5) than VRP26 (relative efficacy 0.28) in vitro, suggesting that the difference in antinociceptive tolerance is not due to greater relative efficacy and receptor reserve. The pharmacokinetic properties of fentanyl and VRP26 should also be considered when discussing the development of antinociceptive tolerance. VRP26 has a slightly shorter duration of action than fentanyl (Mosberg et al. 2014), and as a result, continuous infusion of fentanyl may achieve a higher steady-state concentration than VRP26, which could contribute to the difference in tolerance development between fentanyl and VRP26. It is also possible that a more aggressive dosing regimen or longer treatment with VRP26 might produce antinociceptive tolerance. These points warrant further investigation with repeated and prolonged treatment regimens using larger doses of VRP26 that produce acute antinociceptive effects. However, based on the current data, we would expect less tolerance to develop to VRP26 than to opioid analgesics such as morphine or fentanyl. Additionally, tolerance to the antinociceptive effects of VRP26 may develop differentially across various pain assays, especially procedures more relevant to clinical settings such as chronic pain or pain-suppressed behavior. Future studies with VRP26 will evaluate longer drug exposures, different types of pain (e.g., chronic pain), and development of cross-tolerance with traditional opioid agonists.
We also examined the development of physical dependence following seven day continuous treatment of fentanyl or VRP26. Fentanyl produced significantly more physical dependence than VRP26 (Figure 3C), as naltrexone failed to precipitate withdrawal signs in VRP26-treated mice. Opioid analgesics with reduced development of physical dependence fulfill an unmet medical need as opioid dependence may lead to misuse and abuse of opioids to avoid withdrawal symptoms (Bailey and Connor 2005; Ballantyne and LaForge 2007; Johnston et al. 2009; Ross and Peselow 2009). In this report, we also explored the rewarding properties of VRP26 by examining its ability to induce conditioned place preference. In contrast to fentanyl, VRP26 produced only modest conditioned place preference and locomotor stimulation, suggesting that VRP26 may be less rewarding than fentanyl and may differentially affect dopaminergic reward pathways.
In conclusion, mixed efficacy compounds, such as VRP26, may be safer alternatives to traditional opioid analgesics as they could provide continued pain relief without tolerance, dependence, and abuse liability. Taken together, these data support the idea that mixed efficacy MOR agonist/DOR antagonist compounds have distinctly different pharmacological actions in vivo as compared with traditional opioid analgesics. VRP26 represents an advancement in the field as it has balanced affinity between MOR and DOR, as well as significantly better bioavailability after peripheral administration when compared to previously published compounds (Dietis et al. 2009; Healy et al. 2013). While the molecular mechanism by which antagonism of DOR regulates MOR-mediated effects is unclear, studies have suggested that these effects may be due to receptor oligomerization, alterations in receptor surface expression or recycling, or changes in intracellular signaling cascades (Breitwieser 2004; Cahill et al. 2007; Gomes et al. 2011; Hanyaloglu et al. 2002; Law et al. 2005; Milligan 2010; Wisler et al. 2014). Regardless of the mechanism, mixed efficacy opioid ligands, such as VRP26, may present an effective analgesic alternative to traditional opioids, thereby meeting a significant unmet medical need for analgesics with limited tolerance, dependence, and abuse liability.
Acknowledgments
Funding. This work was funded by NIH Grant DA003910 and R01 DA039997 J.P.A. was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32 DA007267. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors thank Dr. Kenner Rice for his generous gift of SNC80 and John. R. Traynor for his generous gift of mu opioid receptor knock-out mice.
Footnotes
Disclosure. The authors have no conflicts to disclose.
References
- Abdelhamid EE, Sultana M, Portoghese PS, Takemori AE. Selective Blockage of the Delta Opioid Receptors Prevents the Development of Morphine Tolerance and Dependence in Mice. The Journal of Pharmacology and Experimental Therapeutics. 1991;258:299–303. [PubMed] [Google Scholar]
- Aceto MD, Harris LS, Negus SS, Banks ML, Hughes LD, Akgun E, Portoghese PS. MDAN-21: a bivalent opioid ligand containing mu-agonist and delta-antagonist pharmacophores and its effects in rhesus monkeys. International Journal of Medicinal Chemistry. 2012;2012 doi: 10.1155/2012/327257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand JP, Purington LC, Pogozheva ID, Traynor JR, Mosberg HI. Modulation of opioid receptor ligand affinity and efficacy using active and inactive state receptor models. Chem Biol Drug Des. 2012;80:763–70. doi: 10.1111/cbdd.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anathan S, Khare NK, Saini SK, Seitz LE, Bartlett JL, Davis P, Dersch CM, Porecca F, Rothman RB, Bilsky EJ. Identification of Opioid Ligands Possessing Mixed Mu Agonist/Delta Antagonist Activity Among Pyridomorphans Derived from Naloxone, Oxymorphone, and Hydromorphone. Journal of Medicinal Chemistry. 2004;47:1400–1412. doi: 10.1021/jm030311v. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Connor M. Opioids: Cellular Mechanisms of Tolerance and Physical Dependence. Current Opinion in Pharmacology. 2005;5:60–8. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Balboni G, Guerrini R, Salvadori S, Bianchi C, Rizzi D, Bryant SD, Lazaruz LH. Evaluation of the Dmt-Tic Pharmacophore: Conversion of a Potent Delta-opioid Receptor Antagonist into a Potent Delta Agonist and Ligands with Mixed Properties. Journal of Medicinal Chemistry. 2002;45:713–720. doi: 10.1021/jm010449i. [DOI] [PubMed] [Google Scholar]
- Ballantyne JC, LaForge KS. Opioid Dependence and Addiction During Opioid Treatment of Chronic Pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Bender AM, Griggs NW, Anand JP, Traynor JR, Jutkiewicz EM, Mosberg HI. Asymmetric Synthesis and in Vitro and in Vivo Activity of Tetrahydroquinolines Featuring a Diverse Set of Polar Substitutions at the 6 Position as Mixed-Efficacy mu Opioid Receptor/delta Opioid Receptor Ligands. ACS Chem Neurosci. 2015;6:1428–35. doi: 10.1021/acschemneuro.5b00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser GE. G protein-coupled receptor oligomerization: implications for G protein activation and cell signaling. Circulation research. 2004;94:17–27. doi: 10.1161/01.RES.0000110420.68526.19. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Holdridge SV, Morinville A. Trafficking of the Delta Opioid Receptors and other G Protein-Coupled Receptors: Implications for Pain and Analgesia. Trends in Pharmacological Sciences. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:19208–13. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietis N, Guerrini R, Calo G, Salvadori S, Rowbotham DJ, Lambert DG. Simultaneous Targeting of Multiple Opioid Receptors: A Strategy to Improve Side-effect Profile. British Journal of Anaesthesia. 2009;103:38–49. doi: 10.1093/bja/aep129. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Schiller PW, Shapiro M, Weltrowska H, Coderre TJ. Attenuation of Morphine Tolerance and Dependence with the Highly Selective Delta Opioid Receptor Antagonist TIPP(psi) European Journal of Pharmacology. 1995;286:105–108. doi: 10.1016/0014-2999(95)00554-x. [DOI] [PubMed] [Google Scholar]
- Gomes I, IJzerman AP, Ye K, Maillet EL, Devi LA. G Protein-Coupled Receptor Heteromerization: A Role in Allosteric Modulation of Ligand Binding. Molecular Pharmacology. 2011;79:1044–1052. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanyaloglu AC, Seeber RM, Kohout TA, Lefkowitz RJ, Eidne KA. Homo- and hetero-oligomerization of thyrotropin-releasing hormone (TRH) receptor subtypes. Differential regulation of beta-arrestins 1 and 2. J Biol Chem. 2002;277:50422–30. doi: 10.1074/jbc.M209340200. [DOI] [PubMed] [Google Scholar]
- Healy JR, Bezawada P, Shim J, Jones JW, Kane MA, Jr, ADM, Coop A, Matsumoto RR. Synthesis, Modeling, and Pharmacological Evaluation of UMB 425, a Mixed μ Agonist/δ Antagonist Opioid Analgesic with Reduced Tolerance Liabilities. ACS Chemical Neuroscience. 2013;4:1256–66. doi: 10.1021/cn4000428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn MJ, Little PJ, Gringas J, Khun CM. Differential Effects of Naltrindole on Morphine-Induced Tolerance and Physical Dependence in Rate. The Journal of Pharmacology and Experimental Therapeutics. 1997;281:1350–1356. [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future: National Survey Results on Drug Use. National Institute on Drug Abuse. 2009;1:1–721. [Google Scholar]
- Law P-Y, Erickzon-Herbrandson LJ, Zha QQ, Solberg J, Chu J, Sarre A, Loh HH. Heterodimerization of the Mu and Delta Opioid Receptros Occurs at the Cell Surface Only and Requires Receptor-G Protein Interactions. J Biol Chem. 2005;280:11152–11164. doi: 10.1074/jbc.M500171200. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. European Journal of Pharmacology. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- Milligan G. The Role of Dimerization in Cellular Trafficking of G-protein-coupled Receptors. Current Opinion in Pharmacology. 2010;10:23–29. doi: 10.1016/j.coph.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Morphy R, Kay C, Rankovic Z. From Magic Bullets to Designed Multiple Ligands. Research Focus Reviews. 2004;9:641–652. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- Morphy R, Rankovic Z. Designing Multiple Ligands - Medicinal Chemistry Strategies and Challenges. Current Pharmaceutical Design. 2009;15:587–600. doi: 10.2174/138161209787315594. [DOI] [PubMed] [Google Scholar]
- Mosberg HI, Yeomans L, Anand JP, Porter V, Sobczyk-Kojiro K, Traynor JR, Jutkiewicz EM. Development of a Bioavailable μ Opioid Receptor (MOPr) Agonist, δ Opioid Receptor (DOPr) Antagonist Peptide That Evokes Antinociception without Development of Acute Tolerance. Journal of Medicinal Chemistry. 2014;57:3148–3153. doi: 10.1021/jm5002088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purington LC, Pohozheva ID, Traynor JR, Mosberg HI. Pentapeptides Displaying Mu Opioid Receptor Agonist and Delta Opioid Receptor Partial Agonist/Antagonist Properties. Journal of Medicinal Chemistry. 2009;52:7724–7731. doi: 10.1021/jm9007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purington LC, Sobczyk-Kojiro K, Pogozheva ID, Traynor JR, Mosberg HI. Development and in Vitro Characterization of a Novel Bifunctional Mu-Agonist/Delta-Antagonist Opioid Tetrapeptide. ACS Chemical Biology. 2011;6:1375–1381. doi: 10.1021/cb200263q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quock RM, Burkey TH, Varga E, Hosohata Y, Hosohata K, Cowell SM, Slate CA, Ehlert FJ, Roeske WR, Yamamura HI. The d-Opioid Receptor: Molecular Pharmacology, Signal Transduction, and the Determination of Drug Efficacy. Pharmacology Reviews. 1999;51:503–532. [PubMed] [Google Scholar]
- Ramabadran K. Effects of N-methylnaloxone and N-methylanaltrexone on nociception and precipitated abstinence in mice. Life Sciences. 1982;31:1253–1256. doi: 10.1016/0024-3205(82)90355-1. [DOI] [PubMed] [Google Scholar]
- Ross S, Peselow E. The Neurobiology of Addicitive Disorders. Clinical Neuropharmacology. 2009;32:269–276. doi: 10.1097/wnf.0b013e3181a9163c. [DOI] [PubMed] [Google Scholar]
- Salvadori S, Guerrini R, Balboni G, Bianchi C, Bryant SD, Cooper PS, Lazaruz LH. Further Studies on the Dmt-Tic Pharmacophore: Hydrophobic Substituents at the C-terminus Endow Delta Antagonists to Manifest Mu Agonism or Mu Antagonism. Journal of Medicinal Chemistry. 1999;42:5010–5019. doi: 10.1021/jm990165m. [DOI] [PubMed] [Google Scholar]
- Schiller PW. Bi- or Multifunctional Opioid Peptide Drugs. Life Sciences. 2009a;86:598–603. doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW. Bi- or multifunctional opioid peptide drugs. Life Sci. 2009b doi: 10.1016/j.lfs.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguten TM-D, Lemieux C, Chung NN, Coderre TJ. The Opioid Mu Agonist/Delta Antagonist DIPP-NH2(psi) Produces a Potent Analgesic Effect, no Physical Dependence and less Tolerance than Morphine in Rats. Journal of Medicinal Chemistry. 1999;42:3520–3526. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- Wisler JW, Xiao K, Thomsen AR, Lefkowitz RJ. Recent developments in biased agonism. Current opinion in cell biology. 2014;27:18–24. doi: 10.1016/j.ceb.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]