Abstract
Purpose
Little is known about the incidence and characteristics of incontinence associated dermatitis (IAD) in community-living individuals with fecal incontinence. The primary aim of this study was to describe the incidence and characteristics of self-reported IAD among community-living individuals with fecal incontinence. The study also examined whether IAD was associated with older age, gender, presence of urinary incontinence, or fecal incontinence severity.
Design
Secondary data analysis using a prospective cohort design.
Subjects and Setting
Community-living adults (N = 98) with fecal incontinence (76% female, 34% aged 65+ years, 90% White) who participated in a study about dietary fiber supplementation and were free of IAD at the start. Thirty five percent also had urinary incontinence.
Methods
Subjects assessed their skin for IAD daily for 52 days reporting types of IAD damage (redness, rash/fungal infection, and skin loss), location of IAD, and symptoms. They reported fecal incontinence on a diary for the first and last 14 study days.
Results
The incidence of IAD was 41% (40/98). The fecal incontinence severity score for subjects developing IAD was 1.2 higher than those who never had IAD (p < .001). There was no significant association of IAD with age, gender, or dual fecal and urinary incontinence. Incontinence Associated Dermatitis developed within 2 weeks and healed in approximately 1 week. The most common sign and symptom were redness (60% patients) and soreness (78%), respectively. Most subjects (85%) had IAD in one location.
Conclusions
Assessing for IAD in community-living patients with fecal incontinence is important as IAD is common and causes discomfort. The relatively mild severity of IAD offers WOC nurses opportunity for improving patient outcomes by preventing and managing this problem.
Introduction
Incontinence-associated dermatitis (IAD) is an inflammation of the skin in the perineal area resulting from fecal and urinary incontinence. IAD manifests as redness, breakdown, fungal infection/rash.1 The skin damage of IAD can occur in various areas of the perineum such as between the buttocks, on the outer buttocks, and thighs, and in the groin area2 and can cause discomfort and pain for patients.3 Understanding the epidemiology and characteristics IAD in various clinical settings provides important information about its scope and assists in planning resources needed for prevention and treatment interventions. In hospitalized patients, the prevalence of IAD was 20%4 while in critically-ill patients, it was as much as 50%.5 In long-term acute care (LTAC), the incidence of IAD was 7.6%2 while in nursing home residents on a skin damage prevention program the incidence was 3.4%.6 In both LTAC2 and nursing homes6, the time to onset of IAD was approximately two weeks while in critically-ill patients onset was only 4 days.7 A previous study8 reported that 52.5% of community-living individuals with fecal or dual incontinence reported a history of IAD and there was no association of a history of IAD with age, gender, or presence of urinary incontinence.
The primary purpose of this study was to describe the incidence and characteristics of IAD among community-living individuals with fecal incontinence. The study also examined whether IAD was associated with older age, gender, presence of urinary incontinence, or severity of fecal incontinence. Another secondary aim was to assess the relationship between individual characteristics of IAD and those of fecal incontinence. Because subjects reported their history of IAD in a previous study,8 we were able to measure the percentage that developed IAD during this prospective follow-up study.
Methods
The study had a prospective cohort design. Subjects were part of a randomized clinical trial investigating the effects of 3 dietary fiber supplements vs. a placebo supplement on fecal incontinence.9 They were recruited from a University-affiliated practice of colorectal surgeons and a health maintenance organization and toileted independently.10 Data collection began after the parent study was launched and approval from the Institutional Review Board of the University of Minnesota for this study was obtained. All subjects assessed their skin in the perineal area daily for 52 days. Subjects who completed the study protocol were included in this analysis in order to use data about fecal incontinence, which was reported during the last 14 days of the study. Subjects completed a survey about a history of IAD; those results were reported elsewhere.8
Due to the lack of a standard and tested IAD assessment instrument at the time of this study, 2 certified Wound, Ostomy, and Continence (WOC) nurse experts provided consultation about the assessment of IAD that subjects reported. Subjects were instructed to observe their skin for incontinence associated damage by study staff in their homes or in another place they preferred to meet. Instructions were given at the start of the study and reviewed at least 3 other times when other data collection instructions were given during the parent study. Data from skin observations were reviewed with the subjects and collected weekly by study staff. The staff were available to answer questions, reinforce instructions, or refer questions to the principal investigator as needed. Additionally, study staff made approximately 25 in-person visits to subjects during the parent study and answered questions or repeated instructions about a variety of study procedures including observation for skin damage. The data collection form was used to guide and support verbal instructions as it contained the information to be assessed and reported; subjects were encouraged to use a mirror for skin observations. Subjects used the data collection form to report the type (i.e., signs) of skin damage which was scored as redness =1, rash (explained as typically caused by a fungal infection of damaged skin) = 2, and skin loss/breakdown = 3 and whether they had the symptom of soreness. A user- friendly checkbox format was provided for responses as well as an opportunity to report any other signs or symptoms in an open-ended blank field for text. Subjects indicated the location of skin damage for these body areas: around anus/between buttocks, on outer buttocks, groin (labia, scrotum, penis), thighs (inner or outer) using a checkbox and again had an open-ended field to write in any other locations damaged. Subjects were asked to perform their usual skin care routines and were not provided with additional instructions. The study did not collect information on skin care routines or products used by the subjects.
Subjects completed a demographics form and a survey about their history of IAD at the start of the parent study. They also maintained a daily diary for fecal incontinence for the first (baseline) and last (placebo or fiber supplement) 14 days of the 52-day parent study.9 Fecal incontinence frequency was reported as the time of each incontinence episode. Fecal incontinence consistency was reported using a 4-level classification schema where 1 = hard and formed, 2 = soft but formed, 3 = loose and unformed, and 4 = liquid stool. This schema has been shown to have good validity and reliability.11–13 Ratings were then averaged over each day. Subjects chose and wore an incontinence absorbent product (absorptive panty-liner, pad, or brief, Kimberly Clark, Irving, TX), which was provided to them, during the two 14-day fecal incontinence data collection periods. The same type of product was worn in both periods. The largest amount of fecal incontinence for each leakage episode was reported using 6 levels where 0 = none, 1 = leakage between buttocks, 2 = on an incontinence absorbent product, 3 = on underwear, 4 = on outerwear, or 5 = on shoes/the floor. Scores were averaged over each day.
Data Analysis
Because all 98 subjects who were eligible for this study were free of skin damage on the first day of assessing their skin, we were able to calculate incidence. Preliminary analyses showed that the incidence of IAD did not differ among the supplement groups (p = .18) and so results are reported for all subjects together. Descriptive statistics are presented as frequency for categorical variables and means with standard deviations or medians and ranges for interval data depending on their distributions. A daily total IAD severity score was calculated as follows: (score for IAD type/d) × (total number of areas with IAD/d); scores had a possible range of 0 to 12. A maximum IAD severity score was calculated as the highest of any daily score available.
A daily total fecal incontinence severity score was calculated as follows: (number of fecal incontinence episodes/d) × (amount of the fecal incontinence episodes/d) × (consistency of the fecal incontinence episodes/d). In preliminary analyses, no significant difference was found when comparing the amount of fecal incontinence among the various incontinence absorbent products (p > .05); therefore, the measure of fecal incontinence amount and the composite score of fecal incontinence severity (which included amount) combined values from all absorbent products together.
Associations between IAD severity and fecal incontinence severity were assessed on those days when both conditions were present using Spearman’s rho for ranked and non-normally distributed data. The daily total IAD severity score was correlated with the daily total fecal incontinence severity score. The individual signs of IAD (type and location) and the symptom of soreness were correlated with the individual characteristics of fecal incontinence (frequency, amount, and consistency).
We ran a mixed model analysis to determine if the overall severity score of fecal incontinence was different for those who developed IAD at any point versus those who never developed IAD. This model controlled for the period of the parent study (baseline and supplement) and the number of days of reporting fecal incontinence. We selected a mixed model because it accommodates the correlated values within a person over time and is the best method to deal with missing values. An autoregressive covariance structure was chosen as the correlations between fecal incontinence severity scores diminished as lag time increased. A first model included an interaction between IAD groups (none vs. any) and period. Because the interaction was not significant (p = .96), it was dropped from the model. Analyses were performed using SPSS v.19 and SAS v 19.2 Proc Mixed for the mixed modeling. Results were considered significant when p < .05.
Results
The cohort comprised 98 subjects; 76% (74/98) were female, 34% (33/98) were aged 65 years or older, and 63% (62/98) were married/partnered. The racial and ethnic background of the subjects was 90% (88/98) White, 5% (5/98) Black, 1% (1/98) American Indian, and 1% (1/98) Asian, and 3% (3/98) more than one race, and 2% (2/98) were Hispanic. Almost all subjects (99%) had a high school education or greater, 53% (52/98) were employed, and 34% (33/98) were retired. Thirty-five percent (34/98) of subjects had dual fecal and urinary incontinence.
The incidence of any IAD was 41% (40/98). Of the 98 subjects who assessed their skin for IAD in this study, 45 had a history of IAD16 and 32 of those (71%) developed IAD during this study. There was no significant difference in the incidence of IAD between men vs women (12% vs 40%, p =.35) and older (≥ 65 years) vs younger subjects (40% vs 37% p =.61). There was no difference in incidence of IAD in those with dual incontinence vs. those who had fecal incontinence only (55% vs 62%, p = .48). However, the subjects that developed IAD had a greater fecal incontinence severity score than those that never developed IAD (β (seβ)) = 1.2 (0.34), p < .001). The fecal incontinence severity score for subjects that developed IAD was 1.2 higher than those who never had IAD. The overall estimated least square means (SE) fecal incontinence severity score from the mixed model analysis were 3.1 (0.26) for the any IAD group and 2.0 (0.22) for the no IAD group.
Of the 40 subjects who developed IAD, the time to onset of the first episode was 14 (0–38) days (median, range) (Figure 1). The total number of days with IAD per subject was 22 days (range 4–48 days), which accounted for 42% of the total days that skin was assessed. The number of days until IAD was healed was 7 (range 3–44). The maximum IAD severity score was 1.6. Of those who developed IAD during the prospective follow-up in this study, 80% (32/40) had reported a history of IAD on a previous survey.16
Figure 1.
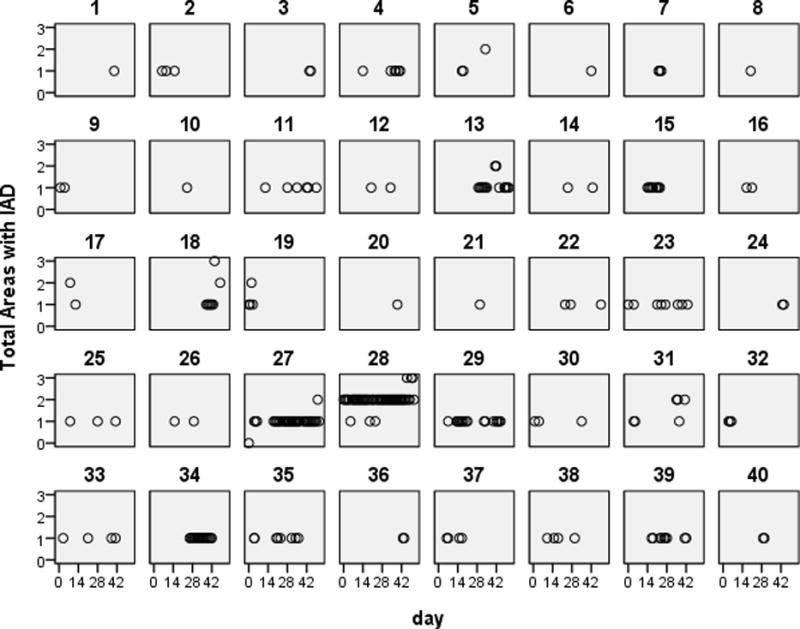
Figure 1 shows the number of body areas that were affected by incontinence associated dermatitis and the days in which IAD developed in community living persons with fecal incontinence. The numbers above the squares (1–40) designate the subjects with IAD, and each circle represents the total number of body locations damaged by incontinence per subject over the course of the study.
The percentages of subjects with various manifestations and locations of IAD are presented in Table 1. Redness was the most common sign of IAD, reported by over half of individuals who experienced skin problems. Five subjects (13%) reported a rash/fungal infection. Five subjects (13%) had skin loss, four of whom reported bleeding. Approximately three-fourths of subjects reported soreness as a symptom of IAD. Other symptoms written in by subjects were itching and burning, the latter which was described as the feeling from a “sunburn” by 1subject. Of the 5 possible body areas for IAD reported, the majority (34/40 85%) of subjects had only 1 area affected, 3 (7.5%) had 2 body areas affected and 3 (7.5%) had areas damaged by IAD (Figure 1). A majority of participants reported that the location of IAD was around the anus/between the buttocks (Table 1). The least common area of IAD damage was on the thighs. No one reported another location of IAD that was not identified on the form. Figure 1 shows that IAD was intermittent recurring in approximately one-third of subjects.
Table 1.
Manifestations of IAD in Community-Dwelling Persons with Fecal Incontinence
| N (%) N = 40 |
|
|---|---|
| Sign | |
| Redness | 24 (60%) |
| Rash | 5 (13%) |
| Skin loss/breakdown | 5 (13%) |
| Bleeding | 4 (10%) |
| Symptom | |
| Soreness | 31 (78%) |
| Itching | 9 (23%) |
| Burning | 6 (15%) |
We evaluated associations between individual signs of IAD and individual characteristics of fecal incontinence on the day of IAD. The total number of body areas affected by IAD was strongly associated with fecal incontinence frequency (rho = 0.66, p < .01) and moderately associated with fecal incontinence amount (rho = 0.31, p = .009). The greater the frequency and amount of fecal incontinence, the more body areas were damaged. The type of IAD was not significantly related to any individual characteristics of fecal incontinence severity on the day of IAD (p > .05). The symptom of soreness from IAD was moderately associated with fecal incontinence frequency on the day of IAD (rho = 0.25, p = .04); the greater the frequency of fecal incontinence, the greater the soreness from IAD. There were no significant associations between soreness and other characteristics of fecal incontinence on the day of IAD (all p > .05). The daily total IAD severity score was not significantly associated with the daily total fecal incontinence severity score (p = .88).
Discussion
This is the first study to our knowledge to report incidence and characteristics of IAD in a community-living population with fecal incontinence. Incontinence-associated dermatitis is a common problem in these individuals as its incidence was 41%. The incidence was associated with the severity of fecal incontinence but not gender or age. The severity of IAD was typically mild; redness was more common than skin loss or rash/fungal infection, and the spread of IAD was limited to one body area in the majority of participants. The mild severity of IAD is not surprising since subjects were healthy enough to live in the community and could cleanse soiled skin without delays from waiting for assistance. Despite its mildness, IAD frequently produced discomfort as 78% of subjects reported soreness and 38% had itching or burning symptoms.
Our findings add to a more complete understanding of IAD in different healthcare settings and patient groups. The time to onset of IAD in this study of community-living adults performing their usual skin care routines was approximately 14 days, which was similar to residents in long-term care on a skin damage prevention program,2,6 but considerably longer than the duration in critically-ill patients who received usual care and had an average onset of 4 days.7 These differences may be due in part to differences in illness severity, frequency or severity of fecal incontinence, skin care routines, and level of independence in perineal hygiene among the various patient groups. Results about the most and least common locations of IAD in this study are consistent with those of Long2 who observed LTAC residents. Both studies showed that the most common area for IAD occurrence seemed to be perianal area and the buttocks, while the thighs were among the least common areas. The common occurrence of IAD on the perianal area and buttocks implies an important role of leaked feces in the development of IAD as proposed by others.14
We found that IAD was intermittent, recurring in at least one-third of community-living individuals. Reports of the duration of IAD are influenced by the length of observation in a study so this outcome is difficult to compare among studies with varying observation times. The prospective design and length of observation in this study enabled observation of the pattern of IAD and time to healing in community-living subjects.
Limitations
There are limitations to this study. Subjects self-assessed IAD. Self-examination of the perineal area can be challenging even with use of a mirror depending on body size, flexibility, and vision; data about the type of IAD were missing for 6 subjects. We were interested in the incidence of IAD among community-living subjects using their regular skin care routines, which may have varied in appropriateness and effectiveness. All subjects wore an absorbent product during the first and last 14 days of the study so results may not be generalizable to individuals who do not wear these products. No instrument for assessing IAD that has undergone testing for reliability with community-living people was available at the time of the study. Because fecal incontinence severity scores were reported for only 28 days, the number of days that IAD severity could be associated with fecal incontinence severity was limited. Associations of IAD signs with fecal incontinence characteristics might be different in patient groups with more severe cases of fecal incontinence.
Clinical Implications
The results of the study offer implications for WOC nursing practice related to care for IAD. Because incontinence is associated with stigma and reluctance in reporting, inquiring about incontinence when performing a health history or assessment of community-living patients has been recommended.15,16 The findings of this study support that inquiring about and assessing for IAD in community-living patients with incontinence is important as well. Supplementing patient teaching about using a mirror for assessing for IAD in the perineal area with written materials with diagrams or practice in the clinic setting may be beneficial. Our findings offer WOC nurses evidenced-based information about the course of IAD; it typically resolves in 1 week, but may be longer in a few cases, and IAD may recur. Although we did not evaluate subjects’ usual skin care routines, such an evaluation would be part of usual WOC nurse practice and a topic for future research. Some community-living patients may require consultation about proper prevention and treatment of IAD.
Conclusions
Results of this study provide new information about IAD in community-dwelling adults. WOC nurses should be aware that IAD affects a large number of community living individuals. In the future, WOC nurses can use this data to educate all clients, regardless of the setting about prevention and management of IAD.
Table 2.
Body Locations of IAD Skin Damage
| N (%) | |
|---|---|
| Around anus/between buttocks | 38 (95%) |
| Outer Buttocks | 5 (13%) |
| Groin (labia, scrotum, penis) | 4 (10%) |
| Thighs (inner or outer) | 1 (3%) |
Acknowledgments
This study was supported by a grant from the National Institute of Nursing Research, NIH, R01NR07756. Kimberly Clark (Irving, TX) donated incontince absorbent products worn by subjects in the study.
Footnotes
Authors have no conflcits of interest.
References
- 1.Gray M, Beeckman D, Bliss DZ, et al. Incontinence-associated dermatitis: A comprehensive review and update. J Wound Ostomy Continence Nurs. 2012;39(1):61–74. doi: 10.1097/WON.0b013e31823fe246. [DOI] [PubMed] [Google Scholar]
- 2.Long MA, Reed LA, Dunning K, Ying J. Incontinence-associated dermatitis in a long-term acute care facility. J Wound Ostomy Continence Nurs. 2012;39(3):318–327. doi: 10.1097/WON.0b013e3182486fd7. [DOI] [PubMed] [Google Scholar]
- 3.Brown D. Perineal dermatitis risk factors: Clinical validation of a conceptual framework. Ostomy Wound Management. 1995;41(10):46–48. 50, 52–53. [PubMed] [Google Scholar]
- 4.Junkin J, Selekof JL. Prevalence of incontinence and associated skin injury in the acute care inpatient. J Wound Ostomy Continence Nurs. 2007;34(3):260–269. doi: 10.1097/01.WON.0000270820.91694.1f. [DOI] [PubMed] [Google Scholar]
- 5.Driver DS. Perineal dermatitis in critical care patients. Crit Care Nurse. 2007;27(4):42–46. [PubMed] [Google Scholar]
- 6.Bliss DZ, Zehrer C, Savik K, Thayer D, Smith G. Incontinence-associated skin damage in nursing home residents: A secondary analysis of a prospective, multicenter study. Ostomy Wound Manage. 2006;52(12):46–55. [PubMed] [Google Scholar]
- 7.Bliss DZ, Savik K, Thorson MA, Ehman SJ, Lebak K, Beilman G. Incontinence-associated dermatitis in critically ill adults: Time to development, severity, and risk factors. J Wound Ostomy Continence Nurs. 2011;38(4):433–445. doi: 10.1097/WON.0b013e318220b703. [DOI] [PubMed] [Google Scholar]
- 8.Rohwer K, Bliss DZ, Savik K. Incontinence-associated dermatitis in community-dwelling individuals with fecal incontinence. J Wound Ostomy Continence Nurs. 2013;40(2):181–184. doi: 10.1097/WON.0b013e31827e8b3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliss DZ, Savik K, Jung H, Whitebird R, Lowry A, Sheng X. Dietary fiber supplementation for fecal incontinence: A randomized clinical trial. doi: 10.1002/nur.21616. In review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitebird R, Bliss DZ, Savik K, Lowry A, Jung HJ. Comparing community and specialty provider-based recruitment for a randomized clinical trial on fecal incontinence: The fiber study. Res Nurs Health. 2010;33(6):500–511. doi: 10.1002/nur.20408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bliss DZ, Larson SJ, Burr JK, Savik K. Reliability of a stool consistency classification system. J Wound Ostomy Continence Nurs. 2001;28(6):305–313. doi: 10.1067/mjw.2001.119013. [DOI] [PubMed] [Google Scholar]
- 12.Bliss DZ, Dhamani KA, Savik K, Kirk K. Tool to classify stool consistency: Content validity and use by persons of diverse cultures. Nurs Health Sci. 2003;5(2):115–121. doi: 10.1046/j.1442-2018.2003.00145.x. [DOI] [PubMed] [Google Scholar]
- 13.Bliss DZ, Savik K, Jung H, Jensen L, LeMoine M, Lowry A. Comparison of subjective classification of stool consistency and stool water content. J Wound Ostomy Continence Nurs. 1999;26(3):137–141. doi: 10.1016/s1071-5754(99)90031-1. [DOI] [PubMed] [Google Scholar]
- 14.Buckingham KW, Berg RW. Etiologic factors in diaper dermatitis: The role of feces. Pediatr Dermatol. 1986;3(2):107–112. doi: 10.1111/j.1525-1470.1986.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 15.Bliss DZ, Norton CA, Miller J, Krissovich M. Directions for future nursing research on fecal incontinence. Nurs Res. 2004;53(6 Suppl):S15–S21. doi: 10.1097/00006199-200411006-00003. [DOI] [PubMed] [Google Scholar]
- 16.Norton C, Chelvanayagam S. A nursing assessment tool for adults with fecal incontinence. J Wound Ostomy Continence Nurs. 2000;27(5):279–291. doi: 10.1067/mjw.2000.109082. [DOI] [PubMed] [Google Scholar]


