Abstract
Calcific diseases of the cardiovascular system, such as atherosclerotic calcification and calcific aortic valve disease, are widespread and clinically significant, causing substantial morbidity and mortality. Vascular cells, like bone cells, interact with their matrix substrate not only through molecular signals, but also through biomechanical signals, such as traction forces transmitted from cytoskeleton to matrix. The interaction of contractile vascular cells with their matrix may be one of the most important factors controlling pathological mineralization of the artery wall and cardiac valves. In many respects, the matricrine and matrix mechanical changes in calcific vasculopathy and valvulopathy resemble those occurring in embryonic bone development and normal bone mineralization. The matrix proteins provide not only a microenvironment for propagation of crystal growth but also provide mechanical cues to the cells that direct differentiation. Small contractions of the cytoskeleton may tug on integrin links to sites on matrix proteins, and thereby sense the stiffness, possibly through deformation of binding proteins causing release of differentiation factors such as products of the members of the transforming growth factor-beta superfamily. Inflammation and matrix characteristics are intertwined: inflammation alters the matrix such as through matrix metalloproteinases, while matrix mechanical properties affect cellular sensitivity to inflammatory cytokines. The adhesive properties of matrix also regulate self-organization of vascular cells into patterns through reaction-diffusion phenomena and left-right chirality. In this review, we summarize the roles of extracellular matrix proteins and biomechanics in the development of inflammatory cardiovascular calcification.
Keywords: aortic valve, vascular, calcification, inflammation, matrix
INTRODUCTION
In 1761, the “father of anatomic pathology,” Giovanni Morgagni, described growths of bone-like tissue within structures of the cardiovascular system,[1] and while this observation was reproduced by pathologists over the next century, it was largely forgotten by about 1930. For the next several decades, calcification in arteries was considered a passive, degenerative process of aging - inevitable and unregulated - and calcification in cardiac valves was considered a result of “wear-and-tear.” Not until the early 1990s was it recognized anew that vascular calcification occurs by an active, cell-mediated process with striking similarities to bone development.[2] Research over the past two decades has shed significant light on the role of mechanical cues - such as substrate stiffness, fluid shear stress, and tissue strain - in the development of inflammatory vascular and valvular calcification. This brief review aims to highlight the clinical relevance of inflammatory cardiovascular calcification, and while there are distinct differences in the mechanisms that drive vascular and valvular calcification, we will focus on their common themes that have been elucidated over the past several years, providing the foundation for future research.
CLINICAL SIGNIFICANCE
Atherosclerotic calcification
The entire cardiovascular system is vulnerable to pathological mineralization. In the arteries, calcification is widespread, increasing in prevalence with age. Over 60% of adults over age 60 have calcium deposits in at least one of the major vascular beds, such as the intrathoracic aorta or the coronary and carotid arteries.[3] The presence and extent of coronary artery calcification are strong predictors of cardiovascular morbidity and mortality, and they are now used for risk stratification in patients with presumed coronary artery disease.[4,5]
The primary adverse effect of vascular calcification is increased vessel stiffness, though atherosclerotic calcification also affects plaque vulnerability, as discussed below. Vascular calcification occurs in two distinct forms: one arises in the intimal layer, which is usually associated with atherosclerosis and mediated by inflammation. The other occurs in the medial layer, usually associated with chronic kidney disease (CKD) and its concomitant hyperphosphatemic, apoptotic, and uremic derangements, but is also present in certain genetic disorders (e.g. Marfan syndrome, generalized arterial calcification of infancy).[6] Vascular calcification in the medial layer associated with CKD appears to occur through different mechanisms than inflammatory, atherosclerotic calcification, and these mechanisms have been reviewed in detail by Shanahan and colleagues [7] and are not addressed further in this review.
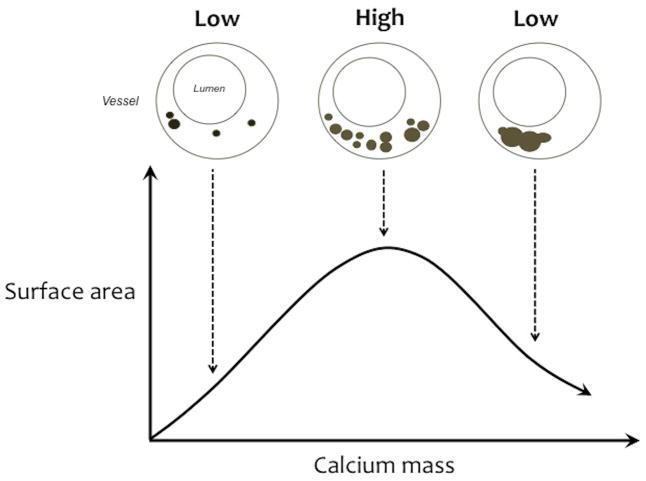
Clinically, the importance of atherosclerotic calcification is its effects on plaque rupture and thrombosis. Biomechanical analyses suggest that mechanical stresses leading to vessel rupture are increased at those interfaces between vascular tissue and calcium deposits that face the direction of vascular stress, whether circumferential, axial, or radial.[8] Since this mechanical phenomenon of compliance mismatch is independent of scale, it therefore applies to calcium deposits of essentially all sizes, including microcalcifications. Interestingly, the interfaces perpendicular to the stress axis have reduced rupture stresses. The degree of compliance mismatch increases with the surface area of calcium deposits exposed to mechanical stress. As calcium deposits progress, the interface area is expected to increase until the deposits coalesce, then interface area is expected to decrease.[9] Thus, clinical risk is expected to have a biphasic relationship with the degree of calcification: increasing risk with increasing calcification at early stages, then decreasing risk with more advanced calcification (Figure 1). This concept was not testable until recent elegant work from Dweck, Newby and colleagues of the University of Edinburgh [10,11] showed that clinical 18F-fluoride positron emission tomography (PET) images identify culprit lesions in the coronary vasculature, sites of plaque rupture in carotid arteries, and sites of progressive calcific disease in the aortic valve. The fluoride appears to bind to hydroxyapatite crystals forming at the growing surface area of calcium deposits. These findings support the theoretical mechanical analysis that surface area and compliance mismatch promote rupture.
Figure 1. Schematic of the biphasic effect of calcification on surface area.
With increasing calcification, the surface area of the deposits increases until they coalesce, at which point surface area decreases. Based on engineering analyses, the rupture stress corresponds with surface area. These phenomena may explain the biphasic clinical risk associated with coronary calcification.
Calcific aortic valvular disease
Another site where calcification is both common and severe in consequence is the aortic valve, which has been termed calcific aortic valvular disease (CAVD). In people over the age of 70, approximately 40% have mild calcification of the aortic valve and over 10% have severe calcification.[12] Otto and colleagues have shown that, even in the absence of hemodynamically significant obstruction of left ventricular outflow, CAVD is associated with higher risk of cardiovascular events.[13] Ultimately, CAVD progresses to severe hemodynamic compromise, with insufficient systemic perfusion due to stiffening of the valve leaflets.
Until recently, CAVD was believed to result from mechanical “wear-and-tear” on the valve leaflets. In a major paradigm shift, compelling evidence now supports an active mechanism involving osteochondrogenic differentiation of valvular interstitial cells (VICs) regulated at the cellular and molecular levels,[14,15] similar to the mechanism of vascular calcification.[16,17] Growing evidence, detailed below, supports the role of inflammation and biomechanical cues from the matrix in driving the pathophysiology of CAVD.
COMPARISON TO BONE MINERALIZATION
As in bone matrix, the mineral deposited in the matrix of diseased vessels and valves has been identified by electron microprobe analysis as hydroxyapatite, Ca10(PO4)6(OH)2,[18] and other forms of bone mineral, such as whitlockite (Ca, Mg)3(PO4)2.[19] Similarly, in cell culture models, the mineral produced by artery wall cells has the stoichiometry of hydroxyapatite mineral.[16] Both vascular and valvular calcification undergo a progressive maturation process with continuous changes in composition, similar to the multistep process of bone formation.[20] It is even conceivable that calcified vascular matrix undergoes the continuous remodeling process seen in bone. In human plaque, calcification takes on the form of mature trabecular bone tissue in 65% of femoral lesions and 15% of carotid lesions and aortic valve nodules.[14,21,22]
In bone, matrix vesicles secreted by osteoblasts serve as initiation sites for mineral crystal formation. In human vascular and valvular calcification, matrix vesicles appear to have the same role [23], and these may be produced by both macrophages [24] and vascular smooth muscle cells.[25] Both vascular-derived and osteoblast-derived matrix vesicles contain calcium-binding and extracellular matrix proteins.[25] Interestingly, the reported diameters of matrix vesicles in cardiovascular tissues range widely from 30 to 5,000 nm.[25,26]
INFLAMMATORY MEDIATORS IN CARDIOVASCULAR CALCIFICATION
Inflammation is associated epidemiologically with increased risk of cardiovascular and all-cause mortality.[27,28] Both atherosclerosis [29] and progression to aortic valve stenosis [30] are associated with higher levels of tumor necrosis factor-alpha (TNF-α), a pro-inflammatory cytokine. In a mouse model of aortic and aortic valve calcification, both TNF-α serum levels and local protein expression in aortic valve leaflets were upregulated.[31,32] Towler and colleagues carried this work to important potential clinical application by showing that treatment with infliximab, a neutralizing antibody to TNF-α, significantly attenuated aortic valve calcification.[31]
Inflammation in the cardiovascular system, as in other organ systems, produces substantial changes in the extracellular matrix.[33] Certain types of matrix proteins increase in expression and secretion, while the existing matrix is removed or remodeled by production of matrix metalloproteinases. In the vasculature, inflammation results when lipoprotein particles accumulate in the subendothelial matrix where they undergo oxidative modification, triggering an immune response, generating inflammation. Inflammatory stimuli, such as cytokines and lipid oxidation products, promote the osteoblastic differentiation of vascular cells in atherosclerosis and valvular cells in CAVD.[31,34,35] Molecular imaging studies demonstrate that inflammation precedes matrix calcification in the vasculature, and inflammatory factors are present in close proximity to intimal arterial calcification.[36] Kapustin and colleagues recently show that production of SMC-derived exosomes is increased by TNF- α.[25] Additionally, cell death by apoptosis accompanies inflammation, and apoptosis accelerates calcification.[37]
MATRIX PROTEINS IN CALCIFIC DISEASE
While normal valve tissue matrix is a “loose” and hydrated network enriched in glycoproteins, in advanced lesions, the matrix becomes more dense and fibrous.[38,39] In culture, the extracellular matrix produced by calcifying vascular cells (CVCs) contains much more collagen I and fibronectin than that of normal vascular smooth muscle cells, but less collagen IV.[40] Thus, vascular cells that mineralize may have a more adhesive and stiff substrate and one more susceptible to mineralization.
Additionally, with advancing atherosclerosis, the intima thickens with increased expression of matrix proteins including thrombospondin, tenascin, osteopontin, osteocalcin, and dentin matrix acidic phosphoprotein 1 (DMP-1). In aortic valves, VICs produce fibronectin in response to injury.[41] Valvular disease evolves with further increases in expression of the matrix proteins osteopontin, osteocalcin,[15] and matrix metalloproteinases, such as MMP-1, MMP-3, and MMP-9, as well as their inhibitors.[42,43]
Inflammatory-mediated changes in the matrix also affect the lipid environment in the vasculature. The higher content of fibronectin promotes retention of lipoproteins, likely through fibronectin’s heparin-binding domain,[44] and proteoglycans also bind lipoproteins.[45]
MATRIX MECHANICS AND CELLULAR DIFFERENTIATION
Effects of substrate stiffness
Integrin connections between cytoskeletal fibers and extracellular matrix proteins allow transmission of forces and have profound effects on cell behavior and shape [46] as well as differentiation. Valve cells, in particular, undergo osteochondrogenic differentiation when grown on a matrix with stiffness similar in degree to that of normal valve tissue (apparent elastic moduli 25–30 kPa),[47]. Matrix of this stiffness has the same effect on differentiation in mesenchymal stem cells.[48,49] Interestingly, valve cells grown on a stiffer matrix (~113 kPa), similar to that of stenotic valve tissue, exhibit myofibroblastic differentiation.[47] Additionally, the Anseth group has elegantly demonstrated that reduction of the substrate modulus can “redirect” valvular myofibroblasts into a more dormant fibroblast phenotype.[50] One potential mechanism is that transforming growth factor-β (TGF-β) superfamily members may be released from the matrix on a stiff, but not compliant, matrix when their binding proteins, strategically located between cellular integrins and other matrix proteins, are stretched open by cellular traction forces.[51] This phenomenon would explain some observations about vascular progenitor cells that have calcification potential: (a) when grown on tissue culture plastic, CVCs undergo osteochondrogenic differentiation and retract locally to form calcified nodules,[16] (b) when treated with TGF-β, CVCs form abundant nodules,[52] and (c) when grown on substrates containing specific extracellular matrix proteins (collagen I, collagen IV, laminin, or fibronectin), the calcification behavior of CVCs [40] and VICs [53] changes dramatically.
Effects of fluid shear stress
Interestingly, calcium deposits usually arise on the aortic surface of the cusps, whereas the ventricular surface is generally spared. Differential hemodynamic forces acting on the two surfaces may at least partially explain this predilection for the aortic surface, as the aortic surface experiences disturbed flow, while the ventricular surface experiences laminar, albeit intermittent, flow. Endothelial cells isolated from the aortic and ventricular surfaces reveal significant differences in genes protective against inflammation and calcification,[54] which suggest the endothelial response to flow as one possible link between the differential hemodynamic forces and side-specific predilection for calcification. One mechanism linking shear stress with calcific disease may be induction of bone morphogenetic proteins and TGF-β by oscillatory flow, as shown in valve leaflets.[55] This may also account for increased calcification at other areas exposed to oscillatory shear stresses, such as the non-coronary cusp of the aortic valve and the lesser curvature of the aortic arch.
Effects of tissue strain
While fluid shear stress primarily affects valvular and vascular endothelial cells, VICs and vascular smooth muscle cells experience solid mechanical stresses as a result of cyclic stretching of the valvular and vascular tissue during each cardiac cycle. Computational modeling by the Yoganathan group recently showed regional strain variations in aortic valve tissue subjected to physiological stresses, with the highest stretch magnitude seen in the base and along the coaptation lines of the valve cusps, which is, interestingly, also where calcification is most often seen.[56] Additionally, in vitro studies by the Merryman group have demonstrated the strain-dependence of apoptosis and nodule formation in valvular cells.[57,58] These studies suggest an important role of tissue stretch in the pathogenesis of valvular calcification, and highlight the complexities of studying cardiovascular cells exposed to a dynamic biomechanical environment.
PATTERN FORMATION
CVCs, which have properties of mesenchymal progenitor or stem cells, have the ability to self-organize, interacting with their matrix substrate through biomechanical signals. They form raised structures (nodules or ridges),[16] apparently by retraction of matrix into condensations, reminiscent of those seen in mesenchymal tissue in embryonic development. Depending on their substrate conditions, they organize into macroscopically visible spot, stripe, trabecular, and labyrinthine patterns, with distinct preferred separation distances, as found in other putative reaction-diffusion phenomena in nature, such as the formation of zebra stripes and leopard spots.[59]
When CVCs form nodules, they are evenly spaced in periodic patterns, which appear to be a result of molecular morphogens interacting in a mechanism termed reaction-diffusion.[59] Depending on conditions, CVCs retract and self-organize into macroscopic aggregates roughly 0.1 to 2 mm in diameter or width in a spectrum of periodic patterns. The type of pattern can be altered, even in a local manner, by cell plating density.[60] The type of pattern produced is also predictable by a mathematical model (a system of partial differential equations) representing reaction-diffusion principles with parameter values based on experimentally established properties of the molecular morphogens. The activator and inhibitor morphogens appear to be bone morphogenetic protein-2 (BMP-2) and matrix gamma-carboxyglutamic acid protein (MGP), respectively, given that addition of inhibitors to these morphogens produce predicted transitions from stripes to spots and from low frequency to double-frequency stripes.[59] Two lines of evidence support the hypothesis that BMP-2 and MGP are the reaction-diffusion morphogens that govern pattern formation in vivo: (1) Exogenous addition of inhibitors of MGP and BMP-2 modify the patterns in a manner predictable by reaction-diffusion partial differential equations, and (2) MGP deficiency in mice results in substantial abnormalities of vascular branching patterns.[61,62] In vivo, the trabecular patterns in endosteal bone and cardiac chambers may arise from reaction-diffusion phenomena.[63]
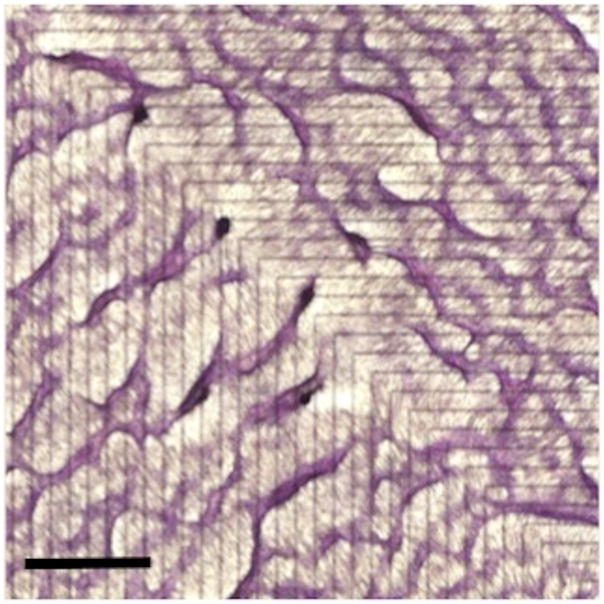
Additionally, CVCs have the capacity to recognize matrix interfaces, with an intrinsic chirality that leads them to preferentially migrate rightward. [64] The cells evidently sense mechanical features of their matrix substrate, detect adhesivity and interfaces between substrates of different adhesivity. They appear to use interfaces in substrate adhesivity as a directional guide for cell migration and self-organization. Remarkably, these cells have the capacity to recognize a micromachined interface between adherent (fibronectin) and non-adherent (polyethylene glycol) surfaces and respond by altering their migration path toward the right, lining up 10–20 degrees counterclockwise relative to the interface axis, and then moving forward or backward into equally spaced ridges.[64] These phenomena produce macroscopic patterns of parallel ridges diagonally crossing the micromachined interfaces (Figure 2). This ability of matrix adhesivity to guide cellular self-organization may be harnessed for applications in tissue engineering purposes. Rather than forcing cells into a desired architecture, this phenomenon can be used to work with the natural cell inclinations to produce radial, concentric patterns of organization, reminiscent of the architecture of various mammalian organs.[65]
Figure 2. Macropattern formation.
Calcifying vascular cells were grown on alternating stripes of micromachined adhesive and non-adhesive substrates (fibronectin and hexamethyldisalazane/polyethylene glycol, respectively). Each stripe was 300 μm wide, and the interfaces between substrate stripes are indicated by black lines on the back (non-plated) side. In this case, a 90° bend was introduced in the configuration. After 10–14 days, the cells were fixed and stained with hematoxylin. Scale bar, 2mm.
The formation of three-dimensional, calcified structures in patterns driven by left-right asymmetry may account for the finding that calcium deposits in CAVD and atherosclerosis occur in spotty, patchy, and nodular patterns [10,11,66] rather than diffuse patterns, despite the fact that the triggers are systemic. In analogy to the “mesenchymal condensations” that are pivotal in embryonic bone formation, it appears that aggregation into patterned structures is a precursor to mineralization.[67] The regularity of the pattern may be less apparent in vivo due to local variations in environmental conditions, such as fluid and solid stresses.
POSITIVE FEEDBACK
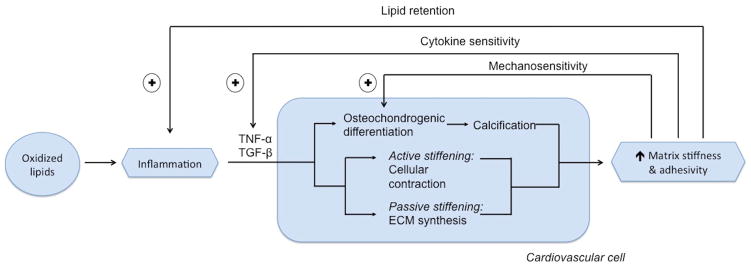
From a systems biology point of view, vascular calcification and CAVD appear to involve positive feedback circuits in which inflammatory cytokines promote matrix changes that increase osteochondrogenic differentiation, cell sensitivity to inflammatory cytokines, and lipid retention, all of which create positive feedback to inflammation and matrix stiffness. Our working model (Figure 3) links inflammation to calcific disease through feedback control circuits: inflammation promotes both passive and active stiffening of the matrix, through changes in production and ultrastructure of extracellular matrix proteins, and through cytoskeletal contraction, respectively. Active and passive stiffening act synergistically, with cell and matrix stress being transmitted both in series and in parallel. Cytoskeletal contraction transmits force to the matrix through the multiple molecular links connecting actin to focal adhesion proteins and integrin to arginine-glycine-aspartate peptide (RGD)-containing proteins, such as fibronectin and discoidin domain receptor tyrosine kinase 2. Conceivably, as proposed by Hinz,[68] a stiff matrix allows tension from cell contraction to open latency-associated peptide to release matrix-bound differentiation factors, such as TGF-β and family member BMP-2. Through this “matricrine” mechanism, differentiation factors activate receptors on the cells and active signaling molecules that lead to osteochondrogenic differentiation or other lineage fate. This paradigm is supported by the important work of Simmons and colleagues showing that the degree of matrix stiffness corresponds with the degree of osteochondrogenic differentiation of valvular cells.[47] A positive feedback loop ensues, as cells undergo osteochondrogenic differentiation, producing collagen and mineral, leading to further increases in matrix stiffness. Two additional positive feedback loops arise because the inflammation-induced changes in matrix adhesivity promote lipid retention,[44,45] and because increased matrix stiffness increases cellular responsiveness to inflammatory factors such as TNF-α and TGF-β.[39,69,70] Since unopposed positive feedback loops are unstable, some form of negative feedback must also arise, such as the induction of inhibitors like MGP.
Figure 3. Schematic diagram of positive feedback loops in inflammatory cardiovascular calcification.
Positive feedback loops may contribute to calcific diseases when oxidatively modified lipids stimulate release of inflammatory cytokines, such as TNF-α, which promote osteochondrogenic differentiation of cardiovascular cells and promote stiffening of their extracellular matrix. The matrix stiffening occurs through both active cytoskeletal contraction and expression and deposition of a less compliant assortment of matrix proteins. The stiffer matrix leads to at least three positive feedback mechanisms: increased release of osteochondrogenic differentiation factors (such as TGF-β and its close relative, bone morphogenetic protein), increased cell sensitivity to TGF-β and inflammatory cytokines, and lipid retention, all of which feed back positively to inflammation and matrix stiffness. Since unopposed positive feedback loops are unstable, some form of negative feedback must also arise, such as the induction of inhibitors, such as matrix GLA protein, by bone morphogenetic protein.
These mechanisms would account for the increased vulnerability to calcific cardiovascular disease in patients with hyperlipidemia and even in those with inflammation due to other conditions, such as rheumatoid arthritis, which is associated with more severe and more prevalent coronary and aortic calcification than age and sex-matched controls.[71] Thus, prolonged inflammation creates a powerful milieu for advancing bone formation in the cardiovascular system.
SUMMARY
Inflammatory cytokines, matrix proteins, and biomechanical cues exert powerful control over vascular and valvular cells, such as cell fate and cell migration into architectural patterns, reminiscent of the influence the matrix has in lineage determination of mesenchymal stem cells in embryonic development. Cells also directly influence their own matrix and mechanical environment by adjusting the amount and types of protein fibrils they produce. The cells interact with their matrix to aggregate into periodic patterns, forming three-dimensional macroscopic nodules, similar to those found in stenotic valves and atherosclerotic lesions. Under inflammatory conditions, the cardiovascular matrix tends to stiffen, which promotes osteochondrogenic differentiation and increased sensitivity to cytokines, both of which promote further stiffening of the extracellular matrix in positive feedback loops.
As new imaging modalities, such as 18F-PET scanning, may allow for both early detection of calcific disease and specific identification of vulnerable plaque, physicians may have the opportunity to intervene at early stages and potentially attenuate and/or reverse disease progression. The continued elucidation of the inflammatory, biomechanical, and matricellular mechanisms underlying vascular and valvular calcification is crucial to developing effective therapies.
Acknowledgments
FUNDING
This work was supported by funding from the Heart Lung and Blood Institute of the National Institutes of Health, (HL114709 and HL121019). J.J.H. was supported by a T32 training grant from the National Institutes of Health (HL007895) and an award from the UCLA Specialty Training and Advanced Research (STAR) Program. J.L. was supported by the UCLA Children’s Discovery and Innovation Institute Harry Winston Fellowship Award.
Footnotes
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in HEART editions and any other BMJPGL products to exploit all subsidiary rights.
References
- 1.Davies P, Bucky NL. Tomography of calcified aortic and mitral valves. Br Heart J. 1959;21:17–22. doi: 10.1136/hrt.21.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demer LL, Tintut Y. Vascular calcification: pathobiology of a multifaceted disease. Circulation. 2008;117:2938–48. doi: 10.1161/CIRCULATIONAHA.107.743161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–6. doi: 10.1161/01.ATV.0000110786.02097.0c. [DOI] [PubMed] [Google Scholar]
- 4.Gepner AD, Young R, Delaney JA, et al. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polonsky TS, McClelland RL, Jorgensen NW, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demer LL, Tintut Y. Inflammatory, metabolic, and genetic mechanisms of vascular calcification. Arterioscler Thromb Vasc Biol. 2014;34:715–23. doi: 10.1161/ATVBAHA.113.302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanahan CM, Crouthamel MH, Kapustin A, et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoshino T, Chow LA, Hsu JJ, et al. Mechanical stress analysis of a rigid inclusion in distensible material: a model of atherosclerotic calcification and plaque vulnerability. Am J Physiol Heart Circ Physiol. 2009;297:H802–810. doi: 10.1152/ajpheart.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arterioscler Thromb Vasc Biol. 2004;24:1161–70. doi: 10.1161/01.ATV.0000133194.94939.42. [DOI] [PubMed] [Google Scholar]
- 10.Joshi NV, Vesey AT, Williams MC, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet Lond Engl. 2014;383:705–13. doi: 10.1016/S0140-6736(13)61754-7. [DOI] [PubMed] [Google Scholar]
- 11.Dweck MR, Jenkins WSA, Vesey AT, et al. 18F-sodium fluoride uptake is a marker of active calcification and disease progression in patients with aortic stenosis. Circ Cardiovasc Imaging. 2014;7:371–8. doi: 10.1161/CIRCIMAGING.113.001508. [DOI] [PubMed] [Google Scholar]
- 12.Lindroos M, Kupari M, Heikkila J, et al. Prevalence of aortic valve abnormalities in the elderly: an echocardiographic study of a random population sample. J Am Coll Cardiol. 1993;21:1220–5. doi: 10.1016/0735-1097(93)90249-z. [DOI] [PubMed] [Google Scholar]
- 13.Otto CM, Lind BK, Kitzman DW, et al. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med. 1999;341:142–7. doi: 10.1056/NEJM199907153410302. [DOI] [PubMed] [Google Scholar]
- 14.Mohler ER, Gannon F, Reynolds C, et al. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–8. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 15.Rajamannan NM, Subramaniam M, Rickard D, et al. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–4. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bostrom K, Watson KE, Horn S, et al. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tintut Y, Parhami F, Bostrom K, et al. cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J Biol Chem. 1998;273:7547–53. doi: 10.1074/jbc.273.13.7547. [DOI] [PubMed] [Google Scholar]
- 18.Daoud AS, Frank AS, Jarmolych J, et al. Ultrastructural and elemental analysis of calcification of advanced swine aortic atherosclerosis. Exp Mol Pathol. 1985;43:337–47. doi: 10.1016/0014-4800(85)90070-x. [DOI] [PubMed] [Google Scholar]
- 19.Reid JD, Andersen ME. Medial calcification (whitlockite) in the aorta. Atherosclerosis. 1993;101:213– 24. doi: 10.1016/0021-9150(93)90118-e. [DOI] [PubMed] [Google Scholar]
- 20.Cottignoli V, Relucenti M, Agrosi G, et al. Biological Niches within Human Calcified Aortic Valves: Towards Understanding of the Pathological Biomineralization Process. BioMed Res Int. 2015;2015:542687. doi: 10.1155/2015/542687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt JL, Fairman R, Mitchell ME, et al. Bone formation in carotid plaques: a clinicopathological study. Stroke J Cereb Circ. 2002;33:1214–9. doi: 10.1161/01.str.0000013741.41309.67. [DOI] [PubMed] [Google Scholar]
- 22.Davaine J-M, Quillard T, Chatelais M, et al. Bone Like Arterial Calcification in Femoral Atherosclerotic Lesions: Prevalence and Role of Osteoprotegerin and Pericytes. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2016;51:259–67. doi: 10.1016/j.ejvs.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Hutcheson JD, Goettsch C, Bertazzo S, et al. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–43. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.New SEP, Goettsch C, Aikawa M, et al. Macrophage-derived matrix vesicles: an alternative novel mechanism for microcalcification in atherosclerotic plaques. Circ Res. 2013;113:72–7. doi: 10.1161/CIRCRESAHA.113.301036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapustin AN, Chatrou MLL, Drozdov I, et al. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–23. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 26.Bertazzo S, Gentleman E, Cloyd KL, et al. Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nat Mater. 2013;12:576–83. doi: 10.1038/nmat3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gondrie MJA, van der Graaf Y, Jacobs PC, et al. The association of incidentally detected heart valve calcification with future cardiovascular events. Eur Radiol. 2011;21:963–73. doi: 10.1007/s00330-010-1995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennenberg RJMW, Kessels AGH, Schurgers LJ, et al. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc Health Risk Manag. 2009;5:185–97. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruunsgaard H, Skinhoj P, Pedersen AN, et al. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–60. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swierszcz J, Dubiel JS, Krzysiek J, et al. One-year observation of inflammatory markers in patients with aortic valve stenosis. J Heart Valve Dis. 2011;20:639–49. [PubMed] [Google Scholar]
- 31.Al-Aly Z, Shao J-S, Lai C-F, et al. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler Thromb Vasc Biol. 2007;27:2589–96. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Lim J, Lu J, et al. Protective Role of Smad6 in Inflammation-Induced Valvular Cell Calcification. J Cell Biochem. 2015;116:2354–64. doi: 10.1002/jcb.25186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712– 23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 34.Parhami F, Morrow AD, Balucan J, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler Thromb Vasc Biol. 1997;17:680–7. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 35.Yu Z, Seya K, Daitoku K, et al. Tumor necrosis factor-α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther. 2011;337:16–23. doi: 10.1124/jpet.110.177915. [DOI] [PubMed] [Google Scholar]
- 36.Aikawa E, Nahrendorf M, Figueiredo J-L, et al. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116:2841–50. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 37.Shroff RC, McNair R, Skepper JN, et al. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol JASN. 2010;21:103– 12. doi: 10.1681/ASN.2009060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mazzone A, Epistolato MC, De Caterina R, et al. Neoangiogenesis, T-lymphocyte infiltration, and heat shock protein-60 are biological hallmarks of an immunomediated inflammatory process in end-stage calcified aortic valve stenosis. J Am Coll Cardiol. 2004;43:1670–6. doi: 10.1016/j.jacc.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Lim J, Ehsanipour A, Hsu JJ, et al. Inflammation drives retraction, stiffening and nodule formation via cytoskeletal machinery in a 3-dimensional culture model of aortic stenosis. Am J Pathol. doi: 10.1016/j.ajpath.2016.05.003. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watson KE, Parhami F, Shin V, et al. Fibronectin and collagen I matrixes promote calcification of vascular cells in vitro, whereas collagen IV matrix is inhibitory. Arterioscler Thromb Vasc Biol. 1998;18:1964–71. doi: 10.1161/01.atv.18.12.1964. [DOI] [PubMed] [Google Scholar]
- 41.Fayet C, Bendeck MP, Gotlieb AI. Cardiac valve interstitial cells secrete fibronectin and form fibrillar adhesions in response to injury. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2007;16:203–11. doi: 10.1016/j.carpath.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Fondard O, Detaint D, Iung B, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–41. doi: 10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 43.Kaden JJ, Dempfle C-E, Grobholz R, et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc Pathol Off J Soc Cardiovasc Pathol. 2005;14:80–7. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Van der Hoek YY, Sangrar W, Cote GP, et al. Binding of recombinant apolipoprotein(a) to extracellular matrix proteins. Arterioscler Thromb J Vasc Biol Am Heart Assoc. 1994;14:1792–8. doi: 10.1161/01.atv.14.11.1792. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien KD, Olin KL, Alpers CE, et al. Comparison of apolipoprotein and proteoglycan deposits in human coronary atherosclerotic plaques: colocalization of biglycan with apolipoproteins. Circulation. 1998;98:519–27. doi: 10.1161/01.cir.98.6.519. [DOI] [PubMed] [Google Scholar]
- 46.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J Off Publ Fed Am Soc Exp Biol. 2006;20:811–27. doi: 10.1096/fj.05-5424rev. [DOI] [PubMed] [Google Scholar]
- 47.Yip CYY, Chen J-H, Zhao R, et al. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–42. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 48.Engler AJ, Sen S, Sweeney HL, et al. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 49.Swift J, Ivanovska IL, Buxboim A, et al. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Haeger SM, Kloxin AM, et al. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PloS One. 2012;7:e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wipff P-J, Hinz B. Integrins and the activation of latent transforming growth factor beta1 - an intimate relationship. Eur J Cell Biol. 2008;87:601–15. doi: 10.1016/j.ejcb.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Watson KE, Bostrom K, Ravindranath R, et al. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest. 1994;93:2106–13. doi: 10.1172/JCI117205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodriguez KJ, Masters KS. Regulation of valvular interstitial cell calcification by components of the extracellular matrix. J Biomed Mater Res A. 2009;90:1043–53. doi: 10.1002/jbm.a.32187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simmons CA, Grant GR, Manduchi E, et al. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–9. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sucosky P, Balachandran K, Elhammali A, et al. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–60. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiler M, Yap CH, Balachandran K, et al. Regional analysis of dynamic deformation characteristics of native aortic valve leaflets. J Biomech. 2011;44:1459–65. doi: 10.1016/j.jbiomech.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisher CI, Chen J, Merryman WD. Calcific nodule morphogenesis by heart valve interstitial cells is strain dependent. Biomech Model Mechanobiol. 2013;12:5–17. doi: 10.1007/s10237-012-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutcheson JD, Venkataraman R, Baudenbacher FJ, et al. Intracellular Ca(2+) accumulation is strain-dependent and correlates with apoptosis in aortic valve fibroblasts. J Biomech. 2012;45:888–94. doi: 10.1016/j.jbiomech.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garfinkel A, Tintut Y, Petrasek D, et al. Pattern formation by vascular mesenchymal cells. Proc Natl Acad Sci U S A. 2004;101:9247–50. doi: 10.1073/pnas.0308436101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng H, Reddy A, Sage A, et al. Focal high cell density generates a gradient of patterns in self-organizing vascular mesenchymal cells. J Vasc Res. 2012;49:441–6. doi: 10.1159/000339568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao Y, Nowak S, Yochelis A, et al. Matrix GLA protein, an inhibitory morphogen in pulmonary vascular development. J Biol Chem. 2007;282:30131–42. doi: 10.1074/jbc.M704297200. [DOI] [PubMed] [Google Scholar]
- 62.Guo Y, Chen T-H, Zeng X, et al. Branching patterns emerge in a mathematical model of the dynamics of lung development. J Physiol. 2014;592:313–24. doi: 10.1113/jphysiol.2013.261099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen T-H, Guo C, Zhao X, et al. Patterns of periodic holes created by increased cell motility. Interface Focus. 2012;2:457–64. doi: 10.1098/rsfs.2012.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen T-H, Hsu JJ, Zhao X, et al. Left-right symmetry breaking in tissue morphogenesis via cytoskeletal mechanics. Circ Res. 2012;110:551–9. doi: 10.1161/CIRCRESAHA.111.255927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen T-H, Zhu X, Pan L, et al. Directing tissue morphogenesis via self-assembly of vascular mesenchymal cells. Biomaterials. 2012;33:9019–26. doi: 10.1016/j.biomaterials.2012.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ehara S, Kobayashi Y, Yoshiyama M, et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: an intravascular ultrasound study. Circulation. 2004;110:3424–9. doi: 10.1161/01.CIR.0000148131.41425.E9. [DOI] [PubMed] [Google Scholar]
- 67.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–6. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 68.Hinz B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol J Int Soc Matrix Biol. 2015;47:54–65. doi: 10.1016/j.matbio.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Chen J-H, Chen WLK, Sider KL, et al. β-catenin mediates mechanically regulated, transforming growth factor-β1-induced myofibroblast differentiation of aortic valve interstitial cells. Arterioscler Thromb Vasc Biol. 2011;31:590–7. doi: 10.1161/ATVBAHA.110.220061. [DOI] [PubMed] [Google Scholar]
- 70.Nakasaki M, Hwang Y, Xie Y, et al. The matrix protein Fibulin-5 is at the interface of tissue stiffness and inflammation in fibrosis. Nat Commun. 2015;6:8574. doi: 10.1038/ncomms9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paccou J, Renard C, Liabeuf S, et al. Coronary and abdominal aorta calcification in rheumatoid arthritis: relationships with traditional cardiovascular risk factors, disease characteristics, and concomitant treatments. J Rheumatol. 2014;41:2137–44. doi: 10.3899/jrheum.140239. [DOI] [PubMed] [Google Scholar]