Abstract
Objective
Treatment algorithms for cardiac arrest are rescuer-centric and vary little from patient to patient. The objective of this study was to determine if cardiopulmonary resuscitation (CPR) targeted to arterial blood pressure and coronary perfusion pressure (CPP) rather than optimal Guideline Care would improve 24-hour survival in a porcine model of ventricular fibrillation (VF) cardiac arrest.
Design
Randomized interventional study
Setting
Preclinical animal laboratory
Subjects
Female 3-month old swine
Interventions/Measurements
After induction of anesthesia and 7 minutes of untreated VF, 16 female 3-month old swine were randomized to: 1) Blood Pressure (BP) care: titration of chest compression (CC) depth to a systolic blood pressure (SBP) of 100 mmHg and vasopressor dosing to maintain CPP >20mmHg or 2) Guideline care: CC depth targeted to 51 mm and standard Guideline vasopressor dosing. Animals received manual CPR for 10 minutes before the first defibrillation attempt and standardized post-resuscitation care for 24 hours.
Main Results
24-hour survival was more likely with BP care versus Guideline care (0/8 versus 5/8, p<0.03), and all survivors had normal neurological examinations. Mean CPP prior to defibrillation was significantly higher with BP care (28±3 mmHg versus 10±6 mmHg, p<0.01). CC depth was lower with BP care (48±0.4 mmHg versus 44±0.5 mmHg, p<0.05) and number of vasopressor doses was higher with BP care (median 3 [range 1-7] versus 2 [range 2-2], p<0.01).
Conclusions
Individualized goal-directed hemodynamic resuscitation targeting SBP of 100 mmHg and CPP >20 mmHg improved 24-hour survival compared to Guideline care in this model of VF cardiac arrest.
Keywords: cardiac arrest, cardiopulmonary resuscitation, coronary perfusion pressure, ventricular fibrillation, swine
Introduction
In-hospital cardiac arrest remains a significant problem with approximately 200,000 patients receiving cardiopulmonary resuscitation (CPR) during their hospitalization.1 Recent studies show that 59% of adults and 93% of children have their in-hospital cardiac arrests in intensive care units (ICU).2, 3 Despite improvements in outcomes over the last decade, less than 25% of adults and 50% of children survive to hospital discharge. 3, 4
Current resuscitation algorithms based on the American Heart Association (AHA) Guidelines recommend chest compressions (CC) targeted to a depth greater than 51 mm.5, 6 The AHA also recommends monitoring resuscitation efforts using arterial blood pressure (BP), when feasible. The most important determinant of return of spontaneous circulation (ROSC) during CPR is myocardial blood flow,7, 8 which is driven by coronary perfusion pressure (CPP) (the difference between the aortic and right atrial diastolic pressures).9, 10 CPP can be measured in patients who have arterial and central venous lines. We have proposed an alternative strategy to the “one size fits all” CC depth guided resuscitation: an individualized approach based on a patient's hemodynamic response to resuscitative efforts.11-13
We have previously shown that individualized BP and CPP targeted care (BP care) to achieve a systolic BP >100 mmHg and a CPP >20 mmHg during CPR for ventricular fibrillation (VF) improved short-term (45 minute) survival compared to standard Guideline care targeted to achieve a CC depth of greater than 51 mm.12 The objective of this study was to assess whether BP care would improve 24-hour survival and 24-hour survival with favorable neurologic outcome compared to Guideline care in a porcine model of VF cardiac arrest.
Materials and Methods
Animal Preparation
The University of Pennsylvania Institutional Animal Care and Use Committee (IACUC) approved the experimental protocol. Sixteen 3-month old female domestic swine were anesthetized and mechanically ventilated on a mixture of room air and titrated isoflurane (∼1.0% to 2.5%) with a tidal volume of 10-12 mL/kg, positive end expiratory pressure of 6 cm H2O, and titration of ventilation rate to maintain end-tidal carbon dioxide (ETCO2) at 38 – 42 mmHg (NICO, Novametrix Medical Systems Inc.). Rectal temperature was monitored continuously and the animals were placed on a warming blanket if needed to maintain normothermia.
Continuous aortic and right atrial pressures were measured using solid-state, micromanometer-tipped catheters (MPC-500, Millar Instruments) advanced through the right femoral artery and external jugular vein into thoracic locations. Cardiac output and pulmonary artery pressure measurements were obtained with a Swan-Ganz thermodilution catheter (Edwards Lifesciences) that was advanced into the pulmonary artery. VF was induced through a bipolar pacing catheter (Edwards Lifesciences). 200 U/kg of unfractionated heparin was provided to prevent catheter clotting. All animals received 20 mL/kg 0.9% normal saline intravenously to replace overnight fasting fluid deficits prior to any baseline measurements.
Measurements
Baseline thermodilution cardiac outputs (ICU monitor: model HP66, Hewlett Packard) were obtained prior to induction of VF. Arterial blood gas specimens were obtained from the thoracic aorta at baseline, at the end of untreated VF and after 6 minutes of CPR. Mean CPP during CPR was calculated by the difference between the aortic pressure and the right atrial pressure during the relaxation phase of CPR (“diastolic period”).
The Philips Heart Start MRx defibrillator with Q-CPR option and a metronome were used to guide and record manual CPR quality. This defibrillator records CPR quality and provides audiovisual feedback to the CC provider for rate (CC/min), depth (mm), and incomplete chest wall recoil (residual leaning force) using force transducer/accelerometer technology.14-17
Experimental Protocol
Overview (Figure 1)
Figure 1.
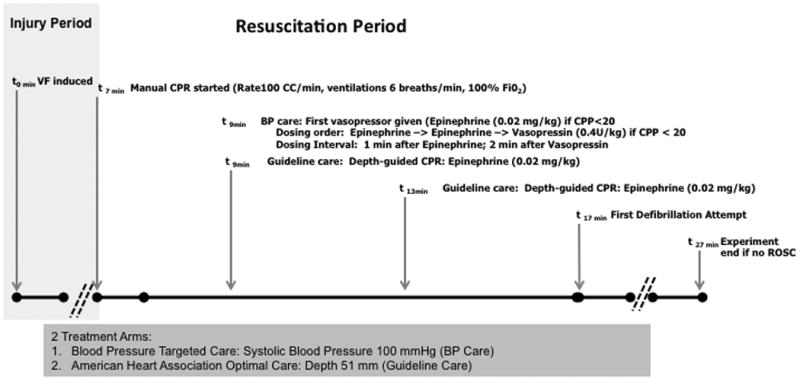
Protocol design. During protocol resuscitation period, animals were randomized to receive one of two resuscitation strategies. SBP indicates systolic blood pressure. Guideline care refers to depth-guided CPR at 51 mm and standard AHA vasopressor dosing intervals. BP care refers to compression depth titrated to systolic blood pressure of 100 mmHg and vasopressor dosing titrated to attain coronary perfusion pressure >20 mmHg.
VF was induced by electrical pacing. After confirmation of VF, the animals remained intubated but were disconnected from mechanical ventilation. Following seven minutes of untreated VF, animals received one of two CPR and advanced life support strategies for a 10-minute duration before attempts at defibrillation. A 10-minute interval of CPR was chosen before defibrillation as a practical approach, because this duration of CPR is necessary to adequately compare CPR techniques. In addition, most in-hospital CPR is at least 10 minutes in duration for both survivors and non-survivors.18, 19 If the animals are defibrillated more promptly, they have immediate ROSC and do not need CPR. The 7 minute untreated VF period was included so that the insult would be severe enough to allow discrimination of outcomes between the two protocols.
In both treatment arms, metronome-guided CCs were provided with a target rate of 100 CC/min. At the initiation of CPR mechanical ventilation was resumed at a rate of 6 breaths per minute with 100% oxygen. CPR was interrupted every two minutes for ≤4 seconds to mimic pulse checks and rhythm analyses. Animals received one of two resuscitation strategies: 1) BP care: titration of CC depth to a systolic BP (SBP) of 100 mmHg and vasopressor dosing to maintain CPP >20mmHg or 2) Guideline care: CC depth targeted to 51 mm and standard Guideline vasopressor dosing. Guideline vasopressor dosing included intravenous epinephrine (0.02 mg/kg) every 4 minutes starting at minute 9 of the protocol (2 minutes after CPR was started). Animals in the BP care group received intravenous vasopressor only if the CPP was <20mmHg, also starting at minute 9 of the protocol. The order of drug administration for BP care was epinephrine (0.02 mg/kg) followed by epinephrine (0.02 mg/kg) and then vasopressin (0.4 U/kg). The dosing interval was 1 minute between epinephrine doses, and 2 minutes after a vasopressin dose. After 10 minutes of CPR (minute 17 of the protocol), an initial 200 J biphasic waveform defibrillation attempt was provided. CPR according to treatment strategy continued until there was sustained ROSC or until minute 27 of the protocol (after an additional 10 minutes of resuscitation post-initial defibrillation attempt). If ROSC was attained, the animals then received post-cardiac arrest care according to protocol. Any animals not achieving ROSC received post-mortem examination for detection of visceral injuries that may have contributed to unsuccessful resuscitation.
Post-cardiac arrest Care
Animals that achieved ROSC received protocolized intensive care treatment which included: 1) titration of oxygen concentration to maintain oxygen saturation 92 - 94%; 2) titration of ventilation (tidal volume (6 – 10 mL/ kg) and ventilation rate) to maintain end tidal carbon dioxide between 38 and 42 mmHg; and 3) intravenous infusions of dopamine (up to 20 mcg/kg/min), as needed, to maintain mean arterial pressure greater than 55 mmHg. Anesthesia was maintained with inhaled isoflurane (∼1.0% to 2.5%), titrated to comfort, during this time period. All animals received buprenorphine (0.02 mg/kg intramuscular every 6 hours) for pain control. By 4 hours post-ROSC, vasopressor support was weaned and discontinued, and the animals were extubated. At 24 hours, all animals were neurologically assessed using the previously validated swine cerebral performance category (SCPC),20, 21 performed by 2 experienced research technicians who agreed on the final score.
Data Analysis / Outcomes
The primary outcome of this study was 24-hour survival. Secondary outcomes were: 1) any ROSC; 2) 45-minute survival; 3) 24-hour survival with favorable neurological outcome; 4) hemodynamic measures (specifically mean CPP); and 5) CPR quality variables. A favorable neurological outcome was defined as a SCPC as 1 - 2 at 24 hours. The Skewness-Kurtosis test was used to assess normality of continuous variables. Mean ± standard error of mean (SEM) were used to describe normally distributed continuous variables which were compared by Student's t-test. Continuous variables that were not normally distributed were described as median and interquartile range (25%, 75%) and evaluated by Wilcoxon Rank Sum. Fisher's exact test was used to evaluate comparisons of dichotomous variables, such as survival outcomes. Differences in CPR quality variables and differences in CPPs over time and between treatment groups as well as between survivors / non-survivors were assessed using generalized estimating equations (GEE) with an identity link. A robust variance estimator with an exchangeable correlation structure was used to account for longitudinal correlation, which arose from collecting observations on the same study animals over time. For our primary outcome, 10 animals were randomly assigned to each treatment protocol to have at least 80% power to detect a difference similar to survival in our short-term survival studies (37.5% in the Guideline care group and 100% in the BP care group). Because there were no survivors in the first 8 animals of the Guideline care control group, our IACUC asked us to analyze the data in an attempt to reduce the number of animals needed in accordance with the National Institutes of Health Animal Use Policy.22 Stata-IC statistical package (Version 12.0, StataCorp, College Station, TX) was used to perform the statistical analysis.
Results
Primary outcome of 24-hour survival and secondary outcomes of any ROSC, 45-minute survival, and 24-hour survival with favorable neurological outcome were all significantly higher in the BP care group compared to Guideline care (Table 1). In a model using generalized estimating equations (GEE), coronary perfusion pressure (Figure 2) was significantly higher in the BP care group compared to Guideline care (point estimate +11.7 mmHg; CI95 4.5 – 18.9 mmHg; p<0.01). Survivors at 24 hours tended to have higher CPPs than non-survivors that were not statistically significant (point estimate +7.8 mmHg; CI95 -1.53 – 17.1 mmHg; p=0.10). All survivors had a SCPC of 1 (normal, no difficulty standing, walking, eating, drinking, alert and fully responsive) at 24 hours. None of the survivors had fever in the post-cardiac arrest phase. Post-mortem examination in non-survivors showed no visceral injuries that may have contributed to unsuccessful resuscitation.
Table 1.
Rates of survival between treatment groups. Guideline care refers to depth-guided CPR at 51 mm and standard AHA vasopressor dosing intervals. BP care refers to compression depth titrated to systolic blood pressure of 100 mmHg and vasopressor dosing titrated to attain coronary perfusion pressure >20 mmHg. Favorable neurological outcome indicates 24 hour survival with favorable neurological outcome (swine cerebral performance category of 1 or 2).
| Guideline Care (n=8) | BP care (n=8) | p | |
|---|---|---|---|
| Survival [n (%)] | |||
| 45 Minute ICU Survival | 0 (0) | 7 (88) | p = 0.001 |
| 24 Hour Survival | 0 (0) | 5 (63) | p = 0.026 |
| Favorable Neurological Outcome | 0 (0) | 5 (63) | p = 0.026 |
Figure 2.
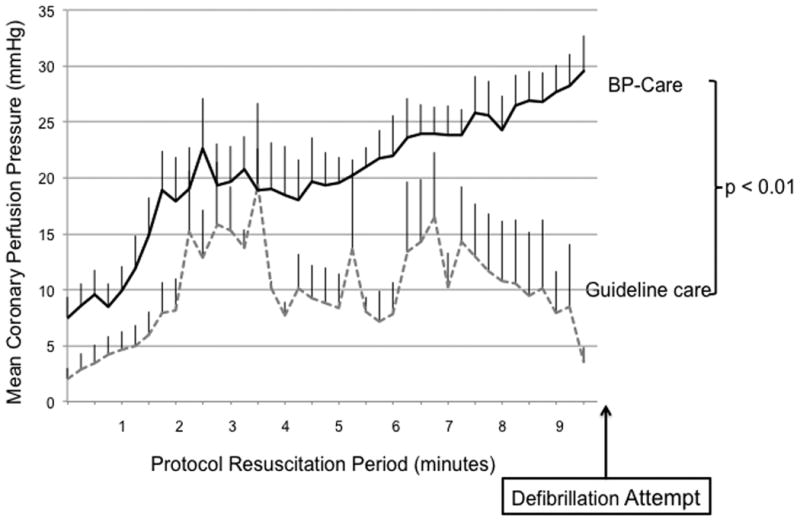
Mean coronary perfusion pressure during each minute of CPR across treatment groups. Error bars represent SEM. Guideline care refers to depth-guided CPR at 51 mm and standard AHA vasopressor dosing intervals. BP care refers to compression depth titrated to systolic blood pressure of 100 mmHg and vasopressor dosing titrated to attain coronary perfusion pressure >20 mmHg.
Resuscitation Variables
Mean CC depth was significantly different among groups: 44 ± 0.5 mm with BP care versus 48 ± 0.4 mm with Guideline care, p=0.049. Other CPR quality variables were not different between the two groups (rate = 100 ± 0.2 CC/min; CC fraction = 97 ± 0.3%; and no CCs delivered had leaning exceeding 2.5 kg). The total number of vasopressor doses administered prior to first defibrillation attempt after 10 minutes of resuscitation was significantly higher in BP care group compared to Guideline care (median 3 [range 1 – 7] vs. 2 [range 2 – 2]; p=0.006). Median number of defibrillation attempts to terminate VF and lead to sustained ROSC in survivors was 1 [range 1 – 3]. Of the 7 animals achieving 45-minute survival, 5 (71%) required vasopressor support to maintain goal blood pressures during the four hour Post-Cardiac Arrest period (dopamine infusion: mean peak dose 12 ± 2 mcg/kg/min). One surviving animal in the BP care group required cardioversion for unstable narrow complex supraventricular tachycardia during the four hour Post-Cardiac Arrest period after ROSC.
Hemodynamics (Table 2) and Arterial Blood Gases (Table 3)
Table 2.
Guideline care refers to depth-guided CPR at 51 mm and standard AHA vasopressor dosing intervals. BP care refers to compression depth titrated to systolic blood pressure of 100 mmHg and vasopressor dosing titrated to attain coronary perfusion pressure >20 mmHg. Data presented as mean (SEM) or median [IQR].
| Guideline care (n=8) | BP care (n=8) | p | |
|---|---|---|---|
| Baseline | |||
| Weight | 32.3 (0.5) | 31.3 (0.5) | 0.27 |
| CO (L/min) | 3.0 (0.3) | 3.0 (0.3) | 0.78 |
| AoS | 109 (4) | 111 (5) | 0.80 |
| AoD | 79 (5) | 81 (5) | 0.86 |
| RAD | 12 (1) | 11 (1) | 0.67 |
| CPP | 72 (5) | 72 (5) | 0.92 |
| End of Untreated VF Period* | |||
| AoS | 22 [18, 28] | 28 [21, 31] | 0.25 |
| AoD | 16 (3) | 19 (4) | 0.54 |
| RAD | 18 [14, 19] | 18 [16, 19] | 0.73 |
| CPP | 1 (1) | 6 (2) | 0.03 |
| End of Resuscitation Period† | |||
| AoS | 93 [75, 109] | 104 [97, 109] | 0.20 |
| AoD | 27 (6) | 42 (3) | 0.04 |
| RAD | 12 (2) | 12 (4) | 0.94 |
| CPP | 9 (6) | 28 (3) | <0.01 |
| ET CO2 | 29 (3) | 28 (2) | 0.86 |
15-second epoch during untreated VF period from 6:30 to 6:45;
15-second epoch during protocol resuscitation period from 16:15 to 16:30.Pressures in mmHg. AoS indicates aortic systolic pressure; AoD, aortic diastolic pressure; RAD, right atrial diastolic pressure; CPP, coronary perfusion pressure; ET CO2, end tidal carbon dioxide.
Table 3. Arterial Blood Gases.
Guideline care refers to depth-guided CPR at 51 mm and standard AHA vasopressor dosing intervals. BP care refers to compression depth titrated to systolic blood pressure of 100 mmHg and vasopressor dosing titrated to attain coronary perfusion pressure >20 mmHg.
| Guideline care (n = 8) | BP care (n = 8) | p | |
|---|---|---|---|
| Baseline | |||
| pH | 7.52 (0.01) | 7.56 (0.01) | 0.03 |
| PCO2 (mmHg) | 42 (2) | 41 (2) | 0.19 |
| PO2 (mmHg) | 120 (7) | 138 (13) | 0.29 |
| End of Untreated VF* | |||
| pH | 7.71 (0.05) | 7.65 (0.05) | 0.40 |
| PCO2 (mmHg) | 21 (4) | 23 (5) | 0.71 |
| PO2 (mmHg) | 95 (11) | 138 (22) | 0.08 |
| After 6 Minutes of CPR† | |||
| pH | 7.39 (0.03) | 7.42 (0.03) | 0.44 |
| PCO2 (mmHg) | 44 (5) | 36 (6) | 0.37 |
| PO2 (mmHg) | 225 (75) | 229 (53) | 0.88 |
Sample drawn after 6min 30s of untreated VF
Sample drawn after 6 minutes of CPR during protocol resuscitation period
At pre-arrest baseline hemodynamic variables were not different. Mean diastolic CPP was higher in the BP care group at the end of the untreated VF period compared to Guideline care: 6 ± 2 mmHg vs. 1 ± 1 mmHg; p=0.03. At the end of the resuscitation period, the BP care group had higher diastolic blood pressure compared to Guideline care (42 ± 3 mmHg vs. 27 ± 6 mmHg, p=0.04) and mean CPP (28 ± 3 mmHg vs. 10 ± 6 mmHg, p<0.01). There were no differences in mean ETCO2 between the groups throughout the CPR period or ETCO2 at the end of the resuscitation period. There were no differences in arterial blood gases obtained at the end of the untreated VF period, or after 6 minutes of CPR; however, the BP care group had a higher pH at baseline (Table 3).
Discussion
In this study we have demonstrated that individualized BP and CPP targeted cardiopulmonary resuscitation improves 24-hour survival and 24-hour survival with favorable neurological outcomes compared to depth targeted AHA Guideline care in a porcine model of VF cardiac arrest. The BP care group attained higher CPP despite slightly lower chest compression depth. As expected, the BP group received more vasopressor doses overall, yet the wide range of doses required highlights the variable resuscitation needs, and some survivors in the BP group only received one vasopressor dose during the entire 10 minutes of CPR.
This animal model was intended to address whether individualized BP and CPP targeted CPR could improve outcomes. We provided CPR for 10 minutes because most in-hospital CPR is provided for at least 10 minutes to both survivors and non-survivors.18, 19 Rather than defibrillate these animals promptly, we left them in VF so that we could provide the same duration of CPR to both the experimental BP care group and the Guideline care group. If we had defibrillated the animals earlier, the animals would have had variable times to ROSC so we would have not been able to compare the chest compression and vasopressor strategies. We had previously shown that BP care improved rates of ROSC and 45-minute survival, but it was possible that this approach would not result in longer duration survival or that the survivors would have been neurologically devastated. These exciting findings provide a proof-in-concept, but further data is necessary for applications in patients.
Most in-hospital cardiac arrests occur in ICUs,2, 3 and >10% of these ICU patients have invasive arterial BP monitoring. 18 For these patients, a BP-directed approach is possible. Yet we do not know the optimal CPP for human CPR. Nevertheless, these data support the biologically plausible concept that individualized hemodynamic titrated CPR can improve outcomes compared to rescuer-centric resuscitation protocols.
The AHA has placed a great emphasis on high quality CPR, which includes CCs at a rate of 100/minute, ventilation of 6 breaths/minute, no residual leaning, and compressing to an optimal depth of 51 mm.6, 23, 24 Previous investigations have shown that deeper compressions are associated with improved outcomes; 25-27 however, in this present study CC depth was slightly lower in the BP targeted group compared to the Guideline care group (i.e., depth alone does not guarantee adequate arterial pressures). The wide range of doses of vasopressors administered to the BP care group, including some animals that received only one vasopressor dose, suggests that an individualized approach to resuscitation may have additional benefits by giving vasopressors only when they are needed. While speculative, the success of the individualized approach may be that it allows rescuers to avert potential harm of both: (a) overly deep compressions and/or (b) vasopressor use when they are not required. By titrating CPR effect to blood pressure, compressions that were too deep for a given “patient” were avoided and unnecessary vasopressors that could adversely affect myocardial bioenergetics during CPR and afterload after ROSC were avoided when they were not needed to maintain CPP during CPR
ETCO2 monitoring has been recommended during resuscitation by the American Heart Association and correlates with cardiac output and resuscitation outcomes.6, 23, 24, 28 In the present study, there were no differences in mean ETCO2 during CPR in the two groups. These data suggests that monitoring invasive hemodynamic measurement of CPP during CPR is superior to ETCO2 monitoring during CPR.
An important finding in our study was the excellent neurological outcome in animals that survived after receiving BP directed CPR. Previous work by our group with cerebral monitoring of intracranial pressure and brain tissue oxygenation has demonstrated higher cerebral perfusion pressures and brain oxygen tension during CPR in animals that received BP care for a VF cardiac arrest compared to Guideline care.29 This finding is significant as hypoxic-ischemic brain injury causes significant morbidity in survivors of cardiac arrest. In the present investigation, neurologic testing was performed using the SCPC, which provides a gross evaluation of overall neurologic function.
This study has several limitations. This is an animal study in anesthetized healthy young swine without coronary artery disease. The findings are only potentially applicable to patients who have invasive monitoring in place at the time of arrest. However, large epidemiological studies have established that the majority of in-hospital cardiac arrests occur in ICUs,2-4 and some of these patients have an arterial line in place at the time of arrest.18 At the end of the untreated VF period the BP targeted group had a statistically higher diastolic CPP (6 mmHg versus 1 mmHg) and higher baseline pH compared to the BP care group (7.56 versus 7.52). The animals were randomized before each study to one or the other group, and it is not unusual to have one of many sets of measurements be statistically different when so many comparisons are analyzed. Importantly, the CPP was similarly quite low in both groups and the pH was not clinically different in the two groups. In the BP care group the overall median number of vasopressors prior to defibrillation was higher compared to the Guideline care group (median 3 [range 1 – 7] vs. 2 [range 2 – 2]; p=0.006), as would be expected because the experimental protocol in the BP care group could result in 0-7 doses of vasopressor per animal whereas the Guideline care group all received 2 doses prior to the first defibrillation attempt. In part, the higher CPPs in the BP group resulted from the titrated higher vasopressor dosage. However, it is important to note that there was a wide range of vasopressor doses administered to the BP titrated group and some of the 24-hour survivors in the BP care group received only one vasopressor dose. Therefore number of doses alone does not explain the differences in outcomes between the two groups. We were not able to compare neurologic outcome between survivors in the BP care group and Guideline care group as there were no survivors in the Guideline care group. Although neurologic outcome was favorable at 24 hours in survivors by the SCPC, complex neurobehavioral testing was not performed. Nevertheless, all survivors were walking normally and drinking normally. Anesthesia was provided with isoflurane which may have protective properties;32, 33 however, isoflurane was provided to all animals in both groups, yet survival only occurred in the BP care group. 30, 31 Finally, our study was not blinded, so objective measures for rate, depth, leaning and ventilation, and strict adherence to a prospective post-cardiac arrest protocol were implemented to minimize risk for bias.
Conclusion
In this animal model, individualized goal-directed resuscitation targeting SBP >100mmHg and CPP >20 mmHg improved 24-hour survival compared to Guideline “one size fits all” rescuer-centric care. This approach is a promising strategy for use in invasively monitored ICU patients.
Acknowledgments
Financial Support: This study was funded by The Russell Raphaely Endowed Chair Fund at The Children's Hospital of Philadelphia. The authors have no conflicts of interest.
Copyright form disclosures: Dr. Sutton received support for article research from the National Institutes of Health (NIH), received funding from Zoll Medical, and disclosed other support (He is a member of the AHA's Get with the Guidelines Pediatric Research Task force and a main author of the 2015 PALS Guidelines). His institution received funding from the NIH NICHD. Dr. Friess received support for article research from the NIH. His institution received funding from the NIH. Dr. Lampe disclosed other support (Ownership Interest in Helar Technologies, Intellectual Property in Resuscitation Devices), received support for article research from the NIH, and received funding from Philips Healthcare. His institution received funding from the NHLBI and ZOLL Medical Corporation. Dr. Becker received funding from Scientific Advisory Board (Nihon Kohden, Tokyo, Japan), received funding from the NIH Data Safety Monitoring Board and Protocol Review Committee (Bethesda, MD), and received funding from NIH New York Mt. Sinai K12 Training Grant. He disclosed other support: 1) INSTITUTIONAL GRANT/RESEARCH SUPPORT (funds go to Northwell Health) from Philips Medical Systems (Seattle, WA), NIH (Bethesda, MD), Zoll Medical Corp (Boston, MA), Medtronic Foundation (Minneapolis, MN), and Nihon Kohden (Tokyo, Japan). 2) SCIENTIFIC CONSULTANT/ADVISORY PANEL (Have received honoraria and payments) from Philips Medical Systems (Seattle, WA), NIH Data Safety Monitoring Board and Protocol Review Committee (Bethesda, MD), NIH Resuscitation Outcomes Consortium (Bethesda, MD), NIH New York Mt. Sinai K12 Training Grant, and Scientific Advisory Board (Nihon Kohden, Tokyo, Japan). 3) PATENTS (Currently have received no payments for these but they have potential royalties) Hypothermia Induction and Reperfusion therapies (Patents including 7 issued patents and several pending patents involving the use of medical slurries as a human coolant, devices to create slurries, and reperfusion cocktails). 4) GOVERNMENT (paid per day at governmental approved rate) NIH Data Safety Monitoring Board and Protocol Review Committee (Bethesda, MD). 5) OWNERSHIP, EQUITY, ROYALTIES IN PRIVATELY HELD COMPANIES Inventor's equity and royalties from Helar, a company started by University of Pennsylvania developing cooling technologies for medical cooling using “slurry” technology. The company was started in 2013. It currently has 00.00 net worth. 6) MEMBERSHIP IN NATIONAL ORGANIZATION THAT HAS A FINANCIAL INTEREST IN OUTCOME OF STUDIES (no personnel payments but income is generated for the American Heart Association) Long standing volunteer member of the American Heart Association (currently serving on several committees) which has a financial interest in the outcome of resuscitation studies being conducted. The AHA sells training materials worldwide on resuscitation techniques. 7) OTHER: SPEAKER HONORARIA, TRAVEL COST SUPPORT OR REIMBURSEMENT (DIRECT COSTS) FOR LECTURING AND TRAVEL Universities for Lecturing, Keio University (Tokyo). His institution received funding (above). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, Groeneveld PW. Incidence of treated cardiac arrest in hospitalized patients in the united states. Crit Care Med. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg RA, Sutton RM, Holubkov R, Nicholson CE, Dean JM, Harrison R, Heidemann S, Meert K, Newth C, Moler F, Pollack M, Dalton H, Doctor A, Wessel D, Berger J, Shanley T, Carcillo J, Nadkarni VM. Ratio of picu versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in-hospital cardiac arrest. The New England journal of medicine. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girotra S, Spertus JA, Li Y, Berg RA, Nadkarni VM, Chan PS. Survival trends in pediatric in-hospital cardiac arrests: An analysis from get with the guidelines-resuscitation. Circulation. Cardiovascular quality and outcomes. 2013;6:42–49. doi: 10.1161/CIRCOUTCOMES.112.967968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Caen AR, Berg MD, Chameides L, Gooden CK, Hickey RW, Scott HF, Sutton RM, Tijssen JA, Topjian A, van der Jagt EW, Schexnayder SM, Samson RA. Part 12: Pediatric advanced life support: 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S526–542. doi: 10.1161/CIR.0000000000000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Link MS, Berkow LC, Kudenchuk PJ, Halperin HR, Hess EP, Moitra VK, Neumar RW, O'Neil BJ, Paxton JH, Silvers SM, White RD, Yannopoulos D, Donnino MW. Part 7: Adult advanced cardiovascular life support: 2015 american heart association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S444–464. doi: 10.1161/CIR.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 7.Halperin HR, Lee K, Zviman M, Illindala U, Lardo A, Kolandaivelu A, Paradis NA. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. The American journal of emergency medicine. 2010;28:195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: A predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 9.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12:871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 11.Sutton RM, Friess SH, Maltese MR, Naim MY, Bratinov G, Weiland TR, Garuccio M, Bhalala U, Nadkarni VM, Becker LB, Berg RA. Hemodynamic-directed cardiopulmonary resuscitation during in-hospital cardiac arrest. Resuscitation. 2014;85:983–986. doi: 10.1016/j.resuscitation.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friess SH, Sutton RM, Bhalala U, Maltese MR, Naim MY, Bratinov G, Weiland TR, 3rd, Garuccio M, Nadkarni VM, Becker LB, Berg RA. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41:2698–2704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton RM, Friess SH, Naim MY, Lampe JW, Bratinov G, Weiland TR, 3, Garuccio M, Nadkarni VM, Becker LB, Berg RA. Patient-centric blood pressure-targeted cardiopulmonary resuscitation improves survival from cardiac arrest. Am J Respir Crit Care Med. 2014;190:1255–1262. doi: 10.1164/rccm.201407-1343OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abella BS, Alvarado JP, Myklebust H, Edelson DP, Barry A, O'Hearn N, Vanden Hoek TL, Becker LB. Quality of cardiopulmonary resuscitation during in-hospital cardiac arrest. JAMA : the journal of the American Medical Association. 2005;293:305–310. doi: 10.1001/jama.293.3.305. [DOI] [PubMed] [Google Scholar]
- 15.Abella BS, Edelson DP, Kim S, Retzer E, Myklebust H, Barry AM, O'Hearn N, Hoek TL, Becker LB. Cpr quality improvement during in-hospital cardiac arrest using a real-time audiovisual feedback system. Resuscitation. 2007;73:54–61. doi: 10.1016/j.resuscitation.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Edelson DP, Litzinger B, Arora V, Walsh D, Kim S, Lauderdale DS, Vanden Hoek TL, Becker LB, Abella BS. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Archives of internal medicine. 2008;168:1063–1069. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 17.Sutton RM, Niles D, Nysaether J, Abella BS, Arbogast KB, Nishisaki A, Maltese MR, Donoghue A, Bishnoi R, Helfaer MA, Myklebust H, Nadkarni V. Quantitative analysis of cpr quality during in-hospital resuscitation of older children and adolescents. Pediatrics. 2009;124:494–499. doi: 10.1542/peds.2008-1930. [DOI] [PubMed] [Google Scholar]
- 18.Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38:101–108. doi: 10.1097/CCM.0b013e3181b43282. [DOI] [PubMed] [Google Scholar]
- 19.Goldberger ZD, Chan PS, Berg RA, Kronick SL, Cooke CR, Lu M, Banerjee M, Hayward RA, Krumholz HM, Nallamothu BK. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: An observational study. Lancet. 2012;380:1473–1481. doi: 10.1016/S0140-6736(12)60862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berg RA, Chapman FW, Berg MD, Hilwig RW, Banville I, Walker RG, Nova RC, Sherrill D, Kern KB. Attenuated adult biphasic shocks compared with weight-based monophasic shocks in a swine model of prolonged pediatric ventricular fibrillation. Resuscitation. 2004;61:189–197. doi: 10.1016/j.resuscitation.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 21.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, Ewy GA. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 22.Silk SB, Hampton LL, Brown PA. What investigators need to know about the use of animals. Ilar J. 2014;54:324–328. doi: 10.1093/ilar/ilt046. [DOI] [PubMed] [Google Scholar]
- 23.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, Passman RS, White RD, Hess EP, Tang W, Davis D, Sinz E, Morrison LJ. Part 8: Adult advanced cardiovascular life support: 2010 american heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2010;122:S729–767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 24.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, Aufderheide TP, Menon V, Leary M. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: A consensus statement from the american heart association. Circulation. 2013;128:417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 25.Kramer-Johansen J, Myklebust H, Wik L, Fellows B, Svensson L, Sorebo H, Steen PA. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: A prospective interventional study. Resuscitation. 2006;71:283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, Merchant RM, Hoek TL, Steen PA, Becker LB. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71:137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Sutton RM, French B, Niles DE, Donoghue A, Topjian AA, Nishisaki A, Leffelman J, Wolfe H, Berg RA, Nadkarni VM, Meaney PA. 2010 american heart association recommended compression depths during pediatric in-hospital resuscitations are associated with survival. Resuscitation. 2014;85:1179–1184. doi: 10.1016/j.resuscitation.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders AB, Kern KB, Otto CW, Milander MM, Ewy GA. End-tidal carbon dioxide monitoring during cardiopulmonary resuscitation. A prognostic indicator for survival. JAMA. 1989;262:1347–1351. [PubMed] [Google Scholar]
- 29.Friess SH, Sutton RM, French B, Bhalala U, Maltese MR, Naim MY, Bratinov G, Arciniegas Rodriguez S, Weiland TR, Garuccio M, Nadkarni VM, Becker LB, Berg RA. Hemodynamic directed cpr improves cerebral perfusion pressure and brain tissue oxygenation. Resuscitation. 2014;85:1298–1303. doi: 10.1016/j.resuscitation.2014.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao P, Ji G, Xue H, Yu W, Zhao X, Ding M, Yang Y, Zuo Z. Isoflurane postconditioning improved long-term neurological outcome possibly via inhibiting the mitochondrial permeability transition pore in neonatal rats after brain hypoxia-ischemia. Neuroscience. 2014;280:193–203. doi: 10.1016/j.neuroscience.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Burchell SR, Dixon BJ, Tang J, Zhang JH. Isoflurane provides neuroprotection in neonatal hypoxic ischemic brain injury. J Investig Med. 2013;61:1078–1083. doi: 10.231/JIM.0b013e3182a07921. [DOI] [PMC free article] [PubMed] [Google Scholar]


