Amplified pulmonary inflammation following intoxication and burn injury is driven by AM cell death, and a heightened pro-inflammatory profile.
Keywords: alcohol, lung, apoptosis, proinflammatory
Abstract
In this study, the role and fate of AMs were examined in pulmonary inflammation after intoxication and injury. Clinical evidence has revealed that half of all burn patients brought to the emergency department are intoxicated at the time of injury. This combined insult results in amplified neutrophil accumulation and pulmonary edema, with an increased risk of lung failure and mortality, relative to either insult alone. We believe that this excessive pulmonary inflammation, which also parallels decreased lung function, is mediated in part by AMs. Restoration of lung tissue homeostasis is dependent on the eradication of neutrophils and removal of apoptotic cells, both major functions of AMs. Thirty minutes after binge ethanol intoxication, mice were anesthetized and given a 15% total body surface area dorsal scald injury. At 24 h, we found a 50% decrease in the total number of AMs (P < 0.05) and observed a proinflammatory phenotype on the remaining lung AMs. Loss of AMs paralleled a 6-fold increase in the number of TUNEL+ lung apoptotic cells (P < 0.05) and a 3.5-fold increase in the percentage of annexin V+ apoptotic cells in BAL (P < 0.05), after intoxication and injury, relative to controls. In contrast to the reduction in the number of cells, AMs from intoxicated and injured mice had a 4-fold increase in efferocytosis (P < 0.05). In summary, these data suggest that loss of AMs may delay resolution of inflammation, resulting in the pulmonary complications and elevated mortality rates observed in intoxicated and burn-injured patients.
Introduction
Binge drinking is an increasingly prevalent activity in the United States and is a frequent drinking pattern among patients with traumatic injury, including burn injury [1–3]. It is defined by a blood alcohol concentration of 0.08% or by the number of alcoholic drinks an individual has within a 2 h time period (4 for women, 5 for men). The consumption of alcohol before injury leads to more adverse outcomes, and clinical evidence suggests that half of the burn patient population is under the influence of alcohol at the time of injury [4, 5]. This combined insult results in greater need for fluid resuscitation, a longer hospital stay, and an increase in the number of days spent on mechanical ventilation, leading to a greater risk of pulmonary complications and mortality [3, 4, 6–9].
ARDS is a common morbidity among severely burn-injured patients, with or without inhalation injury [10, 11]. Infiltration of inflammatory cells, primarily neutrophils, and increased pulmonary edema lead to impaired gas exchange and increase the risk of pulmonary failure [10, 12, 13], resulting in 42% of mortality linked to pulmonary complications after burn injury [14, 15]. We and others have established in a mouse model of intoxication and injury that lung inflammation mirrors the characteristic pathology of ARDS. Within the distal airways of the lung, intoxicated mice subjected to a moderate-sized scald injury had a profound increase in alveolar wall thickening, neutrophil accumulation in the interstitium, and heightened levels of neutrophil chemokines CXCL1 (KC) and MIP-2, relative to either ethanol exposure or burn injury alone [16–21]. Elevated levels of the proinflammatory cytokine IL-6 were also seen, and this excessive pulmonary inflammation paralleled a decrease in lung function and an increase in mortality [16, 17, 21]. The lungs are often the first organ to fail after traumatic injury [11]; hence, identification of therapeutic targets in the lung to help manage the excessive inflammatory response after remote injury is likely to reduce elevated morbidity and mortality in intoxicated and burn-injured patients.
During an inflammatory response, mediators produced by macrophages, neutrophils, or other activated lung cells, can result in lung tissue damage if not properly regulated [22]. Thus, lung inflammation needs to be a highly monitored process. Resident AMs play a critical role in both the onset and resolution of pulmonary inflammation [23–25]. Macrophages can readily activate into a proinflammatory M1 macrophage or an anti-inflammatory M2 macrophage, depending on mediators present within the microenvironment. Resolution of inflammation is achieved, in part, through pathogen clearance, downregulation of neutrophil chemokines and the removal of apoptotic cells, all potential functions AMs [26]. Others have reported an increase in apoptosis in multiple cell populations in lung tissue after burn injury alone [27–29], but specific subsets of cells undergoing apoptosis were not analyzed in detail. The removal of apoptotic cells and the emigration of neutrophils from the lung tissue is key to resolving pulmonary inflammation. The role of AMs in coordinating on-going inflammation and the timely initiation of resolution emphasizes that the activation profile and fate of AMs during inflammation after intoxication and injury are critical to restoring lung homeostasis.
Because intoxication at the time of injury results in greater pulmonary complications and mortality rates than burn injury alone [21], we chose to examine the role of AMs in pulmonary inflammation after intoxication and burn injury, in comparison to sham vehicle control. At 24 h after injury, there was a 50% reduction in the total number of AMs present within the lung tissue, paralleling an increase in AM apoptosis. Furthermore, the remaining AMs had a proinflammatory phenotype. We conclude that, in this model of combined insult, the role of AMs in clearing apoptotic cells in the lung is limited by the reduction in the number of cells as a result of apoptosis by AMs themselves. In the absence of optimal clearance, resolution of inflammation in the lungs of intoxicated and injured mice is delayed, resulting in excessive tissue damage relative to either insult alone.
MATERIALS AND METHODS
Mice
Male (C57BL/6) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and used at 8–10 wk of age. The mice were housed in sterile microisolator cages in specific pathogen-free conditions in the Loyola University Chicago Comparative Medicine facility. All experiments were conducted in accordance with the Institutional Animal Care and Use Committee. Mice weighing between 22 and 27 g were used in these studies.
Murine model of binge ethanol and burn injury
A murine model of binge ethanol intoxication and burn injury was used, with single-dose oral gavage [30–32]. Animals were given 400 μl of 10% (v/v) ethanol solution (1.6 g/kg) or water control by gavage at a dose designed to elevate the blood alcohol concentration to 150 mg/dl at 30 min after ethanol exposure [33]. The mice were then anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine) and their dorsum shaved. They were placed in a plastic template exposing 15% of the TBSA and were subjected to a scald injury in a 92–95°C water bath or to sham injury in room-temperature water [30]. The scald injury results in an insensate, full-thickness burn [34]. The mice were then resuscitated with 1.0 ml saline and allowed to recover on warming pads. All experiments were performed between 8 and 9 AM, to avoid confounding factors related to circadian rhythms. The animals were euthanized at 24 h.
Bronchoalveolar lavage
The lungs of the mice were lavaged 5 times with 0.9 ml of PBS to obtain the BAL fluid [35]. All lavage washes were pooled per animal and centrifuged for 1200 rpm for 5 min. Lavage fluid was removed from pelleted cells, stored at −80°C, and analyzed for SP-D via ELISA (R&D Systems, Minneapolis, MN, USA). RBCs in the cell pellet were lysed using ACK lysis buffer (Thermo Fisher Scientific, Grand Island, NY, USA) and were counted with a hemocytometer and either analyzed by flow cytometry or cytocentrifuged onto slides.
Enzymatic lung tissue dissociation
The upper left lung lobe was removed and cut into small pieces with a razor. The lung tissue was then transferred to a C-tube (Miltenyi Biotec, Auburn, CA, USA) and processed using digestion buffer containing 1 mg/ml of collagenase D and 0.1 mg/ml DNase I (Roche, Indianapolis, IN, USA) in HBSS and a GentleMACS dissociator (Miltenyi Biotec), according to the manufacturer’s instructions. The homogenates were then filtered through a 70 μm nylon cell strainer to obtain a single-cell suspension [36]. RBCs were lysed with ACK lysis buffer. Cells were counted by trypan blue exclusion.
Flow cytometry analysis of AMs
To assess AMs, 1 × 106 lung cells or total recovered BAL cells (<2 × 105 cells) were first incubated with anti-CD16/32 (eBioscience, San Diego, CA, USA) to block nonspecific binding to the Fcy II/III receptor [21]. Cells were then immunostained with rat anti-mouse antibodies: CD11c APC-eFluor 780, CD11b eFluor 450, F4/80 APC, Gr-1 PE Cy7, MHC II (I-A/I-E) V500, TLR4/MD-2 APC (eBioscience); Siglec-F PE-CF594 (BD Biosciences, San Jose, CA, USA); and MARCO FITC (AbD Serotec, Raleigh, NC, USA) [36]. Antibody incubation was performed for 30 min at 4°C, and the cells were washed and fixed [37, 38]. In separate experiments, apoptosis and cell death was assessed in BAL cells by using an AlexaFluor 488 Annexin V/Dead Cell Apoptosis Kit (Thermo Fisher Scientific), according to the manufacturer’s protocol. Samples were run on a BD Fortessa cytometer (BD Biosciences). Data analysis was performed using Flow Jo FCS analysis software (Treestar, Ashland, OR, USA).
TUNEL immunofluorescent staining
The upper right lobe of the lung was inflated with 10% formalin, fixed overnight, embedded in paraffin, and sectioned at 5 μm [19]. A Click-it Plus in situ TUNEL Alexa Fluor 488 assay was performed according to the manufacturer’s protocol (Thermo Fisher Scientific) to detect apoptotic cells. Lung sections were evaluated using fluorescence microscopy (EVOS; Thermo Fisher Scientific), and micrographs were obtained at ×100 and ×200. TUNEL+ cells were counted in a blinded fashion in 10 low-power fields (×100), with a Java-based imaging program ImageJ (National Institutes of Health, Bethesda, MD, USA). The images were converted to binary to differentiate TUNEL+ from nonfluorescent cells and then analyzed for the number of TUNEL+ cells in each field of view.
Histology of AMs
BAL cells were centrifuged onto slides with a Shandon Cytospin 2 (Block Scientific, Bellport, NY, USA). In brief, 30,000 BAL cells were resuspended in 200 μl PBS and centrifuged at 400 rpm for 5 min. Slides were stained with HEMA 3 differential stain and visualized by light microscopy (EVOS; Thermo Fisher Scientific). Micrographs of cells were obtained at ×400 and ×1000 magnification. Phagocytic AMs were counted in a blinded fashion in 5 high power fields (×400). Slides were stained with antibody to rabbit anti-mouse active-caspase 3 (Abcam, Cambridge, MA, USA) and donkey anti-rabbit 594 secondary antibody (Thermo Fisher Scientific). Slides were air dried and mounted with Prolong DAPI Gold antifade medium (Thermo Fisher Scientific). Immunofluorescent micrographs were obtained at ×1000, with an AxioVert microscope (Zeiss, Thornwood, NY, USA).
Statistical analysis
Statistical comparisons were made between the sham vehicle and burn ethanol treatment groups. The unpaired t test was used and results were statistically significant at P < 0.05. Data are reported as means ± sem. Each data set is representative of 2 independent experiments (n = 3–4 sham vehicle and n = 5–6 burn ethanol animals per experiment).
RESULTS
Loss of AMs after intoxication and injury
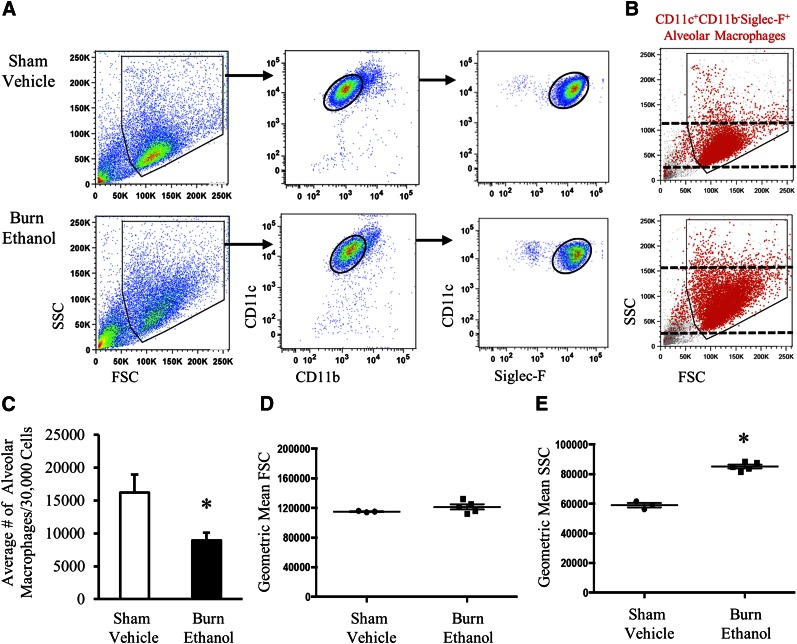
After intoxication and injury, we observed an ∼50% reduction in the number of cells recovered from the BAL fluid, where 164,500 ± 25,239 cells were recovered from sham vehicle mice and 83,300 ± 14,866 cells after intoxication and injury (P < 0.05). AMs have a phenotype distinct from other tissue-resident macrophage populations and can be characterized as CD11c+CD11b−Siglec-F+, in addition to F4/80+, with low to negative expression of MHC II [36, 39, 40] and low expression of the scavenger receptor, MARCO, a receptor that binds nonopsonized inhaled particles and bacteria in the lung [41]. Using flow cytometry, we identified BAL AMs as CD11c+CD11b−Siglec-F+ (Fig. 1A) [36] and found that they represented >82% of total BAL cells, regardless of treatment. In addition, we confirmed that these cells were F4/80+ (data not shown). We quantified AMs and confirmed a 50% decrease in the absolute number of CD11c+CD11b−Siglec-F+ BAL AMs (P < 0.05; Fig. 1C) after injury. We next analyzed the size and granularity of AMs using FSC and SSC, respectively (Fig. 1B). Geometric mean analysis of FSC (Fig. 1D) did not show a significant difference in cell size between groups; however, there was slight variability in comparison to sham vehicle. Analysis of SSC (Fig. 1E) revealed that AMs had a higher level of granularity (P < 0.05), which is a characteristic of activated macrophages.
Figure 1. Decreased number of CD11c+CD11b−Siglec-F+ AMs after intoxication and injury.
(A) Representative gating of CD11c+CD11b−Siglec-F+ AMs in BAL fluid. Total lung cells were gated to include large myeloid cells (polygon gate) and to exclude dead cells. AMs were first selected for CD11c+CD11b− expression, then expression of Siglec-F (black circular gates). (B) FSC and SSC characteristics of CD11c+CD11b−Siglec-F+ AMs (red) in both treatment groups. Black dashed lines indicate range of SSC. (C) Average number of CD11c+CD11b−Siglec-F+ cells recovered from BAL fluid. Data are representative of flow cytometric cell counts per 30,000 BAL cells. Geometric mean of FSC (D) and SSC (E) on CD11c+CD11b−Siglec-F+ AMs. Data are means ± sem. Each dot represents an individual animal. *P < 0.05 by unpaired t test.
We also noted an increase in the population to the left of the CD11c+CD11b−Siglec-F+AMs, and identified these cells as CD11c+CD11b−Siglec-F−. Analysis of FSC and SSC suggested that these were dead or apoptotic cells (Supplemental Fig. 1A, B), a conclusion that was further supported by histologic analysis of sorted CD11c+CD11b−Siglec-F− cells (Supplemental Fig. 1C). Quantification of this population showed an increase in dead cells after intoxication and injury (Supplemental Fig. 1D). Preliminary data from our laboratory suggest that AMs lose Siglec-F as they undergo apoptosis, and therefore these data would support our findings that intoxication and injury lead to a loss of AMs 24 h after injury.
Similar findings were identified in enzymatic-dissociated lung tissue that contained a heterogeneous population of lung cells, including leukocytes. We saw a decrease in the density of the AM population in flow cytometry plots of total lung tissue and quantified a 50% decrease in the absolute number of CD11c+CD11b−Siglec-F+ AMs (P < 0.05) (Supplemental Fig. 2). These data suggest that the observed 50% reduction in the number of AMs isolated from the BAL fluid is not a result of AMs adhering to the alveolar wall after intoxication and injury but is probably related to a loss of the AM population within the lungs. Of note, we did not see neutrophil infiltration into the alveolar space (Fig. 1A); however, we did observe neutrophil accumulation in the lung tissue (interstitium) (Supplemental Fig. 2A, C, D), as previously described by our laboratory [16–21]. Together, these data suggest that intoxication and injury lead to a decrease in the number of AMs 24 h after injury, but the remaining AMs are activated.
AMs upregulate a proinflammatory phenotype
The upregulation of cell surface receptors can define AM function at different stages of inflammation. MARCO is a marker of proinflammatory M1 macrophages. It binds nonopsonized inhaled particles and bacteria in the lung and has been shown to aid in the clearance of apoptotic cells through efferocytosis, a process crucial to the inflammatory response [41, 42]. To determine the activation state, levels of surface receptors expression were assessed on BAL-derived AMs by measuring MFI. Consistent with the literature, we confirmed that AMs are MHC IIlow/−, MARCO+, and CD11b− (Fig. 2B–D). In comparison to AMs from sham vehicle animals, we found intoxication and injury increased levels of TLR4/MD-2, MHC II, MARCO, and CD11b expression (P < 0.05; Fig. 2). Because AM FSC was not significantly different after intoxication and injury, relative to sham, our findings suggests the upregulation of these receptors is a result of the activation of AMs and not to an increase in cell size and subsequent receptor expression.
Figure 2. AMs upregulate cell surface receptors after intoxication and injury.
Histogram overlay (top) and scatter plot graph representation (bottom) of average MFI on AMs from sham vehicle (blue) and burn ethanol (red) groups, in comparison to FMO controls (gray) for TLR4/MD-2 (A), MHC II (B), MARCO (C), and CD11b (D). Each dot represents an individual animal. *P < 0.05 by unpaired t test. Data are means ± sem.
A change in TLR4/MD-2 and MHC II expression at 24 h suggests AMs are activated by TLR4 ligands, such as LPS or endogenous danger signals, as a result of the cutaneous injury. In addition, MARCO is an important receptor in efferocytosis. CD11b has a role in leukocyte adhesion and also aids in the removal of iC3b-opsonized apoptotic cells through the complement receptor 3 [43]. Because naïve AMs are identified as CD11b−, we questioned whether the increase in MFI was related to receptor upregulation or the replenishment of AMs by CD11b+ monocytes. Our data indicate that there was a shift in CD11b expression on AMs after injury (Supplemental Fig. 3), supporting our observation that AMs are acquiring CD11b during activation. AMs express high levels of mannose receptor CD206 [36, 39], which has been characterized as an anti-inflammatory macrophage surface marker [25, 44–46]. We examined expression of CD206 on AMs and found no significant difference between treatment groups (data not shown). Together, these markers are characteristic of an activated proinflammatory AM phenotype after intoxication and injury.
Intoxication and injury results in AM apoptosis
AMs comprise only 10% of the total cells found in the alveoli, yet their ability to balance pro- and anti-inflammatory responses is critical for lung homeostasis. The loss of AMs after intoxication and injury led us to investigate the fate of these regulatory cells 24 h after injury. Using immunofluorescent TUNEL staining, we assessed lung tissue for apoptotic cells. We observed an increase in the number of fluorescent punctate cells in the lung tissue (Fig. 3A, B) and determined that there were 6-fold more apoptotic cells after intoxication and injury (P < 0.05) (Fig. 3C), relative to those in sham vehicle mice. Others have suggested that there is an increase in the apoptosis of multiple lung cell populations, including AMs, neutrophils, epithelial, and endothelial cells [27–29]. To determine whether AMs were undergoing apoptosis, BAL cells were isolated and analyzed for apoptosis marker, annexin V, and dead cell marker, propidium iodide (Fig. 3D–F). Intoxicated and injured animals had an average 3.5-fold increase in the percentage of apoptotic cells (P < 0.05), in comparison to the sham vehicle group (Fig. 3F). The FSC and SSC of annexin V+ cells in the total BAL cell gate (Fig. 3E) mirrors the FSC and SSC of CD11c+CD11b−Siglec-F+ AMs in both treatment groups (Fig. 1). These data suggest that AMs are susceptible to an increase in apoptosis and are at least one population of apoptotic cells in the lung after intoxication and injury.
Figure 3. Amplified AM apoptosis.
An immunofluorescent in situ TUNEL assay was used to identify apoptotic cells in lung tissue. Representative images of TUNEL+ cells (green) after sham vehicle (A) and burn ethanol (B); nuclei are blue (magnification, ×200). (C) Average number of apoptotic cells per ×100 field after intoxication and injury. Flow cytometry was used to assess apoptosis of BAL cells using propidium iodide and annexin V staining. (D) Representative gating of propidium iodide and annexin V+ BAL cells. (E) FSC and SSC characteristics of annexin V+ cells (red). (F) Percent apoptotic cells out of total BAL cells. *P < 0.05 by unpaired t test. Data are means ± sem.
Heightened AM phagocytosis after intoxication and injury
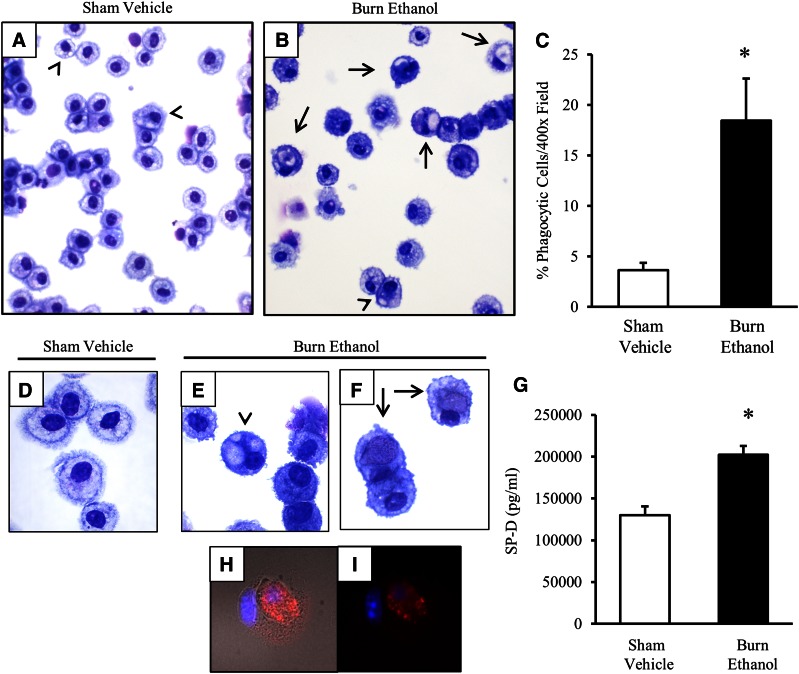
Phagocytic removal of apoptotic cells is an important function of AMs to restore lung tissue to homeostasis. We have shown that AMs from intoxicated and injured mice have heightened levels of phagocytosis-mediating receptors and that there is an increase in apoptotic cells, including AMs, in lung tissue. These data led us to determine whether AMs from intoxicated and injured animals actively phagocytose apoptotic cells. Analysis of the morphology of AMs revealed that cells in sham animals were healthy (Fig. 4A, D), whereas those from intoxicated and injured animals had an increase in phagocytic capacity (Fig. 4B, E, F). The appearance of phagocytic AMs ranged from small, translucent, lipid-like vesicles (Fig. 4B, E), to larger cellular-like bodies within AMs (Fig. 4B, F). Quantification of these cells revealed that ∼20% of AMs were phagocytic after intoxication and injury, in comparison to those of sham vehicle mice (P < 0.05; Fig. 4C).
Figure 4. Phagocytic AMs after intoxication and injury.
BAL cells were centrifuged onto slides and stained with a HEMA 3 differential stain. Images demonstrate the predominance of AMs in BAL from sham vehicle (A) and burn ethanol (B) treatment groups (magnification, ×400). (C) Quantification of the percentage of phagocytic cells per ×400 field. (D–F) BAL cells in sham vehicle (D) and intoxication and injury (E–F) (magnification ×1000). Arrowheads: AM engulfment of translucent vesicles; arrows: AM engulfment of cellular-like bodies. (G) BAL fluid was analyzed for SP-D concentrations. Data are mean picograms per milliliter ± sem. Fluorescence microscopy of BAL cell engulfment of an apoptotic cell from burn ethanol using active-caspase-3 (red) and DAPI (blue) staining with (H) and without (I) DIC (×1000 magnification). *P < 0.05 by unpaired t test.
In addition, there was a 55% increase in SP-D in the BAL fluid, in comparison to sham vehicle mice (P < 0.05; Fig. 4G), supporting our observations that a portion of phagocytic AMs are taking up lipid-like molecules after intoxication and injury. Furthermore, other phagocytic AMs engulfed apoptotic cells, as demonstrated using an antibody against active-caspase-3 (Fig. 4H, I). The DIC channel outlines a caspase-3+ cell engulfed by another cell (Fig. 4H). Morphologic analysis of AMs did not visually indicate an increase in cell size between treatment groups, but the slight variability in FSC observed in AMs from intoxicated and injured mice (Fig. 1D) may be a result of AMs engulfing lipid molecules and apoptotic cells.
DISCUSSION
Intoxication at the time of cutaneous burn injury results in adverse effects in multiple major organ systems, including gastrointestinal, hepatic, and pulmonary damage [47–49]. Our laboratory has shown that there is an increase in bacterial translocation and LPS release from the intestines after intoxication and injury [50]. This effect may lead to a greater hepatic response [20, 51] that contributes to the exacerbated levels of systemic IL-6 observed after intoxication and injury [52]. Clinical data revealed that isolated burn injury leads to increased levels of endotoxins in the bloodstream, peaking between 7 and 12 h after injury [53]. Elevated levels of circulating endotoxin after burn injury also correlate with an increased incidence of multiple organ failure [54]. Although the effect of alcohol intoxication and burn injury on circulating endotoxin levels has not been determined, we postulate that increased bacterial translocation from the gut likely results in heightened levels of endotoxins in the blood stream. Furthermore, clinical studies have suggested that apoptosis of AMs and epithelial cells in acute lung injury is induced by endotoxins and not hypoxemia [55, 56]. In the present study, TLR4/MD-2 was upregulated 24 h after injury, indicative of AM activation. We speculate that if endotoxin levels peak by 12 h, then endotoxin-induced apoptosis is a possible mechanism that contributes to an overall decrease in the number of AMs, increased AM apoptosis, and an activated proinflammatory phenotype at 24 h after injury.
Burn injury is associated with increased apoptosis in both lymphoid and parenchymal tissue, including the thymus, spleen, and lungs [27–29]. Mesenteric lymph nodes are a likely source of gut-derived factors that initiate inflammation in the lungs after burn injury [29, 57]. In a rodent model of 40% TBSA scald injury, an increase in apoptotic cells in the lungs was observed at 3 h, but surgically redirecting the mesenteric lymph to the peritoneal cavity before burn injury decreased lung apoptosis, in comparison to burn-injured control counterparts [57]. This result further supports the hypothesis that systemic inflammatory mediators are responsible for increased pulmonary inflammation after burn injury. In contrast, other studies have assessed apoptosis in the lungs at 3 and 8 h after a 20% scald injury in mice, but have found various results regarding the presence of apoptotic cells in the lung tissue [27, 28, 58]. Fukuzuka et al. [27, 28] reported an increase in apoptosis in the thymus and spleen 3 and 24 h after burn injury, but not in the lungs and liver. Using various methods to detect apoptosis, including TUNEL staining, measurement of caspase-3 activity, and mRNA quantification of TNFα and Fas ligand levels, they found that the apoptosis observed in lymphoid organs may be a result of increased caspase-3 activity and was not dependent on LPS or TNFα [28]. Another study examined apoptosis in the lung at 8 h after burn injury and found there was an increase in caspase-3 activity, but not in TUNEL+ cells in lung tissue [58]. As described by Lutmer et al. [58], the discrepancies between reported results are likely to be related to differences in the model of burn injury, as well as the methods of apoptotic cell detection. Of note, these studies did not analyze specific cell types undergoing apoptosis in any of the organs, including the lung. Because AMs can regulate neutrophil recruitment and are also key mediators of inflammation resolution, determining the fate of these cells after intoxication and injury is important.
Clinical studies have revealed that patients who are intoxicated at the time of burn injury have an increased risk of pulmonary complications and failure [10, 11, 14, 15]. Recently, we reported that heightened pulmonary inflammation parallels abnormal breathing patterns, with a correlation between an increased number of neutrophils and impaired lung function [21]. Heightened infiltration of neutrophils or ineffective removal of apoptotic cells can prolong the inflammatory response, whereas damage to epithelial cells at the capillary–alveolar interface can lead to increased lung permeability. The resolution of pulmonary inflammation is influenced by efferocytosis of apoptotic cells by AMs [59, 60]. A defect in this process can substantially prolong the inflammatory response, leading to greater tissue damage. A functional population of AMs is therefore needed to regulate pulmonary inflammation.
In the experiments herein, AMs from intoxicated and injured mice display higher SSC granularity and elevated levels of TLR4/MD-2, MHC II, MARCO, and CD11b receptors, suggesting that AMs from intoxicated and injured mice have an activated proinflammatory phenotype. The increased surface expression of MARCO and CD11b on AMs, both important receptors in efferocytosis, coincides with an elevated number of apoptotic cells in lung tissue after injury. We determined that AMs are one population of these apoptotic cells, whereas other apoptotic cells are likely to include epithelial or endothelial cells. Furthermore, morphologic analysis revealed a percentage of AMs from intoxicated and injured mice could effectively take up these apoptotic cells, leading us to believe intoxication at the time of injury does not completely inhibit the function of AM efferocytosis. Previous studies by our laboratory demonstrated that ethanol exposure alone impaired AM phagocytosis of Pseudomonas aeruginosa, which was facilitated by a decrease in macrophage Fcγ receptors after in vitro or in vivo ethanol exposure [35, 61]. Efferocytosis is a separate and distinct process from the phagocytosis of pathogens; therefore, it is plausible that alcohol can have differential effects on both of these functions. This study only examined the mean surface expression of proinflammatory markers on the entire isolated population of AMs. The expression of markers on a small subset (<20%) of AMs phagocytizing apoptotic cells was not determined in the present investigation. More studies are warranted to determine whether the AMs capable of phagocytosis are proinflammatory or transition into an anti-inflammatory phenotype.
The literature suggests that efferocytosis by AMs initiates the transition from an inflammatory phase toward a resolution phase by stimulating the release of IL-10 and TGFβ [60, 62–64]. Loss of AMs, in addition to minimal AM efferocytosis of the abundant apoptotic cells, may result in deficient levels of anti-inflammatory mediators that can initiate the resolution phase. We speculate that a lack of mediators released in response to efferocytosis by AMs (i.e., IL-10 or TGFβ) is responsible for the amplified neutrophil accumulation, resulting in a prolonged proinflammatory phase. In addition, excessive production of proinflammatory mediators has been shown to inhibit apoptosis of neutrophils and prolong their stay in tissue [65], and our laboratory has reported in a lung infection model that intoxication, before burn injury, leads to decreased neutrophil apoptosis and neutrophil retention in the airways [38]. These factors support an extended proinflammatory phase that can result in tissue damage and impair lung function.
Recently, the major macrophage research investigators collaborated and addressed the macrophage classification terminology [44]. Our data indicate that AMs may be activated by circulating endotoxins [20] and upregulate MARCO expression. Also, we have observed heightened levels of KC and IL-6 in lung tissue, but not increased IFNγ. Using the recent macrophage nomenclature guidelines, these data suggest that AMs from intoxicated and injured mice could be classified as M(LPS) [44].
Taken together, our data demonstrate that both the loss of AMs and a heightened proinflammatory profile on remaining AMs may prolong pulmonary inflammation after intoxication and injury, indicating a mechanism for impaired lung function and increased mortality incidence. Finally, the diverse functions of AMs and their changeable activation state present an opportune therapeutic target to help control pulmonary inflammation in all burn patients.
AUTHORSHIP
J.A.S. performed the experiments, interpreted results, and wrote the manuscript. B.J.C. assisted with assay troubleshooting and revising the manuscript. D.M.B. assisted with tissue processing and revising the manuscript. L.R. aided in animal handling. E.J.K. supervised the design, interpretation, and execution of the studies and edited the manuscript.
ACKNOWLEDGMENTS
Research for this study was supported by U.S. National Institutes of Health (NIH), National Institute of Alcohol Abuse and Alcoholism Grants R01 AA012034, R21 AA023193, and T32 AA013527 (to E.J.K.) and Grant F31 AA022566 (to J.A.S); NIH National Institute of General Medical Sciences Grant R01 GM115257 (to E.J.K.); and by the Marian and Ralph C. Falk Medical Research Trust (to E.J.K.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Patricia Simms for technical assistance in experiments involving flow cytometry, and Drs. Pamela Witte and Stephanie Watkins for assistance with data analysis.
Glossary
- AM
alveolar macrophage
- ARDS
acute respiratory distress syndrome
- BAL
bronchoalveolar lavage
- DIC
differential interference contrast
- FMO
fluorescence minus one
- FSC
forward scatter
- KC
CXCL1
- MARCO
macrophage receptor with collagenous structure
- MFI
mean fluorescence intensity
- MHC-II
major histocompatibility complex class II
- MIP
macrophage inflammatory protein
- OCT
optimal cutting temperature
- SP-D
surfactant protein D
- SSC
side scatter
- TBSA
total body surface area
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Naimi T. S., Brewer R. D., Mokdad A., Denny C., Serdula M. K., Marks J. S. (2003) Binge drinking among US adults. JAMA 289, 70–75. [DOI] [PubMed] [Google Scholar]
- 2.Savola O., Niemelä O., Hillbom M. (2005) Alcohol intake and the pattern of trauma in young adults and working aged people admitted after trauma. Alcohol Alcohol. 40, 269–273. [DOI] [PubMed] [Google Scholar]
- 3.Brezel B. S., Kassenbrock J. M., Stein J. M. (1988) Burns in substance abusers and in neurologically and mentally impaired patients. J. Burn Care Rehabil. 9, 169–171. [DOI] [PubMed] [Google Scholar]
- 4.Silver G. M., Albright J. M., Schermer C. R., Halerz M., Conrad P., Ackerman P. D., Lau L., Emanuele M. A., Kovacs E. J., Gamelli R. L. (2008) Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. J. Burn Care Res. 29, 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grobmyer S. R., Maniscalco S. P., Purdue G. F., Hunt J. L. (1996) Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. J. Burn Care Rehabil. 17, 532–539. [DOI] [PubMed] [Google Scholar]
- 6.Raff T., Germann G., Barthold U. (1996) Factors influencing the early prediction of outcome from burns. Acta Chir. Plast. 38, 122–127. [PubMed] [Google Scholar]
- 7.Davis C. S., Esposito T. J., Palladino-Davis A. G., Rychlik K., Schermer C. R., Gamelli R. L., Kovacs E. J. (2013) Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. J. Burn Care Res. 34, 120–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadjizacharia P., O’Keeffe T., Plurad D. S., Green D. J., Brown C. V., Chan L. S., Demetriades D., Rhee P. (2011) Alcohol exposure and outcomes in trauma patients. Eur. J. Trauma Emerg. Surg. 37, 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelley D., Lynch J. B. (1992) Burns in alcohol and drug users result in longer treatment times with more complications. J. Burn Care Rehabil. 13, 218–220. [DOI] [PubMed] [Google Scholar]
- 10.Liffner G., Bak Z., Reske A., Sjöberg F. (2005) Inhalation injury assessed by score does not contribute to the development of acute respiratory distress syndrome in burn victims. Burns 31, 263–268. [DOI] [PubMed] [Google Scholar]
- 11.Turnage R. H., Nwariaku F., Murphy J., Schulman C., Wright K., Yin H. (2002) Mechanisms of pulmonary microvascular dysfunction during severe burn injury. World J. Surg. 26, 848–853. [DOI] [PubMed] [Google Scholar]
- 12.Dancey D. R., Hayes J., Gomez M., Schouten D., Fish J., Peters W., Slutsky A. S., Stewart T. E. (1999) ARDS in patients with thermal injury. Intensive Care Med. 25, 1231–1236. [DOI] [PubMed] [Google Scholar]
- 13.Steinvall I., Bak Z., Sjoberg F. (2008) Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns 34, 441–451. [DOI] [PubMed] [Google Scholar]
- 14.Phillips A. W., Cope O. (1962) Burn therapy, II: the revelation of respiratory tract damage as a principal killer of the burned patient. Ann. Surg. 155, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Achauer B. M., Allyn P. A., Furnas D. W., Bartlett R. H. (1973) Pulmonary complications of burns: the major threat to the burn patient. Ann. Surg. 177, 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bird M. D., Morgan M. O., Ramirez L., Yong S., Kovacs E. J. (2010) Decreased pulmonary inflammation after ethanol exposure and burn injury in intercellular adhesion molecule-1 knockout mice. J. Burn Care Res. 31, 652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bird M. D., Zahs A., Deburghgraeve C., Ramirez L., Choudhry M. A., Kovacs E. J. (2010) Decreased pulmonary inflammation following ethanol and burn injury in mice deficient in TLR4 but not TLR2 signaling. Alcohol. Clin. Exp. Res. 34, 1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen M. M., Bird M. D., Zahs A., Deburghgraeve C., Posnik B., Davis C. S., Kovacs E. J. (2013) Pulmonary inflammation after ethanol exposure and burn injury is attenuated in the absence of IL-6. Alcohol 47, 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel P. J., Faunce D. E., Gregory M. S., Duffner L. A., Kovacs E. J. (1999) Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. Am. J. Respir. Cell Mol. Biol. 20, 1229–1237. [DOI] [PubMed] [Google Scholar]
- 20.Chen M. M., Zahs A., Brown M. M., Ramirez L., Turner J. R., Choudhry M. A., Kovacs E. J. (2014) An alteration of the gut-liver axis drives pulmonary inflammation after intoxication and burn injury in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G711–G718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shults J. A., Curtis B. J., Chen M. M., O’Halloran E. B., Ramirez L., Kovacs E. J. (2015) Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol 49, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., Weiss J. M., Wlaschek M., Sunderkötter C., Scharffetter-Kochanek K. (2011) An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J. Clin. Invest. 121, 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herold S., Mayer K., Lohmeyer J. (2011) Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front. Immunol. 2, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal N. R., King L. S., D’Alessio F. R. (2014) Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Lung Cell. Mol. Physiol. 306, L709–L725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porcheray F., Viaud S., Rimaniol A. C., Léone C., Samah B., Dereuddre-Bosquet N., Dormont D., Gras G. (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin. Exp. Immunol. 142, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ariel A., Maridonneau-Parini I., Rovere-Querini P., Levine J. S., Mühl H. (2012) Macrophages in inflammation and its resolution. Front. Immunol. 3, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fukuzuka K., Edwards C. K. III, Clare-Salzler M., Copeland E. M. III, Moldawer L. L., Mozingo D. W. (2000) Glucocorticoid-induced, caspase-dependent organ apoptosis early after burn injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 278, R1005–R1018. [DOI] [PubMed] [Google Scholar]
- 28.Fukuzuka K., Rosenberg J. J., Gaines G. C., Edwards C. K. III, Clare-Salzler M., MacKay S. L., Moldawer L. L., Copeland E. M. III, Mozingo D. W. (1999) Caspase-3-dependent organ apoptosis early after burn injury. Ann. Surg. 229, 851–858, discussion 858–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magnotti L. J., Upperman J. S., Xu D. Z., Lu Q., Deitch E. A. (1998) Gut-derived mesenteric lymph but not portal blood increases endothelial cell permeability and promotes lung injury after hemorrhagic shock. Ann. Surg. 228, 518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faunce D. E., Gregory M. S., Kovacs E. J. (1997) Effects of acute ethanol exposure on cellular immune responses in a murine model of thermal injury. J. Leukoc. Biol. 62, 733–740. [DOI] [PubMed] [Google Scholar]
- 31.Qin Y., Hamilton J. L., Bird M. D., Chen M. M., Ramirez L., Zahs A., Kovacs E. J., Makowski L. (2014) Adipose inflammation and macrophage infiltration after binge ethanol and burn injury. Alcohol. Clin. Exp. Res. 38, 204–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Messingham K. A., Fontanilla C. V., Colantoni A., Duffner L. A., Kovacs E. J. (2000) Cellular immunity after ethanol exposure and burn injury: dose and time dependence. Alcohol 22, 35–44. [DOI] [PubMed] [Google Scholar]
- 33.Murdoch E. L., Brown H. G., Gamelli R. L., Kovacs E. J. (2008) Effects of ethanol on pulmonary inflammation in postburn intratracheal infection. J. Burn Care Res. 29, 323–330. [DOI] [PubMed] [Google Scholar]
- 34.Faunce D. E., Llanas J. N., Patel P. J., Gregory M. S., Duffner L. A., Kovacs E. J. (1999) Neutrophil chemokine production in the skin following scald injury. Burns 25, 403–410. [DOI] [PubMed] [Google Scholar]
- 35.Karavitis J., Murdoch E. L., Gomez C. R., Ramirez L., Kovacs E. J. (2008) Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. J. Interferon Cytokine Res. 28, 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misharin A. V., Morales-Nebreda L., Mutlu G. M., Budinger G. R., Perlman H. (2013) Flow cytometric analysis of macrophages and dendritic cell subsets in the mouse lung. Am. J. Respir. Cell Mol. Biol. 49, 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boehmer E. D., Goral J., Faunce D. E., Kovacs E. J. (2004) Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J. Leukoc. Biol. 75, 342–349. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch E. L., Karavitis J., Deburghgraeve C., Ramirez L., Kovacs E. J. (2011) Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock 35, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guth A. M., Janssen W. J., Bosio C. M., Crouch E. C., Henson P. M., Dow S. W. (2009) Lung environment determines unique phenotype of alveolar macrophages. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L936–L946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Von Garnier C., Filgueira L., Wikstrom M., Smith M., Thomas J. A., Strickland D. H., Holt P. G., Stumbles P. A. (2005) Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J. Immunol. 175, 1609–1618. [DOI] [PubMed] [Google Scholar]
- 41.Palecanda A., Paulauskis J., Al-Mutairi E., Imrich A., Qin G., Suzuki H., Kodama T., Tryggvason K., Koziel H., Kobzik L. (1999) Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 189, 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rogers N. J., Lees M. J., Gabriel L., Maniati E., Rose S. J., Potter P. K., Morley B. J. (2009) A defect in Marco expression contributes to systemic lupus erythematosus development via failure to clear apoptotic cells. J. Immunol. 182, 1982–1990. [DOI] [PubMed] [Google Scholar]
- 43.Mevorach D., Mascarenhas J. O., Gershov D., Elkon K. B. (1998) Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188, 2313–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray P. J., Allen J. E., Biswas S. K., Fisher E. A., Gilroy D. W., Goerdt S., Gordon S., Hamilton J. A., Ivashkiv L. B., Lawrence T., Locati M., Mantovani A., Martinez F. O., Mege J. L., Mosser D. M., Natoli G., Saeij J. P., Schultze J. L., Shirey K. A., Sica A., Suttles J., Udalova I., van Ginderachter J. A., Vogel S. N., Wynn T. A. (2014) Macrophage activation and polarization: nomenclature and experimental guidelines (published correction in Immunity 41, 339–340). Immunity 41, 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rőszer T. (2015) Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015, 816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein M., Keshav S., Harris N., Gordon S. (1992) Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choudhry M. A., Chaudry I. H. (2008) Alcohol, burn injury, and the intestine. J. Emerg. Trauma Shock 1, 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeschke M. G. (2009) The hepatic response to thermal injury: is the liver important for postburn outcomes? Mol. Med. 15, 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pruitt B. A. Jr., Erickson D. R., Morris A. (1975) Progressive pulmonary insufficiency and other pulmonary complications of thermal injury. J. Trauma 15, 369–379. [PubMed] [Google Scholar]
- 50.Zahs A., Bird M. D., Ramirez L., Turner J. R., Choudhry M. A., Kovacs E. J. (2012) Inhibition of long myosin light-chain kinase activation alleviates intestinal damage after binge ethanol exposure and burn injury. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G705–G712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emanuele N. V., Emanuele M. A., Morgan M. O., Sulo D., Yong S., Kovacs E. J., Himes R. D., Callaci J. J. (2009) Ethanol potentiates the acute fatty infiltration of liver caused by burn injury: prevention by insulin treatment. J. Burn Care Res. 30, 482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Faunce D. E., Gregory M. S., Kovacs E. J. (1998) Acute ethanol exposure prior to thermal injury results in decreased T-cell responses mediated in part by increased production of IL-6. Shock 10, 135–140. [DOI] [PubMed] [Google Scholar]
- 53.Dobke M. K., Simoni J., Ninnemann J. L., Garrett J., Harnar T. J. (1989) Endotoxemia after burn injury: effect of early excision on circulating endotoxin levels. J. Burn Care Rehabil. 10, 107–111. [DOI] [PubMed] [Google Scholar]
- 54.Yao Y. M., Sheng Z. Y., Tian H. M., Yu Y., Wang Y. P., Yang H. M., Guo Z. R., Gao W. Y. (1995) The association of circulating endotoxaemia with the development of multiple organ failure in burned patients. Burns 21, 255–258. [DOI] [PubMed] [Google Scholar]
- 55.Bingisser R., Stey C., Weller M., Groscurth P., Russi E., Frei K. (1996) Apoptosis in human alveolar macrophages is induced by endotoxin and is modulated by cytokines. Am. J. Respir. Cell Mol. Biol. 15, 64–70. [DOI] [PubMed] [Google Scholar]
- 56.Z’graggen B. R., Tornic J., Müller-Edenborn B., Reyes L., Booy C., Beck-Schimmer B. (2010) Acute lung injury: apoptosis in effector and target cells of the upper and lower airway compartment. Clin. Exp. Immunol. 161, 324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Magnotti L. J., Xu D. Z., Lu Q., Deitch E. A. (1999) Gut-derived mesenteric lymph: a link between burn and lung injury. Arch. Surg. 134, 1333–1340, discussion 1340–1341. [PubMed] [Google Scholar]
- 58.Lutmer J., Watkins D., Chen C. L., Velten M., Besner G. (2013) Heparin-binding epidermal growth factor-like growth factor attenuates acute lung injury and multiorgan dysfunction after scald burn. J. Surg. Res. 185, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fadok V. A., Bratton D. L., Konowal A., Freed P. W., Westcott J. Y., Henson P. M. (1998) Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Invest. 101, 890–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voll R. E., Herrmann M., Roth E. A., Stach C., Kalden J. R., Girkontaite I. (1997) Immunosuppressive effects of apoptotic cells. Nature 390, 350–351. [DOI] [PubMed] [Google Scholar]
- 61.Karavitis J., Murdoch E. L., Deburghgraeve C., Ramirez L., Kovacs E. J. (2012) Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcγR-mediated phagocytosis. Cell. Immunol. 274, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S., Elkon K. B., Ma X. (2004) Transcriptional suppression of interleukin-12 gene expression following phagocytosis of apoptotic cells. Immunity 21, 643–653. [DOI] [PubMed] [Google Scholar]
- 63.Ariel A., Serhan C. N. (2012) New lives given by cell death: macrophage differentiation following their encounter with apoptotic leukocytes during the resolution of inflammation. Front. Immunol. 3, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Freire-de-Lima C. G., Xiao Y. Q., Gardai S. J., Bratton D. L., Schiemann W. P., Henson P. M. (2006) Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem. 281, 38376–38384. [DOI] [PubMed] [Google Scholar]
- 65.Lee A., Whyte M. K., Haslett C. (1993) Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J. Leukoc. Biol. 54, 283–288. [PubMed] [Google Scholar]