Nicotine induces neutrophils to release DNA traps via activation of Akt, but independently of NADPH oxidase.
Keywords: tobacco, protein kinase B, ROS, nicotinic acetylcholine receptor
Abstract
NETs serve to ensnare and kill microbial pathogens. However, NETs can at the same time contribute to tissue damage and excessive inflammation. Nicotine is a major toxic agent and has been associated with exacerbated inflammatory diseases. The current study aimed at investigating the role of nicotine, the addictive component of tobacco and electronic cigarettes, on triggering NET formation. We report that nicotine induces neutrophils to release NETs in a dose-dependent manner. Nicotine-induced NET formation is mediated via nicotine acetylcholine receptors, depends on Akt and PAD4 activation, but is Nox2-independent, as demonstrated by pharmacological inhibition of Nox2 and by use of Nox2-deficient mouse neutrophils. These findings demonstrate that nicotine induces NETs, which may in turn contribute to smoking-related diseases.
Introduction
Neutrophils are terminally differentiated cells and, as the first line of defense, play a central role in innate immunity. Their potentially assaultive antimicrobial arsenal is controlled by a preprogrammed short lifespan of several hours in circulation and up to 5 days in tissue after recruitment to the locus of infection [1, 2]. Recruitment to infection sites is controlled by chemokines secreted by endothelial and epithelial cells, as well as by macrophages in the vicinity of the afflicted areas. Apoptosis is the default mode of neutrophil death. Daily turnover ranges between 0.8 and 1.6 × 109 cells/kg body weight. Apoptotic neutrophils are recognized, engulfed, and cleared by macrophages in a process called efferocytosis [3].
Engulfment of microbes can lead to activation of the phagocyte Nox2, resulting in ROS production. Contents of vesicular granules including antimicrobial peptides and enzymes are released into phagocytic vacuoles. ROS and antimicrobial proteins cooperate to destroy the internalized microbes [4]. Besides this phagocytic activity, microbe-stimulated neutrophils can release NETs, which are composed of decondensed chromatin associated with granular and cytoplasmic proteins [5, 6]. NETs are critical for antimicrobial defense. For instance, they ensnare and kill pathogenic fungi, such as Candida [7] and Aspergillus species [8], but may also cause exacerbated inflammation in case of dysregulation [9]. Patients with COPD, an inherited disorder of Nox2, suffer from recurrent, severe microbial infections, such as aspergillosis. Nox2-deficient phagocytes fail to produce an oxidative burst, and, depending on the nature of the stimulus, may have defective NET release [10]. Restoration of NET formation in these patients could enhance host defense against aspergillosis [8, 11]. NETs can effectively entrap microbes and promote their extracellular killing. However, NET formation can also be associated with autoimmunity and vascular diseases, including systemic lupus erythematosus, small vessel vasculitis, deep venous thrombosis, inflammatory lung diseases, and acute lung injury [12–15]. Neutrophilic airway inflammation can additionally be exacerbated by exposure to other proinflammatory stimuli, including tobacco smoking [16, 17].
Nicotine is the major addictive component of tobacco, contributing 1.5% by weight and comprising ∼95% of its alkaloid content. Tobacco use is a considerable cause of disease, disability, and death. According to the Centers for Disease Control and Prevention, cigarette smoking results in ∼480,000 premature deaths anually in the United States [18]. In persons who smoke or chew tobacco, considerable amounts of the nicotine are immediately absorbed in the blood and subsequently reach the brain. In addition, tobacco and electronic cigarette smoking expose lung tissues to high levels of nicotine [19, 20]. Residual nicotine levels vary in circulation and different tissues. In blood, nicotine concentrations of tobacco users were reported to range between 0.2 and 1 µM with fewer references pointing to peak concentrations of 20 µM [21], and nicotine levels in saliva and urine of heavy smokers can reach concentrations of ∼10 µM [22–24].
Nicotine contributes to disease by several pathways, including stimulation of inflammation [25]. Nicotine has been demonstrated to suppress phagocytic activity in neutrophils while enhancing degranulation as determined by neutrophil elastase release without affecting superoxide production [3, 26]. Nicotine also has the potential to react with HOCl, a product of Nox2 activation, to produce nicotine chloramine, which is membrane permeable and capable of causing molecular protein damage to nuclei of intact cells [27]. Therefore, nicotine can impair phagocytic host defense while also increasing ROS-associated injury.
Several NET-inducing mechanisms have been described. Reports described fast and slow NETosis originating from either dying cells that burst open or from living cells that keep functioning as nuclear cell ghosts [28–30]. PMA is a very potent inducer of NETs and a strong stimulus of Nox2-dependent ROS production. Other proinflammatory triggers of NET release have been described, including IL-8, TNF-ɑ, LPS, fMLP, and H2O2 [31–33]. Regulation of these pathways is less well understood. It has been proposed that the PKB, Akt, is essential in the induction of NET formation and acts as a molecular switch regulating the NETosis-apoptosis axis. PMA-induced NET release is mediated by Akt activation, and inhibition of Akt results in induction of caspase-dependent apoptosis in neutrophils. In addition, Akt is a well-known inhibitor of apoptosis [34]. Together, these findings indicate that Akt plays a key role in modulating neutrophil death [14].
Nicotine has been shown to block Akt deactivation and reduce spontaneous death of neutrophils [3]. Based on these findings, we hypothesized that nicotine may induce NETs through Akt-dependent signaling. We found that nicotine-induced NET formation was dependent on Akt activation, but was Nox2 independent. These findings point to the ability of nicotine to induce NETs, which may in turn contribute to smoking-related inflammation and injury.
MATERIALS AND METHODS
Isolation of human and mouse neutrophils
Blood was collected from healthy individuals for whom tobacco usage habits were not assessed. Human neutrophil isolation was performed as described previously [35]. Bone marrow-derived mouse neutrophils were isolated from male gp91phox−/− (B6.129S-Cybbtm1Din/J) and wild-type C57BL/6 mice. Neutrophils isolation was then performed [36]. Animals were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and kept in the animal facility at the Umeå Center for Comparative Biology.
Extracellular DNA fluorescence assay
Presence of extracellular DNA was measured as has been described [36, 37]. In brief, 5 × 104 cells per well were seeded in a black-bottom 96-well plate. After addition of Sytox Green (2.5 µM; Thermo Fisher Scientific, Waltham, MA, USA), neutrophils were stimulated with different concentrations of nicotine (cat. no. N3876; Sigma-Aldrich, St. Louis, MO, USA) or PMA (100 nM final concentration); untreated or lysed with 1% Triton X-100 (Merck, Darmstadt, Germany) as a 100% lysis control; or treated with 10 µM Akt inhibitor XI (Merck Millipore, Billerica, MA, USA), 200 µM PAD4 inhibitor (Cl-amidine, provided by P. Thompson), or 10 µM nAChR inhibitor (MG 624; Tocris Bioscience, Bristol, UK). Fluorescence was measured every 10 min by a fluorescence spectrometer (Fluostar Ω; BMG Labtech, Cary, NC, USA) for a period of 10 h in cell culture conditions (37°C; 5% CO2). Fluorescence was calculated as a percentage of lysis control. Data were presented as a percentage of extracellular DNA.
ROS quantification
ROS production of human neutrophils was quantified by a luminometric assay [37]. Neutrophils were seeded into a white 96-well plate (Nunc; Sigma-Aldrich) and incubated with 50 μM luminol (Sigma-Aldrich) and 1.2 u/ml horseradish peroxidase (Sigma-Aldrich) for 15 min. ROS was measured in triplicate from every donor for 3 h (infinite 200; Tecan, Männedorf, Switzerland) at 37°C and 5% CO2. Amounts of ROS are presented by calculating the integral of the area under the curve (AUC) from measured relative light units for 3 h.
Immunostaining of neutrophils
Immunostaining was performed as described elsewhere [37]. In brief, 105 cells were seeded on 0.01% poly-lysine (Sigma-Aldrich)-precoated glass coverslips in 24-well plates after incubation with different concentrations of nicotine or PMA at 37°C and 5% CO2. The cells were fixed with paraformaldehyde (2%). Samples were incubated with primary antibodies directed against human neutrophil elastase (Acris Antibodies, San Diego, CA, USA), cleaved caspase-3 (Cell Signaling Technology, Danvers, MA, USA), and Cit-H3 (Abcam, Cambridge, MA, USA), conjugated with secondary antibodies Cy2 and Cy3 (Jackson ImmunoResearch, West Grove, PA, USA), and stained with DAPI (Sigma-Aldrich) to detect DNA. Microscopy was performed by using a confocal microscope (C1; Nikon, Tokyo, Japan) with NIS-Elements AR, version 3.2.0 software (Nikon Instruments, Inc., Melville, NY, USA). .
Microscopic image analysis
NETs were quantified microscopically [37]. More than 250 neutrophils were analyzed by measuring DNA area (DAPI stain). Microscopic images were analyzed by using ImageJ version 1.44p software [National Institutes of Health (NIH), Bethesda, MD, USA]. Human neutrophils have an average diameter of 10 μm; hence, their average area in an unstimulated stage is ∼80 μm2 (assuming a circle and using πr2). To quantify NET formation events, nuclei that exceeded 100 μm2 and thus were larger than the whole intact cell area were considered to be decondensed nuclei, an essential step before NETosis, or as released NETs.
Ethics statements
Human neutrophils were isolated from peripheral blood of healthy volunteers in accordance with the recommendations of the local ethics committee (Regionala etikprövningsnämnden i Umeå), as previously approved in permit Dnr 09-210M. Fully informed, written consent was obtained, and all investigations were conducted according to the principles expressed in the Declaration of Helsinki. Blood donors were not followed up or asked about tobacco use. All animal experiments in this study were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (NIH) and conformed to Swedish animal protection laws and applicable guidelines (djurskyddslagen 1988:534; djurskyddsförordningen 1988:539; djurskyddsmyndigheten DFS 2004:4). Isolation of cells from mice was in strict compliance with the Swedish animal protection law in a protocol approved by the local ethics committee (Umeå djurförsöksetiska nämnd, permit number 79-14). The animals showed no signs of disease or pain before they were euthanized.
Statistical analysis
Prism version 5.00 (Graph Pad, San Diego, CA, USA) was used for all analyses. Data were presented as means ± sd. One-way ANOVA followed by post hoc multiple comparison Dunnett’s test was used to compare significance with a single control. Differences were significant at P < 0.05.
RESULTS AND DISCUSSION
Nicotine induces NETosis but not apoptosis in human neutrophils
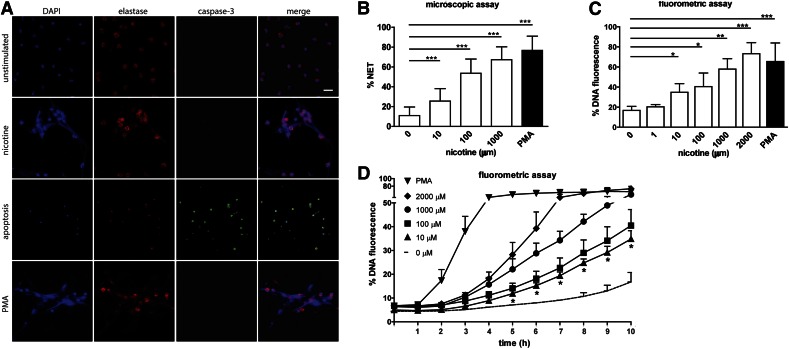
Nicotine has been described as a chemotactic factor for neutrophils, the most abundant white blood cell in circulation [38]. We therefore investigated how human neutrophils respond to direct contact with nicotine by incubating the cells with different concentrations of nicotine. Immunofluorescence revealed that nicotine induces NET formation (Fig. 1A). In this study, we show NET formation after 10 h stimulation with 1 mM nicotine. NETs are web-like, fibrous structures spanning large areas of the panel that stained for DNA and elastase (Fig. 1A; 2nd row). The nicotine-induced NETs were morphologically indistinguishable from NETs triggered by PMA (Fig. 1A; 4th row). To discriminate this activation from apoptosis, we additionally stained neutrophils for cleaved caspase 3, indicative of apoptosis (Fig. 1A). We found that in contrast to NETosis, apoptosis was virtually absent in nicotine-stimulated and PMA-stimulated neutrophils after 10 h. After 24 h without stimulus, significant amounts of neutrophils underwent apoptosis, as demonstrated by robust cleaved caspase 3 staining (Fig. 1A; 3rd row). To quantify NETs microscopically, we applied Image J analysis by using a published method [37]. Indeed, we found that nicotine concentrations of 10 μM to 1 mM induced robust NET generation. At concentrations of 1 mM and above, nicotine resulted in amounts of NETs similar to those induced by PMA after 10 h of stimulation (Fig. 1B).
Figure 1. Nicotine induces NET formation in human neutrophils in a dose-dependent manner.
(A) Immunofluorescent staining of neutrophils that were unstimulated, treated with 1 mM nicotine or 100 nM PMA for 10 h, or remained without stimulus 24 h to induce apoptosis. NET formation was visualized by using DAPI (blue channel) to stain DNA and primary antibody directed against human neutrophil elastase (red channel). Apoptotic cells were stained by applying antibody against cleaved caspase-3 (green channel). (B) NET formation at 10 h was quantified from microscopic images by measuring DNA-stained area >100 μm2 in each PMN. Scale bar, 20 μm. (C, D) Dose dependence and kinetics of NET generation were determined by DNA release of neutrophils using the cell-impermeable fluorescent DNA dye Sytox green in a fluorometric plate-based assay. Human neutrophils were incubated with different concentrations of nicotine or with PMA (100 nM) as a positive control. Untreated neutrophils served as negative control. Values are presented as percentage of DNA fluorescence compared to Triton X100 lysis control (100%). (C) NET generation at 10 h using various concentrations of nicotine. (D) Kinetics of NET generation using various concentrations of nicotine. Data are shown as means ± sd from 6 independent donors, each in 3 technical replicates. Significance of data sets was analyzed by 1-way ANOVA followed by Dunnett’s multiple-comparison test. Statistical significance of 10 μM nicotine compared to unstimulated control is indicated by asterisks below the symbols of the time points. The onset of NET release is significant above background at 5 h onward. *P < 0.05; **P < 0.01; ***P < 0.001.
To confirm quantitative analysis based on microscopic images, we also performed an online, plate-based DNA fluorescence assay. In these assays, NET induction very similarly increased at 10 µM nicotine and was significantly different from background at concentrations equal to and above 10 μM. At nicotine concentrations between 10 µM and 1 mM, DNA fluorescence corresponded to ∼40–60% of lysis control (Fig. 1C).
Another report showed that 1 μM nicotine can delay neutrophil apoptosis [3]. We could not detect any NET formation at nicotine concentration of 1 µM or below. Because quantification of nicotine-induced NET formation by microscopic and online DNA fluorescence led to similar results, we analyzed the kinetics of nicotine-induced NET release using the DNA fluorometric assay (Fig. 1D). The dynamics of nicotine-induced NETosis was slightly delayed compared to PMA-induced NETosis (Fig. 1D). The onset of nicotine-stimulated NET generation occurred after 2–4 h and steadily increased over the course of 10 h. At nicotine concentrations within physiologic range (10 µM) NET formation was significantly above background starting from 5 h onward. At higher nicotine concentrations significance shifted to earlier time points (not shown) and a maximum started to be reached around 10 h when using higher nicotine concentrations (Fig. 1D). Taken together, these results suggest that, at low concentrations, nicotine may enhance neutrophilic inflammation by delaying apoptosis, whereas higher nicotine concentrations within the physiologic range of 10 µM [21–24] can induce NET generation, which is predicted to increase tissue injury.
Constituents of cigarette smoke, such as nicotine, have been shown to inhibit or delay apoptosis in human oral cancer cells [39]. In addition, the reduction in spontaneous apoptosis of neutrophils was described for tobacco-induced lung-related inflammatory disease in patients with COPD [3, 40]. Our results raise the notion that nicotine-induced NET contribute to smoking-related oral and lung disease.
Nicotine induces NET formation via activation of nAChR and is dependent on Akt activation
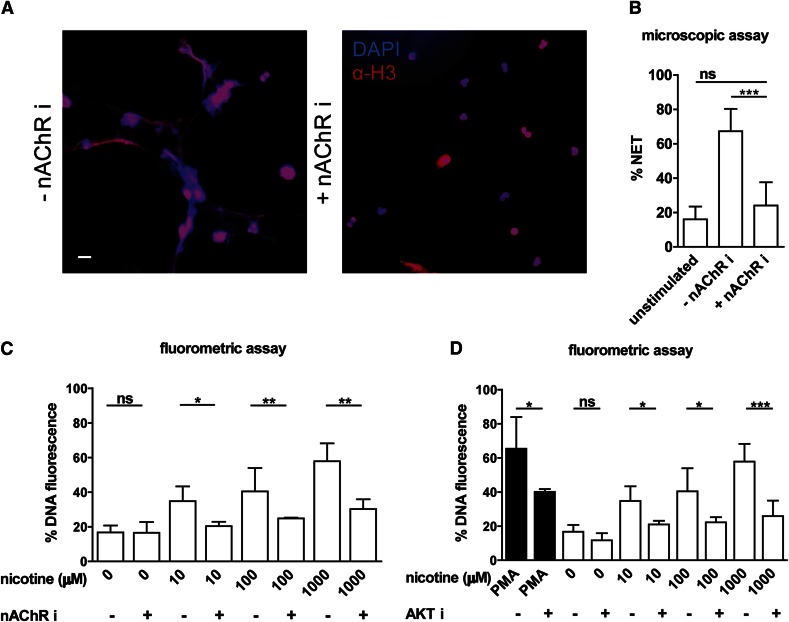
Neutrophils express the α7 subunit of nAChRs that are activated by nicotine [41, 42]. These receptors display high affinity for nicotine. We reasoned that these receptors mediate the signaling required for nicotine-induced NET formation. Indeed, the blockage of nAChRs on neutrophils with a specific antagonist for α7 subunit containing nAChR subtypes abrogated induction of NETs by nicotine stimulation (Fig. 2A). Microscopic quantification revealed that treatment with an nAChR antagonist reduced NET release to the levels equivalent to those in the unstimulated control (Fig. 2B). This finding indicates that NET release observed during nicotine exposure is mediated by specific signaling via nAChRs, rather than by nonspecific toxicity. To confirm microscopic quantification and to be able to test more conditions, we applied fluorometric assays based on membrane-impermeable DNA dye to assess NET release. Blockage of nAChR led to significant abrogation of NET release at nicotine concentrations of 10 μM or more (Fig. 2C).
Figure 2. Nicotine induces NET formation in human neutrophils via nAChR and is Akt dependent.
(A) Immunofluorescent staining of neutrophils treated for 10 h with 1 mM nicotine in the presence or absence of nAChR inhibitor. Samples were stained for DNA (blue channel) and histone H3 (red channel). (B) NET formation at10 h was quantified from microscopic images by measuring DNA-stained area >100 μm2 in individual PMNs. Scale bar, 10 μm. (C) DNA release of neutrophils using the cell-impermeable fluorescent DNA dye Sytox green was measured by a fluorometric assay. Human neutrophils were incubated with different concentrations of nicotine, with or without pretreatment with the nAChR inhibitor (10 μM) or (D) Akt inhibitor (10 μM). PMA (100 nM) was used as a positive control (maximum after 10 h, solid line). Values are presented as the percentage of DNA fluorescence compared with the Triton X100 lysis control (100%). Data are shown as means ± sd and are from at least 6 independent donors, each in 3 technical replicates. *P <0.05; **P < 0.01; ***P < 0.001.
Akt was identified as a decisive switch for a “cellular decision” either toward apoptosis or toward NETosis, and inhibition of Akt suppressed PMA-induced NET generation [14]. We therefore asked whether Akt signaling stimulates nicotine-induced NETosis. NET formation after nicotine stimulation was monitored by the fluorometric assay. To test whether NET induction by nicotine is regulated by Akt, we used the specific Akt inhibitor XI. Akt-i abrogated nicotine-induced NET release over a period of 10 h (Fig. 2D), demonstrating the requirement for Akt signaling for NET generation.
Nicotine-induced NET formation does not require Nox2-dependent ROS production and leads to PAD4 activation
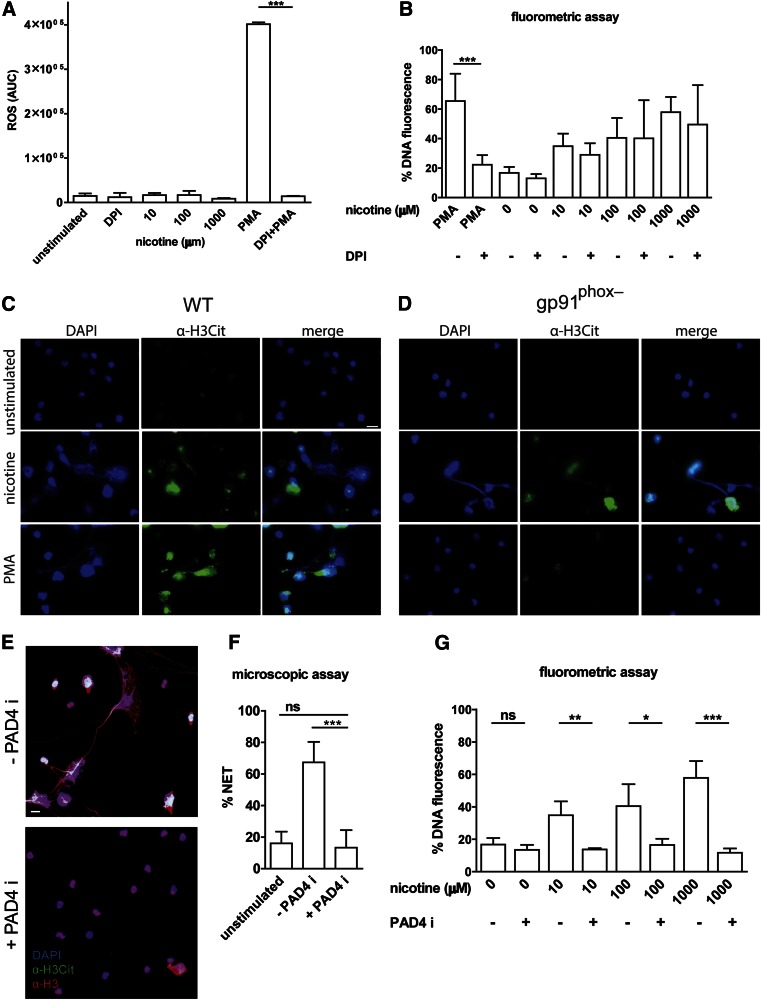
ROS are known to be necessary for NET generation after various stimuli, including PMA and bacterial and fungal infection [43]. We investigated whether nicotine stimulates ROS production in neutrophils. Neutrophils were stimulated with different concentrations of nicotine (10 µM–1 mM), and ROS generation was quantified with a luminol-based assay. Consistent with prior studies [44–46], different concentrations of nicotine did not induce neutrophil ROS production (Fig. 3A).
Figure 3. Induction of NET formation by nicotine is independent of ROS.
(A) Total ROS produced by neutrophils over 3 h were calculated as the AUC. Neutrophils were unstimulated or were stimulated with different concentrations of nicotine or PMA (100 nM) as a positive control for Nox2-dependent NET generation. DPI (10 μM) was used to inhibit Nox2. ROS production was plotted. (B) NET generation was quantified based on DNA release from neutrophils by fluorometric assay. Human neutrophils were incubated with or without DPI (10 μM) before stimulation with different concentrations of nicotine or PMA for 10 h. Values are presented as the percentage of DNA fluorescence compared with the Triton X100 lysis control (100%). Data are shown as means ± sd and are from at least 6 independent donors, each in 3 technical replicates. (C, D) Immunofluorescence staining of C57BL/6 and gp91phox−/− mouse neutrophils. Cells were unstimulated (first row) or were treated with 2 mM nicotine (second row) or 100 nM PMA (third row) for 16 h. NET formation was visualized by using DAPI (blue channel) and primary antibody directed against Cit-H3 (green channel). Representatives from 2 independent experiments are shown. Scale bar, 10 µm. (E) Immunofluorescent staining of neutrophils that were treated with 1 mM nicotine for 10 h in the presence or absence of PAD4 inhibitor (200 μM). Samples were stained for DNA (blue channel), histone H3 (red channel), and Cit-H3 (green channel). (F) NET formation at 10 h was quantified from microscopic images by measuring DNA stained area above 100 μm2 of individual PMNs. Scale bar, 10 μm. (G) DNA release from neutrophils was measured by a fluorometric assay. Human neutrophils were incubated with different concentrations of nicotine in presence or absence of PAD4 inhibitor (200 μM). Values are presented as percentage of DNA fluorescence compared with the Triton X100 lysis control (100%). Data are means ± sd and are from at least 6 independent donors, each in 3 technical replicates. *P <0.05; **P < 0.01; ***P < 0.001.
To specifically address whether nicotine-induced NET generation is dependent on Nox2, we used DPI, a small-molecule inhibitor of Nox2. After 10 h incubation of neutrophils with nicotine, we observed that DPI did not affect the level of NET induction to any significant level (Fig. 3B). These results imply that nicotine-induced NETosis is ROS-independent (Fig. 3B). To verify that nicotine-induced NET formation is Nox2-independent, we used gp91phox−/− mice, in which phagocytes have a defective oxidative burst. Nicotine-induced NET generation was similar in wild-type and gp91phox−/− neutrophils (Fig. 3C, D, second row). On the other hand, PMA-treated neutrophils from gp91phox−/− mice failed to form NETs (Fig. 3C and D, third row). These results show that induction of NETs by nicotine is not restricted to human neutrophils, but to a more general feature, conserved in neutrophils from other species as well. Our results do not exclude the possibility that Nox2-independent ROS contributes to nicotine-induced NETs. Recently, involvement of mitochondrial ROS in NET formation triggered by calcium influx has been demonstrated [47]. In addition, mitochondrial ROS is relevant in dysregulated NET formation in systemic lupus erythematosus [48].
Conversion of arginine residues into citrulline amino acid is mediated by PAD4 in granulocytes and is essential for the formation of NETs in response to several stimuli [49]. Tobacco smoking has also been associated with increased PAD4 activity in the airways of patients with COPD, causing protein citrullination [50]. To stain for mouse NETs we used antibodies directed against citrullinated histone H3 (Cit-H3), which result in a bright staining pattern on NETs, whereas compact nuclei remain virtually unstained. We further evaluated PAD4 involvement in nicotine-induced NET formation in human neutrophils. Blockage of PAD4 using specific inhibitor Cl-amidine resulted in abrogation of NET release (Fig. 3E) to background levels, as assessed by microscopic quantification (Fig. 3F), and by fluorometric assays of different nicotine concentrations (Fig. 3G).
Taken together, our results demonstrate that nicotine triggers neutrophils to release NETs via nAChRs. This pathway is mediated by Akt activation without affecting or requiring the activity of Nox2. Our findings serve as a good starting point to investigate whether nicotine use may exacerbate unwanted inflammation and injury in tissues due to increased NET formation. This process in turn could contribute to smoking-related diseases by chronic inflammation. Inhibition of Akt could provide a novel therapeutic target to mitigate nicotine-related diseases.
AUTHORSHIP
A. H. and C.F.U. conceived and designed the experiments. A.H. performed the experiments. P.T. provided reagents and critically read the manuscript. A.H., B.H.S., and C.F.U. analyzed the data. A.H., B.H.S., and C.F.U. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Research Council VR-M, the Åke Wiberg Foundation, the Laboratory for Molecular Medicine Sweden (MIMS), and the Medial Faculty Umeå (to C.F.U.) and from U.S. National Institutes of Health, National Cancer Institute Grant 1R01CA188900-01A1 (to B.H.S.). A.H. acknowledges financial support from the J. C. Kempes Memorial Fund.
Glossary
- Akt-I
Akt inhibitor XI
- AUC
area under the curve
- COPD
chronic obstructive pulmonary disease
- Cit-H3
citrullinated histone
- DPI
diphenylene iodonium
- nAChR
nicotinic acetylcholine receptor
- Nox2
phagocyte NADPH oxidase
- NET
neutrophil extracellular trap
- PAD4
peptidylarginine deiminase 4
- ROS
reactive oxygen species
DISCLOSURE
The authors declare no conflicts of interest.
REFERENCES
- 1.Amulic B., Cazalet C., Hayes G. L., Metzler K. D., Zychlinsky A. (2012) Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. [DOI] [PubMed] [Google Scholar]
- 2.Pillay J., den Braber I., Vrisekoop N., Kwast L. M., de Boer R. J., Borghans J. A., Tesselaar K., Koenderman L. (2010) In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116, 625–627. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y., Li H., Bajrami B., Kwak H., Cao S., Liu P., Zhou J., Zhou Y., Zhu H., Ye K., Luo H. R. (2013) Cigarette smoke (CS) and nicotine delay neutrophil spontaneous death via suppressing production of diphosphoinositol pentakisphosphate. Proc. Natl. Acad. Sci. USA 110, 7726–7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klebanoff S. J. (2005) Myeloperoxidase: friend and foe. J. Leukoc. Biol. 77, 598–625. [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535. [DOI] [PubMed] [Google Scholar]
- 6.Urban C. F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P. R., Zychlinsky A. (2009) Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 5, e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban C. F., Reichard U., Brinkmann V., Zychlinsky A. (2006) Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell. Microbiol. 8, 668–676. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi M., Hakkim A., Brinkmann V., Siler U., Seger R. A., Zychlinsky A., Reichenbach J. (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114, 2619–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Branzk N., Lubojemska A., Hardison S. E., Wang Q., Gutierrez M. G., Brown G. D., Papayannopoulos V. (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 15, 1017–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal B. H., Veys P., Malech H., Cowan M. J. (2011) Chronic granulomatous disease: lessons from a rare disorder. Biol. Blood Marrow Transplant. 17(1, Suppl)S123–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi M., Niemiec M.J., Siler U., Urban C.F., Reichenbach J. (2011) Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J. Allergy Clin. Immunol. 127, 1243–1252 e7. [DOI] [PubMed] [Google Scholar]
- 12.Chen K., Nishi H., Travers R., Tsuboi N., Martinod K., Wagner D. D., Stan R., Croce K., Mayadas T. N. (2012) Endocytosis of soluble immune complexes leads to their clearance by FcγRIIIB but induces neutrophil extracellular traps via FcγRIIA in vivo. Blood 120, 4421–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng O. Z., Palaniyar N. (2013) NET balancing: a problem in inflammatory lung diseases. Front. Immunol. 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douda D. N., Yip L., Khan M. A., Grasemann H., Palaniyar N. (2014) Akt is essential to induce NADPH-dependent NETosis and to switch the neutrophil death to apoptosis. Blood 123, 597–600. [DOI] [PubMed] [Google Scholar]
- 15.Demers M., Krause D. S., Schatzberg D., Martinod K., Voorhees J. R., Fuchs T. A., Scadden D. T., Wagner D. D. (2012) Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 109, 13076–13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes P. J. (2007) New molecular targets for the treatment of neutrophilic diseases. J. Allergy Clin. Immunol. 119, 1055–1062, quiz 1063–1064. [DOI] [PubMed] [Google Scholar]
- 17.Di Stefano A., Capelli A., Lusuardi M., Balbo P., Vecchio C., Maestrelli P., Mapp C. E., Fabbri L. M., Donner C. F., Saetta M. (1998) Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am. J. Respir. Crit. Care Med. 158, 1277–1285. [DOI] [PubMed] [Google Scholar]
- 18.Jamal A., Agaku I. T., O’Connor E., King B. A., Kenemer J. B., Neff L. (2014) Current cigarette smoking among adults: United States, 2005–2013. MMWR Morb. Mortal. Wkly. Rep. 63, 1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz N.L., Hukkanen J., and Jacob P. 3rd (2009). Nicotine chemistry, metabolism, kinetics and biomarkers. In: Nicotine Psychopharmacology (Henningfield J. E., London E. D., Pogun S., eds.) Springer, Berlin, Germany, 192, 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flouris A. D., Chorti M. S., Poulianiti K. P., Jamurtas A. Z., Kostikas K., Tzatzarakis M. N., Wallace Hayes A., Tsatsakis A. M., Koutedakis Y. (2013) Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhal. Toxicol. 25, 91–101. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury B. (1998) Studies on the blood nicotine level in the oral and oropharyngeal cancers of habitual tobacco users. Indian J. Otolaryngol. Head Neck Surg. 50, 230–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feyerabend C., Higenbottam T., Russell M. A. (1982) Nicotine concentrations in urine and saliva of smokers and non-smokers. Br. Med. J. (Clin. Res. Ed.) 284, 1002–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iho S., Tanaka Y., Takauji R., Kobayashi C., Muramatsu I., Iwasaki H., Nakamura K., Sasaki Y., Nakao K., Takahashi T. (2003) Nicotine induces human neutrophils to produce IL-8 through the generation of peroxynitrite and subsequent activation of NF-kappaB. J. Leukoc. Biol. 74, 942–951. [DOI] [PubMed] [Google Scholar]
- 24.Benowitz N. L., Jacob P. III (1984) Daily intake of nicotine during cigarette smoking. Clin. Pharmacol. Ther. 35, 499–504. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii M., Iijima H., Nishida T., Takehara T. (2013) [Smoking and alimentary diseases]. Nippon Rinsho 71, 436–442. [PubMed] [Google Scholar]
- 26.Seow W. K., Thong Y. H., Nelson R. D., MacFarlane G. D., Herzberg M. C. (1994) Nicotine-induced release of elastase and eicosanoids by human neutrophils. Inflammation 18, 119–127. [DOI] [PubMed] [Google Scholar]
- 27.Salama S. A., Arab H. H., Omar H. A., Maghrabi I. A., Snapka R. M. (2014) Nicotine mediates hypochlorous acid-induced nuclear protein damage in mammalian cells. Inflammation 37, 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Branzk N., Papayannopoulos V. (2013) Molecular mechanisms regulating NETosis in infection and disease. Semin. Immunopathol. 35, 513–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilsczek F. H., Salina D., Poon K. K., Fahey C., Yipp B. G., Sibley C. D., Robbins S. M., Green F. H., Surette M. G., Sugai M., Bowden M. G., Hussain M., Zhang K., Kubes P. (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 185, 7413–7425. [DOI] [PubMed] [Google Scholar]
- 30.Yousefi S., Mihalache C., Kozlowski E., Schmid I., Simon H. U. (2009) Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 16, 1438–1444. [DOI] [PubMed] [Google Scholar]
- 31.Remijsen Q., Kuijpers T. W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. (2011) Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruger P., Saffarzadeh M., Weber A. N., Rieber N., Radsak M., von Bernuth H., Benarafa C., Roos D., Skokowa J., Hartl D. (2015) Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. 11, e1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu D., Hattori H., Jo H., Jia Y., Subramanian K. K., Loison F., You J., Le Y., Honczarenko M., Silberstein L., Luo H. R. (2006) Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc. Natl. Acad. Sci. USA 103, 14836–14841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosseinzadeh A., Urban C. F. (2013) Novel insight into neutrophil immune responses by dry mass determination of Candida albicans morphotypes. PLoS One 8, e77993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ermert D., Urban C. F., Laube B., Goosmann C., Zychlinsky A., Brinkmann V. (2009) Mouse neutrophil extracellular traps in microbial infections. J. Innate Immun. 1, 181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hosseinzadeh A., Messer P. K., Urban C. F. (2012) Stable redox-cycling nitroxide tempol inhibits NET formation. Front. Immunol. 3, 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Totti N. III, McCusker K. T., Campbell E. J., Griffin G. L., Senior R. M. (1984) Nicotine is chemotactic for neutrophils and enhances neutrophil responsiveness to chemotactic peptides. Science 223, 169–171. [DOI] [PubMed] [Google Scholar]
- 39.Xu J., Huang H., Pan C., Zhang B., Liu X., Zhang L. (2007) Nicotine inhibits apoptosis induced by cisplatin in human oral cancer cells. Int. J. Oral Maxillofac. Surg. 36, 739–744. [DOI] [PubMed] [Google Scholar]
- 40.Pletz M. W., Ioanas M., de Roux A., Burkhardt O., Lode H. (2004) Reduced spontaneous apoptosis in peripheral blood neutrophils during exacerbation of COPD. Eur. Respir. J. 23, 532–537. [DOI] [PubMed] [Google Scholar]
- 41.Xu M., Scott J. E., Liu K. Z., Bishop H. R., Renaud D. E., Palmer R. M., Soussi-Gounni A., Scott D. A. (2008) The influence of nicotine on granulocytic differentiation - inhibition of the oxidative burst and bacterial killing and increased matrix metalloproteinase-9 release. BMC Cell Biol. 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebargy F., Benhammou K., Morin D., Zini R., Urien S., Brée F., Bignon J., Branellec A., Lagrue G. (1996) Tobacco smoking induces expression of very-high-affinity nicotine binding sites on blood polymorphonuclear cells. Am. J. Respir. Crit. Care Med. 153, 1056–1063. [DOI] [PubMed] [Google Scholar]
- 43.Röhm M., Grimm M. J., D’Auria A. C., Almyroudis N. G., Segal B. H., Urban C. F. (2014) NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect. Immun. 82, 1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews J. B., Chen F. M., Milward M. R., Wright H. J., Carter K., McDonagh A., Chapple I. L. (2011) Effect of nicotine, cotinine and cigarette smoke extract on the neutrophil respiratory burst. J. Clin. Periodontol. 38, 208–218. [DOI] [PubMed] [Google Scholar]
- 45.Sasagawa S., Suzuki K., Sakatani T., Fujikura T. (1985) Effects of nicotine on the functions of human polymorphonuclear leukocytes in vitro. J. Leukoc. Biol. 37, 493–502. [DOI] [PubMed] [Google Scholar]
- 46.Pabst M. J., Pabst K. M., Collier J. A., Coleman T. C., Lemons-Prince M. L., Godat M. S., Waring M. B., Babu J. P. (1995) Inhibition of neutrophil and monocyte defensive functions by nicotine. J. Periodontol. 66, 1047–1055. [DOI] [PubMed] [Google Scholar]
- 47.Douda D. N., Khan M. A., Grasemann H., Palaniyar N. (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 112, 2817–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lood C., Blanco L. P., Purmalek M. M., Carmona-Rivera C., De Ravin S. S., Smith C. K., Malech H. L., Ledbetter J. A., Elkon K. B., Kaplan M. J. (2016) Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li P., Li M., Lindberg M. R., Kennett M. J., Xiong N., Wang Y. (2010) PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kilsgård O., Andersson P., Malmsten M., Nordin S. L., Linge H. M., Eliasson M., Sörenson E., Erjefält J. S., Bylund J., Olin A. I., Sørensen O. E., Egesten A. (2012) Peptidylarginine deiminases present in the airways during tobacco smoking and inflammation can citrullinate the host defense peptide LL-37, resulting in altered activities. Am. J. Respir. Cell Mol. Biol. 46, 240–248. [DOI] [PubMed] [Google Scholar]