PKCδ inhibition significantly reduces neutrophil-endothelial interaction during acute inflammation suggesting a therapeutic strategy for treating inflammatory disease.
Keywords: inflammation, adhesion, biomimetic, leukocytes, transmigration
Abstract
A key step in neutrophil-mediated tissue damage is the migration of activated neutrophils across the vascular endothelium. Previously, we identified protein kinase C δ as a critical regulator of neutrophil migration in sepsis but did not identify specific steps in migration. In this study, we used our novel biomimetic microfluidic assay to delineate systematically the mechanism by which protein kinase C δ regulates individual steps in human neutrophil–endothelial interaction during inflammation. The biomimetic microfluidic assay includes a network of vascular channels, produced from in vivo images connected to a tissue compartment through a porous barrier. HUVECs cultured in vascular channels formed a complete lumen under physiologic shear flow. HUVECs were pretreated with TNF-α ± a protein kinase C δ inhibitor, and the tissue compartment was filled with a chemoattractant (fMLP or IL-8). Under physiologic shear flow, the role of protein kinase C δ on spatial and temporal neutrophil adherence/migration was quantified. Protein kinase C δ inhibition significantly reduced neutrophil adhesion in response to fMLP and IL-8 only under low shear rate and near bifurcations. Protein kinase C δ inhibition also decreased adherence to nonactivated HUVECs in response to fMLP or IL-8. Protein kinase C δ inhibition reduced neutrophil migration into the tissue compartment in response to fMLP and to a lesser degree, to IL-8. Antibody-coated microparticles demonstrated that protein kinase C δ inhibition down-regulated E-selectin and ICAM-1 but not VCAM-1 expression. With the use of a physiologically relevant in vitro model system, we demonstrate that protein kinase C δ plays an important role in the regulation of neutrophil adherence/migration during inflammation and identifies key steps regulated by protein kinase C δ in neutrophil–endothelial interactions.
Introduction
Sepsis is one of the leading causes of death in Intensive Care Units and causes >200,000 deaths/yr in the United States [1–3]. Sepsis is characterized by an intense systemic inflammatory response that develops in response to pathogen-associated molecular patterns, which are released during infection. This systemic inflammation activates a cascade of proinflammatory events, resulting in neutrophil dysregulation and host tissue damage.
Neutrophil dysfunction damages the host tissue through the release of proteases and oxygen radicals. A key step in neutrophil-mediated tissue damage is the migration of activated neutrophils across the vascular endothelium [4, 5]. Whereas there are no specific pharmacologic therapies for treating this damage, we have identified PKCδ as a critical regulator of the inflammatory response controlling recruitment and activation of inflammatory cells [6]. Our previous studies indicated a role for PKCδ in regulating neutrophil migration but did not address specific mechanisms or identify specific steps by which PKCδ impacts the interaction of neutrophils and endothelial cells during inflammation [6–8].
Neutrophil recruitment during sepsis is a multistep dynamic phenomenon that requires real-time monitoring for mechanistic understanding. The vascular endothelium is involved in the pathogenesis of sepsis and is an active participant in the recruitment and activation of neutrophils through the production of chemokines/cytokines and expression of adhesion molecules. The crosstalk between neutrophils and endothelial cells is composed of a series of interactions that orchestrate rolling, adhesion, and transmigration. Firmly adhered neutrophils migrate across endothelial cells into the inflamed tissues via a multistep process controlled by concurrent chemoattractant-dependent signals, adhesive events, and hemodynamic shear forces. Moreover, neutrophil recruitment involves integrin-dependent and integrin-independent pathways [9]. Although both pathways may function simultaneously, the relative importance of a specific pathway is stimulus dependent [6, 10, 11]. Whether PKCδ regulates β2-integrin-dependent and/or β2-integrin-independent pathways under flow conditions is not known.
Traditionally, most studies involving inflammatory pathways have been performed using in vitro static well plates or animal experiments. To study neutrophil–endothelial cell interactions under shear conditions, several flow-based devices have been developed [12–16]. However, these devices are typically idealized and lack correspondence with the in vivo physiologic conditions, such as geometry and microenvironment [17]. To address this important limitation, in a previous study, we have developed a bMFA for studying the entire neutrophil adhesion cascade, encompassing circulation, rolling, adhesion, and migration of neutrophils [18–22]. This assay reproduces a physiologically realistic (geometry and shear conditions), 3D microvascular environment that can be used to study the complete adhesion/transmigration cascade.
In this study, we investigated the role of PKCδ in human neutrophil–endothelial interaction during the inflammatory response under shear flow conditions. With the use of a bMFA, we investigated the effect of PKCδ inhibition on temporal and spatial distribution of adhering/migrating neutrophils through endothelium in response to β2-integrin-dependent vs. β2-integrin-independent signaling pathways.
MATERIALS AND METHODS
Antibodies and reagents
A mouse anti-human ICAM-1 mAb (Cat. No. sc-7891), a mouse anti-human VCAM-1 mAb (Cat. No. sc-18854), a mouse anti-human E-selectin mAb (Cat. No. sc-137054), and a mouse IgG mAb (Cat. No. sc-2025) were purchased from Santa Cruz Biotechnology (Dallas, TX, USA) and a rabbit anti-goat IgG2b antibody was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Human fibronectin was obtained from BD Biosciences (San Jose, CA, USA). Recombinant human TNF-α was purchased from BioVision (Milpitas, CA, USA). Protein A was purchased from Thermo Fisher Scientific. Fluorescent, 9.9 μm microparticles (green: excitation 468 nm, emission 508 nm) were purchased from Thermo Fisher Scientific. BSA was purchased from Sigma-Aldrich (St. Louis, MO, USA). HBSS was obtained from Mediatech (Manassas, VA, USA). Trypsin/EDTA was purchased from Cell Biologics (Chicago, IL, USA). HUVECs were purchased from Lonza (Walkersville, MD, USA). Fluorescence visualization was achieved using a streptavidin Alexa Fluor 594 conjugate from Thermo Fisher Scientific, MPO from Santa Cruz Biotechnology (L-20), CFDA/SE probe from Thermo Fisher Scientific, Draq5 from Thermo Fisher Scientific, and Alexa Fluor 488 Phalloindin from Thermo Fisher Scientific.
Fabrication of the bMFA
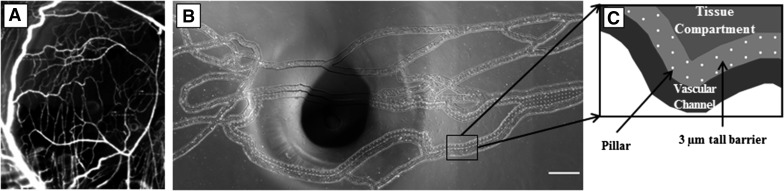
Previously, our group has developed a methodology for digitization and fabrication of microfluidic devices based on in vivo microvascular networks collected by intravital microscopy (Fig. 1A) [23]. A modified geographic information system approach was used to digitize the microvascular networks. In brief, the fabrication of the microfluidic devices starts with lithographically patterning SU-8 photoresist on Si wafers. To achieve the multiple heights associated with the vascular channels, barrier, and tissue compartment area, multiple layers of SU-8 were spin coated and patterned. Microfabricated pillars (10 μm diameter) were used to fabricate the 3 μm pores with a width of 100 μm connecting the vascular channels and tissue compartments. Once the SU-8 microfluidic features were patterned, the 1:10 w/w ratio of Sylgard 184 silicone elastomer base and curing agent (Dow Corning, Midland, MI, USA) was poured over the master and cured. Subsequently, the cured PDMS was peeled from the SU-8 master, followed by punching of inlet/outlet ports, and plasma bonded to a glass slide cleaned to remove organic species. As a result, the bMFA assay has a network of vascular channels (Fig. 1B) connected via a 3 μm porous barrier to a tissue compartment (Fig. 1C), an optimum size for neutrophil migration. In agreement with the flow parameters measured in the vessels of original microvascular network in vivo [20], shear rates used in the vascular channels of bMFA ranged from 0 to 280 s−1.
Figure 1. Overview of the bMFA.
Images of mouse cremaster muscle (A) are used to fabricate the network on PDMS in the bMFA (B; original scale bar, 500 µm). (C) In the bMFA, vascular channels are connected to the tissue compartment through a 3 μm barrier. The dark spot in the middle of B is from the tube connecting the tissue compartment to the syringe pump.
Seeding of HUVECs into the bMFA
With the use of our established protocol [20], microvascular networks were coated with fibronectin, and endothelial cells were cultured under shear flow (inlet flow rate of 0.5 μl/min) at 37°C and 5% CO2 for 24 h to form a 3D lumen in the vascular channels. In the current study (see Fig. 3), as well as in previous studies [24], we have demonstrated that in the bMFA, endothelial cells form a confluent lumen with tight junctions and minimal leukocyte migration in the absence of cell activation (see Fig. 4C). We have also shown previously that these devices have high transendothelial electrical resistance and that dextran permeation is reduced significantly when the vascular compartment is cultured with endothelial cells [24]. The formation of endothelial lumen in the bMFA is assessed before the start of experimental protocols, and devices are discarded if any sections are observed with disrupted lumen or if neutrophils rapidly entered the tissue compartment without attachment to vascular channel walls.
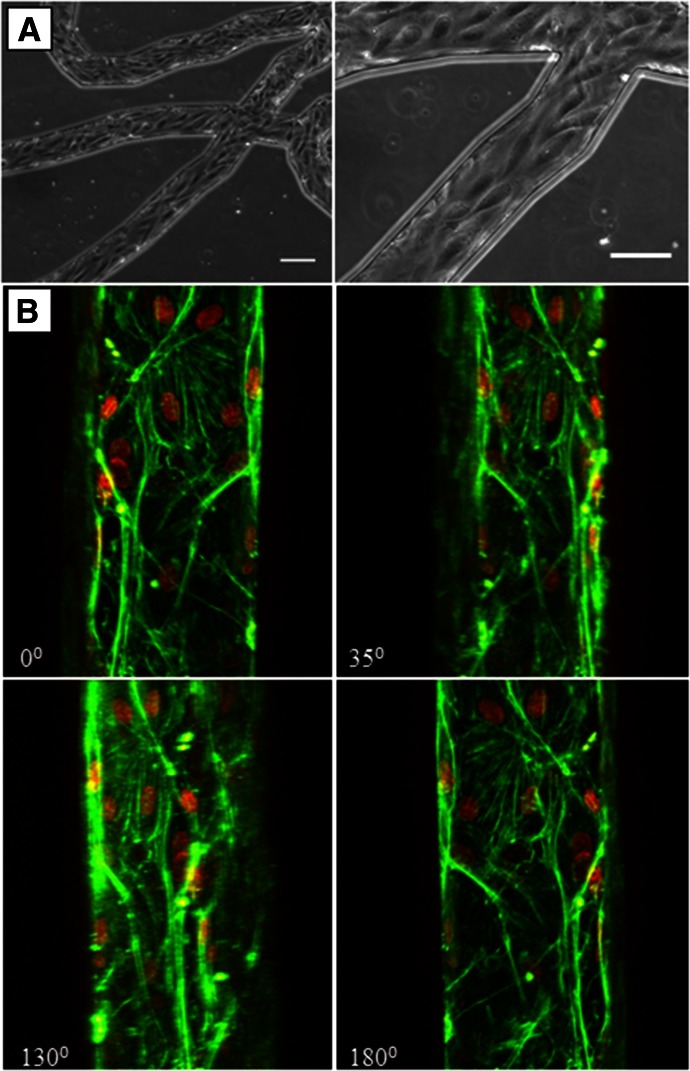
Figure 3. Endothelial cells form a complete lumen in the bMFA.
Phase contrast images show that endothelial cells are lined up in the direction of flow (A; original scale bars, 100 µm). (B) Confocal micrograph of endothelial cells showing a complete 3D lumen formation in the vascular channel; F-actin is labeled in green, and nuclei is labeled in red.
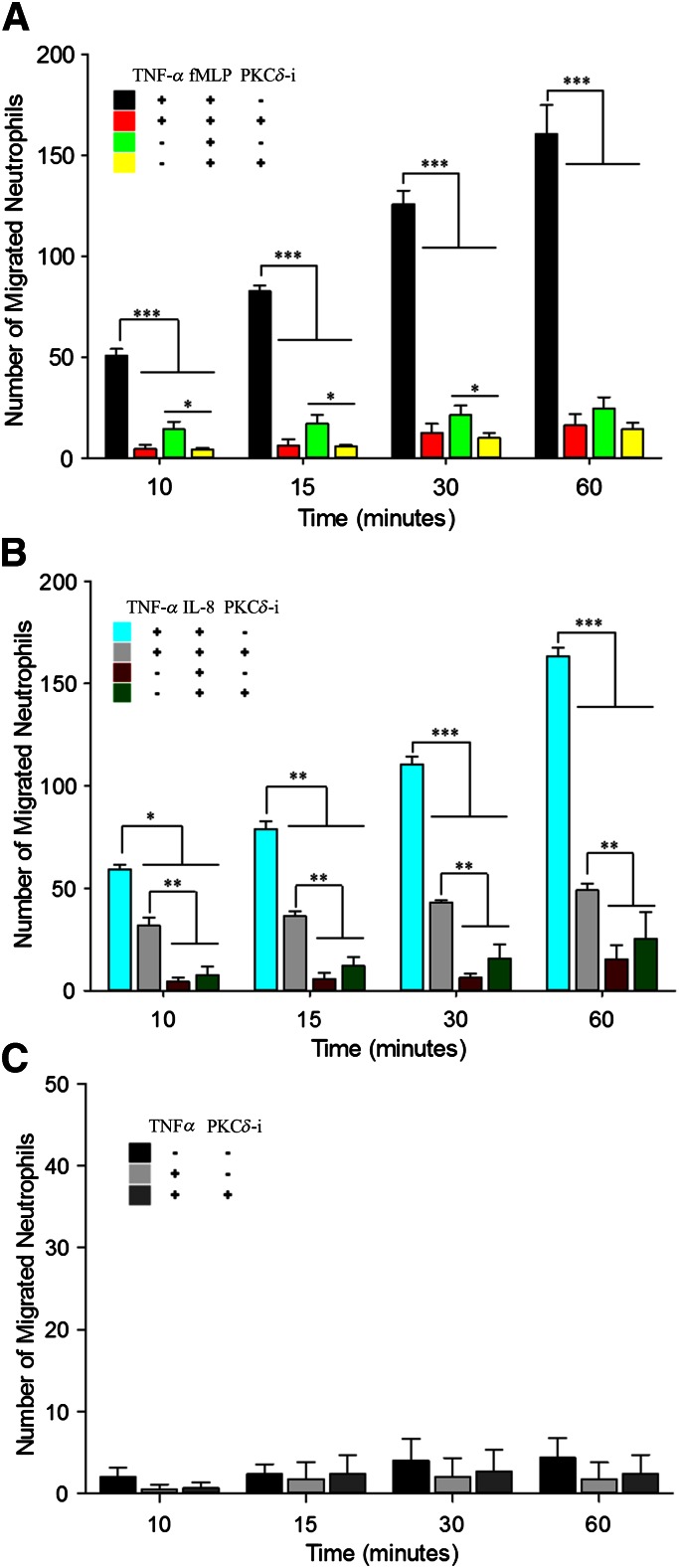
Figure 4. Neutrophil migration is stimulus dependent and regulated by PKCδ.
PKCδ inhibition reduces migration of neutrophils from the vascular channels, across the activated endothelium, into the tissue compartment. There is a general increase in the number of migrated neutrophils across endothelium during the course of experiments. (A) PKCδ inhibition significantly reduces neutrophil transmigration across activated endothelium in response to fMLP. PKCδ inhibition significantly decreases neutrophil transmigration across nonactivated endothelial cells in response to fMLP, up to 30 min. (B) PKCδ inhibition significantly reduces neutrophil transmigration across activated endothelium in response to IL-8 but by a lesser degree compared with fMLP. Treatment with PKCδ-i does not result in a significant change in transmigration of neutrophils across nonactivated endothelial cells in response to IL-8. (C) In the absence of chemoattractants, neutrophil migration across TNF-α-activated endothelial is not significantly different from background levels (i.e., no TNF-α activation and no chemoattractant in the tissue compartment). Under these conditions, PKCδ inhibition does not significantly change neutrophil transmigration across activated endothelium (means ± sem; n = 3; *P < 0.05, **P < 0.01, ***P < 0.001, 2-way ANOVA).
Endothelial cells were activated with a solution of TNF-α (10 U/ml) in endothelial growth media injected into the vascular channels network. For PKCδ-i treatment, a solution of TNF-α and PKCδ-i was injected into the network. At 4 or 24 h post-TNF-α, with or without PKCδ-i treatment, the tissue compartment was filled with buffer (control), fMLP (1 μM; β2-integrin-dependent chemoattractant), or IL-8 (20 nM; β2-integrin-independent chemoattractant) before injecting neutrophils in the vascular compartment. Therefore, endothelial cells were treated with TNF-α, with or without the PKCδ-i, followed by the addition of a chemoattractant (fMLP or IL-8) or buffer.
Neutrophil isolation and labeling
Human blood was obtained via venipuncture from healthy adult donors and collected into a sterile tube containing sodium heparin (BD Biosciences), after informed consent was obtained, as approved by the Institutional Review Board of Temple University. Human neutrophils were isolated by standard techniques using Ficoll-Hypaque separation, dextran sedimentation, and hypotonic lysis to remove erythrocytes [7, 11]. After isolation, neutrophils were counted and suspended in HBSS (5 × 106 cells/ml). Neutrophils were labeled in suspension using a CFDA/SE probe for 10 min at room temperature. Human neutrophils were also treated with the solution of TNF-α alone or TNF-α + PKCδ-i for 10 min before injection into the microfluidic assay. Neutrophils were introduced in vascular channels at a flow rate of 1 μl/min at the entrance of the network.
PKCδ-i treatment
As described previously, PKCδ activity was selectively inhibited by a peptide antagonist [6]. This antagonist consists of a peptide derived from the first unique region (V1) of PKCδ (SFNSYELGSL; aa 8–17), coupled via an N-terminal Cys–Cys bond to a membrane-permeant peptide sequence in the HIV TAT gene product (YGRKKRRQRRR; aa 47–57 of TAT) [25]. The coupling of this inhibitor to a membrane-permeant TAT peptide sequence permits effective intracellular delivery into target cells. Both HUVECs and neutrophils were treated with the inhibitor (5 μM) before injection of neutrophils to bMFA.
To study systematically the impact of PKCδ inhibition on neutrophil–endothelial interaction, endothelial cells were activated with TNF-α for 4 or 24 h. Adhesion and transmigration of neutrophils activated with TNF-α for 10 min in bMFA were then measured in the presence of fMLP or IL-8 in the tissue compartment. Control experiments were performed with endothelial cells and neutrophils treated with media and/or tissue compartment filled with media.
Preparation of antibody-coated microparticles
With the use of our established methodology [26], fluorescent polystyrene spherical microparticles were used to measure the impact of PKCδ treatment on the up-regulation of adhesion molecules. In brief, 9.9 μm fluorescent polystyrene mircoparticles were washed with a sodium bicarbonate buffer and coated with protein A (300 μg/ml) via passive adsorption and incubated overnight at room temperature. Mircoparticles were then washed and incubated in a blocking buffer (HBSS, supplemented with 1% BSA) at room temperature. Microparticles were counted and diluted in buffer (HBSS containing Ca2+, Mg2+, and 1% BSA) to achieve a concentration of 5 × 106 particles/ml. Then, the particles were incubated with different antibodies of anti-ICAM-1, anti-VCAM-1, and anti-E-selectin for 30 min. The total concentration of antibody in the coating solution used to generate the particles was the same for all mAb ratios and greater than that needed to saturate the particles [26]. Antibody-coated microparticles were then suspended in the endothelial growth media and introduced to the network inlet with a programmable syringe pump. The level of adhesion of a given antibody (e.g., anti-ICAM-1)-coated microparticle to endothelial cells was used as an index of the level of up-regulation of that adhesion molecule [26].
Animal protocols
All animal handling and care adhered to the U.S. National Institutes of Health standards and were approved by the Institutional Animal Care and Use Committee at Temple University School of Medicine. Male Sprague-Dawley rats (225–250 g; Charles River Laboratories, Wilmington, MA, USA) were used in all experiments. Rats were acclimated for at least 1 wk in a climate-controlled facility and given free access to food and water.
CLP model
Sepsis was induced by the CLP method, as described previously [6, 7, 27]. Sham controls underwent a laparotomy without cecal ligation or puncture. Following CLP or Sham surgery, the abdominal incision was closed, and the animals were orally intubated with a 16-gauge intravenous cannula. Animals were randomized to receive either the PKCδ-i peptide (200 μg/kg in 200 μl PBS) or a like volume of PBS (vehicle).
At 24 h after surgery, animals were euthanized. The lungs were gravity fixed with 10% neutral-buffered formalin instillation into the airways; the trachea was then tied off to maintain inflation during fixation. Lungs were fixed for 2–3 h at room temperature and then held overnight in formalin at 4°C. After fixation, lungs were washed several times with PBS and stored in 70% ethanol at 4°C. Lung tissue was paraffin embedded, cut into sections (8–10 μm thick), and stained with specific antibodies for immunohistochemical detection of MPO, as described previously [7].
Lung tissue MPO staining
Rat lung tissue sections were deparaffinized and antigen retrieval achieved by microwaving the tissue slides for 4 min in citrate buffer, pH 6.0. MPO levels were determined using a goat polyclonal antibody against MPO. The slides were washed and incubated with biotinylated rabbit anti-goat IgG2b antibody. The slides were mounted in Vectashield with DAPI (DNA stain) and imaged using fluorescence microscopy. ImageJ was used to determine the ratio of MPO-positive cells to total cells (DAPI-positive cells).
Data analysis
As bMFA can resolve the effect of shear rate on adhesion in bifurcations and various linear sections in vascular channels, we used our CFD-based model to calculate various flow parameters (e.g., shear stress) at different locations in the network [23]. Cells that did not move for 30 s were considered adherent. Adhesion level of neutrophils to the endothelium reached steady state after 10 min of flow and was quantified by scanning the entire network. Analysis of neutrophil migration was quantified using time-lapse imaging by obtaining images from the assay every 3 min for 60 min. Results were used to measure the number of migrated neutrophils at time points of 10, 15, 30, and 60 min. Nikon Elements and ImageJ software were used to collect and analyze the data. Data are presented as means ± sem, and statistically significant differences were determined by 1- or 2-way ANOVA using SigmaPlot software. Differences were considered statistically significant if P < 0.05.
RESULTS
In vivo administration of the PKCδ-i decreases neutrophil migration into the lung in septic animals
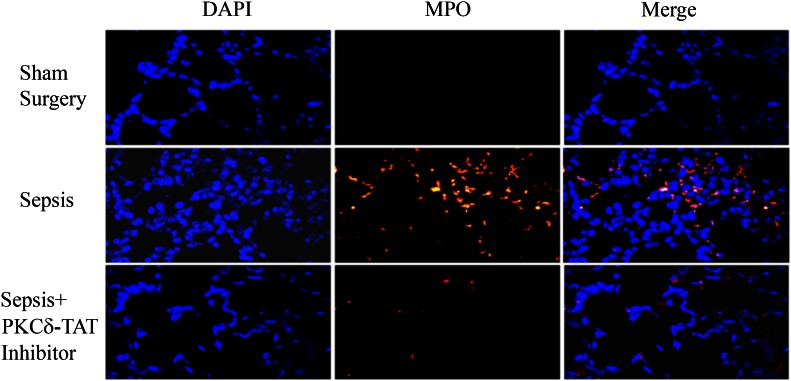
Intra-abdominal sepsis produces systemic inflammation, which leads to the influx of neutrophils into the lungs and the development of acute lung injury [28, 29]. In support of our previous studies, we found that 24 h post-CLP surgery, there was a significant influx of neutrophils into the lung compared with sham surgery animals (Fig. 2). The lungs of sham surgery rats had very low numbers of MPO-positive cells (3.4 ± 1.9% of total cells). Conversely, the lungs of septic animals treated with vehicle (Sepsis + PBS) contained a significantly greater number of MPO-positive cells (17.0 ± 6.0% of total cells, P < 0.02 vs. sham surgery rats). Treatment with the PKCδ-i resulted in a significant decrease in MPO-positive cells compared with septic rats treated with vehicle alone (6.6 ± 2.8% of total cells, P < 0.04 vs. CLP + PBS vehicle). Thus, systemic inflammation triggers a significant increase in neutrophil influx into the lung, which is attenuated by treatment with the PKCδ-i.
Figure 2. PKCδ inhibition decreases neutrophil migration into the lung in a rodent sepsis model.
Immunohistochemical detection of MPO in representative lung tissue sections from 24 h postsurgery (n = 6–8 animals/group). Sham Surgery: only a few MPO-positive cells were seen in each field. Sepsis: following CLP surgery and intratracheal (IT) administration of PBS vehicle, sepsis induces the infiltration of numerous MPO-positive cells throughout the lung parenchyma. Sepsis + PKCδ-TAT Inhibitor: following CLP surgery and IT administration of the PKCδ-i, there was significant reduction of sepsis-induced, MPO-positive cell numbers in the lung. Original magnification, 400×.
HUVECs form a complete lumen along the walls of vascular channels of bMFA
Endothelial cells were cultured in bMFA and under shear flow, formed a complete 3D lumen in the vascular channels. As shown in Fig. 3A, endothelial cells covered the surface of the vascular channels in bMFA and aligned in the direction of flow in these channels. Confocal microscopy (Fig. 3B) indicated that endothelial cells in bMFA covered all surfaces of the vascular channels and formed a complete lumen [24]. Therefore, endothelial cells form a complete lumen in bMFA under physiologic conditions.
Migration of neutrophils across activated endothelial cells in response to chemoattractants decreases after inhibition of PKCδ
In bMFA, there was a time-dependent increase in the number of neutrophils that migrate across TNF-α-activated HUVECs in response to the chemoattractants in the tissue compartment over a 60 min time course (Fig. 4). There was significant migration of neutrophils across TNF-α-activated endothelial cells in the presence of a chemoattractant (fMLP or IL-8), which increased over the course of the experiment. The choice of fMLP (β2-integrin-dependent pathway) or IL-8 (β2-integrin-independent pathway) did not significantly affect the maximum number of migrated neutrophils across activated endothelial cells. However, there was very little migration of neutrophils in response to fMLP or IL-8 alone (Fig. 4A and B). Migration of neutrophils across activated endothelial cells in response to chemoattractant, either fMLP or IL-8, was reduced significantly after treatment with the PKCδ-i (Fig. 4A and B). Neutrophil migration across TNF-activated endothelial cells in response to fMLP was inhibited by 85–92% over the 60 min experiment (Fig. 4A). In contrast, inhibition of neutrophil migration across activated endothelial cells in response to IL-8 by the PKCδ-i was less pronounced (between 32 and 78%) during the time course of the experiment (Fig. 4B). Neutrophil migration in response to fMLP across nonactivated endothelium was decreased significantly in response to PKCδ inhibition at 10, 15, and 30 min but not at 60 min (Fig. 4A). In contrast, treatment with the PKCδ-i did not cause a significant change in transmigration of neutrophils across nonactivated endothelium in response to IL-8 (Fig. 4B). Consistent with in vivo observations, in the absence of cytokines (no treatment with TNF-α and no chemoattractant in the tissue compartment), there was negligible migration of neutrophils across endothelial cells, demonstrating a confluent endothelial layer in the lumen (Fig. 4C). In the absence of chemoattractants, treatment with PKCδ-i did not result in a significant change in the level of neutrophil transmigration across TNF-α-activated endothelium (Fig. 4C). Thus, inhibition of PKCδ attenuated neutrophil migration across endothelial cells in the presence of fMLP or IL-8.
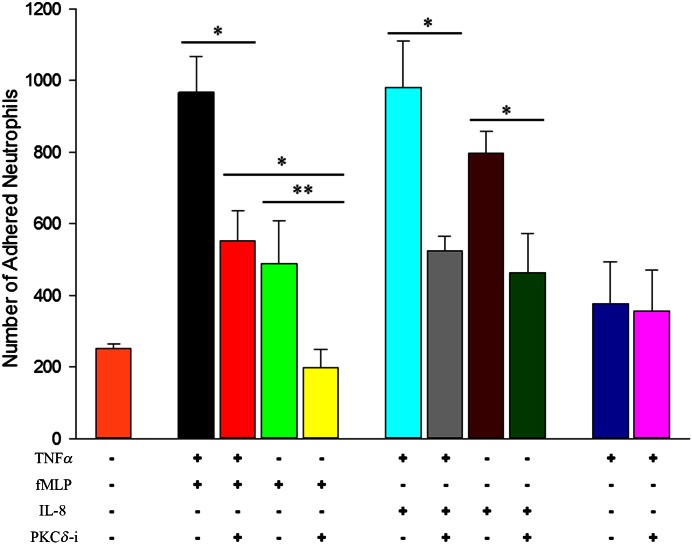
PKCδ inhibition decreases adhesion of neutrophils to the activated endothelium in the presence of chemoattractants
To explore further the effect of PKCδ inhibition on neutrophil–endothelium interaction, we investigated neutrophil adhesion to endothelial cells under different conditions. As shown in Fig. 5, the presence of both TNF-α and chemoattractant resulted in significant adhesion of neutrophils to endothelial cells compared with adhesion in response to no treatment, TNF-α treatment, or chemoattractant alone. The number of adhered neutrophils to endothelial cells in the entire network decreased by ∼46% after treatment with the PKCδ-i in the presence of fMLP or IL-8. In the absence of activation with TNF-α, the PKCδ-i significantly reduced the number of adhered neutrophils in response to fMLP and IL-8. In the absence of a chemoattractant, PKCδ inhibition did not cause a significant change in the number of adhered neutrophils to the activated endothelium (Fig. 5). In the absence of cytokines (no treatment with TNF-α and no chemoattractant in the tissue compartment), there was only a background level of adhesion of neutrophils to endothelial cells (Fig. 5). Thus, PKCδ activity is stimulus dependent and may engage β2-integrin-dependent and -independent signaling pathways. These observations indicate that adhesion of neutrophils to activated endothelial cells in the presence of fMLP or IL-8 was reduced after PKCδ inhibition.
Figure 5. Neutrophil adhesion is also stimulus dependent and regulated by PKCδ.
Adhesion of neutrophils to both activated and nonactivated endothelium decreases significantly after treatment with PKCδ-i with fMLP or IL-8. In the absence of chemoattractants, treatment with PKCδ-i does not significantly affect adhesion of neutrophils to the endothelial cells (means ± sem; n = 3; *P < 0.05, **P < 0.01, 2-way ANOVA).
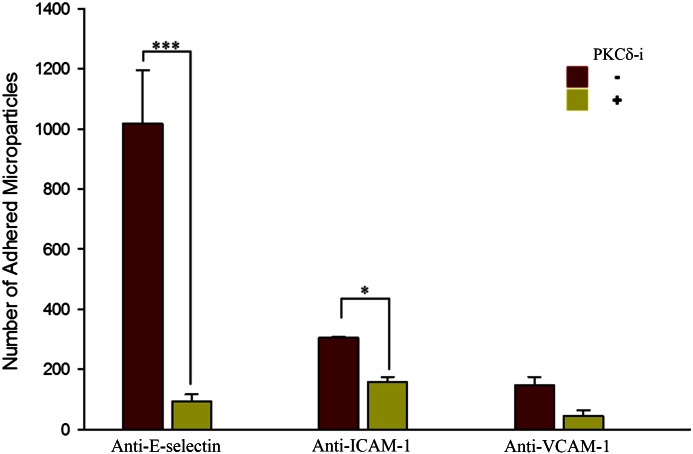
Inhibition of PKCδ down-regulates expression of E-selectin and ICAM-1 on endothelial cells
We used antibody-coated microparticles [18, 19] to determine the degree by which PKCδ impacts the up-regulation of adhesion molecules on endothelial cells during acute inflammation. The interaction of microparticles coated with antibodies to E-selectin, ICAM-1, or VCAM-1 with TNF-α-activated endothelial cells was measured in the presence of fMLP under the same experimental conditions used to measure neutrophil adherence and migration. Interaction of IgG-coated microparticles with endothelial cells was measured and used as control (data not shown). Our findings indicate that in the presence of fMLP, PKCδ inhibition significantly reduced the adhesion of anti-E-selectin- and anti-ICAM-1-coated microparticles to TNF-α-activated endothelial cells (Fig. 6). In contrast, PKCδ inhibition did not significantly alter adhesion of anti-VCAM-1-coated microparticles to TNF-α-activated endothelial cells. Similar to results obtained with neutrophils, inhibition of PKCδ selectively down-regulated the adhesion of anti-E-selectin- and anti-ICAM-1-coated microparticles. Hence, ICAM-1 and E-selectin expression was down-regulated after PKCδ inhibition in endothelial cells.
Figure 6. PKCδ regulates expression of adhesion molecules.
In the presence of fMLP, PKCδ inhibition significantly reduces the adhesion of anti-E-selectin- and anti-ICAM-1-coated microparticles to 4 h TNF-α-treated endothelial cells (means ± sem; n = 3; *P < 0.05, ***P < 0.001, 2-way ANOVA).
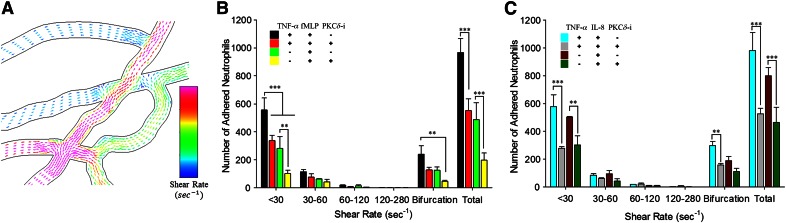
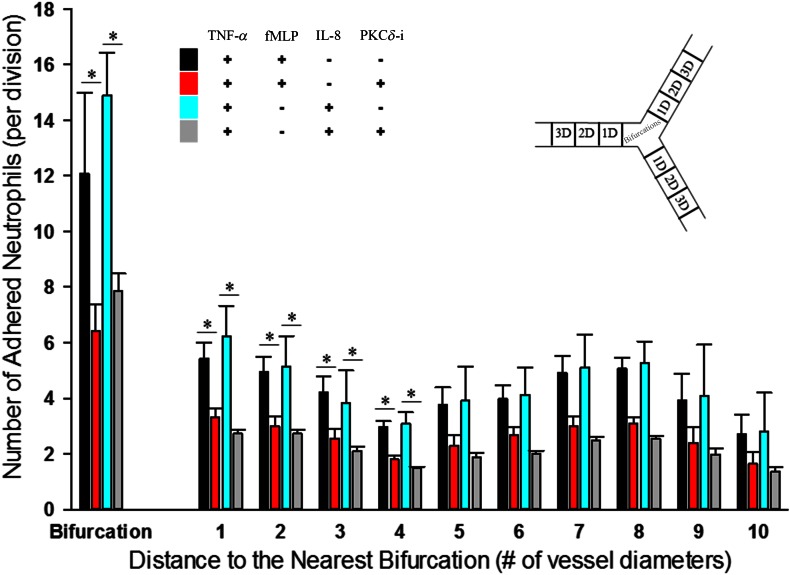
Shear flow and vascular geometry regulate the impact of PKCδ on neutrophil–endothelial interaction
Flow conditions, including shear rate and variability of flow patterns near bifurcations, are important regulators of leukocyte–endothelium interaction in vivo [23, 30–34], and we have shown that the unique design of bMFA allows us to study these parameters in a physiologically realistic environment in vitro [17, 18, 21, 22]. Figure 7A shows the complexity of flow in vascular channels of bMFA, as simulated by CFD modeling [18, 23], where irregular flow patterns around bifurcations increase the probability of cell–cell interaction, whereas flow patterns become more uniform with increasing distance from bifurcations. Previously, we have shown that bifurcations and low shear regions are focal points of leukocyte adhesion in vivo and in vitro [20, 21, 35]. As shown in Figs. 7 and 8, neutrophils preferentially adhered to the endothelial cells in channels with low shear rate and near bifurcations. PKCδ inhibition significantly reduced the number of adhered neutrophils to the activated or nonactivated endothelium in the presence of fMLP or IL-8, only in channels with low shear rate flow (γ < 30 1/s) and at bifurcations. Treatment with PKCδ-i significantly decreased the number of adhered neutrophils to activated endothelium, up to 4 diameters from bifurcations in the presence of fMLP or IL-8 (Fig. 8), suggesting that inhibition of PKCδ reduces neutrophil–endothelial interaction in focal points of adhesion in the microvasculature in low shear areas and near bifurcations. Therefore, the impact of PKCδ in neutrophil–endothelial interaction was affected by shear flow and geometry of the vascular channels.
Figure 7. Similar to in vivo observations, there are spatial variations in flow conditions in vessels of bMFA.
CFD modeling indicates that in vascular networks, shear rate (blue is low shear rate, and red is high shear rate) is different in different vessels and is often variable at bifurcations (A). PKCδ inhibition significantly reduces the adhesion of neutrophils to the endothelium in low shear regions and near bifurcations in bMFA in the presence of fMLP (β2-integrin-dependent; B) or IL-8 (β2-integrin-independent; C; means ± sem; n = 3; **P < 0.01, ***P < 0.001, 2-way ANOVA).
Figure 8. Bifurcations are focal points of action of the PKCδ-i.
PKCδ inhibition preferentially reduces neutrophil adhesion to activated endothelial cells only within 4 channel diameters from the nearest bifurcation. This spatial pattern of PKCδ inhibition of neutrophil adhesion is observed in the presence of fMLP or IL-8 (means ± sem; n = 3; *P < 0.05, 2-way ANOVA).
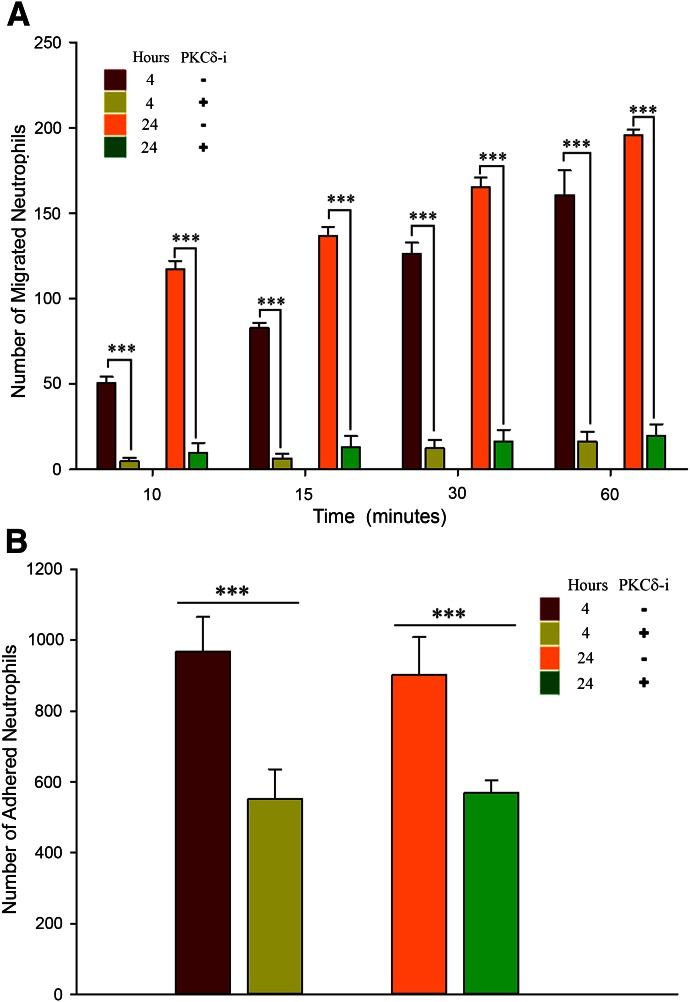
PKCδ regulation of neutrophil–endothelium interaction persists over time
We compared the impact of PKCδ inhibition on neutrophil–endothelial interactions after 24 h of activation with TNF-α and compared that with 4 h activation in the presence of fMLP. PKCδ inhibition significantly reduced adhesion and migration of neutrophils in response to fMLP after 4 or 24 h treatment of endothelial cells with TNF-α (Fig. 9). These observations indicate that PKCδ inhibition of neutrophil–endothelial interaction persists over time.
Figure 9. The impact of the PKCδ-i persists over time.
PKCδ inhibition significantly reduced neutrophil migration (A) and adhesion (B) in response to fMLP across endothelium treated with TNF-α for 4 or 24 h (means ± sem; n = 3; ***P < 0.001, 2-way ANOVA).
DISCUSSION
Almost all drugs recently developed to treat inflammatory disease have failed in clinical trials [5]. Therefore, there is a significant need for understanding the mechanisms by which neutrophils interact with endothelial cells during inflammation and providing a roadmap for rational design of future therapeutics for acute inflammatory diseases. Previously, our group has introduced a novel therapeutic paradigm that uses a peptide antagonist to inhibit selectively PKCδ activity and neutrophil–endothelial interactions to protect vascular endothelial integrity and attenuate sepsis-induced tissue damage [6–8]. We have also shown that administration of PKCδ-i in a rat sepsis model reduced pulmonary and circulating chemokine levels (e.g., cytokine-induced neutrophil chemoattractant 1 and MIP-2) [6]. The role of PKCδ in rodent sepsis models and in neutrophils or endothelial is shown to be stimulus dependent and organ specific [7, 8, 36, 37]. However, the mechanisms by which neutrophils adhere and migrate across endothelial cells during inflammation are not clearly understood, in part, as a result of the lack of realistic fluidic models for in vitro reconstitution of disease-related cell types and tissues [38].
In the current study, we have used a physiologically realistic in vitro environment to delineate mechanisms by which PKCδ inhibition impacts neutrophil–endothelial interaction during acute inflammation. With the use of a well-known rodent model of sepsis, we showed that the inhibition of PKCδ attenuates neutrophil migration into the lung tissue in vivo (Fig. 2). We then used a bMFA to show that HUVECs form a 3D lumen in the bMFA to provide a physiologically realistic environment to study cell–cell interactions (Fig. 3). Our results demonstrate that PKCδ is a critical regulator of signaling mechanisms of neutrophil–endothelium interaction during acute inflammation. Inhibition of PKCδ in human endothelial cells and neutrophils significantly reduced migration of neutrophils across TNF-α-activated endothelium in the presence of β2-integrin-dependent and β2-integrin-independent chemoattractants (Fig. 4). Inhibition of PKCδ significantly attenuated expression of E-selectin and ICAM-1 adhesion molecules on activated endothelium (Fig. 6), suggesting a specific and crucial role for PKCδ in regulation of specific adhesion molecules, both selectins and Igs, on neutrophil–endothelial interaction. Furthermore, we showed that shear rate and vascular geometry are significant regulators of the impact of PKCδ inhibition of neutrophil adhesion to endothelial cells (Figs. 7 and 8). These latter studies would not be possible with traditional in vitro fluidic models, such as the parallel plate flow chamber (Figs. 7 and 8). Moreover, we showed that the therapeutic effects of PKCδ inhibition during acute inflammation persist at least for 24 h (Fig. 9).
Adhesion molecules, selectins and Igs, are well known to be the first contact point of neutrophils for adhesion and later migration [39]. In previous studies using a static Transwell system, our group demonstrated a role for PKCδ in fMLP but not IL-8-mediated transmigration [7]. However, in the realistic physiologic environment of the bMFA used in these studies, we demonstrate that PKCδ inhibition results in a significant decrease in migration and adhesion in the presence of fMLP and IL-8, which was dependent on shear rate and spatial distribution. These studies underscore the importance of in vitro modeling under physiologically relevant conditions, accounting for 3D interactions, shear forces, and altered flow at bifurcations.
On endothelial cells, selectins (e.g., E-selectin) are responsible for neutrophil capture and rolling, whereas adhesion molecules ICAM-1, VCAM-1, PECAM-1, and junctional adhesion molecule C are critical regulators of neutrophil firm attachment and migration [40–43]. A number of the molecules involved in the neutrophil adhesion cascade are involved in sepsis-induced tissue damage. In patients who died from sepsis-induced Acute Respiratory Distress Syndrome, lung ICAM-1, VCAM-1, and E-selectin expression is up-regulated [44]. ICAM-1 and VCAM-1 levels are also elevated in animal models, and the blockage of ICAM-1 protects against lung injury [41, 45, 46]. In this study, we determined the impact of PKCδ inhibition on expression of E-selectin, ICAM-1, and VCAM-1 in endothelial cells cultured in the physiologically realistic, 3D cell culture environment of bMFA. We found that 4 h exposure to the PKCδ-i resulted in decreased expression of E-selectin and ICAM-1 but not VCAM-1. This decreased expression was associated with decreased adherence and migration through activated endothelial cells in response to fMLP and IL-8. Of interest fMLP-induced migration is β2-integrin dependent and requires ICAM-1 [47–49]. In contrast, IL-8-mediated migration is β2-integrin independent and does not require ICAM-1 or VCAM-1 [43, 50–52]. Thus, other adhesion molecules may also be regulated by PKCδ and further studies are required to ascertain the effect of PKCδ inhibition on adhesion molecules regulating β2-integrin-independent adhesion and migration.
In summary, we have used a novel bMFA to delineate the role of PKCδ in regulating human neutrophil–endothelium interaction. Our findings further indicate that PKCδ inhibition significantly reduces neutrophil interactions with the endothelium during acute inflammation and may offer an important approach for treating inflammatory disease. Our novel bMFA provides a rapid screening system for testing the specific response of novel therapeutics.
AUTHORSHIP
F.S., B.P., L.E.K., M.F.K., and S.D. provided the research design. F.S., T.Z., and D.J.K. conducted experiments and performed data analysis. F.S., Y.T., S.D., B.P., L.E.K., and M.F.K. wrote or contributed to the writing of the manuscript.
ACKNOWLEDGMENTS
This work was supported, in part, by U.S. National Institutes of Health (Grants HL111552 to L.E.K. and GM114359 to M.F.K. and L.E.K.) and Shriners Hospitals for Children (Grant 86400).
Glossary
- 3D
3-dimensional
- bMFA
biomimetic microfluidic assay
- CFD
computational fluid dynamics
- CFDA/SE
carboxyfluorescein diacetate succinimidyl ester
- CLP
cecal ligation and puncture
- MPO
myeloperoxidase
- PDMS
polydimethylsiloxane
- PKC
protein kinase C
- PKCδ-i
protein kinase C δ-TAT inhibitor
- TAT
transactivator of transcription
DISCLOSURES
L.E.K. is listed as an inventor on U.S. Patent #8,470,766, entitled “Novel Protein Kinase C Therapy for the Treatment of Acute Lung Injury,” which is assigned to Children's Hospital of Philadelphia and the University of Pennsylvania.
REFERENCES
- 1.Angus D. C., Wax R. S. (2001) Epidemiology of sepsis: an update. Crit. Care Med. 29 (7 Suppl), S109–S116. [DOI] [PubMed] [Google Scholar]
- 2.Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson E. K., Rubenstein A. R., Radin G. T., Wiener R. S., Walkey A. J. (2014) Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit. Care Med. 42, 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillipson M., Kubes P. (2011) The neutrophil in vascular inflammation. Nat. Med. 17, 1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick L. E., Standage S. W., Li H., Raj N. R., Korchak H. M., Wolfson M. R., Deutschman C. S. (2011) Protection against sepsis-induced lung injury by selective inhibition of protein kinase C-δ (δ-PKC). J. Leukoc. Biol. 89, 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mondrinos M. J., Zhang T., Sun S., Kennedy P. A., King D. J., Wolfson M. R., Knight L. C., Scalia R., Kilpatrick L. E. (2014) Pulmonary endothelial protein kinase C-delta (PKCδ) regulates neutrophil migration in acute lung inflammation. Am. J. Pathol. 184, 200–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mondrinos M. J., Knight L. C., Kennedy P. A., Wu J., Kauffman M., Baker S. T., Wolfson M. R., Kilpatrick L. E. (2015) Biodistribution and efficacy of targeted pulmonary delivery of a protein kinase C-δ inhibitory peptide: impact on indirect lung injury. J. Pharmacol. Exp. Ther. 355, 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van den Berg J. M., Mul F. P., Schippers E., Weening J. J., Roos D., Kuijpers T. W. (2001) Beta1 integrin activation on human neutrophils promotes beta2 integrin-mediated adhesion to fibronectin. Eur. J. Immunol. 31, 276–284. [DOI] [PubMed] [Google Scholar]
- 10.Mondrinos M. J., Kennedy P. A., Lyons M., Deutschman C. S., Kilpatrick L. E. (2013) Protein kinase C and acute respiratory distress syndrome. Shock 39, 467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kilpatrick L. E., Sun S., Li H., Vary T. C., Korchak H. M. (2010) Regulation of TNF-induced oxygen radical production in human neutrophils: role of delta-PKC. J. Leukoc. Biol. 87, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camp J. P., Capitano A. T. (2007) Induction of zone-like liver function gradients in HepG2 cells by varying culture medium height. Biotechnol. Prog. 23, 1485–1491. [DOI] [PubMed] [Google Scholar]
- 13.Cokelet G. R., Soave R., Pugh G., Rathbun L. (1993) Fabrication of in vitro microvascular blood flow systems by photolithography. Microvasc. Res. 46, 394–400. [DOI] [PubMed] [Google Scholar]
- 14.Frame M. D., Sarelius I. H. (1995) A system for culture of endothelial cells in 20–50-microns branching tubes. Microcirculation 2, 377–385. [DOI] [PubMed] [Google Scholar]
- 15.Shin J. W., Huggenberger R., Detmar M. (2008) Transcriptional profiling of VEGF-A and VEGF-C target genes in lymphatic endothelium reveals endothelial-specific molecule-1 as a novel mediator of lymphangiogenesis. Blood 112, 2318–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou X., Shinde Patil V. R., Dagia N. M., Smith L. A., Wargo M. J., Interliggi K. A., Lloyd C. M., Tees D. F. J., Walcheck B., Lawrence M. B., Goetz D. J. (2005) PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. Am. J. Physiol. Cell Physiol. 289, C415–C424. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakarpandian B., Shen M. C., Pant K., Kiani M. F. (2011) Microfluidic devices for modeling cell-cell and particle-cell interactions in the microvasculature. Microvasc. Res. 82, 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prabhakarpandian B., Wang Y., Rea-Ramsey A., Sundaram S., Kiani M. F., Pant K. (2011) Bifurcations: focal points of particle adhesion in microvascular networks. Microcirculation 18, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamberti G., Tang Y., Prabhakarpandian B., Wang Y., Pant K., Kiani M. F., Wang B. (2013) Adhesive interaction of functionalized particles and endothelium in idealized microvascular networks. Microvasc. Res. 89, 107–114. [DOI] [PubMed] [Google Scholar]
- 20.Lamberti G., Prabhakarpandian B., Garson C., Smith A., Pant K., Wang B., Kiani M. F. (2014) Bioinspired microfluidic assay for in vitro modeling of leukocyte-endothelium interactions. Anal. Chem. 86, 8344–8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosano J. M., Tousi N., Scott R. C., Krynska B., Rizzo V., Prabhakarpandian B., Pant K., Sundaram S., Kiani M. F. (2009) A physiologically realistic in vitro model of microvascular networks. Biomed. Microdevices 11, 1051–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamberti G., Soroush F., Smith A., Kiani M. F., Prabhakarpandian B., Pant K. (2015) Adhesion patterns in the microvasculature are dependent on bifurcation angle. Microvasc. Res. 99, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prabhakarpandian B., Pant K., Scott R. C., Pattillo C. B., Irimia D., Kiani M. F., Sundaram S. (2008) Synthetic microvascular networks for quantitative analysis of particle adhesion. Biomed. Microdevices 10, 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deosarkar S. P., Prabhakarpandian B., Wang B., Sheffield J. B., Krynska B., Kiani M. F. (2015) A novel dynamic neonatal blood-brain barrier on a chip. PLoS One 10, e0142725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L., Hahn H., Wu G., Chen C. H., Liron T., Schechtman D., Cavallaro G., Banci L., Guo Y., Bolli R., Dorn G. W. II, Mochly-Rosen D. (2001) Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. USA 98, 11114–11119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiani M. F., Yuan H., Chen X., Smith L., Gaber M. W., Goetz D. J. (2002) Targeting microparticles to select tissue via radiation-induced upregulation of endothelial cell adhesion molecules. Pharm. Res. 19, 1317–1322. [DOI] [PubMed] [Google Scholar]
- 27.Rittirsch D., Huber-Lang M. S., Flierl M. A., Ward P. A. (2009) Immunodesign of experimental sepsis by cecal ligation and puncture. Nat. Protoc. 4, 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamim C. F., Hogaboam C. M., Kunkel S. L. (2004) The chronic consequences of severe sepsis. J. Leukoc. Biol. 75, 408–412. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard W. J., Choudhry M., Schwacha M. G., Kerby J. D., Rue L. W. III, Bland K. I., Chaudry I. H. (2005) Cecal ligation and puncture. Shock 24 (Suppl 1), 52–57. [DOI] [PubMed] [Google Scholar]
- 30.Lu H., Koo L. Y., Wang W. M., Lauffenburger D. A., Griffith L. G., Jensen K. F. (2004) Microfluidic shear devices for quantitative analysis of cell adhesion. Anal. Chem. 76, 5257–5264. [DOI] [PubMed] [Google Scholar]
- 31.Sundd P., Gutierrez E., Koltsova E. K., Kuwano Y., Fukuda S., Pospieszalska M. K., Groisman A., Ley K. (2012) ‘Slings’ enable neutrophil rolling at high shear. Nature 488, 399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sundd P., Pospieszalska M. K., Ley K. (2013) Neutrophil rolling at high shear: flattening, catch bond behavior, tethers and slings. Mol. Immunol. 55, 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiani M. F., Pries A. R., Hsu L. L., Sarelius I. H., Cokelet G. R. (1994) Fluctuations in microvascular blood flow parameters caused by hemodynamic mechanisms. Am. J. Physiol. 266, H1822–H1828. [DOI] [PubMed] [Google Scholar]
- 34.Von Andrian U. H., Hansell P., Chambers J. D., Berger E. M., Torres Filho I., Butcher E. C., Arfors K. E. (1992) L-Selectin function is required for beta 2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am. J. Physiol. 263, H1034–H1044. [DOI] [PubMed] [Google Scholar]
- 35.Tousi N., Wang B., Pant K., Kiani M. F., Prabhakarpandian B. (2010) Preferential adhesion of leukocytes near bifurcations is endothelium independent. Microvasc. Res. 80, 384–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossaint J., Nadler J. L., Ley K., Zarbock A. (2012) Eliminating or blocking 12/15-lipoxygenase reduces neutrophil recruitment in mouse models of acute lung injury. Crit. Care 16, R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lokuta M. A., Huttenlocher A. (2005) TNF-alpha promotes a stop signal that inhibits neutrophil polarization and migration via a p38 MAPK pathway. J. Leukoc. Biol. 78, 210–219. [DOI] [PubMed] [Google Scholar]
- 38.Inflammation and Host Response to Injury, Large Scale Collaborative Research Program (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689. [DOI] [PubMed] [Google Scholar]
- 40.Chavakis T., Keiper T., Matz-Westphal R., Hersemeyer K., Sachs U. J., Nawroth P. P., Preissner K. T., Santoso S. (2004) The junctional adhesion molecule-C promotes neutrophil transendothelial migration in vitro and in vivo. J. Biol. Chem. 279, 55602–55608. [DOI] [PubMed] [Google Scholar]
- 41.Reutershan J., Ley K. (2004) Bench-to-bedside review: acute respiratory distress syndrome—how neutrophils migrate into the lung. Crit. Care 8, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reutershan J., Stockton R., Zarbock A., Sullivan G. W., Chang D., Scott D., Schwartz M. A., Ley K. (2007) Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am. J. Respir. Crit. Care Med. 175, 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doerschuk C. M., Tasaka S., Wang Q. (2000) CD11/CD18-dependent and -independent neutrophil emigration in the lungs: how do neutrophils know which route to take? Am. J. Respir. Cell Mol. Biol. 23, 133–136. [DOI] [PubMed] [Google Scholar]
- 44.Müller A. M., Cronen C., Müller K.-M., Kirkpatrick C. J. (2002) Heterogeneous expression of cell adhesion molecules by endothelial cells in ARDS. J. Pathol. 198, 270–275. [DOI] [PubMed] [Google Scholar]
- 45.Guo R.-F., Riedemann N. C., Laudes I. J., Sarma V. J., Kunkel R. G., Dilley K. A., Paulauskis J. D., Ward P. A. (2002) Altered neutrophil trafficking during sepsis. J. Immunol. 169, 307–314. [DOI] [PubMed] [Google Scholar]
- 46.Laudes I. J., Guo R. F., Riedemann N. C., Speyer C., Craig R., Sarma J. V., Ward P. A. (2004) Disturbed homeostasis of lung intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 during sepsis. Am. J. Pathol. 164, 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGettrick H. M., Lord J. M., Wang K. Q., Rainger G. E., Buckley C. D., Nash G. B. (2006) Chemokine- and adhesion-dependent survival of neutrophils after transmigration through cytokine-stimulated endothelium. J. Leukoc. Biol. 79, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yan S. R., Sapru K., Issekutz A. C. (2004) The CD11/CD18 (beta2) integrins modulate neutrophil caspase activation and survival following TNF-alpha or endotoxin induced transendothelial migration. Immunol. Cell Biol. 82, 435–446. [DOI] [PubMed] [Google Scholar]
- 49.Lee W. L., Downey G. P. (2001) Neutrophil activation and acute lung injury. Curr. Opin. Crit. Care 7, 1–7. [DOI] [PubMed] [Google Scholar]
- 50.Mackarel A. J., Russell K. J., Ryan C. M., Hislip S. J., Rendall J. C., FitzGerald M. X., O’Connor C. M. (2001) CD18 dependency of transendothelial neutrophil migration differs during acute pulmonary inflammation. J. Immunol. 167, 2839–2846. [DOI] [PubMed] [Google Scholar]
- 51.Doerschuk C. M. (2001) Mechanisms of leukocyte sequestration in inflamed lungs. Microcirculation 8, 71–88. [PubMed] [Google Scholar]
- 52.Mackarel A. J., Russell K. J., Brady C. S., FitzGerald M. X., O’Connor C. M. (2000) Interleukin-8 and leukotriene-B(4), but not formylmethionyl leucylphenylalanine, stimulate CD18-independent migration of neutrophils across human pulmonary endothelial cells in vitro. Am. J. Respir. Cell Mol. Biol. 23, 154–161. [DOI] [PubMed] [Google Scholar]