Sepsis activates memory CD8 T cells independent of cognate antigen, and likely through cytokine-mediated signaling; the loss of unactivated cells requires direct TCR:MHC interactions.
Keywords: lymphocyte activation, TCR, bystander activation, cytokine
Abstract
CD8 T cell loss and dysfunction have been implicated in the increased susceptibility to opportunistic infections during the later immunosuppressive phase of sepsis, but CD8 T cell activation and attrition in early sepsis remain incompletely understood. With the use of a CLP model, we assessed CD8 T cell activation at 5 consecutive time points and found that activation after sepsis results in a distinct phenotype (CD69+CD25intCD62LHI) independent of cognate antigen recognition and TCR engagement and likely through bystander-mediated cytokine effects. Additionally, we observed that sepsis concurrently results in the preferential depletion of a subset of memory-phenotype CD8 T cells that remain “unactivated” (i.e., fail to up-regulate activation markers) by apoptosis. Unactivated CD44HI OT-I cells were spared from sepsis-induced attrition, as were memory-phenotype CD8 T cells of mice treated with anti-LFA-1 mAb, 1 h after CLP. Perhaps most importantly, we demonstrate that attrition of memory phenotype cells may have a pathologic significance, as elevated IL-6 levels were associated with decreased numbers of memory-phenotype CD8 T cells in septic mice, and preservation of this subset after administration of anti-LFA-1 mAb conferred improved survival at 7 d. Taken together, these data identify potentially modifiable responses of memory-phenotype CD8 T cells in early sepsis and may be particularly important in the application of immunomodulatory therapies in sepsis.
Introduction
Sepsis is the leading cause of morbidity and mortality in Intensive Care Units in the United States and has an incidence estimated to be as high as 3 million cases/yr [1, 2]. Resuscitation with i.v. fluids and antibiotics remains the mainstays of therapy, and despite advances in supportive care, most current sepsis trials still cite a 20–30% all-cause mortality rate [3]. Recently, investigators have turned to modulation of the adaptive immune response as a possible therapeutic strategy in sepsis. Although the pathophysiology of sepsis is complex and incompletely understood, it is now recognized that lymphoid cell loss and dysfunction are critical processes in the high morbidity and mortality [4, 5]. Additionally, recent reports demonstrating increased T cell expression of coinhibitory molecules, a shift toward an immunosuppressive cytokine profile, and decreased TCR heterogeneity in patients with sepsis have implicated T cell exhaustion and anergy as critical mediators of sepsis-induced immunosuppression [6–8]. Whereas these studies highlight the potential role of novel therapeutics to enhance immune cell function, particularly with regards to secondary infection, CD8 T cell activation in the primary response to sepsis remains poorly defined.
In canonical models of CD8 T cell activation, infection by MHC class I-restricted pathogens results in an activation program that is initiated upon T cell recognition of its cognate antigen [9]. LFA-1, a β2 integrin expressed by multiple leukocyte subsets, including CD8 T cells, assists in stabilizing the physical interaction between T cells and APCs and results in enhanced TCR:MHC signal transduction [10, 11]. Memory CD8 T cells have also been shown to acquire cytotoxic function without the involvement of a strong agonist TCR signal; in this model, upon detection of microbial-derived danger signals (pathogen-associated molecular patterns and danger-associated molecular patterns), innate immune cells provide inflammatory cytokines that directly mediate memory CD8 T cell activation [12–14]. Studies suggest that 1 particular subset of CD8 T cells, memory-phenotype cells or “virtual memory cells,” named because they arise from homeostatic mechanisms rather than true exposure to cognate antigen, may serve as the primary executors of these antigen-independent responses [15].
In the current study, we explore the responses of these naturally occurring memory-phenotype (CD44HI CD11aHI) CD8 T cells in early sepsis using a moderate-severity model of CLP. We show that sepsis results in phenotypic changes in memory-phenotype polyclonal CD8 T cells, as well as TCR-transgenic OT-I cells, consistent with cytokine-driven bystander activation. Furthermore, we reveal that activation is accompanied by apoptosis of memory-phenotype cells that fail to up-regulate activation markers. Importantly, administration of αLFA-1 mAb after CLP abrogates this loss and confers improved 7 d survival without dampening the activation response or significantly altering the cytokine environment.
MATERIALS AND METHODS
Ethics statement
All experiments were performed in accordance with the U.S. National Institutes of Health Guidelines for the Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at Emory University School of Medicine (Protocol 2001875-082815BN).
Mice
Adult, male 9- to 13-wk-old B6 and RAG1 knockout (B6.129S7-Rag1tm1Mom/J) mice (used for survival studies) were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). OT-I transgenic mice, purchased from Taconic Farms (Hudson, NY, USA), were bred to a Thy1.1+ background at Emory University (Atlanta, GA, USA). mOVA mice (B6 background, H-2b [16]) were a gift from Marc Jenkins (University of Minnesota, Minneapolis, MN, USA). All mice were maintained in the same facilities and allowed to acclimate at least 1 wk before surgery. Single-gender mice were used in this study to avoid the confounding impact of the estrus cycle-related hormonal influence on immunologic responses in female septic mice and to understand the baseline physiologic effects in sepsis [17].
CLP
Sepsis was induced using CLP, a murine model of polymicrobial sepsis. Injury was titrated to achieve a 30–50%, 14 d mortality to mimic the clinical scenario of moderate-severe sepsis and performed as described in previously published protocols [18]. In brief, B6 mice were anesthetized using isoflurane and underwent laparotomy; the cecum was exteriorized, ligated distal to the ileocecal valve, and punctured twice with a 25-gauge needle. Sham-operated animals underwent laparotomy and exteriorization of the cecum only. All mice received a 1 ml s.c. injection of saline postoperatively to replace insensible fluid losses. For experiments evaluating LFA-1 blockade, mice designated to the CLP+ αLFA-1 group received s.c. injections of (250 μg/1 ml dose) 10 mg/kg αLFA-1 antibody (m17/4; Bio X Cell, West Lebanon, NH, USA) in 1 ml saline, 1 h after closure. Mice were euthanized by CO2 asphyxiation at designated time points. For survival studies, mice were observed daily for 7 d after surgery and subsequently euthanized.
Immunophenotyping by flow cytometry
Groups of mice were euthanized at the following time points after surgery: 6, 24, 48, 72, and 120 h. An unmanipulated mouse was also euthanized at each time point for use as an internal control. Spleens were removed aseptically, placed in a 10 ml culture dish containing 5 ml PBS (Mediatech, Corning Life Sciences, Tewksbury, MA, USA), and disrupted using the rubber end of a sterile 3 ml syringe (BD Falcon; BD Biosciences, San Jose, CA, USA). Cells were passed through a 70 μm mesh filter (BD Falcon), and single cell suspensions were centrifuged and resuspended in 10 ml PBS. Each sample was counted by Trypan blue exclusion staining, and suspensions adjusted to a concentration of 1 × 107 cells/ml. Aliquots containing 200 μl were apportioned into FACS tubes, incubated in 50 μl HYL solution (Thermo Fisher Scientific, Waltham, MA, USA) for 5 min, and then centrifuged and resuspended in 100 μl FACS buffer (PBS + 2% FCS + 0.1% sodium azide). Samples were preincubated with anti-CD16/CD32 mAb (BD Biosciences) for 15 min, followed by 30 min incubation with the following fluorochrome-conjugated mAb: Gr-1 (Rb6-8C5; eBioscience, San Diego, CA, USA); CD3 (17A2; BioLegend, San Diego, CA, USA); and CD19 (1D3), CD4 (RM4.5), CD44 (IM7), CD8 (53-6.7), CD62L (MEL-14), CD69 ([1H].2F3), CD11a (2D7), CD25 (7D4), B220 (53-6.7), and 7 aminoactinomycin D (all from BD Biosciences). Accucheck Counting beads (Thermo Fisher Scientific) were added before data collection, per the manufacturer’s protocols, and samples were analyzed on a BD LSR II cytometer (BD Biosciences). Flow cytometric data were analyzed using FlowJo Vx software (Tree Star, Ashland, OR, USA). Absolute cell counts (per sample) were determined by factoring in the absolute number of cells per spleen enumerated at the time of harvest, according to previously published protocols [19].
Annexin V staining
Sham and CLP groups were euthanized at 24 h after surgery, and spleens were processed as detailed above, with the exception of HYL incubation. After preincubation with anti-CD16/CD32 mAb, single-cell suspensions were stained for the following extracellular antibodies: CD3 (17A2), CD4 (RM4-5), CD8 (53-6.7), and CD44 (IM7; all from BD Biosciences). Subsequently, the Annexin V-FITC kit (Thermo Fisher Scientific) was used, per the manufacturer’s protocols, to quantify apoptosis by flow cytometry.
T cell adoptive transfer experiments
For adoptive transfers of TCR-transgenic T cells, spleens and mesenteric lymph nodes isolated from Thy1.1+ OT-I mice were processed and stained with mAb for CD8 (Thermo Fisher Scientific), Thy1.1, Vα2, and Vβ5 (BD Biosciences) for quantification of OT-I cell number by flow cytometric analysis. Appropriate volumes were then resuspended in PBS and adjusted to a concentration of 2.0 × 106 Thy1.1+ OT-I cells/ml for injection of 0.5 ml i.v. into B6 recipients, performed 24 h before CLP or sham operations.
Cytokine quantification
Sham, CLP, and CLP + αLFA-1 mice were euthanized at 24 h following surgery, and whole blood was aspirated via cardiac puncture. Following 30 min of incubation, samples were centrifuged (1000 g × 10 min), and supernatant (serum) was apportioned into 100 μl aliquots and stored at −20°C until use. Serum cytokines were evaluated using the Bio-Plex suspension array system and Bio-Plex Mouse Cytokine 23-Plex Panel, according to the manufacturer’s instructions (both Bio-Rad Laboratories, Marnes-La-Coquette, France). Cytokine assays included antibodies for the following: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-17, eotaxin, G-CSF, GM-CSF, IFN-γ, KC, MCP-1, MIP-1α, MIP-1β, RANTES, and TNF-α. Results were analyzed using Bio-Plex Manager 3.0 software with 5 parameter logistic (5PL) curve fitting for determination of serum concentrations (pg/ml) of individual cytokines per sample.
Statistical analysis
Data were analyzed using the statistical software Prism V; all data are reported as means ± sem. For comparison between sham and CLP groups at individual time points, Student’s t test was used after confirmation of Gaussian distribution. Intragroup comparison of data collected across multiple time points was analyzed using regular two-way ANOVA and Sidak’s test to correct for multiple comparisons. For comparison of cytokine concentrations across 3 groups, one-way ANOVA and Tukey’s post-test were used. Survival studies were analyzed by χ2 analysis. For all data, a confidence interval of ≥95% was used to determine significance (P ≤ 0.05).
RESULTS
The first 48 h after sepsis is characterized by attrition of memory-phenotype (CD44HICD11aHI) CD8 T cells
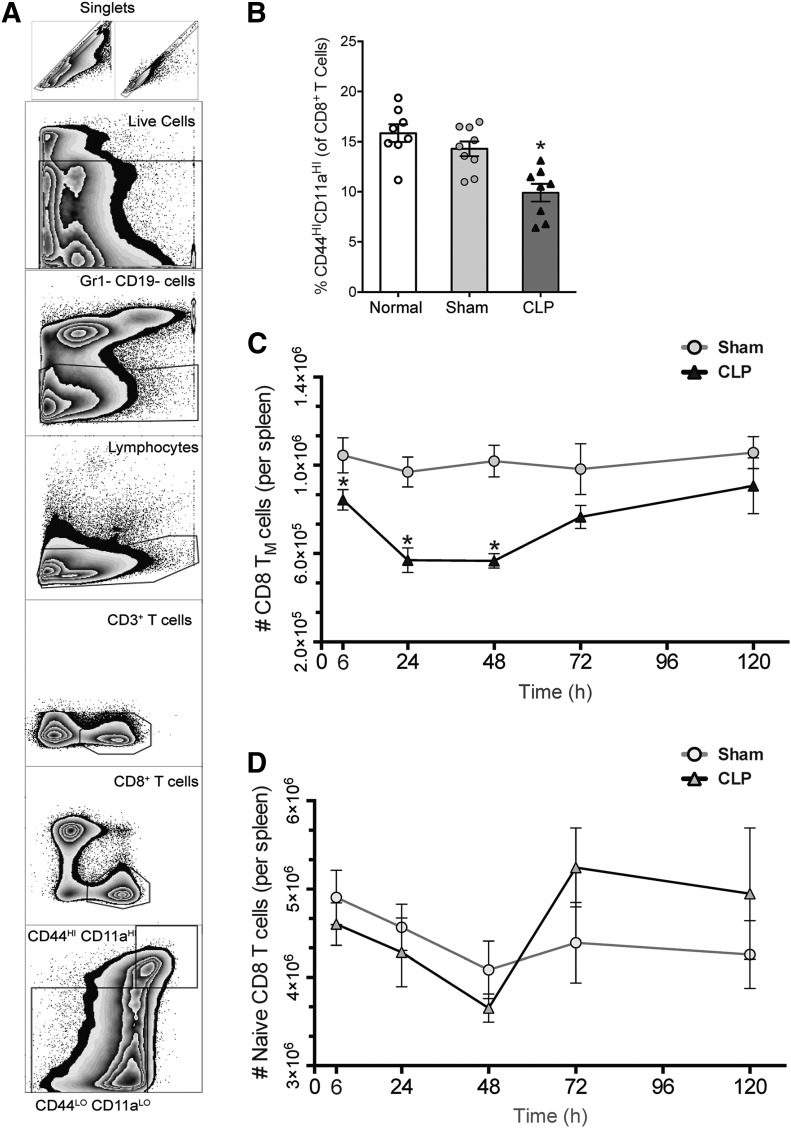
To evaluate the effect of sepsis on memory-phenotype CD8 T cells, splenocytes from WT B6 mice that had undergone either CLP or sham operations were analyzed by flow cytometry. CD3+ CD8a+ T cells were then subdivided into memory-phenotype (CD44HICD11aHI) and naïve (CD44LOCD11aLO) subsets (see Fig. 1A for gating strategy). At all time points evaluated, the frequency of CD44HICD11aHI cells in sham-operated mice ranged from 14 to 20%, similar to that of unmanipulated mice and consistent with reports from other studies of mice raised in pathogen-free conditions [15, 20]. However, septic mice demonstrated significantly lower frequencies of CD44HICD11aHI cells, with the most pronounced difference occurring at 24 h (Fig. 1B). To distinguish between the absolute reduction of CD44HICD11aHI cells rather than a proportional increase in the CD44LOCD11aLO (naïve) population, absolute cell counts per spleen were calculated. Septic mice had significantly fewer CD44HICD11aHI cells as early as 6 h compared with sham; this reduction was most pronounced at 24 h and was sustained until 48 h (Fig. 1C). By 120 h, the number of CD44HICD11aHI cells was equal between groups, suggesting repletion of this subset. In contrast, numbers of CD44LOCD11aLO cells remained similar between sham and sepsis at all time points and in fact, underwent expansion at 48 and 72 h in sepsis (Fig. 1D). To confirm that our gating strategy was not artificially representing our results by failing to reflect potential increases in the expression of CD44 and CD11a, known to accompany antigen-specific activation [21], CD8 T cell expression of CD44 and CD11a was evaluated by MFI. CD8 T cells from septic mice demonstrated significantly lower expression of CD44 at 6, 24, 48, and 72 h and of CD11a at 24, 48, and 72 h (data not shown), corroborating the results of our quantitative analysis.
Figure 1. Sepsis results in attrition of memory-phenotype (CD44HICD11aHI) CD8 T cells from 6 to 72 h after onset.
(A) Gating strategy for identification of CD44HICD11aHI and CD44LOCD11aLO CD8 T cells. (B) Percentages of memory-phenotype (CD44HICD11aHI) CD8 T cells are significantly reduced at 24 h after sepsis relative to sham (14.29% in sham vs. 9.91% in CLP; *P < 0.05), whereas normal (unmanipulated) and sham mice are similar. (C) Septic mice demonstrate decreased numbers of CD44HICD11aHI CD8 T cells at 6, 24, and 48 h (*P < 0.05 for all) compared with sham mice. The maximum cell loss in the spleen occurred at 24 h, at which point, the population of memory-phenotype (TM) cells was reduced by 41%. Thereafter, numbers of CD44HICD11aHI CD8 T cells began to recover, with reductions of 38% at 48 h and 22% at 72 h; however, the difference at the latter time point was not statistically significant. (D) Numbers of naive (CD44LOCD11aLO) CD8 T cells are similar between sham and CLP groups at all time points, and in fact, accompanying the recovery of memory-phenotype cells between 48 and 72 h, numbers of CD44LOCD11aLO CD8 T cells also underwent a significant expansion in sepsis. Results represent independent replicate experiments (n = 5–8/group) and are presented as means ± sem.
Despite attrition of the memory compartment, numbers of activated memory-phenotype CD8 T cells increase as early as 6 h after sepsis
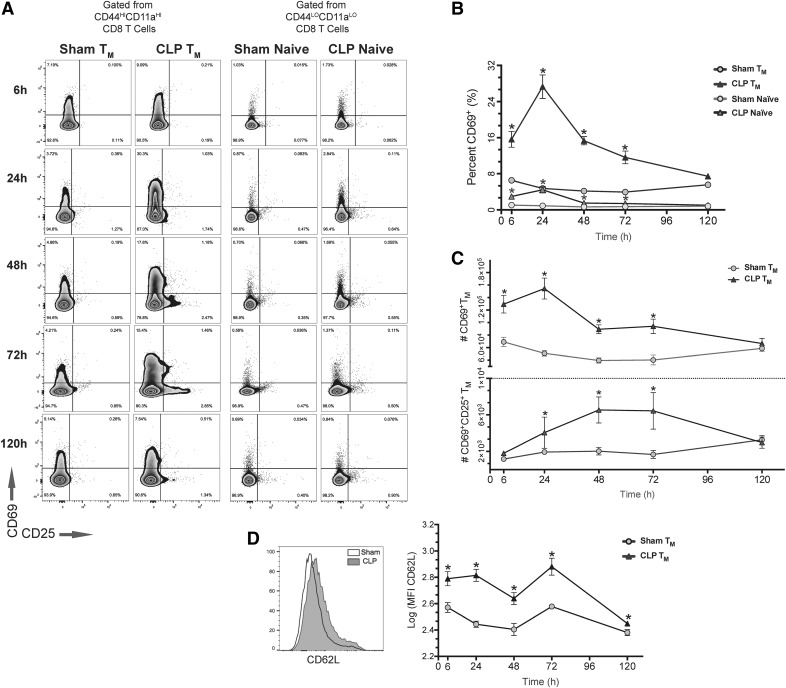
Activated T cells can be identified by characteristic phenotypic changes: increased CD8 T cell expression of CD69 has been shown early after recruitment in antigen-specific and cytokine-driven activation responses [22], whereas pronounced up-regulation of CD25 (IL-2Rα) has been shown only after sustained APC contact with T cells after recognition of cognate antigen [23, 24]. Thus, by quantifying the populations of CD69+ and CD69+CD25+ memory-phenotype (CD44HICD11aHI) CD8 T cells, we were able to assess for the presence of activation and gain insight into the underlying mechanism (see Fig. 2A for gating strategy). As early as 6 h after sepsis, 16% of CD44HICD11aHI cells from septic mice displayed a CD69+ phenotype compared with only 6.5% in sham. This proportion remained greater in septic mice at 24, 48, and 72 h compared with sham. Although analysis of naïve (CD44LOCD11aLO) CD8 T cells revealed similar kinetics of CD69+ up-regulation after sepsis, memory-phenotype cells demonstrated a heightened response at all time points measured (Fig. 2B). Absolute cell counts of CD69+ cells paralleled the results of our qualitative analysis, and despite the reduction in number of memory-phenotype cells, numbers of CD69+ CD44HICD11aHI cells increased significantly compared with sham (Fig. 2C, upper). In fact, coinciding with the height of sepsis-induced attrition, recruitment was greatest at 24 h when septic mice demonstrated a 3-fold increase in number of CD69+ cells. From 24 to 72 h, septic mice also demonstrated a significant increase in numbers of CD69+CD25+ CD44HICD11aHI CD8 T cells compared with sham (Fig. 2C, lower). Likewise, numbers of CD69+ naïve CD8 T cells increased after sepsis from 6 to 72 h compared with sham, whereas numbers of further-differentiated CD69+CD25+ naïve CD8 T cells increased significantly only at 72 h (data not shown).
Figure 2. Despite attrition of the memory compartment, numbers of activated memory-phenotype cells increase as early as 6 h after sepsis.
(A) Representative flow cytometry plots demonstrating expression of CD69 and CD25 at all time points after gating on memory-phenotype (CD44HICD11aHI, left) or naive (CD44HICD11aHI, right) CD8 T cells from sham and septic mice. (B) A significantly greater proportion of both memory-phenotype and naive CD8 T cells is CD69+ at 6–72 h after sepsis compared with sham (*P < 0.05 for all, compared with sham). Additionally, memory-phenotype cells demonstrate a more robust CD69 response relative to their naive counterparts at all time points after CLP (P < 0.05). (C, Upper) Despite the decrease in CD44HICD11aHI CD8 T cells after sepsis, numbers of CD69+ cells increased significantly from 6 to 24 h after CLP compared with sham and remained greater through 72 h, a maximum expansion at 24 h (*P < 0.05 for all). (Lower) From 24 to 72 h after CLP, numbers of CD69+CD25+ CD44HICD11aHI CD8 T cells increased relative to sham (*P < 0.05). (D) CD44HICD11aHI CD8 T cells from septic mice had significantly greater expression of CD62L compared with sham at all time points evaluated. Results represent independent replicate experiments (n = 5–8/group) and are presented as means ± sem.
We also evaluated memory-phenotype CD8 T cells for expression of CD62L, a homing marker that distinguishes central (CD62LHI) from effector (CD62LLO) memory CD8 T cells [25]. Whereas a preference for a CD62LLO phenotype is a well-known characteristic of CD8 T cells activated after antigen recognition, studies have shown that memory CD8 T cells activated via cytokine-driven bystander effects preferentially differentiate into a CD62LHI phenotype [20, 26]. Analysis of CD62L expression revealed that CD44HICD11aHI CD8 cells from septic mice had significantly greater expression of CD62L at all time points compared with sham (Fig. 2D).
Memory cells that fail to up-regulate markers of activation are preferentially depleted early in sepsis
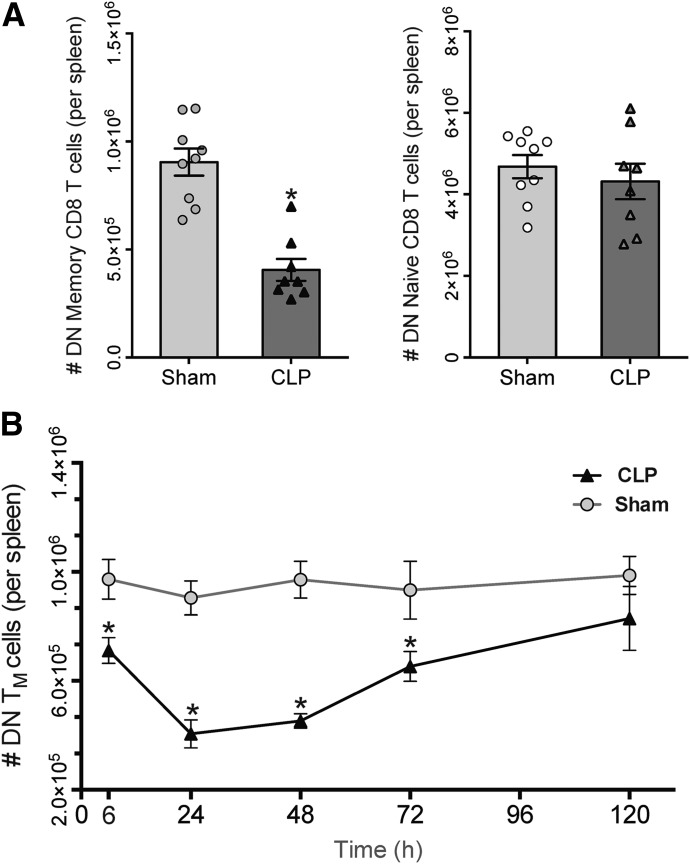
The increasing numbers of CD69+ and CD69+CD25+ memory-phenotype CD8 T cells in the face of sepsis-induced attrition of the memory compartment suggested that CD44HICD11aHI cells that had up-regulated either activation marker were not subject to depletion. These findings prompted us to consider that “unactivated” memory-phenotype cells—those that failed to increase CD69 or CD25 expression after CLP—were preferentially lost. Numbers of unactivated, CD69−CD25− DN CD44HICD11aHI and naïve CD8 T cells were quantified at 24 h, the height of cell loss. Whereas septic mice demonstrated a profound reduction in numbers of DN CD44HICD11aHI cells compared with sham, numbers of DN naïve CD8 T cells remained similar between groups (Fig. 3A). Although we anticipated that conversion of memory-phenotype cells to an activated phenotype would yield a smaller population of unactivated cells after sepsis, we found that phenotypic conversion alone did not account for the 55% reduction in cell number that we observed. Furthermore, analysis of other time points revealed a significantly lower number of DN CD44HICD11aHI cells in septic mice at 6–72 h (Fig. 3B). At each time point, the difference in the number of DN CD44HICD11aHI cells between sham and CLP groups was approximately equal to the difference we had observed in the size of the overall memory compartment. These data indicated that the preferential loss of unactivated cells was responsible for the reduction in numbers of memory-phenotype cells after sepsis.
Figure 3. Memory-phenotype CD8 T cells that fail to express CD69 or CD25 are preferentially depleted early in sepsis.
(A) At the height of CD44HI CD8 T cell attrition (24 h), CLP mice demonstrate a significant decrease in the number of DN memory CD8 T cells (left; *P < 0.05), whereas numbers of DN naïve CD8 T cells remain unchanged between sham and sepsis (right). (B) Numbers of DN CD44HI CD8 T cells are decreased at 6, 24, 48, and 72 h after CLP, mirroring our observations of total numbers of CD44HI CD8 T cells between sham and sepsis. Results represent independent replicate experiments (n = 5–8/group) and are presented as means ± sem. (*P < 0.05 for all, compared with sham).
Apoptosis accounts for the loss of memory-phenotype cells
We next sought to delineate the cause of the rapid disappearance of CD44HICD11aHI CD8 T cells from the spleens of septic mice. As cell loss can either be the result of egress from the spleen or cell death, we evaluated the population of memory-phenotype CD8 T cells in 2 other compartments at 24 h after surgery: the blood and peritoneal fluid. In the blood, we found a 2-fold reduction in numbers of circulating CD44HICD11aHI cells after CLP (Fig. 4A), indicating that entry into the circulation is not the cause of reduced numbers of splenic memory-phenotype CD8 T cells. Analysis of peritoneal fluid of both groups also demonstrated that septic mice contained significantly fewer memory-phenotype cells (data not shown).
Figure 4. Apoptosis causes the loss of memory CD8 T cells early in sepsis.
(A) Blood taken 24 h after surgery showed significantly reduced numbers of CD44HICD11aHI CD8 T cells in septic mice compared to sham (*P < 0.005). (B) Representative histograms demonstrating Annexin expression after gating on CD44HI CD8 T cells from sham (upper) and septic mice (lower). (C) A significantly greater proportion of CD44HI CD8 T cells from septic mice was reactive to Annexin relative to sham mice, whereas Annexin reactivity was similar between CD44LO CD8 T cells from both groups (*P < 0.05). Results represent independent replicate experiments (n = 4–6/group) and are presented as means ± sem.
As trafficking was less likely to be the cause of the reduction in number of memory-phenotype cells, we next investigated cell death. Lymphocyte apoptosis has been well described in sepsis literature, but there is little data that specifically address the process in memory versus naïve CD8 T cells early after sepsis. With the use of Annexin V, which captures the externalization of phosphatidylserine during midapoptosis [27], we evaluated apoptosis in memory-phenotype and naive CD8 T cells. Notably, although prior experiments used CD11a in conjunction with CD44 to delineate memory-phenotype cells, reanalysis of these data using only a CD44HI and CD44LO gates yielded the same results; thus, to minimize unnecessary spillover, in the subsequent experiments, memory CD8 T cells were identified by CD44 expression alone. Memory-phenotype cells of septic mice exhibited a greater increase in Annexin reactivity relative to CD44HI cells of sham mice and to CD44LO cells of septic mice (Fig. 4C). Together, these data support programmed cell death as the cause of the attrition of memory-phenotype CD8 T cells early in sepsis.
Cognate antigen recognition is not required for the early phenotypic activation of memory CD8 T cells observed in sepsis but is implicated in the deletion of unactivated cells
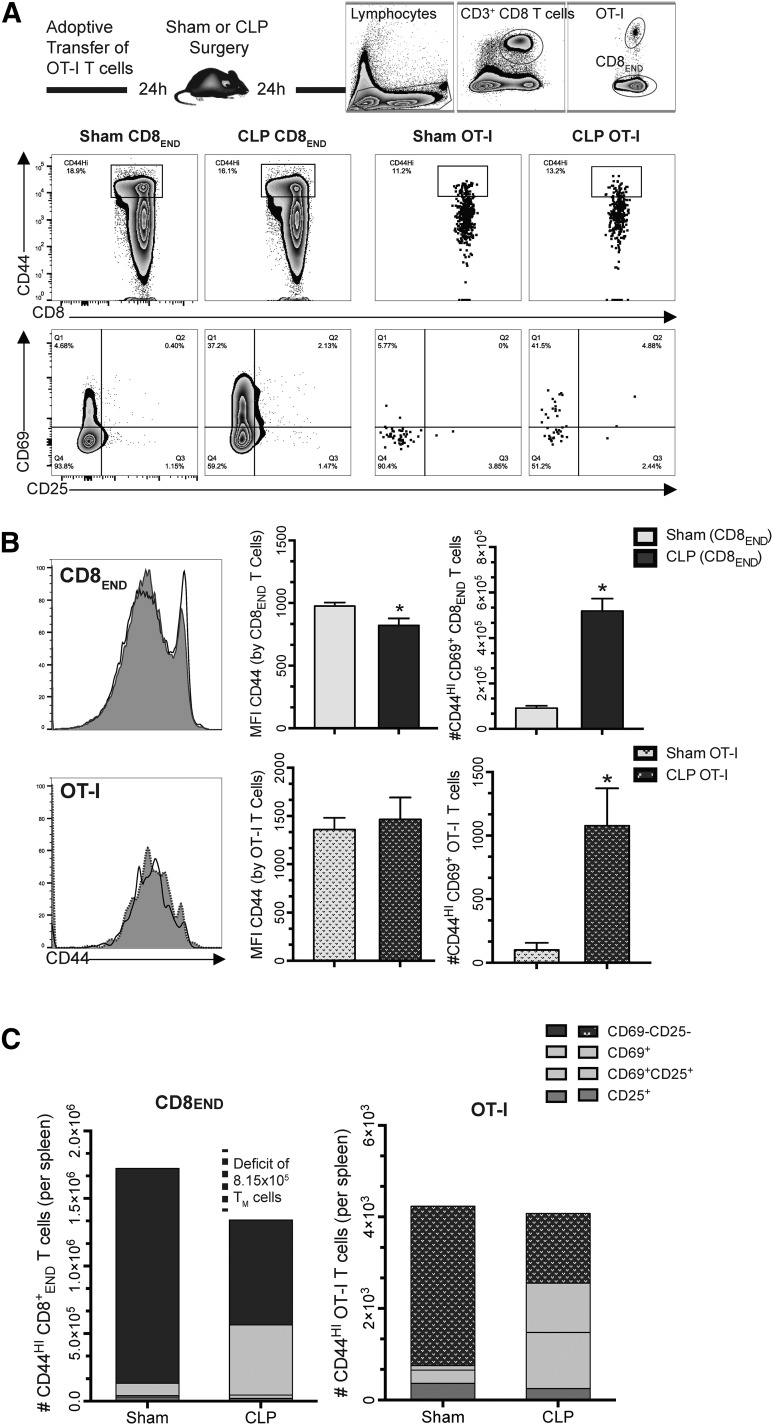
To determine if cognate antigen recognition is required for acquisition of an activated phenotype after sepsis, we assessed the activation responses of TCR transgenic T cells specific for a class I-restricted epitope, irrelevant in our CLP model of sepsis after adoptive transfer [28, 29]. Groups of B6 mice received 106 OT-I T cells followed by sham or CLP operations. Transferred memory-phenotype OT-I cells and endogenous polyclonal memory-phenotype CD8 T cells from recipient mice were compared in the setting of sepsis (see Fig. 5A for gating strategy).
Figure 5. Cognate antigen is not required for activation of memory phenotype CD8 T cells after sepsis, but TCR-mediated signaling is implicated in the attrition of unactivated cells.
For adoptive transfer experiments, WT B6 mice were transferred 106 OT-I mOVA-specific cells, 1 d before undergoing sham or CLP operation; 24 h later, spleens were harvested, and responses of antigen-inexperienced CD44HI OT-I cells and CD44HI endogenous CD8 T cells (CD8END) were compared between sham and septic mice (A–D). (A, Upper) Representative flow cytometry plots demonstrating gating of OT-I and CD8END populations. (Lower) CD44HI cells were identified from their respective populations (upper, CD44 × CD8a) and subsequently evaluated for expression of CD69 and CD25 to determine numbers of activated and unactivated cells (lower, CD69 × CD25). (B) Endogenous CD8 T cells (upper) and OT-I T cells (lower) were evaluated for expression of CD44 and for numbers of activated (CD69+) memory cells (*P < 0.005 for both). (C) Full columns represent total population of CD44HI cells; the absolute decrease in CD44HI CD8END cell numbers after sepsis is a result of a selective loss of the CD69−CD25− subset. However, numbers of CD44HI OT-I T cells remain unchanged between groups, suggesting that phenotypic conversion accounts for the observed reduction in CD69−CD25− CD44HI OT-I cells after sepsis. Results represent independent replicate experiments (n = 6–8/group) and are presented as means ± sem.
Consistent with the findings presented above, septic mice demonstrated significantly greater numbers of endogenous CD69+ CD44HI CD8 T cells. Phenotypic activation of antigen-inexperienced CD44HI OT-I T cells after sepsis paralleled the endogenous response: a CD69+ phenotype was demonstrated by 40% of endogenous CD44HI cells and 32% of CD44HI OT-I cells (Fig. 5B). Furthermore, numbers of CD69+ CD44HI OT-I T cells increased 10-fold after sepsis, indicating that CD69 up-regulation is not dependent on cognate antigen recognition and subsequent TCR-MHC signal transduction. Frequencies and numbers of CD69+CD25+ endogenous CD44HI CD8 T cells and CD69+CD25+ CD44HI OT-I T cells demonstrated a trend toward an increase in sepsis compared with sham, but this difference was not significant in either population (data not shown). To discern whether TCR specificity is implicated in sepsis-induced attrition, we investigated whether CD44HI OT-I cells were equally susceptible to depletion as their endogenous counterparts. Analysis of total numbers of endogenous CD44HI CD8 T cells again revealed a significant loss of cells after sepsis; however, the size of the CD44HI OT-I population was unchanged between septic and sham mice (Fig. 5C). Quantification of numbers of activated and unactivated CD44HI cells of endogenous and OT-I populations revealed that cell numbers of endogenous DN CD44HI CD8 T cells and DN CD44HI OT-I cells were significantly lower in sepsis compared with sham (Fig. 5C). However, unlike the absolute loss of DN CD44HI cells in the endogenous population, the loss of DN CD44HI cells was approximately equivalent to the gain in CD69+ CD44HI OT-I cells, suggesting that phenotypic conversion to an activated phenotype was responsible for the observed decrease. Additionally, the MFI of CD44 by OT-I T cells was unchanged between sham and sepsis, providing further evidence that CD44HI OT-I cells were protected from depletion in sepsis (Fig. 5B).
Inhibition of the IS by LFA-1 blockade prevents the loss of unactivated memory-phenotype CD8 T cells after sepsis but does not alter phenotypic activation
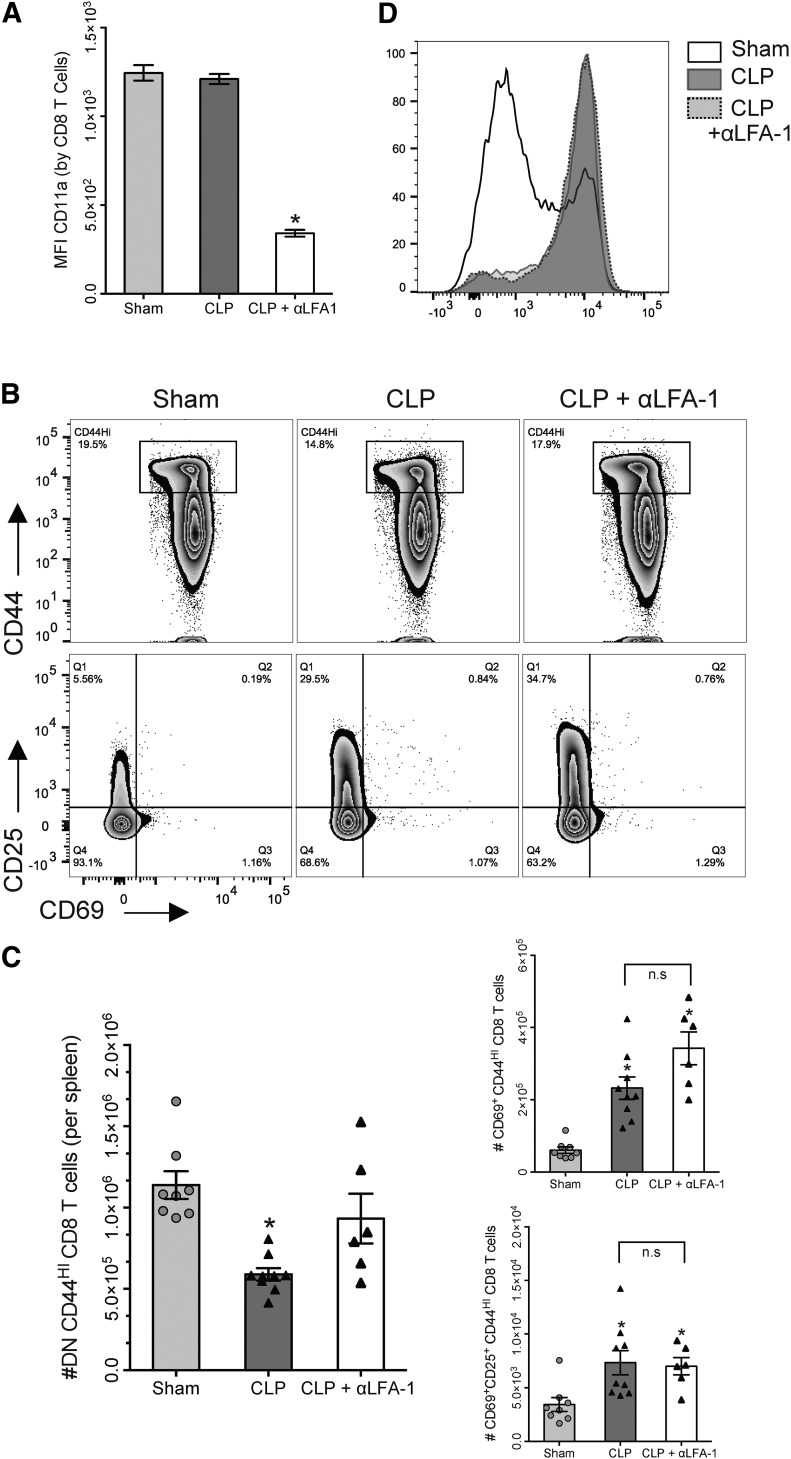
As our adoptive transfer experiments suggested that loss of memory-phenotype CD8 T cells was dependent on signaling through the TCR:MHC complex, we next investigated the involvement of the IS in activation and attrition. B6 mice were divided into 3 groups: sham, CLP, and CLP + αLFA-1 (received s.c. injection of αLFA-1 mAb 1 h post-CLP), and following euthanasia at 24 h, splenocytes were stained and analyzed by flow cytometry. To ensure that administration of αLFA-1 mAb resulted in inhibition of LFA-1 on CD8 T cells, we assessed CD11a (LFA-1) expression by CD8 T cells using an antibody for the 2D7 clone of CD11a, which would not be affected by the binding of αLFA-1 mAb.
As expected, the CLP + αLFA-1 group demonstrated significantly diminished CD8 T cell expression of CD11a compared with the sham or CLP group (Fig. 6A). We then assessed the effects of LFA-1 blockade on memory-phenotype CD8 T cell responses in sepsis. Interestingly, LFA-1 blockade did not alter the expression of CD69 or CD25, and both CLP and CLP + αLFA-1 groups demonstrated similar frequencies and numbers of CD69+ and CD69+CD25+ CD44HI CD8 T cells (Fig. 6B and C). Sepsis resulted in a significant reduction in the number of unactivated DN CD44HI cells, and administration of αLFA-1 abrogated this loss, such that numbers of unactivated CD44HI cells in the CLP + αLFA-1 group were similar to those of sham mice. As LFA-1:ICAM interactions have been shown to facilitate lymphocyte entry into lymph nodes [10, 30], we questioned whether LFA-1 blockade was also altering the expression of CD62L on memory cells. We found that memory CD8 cells from both CLP and CLP + αLFA-1 groups had similar increases in CD62L expression when compared with sham (Fig. 6D).
Figure 6. Inhibition of LFA-1 early after sepsis prevents the loss of unactivated memory-phenotype CD8 T cells but does not affect activation.
(A) Expression of CD11a on CD8 T cells was significantly reduced in septic mice treated with αLFA-1 mAb relative to sham and CLP groups (*P < 0.005). (B, Upper) Memory (CD44HI) CD8 T cells were gated on and subsequently analyzed for expression of CD69 and CD25 (lower). (C, Left) Numbers of unactivated (CD69−CD25−) memory CD8 T cells are reduced by 31% in CLP mice. Administration of αLFA-1 abrogates this loss; numbers of unactivated memory cells in the CLP+ αLFA-1 group are similar to sham. (Right) Numbers of CD69+ (upper) and CD69+CD25+ (lower) memory CD8 T cells are similar between CLP and CLP + αLFA-1 groups (*P < 0.01 for all). (D) Representative histograms demonstrating that αLFA-1 does not alter CD62L expression by CD44HI CD8 T cells. Results represent independent replicate experiments (n = 6–8/group) and are presented as means ± sem.
Circulating cytokines at 24 h after sepsis are consistent with bystander-driven activation of memory CD8 T cells; addition of LFA-1 blockade does not dampen cytokine response
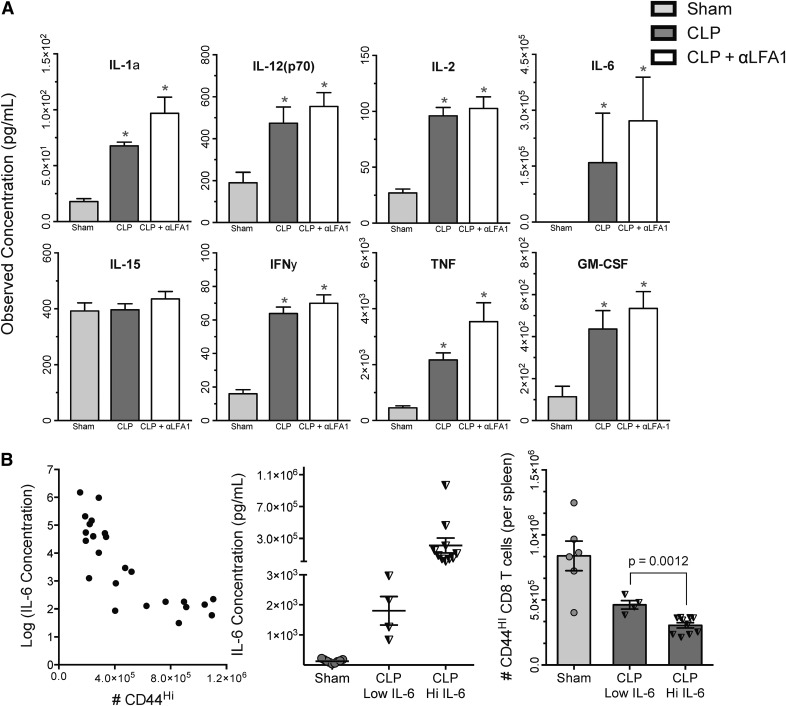
Multiple cytokines have been identified as key regulators in CD8 T cell activation, possessing the ability to elicit a CD8 activation response in a TCR-independent manner, including IL-1a and IL-1b, IL-2, IL-6, IL-12 (p40 and p70), IL-15, and TNF [31]. We sought to confirm that cytokines capable of eliciting CD8 T cell activation were present in the inflammatory environment evoked by sepsis. We evaluated sham mice, septic mice, and septic mice that were given αLFA-1 mAb to determine if LFA-1 blockade may have been acting at an innate level. Consistent with other studies in mouse and human sepsis, at 24 h after surgery, CLP mice demonstrated significantly higher concentrations of almost every mediator analyzed compared with sham, including those known to direct T cell activation (Fig. 7A). Administration of αLFA-1 did not result in any significant changes in the cytokine microenvironment, and concentrations of all cytokines analyzed remained similar between CLP and CLP + αLFA-1 groups. Notably, the only mediator that was not elevated after sepsis was IL-15, a regulator of memory cell longevity and homeostasis.
Figure 7. Sepsis increases serum concentrations of multiple cytokines capable of initiating CD8 T cell activation in the absence of cognate antigen.
(A) CLP and CLP + αLFA-1 groups demonstrated significant increases in serum concentrations of cytokines, except IL-15, compared with sham (*P < 0.01). (B, Left) For each animal, the number of CD44HI CD8 T cells was plotted against its corresponding circulating IL-6 level (IL-6 concentrations were logarithmically transformed to improve interpretability). (Middle) Septic mice were stratified into CLP-IL-6 Low (<6650 pg/ml) and CLP-IL-6 High (>6650 pg/ml) groups. (Right) Absolute numbers of splenic CD44HI CD8 cells were compared among Sham, CLP-IL-6 Low, and CLP-IL-6 High groups. In septic mice, high IL-6 levels were associated with a 34.2% reduction in the absolute number of CD44HI CD8 cells compared with septic mice with low IL-6 levels (P = 0.0012). Results represent independent replicate experiments (n = 6–10/group) and are presented as means ± sem.
Circulating IL-6 levels inversely correlate with numbers of memory-phenotype CD8 T cells at 24 h after sepsis
Multiple studies in the past decades have shown that plasma levels of IL-6 correlate with mortality in murine models of sepsis, as well as septic patients [8, 32, 33]. Thus, we evaluated whether a relationship existed between serum concentrations of IL-6 and attrition of memory-phenotype CD8 T cells. We hypothesized that attrition, too, may be a marker of severity, such that septic mice with fewer numbers of memory-phenotype CD8 T cells would also have higher levels of circulating IL-6. As spleens were also collected at the time of euthanasia, we were able to match IL-6 levels from our cytokine studies to absolute numbers of CD8 T cells from the same mouse. In keeping with the cited studies, we found a significant elevation in the levels of circulating IL-6 in septic mice compared with sham, regardless of therapy with LFA-1 (data shown in Fig. 7A). For each animal, we then plotted concentrations of IL-6 against CD44HI counts to determine if a correlative relationship exists between the 2 variables (Fig. 7B). Once this was confirmed, receiver operating characteristic curves were used to generate IL-6 discrimination values to stratify septic mice into low and high groups using an IL-6 concentration of 6650 pg/ml as a cutoff value; the groups were named “CLP–IL-6 Low” and “CLP–IL-6 High.” Absolute numbers of CD44HI CD8 cells were then compared among Sham, CLP–IL-6 Low, and CLP–IL-6 High groups; as expected, sham mice had the lowest levels of circulating IL-6 and also, the highest numbers of CD44HI CD8 T cells of all 3 groups. Mice in the CLP–IL-6 Low group had fewer CD44HI cells than sham but significantly greater numbers than mice from the CLP–IL-6 High group. With the analysis of the 2 CLP groups, we found that mice with higher levels of IL-6 had significantly fewer numbers of CD44HI CD8 T cells.
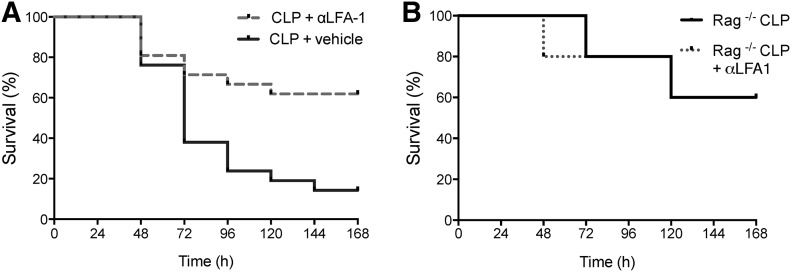
LFA-1 blockade improves 7 d survival in sepsis in a lymphocyte-dependent manner
Whereas we had previously used αLFA-1 mAb as a tool to determine the effects of IS stability on activation in sepsis, our results prompted us to investigate if prevention of memory CD8 T cell attrition in sepsis with LFA-1 blockade conveyed any effects on mortality. To analyze 7 d survival, all mice were subjected to CLP operations and then 1 h later, received either 1 ml NaCl (CLP group) or 1 ml αLFA-1 mAb (CLP + αLFA-1) as before. Administration of αLFA-1 reduced mortality from 61.9 to 14.2% (Fig. 8A). As sepsis alters both the innate and adaptive responses, we sought to confirm that the survival benefit conferred by the LFA-1 blockade was a result of its effect on lymphocytes. Survival studies were repeated using RAG−/− mice, known to lack mature T cells. Unlike our findings in WT mice, mortality between RAG−/− CLP and RAG−/− CLP + αLFA-1 groups was similar, suggesting that the survival benefit conferred by treatment with αLFA-1 mAb is dependent on the presence of lymphocytes (Fig. 8B). These data provide evidence that the administration of αLFA-1 and subsequent preservation of CD44HI CD8 T cells improve mortality in sepsis and implicate attrition of unactivated memory-phenotype CD8 T cells as a possible pathophysiologic mechanism of early sepsis.
Figure 8. Inhibition of LFA-1 improves survival in sepsis and acts in a lymphocyte dependent manner.
(A) Kaplan-Meier survival curve displaying mortality of B6 mice given with vehicle control or αLFA-1 mAb, 1 h after CLP. In WT B6 mice, treatment with αLFA-1 mAb decreased 7 d mortality from 61.9 to 14.2% (P < 0.005). Results represent independent replicate experiments (n = 21/group). (B) Survival studies were repeated using RAG−/− mice, known to lack mature T cells. Unlike our findings in WT mice, mortality between RAG−/− CLP and RAG−/− CLP + αLFA-1 groups was similar (P = 0.9539), suggesting that the survival benefit conferred by treatment with αLFA-1 mAb is dependent on the presence of lymphocytes.
DISCUSSION
Recently, memory CD8 T cell loss and dysfunction have been implicated in the increased susceptibility to secondary infections after sepsis [34]; however, the interplay between memory CD8 T cell activation and apoptosis in the primary immune response to sepsis was previously unexplored. This study demonstrates that polymicrobial sepsis initiates phenotypic activation of memory-phenotype CD8 T cells independent of cognate antigen; cells that fail to engage in this primary response are preferentially depleted, resulting in attrition of the memory compartment within the first 48 h after injury. Furthermore, we have shown that LFA-1 blockade prevents this loss without affecting activation and confers improved survival at 7 d.
Activation of memory CD8 T cells in sepsis
Activation of CD8 T cells has been reported in several other studies of sepsis: increased expression of CD69 by CD8 T cells was observed within 24 h of Escherichia coli endotoxin-induced acute lung injury and Pseudomonas aeruginosa-induced pneumonia, and in serum samples collected from patients, expression of CD69 within 24 h of diagnosis was able to reliably distinguish between septic and nonseptic critical illness [35, 36]. Here, we have shown that recruitment of CD8 T cells is markedly efficient, as phenotypically activated CD8 T cells were found in the spleen as early as 6 h after an intra-abdominal insult. The numbers of CD69+ memory-phenotype cells peaked at 24 h after sepsis but returned to baseline by d 5. In isolation, these findings may aid in the ability to use lymphocyte phenotyping to identify patients with sepsis. However, our study also sheds light on the mechanism that drives CD8 T cell activation in sepsis, which was previously unexplored. We provide several key pieces of data that indicate that activation in sepsis is primarily a consequence of cytokine-mediated bystander effects, rather than engagement of the TCR.
We show that sepsis triggers the production of multiple cytokines known to be potent inducers of cytotoxic CD8 T cells, including IL-2, IL-12, and TNF [37]. In fact, a recent study evaluated >1800 cytokine combinations and demonstrated that the synergistic effects of IL-12 and TNF and IL-2 and IL-12 were among the most effective at inducing antigen-independent effector function [31]. Moreover, the phenotypic changes we observed in endogenous memory-phenotype CD8 T cells after CLP bear several key similarities to the cytotoxic memory CD8 T cells activated by cytokine-mediated effects: namely, rapid (<8 h) up-regulation of CD69 [38], modest increases in CD25 expression [39], and a preference toward differentiation into a central memory phenotype [40]. In keeping with our hypothesis, TCR transgenic OT-I memory-phenotype cells paralleled their endogenous counterparts in the expression of CD69 and CD25 after sepsis, despite the absence of a cognate antigen.
Although our results are consistent with other mouse and human studies reporting CD8 T cell activation after sepsis, a recent study by Badovinac and coworkers [34] evaluating functional memory CD8 T cell responses after CLP failed to demonstrate a difference in activation phenotypes between sham and septic mice at 48 h after injury. We believe this discrepancy is likely a result of 2 important distinctions. First, the referenced study used a 5–10% mortality model of CLP, whereas we used a 30–50% mortality model aimed to simulate clinically moderate to severe sepsis. It is highly possible that our models yielded distinct cytokine environments and that only more severe injuries elicit the inflammatory cues capable of recruiting CD8 T cells early in the host response. Secondly, the mentioned study evaluated the effect of sepsis on transferred bona fide antigen-experienced memory CD8 T cells; our experiments focused on endogenous, polyclonal CD8 T cells in B6 mice and unimmunized OT-I mice with a memory phenotype. In other studies, these virtual memory cells [41, 42] have been shown to possess unique functional properties distinct from their antigen-primed counterparts, providing a possible explanation for the discrepancy in activation responses that we observed versus those of Badovinac and coworkers [34].
Attrition of unactivated memory-phenotype CD8 T cells
We also noticed the preferential depletion of memory-phenotype endogenous CD8 cells with an unactivated phenotype, which seemed to coincide with activation. Interestingly, OT-I counterparts, as well as unactivated memory-phenotype CD8 T cells from septic mice treated with the LFA-1 blockade, were spared from this attrition. Studies using MPM have shown that after infection, T cell:APC interactions within lymph nodes are first marked by short stop-and-go contact, which can occur independent of antigen and results in increased expression of CD69 and CD44 [43]. Subsequently, TCR avidity directs long-lasting (>1 h) APC–T cell interactions, resulting in full activation and effector function via signaling through the TCR:MHC complex [44]. LFA-1/ICAM-1 interactions are particularly important in this second phase, allowing for stabilization of T cell–APC contact and maturation of the IS, as well as facilitating TCR-MHC signal transduction and decreasing the threshold for activation, proliferation, and effector cell induction [45–47]. We suspect that both OT-I and CD8 T cells from mice receiving anti-LFA-1 were more prone to form short contacts with APCs, either as a result of TCR nonrecognition or IS instability. These data suggest an association between the formation of long, stable interactions and the delivery of apoptotic signals that mediate sepsis-induced attrition of unactivated cells. Whereas this model suggests that apoptosis is dependent on stable T cell–APC contact, it is difficult to imagine that this accounts for the substantial amount of loss that we observed, and other mechanisms may also be involved. We hope to reconcile this with future experiments using Nur77-GFP reporter mice to determine the role of TCR engagement, as well as our own MPM studies to visualize these interactions directly.
Nonetheless, our study raises multiple questions regarding why unactivated memory-phenotype cells are depleted early in the course of sepsis and whether this loss has functional implications on outcome. A profound lymphopenia that preferentially affects memory (CD44HI) CD8 T cells has been reported after treatment with TLR agonists (polyinosinic:polycytidylic acid) and in early stages of viral infections as a consequence of apoptosis mediated by IFN signaling [27]. Similar to the kinetics that we observed in sepsis, IFN-induced apoptosis results in the significant loss of splenic CD44HI CD8 T cells from 6 to 48 h after infection, with repletion of the subset by 3 d [27, 48, 49]. These investigators went on to surmise that attrition may represent an integral part of the response to infection, allowing the antigen-specific T cell population to expand without competition for APCs, cytokines, and growth factors. Although further experiments are necessary to determine if apoptosis in sepsis is mediated by IFN, a similar teleological argument may explain the loss of unactivated memory-phenotype cells that we observed. In this sense, the apoptosis of cells that are unable to assist in clearing the primary infection may permit unrestricted responses by those that can and allow for the expansion of CD8 T cells with specificity for immunodominant epitopes. Accordingly, we observed repletion of memory-phenotype cells by 120 h after sepsis, but as a CD44HICD11aHI phenotype can be a feature of newly differentiated effectors, memory cells, and cells proliferating in response to lymphopenia [20], the mechanism responsible is unclear. Nonetheless, several studies on sepsis have reported impaired CD8 T cell function and decreased TCR heterogeneity, even months after the initial insult, calling into question the enhanced protection that this repletion actually confers. It may be that in the context of the overwhelming impairment of immune functions elicited by sepsis, the loss of unactivated memory-phenotype CD8 T cells no longer serves as advantageous and in fact, may be deleterious. IL-6 levels have been shown to correlate with increased severity and mortality in sepsis. In fact, in a study in patients with sepsis, IL-6 levels were significantly higher in septic patients who subsequently died than in those who survived, suggesting a role for the cytokine as a prognostic biomarker [50]. Our study revealed an inverse correlation between numbers of CD44HI CD8 T cells and levels of IL-6, suggesting that CD8 T cell attrition may be an important immunopathologic derangement associated with worsened outcomes in sepsis.
The improved survival associated with the rescue of unactivated memory CD8 T cells with LFA-1 blockade further suggests that attrition may be maladaptive in sepsis but also carries certain implications regarding the use of immunomodulatory therapies. In this study, αLFA-1 mAb was administered 1 h after CLP—almost 1 d before the height of memory CD8 T cell attrition—but conferred the greatest survival benefit at 48–72 h. Thus, even in the hyper-acute phase of sepsis, a developmental program that determines CD8 T cell fate, may already be in effect. In fact, a similar “blueprint for differentiation” has been described in models of antigen-specific activation, whereby the fate of a naïve T cell, including proliferation and differentiation, is programmed within hours of the initial antigen encounter [51]. In our CLP model, the signals targeting CD8 T cells for activation and attrition had long been generated by the time we observed the greatest loss in memory CD8 T cells, cautioning the use of lymphocyte numbers alone as a means to select and initiate therapy. Whereas we are still investigating the mechanisms by which LFA-1 blockade improves survival, we predict that delayed administration beyond the given (unknown) treatment window may not have a beneficial effect and may result in the unintentional modulation of an altogether different set of immune responses.
This study has several limitations. As in most models of sepsis, our study required euthanizing animals at predetermined time points after sepsis, thus introducing a selection bias in which analysis was limited to mice that were destined to live beyond that time point. As much of our data were collected at 24 h, some error was avoided, as at this time, the CLP group was still comprised of mice of heterogeneous fates. However, our findings in later time points (particularly the repletion of memory CD8 T cells at 120 h) may be characteristic only of mice destined to survive the primary injury. Additionally, we recognize that LFA-1 plays an important role in multiple innate and adaptive immune cell functions and that the administration of αLFA-1 mAb may have had additional effects beyond destabilization of memory CD8 T cell–TCR:MHC interactions. Although surface expression of LFA-1 has also been demonstrated by neutrophils, T cells, and B cells [11], our survival experiments in RAG−/− mice indicate that the survival benefit conferred by LFA-1 blockade after CLP is lymphocyte mediated, and the similar cytokine environments between CLP and CLP + αLFA-1 mAb groups suggest that innate immune functions are grossly unaffected. Whereas similar CD62L expression between memory CD8 T cells of treated and untreated mice suggests that LFA-1 blockade did not significantly alter T cell trafficking patterns, we cannot exclude the possibility that LFA-1 blockade may have also impacted TCR:MHC interactions of naïve CD8 T cells. Whereas the loss of cells that we see in our studies appears to be attributed to apoptosis, it is also possible that receptor down-regulation or cell trafficking contributes to this process. We are currently characterizing additional lymphoid compartments to clarify this further.
Despite these limitations, this study reveals important features of memory CD8 T cell activation and attrition in the days following polymicrobial sepsis. Further exploration into the protection conferred by “innate-like” CD8 T cells and the functional implications of memory cell loss are warranted, as both offer new perspectives of possible therapeutic strategies in sepsis. However, perhaps more importantly, this study clarifies some of the complex and dynamic features of the responses of innate-like memory CD8 T cells early in sepsis and underscores the difficulty that sepsis researchers face. Timing, antigen, prior T cell memory, severity of infection, age, and genetics each plays a role in the pathologic immune response seen in sepsis and may contribute to the outcome of the disease. This study adds to our understanding of the host response to sepsis and may have applications in the identification of which patients and at which point in their presentation would most likely benefit from the immunomodulatory therapies on the horizon.
AUTHORSHIP
M.A.S. and K.W.M. conceived of the idea, designed the experiments, oversaw the entire project, and conducted experiments. M.A.S. and K.M.R. prepared this manuscript. A.H., J.D.L., R.M., Z.L., and L.M.M. performed adoptive transfer experiments of TCR-transgenic Thy1.1+ OT-I T cells in WT recipient mice to determine if cognate antigen recognition is required for acquisition of an activated phenotype in sepsis. Additionally, these authors performed cytokine quantification analysis, as well as data analysis of flow cytometric apoptosis experiments and manuscript revision. M.L.F. and C.M.C. provided mentorship for this K Award and manuscript revision.
ACKNOWLEDGMENTS
This work was supported by the Shock Society Research Fellowship for Early Career Investigators and U.S. National Institutes of Health Grant 1K08GM110537-01 (to K.W.M.). The authors give special thanks to the Emory University Department of Surgery for its support with this research project.
Glossary
- B6
C57BL/6
- CD62L
cluster of differentiation 62 ligand
- CLP
cecal ligation and puncture
- DN
double-negative
- HYL
high-yield lyse
- IS
immunologic synapse
- i.v.
intravenous
- MFI
median fluorescence intensity
- mOVA
murine OVA
- MPM
multiphoton microscopy
- RAG1
recombination activating gene 1
- s.c.
subcutaneous
- WT
wild-type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Surviving Sepsis Campaign Guidelines Committee including the Pediatric Subgroup (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 41, 580–637. [DOI] [PubMed] [Google Scholar]
- 2.Gaieski D. F., Edwards J. M., Kallan M. J., Carr B. G. (2013) Benchmarking the incidence and mortality of severe sepsis in the United States. Crit. Care Med. 41, 1167–1174. [DOI] [PubMed] [Google Scholar]
- 3.Marshall J. C. (2014) Why have clinical trials in sepsis failed? Trends Mol. Med. 20, 195–203. [DOI] [PubMed] [Google Scholar]
- 4.Hutchins N. A., Unsinger J., Hotchkiss R. S., Ayala A. (2014) The new normal: immunomodulatory agents against sepsis immune suppression. Trends Mol. Med. 20, 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stearns-Kurosawa D. J., Osuchowski M. F., Valentine C., Kurosawa S., Remick D. G. (2011) The pathogenesis of sepsis. Annu. Rev. Pathol. 6, 19–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K., Svabek C., Vazquez-Guillamet C., Sato B., Rasche D., Wilson S., Robbins P., Ulbrandt N., Suzich J., Green J., Patera A. C., Blair W., Krishnan S., Hotchkiss R. (2014) Targeting the programmed cell death 1: programmed cell death ligand 1 pathway reverses T cell exhaustion in patients with sepsis. Crit. Care 18, R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotchkiss R. S., Monneret G., Payen D. (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 13, 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osuchowski M. F., Connett J., Welch K., Granger J., Remick D. G. (2009) Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit. Care Med. 37, 1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi N. S., Kaech S. M. (2008) Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 180, 1309–1315. [DOI] [PubMed] [Google Scholar]
- 10.Hogg N., Smith A., McDowall A., Giles K., Stanley P., Laschinger M., Henderson R. (2004) How T cells use LFA-1 to attach and migrate. Immunol. Lett. 92, 51–54. [DOI] [PubMed] [Google Scholar]
- 11.Nicolls M. R., Gill R. G. (2006) LFA-1 (CD11a) as a therapeutic target. Am. J. Transplant. 6, 27–36. [DOI] [PubMed] [Google Scholar]
- 12.Sun S., Zhang X., Tough D. F., Sprent J. (1998) Type I interferon-mediated stimulation of T cells by CpG DNA. J. Exp. Med. 188, 2335–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tough D. F., Sun S., Sprent J. (1997) T cell stimulation in vivo by lipopolysaccharide (LPS). J. Exp. Med. 185, 2089–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasaki A., Medzhitov R. (2004) Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5, 987–995. [DOI] [PubMed] [Google Scholar]
- 15.Jameson S. C., Lee Y. J., Hogquist K. A. (2015) Innate memory T cells. Adv. Immunol. 126, 173–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehst B. D., Ingulli E., Jenkins M. K. (2003) Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am. J. Transplant. 3, 1355–1362. [DOI] [PubMed] [Google Scholar]
- 17.Angele M. K., Schwacha M. G., Ayala A., Chaudry I. H. (2000) Effect of gender and sex hormones on immune responses following shock. Shock 14, 81–90. [DOI] [PubMed] [Google Scholar]
- 18.Coopersmith C. M., Chang K. C., Swanson P. E., Tinsley K. W., Stromberg P. E., Buchman T. G., Karl I. E., Hotchkiss R. S. (2002) Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit. Care Med. 30, 195–201. [DOI] [PubMed] [Google Scholar]
- 19.Gilson C. R., Milas Z., Gangappa S., Hollenbaugh D., Pearson T. C., Ford M. L., Larsen C. P. (2009) Anti-CD40 monoclonal antibody synergizes with CTLA4-Ig in promoting long-term graft survival in murine models of transplantation. J. Immunol. 183, 1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung K. P., Yang E., Goldrath A. W. (2009) Memory-like CD8+ T cells generated during homeostatic proliferation defer to antigen-experienced memory cells. J. Immunol. 183, 3364–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldrath A. W., Bogatzki L. Y., Bevan M. J. (2000) Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J. Exp. Med. 192, 557–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slifka M. K., Whitton J. L. (2000) Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 164, 208–216. [DOI] [PubMed] [Google Scholar]
- 23.Kalia V., Sarkar S., Subramaniam S., Haining W. N., Smith K. A., Ahmed R. (2010) Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity 32, 91–103. [DOI] [PubMed] [Google Scholar]
- 24.Létourneau S., Krieg C., Pantaleo G., Boyman O. (2009) IL-2- and CD25-dependent immunoregulatory mechanisms in the homeostasis of T-cell subsets. J. Allergy Clin. Immunol. 123, 758–762. [DOI] [PubMed] [Google Scholar]
- 25.Badovinac V. P., Harty J. T. (2006) Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 211, 67–80. [DOI] [PubMed] [Google Scholar]
- 26.Raué H. P., Beadling C., Haun J., Slifka M. K. (2013) Cytokine-mediated programmed proliferation of virus-specific CD8(+) memory T cells. Immunity 38, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bahl K., Hüebner A., Davis R. J., Welsh R. M. (2010) Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to Toll-like receptor agonists. J. Virol. 84, 4866–4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mittal R., Wagener M., Breed E. R., Liang Z., Yoseph B. P., Burd E. M., Farris A. B. III, Coopersmith C. M., Ford M. L. (2014) Phenotypic T cell exhaustion in a murine model of bacterial infection in the setting of pre-existing malignancy. PLoS One 9, e93523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogquist K. A., Gavin M. A., Bevan M. J. (1993) Positive selection of CD8+ T cells induced by major histocompatibility complex binding peptides in fetal thymic organ culture. J. Exp. Med. 177, 1469–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisman N. M., Floyd T. L., Wagener M. E., Kirk A. D., Larsen C. P., Ford M. L. (2011) LFA-1 blockade induces effector and regulatory T-cell enrichment in lymph nodes and synergizes with CTLA-4Ig to inhibit effector function. Blood 118, 5851–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman B. E., Hammarlund E., Raué H. P., Slifka M. K. (2012) Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc. Natl. Acad. Sci. USA 109, 9971–9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiswick E. L., Mella J. R., Bernardo J., Remick D. G. (2015) Acute-phase deaths from murine polymicrobial sepsis are characterized by innate immune suppression rather than exhaustion. J. Immunol. 195, 3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Remick D. G., Bolgos G., Copeland S., Siddiqui J. (2005) Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect. Immun. 73, 2751–2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duong S., Condotta S. A., Rai D., Martin M. D., Griffith T. S., Badovinac V. P. (2014) Polymicrobial sepsis alters antigen-dependent and -independent memory CD8 T cell functions. J. Immunol. 192, 3618–3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwulst S. J., Muenzer J. T., Chang K. C., Brahmbhatt T. S., Coopersmith C. M., Hotchkiss R. S. (2008) Lymphocyte phenotyping to distinguish septic from nonseptic critical illness. J. Am. Coll. Surg. 206, 335–342. [DOI] [PubMed] [Google Scholar]
- 36.Roger P. M., Hyvernat H., Ticchioni M., Kumar G., Dellamonica J., Bernardin G. (2012) The early phase of human sepsis is characterized by a combination of apoptosis and proliferation of T cells. J. Crit. Care 27, 384–393. [DOI] [PubMed] [Google Scholar]
- 37.Lertmemongkolchai G., Cai G., Hunter C. A., Bancroft G. J. (2001) Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166, 1097–1105. [DOI] [PubMed] [Google Scholar]
- 38.Soudja S. M., Ruiz A. L., Marie J. C., Lauvau G. (2012) Inflammatory monocytes activate memory CD8(+) T and innate NK lymphocytes independent of cognate antigen during microbial pathogen invasion. Immunity 37, 549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maroof A., Beattie L., Kirby A., Coles M., Kaye P. M. (2009) Dendritic cells matured by inflammation induce CD86-dependent priming of naive CD8+ T cells in the absence of their cognate peptide antigen. J. Immunol. 183, 7095–7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gagnon J., Ramanathan S., Leblanc C., Cloutier A., McDonald P. P., Ilangumaran S. (2008) IL-6, in synergy with IL-7 or IL-15, stimulates TCR-independent proliferation and functional differentiation of CD8+ T lymphocytes. J. Immunol. 180, 7958–7968. [DOI] [PubMed] [Google Scholar]
- 41.Lee J. Y., Hamilton S. E., Akue A. D., Hogquist K. A., Jameson S. C. (2013) Virtual memory CD8 T cells display unique functional properties. Proc. Natl. Acad. Sci. USA 110, 13498–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Renkema K. R., Li G., Wu A., Smithey M. J., Nikolich-Žugich J. (2014) Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J. Immunol. 192, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henrickson S. E., von Andrian U. H. (2007) Single-cell dynamics of T-cell priming. Curr. Opin. Immunol. 19, 249–258. [DOI] [PubMed] [Google Scholar]
- 44.Mempel T. R., Henrickson S. E., Von Andrian U. H. (2004) T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. [DOI] [PubMed] [Google Scholar]
- 45.Dustin M. L. (2014) The immunological synapse. Cancer Immunol. Res. 2, 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki J., Yamasaki S., Wu J., Koretzky G. A., Saito T. (2007) The actin cloud induced by LFA-1-mediated outside-in signals lowers the threshold for T-cell activation. Blood 109, 168–175. [DOI] [PubMed] [Google Scholar]
- 47.Perez O. D., Mitchell D., Jager G. C., South S., Murriel C., McBride J., Herzenberg L. A., Kinoshita S., Nolan G. P. (2003) Leukocyte functional antigen 1 lowers T cell activation thresholds and signaling through cytohesin-1 and Jun-activating binding protein 1. Nat. Immunol. 4, 1083–1092. [DOI] [PubMed] [Google Scholar]
- 48.Jiang J., Lau L. L., Shen H. (2003) Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J. Immunol. 171, 4352–4358. [DOI] [PubMed] [Google Scholar]
- 49.Bahl K., Kim S. K., Calcagno C., Ghersi D., Puzone R., Celada F., Selin L. K., Welsh R. M. (2006) IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 176, 4284–4295. [DOI] [PubMed] [Google Scholar]
- 50.Hack C. E., De Groot E. R., Felt-Bersma R. J., Nuijens J. H., Strack Van Schijndel R. J., Eerenberg-Belmer A. J., Thijs L. G., Aarden L. A. (1989) Increased plasma levels of interleukin-6 in sepsis. Blood 74, 1704–1710. [PubMed] [Google Scholar]
- 51.Kaech S. M., Ahmed R. (2001) Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat. Immunol. 2, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]