ADAM17 activity in leukocytes during sepsis tips the balance between efficient and impaired neutrophil migration into sites of infection.
Keywords: inflammation, microbiota, TACE
Abstract
A rapid and robust recruitment of circulating neutrophils at sites of infection is critical for preventing bacterial spread. The efficiency of this process, however, is greatly diminished during sepsis, a severe systemic inflammatory response to infection. The proteolytic activity of a disintegrin and metalloprotease-17 is induced in the cell membrane of leukocytes upon their activation, resulting in the conversion of membrane to soluble TNF-α and the release of assorted receptors from the surface of neutrophils important for their effector functions. We show that conditional knockout mice lacking a disintegrin and metalloprotease-17 in all leukocytes had a survival advantage when subjected to polymicrobial sepsis. Bacteremia and the levels of circulating proinflammatory cytokines, key determinants of sepsis severity, were significantly reduced in conditional a disintegrin and metalloprotease-17 knockout mice during sepsis. Although cecal bacterial microbiota and load were similar in unmanipulated conditional a disintegrin and metalloprotease-17 knockout and control mice, peritoneal spread of bacteria was significantly reduced in conditional a disintegrin and metalloprotease-17 knockout mice following sepsis induction, which was associated with an amplified recruitment of neutrophils. Taken together, our findings suggest that extensive a disintegrin and metalloprotease-17 induction during sepsis may tip the balance between efficient and impaired neutrophil recruitment.
Introduction
Neutrophils account for the majority of circulating leukocytes in the bloodstream of humans and are poised for a rapid influx into tissue locations during microbial infection [1]. This process, however, is diminished during excessive inflammation [2, 3], which is well established in animal models and patients with sepsis [4, 5]. Such impairment of circulating neutrophils is associated with a reduction in surface levels of adhesion proteins and chemokine receptors important for their migration [4, 6–8] and a corresponding increase in the blood levels of soluble adhesion molecules [9]. At this time, little is known about central regulators of neutrophil impairment during sepsis, which may provide new targets for host-directed therapeutics.
ADAM17 is a constitutively expressed, membrane-associated protease that functions in the microenvironment of the cell surface by cleaving membrane-associated proteins [10]. ADAM17 was initially shown to convert transmembrane TNF-α to its soluble form; hence, its original name, TNF-α-converting enzyme (TACE) [11]. ADAM17 tends to have a low level of activity in resting leukocytes that undergoes rapid induction upon cell activation by numerous stimuli [12–14]. This induction process occurs through assorted intrinsic changes in ADAM17, including redox modification of its extracellular region [12], phosphorylation of its cytoplasmic region [15], and intermolecular interactions [14, 16].
ADAM17 is now recognized to cleave a variety of substrates [17], including adhesion molecules and chemokine receptors on leukocytes and endothelial cells that regulate cell–cell adhesion [18–23]. For instance, L-selectin, CD16b, and CXCR2 on circulating neutrophils facilitate their attachment and migration through the vascular wall [24, 25] and undergo a rapid down-regulation in expression by ADAM17 upon neutrophil activation [18, 22, 23]. We [26, 27] and others [28] have shown that an ADAM17 deficiency in leukocytes increases neutrophil recruitment at sites of inflammation and Escherichia coli infection in mice. We extend these findings by showing that conditional ADAM17 knockout mice lacking ADAM17 in all leukocytes had a significant survival advantage during severe polymicrobial sepsis induced by CLP, which was associated with enhanced neutrophil recruitment at the infectious locus along with decreased bacterial spread and circulating levels of proinflammatory factors.
MATERIALS AND METHODS
Animals
Mice were housed in a specified pathogen-free facility, and all procedures performed were done in accordance with protocols approved by the Animal Care and Use Committee of the University of Minnesota. Mice used in this study were Adam17flox/flox mice (Adam17tm1.2Bbl/J) and Vav1-Cre mice (B6.Cg-Tg(Vav1-cre)A2Kio/J) from The Jackson Laboratory (Bar Harbor, ME, USA). The Adam17flox/flox and Vav1-Cre mice were crossed to the C57BL/6J genetic background (both ≥98.4%) and then crossed together to generate Adam17flox/flox/Vav1-Cre mice and littermate Adam17flox/flox mice, as we have described previously [23]. ADAM17 knockout mice are embryonic or perinatal lethal [29], whereas Adam17flox/flox/Vav1-Cre mice deficient in ADAM17 in all leukocytes are viable and lack any obvious developmental abnormalities [26, 27, 30]. Adam17flox/flox/Vav1-Cre mice and Adam17flox/flox mice are referred to below as conditional ADAM17 knockout and control mice, respectively.
CLP
CLP was performed as described by others with some modifications [31]. Conditional ADAM17 knockout and control mice (26–30 g in weight) were anesthetized with ketamine and xylazine (100 mg/kg and 10 mg/kg, respectively) administered intraperitoneally, their abdomen shaved, and disinfected with iodine, and ophthalmic ointment was applied to the eyes. A midline abdominal incision through the skin and then abdominal musculature was made. The cecum was identified and then ligated below the ileum with sterile, nonabsorbable silk thread (Teleflex Medical, Coventry, CT, USA). The distal end of the cecum was punctured with a sterile needle. This was done with a 21-G, 1-puncture or an 18-G, 2-puncture. Fecal material was not manipulated out of the puncture sites, as we found this increased variability in bacteremia and internal bleeding. The ligated and punctured cecum was placed back into the abdomen, the peritoneum and skin were closed with sterile 4-0 Vicryl sutures (Ethicon, Somerville, NJ, USA), and 1 ml saline was injected subcutaneously for resuscitation. Sham surgery consisted of the same steps, except that the cecum was not punctured. All mice following surgery were gently placed on a thermal blanket and monitored. Once sternal and fully recovered from anesthesia, mice were placed back in their cages with accessible food and water. For this sepsis model, establishment of an intra-abdominal infection required ∼12 h. Mice were evaluated post-CLP using a murine sepsis scoring system, adapted from ref. [32]. Eight individual criteria were assessed; appearance, consciousness, activity, response to stimulus, eyes, respiration rate, respiration quality, and diarrhea. Animal survival was monitored for 5 d. Moribund animals, exhibiting an inability to move, unresponsiveness, and stressed breathing, were euthanized. Survival comparison between conditional ADAM17 knockout and control mice was done by a log-rank (Mantel-Cox) test with P < 0.05 taken as statistically significant.
Multiplexed ELISA
Plasma cytokine and chemokine concentrations were measured by quantitative ELISA, as we have described previously [19, 30], with some modifications. For these studies, a mouse inflammation multiplex kit was used to detect 11 cytokines and chemokines (TNF-α, IFN-γ, IL-1α, IL-1β, IL-6, IL-10, IL-12p70, IL-23p19, KC, MCP-1, and IP-10), performed according to the manufacturer’s protocol (Antigenix America, Huntington Station, NY, USA). MFIs of the samples and standards were determined using a FACSCanto instrument (BD Biosciences, San Jose, CA, USA). Cytokine concentrations in the samples were a function of MFI, determined using the manufacturer’s recommended standard curves and dilution factors. Samples were not diluted, and MFIs for all cytokines fell within the range of the standard curve, except for TNF-α, which was below the standard curve for some samples. A comparison of conditional ADAM17 knockout and control mice was done by Student’s t test, with P < 0.05 taken as statistically significant.
Cecal bacterial microbiota
Aseptic technique was used throughout the sampling process. Cecal samples from unmanipulated conditional ADAM17 knockout and control mice were collected separately and diluted into 5 ml sterile water, and DNA extraction was performed using a QIAamp DNA Stool Kit, according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). Amplification of the 16S rRNA gene was performed using KAPA HiFi HotStart Polymerase (Kapa Biosystems, Wilmington, MA, USA) for 2 rounds of PCR at the University of Minnesota Genomics Center (Minneapolis, MN, USA). For the first round, the V5 forward (5′-tcgtcggcagcgtcagatgtgtataagagacagrggattagataccc-3′) and V6 reverse (5′-gtctcgtgggctcggagatgtgtataagagacagcgacrrccatgcancacct-3′) Nextera primers (Integrated DNA Technologies, Coralville, IA, USA) were used to amplify the V5–V6 hypervariable region using the following cycling parameters: 1 cycle of 95°C for 5 min, followed by 20 cycles of 98°C for 20 s, 55°C for 15 s, and 72°C for 1 min. The products were then diluted 1:100, and 5 μl was used in a second round of PCR using forward (5′-aatgatacggcgaccaccgagatctacac[i5]tcgtcggcagcgtc-3′) and reverse (5′-caagcagaagacggcatacgagat[i7]gtctcgtgggctcgg-3′) indexing primers (Integrated DNA Technologies). The second PCR used the following cycling parameters: 1 cycle at 95°C for 5 min, followed by 10 cycles of 98°C for 20 s, 55°C for 15 s, and 72°C for 1 min. Samples were denatured with NaOH, diluted to 8 pM in HT1 buffer (Illumina, San Diego, CA, USA), spiked with 15% PhiX, and heat denatured at 96°C for 2 min immediately before loading. A MiSeq 600 cycle v3 kit (Illumina) was used to sequence the sample.
Following sequencing, random subsets of 40,000 high-quality reads per sample were selected using the subsample approach in Mothur [33]. Subsequent data analysis was performed using the QIIME software program [34]. A de novo OTU picking approach was performed using UCLUST [35]. OTUs containing <10 sequences were removed to eliminate possible spurious OTUs as a result of sequencing error. QIIME was used for assessments of α and β diversity using UniFrac and phylogenetic classifications using the Ribosomal Database Project (RDP) database [36]. Statistical comparisons between groups of mice were performed in QIIME using the Analysis of Similarity (ANOSIM) method for distance matrices and nonparametric t test for α diversity.
Cecal bacterial load
DNA extraction from cecal samples was performed as described above, and qPCR was performed for quantification of bacterial 16S and mammalian 18S rRNA genes. For conditional ADAM17 knockout and control mice, the CT values for 16S rRNA gene qPCR were 13.84 ± 0.62 and 14.29 ± 1.022, respectively, and for 18S rRNA gene qPCR, they were 11.38 ± 0.78 and 10.73 ± 0.24 (means ± sd, n = 4 mice in each group). Mice constitutively slough intestinal epithelial cells, and CT values of the 18S rRNA gene were determined for normalization in calculating the relative fold change in 16S rRNA gene levels using the 2−ΔΔ CT method [37]. All qPCR assays were performed using an ABI 7500 Fast Real-Time PCR System (Thermo Fisher Scientific Life Sciences, Waltham, MA, USA). The PCR reaction mixture included 10 μl TaqMan Gene Expression MasterMix (Thermo Fisher Scientific Life Sciences), 3 µl cecal DNA, 1 μl FAM-labeled TaqMan probe for conserved sequences within the 16S rRNA gene (Pa04230899_s1; Thermo Fisher Scientific Life Sciences), or 1 μl FAM-labeled TaqMan probe for the 18S rRNA gene (Mm03928990_g1; Thermo Fisher Scientific Life Sciences). The following cycling parameters were performed, according to the manufacturer’s instructions: 1 cycle of 50°C for 2 min and 1 cycle of 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s.
Enumeration of blood and peritoneal neutrophils and bacterial CFUs
Bacterial counts were determined by plating 10-fold serial dilutions of blood or peritoneal lavage fluid, as previously described [27, 30]. All samples were plated on brain heart infusion agar plates (Becton Dickinson, Sparks, MD, USA). Enumeration of blood and peritoneal levels of neutrophils were performed as previously described [23]. Leukocyte differentials were performed using peripheral blood and peritoneal fluid smears that were prepared and stained with Wright-Giemsa using an automated stainer (Aerospray 7120, Wescor, ELITechGroup Solutions, Logan, UT, USA). For each sample, 100 cell differentials were performed. Comparison between conditional ADAM17 knockout and control mice was done by Student’s t test, with P < 0.05 taken as statistically significant.
RESULTS AND DISCUSSION
ADAM17 deficiency in leukocytes improves survival during polymicrobial sepsis
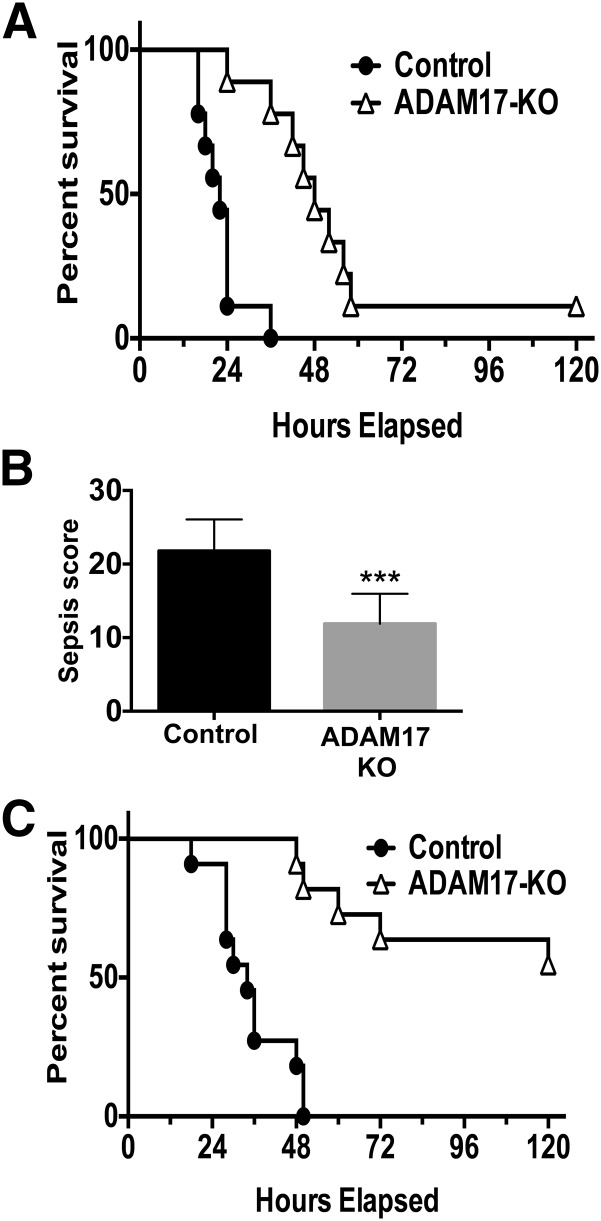
We explored the role of leukocyte ADAM17 on outcome during severe sepsis by using Adam17flox/flox/Vav1-Cre mice (conditional ADAM17 knockout mice). Severe sepsis was induced in conditional ADAM17 knockout and control mice (Adam17flox/flox) by CLP (18-G, 2-puncture). By 36 h post-CLP, control mice demonstrated 100% mortality, whereas 78% of conditional ADAM17 knockout mice remained alive (Fig. 1A). This decreased to 11% survival by 5 d post-CLP (Fig. 1A). Sepsis severity was also evaluated by a murine sepsis scoring system to assess various early physiologic parameters. At 12 h post-CLP, a time point past potential anesthesia interference (e.g., cardiac depression [38]) and before a moribund state by mice in either group, conditional ADAM17 knockout mice demonstrated significantly lower sepsis scores than control mice (Fig. 1B), which corresponded with their enhanced survival.
Figure 1. Improved survival by conditional ADAM17 knockout mice during sepsis.
Conditional ADAM17 knockout (KO) and control mice were subjected to CLP and monitored for 5 d for survival. (A) 18-G, 2-puncture CLP; n = 9 mice per group; P < 0.002, log-rank test. (B) Conditional ADAM17 knockout and control mice were subjected to CLP (18-G, 2-puncture), and sepsis severity was assessed at 12 h using a murine sepsis scoring system to evaluate various physiologic variables, as described in Materials and Methods; n = 9 mice per group; ***P < 0.001. (C) 21-G, 1-puncture CLP; n = 11 mice per group; P < 0.0001, log-rank test.
The difference in sepsis resistance between conditional ADAM17 knockout and control mice was more evident with less-severe CLP. Control mice subjected to a 21-G, 1-puncture survived marginally longer than mice subjected to 18-G, 2-puncture CLP, demonstrating 100% mortality by 48 h. In contrast, conditional ADAM17 knockout mice demonstrated only 18% mortality at this time point (Fig. 1C). At the end of the 5 d observation period, 55% of conditional ADAM17 knockout mice remained alive (Fig. 1C). All mice in both groups subjected to sham surgery survived during the 5 d observation period (data not shown). Taken together, our data indicate that the targeting of ADAM17 in leukocytes provides a survival advantage during the early stages of severe sepsis.
Leukocyte ADAM17 increases bacterial spread and circulating levels of proinflammatory cytokines during sepsis
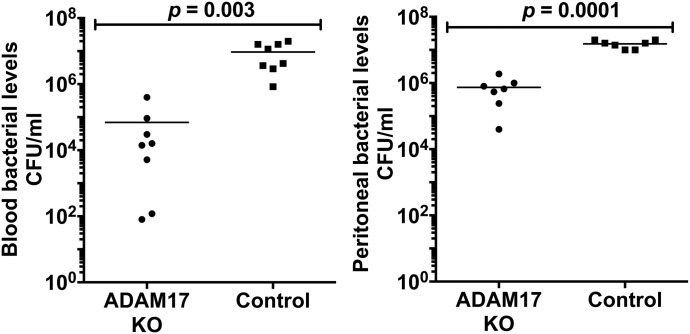
Sepsis pathogenesis increases with higher circulating levels of proinflammatory cytokines and bacteria [39]. Conditional ADAM17 knockout and control mice were compared for these determinants of sepsis severity. The time point of maximum contrast in sepsis susceptibility between conditional ADAM17 knockout and control mice, at which surviving mice in both groups could be examined, was at 24 h post-CLP (18-G, 2-puncture; Fig. 1A). Of interest was that blood and peritoneal bacterial levels were markedly lower in conditional ADAM17 knockout mice (Fig. 2).
Figure 2. Decreased bacterial spread in conditional ADAM17 knockout mice during sepsis.
Conditional ADAM17 knockout and control mice were subjected to CLP (18-G, 2-puncture). CFUs in the blood and peritoneal cavity, as indicated, were determined 24 h post-CLP.
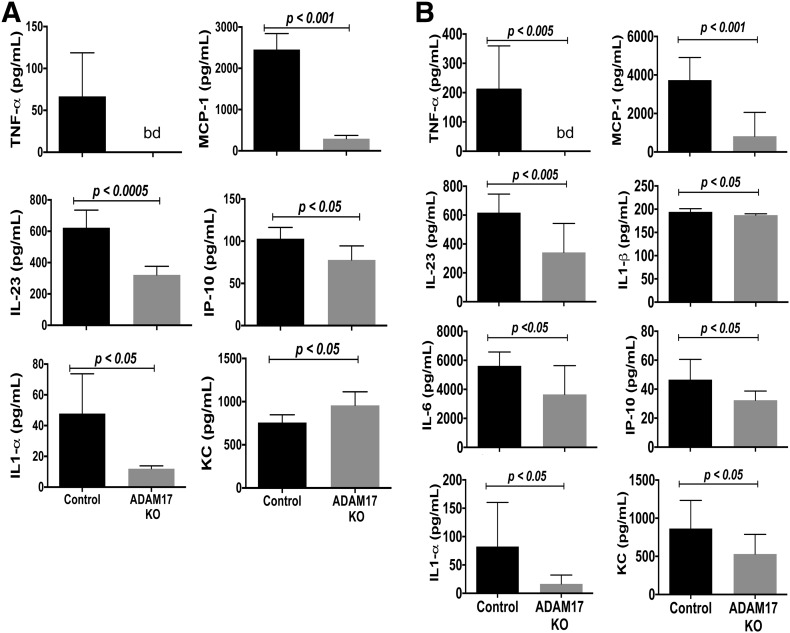
In addition to increased bacterial infection, the systemic levels of various proinflammatory factors were significantly higher in control mice than conditional ADAM17 knockout mice during sepsis. We determined the plasma concentrations of various inflammation-regulating cytokines and chemokines (IL-1α, IL-1β, IL-6, IL-23p19, IFN-γ, IL-12p70, IL-10, KC, MCP-1, and IP-10). At 24 h post-CLP, the plasma levels of TNF-α, IL-1α, IL-1β, IL-6, IL-23p19, KC, MCP-1, and IP-10 were reduced significantly in conditional ADAM17 knockout mice, although some were more pronounced than others (Fig. 3B). TNF-α levels, for instance, were strikingly lower in conditional ADAM17 knockout mice. The plasma levels of IFN-γ, IL-12p70, and IL-10, however, did not differ significantly in the 2 groups of mice (data not shown). Even early during abdominal infection (12 h post-CLP), some cytokines and chemokines were found to be significantly lower in conditional ADAM17 knockout mice, including TNF-α, IL-1α, IL-23p19, MCP-1, and IP-10 (Fig. 3A). None of the cytokines examined demonstrated higher levels in conditional ADAM17 knockout mice following CLP, except for the chemokine KC, which was increased significantly at 12 h post-CLP and decreased significantly at 24 h when compared with control mice (Fig. 3). The reasons for this are currently unclear.
Figure 3. Reduced circulating levels of proinflammatory cytokines in conditional ADAM17 knockout mice during sepsis.
Conditional ADAM17 knockout and control mice were subjected to CLP (18-G, 2-puncture). (A) Plasma cytokine levels were quantified 12 h post-CLP; n = 6 mice per group. (B) Plasma cytokine levels were quantified 24 h post-CLP; n = 8 mice per group. bd, Below the detection limit of the standard curve.
Several sheddase mechanisms have been reported to contribute to soluble TNF-α production [40–42]. The very low-circulating levels of TNF-α in conditional ADAM17 knockout mice further demonstrate a key role by ADAM17 [11, 19]. The diminished levels of other circulating cytokines and chemokines in conditional ADAM17 knockout mice, which are not known substrates of the sheddase, were likely a result of reduced bacterial infection, and/or their expression occurs downstream of TNF-α signaling. For instance, the circulating levels of MCP-1 were appreciably reduced in conditional ADAM17 knockout mice during sepsis at the time points examined (Fig. 3), and TNF-α has been reported to increase its expression in neutrophils [43].
ADAM17 activity impedes neutrophil infiltration at the infectious locus during sepsis
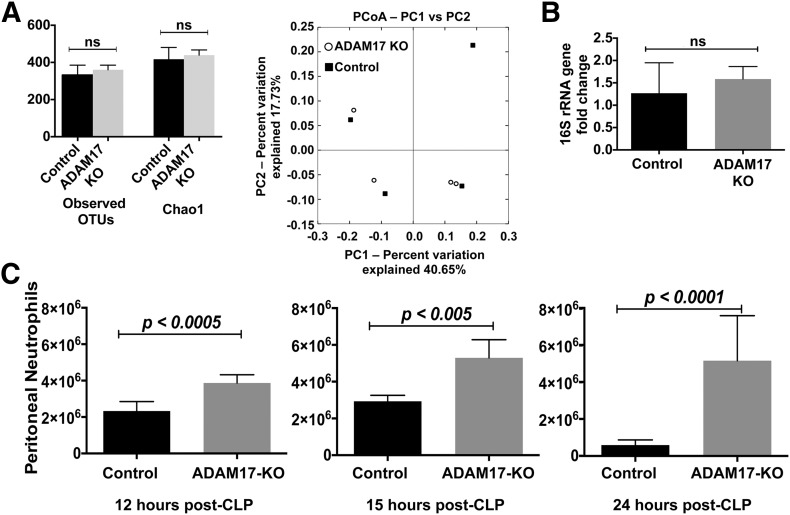
As shown in Fig. 2, bacteria levels in the blood and peritoneal cavity of conditional ADAM17 knockout mice were distinctly lower than in control mice post-CLP. To establish a mechanism underlying the reduced spread of bacteria in conditional ADAM17 knockout mice, we initially examined whether gene-targeting ADAM17 in leukocytes altered in some manner the baseline bacterial community and/or bacterial load in the cecum. To determine this, we performed high-throughput DNA sequencing of the V5–V6 hypervariable region of the 16S rRNA gene to assess the cecal microbiota in unmanipulated conditional ADAM17 knockout and control mice. These analyses revealed that the cecal bacterial communities were not significantly different in the 2 groups of mice, based on α diversity (OTU distribution and richness) and β diversity (PCoA; Fig. 4A). Moreover, bacterial plating (data not shown) and 16S rRNA gene qPCR indicated that the relative abundance of cecal bacteria was also not significantly different in conditional ADAM17 knockout and control mice (Fig. 4B). Hence, the lower levels of bacterial spread in conditional ADAM17 knockout mice following CLP were not a result of baseline differences in their cecal microbiota, although we cannot rule out undetected bacterial species or changes that may have occurred after CLP.
Figure 4. ADAM17 deficiency in leukocytes increases neutrophil recruitment during sepsis.
(A) High-throughput DNA sequencing of the V5–V6 hypervariable regions of the 16S rRNA gene was performed on DNA samples isolated from the cecal contents of unmanipulated, conditional ADAM17 knockout and control mice. Taxa distribution (Observed OTUs) and richness (Chao1) within cecal bacterial communities from conditional ADAM17 knockout and control mice (left), as well as their overlap (right) were determined; n = 4 mice per group. ns, Not significant; PC1/2, principal coordinates 1/2. (B) qPCR of the 16S rRNA gene in the cecal contents of unmanipulated conditional ADAM17 knockout and control mice; n = 4 mice per group. (C) Conditional ADAM17 knockout and control mice were subjected to CLP (18-G, 2-puncture). Peritoneal lavage was performed at the indicated time points after CLP and neutrophil counts determined: 12 h post-CLP, n = 6 mice per group; 15 h post-CLP, n = 4 mice per group; 24 h post-CLP, n = 9 mice per group.
Neutrophils are critical, early responders during bacterial infection [1]. Blockage of ADAM17 can amplify neutrophil infiltration at sites of inflammation [23, 26, 28, 30], although this has not been examined during polymicrobial sepsis, which is known to suppress neutrophil recruitment [4, 5]. In unmanipulated, conditional ADAM17 knockout and control mice, peritoneal neutrophil counts were very low and did not differ significantly (6125 ± 5303 and 7587 ± 1749, respectively; means ± sd, n = 3 mice in each group). Neutrophil absolute and differential counts in the blood of these mice were equivalent as well (data not shown), as reported previously [26–28]. Conditional ADAM17 knockout mice, however, exhibited significantly higher levels of peritoneal neutrophils at all time points examined post-CLP (Fig. 4C), revealing that the targeting of ADAM17 in leukocytes increases neutrophil recruitment during sepsis. Sepsis also results in the efflux of mature and immature (band) neutrophils from the bone marrow [44]. We found that their proportions in the blood did not significantly vary in the 2 groups of mice post-CLP (data not shown). Unlike neutrophils, we did not observe increased levels of peritoneal macrophages in conditional ADAM17 knockout mice at any of the time points examined post-CLP (data not shown), which is consistent with previous findings for thioglycollate-induced peritonitis [28].
In consideration of ADAM17’s diverse substrates [10], a combination of causal mediators likely contributed to the amplification of neutrophil recruitment and increased bacterial clearance in conditional ADAM17 knockout mice during sepsis. For instance, blockage of ADAM17 prevents the down-regulation of various receptors on the surface of leukocytes, as described in Introduction. High circulating levels of TNF-α can also impair vascular function and neutrophil migration during sepsis [4], which was markedly reduced in conditional ADAM17 knockout mice. The blockage of TNF-α signaling, however, is also known to impair the antibacterial host response during sepsis [45–48]. Of importance is that blocking TNF-α and blocking ADAM17 are not equivalent events, as the latter disrupts TNF-α conversion to its soluble form but not necessarily its function. Indeed, membrane TNF-α has been shown to provide localized antimicrobial activity [49, 50].
In conclusion, our findings suggest that extensive ADAM17 induction during sepsis may tip the balance between efficient and impaired neutrophil recruitment. Therefore, it will be interesting to examine the targeting of ADAM17 as a host-directed therapeutic approach for sepsis. Of note is that ADAM17 knockout mice are embryonic or perinatal lethal [29], and a loss-of-function mutation in ADAM17 in humans has been reported to cause inflammatory diseases [51]. However, pharmacological inhibitors of ADAM17 have progressed to clinical trials for cancer and have been shown to be safe and well tolerated [52, 53]. Thus, the temporary targeting of ADAM17 with highly specific inhibitors may not result in significant adverse effects or toxicity.
AUTHORSHIP
H.K.M. designed and performed the research, analyzed data, and helped with manuscript preparation. T.J.J. helped perform the research and analyze the data. D.M.S is a board-certified veterinary clinical pathologist, and he helped analyze the data. B.W. designed the research, analyzed and interpreted data, and helped with manuscript preparation.
ACKNOWLEDGMENTS
This study was supported by Grants AI103328, AI107543, and HL128580 (to B.W.) from the U.S. National Institutes of Health.
Glossary
- 18-G, 2-puncture
2 through-and-through punctures of the cecum with an 18 G needle
- 21-G, 1-puncture
1 through-and-through puncture of the cecum with a 21 G needle
- ADAM17
a disintegrin and metalloprotease
- CLP
cecal ligation and puncture
- CT
cycle threshold
- FAM
fluorescein amidite
- IP-10
IFN-γ-inducible protein 10
- KC
keratinocyte-derived chemokine
- MFI
mean fluorescence intensity
- OTU
operational taxonomic unit
- PCoA
principal coordinates analysis
- QIIME
Quantitative Insights into Microbial Ecology
- qPCR
quantitative PCR
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Mayadas T. N., Cullere X., Lowell C. A. (2014) The multifaceted functions of neutrophils. Annu. Rev. Pathol. 9, 181–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quaid G. A., Cave C., Robinson C., Williams M. A., Solomkin J. S. (1999) Preferential loss of CXCR-2 receptor expression and function in patients who have undergone trauma. Arch. Surg. 134, 1367–1371, discussion 1371–1372. [DOI] [PubMed] [Google Scholar]
- 3.Kordonowy L. L., Burg E., Lenox C. C., Gauthier L. M., Petty J. M., Antkowiak M., Palvinskaya T., Ubags N., Rincón M., Dixon A. E., Vernooy J. H., Fessler M. B., Poynter M. E., Suratt B. T. (2012) Obesity is associated with neutrophil dysfunction and attenuation of murine acute lung injury. Am. J. Respir. Cell Mol. Biol. 47, 120–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sônego F., Alves-Filho J. C., Cunha F. Q. (2014) Targeting neutrophils in sepsis. Expert Rev. Clin. Immunol. 10, 1019–1028. [DOI] [PubMed] [Google Scholar]
- 5.Lerman Y. V., Kim M. (2015) Neutrophil migration under normal and sepsis conditions. Cardiovasc. Hematol. Disord. Drug Targets 15, 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves-Filho J. C., Freitas A., Souto F. O., Spiller F., Paula-Neto H., Silva J. S., Gazzinelli R. T., Teixeira M. M., Ferreira S. H., Cunha F. Q. (2009) Regulation of chemokine receptor by Toll-like receptor 2 is critical to neutrophil migration and resistance to polymicrobial sepsis. Proc. Natl. Acad. Sci. USA 106, 4018–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves-Filho J. C., Sônego F., Souto F. O., Freitas A., Verri W. A. Jr, Auxiliadora-Martins M., Basile-Filho A., McKenzie A. N., Xu D., Cunha F. Q., Liew F. Y. (2010) Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat. Med. 16, 708–712. [DOI] [PubMed] [Google Scholar]
- 8.Spiller F., Orrico M. I., Nascimento D. C., Czaikoski P. G., Souto F. O., Alves-Filho J. C., Freitas A., Carlos D., Montenegro M. F., Neto A. F., Ferreira S. H., Rossi M. A., Hothersall J. S., Assreuy J., Cunha F. Q. (2010) Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am. J. Respir. Crit. Care Med. 182, 360–368. [DOI] [PubMed] [Google Scholar]
- 9.Zonneveld R., Martinelli R., Shapiro N. I., Kuijpers T. W., Plötz F. B., Carman C. V. (2014) Soluble adhesion molecules as markers for sepsis and the potential pathophysiological discrepancy in neonates, children and adults. Crit. Care 18, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gooz M. (2010) ADAM-17: the enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 45, 146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Black R. A., Rauch C. T., Kozlosky C. J., Peschon J. J., Slack J. L., Wolfson M. F., Castner B. J., Stocking K. L., Reddy P., Srinivasan S., Nelson N., Boiani N., Schooley K. A., Gerhart M., Davis R., Fitzner J. N., Johnson R. S., Paxton R. J., March C. J., Cerretti D. P. (1997) A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 385, 729–733. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y., Herrera A. H., Li Y., Belani K. K., Walcheck B. (2009) Regulation of mature ADAM17 by redox agents for L-selectin shedding. J. Immunol. 182, 2449–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y., Robertson J. D., Walcheck B. (2011) Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation. J. Biol. Chem. 286, 38980–38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maretzky T., McIlwain D. R., Issuree P. D., Li X., Malapeira J., Amin S., Lang P. A., Mak T. W., Blobel C. P. (2013) iRhom2 controls the substrate selectivity of stimulated ADAM17-dependent ectodomain shedding. Proc. Natl. Acad. Sci. USA 110, 11433–11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lamour N. F., Wijesinghe D. S., Mietla J. A., Ward K. E., Stahelin R. V., Chalfant C. E. (2011) Ceramide kinase regulates the production of tumor necrosis factor α (TNFα) via inhibition of TNFα-converting enzyme. J. Biol. Chem. 286, 42808–42817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu P., Liu J., Sakaki-Yumoto M., Derynck R. (2012) TACE activation by MAPK-mediated regulation of cell surface dimerization and TIMP3 association. Sci. Signal. 5, ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheller J., Chalaris A., Garbers C., Rose-John S. (2011) ADAM17: a molecular switch to control inflammation and tissue regeneration. Trends Immunol. 32, 380–387. [DOI] [PubMed] [Google Scholar]
- 18.Li Y., Brazzell J., Herrera A., Walcheck B. (2006) ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood 108, 2275–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell J. H., Herrera A. H., Li Y., Walcheck B. (2007) Role of ADAM17 in the ectodomain shedding of TNF-alpha and its receptors by neutrophils and macrophages. J. Leukoc. Biol. 82, 173–176. [DOI] [PubMed] [Google Scholar]
- 20.Weskamp G., Mendelson K., Swendeman S., Le Gall S., Ma Y., Lyman S., Hinoki A., Eguchi S., Guaiquil V., Horiuchi K., Blobel C. P. (2010) Pathological neovascularization is reduced by inactivation of ADAM17 in endothelial cells but not in pericytes. Circ. Res. 106, 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dreymueller D., Martin C., Kogel T., Pruessmeyer J., Hess F. M., Horiuchi K., Uhlig S., Ludwig A. (2012) Lung endothelial ADAM17 regulates the acute inflammatory response to lipopolysaccharide. EMBO Mol. Med. 4, 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y., Wu J., Newton R., Bahaie N. S., Long C., Walcheck B. (2013) ADAM17 cleaves CD16b (FcγRIIIb) in human neutrophils. Biochim. Biophys. Acta 1833, 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mishra H. K., Long C., Bahaie N. S., Walcheck B. (2015) Regulation of CXCR2 expression and function by a disintegrin and metalloprotease-17 (ADAM17). J. Leukoc. Biol. 97, 447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuboi N., Asano K., Lauterbach M., Mayadas T. N. (2008) Human neutrophil Fcgamma receptors initiate and play specialized nonredundant roles in antibody-mediated inflammatory diseases. Immunity 28, 833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175. [DOI] [PubMed] [Google Scholar]
- 26.Arndt P. G., Strahan B., Wang Y., Long C., Horiuchi K., Walcheck B. (2011) Leukocyte ADAM17 regulates acute pulmonary inflammation. PLoS One 6, e19938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long C., Hosseinkhani M. R., Wang Y., Sriramarao P., Walcheck B. (2012) ADAM17 activation in circulating neutrophils following bacterial challenge impairs their recruitment. J. Leukoc. Biol. 92, 667–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J., Zarbock A., Gomez I., Wilson C. L., Lefort C. T., Stadtmann A., Bell B., Huang L.-C., Ley K., Raines E. W. (2011) Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood 118, 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284. [DOI] [PubMed] [Google Scholar]
- 30.Long C., Wang Y., Herrera A. H., Horiuchi K., Walcheck B. (2010) In vivo role of leukocyte ADAM17 in the inflammatory and host responses during E. coli-mediated peritonitis. J. Leukoc. Biol. 87, 1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen H. (2013) Sepsis induced by cecal ligation and puncture. Methods Mol. Biol. 1031, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shrum B., Anantha R. V., Xu S. X., Donnelly M., Haeryfar S. M., McCormick J. K., Mele T. (2014) A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 7, 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schloss P. D., Westcott S. L., Ryabin T., Hall J. R., Hartmann M., Hollister E. B., Lesniewski R. A., Oakley B. B., Parks D. H., Robinson C. J., Sahl J. W., Stres B., Thallinger G. G., Van Horn D. J., Weber C. F. (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caporaso J. G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F. D., Costello E. K., Fierer N., Peña A. G., Goodrich J. K., Gordon J. I., Huttley G. A., Kelley S. T., Knights D., Koenig J. E., Ley R. E., Lozupone C. A., McDonald D., Muegge B. D., Pirrung M., Reeder J., Sevinsky J. R., Turnbaugh P. J., Walters W. A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. (2010) QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar R. C. (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. [DOI] [PubMed] [Google Scholar]
- 36.Cole J. R., Wang Q., Cardenas E., Fish J., Chai B., Farris R. J., Kulam-Syed-Mohideen A. S., McGarrell D. M., Marsh T., Garrity G. M., Tiedje J. M. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–D145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 38.Roth D. M., Swaney J. S., Dalton N. D., Gilpin E. A., Ross J. Jr (2002) Impact of anesthesia on cardiac function during echocardiography in mice. Am. J. Physiol. Heart Circ. Physiol. 282, H2134–H2140. [DOI] [PubMed] [Google Scholar]
- 39.Schulte W., Bernhagen J., Bucala R. (2013) Cytokines in sepsis: potent immunoregulators and potential therapeutic targets—an updated view. Mediators Inflamm. 2013, 165974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haro H., Crawford H. C., Fingleton B., Shinomiya K., Spengler D. M., Matrisian L. M. (2000) Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J. Clin. Invest. 105, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng Y., Saftig P., Hartmann D., Blobel C. (2004) Evaluation of the contribution of different ADAMs to tumor necrosis factor alpha (TNFalpha) shedding and of the function of the TNFalpha ectodomain in ensuring selective stimulated shedding by the TNFalpha convertase (TACE/ADAM17). J. Biol. Chem. 279, 42898–42906. [DOI] [PubMed] [Google Scholar]
- 42.Vandenbroucke R. E., Dejonckheere E., Van Hauwermeiren F., Lodens S., De Rycke R., Van Wonterghem E., Staes A., Gevaert K., López-Otin C., Libert C. (2013) Matrix metalloproteinase 13 modulates intestinal epithelial barrier integrity in inflammatory diseases by activating TNF. EMBO Mol. Med. 5, 932–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashiro S., Kamohara H., Yoshimura T. (2000) Alteration in the responsiveness to tumour necrosis factor-alpha is crucial for maximal expression of monocyte chemoattractant protein-1 in human neutrophils. Immunology 101, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delano M. J., Kelly-Scumpia K. M., Thayer T. C., Winfield R. D., Scumpia P. O., Cuenca A. G., Harrington P. B., O’Malley K. A., Warner E., Gabrilovich S., Mathews C. E., Laface D., Heyworth P. G., Ramphal R., Strieter R. M., Moldawer L. L., Efron P. A. (2011) Neutrophil mobilization from the bone marrow during polymicrobial sepsis is dependent on CXCL12 signaling. J. Immunol. 187, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Echtenacher B., Falk W., Männel D. N., Krammer P. H. (1990) Requirement of endogenous tumor necrosis factor/cachectin for recovery from experimental peritonitis. J. Immunol. 145, 3762–3766. [PubMed] [Google Scholar]
- 46.Eskandari M. K., Bolgos G., Miller C., Nguyen D. T., DeForge L. E., Remick D. G. (1992) Anti-tumor necrosis factor antibody therapy fails to prevent lethality after cecal ligation and puncture or endotoxemia. J. Immunol. 148, 2724–2730. [PubMed] [Google Scholar]
- 47.Zeni F., Freeman B., Natanson C. (1997) Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit. Care Med. 25, 1095–1100. [DOI] [PubMed] [Google Scholar]
- 48.Echtenacher B., Weigl K., Lehn N., Männel D. N. (2001) Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 69, 3550–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olleros M. L., Guler R., Vesin D., Parapanov R., Marchal G., Martinez-Soria E., Corazza N., Pache J. C., Mueller C., Garcia I. (2005) Contribution of transmembrane tumor necrosis factor to host defense against Mycobacterium bovis bacillus Calmette-Guerin and Mycobacterium tuberculosis infections. Am. J. Pathol. 166, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torres D., Janot L., Quesniaux V. F., Grivennikov S. I., Maillet I., Sedgwick J. D., Ryffel B., Erard F. (2005) Membrane tumor necrosis factor confers partial protection to Listeria infection. Am. J. Pathol. 167, 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blaydon D. C., Biancheri P., Di W. L., Plagnol V., Cabral R. M., Brooke M. A., van Heel D. A., Ruschendorf F., Toynbee M., Walne A., O’Toole E. A., Martin J. E., Lindley K., Vulliamy T., Abrams D. J., MacDonald T. T., Harper J. I., Kelsell D. P. (2011) Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med. 365, 1502–1508. [DOI] [PubMed] [Google Scholar]
- 52.Duffy M. J., Mullooly M., O’Donovan N., Sukor S., Crown J., Pierce A., McGowan P. M. (2011) The ADAMs family of proteases: new biomarkers and therapeutic targets for cancer? Clin. Proteomics 8, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman S., Levy R., Garrett W., Doval D., Bondarde S., Sahoo T., Lokanatha D., Julka P., Shenoy K., Nagarkar R., Bhattacharyya G., Kumar K., Nag S., Mohan P., Narang N., Raghunadharao D., Walia M., Yao W., Li J., Emm T., Yeleswaram S., Scherle P., Newton R. (2009) Clinical benefit of INCB7839, a potent and selective inhibitor of ADAM10 and ADAM17, in combination with trastuzumab in metastatic HER2 positive breast cancer patients. Cancer Res. 69, 5056 (abs.). [Google Scholar]