Review on the molecular control of TLR signaling by chaperones, co-receptor molecules, intracellular regulators, and post-translational modifications.
Keywords: innate immunity, inflammation, pattern recognition receptors, macrophages, signal transduction
Abstract
TLRs play a critical role in the detection of microbes and endogenous “alarmins” to initiate host defense, yet they can also contribute to the development and progression of inflammatory and autoimmune diseases. To avoid pathogenic inflammation, TLR signaling is subject to multilayer regulatory control mechanisms, including cooperation with coreceptors, post-translational modifications, cleavage, cellular trafficking, and interactions with negative regulators. Nucleic acid-sensing TLRs are particularly interesting in this regard, as they can both recognize host-derived structures and require internalization of their ligand as a result of intracellular sequestration of the nucleic acid-sensing TLRs. This review summarizes the regulatory mechanisms of TLRs, including regulation of their access to ligands, receptor folding, intracellular trafficking, and post-translational modifications, as well as how altered control mechanism could contribute to inflammatory and autoimmune disorders.
Introduction
TLRs are membrane-associated innate-immune sensors that recognize conserved microbial-associated molecular patterns of invading pathogens (e.g., LPS) or mislocalized commensal bacteria. They also detect host danger-associated molecular patterns or alarmins, such as HMGB1, endogenous RNA and DNA that are normally hidden in TLR-inaccessible compartments but become exposed and are released during cell stress, inflammation, or infection [1–4]. TLRs contain an ectodomain with multiple LRRs involved in ligand detection and coreceptor interactions, a transmembrane portion, and an intracellular TIR homology domain essential for signaling [1–4]. TLRs are expressed by macrophages, neutrophils, DCs, NK cells, and mast cells of the innate-immune system; T- and B-lymphocytes of the adaptive branch; as well as by some nonimmune cells, such as epithelial and endothelial cells [1–4]. TLRs are expressed on the plasma membrane (e.g., TLR1, TLR5, TLR6, and TLR10), in intracellular endosomes (e.g., TLR3, TLR7–9, TLR11), or in both compartments (e.g., TLR2 and TLR4) [5], and their localization critically regulates TLR signaling.
In this review, we discuss mechanisms that regulate TLR expression, trafficking, and signal transduction. The regulatory mechanisms fall into several main categories, including access to ligand, assembly of active signaling complexes, and post-translational modifications of the TLRs, as well as APs and kinases involved in the signaling cascades. There are some conserved regulatory mechanisms among all TLRs, but nucleic acid-sensing TLRs have additional layers of unique and multifaceted regulation.
TLR STRUCTURE AND LIGAND BINDING
TLR ectodomains form a solenoid-shaped structure with the inner-concave surface comprised of β sheets with more unstructured loops forming the outer edge [6–8]. TLRs bind to a number of diverse molecular structures, including lipids (e.g., TLR4: LPS via MD2; TLR2: lipoproteins), proteins (e.g., TLR5: flagellin; TLR2 and TLR4: HMGB1), and nucleic acids (e.g., TLR3: dsRNA; TLR7/8: ssRNA; and TLR9: unmethylated CpG motifs in bacterial, viral, and fungal DNA) [5]. Several TLRs require cooperation with coreceptors for ligand binding, such as TLR1 or TLR6 for TLR2, MD2 for TLR4, and CD14 for TLR2, TLR4, and TLR3 [9]. The molecular interaction of ligands and TLRs could involve different molecular interfaces [7, 8]. For example, TLR3 binds dsRNA along the concave dimerization interface contacting 2 different sites on each monomer of the TLR3 dimer—one at the N-terminus and the other at the C-terminus of the solenoid [10–12]. In contrast, both components of the TLR1/TLR2 heterodimer contribute to lipopeptide binding at the convex dimerization interface [13]. For TLR5, there is a 2:2 conformation with a dimer of flagellin binding to a dimer of TLR5, but the flagellin binds on the lateral side of TLR5, not the side that forms the dimer interface [14–17]. The ectodomain of membrane-associated TLRs is required for ligand binding and formation of functional dimers for signaling.
TLR FOLDING AND TRAFFICKING DEPEND ON ACCESSORY PROTEINS THAT ARE IMPORTANT FOR TLR EXIT FROM THE ER
TLRs must be properly folded to traffic to their final destination. Some TLRs (TLR2, TLR4, TLR5, TLR6) traffic to the cell surface, whereas nucleic acid-sensing TLRs (TLR3, TLR7, TLR8, and TLR9) remain primarily sequestered in the ER with low-level, continuous exit from the ER and trafficking to the endosomal system to survey for ligands [18–21]. The folding of TLRs depends on several chaperoning proteins, including gp96 (also known as glucose-regulated protein 94 and heat shock protein 90b1), CNPY3 and CNPY4 (also known as PRAT4A and PRAT4B, respectively), and UNC93B1 (Fig. 1).
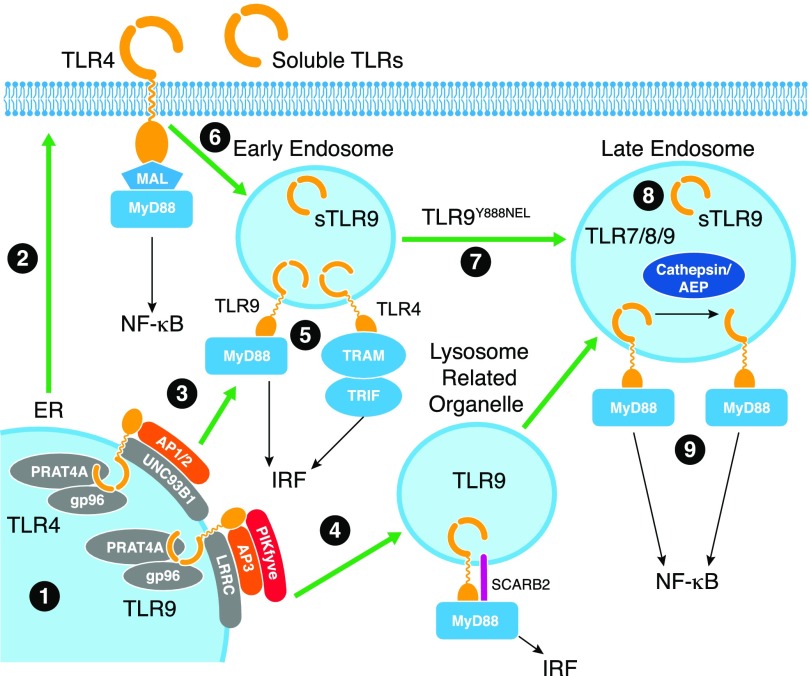
Figure 1. Regulation of TLR folding, trafficking, and cleavage.
1) TLRs are synthesized in the ER, and their correct folding requires gp96 and PRAT4A chaperones. 2) Surface TLRs follow traditional secretory pathways to access the cell surface. 3) Nucleic acid-sensing TLRs associate with UNC93B1, which itself associates with LRRC, and assists in trafficking to the early endosome. 4) Trafficking to endosomes in DCs depends on SCARB2, AP3, and PIKfyve to access the lysosome-related organelle. 5) Encounter with ligand in early endosomes or the lysosome-related organelle will elicit a signaling pathway, resulting in type I IFN production. In the early endosome, sTLR9 is generated that negatively regulates TLR9 signaling. 6) Surface TLR4 initiates signaling to the NF-κB pathway at the surface and upon internalization into the early endosome, reassembles a TRAM/TRIF complex on the TIR domain to elicit type I IFN production. 7) TLR9 trafficking from the early endosome to the lysosome depends on tyrosine 888 within a YNEL motif (single amino acids code). 8) Upon entry into this compartment, TLR9 and other nucleic acid-sensing TLRs are proteolytically cleaved by cathepsins and AEP to generate a mature form. 9) It is in this compartment that signaling leads to activation of the NF-κB pathway.
gp96 binds and facilitates folding of several surface and endosomal TLRs [22, 23]. Macrophage-specific deletion of gp96 compromised signaling through multiple TLRs, including both surface (TLR2 and TLR4) and endosomal (TLR7 and TLR9) receptors, as a result of the failure of TLRs to traffic to their corresponding cellular compartments [24]. Interestingly, gp96 also chaperones integrins [22, 25], known regulators of TLR signaling [25–27]. There is evidence to suggest that gp96 also requires a cochaperone for proper TLR folding [23]. Whereas gp96 was originally noted to be itself an agonist for TLR2 and TLR4, later studies identified endotoxin or lipoprotein contamination of gp96 preparations that could be responsible for this phenomenon [28]. Thus, further studies are needed to determine whether gp96 could directly stimulate TLRs. Preincubation of LPS (TLR4 agonist) or Pam3CSK4 (TLR2 agonist) with low amounts of purified gp96 increased TLR signaling, suggesting that gp96 could act by stabilizing TLR ligands [29].
CNPY3 (PRAT4A) is also important for regulating TLR folding and trafficking [30, 31] (Fig. 1). CNPY3 and CNPY4 comprise a family of ER-resident chaperone proteins, and although both have been implicated in trafficking and surface expression of TLR1 [32], CNPY3 plays a more prominent role in regulating TLRs. CNPY3 is required for surface expression of TLR1, TLR2, TLR4, and TLR5 [33], as well as for TLR9 signaling [34]. TLR3 expression and signaling are not dependent on CNPY3 [34], and interaction of endogenous CNPY3 with a TLR was only demonstrated for TLR4; thus, it remains unclear whether all TLRs are bona fide client proteins of CNPY3 [34].
UNC93B is a 12-segment transmembrane protein that is selectively required for nucleic acid-sensing TLRs [35]. With the use of a reverse genetic approach of mutagen-induced screening of mice, Beutler and colleagues [35] discovered a recessive 3d mutant strain that failed to respond to TLR3, TLR7, and TLR9 agonists. Positional cloning identified a nonsynonymous mutation in UNC93B (H412R) that introduced a positive charge in the ninth transmembrane domain. Further studies showed that UNC93B associates directly with TLR3, TLR7, and TLR9, and the H412R mutation disrupts signaling [36]. Sites in the transmembrane domain [36] and in the juxtamembrane region N-terminal to the transmembrane domain contribute to UNC93B binding to TLRs [37]. The mechanism by which UNC93B regulates TLRs is different than that of CNPY3 or gp96. In the absence of UNC93B or in the presence of the 3d mutant of UNC93B, TLR3, TLR7, TLR9, and TLR11 and TLR13 (mouse) are retained in the ER and do not traffic to endosomes [38–40]. This is because unlike UNC93B trafficking with TLRs to the endosomal compartment, the 3d mutant is itself defective in endosomal trafficking [38]. TLR5 trafficking to the cell surface also requires UNC93B1, suggesting that the chaperoning function of UNC93B1 is not restricted to nucleic acid-sensing TLRs [41].
Although UNC93B binds to all nucleic acid-sensing TLRs, including human TLR8 [42], it preferentially associates with TLR9. A single-point mutation D34A causes increased binding of UNC93B1 to TLR7, increased trafficking of TLR7 to endosomes, and higher levels of TLR7 signaling. Thus, D34 on UNC93B1 is located in a region that normally suppresses TLR7 signaling [43]. In contrast, the same mutation of D34 on UNC93B1 reduced association of UNC93B1 with TLR9, reduced TLR9 trafficking to endosomes, and reduced TLR9 signaling [43]. These results demonstrate that the interaction of TLR7 and TLR9 with UNC93B1 is reciprocal and that UNC93B1 preferentially associates with TLR9 to regulate systemic inflammation that would otherwise be mediated by TLR7 [39]. In addition to the D34 region, a tyrosine motif at aa 539–542 of UNC93B1 was shown to bind to the trafficking AP2 protein in mouse [40] and both AP1 and AP2 in human cells [44]. The binding to AP1 and AP2 was required for appropriate endosomal trafficking of UNC93B1; however, disruption of the tyrosine motif did not lead to global loss of endosomal TLR signaling. In HEK293 cells, mutation of the tyrosine motif in human UNC93B1 abolished signaling by human TLR9 to CpG DNA and human TLR7 to R848; however, signaling by human TLR8 to R848 was, in fact, augmented [44]. Likewise, in THP-1 cells that express TLR7 and TLR8, the response to R848 that preferentially activates TLR7 was lower, whereas the response to CL075 that preferentially activates TLR8 was unaffected by expression of the UNC93B1 tyrosine mutant [44]. These data suggest that in addition to preferential association of UNC93B1 with TLR9 over TLR7, there exists more subtle selectivity of UNC93B1 for TLRs that depends on a specific tyrosine motif. It also appears that UNC93B1 cooperates with LRRC59 [45] (Fig. 1). Endocytosis of poly(I:C), CL075, Pam2CSK4, LPS, or even polystyrene beads or OVA all led to LRRC59 binding to UNC93B1, which suggests that this is an initial response to endocytosis. Yet, the interaction between LRRC59 and UNC93B1 is likely required for translocation of TLR3 from the ER to endosomes, as RNA silencing of LRRC59 prevented this translocation event [45]. Exactly how these interactions regulate TLR trafficking and signaling requires additional study.
Several examples demonstrate the importance of folding and trafficking of TLRs for balanced regulation of inflammatory responses. Forced surface expression of gp96 causes systemic autoimmune-like inflammation that is dependent on MyD88, suggesting that TLRs are likely mediating the response [46, 47]. Patients with RA were reported to express elevated levels of gp96 in the synovial fluid, correlating with increased TLR2 expression and enhanced production of TNF-α and IL-8 by synovial macrophages of RA patients compared with non-RA patients and healthy volunteers [48]. Furthermore, antibody neutralization of gp96 in synovial fluid of RA patients abrogated its capacity to up-regulate proinflammatory cytokines in macrophages [49]. Thus, gp96 acts as a critical chaperone protein for folding of TLRs and integrins, and gp96 likely also fine tunes TLR signaling by stabilizing TLR ligands and promoting their interactions with receptors (Fig. 1). Mice deficient in CNPY3 were resistant to LPS-induced endotoxic shock but were highly impaired in response to whole bacterial challenge and failed to mount an appropriate Th1 immune response [31]. Patients with the autoimmune disease SLE have up-regulated UNC93B1 [50], and mice with the UNC93B1 3d mutant had reduced autoantibody production, as well as clinical manifestations of lupus [51]. Mice expressing the UNC93B1 D34A mutant, which binds better to TLR7 and potentiates TLR7 signaling, exhibited robust systemic inflammation that depended on B cells [39]. Together, these studies illustrate the tight regulation of TLRs necessary to elicit protective inflammation while restricting autoinflammation.
TLR TRAFFICKING REGULATES ACCESS TO LIGANDS AND SIGNALING
Intracellular trafficking plays a multifaceted role in the regulation of signaling by both nucleic acid-sensing, endosomal TLRs and cell-surface TLRs. The same TLR can initiate different signaling pathways from different intracellular compartments. For instance, TLR4 induces a TIRAP–MyD88-dependent signaling pathway from the cell surface that results in downstream activation of MAPKs, IKK, NF-κB activation, and induction of proinflammatory cytokines [52]. Upon subsequent endocytosis, TLR4 triggers a TRAM–TRIF-dependent signaling pathway that results in the activation of TBK1, IRF3, and induction of type I IFNs [53] (Fig. 1). Trapping TLR4 at the surface following LPS stimulation with the dynamin inhibitor dynasore reduced signaling through the TRIF-dependent pathway, yet the MyD88-dependent pathway was not inhibited [54].
TLR2 has also been reported to initiate signaling from both the plasma membrane and endosomal compartments to activate NF-κB and proinflammatory cytokines in macrophages and DCs [55–57]. In addition to proinflammatory cytokine production, lipoteichoic acid from Staphylococcus aureus and infection with Listeria monocytogenes or Lactobacillus acidophilus stimulated type I IFN through TLR2 [58–60]. DNA virus infection, such as VACV in inflammatory monocytes or mouse gammaherpesvirus-68 in HEK293 cells, also induced type I IFNs [61, 62]. However, TLR2 was not required for virus-induced type I IFN production or protection from VACV challenge in vivo [63]. Most reports found that TLR2 uses the MyD88–IRAK4–IRAK1 signaling module to induce proinflammatory cytokines. Interestingly, similar to TLR4, TLR2 has also been reported to assemble a signaling complex, including TRAM and TRIF, in the endosomal compartment, which mediates the induction of CCL5 via TBK1 and IRF3 [64]. Future studies are required to determine the exact mechanisms of spatial-temporal organization of TLR2 signaling, use of adapter-kinase modules in the plasma membrane, endosomal and phagolysosomal compartments to signal induction of proinflammatory cytokines, and type I IFNs in vitro and in vivo in different cell types and the role of type I IFN induction through TLR2 in host defense. Together, these data show that regulation of TLR signaling depends on parallel and sequential assembly of signaling complexes in different intracellular compartments and may function in a cell- and microbe-specific manner (Fig. 1).
TLR4 trafficking is governed by several endosome-associated Rab proteins. Rab11 can target TLR4 to a recycling endosome compartment, where it engages IRF3 and signals to the IFN pathway [65]. Following LPS stimulation, TLR4 is internalized to a Rab7-positive compartment, which also contains lysosomal-associated membrane protein 1. The silencing of Rab7 led to trafficking of TLR4 to early endosomes but resulted in its failure to access the late endosomes and enhanced both proinflammatory and type I IFN production. Overexpression of Rab7 enhanced the degradation and turnover of TLR4 [66]. Thus, inhibition of TLR4 access to the lysosomal compartment enhances TLR4 signaling, whereas the promotion of targeted trafficking to lysosomes reduces signaling.
Similar regulation occurs at the level of trafficking for prototypical endosomal TLR, TLR9, which traffics through the Golgi compartment, where it is glycosylated, but much of TLR9 remains either in the ER or the Golgi without stimulation [18, 19, 21] (Fig. 1). TLR9 trafficking to the endosomal compartment is dependent on specific sequences in the cytoplasmic tail of TLR9 [20] (Fig. 1), although the transmembrane domain has also been implicated [67]. For TLR3, the ectodomain has been implicated in trafficking to endosomes [68, 69]. Thus, exact mechanisms governing intracellular trafficking require further study.
The outcome of TLR9 signaling depends on the intracellular compartment, where TLR9 encounters its ligand, CpG DNA [70, 71] (Fig. 1). CpG DNAs that primarily induce type I IFN production are preferentially retained in early endosomes, whereas CpG DNAs that induce inflammatory cytokines traffic to late endosomes/lysosomes [71]. Inflammatory cytokine-inducing CpG DNAs could be changed to type I IFN-inducing ligands by complexing with microparticles that retained them in early endosomes [70]. In contrast, encapsulation in liposomes that only released their contents at a pH lower than 5.75 eliminated type I IFN production [70]. A tyrosine Y888 in the cytoplasmic tail of TLR9 that was selectively required for proinflammatory cytokine production and the Y888A mutant trafficked to early but not late endosomes [72], supporting the idea that type I IFN production is induced from early endosomes, similar to TLR4, and that proinflammatory cytokines are induced from late endosomes/lysosomes. However, there are data that suggest that signaling to the IFN pathway is induced from a specialized lysosomal compartment [73]. Regardless of the mechanism, AP3 plays a critical role in licensing TLR9 to access the compartment from which the signaling pathway, activating expression of type I IFNs, is induced [73, 74]. AP3-deficient mice have defective induction of type I IFNs but normal IL-12 production in response to TLR9 stimulation. Mechanistically, this is explained by a failure of TLR9 to access the IFN signaling compartment in the absence of AP3 [73]. In addition to AP3, PIKfyve is important for TLR9 trafficking to the endosomal compartment, where signaling for type I IFN production occurs, but this may be specific for DCs and not macrophages [75]. A recent study demonstrated that SCARB2/lysosome membrane protein 2 is highly expressed in plasmacytoid DCs and controls both internalization of CpG DNA and endosomal translocation of TLR9 for type I IFN production [76]. As SCARB2 is also important for endosome generation and organization [76], its exact role in TLR9 signaling is not yet clear.
Whereas there is still much to learn about how regulation of TLR trafficking dysregulation may contribute to disease, there are several examples in both mouse and human that we can use to infer the importance. Mice with surface expression of TLR9, as a result of mutations in the transmembrane domain, gain the ability to respond to host DNA and exhibit a systemic inflammatory response [77]. Humans with a mutation in the Β3a subunit of AP3, which is important for TLR9 trafficking (Fig. 1), have recurrent bacterial infections [78]. Mice deficient in AP3 (Pearl mice) are highly susceptible to challenge with L. monocytogenes and have reduced DC-mediated, IFN-γ-specific CD4+ T cells that are required for efficient bacterial clearance [79]. More studies with specific mutants of TLRs that exhibit defective signaling will provide additional insight into how the induction of different signaling pathways from different intracellular compartments contributes to host defense.
PROTEOLYTIC CLEAVAGE OF NUCLEIC ACID-SENSING TLRS OCCURS IN ENDOSOMES AND REGULATES TLR SIGNALING
Another level of regulation comprises a complex set of proteolytic events that occurs after nucleic acid-sensing TLRs have trafficked to the endosomal compartment (Fig. 1). TLR9 was the first TLR for which endosomal proteolytic cleavage was proposed as a regulatory mechanism. TLR9 in macrophages is present primarily as a lower molecular weight form, generated via its specific and targeted proteolysis by cathepsins and AEP within the unstructured loop region that connects LRR14 and LRR15 (aa 434–473) [80–83]. Similar to TLR9, other TLRs, including TLR3, TLR7, and TLR8, are also proteolytically cleaved [84–86].
The current model is that proteolytic cleavage of nucleic acid-sensing TLRs is a prerequisite to restrain inappropriate signaling in response to host DNA/RNA [81, 82]. However, data from different publications conflict with this notion [86], and it remains to be determined whether proteolytic cleavage of TLR3, TLR7, and TLR9 is required for signaling. In one study, TLR3 cleavage was not required for signaling, as inhibition of cleavage and generation of uncleavable mutants did not disrupt signaling [85]. However, in another study, data supported a role for cleavage of TLR3, although the need for cleavage may be cell-type specific, as this study used retinal pigment epithelial cells [86]. The swapping of the transmembrane domain of TLR9 for that of TLR3 resulted in a TLR9 chimeric protein that was not proteolytically cleaved, signaled well in response to CpG DNA, was not inhibited by the cysteine protease inhibitor benzyloxycarbonyl-phenylalanyl-alanyl-fluoromethyl ketone or endosomal acidification inhibitor bafilomycin, and exhibited low-level surface expression, suggesting that cleavage is not required for signaling [77]. Mice expressing the mutant TLR9 developed a systemic inflammatory response, mediated, at least in part, by TNF-α [77]. Onji and colleagues [87] could not restore signaling with the proteolytically cleaved form of TLR9, unless the N-terminal fragment, removed during cleavage, was added back. In HEK293 cells, Pohar and colleagues [69] used a mutant of TLR9 that contained the ectodomain and cytoplasmic tail of TLR9 connected by the transmembrane domain of TLR3 and observed higher NF-κB activation but no type I IFN response, suggesting that the transmembrane domain may be important for appropriate signaling to the IFN-inducing compartment. It is important to note that without UNC93B1 or in the presence of mutant UNC93-3d, nucleic acid-sensing TLRs do not signal as a result of failure to access the endosomal compartment [35, 36], and they are not cleaved. Thus, cleavage could positively or negatively regulate nucleic acid-sensing TLR signaling.
Soluble ectodomains are generated for a number of different TLRs, including TLR2, TLR4, and TLR9, by several mechanisms, including alternative splicing of mRNAs and proteolytic cleavage [88–93]. This is independent of the proteolytic cleavage between LRR14 and LRR15 for TLR9 described above. Endogenous human TLR9 is proteolytically cleaved between aa 724 and 735, near the transmembrane domain, to generate a soluble, negative regulatory form [94] (Fig. 1). Importantly, sTLR9 was generated from endogenous TLR9, not a tagged, overexpressed TLR9. sTLR9 bound the CpG DNA ligand and could thus act as a competitor with full-length TLR9 to bind, but sTLR9 also bound to full-length TLR9 and therefore, could be a bona fide dominant-negative regulator of signaling by disrupting formation of functional dimers. When an N-terminal fragment (aa 1–440) was fused to a transmembrane domain with an intervening cleavage site, the N-terminal fragment was released in endosomes and down-modulates TLR9 signaling similar to sTLR9 [95]. This inhibitory activity likely occurs through binding directly to functional TLR9 and reducing TLR9 stability [95]. A sTLR2 ectodomain is secreted from cells into saliva, plasma, amniotic fluid, and breast milk to modulate TLR2 signaling [88, 89, 91]. sTLR4 is also present in saliva and can bind to MD2 and regulate TLR4 signaling by binding to LPS [90, 92, 96] (Fig. 1). sTLR2 and sTLR4 ectodomains are generated via cleavage by metalloproteinases [97] or by alternative splicing of the corresponding mRNAs [90], respectively. Both sTLR2 and sTLR4 act as “decoy” negative regulators of membrane-associated, signaling-competent TLR2 and TLR4 and have shown associations with diseases, such as heart failure, after myocardial infarction and HIV infection [98, 99]. An entirely different regulatory mechanism is demonstrated by avian TLR15, where the receptor is auto inhibited by the ectodomain. Upon cleavage of the ectodomain at LRR9, the receptor is active. A full ectodomain deletion mutant was constitutively active, supporting the auto-inhibition model [100]. Together, these studies demonstrate that TLR cleavage plays an important role in regulating signaling, and the generation of soluble ectodomains may be a conserved regulatory mechanism. Whereas we can infer that this regulation will be important in vivo, we do not yet have mouse models or data from humans to explain the importance of proteolytic cleavage on host defense or autoimmune disease. For example, there could be aberrant proteolytic cleavage of TLR7 and TLR9 or generation of soluble negative regulators in SLE.
REGULATING PROTEINS ASSOCIATE WITH TLR ECTODOMAINS
Multiple proteins interact with TLR ectodomains and contribute to protein stability, cleavage, and signaling. In addition to its role for folding TLRs, gp96 remains associated with TLR9 during trafficking to the endosome and regulates TLR9 protein stability [101]. A yeast 2-hybrid screen using the second half of the TLR9 ectodomain as a bait-identified grancalcin, which binds directly to TLR9 and is required for full TLR7- and TLR9-mediated signaling through both NF-κB and IRF7 [102].
CD14 is a glycosylphosphatidylinositol-anchored membrane protein that was originally identified as part of the LPS receptor, which includes TLR4 [103]. More recently, CD14 has also been implicated in regulating nucleic acid-sensing TLRs. In the case of TLR3, CD14 deficiency reduces poly(I:C)-induced NF-κB activation in mouse bone marrow-derived DCs and in vivo [104]. Exogenous supplementation of CD14 enhanced TLR signaling, including type I IFN production [104]. The effect may be a result of a chaperoning function of CD14 on poly(I:C) uptake. CD14 may also be important for TLR7 and TLR9 by directly binding to both nucleic acid-sensing receptors and enhancing the uptake of their ligands [105]. Interestingly, CD14 is expressed in low copy numbers by nonmyeloid cells, such as endothelial cells of the vasculature and eye, as well as epithelial cells of the skin, intestine, and respiratory tract, expanding the range of cells that may use CD14 as a coreceptor for TLRs [106]. It is important to note that bovine serum contains abundant soluble CD14, which can enhance and/or endow LPS responses, and may modulate signaling by TLRs [107, 108].
SIGIRR is a shared negative regulator of the IL-1R and TLR signaling pathways. SIGIRR binds directly to IL-1R or TLR4 in a ligand-dependent manner, sequesters signaling components MyD88, IRAK, and TRAF6, and blocks response to IL-1 and LPS [109]. Interestingly, it has been suggested that the Ig domain of SIGIRR interferes with the IL-1R/IL-1RAcP dimerization, whereas the intracellular TIR domain of SIGIRR inhibits the recruitment of receptor-proximal signaling components to IL-1R/TLR4 [109]. The role of SIGIRR in regulating other TLRs is less clear.
Mice deficient in SIGIRR are hyper-responsive to LPS and thus, exhibit a higher mortality following LPS challenge [109]. SIGIRR expression in colonic epithelial cells plays an important homeostatic role in the gut and may involve TLRs other than TLR4. In the absence of SIGIRR, there are high levels of inflammation, and an exaggerated response to dextran sulfate sodium challenge and a combination of dextran sulfate sodium and azoxymethane induced tumor formation [110], which involve a number of TLRs, including TLR9 [111, 112]. These studies demonstrate that competition for, or sequestration of, downstream signaling proteins may be an important mechanism to regulate TLRs in vivo.
RP105, a TLR family member that lacks a signaling domain, regulates TLR signaling. Analogous to TLR4 association with MD2, RP105 associates with MD1. RP105 is often coexpressed with TLR4 on APCs and negatively regulates TLR4 signaling in HEK293 cells and DCs [113]. Interestingly, in B cells, the absence of the RP105/MD1 complex attenuates signaling by LPS [114] and reduces antibody responses and proliferation in response to TLR2 and TLR4 ligands [115]. RP105/MD1 also played a positive role in promoting the TLR2 response to Mycobacterium tuberculosis infection of macrophages [116]. Together, these studies show that RP105/MD1 plays a positive regulatory role in B cells compared with the negative regulatory role in DCs and hint at the complex and differential regulatory role that individual proteins can play in regulation of TLR signaling.
GLYCOSYLATION AND UBIQUITINATION AS REGULATORS OF TLR SIGNALING
N-linked glycosylation of TLR ectodomains orchestrates localization, assembly, and signaling capacities of TLRs. Site-directed mutagenesis studies and the use of chemical inhibitors have established the importance of glycosylation for assembly of the complete TLR signaling complex (signalosome), as well as for proper function [117]. Furthermore, glycosylation of coreceptor molecules, such as MD2 for TLR4 (at Asn26 and Asn114) [118], is necessary for enabling their interactions with TLRs and imparting TLR signaling. In the case of TLR2, 4 N-linked glycosylation sites within the ectodomain are required for biosynthesis, trafficking, and secretion [119]. TLR4 and MD2 are α-2-6- and α-2,3-sialylated glycoproteins, and removal of sialyl residues after neuraminidase treatment of 293/TLR4/MD2 cells enhances LPS-driven NF-κB activation and cytokine expression and increases TLR4 dimerization and recruitment of MyD88 [120, 121]. Furthermore, sialidase Neu 1 associates with TLR2, TLR3, and TLR4, and its activity is enhanced following agonist stimulation [121], using G protein-coupled receptor signaling via membrane Gα(i) subunit proteins and matrix metalloproteinase-9 [122]. TLR3 contains N-linked glycoprotein moieties, comprising ∼30% of the ectodomain [123]. Mutagenesis of N-linked glycosylation sites blocked TLR3 signaling, and treatment with tunicamycin, an inhibitor of N-linked glycosylation, also suppressed TLR3-driven responses [123]. These results strongly suggest a role for sialylation in regulating TLR4/MD2 signaling and possibly, signaling by TLR2 and TLR3. A TLR7-inactivating mutation at an N-linked glycosylation site N66–N69 was predicted to preclude conjugation of glycans, and mutagenesis of the N68 glycosylation site blocked the capacity of TLR7 to activate NF-κB [124]. Proteolytically cleaved TLR9 bound to CpG has N-linked glycan modifications characteristic of Golgi processing, e.g., the trimming of mannose residues and the incorporation of N-acetylglucosamine, galactose, and N-acetylneuraminic acid, suggesting that such glycan modifications may be important for optimal trafficking [18]. Thus, glycosylation of TLRs and coreceptors is important for their trafficking, assemblies, and signal transduction (Fig. 2).
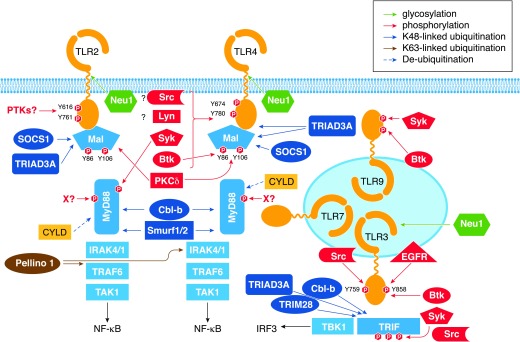
Figure 2. Control of TLR signaling by post-translational modifications.
Assembly of TLR complexes with adapters and kinases and formation of downstream signaling modules are regulated by multiple post-translational modifications. These include glycosylation of TLR2, TLR4, and TLR3 by Neu1; tyrosine phosphorylation of TLR2, TLR3, TLR4, TLR9, MyD88, and Mal by several kinases, including specific involvement of Src, Btk, Syk, Lyn, and EGFR (see text); and serine/threonine phosphorylation events regulating activity of TRIF and IRAK kinases. Several E3 Ub ligases regulate signaling via attachment of K63-linked polyubiquitination moieties to the respective substrates, such as IRAK1 and TBK1 (Pellino-1), promoting protein–protein interactions and downstream signaling or by conjugating K48-linked polyubiquitination residues to TLRs, Mal, MyD88, and TRIF (TRIAD3A, SOCS1, Smurf1/2, Cbl-b), targeting them for proteasomal degradation. CYLD deubiquitinating enzymes removes Ub residues from protein substrates, further fine-tuning TLR signaling.
Ubiquitination, a process of attachment of 8.6 kDa Ub residues, linked together as di- and polymer chains (K48 or K63 linked) to protein substrates, is catalyzed by 3 sequential steps [125]. An E1 Ub-activating enzyme mediates adenylation of the C-terminal glycine of Ub, followed by the conversion into a covalent thioester bond and attachment of Ub to the active cysteine of an E2 Ub-conjugating enzyme. An E3 Ub ligase transfers the E2-Ub complex to a substrate acceptor site, forming an isopeptide bond. This targets K48-ubiquitinated protein substrates for proteasomal degradation, whereas K63-linked, linear ubiquitination or generation of unanchored poly-Ub chains promotes protein–protein interactions and signaling [125]. Deubquitination enzymes, such as CYLD or A20, remove K63-linked polyubiquitination moieties, providing negative regulation [126, 127]. Ubiquitination regulates expression and functions of multiple TLRs. TLR4 and TLR9, but not TLR2, were shown to be subject to K48-linked ubiquitination by an E3 Ub ligase, TRIAD3A, resulting in their proteasomal degradation and decreased cell-surface expression, limiting signaling via the respective TLRs [128] (Fig. 2). Interestingly, whereas APs and kinases within the TLR pathways are subject to K48- or K63-linked polyubiquitination, as detailed in the sections below, TLRs have not been reported to undergo K63-linked polyubiquitination.
Failures in regulation of ubiquitination and deubiquitination of components in the TLR signaling pathways can lead to systemic inflammation [129–131]. In mice, deficiency in A20 causes spontaneous neuroinflammation associated with increased expression of adhesion molecules ICAM-1, VCAM-1, E-selectin in the brain endothelium, oxidative/nitrosative stress, and increased cytokine levels [132]. Mice with myeloid cell-specific ablation of A20 die from otherwise nonlethal LPS doses [133], and they develop features of SLE, including nephritis, the antiphospholipid syndrome, lymphosplenomegaly. A20-deficient DCs exhibit increased sensitivity to CD40 ligand and receptor activator for NF-κB ligand prosurvival signals and up-regulation of antiapoptotic proteins Bcl-2 and Bcl-x [134] Mice deficient in the E3 Ub ligase Pellino-1 are resistant to LPS- or poly(I:C)-induced septic shock [135] and have reduced manifestations of experimental autoimmune encephalomyelitis as a result of the role of Pellino-1 in promoting degradation of TRAF3, a negative regulator of MAPK activation and cytokine induction [136]. Furthermore, in humans, polymorphisms within A20 and Pellino-1 showed association with a risk of lupus and RA [137–139], Pellino-3−/− mice had exacerbated colitis, and patients with Crohn’s disease expressed lower levels of Pellino-3 [140]. Collectively, these results provide evidence to support the importance of regulators of ubiquitination for immunopathology.
REGULATION OF TLR SIGNALING BY RECEPTOR PHOSPHORYLATION
Phosphorylation and dephosphorylation of several TLRs regulate their signaling competence. Yet, the kinases and phosphatases involved in these processes are largely unknown, and the molecular mechanisms remain incompletely understood. Agonist stimulation induces TLR2 phosphorylation [141–143], and mutagenesis of Y616 and Y761 tyrosine residues within the TIR domain of TLR2 blocks its capacity to elicit NF-κB activation. Furthermore, tyrosine phosphorylation of TLR2 was required for recruitment of PI3K and Rac-1 [141]. TLR3 also undergoes phosphorylation in its TIR domain on Y759 and Y858 in response to stimulation with dsRNA that is important for TRIF recruitment and activation of PI3K and TBK1 [144, 145] (Fig. 2).
Several candidate PTKs have been implicated in TLR3 phosphorylation, including Btk [146], EGFR, and c-Src [147]. It is possible that these kinases act cooperatively to phosphorylate tyrosine residues in TLR3 that play nonredundant roles. Y759 is critical for TLR3-elicited p65 NF-κB phosphorylation and binding to NF-κB-dependent promoters (e.g., A20) but is dispensable IκB-α degradation and NF-κB nuclear translocation [148]. Both Y759 and Y858 are necessary for optimal activation of IRF3 via PI3K and TBK1 [144]. LPS stimulation leads to tyrosine phosphorylation of TLR4, and Y674 and Y680 are critical tyrosine residues within the TIR domain required for TLR4-elicited activation of MAPKs, NF-κB, and expression of proinflammatory cytokines [143, 149, 150]. Signaling-incompetent Y674A and Y680A TLR4 mutants exhibit altered MyD88–TLR4 interactions, increased associations with a short IRAK1 isoform, and decreased amounts of activated IRAK1. Endotoxin tolerance also impairs LPS-driven TLR4 tyrosine phosphorylation [143] (Fig. 2).
Inhibitors of PTKs, Src kinases, and Btk reduce TLR4 tyrosine phosphorylation and signaling, and c-Src, Lyn, and Btk associate with TLR4 in a ligand-dependent manner [143, 149, 151]. LPS-tolerized human monocytes and THP1 cells showed impaired Lyn and c-Src recruitment to TLR4, and the inhibition of their phosphorylation correlated with increased expression of PP2A, PTP1B, and MKP-1 phosphatases and enhanced total phosphatase activity [151]. Interestingly, Syk blocks TLR4-driven, MyD88-dependent signaling from the plasma membrane yet promotes signaling of TLR4 in the endosomal compartment to elicit the TRIF-dependent pathway [152]. Mechanistically, these opposing effects were explained by the ability of Syk to impair K63-linked ubiquitination of MyD88-linked TRAF6, while promoting K63-linked ubiquitination of TRIF-coupled TRAF3 [152].
Supporting the importance of tyrosine phosphorylation in TLR signaling, TLR5, TLR7, and TLR9 are also tyrosine phosphorylated in response to agonist stimulation [153–157]. Mass spectrometric analyses on flagellin-treated cells identified Y798 in the TIR domain as the site of TLR5 phosphorylation, and mutation of Y798 impaired TLR5-driven NF-κB and p38 MAPK activation [153]. Bioinformatics modeling suggests that phosphorylation of Y798 provides a docking site for the interaction of TLR5 with PI3K and AKT, promoting downstream signaling [153]. Site-directed mutagenesis studies have identified tyrosine residues Y898, Y904, and Y1048 of human TLR8 as critical for PI3K recruitment and NF-κB activation [154], but the exact identities of kinases and phosphatases regulating these processes are unknown. CpG DNA stimulation triggers phosphorylation within the TLR9 TIR domain in human B-lymphocytes, monocytes, human monocytic THP-1 cells, mouse bone marrow-derived macrophages, and mouse macrophage RAW264.7 cells, and this phosphorylation elicits activation and recruitment of Btk and Syk to TLR9 [72, 155–157] (Fig. 2). Inhibition of both Src and Syk blocks CpG-induced TLR9 tyrosine phosphorylation and Syk activation and recruitment to TLR9 [157]. A 4 aa region 888–891 containing Y888 within the TLR9 TIR domain is specifically required for TLR9 to trigger NF-κB activation but is dispensable for the induction of type I IFNs [72]. A substitution of Y888 in the TLR9 TIR domain to alanine inhibits tyrosine phosphorylation of mature, proteolytically processed TLR9, and although Y888 is not directly phosphorylated itself, it is structurally required for TLR9 phosphorylation and signaling [72]. Additional tyrosine mutations reveal complex regulation of TLR9 signaling. A Y870F mutant in the cytoplasmic tail of TLR9 had wild-type levels of signaling, but a Y980F mutant exhibited much reduced signaling. Mutation of Y870 or Y980 to alanine completely destroyed signaling [158]. Furthermore, stress-inducing stimuli caused destabilization of TLR9, which may depend on phosphorylation. Further studies are required to identify protein kinases and phosphatases involved in regulation of TLR phosphorylation and to dissect molecular details of how tyrosine phosphorylation of TLRs regulates their interactions with coreceptor molecules and adapter/kinase modules and impacts MyD88- and TRIF-dependent signaling pathways.
Phosphorylation of TLRs is important for host defense. An SNP located in the TIR domain of TLR2 (SNP R753Q) is linked to an increased risk of infection with M. tuberculosis [159–162]. The R753Q SNP impaired tyrosine phosphorylation of TLR2 and its dimerization with TLR6 in response to activation with Pam2CSK4 or heat-killed M. tuberculosis [142], suggesting that phosphorylation is required for full TLR2-mediated host defense. Btk is a critical kinase for TLR phosphorylation in host defense, as PBMCs from patients with X-linked agamma-globulinemia, who express a dysfunctional Btk, fail to up-regulate IL-6 in response to the TLR9 ligand CpG-DNA [156]. Thus, phosphorylation is a key mechanism to regulate TLR signaling.
ASSEMBLY OF ADAPTER-KINASE COMPLEXES AND REGULATION OF DOWNSTREAM SIGNALING BY PHOSPHORYLATION AND UBIQUITINATION
In addition to the direct regulation of TLR signaling by associated proteins, trafficking, cleavage phosphorylation, and ubiquitination, TLR signaling depends on downstream signaling pathways that are also regulated in a number of different ways. Alternatively spliced APs disrupt signaling complexes (e.g., MyD88s; see below). Furthermore, inhibitors of signaling kinases regulate TLR signaling by altering the balance of both phosphorylation/dephosphorylation and ubiquitination/deubiquitination of signaling intermediates.
The initial step in signal transduction is the recruitment of APs to TLRs, which can be dependent on intracellular localization of TLRs. The 2 main pathways are MyD88 dependent and TRIF dependent. All TLRs except TLR3 use the MyD88-dependent pathway to initiate signaling, whereas TLR3 exclusively uses the TRIF-dependent pathway to elicit induction of both proinflammatory cytokines and type I IFNs [5]. TLR4 is the only receptor that engages all 4 APs, signaling at the cell surface by coupling to MyD88 via a bridging adapter Mal (also termed TIRAP), while using the bridging adapter TRAM to couple with TRIF in the endosomal compartment [5, 54, 163]. After recruitment to TLRs, MyD88 molecules cluster and recruit IRAKs through homotypic death–death domain interactions to form a “Myddososome” [164]. An alternatively spliced form of MyD88 (MyD88s) lacks a short linker sequence between the death domain and the TIR domain, binds to the TIR domain of TLRs, but fails to recruit IRAK1, thereby inhibiting signaling [165]. TAG is a splice variant of TRAM [166]. TAG competes for TRAM binding, thereby disrupting TRAM–TRIF assembly required specifically for TLR4 signaling. Sterile-α and armadillo-motif-containing protein was reported to bind directly to MyD88 and TRIF and sequester these adapters, inhibiting both pathways, and thus, it is likely a broad regulator of TLR signaling [167, 168].
Following adapter association with TLRs, IRAK4 and IRAK1 kinases are recruited to initiate a signaling cascade. Clustered IRAK4 undergoes conformational changes and trans-autophosphorylation, which activates IRAK4 kinase activity and leads to IRAK4-mediated phosphorylation and activation of IRAK1 [169]. Active IRAK1 interacts with TRAF6 and phosphorylates an E3 Ub ligase, Pellino-1, which promotes conjugation of K63-linked polyubiquitination moieties to IRAK1 (Fig. 2). These processes facilitate recruitment and activation of TAK1 and IKKs, which activate MAPKs and transcription factors (NF-κB, AP1), leading to transcription of proinflammatory cytokine genes. Expression of other regulatory kinases within the IRAK family is induced following TLR signaling. Increased expression of IRAK-M or alternatively spliced variants of IRAK1 (e.g., IRAK1c) suppressed TLR signaling [170, 171]. IRAK-M was originally reported to prevent dissociation of IRAK and IRAK4 from MyD88 and to block engagement of TRAF6, inhibiting signaling [170]. However, later studies demonstrated the ability of IRAK-M to form the Myddososome and engage a separate MEK kinase 3-dependent signaling pathway of NF-κB activation. This pathway leads to IκB-α phosphorylation but not degradation and controls a limited set of inflammatory cytokines and negative regulators (SOCS1, SHIP-1, A20) whose expression is not controlled by mRNA stability [172]. IRAK1c is an alternatively spliced form of IRAK that lacks a region encoded by exon 11 of the IRAK1 gene, resulting in a kinase-inactive form of IRAK [170, 171]. IRAK1c can heterodimerize with IRAK, thereby fine-tuning the level of IRAK activity.
APs engaged in MyD88- and TRIF-dependent signaling pathways also become phosphorylated, and these events play a critical role in TLR signaling. Mal undergoes phosphorylation in response to cell stimulation with TLR2 or TLR4 agonists [173–175]. LPS tolerization of human monocytes and macrophages impairs Mal phosphorylation, suggesting that this is an important mechanism to regulate LPS signaling [173]. Site-directed mutagenesis of Y86, Y106, and Y159 led to the loss of tyrosine phosphorylation of Mal. Overexpression of Y86A and Y106A Mal mutants failed to trigger phosphorylation of p38 MAPK and activation of NF-κB. This led to reduced induction of proinflammatory cytokines. Thus, the Mal mutants acted as inhibitors of LPS-induced NF-κB and p38 activation. These data demonstrate the importance of Mal tyrosine phosphorylation for signaling competence. Notably, Y86A and Y106A Mal mutants exhibit increased association with TLR4 and inhibit TLR4-driven activation of p38 MAPK and NF-κB [173]. These results suggest that tyrosine-deficient Mal can compete with signaling-sufficient Mal species in TLR4-tolerant cells, substituting them from TLR4 signaling complexes and leading to reprogramming of TLR4 responses [173]. PKC-δ and Btk interact with Mal [149, 173–176], and upon association mediate Mal phosphorylation. Chemical inhibition of Btk diminishes Mal phosphorylation [174], and induction of endotoxin tolerance disrupts Mal–Btk interactions [173]. Computer modeling, docking, and physicochemical studies suggested that Btk phosphorylates Y86 and Y106 within Mal, whereas PKC-δ preferentially phosphorylates Y106, although both kinases are thought to mediate cooperatively optimal Mal phosphorylation [176]. Mal tyrosine phosphorylation was reported to target Mal for interactions with the E3 Ub ligase SOCS1, leading to K48-linked ubiquitination and proteasomal degradation of Mal, providing negative regulation of TLR signaling [177] (Fig. 2).
Signaling adapters TRIF and MyD88 undergo tyrosine phosphorylation in macrophages following TLR-mediated activation of integrin αM (CD11b) via PI3K, CD11b-mediated activation of Syk and Src, and Syk-mediated phosphorylation of the APs [26]. Phosphorylation of MyD88 and TRIF creates docking platforms to recruit the E3 Ub ligase Cbl-b, K48-linked ubiquitination of adapters, and their proteasomal degradation [26] (Fig. 2). PYK2 and Etk/BMX were also shown to interact with MyD88 in response to LPS stimulation and to regulate MyD88-inducible NF-κB activation and IL-6 expression, respectively [178, 179]. It is unknown whether PYK2 and Ets directly phosphorylate MyD88. TRIF is also subject to serine/threonine phosphorylation. TLR3 or TLR4 dimerization leads to recruitment of TRIF and TRAF3, which promote K63-linked ubiquitination and activation of TBK1 [180]. Activated TBK1 phosphorylates TRIF, facilitating recruitment and binding of IRF3 to phospho-TRIF. This leads to subsequent phosphorylation and activation of IRF3 and downstream signaling [180]. Caveolin-1, a component of TLR4 signaling required for TLR4–MyD88 interactions [181], is phosphorylated on Y14 in response to LPS stimulation, and complementation of caveolin-1-deficient cells with the Y14F caveolin-1 mutant led to impaired, TLR4-driven, MyD88-dependent NF-κB activation [181]. Protein phosphatases, which remove the activating phosphate groups, also regulate TLR signaling. For example, endotoxin tolerance is associated with increased expression and activity of PTP1B, PP2A, PTPN22, and MKP-1 phosphatases [151], but it remains unclear which phosphatases dephosphorylate TLRs, Mal, MyD88, and TRIF.
Several E3 Ub ligases control expression and signaling of TIR-containing adapter molecules. In response to TGF-β, MyD88 undergoes K48-linked polyubiquitination by E3 Ub ligases Smurf-1 and Smurf-2 in SMA and mother against decapentaplegic (MAD)-related protein-dependent manner [182] (Fig. 2). K48-polyubiquitinated MyD88 is proteasomally degraded, contributing to negative regulation of TLR4 signaling. During infection of human epithelial cells with nontypeable Haemophilus influenzae, MyD88 undergoes K63-linked polyubiquitination by an unknown E3 Ub ligase, followed by subsequent deubiquitination by the deubiquitination enzyme CYLD [183]. TRIM28 is an E3 Ub ligase that catalyzes the conjugation of K48-linked polyubiquitination moieties to TRIF, targeting TRIF to proteasomal degradation and attenuating TLR3 signaling and type I IFN expression in response to stimulation with poly(I:C) [184].
Expression and functions of APs and kinases further downstream within the TLR signaling cascades are also regulated by ubiquitination. Pellinos, a family of E3 Ub ligases containing 3 members—Pellino-1, Pellino-2, and Pellino-3—exerts nonredundant functions in IL-1R and TLR signaling [185–187]. Pellino-1 becomes phosphorylated by multiple kinases, including activated IRAK1, IRAK4 [188], TBK1, and IKK-ε [189]. Phosphorylated Pellino-1, in turn, mediates K63-linked polyubiquitination of IRAK1, RIP1, and TRAF6, promoting IRAK1–TRAF6–TAK1–IKK complex assembly and exerting positive regulation of IL-1R/TLR signaling [188, 190, 191]. Overexpression of Pellino-1 promotes LPS-inducible phosphorylation of IRAK1, TBK1, and IRF3; NF-κB activation; and expression of MyD88- and TRIF-dependent cytokines [191]. In contrast, knockdown of Pellino-1 attenuated these responses [191]. Pellino-2 interacts with IRAK4, IRAK1, TRAF6, and TAK1, promoting activation of MAPKs and the transcription factors AP1 and Elk-1 [192, 193]. IRAK4 and IRAK1 phosphorylate and activate Pellino-2, and Pellino-2 promotes NF-κB activation by interacting with Bcl-10 that is recruited to TLR4 in response to LPS stimulation [192, 193]. SOCS3 was shown to block TLR4–Bcl-10–Pellino-2 interactions, exerting a negative regulatory effect on LPS-induced NF-κB activation [192, 193]. However, little information is available regarding the molecular mechanisms by which Pelino-2 fine tunes TLR signaling and cross talks with other members of the Pellino family in vivo. In contrast, Pellino-3 overexpression blocked, whereas its knockdown up-regulated TLR4-driven IRAK1 modifications, TBK1 and IRF3 phosphorylation, NF-κB activation, and induction of proinflammatory cytokines, indicating that Pellino-3 is a negative regulator [194]. Pellino-3 was reported to inhibit TLR3 signaling by promoting ubiquitination of TRAF6, blocking TRAF6-assisted phosphorylation and activation of IRF7 and induction of type I IFNs [195]. Furthermore, IRAK1-activated Pellino-3 blocks TLR4-mediated expression of type I IFNs in response to oxidized LDLs by mediating monoubiquitination and activation of TRAF family member-associated NF-κB activator (TANK) [196], a negative regulator of TLR signaling [197].
A20 is a dual Ub-editing enzyme that facilitates the attachment of K48-linked polyubiquitin chains to certain substrates, such as RIP1, leading to RIP1 degradation. A20 also disables downstream TLR signaling by removing K63-linked polyubiquitin moieties from multiple substrates, e.g., TRAF6, TAK1, and IKK-γ [129, 131]. K48-linked polyubiquitination of IRF8 by Cbl-b generally blocks IL-12 production [198]. TRIAD3A also ubiquitinates TIRAP, TRIF, and RIP1, leading to their degradation [199]. TRIM21, an E3 Ub ligase shared by the TLR3 and TLR4 signaling pathways, regulates IRF3 activation and type I IFN expression in response to viral infections and is an autoantigen in patients with SLE. TRIM21 activity is regulated by phosphorylation of 3 tyrosine residues: Y343, Y388, and Y393. Phosphorylation is required for TRIM21 to function as a negative regulator of IRF3 activation and type I IFN expression [200]. The importance of appropriate regulation of ubiquitination and deubiquitination is exemplified in mice deficient in A20, which develop severe inflammatory disease as a result of prolonged and excessive signaling through the NF-κB pathway [130]. Thus, phosphorylation and ubiquitination are important modifications regulating the TLR signaling pathways.
CONCLUDING REMARKS
As a result of their critical role in initiating inflammation, TLR signaling is regulated at multiple levels. These include access of TLRs to ligands, cooperation with coreceptor molecules and dimerization, intracellular trafficking following ligand binding, assembly of parallel and sequential adapter-kinase complexes, and propagation of signaling downstream. These processes are regulated by several post-translational modifications, including glycosylation, phosphorylation, and ubiquitination. Although extensive discussion of all negative-feedback mechanisms lies outside of the scope of the current review, they also play an important part in TLR regulation. These mechanisms include induction of negative regulatory coreceptors and intracellular intermediates, such as the RP105/MD1 complex [113, 201], DC-specific intercellular adhesion molecule-3-grabbing nonintegrin[202–204], the Tyro3/Axl/Mer family [205], DNAX-activating protein of 12 kDa (DAP12) [206–208], triggering receptor expressed by myeloid cell 2 (TREM-2), SHIP-1, deubiquitination of TLR intermediates, and fine-tuning of TLR signaling by noncoding RNAs, such as micro RNAs and long noncoding RNAs [209–213]. Together, the complex and interactive mechanisms positively and negatively regulate the activity of TLRs. As studies on these TLRs continue, additional regulatory pathways are likely to be discovered.
AUTHORSHIP
C.A.L. and A.E.M. wrote the manuscript.
ACKNOWLEDGMENTS
Research in the authors’ laboratories was supported by grants from the U.S. National Institutes of Health (R01AI076588, R01AI076588S1, and R03AI097671 to C.A.L. and R01AI059524 to A.E.M. The authors thank Erika Gruber and Christa Heyward for critical reading of the manuscript.
Glossary
- 3d
triple D
- AEP
asparagine endopeptidase
- AP
adapter protein
- Bcl
B-cell lymphoma 2
- Btk
Bruton’s tyrosine kinase
- Cbl-b
Casitas B-lineage lymphoma proto-oncogene b
- CNPY
canopy fibroblast growth factor signaling regulator
- c-Src
rous sarcoma virus homolog
- CYLD
cylindromatosis
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- ER
endoplasmic reticulum
- gp96
glycoprotein 96
- HEK
human embryonic kidney
- HMGB1
high-mobility group box 1
- IKK
inhibitor of NF-κB kinase
- IRAK
IL-1R-associated kinase
- IRF
IFN regulatory factor
- LRR
leucine-rich repeat
- LRRC59
leucine-rich repeat-containing protein 59
- MAL
MyD88 adapter-like (same as TIRAP)
- MD2
myeloid differentiation factor 2
- MKP-1
MAPK phosphatase 1
- Pam2/3CSK4
palmitoyl-2/3-Cys-Ser-(Lys)4
- PIKfyve
phosphoinositide kinase, FYVE finger containing
- PKC
protein kinase C
- poly(I:C)
polyinosinic:polycytidylic acid
- PP2
protein phosphatase 2
- PRAT4A
protein associated with TLR4 A
- PTK
protein tyrosine kinase
- PTP
protein-tyrosine phosphatase
- PYK2
proline-rich tyrosine kinase 2
- RA
rheumatoid arthritis
- Rab
Ras-associated binding
- RIP1
receptor-interacting protein
- RP105
radioprotective 105 kDa
- SCARB2
scavenger receptor class B, member 2
- SIGIRR
single Ig IL-1R-related molecule
- SLE
systemic lupus erythematosis
- SNP
single nucleotide polymorphism
- SOCS
suppressor of cytokine signaling
- sTLR
soluble TLR
- Syk
spleen tyrosine kinase
- TAG
Toll-IL-1R homology domain-containing adapter-inducing IFN-β-related adaptor molecule adapter with a Golgi dynamics domain
- TAK1
TGF-activated kinase 1
- TBK1
TNFR-associated factor family member-associated NF-kappa-B activator-binding kinase 1
- TIR
Toll-IL-1R resistance domain
- TIRAP
TIR domain-containing adapter protein (same as MAL)
- TRAF
TNFR-associated factor
- TRAM
TIR domain-containing adapter-inducing IFN-β-related adapter molecule
- TRIF
Toll-IL-1R homology domain-containing adapter-inducing IFN-β
- TRIM
tripartite motif
- Ub
ubiquitin
- UNC93B1
uncoordinated 93 homolog B1
- VACV
vaccinia virus
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Doyle S. L., O’Neill L. A. (2006) Toll-like receptors: from the discovery of NFkappaB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 72, 1102–1113. [DOI] [PubMed] [Google Scholar]
- 2.Medzhitov R., Janeway C. A. Jr. (1997) Innate immunity: the virtues of a nonclonal system of recognition. Cell 91, 295–298. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R., Janeway C. Jr. (2000) Innate immunity. N. Engl. J. Med. 343, 338–344. [DOI] [PubMed] [Google Scholar]
- 4.Pasare C., Medzhitov R. (2005) Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 560, 11–18. [DOI] [PubMed] [Google Scholar]
- 5.Brubaker S. W., Bonham K. S., Zanoni I., Kagan J. C. (2015) Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 33, 257–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell J. K., Mullen G. E., Leifer C. A., Mazzoni A., Davies D. R., Segal D. M. (2003) Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 24, 528–533. [DOI] [PubMed] [Google Scholar]
- 7.Botos I., Segal D. M., Davies D. R. (2011) The structural biology of Toll-like receptors. Structure 19, 447–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kang J. Y., Lee J. O. (2011) Structural biology of the Toll-like receptor family. Annu. Rev. Biochem. 80, 917–941. [DOI] [PubMed] [Google Scholar]
- 9.Lee C. C., Avalos A. M., Ploegh H. L. (2012) Accessory molecules for Toll-like receptors and their function. Nat. Rev. Immunol. 12, 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell J. K., Askins J., Hall P. R., Davies D. R., Segal D. M. (2006) The dsRNA binding site of human Toll-like receptor 3. Proc. Natl. Acad. Sci. USA 103, 8792–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell J. K., Botos I., Hall P. R., Askins J., Shiloach J., Segal D. M., Davies D. R. (2005) The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc. Natl. Acad. Sci. USA 102, 10976–10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choe J., Kelker M. S., Wilson I. A. (2005) Crystal structure of human Toll-like receptor 3 (TLR3) ectodomain. Science 309, 581–585. [DOI] [PubMed] [Google Scholar]
- 13.Jin M. S., Kim S. E., Heo J. Y., Lee M. E., Kim H. M., Paik S. G., Lee H., Lee J. O. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130, 1071–1082. [DOI] [PubMed] [Google Scholar]
- 14.Andersen-Nissen E., Smith K. D., Bonneau R., Strong R. K., Aderem A. (2007) A conserved surface on Toll-like receptor 5 recognizes bacterial flagellin. J. Exp. Med. 204, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizel S. B., West A. P., Hantgan R. R. (2003) Identification of a sequence in human Toll-like receptor 5 required for the binding of Gram-negative flagellin. J. Biol. Chem. 278, 23624–23629. [DOI] [PubMed] [Google Scholar]
- 16.Yoon S. I., Kurnasov O., Natarajan V., Hong M., Gudkov A. V., Osterman A. L., Wilson I. A. (2012) Structural basis of TLR5-flagellin recognition and signaling. Science 335, 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leifer C. A., McConkey C., Li S., Chassaing B., Gewirtz A. T., Ley R. E. (2014) Linking genetic variation in human Toll-like receptor 5 genes to the gut microbiome’s potential to cause inflammation. Immunol. Lett. 162 (2 Pt A), 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chockalingam A., Brooks J. C., Cameron J. L., Blum L. K., Leifer C. A. (2009) TLR9 traffics through the Golgi complex to localize to endolysosomes and respond to CpG DNA. Immunol. Cell Biol. 87, 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latz E., Schoenemeyer A., Visintin A., Fitzgerald K. A., Monks B. G., Knetter C. F., Lien E., Nilsen N. J., Espevik T., Golenbock D. T. (2004) TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5, 190–198. [DOI] [PubMed] [Google Scholar]
- 20.Leifer C. A., Brooks J. C., Hoelzer K., Lopez J., Kennedy M. N., Mazzoni A., Segal D. M. (2006) Cytoplasmic targeting motifs control localization of Toll-like receptor 9. J. Biol. Chem. 281, 35585–35592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leifer C. A., Kennedy M. N., Mazzoni A., Lee C., Kruhlak M. J., Segal D. M. (2004) TLR9 is localized in the endoplasmic reticulum prior to stimulation. J. Immunol. 173, 1179–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B., Li Z. (2008) Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood 112, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu B., Yang Y., Qiu Z., Staron M., Hong F., Li Y., Wu S., Li Y., Hao B., Bona R., Han D., Li Z. (2010) Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat. Commun. 1, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y., Liu B., Dai J., Srivastava P. K., Zammit D. J., Lefrançois L., Li Z. (2007) Heat shock protein gp96 is a master chaperone for Toll-like receptors and is important in the innate function of macrophages. Immunity 26, 215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L., Gordon R. A., Huynh L., Su X., Park Min K. H., Han J., Arthur J. S., Kalliolias G. D., Ivashkiv L. B. (2010) Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity 32, 518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han C., Jin J., Xu S., Liu H., Li N., Cao X. (2010) Integrin CD11b negatively regulates TLR-triggered inflammatory responses by activating Syk and promoting degradation of MyD88 and TRIF via Cbl-b. Nat. Immunol. 11, 734–742. [DOI] [PubMed] [Google Scholar]
- 27.Yee N. K., Hamerman J. A. (2013) β(2) Integrins inhibit TLR responses by regulating NF-κB pathway and p38 MAPK activation. Eur. J. Immunol. 43, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed R. C., Berwin B., Baker J. P., Nicchitta C. V. (2003) GRP94/gp96 elicits ERK activation in murine macrophages. A role for endotoxin contamination in NF-kappa B activation and nitric oxide production. J. Biol. Chem. 278, 31853–31860. [DOI] [PubMed] [Google Scholar]
- 29.Warger T., Hilf N., Rechtsteiner G., Haselmayer P., Carrick D. M., Jonuleit H., von Landenberg P., Rammensee H. G., Nicchitta C. V., Radsak M. P., Schild H. (2006) Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J. Biol. Chem. 281, 22545–22553. [DOI] [PubMed] [Google Scholar]
- 30.Wakabayashi Y., Kobayashi M., Akashi-Takamura S., Tanimura N., Konno K., Takahashi K., Ishii T., Mizutani T., Iba H., Kouro T., Takaki S., Takatsu K., Oda Y., Ishihama Y., Saitoh S., Miyake K. (2006) A protein associated with Toll-like receptor 4 (PRAT4A) regulates cell surface expression of TLR4. J. Immunol. 177, 1772–1779. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi K., Shibata T., Akashi-Takamura S., Kiyokawa T., Wakabayashi Y., Tanimura N., Kobayashi T., Matsumoto F., Fukui R., Kouro T., Nagai Y., Takatsu K., Saitoh S., Miyake K. (2007) A protein associated with Toll-like receptor (TLR) 4 (PRAT4A) is required for TLR-dependent immune responses. J. Exp. Med. 204, 2963–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hart B. E., Tapping R. I. (2012) Cell surface trafficking of TLR1 is differentially regulated by the chaperones PRAT4A and PRAT4B. J. Biol. Chem. 287, 16550–16562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shibata T., Takemura N., Motoi Y., Goto Y., Karuppuchamy T., Izawa K., Li X., Akashi-Takamura S., Tanimura N., Kunisawa J., Kiyono H., Akira S., Kitamura T., Kitaura J., Uematsu S., Miyake K. (2012) PRAT4A-dependent expression of cell surface TLR5 on neutrophils, classical monocytes and dendritic cells. Int. Immunol. 24, 613–623. [DOI] [PubMed] [Google Scholar]
- 34.Kiyokawa T., Akashi-Takamura S., Shibata T., Matsumoto F., Nishitani C., Kuroki Y., Seto Y., Miyake K. (2008) A single base mutation in the PRAT4A gene reveals differential interaction of PRAT4A with Toll-like receptors. Int. Immunol. 20, 1407–1415. [DOI] [PubMed] [Google Scholar]
- 35.Tabeta K., Hoebe K., Janssen E. M., Du X., Georgel P., Crozat K., Mudd S., Mann N., Sovath S., Goode J., Shamel L., Herskovits A. A., Portnoy D. A., Cooke M., Tarantino L. M., Wiltshire T., Steinberg B. E., Grinstein S., Beutler B. (2006) The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat. Immunol. 7, 156–164. [DOI] [PubMed] [Google Scholar]
- 36.Brinkmann M. M., Spooner E., Hoebe K., Beutler B., Ploegh H. L., Kim Y. M. (2007) The interaction between the ER membrane protein UNC93B and TLR3, 7, and 9 is crucial for TLR signaling. J. Cell Biol. 177, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim J., Huh J., Hwang M., Kwon E. H., Jung D. J., Brinkmann M. M., Jang M. H., Ploegh H. L., Kim Y. M. (2013) Acidic amino acid residues in the juxtamembrane region of the nucleotide-sensing TLRs are important for UNC93B1 binding and signaling. J. Immunol. 190, 5287–5295. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y. M., Brinkmann M. M., Paquet M. E., Ploegh H. L. (2008) UNC93B1 delivers nucleotide-sensing Toll-like receptors to endolysosomes. Nature 452, 234–238. [DOI] [PubMed] [Google Scholar]
- 39.Fukui R., Saitoh S., Kanno A., Onji M., Shibata T., Ito A., Onji M., Matsumoto M., Akira S., Yoshida N., Miyake K. (2011) Unc93B1 restricts systemic lethal inflammation by orchestrating Toll-like receptor 7 and 9 trafficking. Immunity 35, 69–81. [DOI] [PubMed] [Google Scholar]
- 40.Lee B. L., Moon J. E., Shu J. H., Yuan L., Newman Z. R., Schekman R., Barton G. M. (2013) UNC93B1 mediates differential trafficking of endosomal TLRs. eLife 2, e00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huh J. W., Shibata T., Hwang M., Kwon E. H., Jang M. S., Fukui R., Kanno A., Jung D. J., Jang M. H., Miyake K., Kim Y. M. (2014) UNC93B1 is essential for the plasma membrane localization and signaling of Toll-like receptor 5. Proc. Natl. Acad. Sci. USA 111, 7072–7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh H., Tatematsu M., Watanabe A., Iwano K., Funami K., Seya T., Matsumoto M. (2011) UNC93B1 physically associates with human TLR8 and regulates TLR8-mediated signaling. PLoS One 6, e28500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukui R., Saitoh S., Matsumoto F., Kozuka-Hata H., Oyama M., Tabeta K., Beutler B., Miyake K. (2009) Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J. Exp. Med. 206, 1339–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelka K., Phulphagar K., Zimmermann J., Stahl R., Schmid-Burgk J. L., Schmidt T., Spille J. H., Labzin L. I., Agrawal S., Kandimalla E. R., Casanova J. L., Hornung V., Marshak-Rothstein A., Höning S., Latz E. (2014) Cutting edge: the UNC93B1 tyrosine-based motif regulates trafficking and TLR responses via separate mechanisms. J. Immunol. 193, 3257–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatematsu M., Funami K., Ishii N., Seya T., Obuse C., Matsumoto M. (2015) LRRC59 regulates trafficking of nucleic acid-sensing TLRs from the endoplasmic reticulum via association with UNC93B1. J. Immunol. 195, 4933–4942. [DOI] [PubMed] [Google Scholar]
- 46.Dai J., Liu B., Ngoi S. M., Sun S., Vella A. T., Li Z. (2007) TLR4 hyperresponsiveness via cell surface expression of heat shock protein gp96 potentiates suppressive function of regulatory T cells. J. Immunol. 178, 3219–3225. [DOI] [PubMed] [Google Scholar]
- 47.Liu B., Dai J., Zheng H., Stoilova D., Sun S., Li Z. (2003) Cell surface expression of an endoplasmic reticulum resident heat shock protein gp96 triggers MyD88-dependent systemic autoimmune diseases. Proc. Natl. Acad. Sci. USA 100, 15824–15829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang Q. Q., Sobkoviak R., Jockheck-Clark A. R., Shi B., Mandelin A. M. II, Tak P. P., Haines G. K. III, Nicchitta C. V., Pope R. M. (2009) Heat shock protein 96 is elevated in rheumatoid arthritis and activates macrophages primarily via TLR2 signaling. J. Immunol. 182, 4965–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Q. Q., Koessler R. E., Birkett R., Dorfleutner A., Perlman H., Haines G. K. III, Stehlik C., Nicchitta C. V., Pope R. M. (2012) Glycoprotein 96 perpetuates the persistent inflammation of rheumatoid arthritis. Arthritis Rheum. 64, 3638–3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakano S., Morimoto S., Suzuki S., Watanabe T., Amano H., Takasaki Y. (2010) Up-regulation of the endoplasmic reticulum transmembrane protein UNC93B in the B cells of patients with active systemic lupus erythematosus. Rheumatology (Oxford) 49, 876–881. [DOI] [PubMed] [Google Scholar]
- 51.Kono D. H., Haraldsson M. K., Lawson B. R., Pollard K. M., Koh Y. T., Du X., Arnold C. N., Baccala R., Silverman G. J., Beutler B. A., Theofilopoulos A. N. (2009) Endosomal TLR signaling is required for anti-nucleic acid and rheumatoid factor autoantibodies in lupus. Proc. Natl. Acad. Sci. USA 106, 12061–12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Latz E., Visintin A., Lien E., Fitzgerald K. A., Espevik T., Golenbock D. T. (2003) The LPS receptor generates inflammatory signals from the cell surface. J. Endotoxin Res. 9, 375–380. [DOI] [PubMed] [Google Scholar]
- 53.Husebye H., Halaas Ø., Stenmark H., Tunheim G., Sandanger Ø., Bogen B., Brech A., Latz E., Espevik T. (2006) Endocytic pathways regulate Toll-like receptor 4 signaling and link innate and adaptive immunity. EMBO J. 25, 683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kagan J. C., Su T., Horng T., Chow A., Akira S., Medzhitov R. (2008) TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat. Immunol. 9, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandt K. J., Fickentscher C., Kruithof E. K., de Moerloose P. (2013) TLR2 ligands induce NF-κB activation from endosomal compartments of human monocytes. PLoS One 8, e80743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dietrich N., Lienenklaus S., Weiss S., Gekara N. O. (2010) Murine Toll-like receptor 2 activation induces type I interferon responses from endolysosomal compartments. PLoS One 5, e10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marre M. L., Petnicki-Ocwieja T., DeFrancesco A. S., Darcy C. T., Hu L. T. (2010) Human integrin α(3)β(1) regulates TLR2 recognition of lipopeptides from endosomal compartments. PLoS One 5, e12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liljeroos M., Vuolteenaho R., Rounioja S., Henriques-Normark B., Hallman M., Ojaniemi M. (2008) Bacterial ligand of TLR2 signals Stat activation via induction of IRF1/2 and interferon-alpha production. Cell. Signal. 20, 1873–1881. [DOI] [PubMed] [Google Scholar]
- 59.Aubry C., Corr S. C., Wienerroither S., Goulard C., Jones R., Jamieson A. M., Decker T., O’Neill L. A., Dussurget O., Cossart P. (2012) Both TLR2 and TRIF contribute to interferon-β production during Listeria infection. PLoS One 7, e33299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiss G., Rasmussen S., Zeuthen L. H., Nielsen B. N., Jarmer H., Jespersen L., Frøkiaer H. (2010) Lactobacillus acidophilus induces virus immune defence genes in murine dendritic cells by a Toll-like receptor-2-dependent mechanism. Immunology 131, 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barbalat R., Lau L., Locksley R. M., Barton G. M. (2009) Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10, 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michaud F., Coulombe F., Gaudreault E., Kriz J., Gosselin J. (2010) Involvement of TLR2 in recognition of acute gammaherpesvirus-68 infection. PLoS One 5, e13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davies M. L., Sei J. J., Siciliano N. A., Xu R. H., Roscoe F., Sigal L. J., Eisenlohr L. C., Norbury C. C. (2014) MyD88-dependent immunity to a natural model of vaccinia virus infection does not involve Toll-like receptor 2. J. Virol. 88, 3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stack J., Doyle S. L., Connolly D. J., Reinert L. S., O’Keeffe K. M., McLoughlin R. M., Paludan S. R., Bowie A. G. (2014) TRAM is required for TLR2 endosomal signaling to type I IFN induction. J. Immunol. 193, 6090–6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Husebye H., Aune M. H., Stenvik J., Samstad E., Skjeldal F., Halaas O., Nilsen N. J., Stenmark H., Latz E., Lien E., Mollnes T. E., Bakke O., Espevik T. (2010) The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity 33, 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Chen T., Han C., He D., Liu H., An H., Cai Z., Cao X. (2007) Lysosome-associated small Rab GTPase Rab7b negatively regulates TLR4 signaling in macrophages by promoting lysosomal degradation of TLR4. Blood 110, 962–971. [DOI] [PubMed] [Google Scholar]
- 67.Barton G. M., Kagan J. C., Medzhitov R. (2006) Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 7, 49–56. [DOI] [PubMed] [Google Scholar]
- 68.Pohar J., Pirher N., Benčina M., Manček-Keber M., Jerala R. (2013) The role of UNC93B1 protein in surface localization of TLR3 receptor and in cell priming to nucleic acid agonists. J. Biol. Chem. 288, 442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pohar J., Pirher N., Benčina M., Manček-Keber M., Jerala R. (2014) The ectodomain of TLR3 receptor is required for its plasma membrane translocation. PLoS One 9, e92391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guiducci C., Ott G., Chan J. H., Damon E., Calacsan C., Matray T., Lee K. D., Coffman R. L., Barrat F. J. (2006) Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 203, 1999–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Honda K., Ohba Y., Yanai H., Negishi H., Mizutani T., Takaoka A., Taya C., Taniguchi T. (2005) Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434, 1035–1040. [DOI] [PubMed] [Google Scholar]
- 72.Chockalingam A., Rose W. A. II, Hasan M., Ju C. H., Leifer C. A. (2012) Cutting edge: a TLR9 cytoplasmic tyrosine motif is selectively required for proinflammatory cytokine production. J. Immunol. 188, 527–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sasai M., Linehan M. M., Iwasaki A. (2010) Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science 329, 1530–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Blasius A. L., Arnold C. N., Georgel P., Rutschmann S., Xia Y., Lin P., Ross C., Li X., Smart N. G., Beutler B. (2010) Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 107, 19973–19978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hayashi K., Sasai M., Iwasaki A. (2015) Toll-like receptor 9 trafficking and signaling for type I interferons requires PIKfyve activity. Int. Immunol. 27, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guo H., Zhang J., Zhang X., Wang Y., Yu H., Yin X., Li J., Du P., Plumas J., Chaperot L., Chen J., Su L., Liu Y., Zhang L. (2015) SCARB2/LIMP-2 regulates IFN production of plasmacytoid dendritic cells by mediating endosomal translocation of TLR9 and nuclear translocation of IRF7. J. Immunol. 194, 4737–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mouchess M. L., Arpaia N., Souza G., Barbalat R., Ewald S. E., Lau L., Barton G. M. (2011) Transmembrane mutations in Toll-like receptor 9 bypass the requirement for ectodomain proteolysis and induce fatal inflammation. Immunity 35, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei M. L. (2006) Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res. 19, 19–42. [DOI] [PubMed] [Google Scholar]
- 79.Mantegazza A. R., Guttentag S. H., El-Benna J., Sasai M., Iwasaki A., Shen H., Laufer T. M., Marks M. S. (2012) Adaptor protein-3 in dendritic cells facilitates phagosomal Toll-like receptor signaling and antigen presentation to CD4(+) T cells. Immunity 36, 782–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ewald S. E., Engel A., Lee J., Wang M., Bogyo M., Barton G. M. (2011) Nucleic acid recognition by Toll-like receptors is coupled to stepwise processing by cathepsins and asparagine endopeptidase. J. Exp. Med. 208, 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ewald S. E., Lee B. L., Lau L., Wickliffe K. E., Shi G. P., Chapman H. A., Barton G. M. (2008) The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature 456, 658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park B., Brinkmann M. M., Spooner E., Lee C. C., Kim Y. M., Ploegh H. L. (2008) Proteolytic cleavage in an endolysosomal compartment is required for activation of Toll-like receptor 9. Nat. Immunol. 9, 1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sepulveda F. E., Maschalidi S., Colisson R., Heslop L., Ghirelli C., Sakka E., Lennon-Duménil A. M., Amigorena S., Cabanie L., Manoury B. (2009) Critical role for asparagine endopeptidase in endocytic Toll-like receptor signaling in dendritic cells. Immunity 31, 737–748. [DOI] [PubMed] [Google Scholar]
- 84.Toscano F., Estornes Y., Virard F., Garcia-Cattaneo A., Pierrot A., Vanbervliet B., Bonnin M., Ciancanelli M. J., Zhang S. Y., Funami K., Seya T., Matsumoto M., Pin J. J., Casanova J. L., Renno T., Lebecque S. (2013) Cleaved/associated TLR3 represents the primary form of the signaling receptor. J. Immunol. 190, 764–773. [DOI] [PubMed] [Google Scholar]
- 85.Qi R., Singh D., Kao C. C. (2012) Proteolytic processing regulates Toll-like receptor 3 stability and endosomal localization. J. Biol. Chem. 287, 32617–32629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Cattaneo A., Gobert F. X., Müller M., Toscano F., Flores M., Lescure A., Del Nery E., Benaroch P. (2012) Cleavage of Toll-like receptor 3 by cathepsins B and H is essential for signaling. Proc. Natl. Acad. Sci. USA 109, 9053–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Onji M., Kanno A., Saitoh S., Fukui R., Motoi Y., Shibata T., Matsumoto F., Lamichhane A., Sato S., Kiyono H., Yamamoto K., Miyake K. (2013) An essential role for the N-terminal fragment of Toll-like receptor 9 in DNA sensing. Nat. Commun. 4, 1949. [DOI] [PubMed] [Google Scholar]
- 88.Kuroishi T., Tanaka Y., Sakai A., Sugawara Y., Komine K., Sugawara S. (2007) Human parotid saliva contains soluble Toll-like receptor (TLR) 2 and modulates TLR2-mediated interleukin-8 production by monocytic cells. Mol. Immunol. 44, 1969–1976. [DOI] [PubMed] [Google Scholar]
- 89.LeBouder E., Rey-Nores J. E., Rushmere N. K., Grigorov M., Lawn S. D., Affolter M., Griffin G. E., Ferrara P., Schiffrin E. J., Morgan B. P., Labéta M. O. (2003) Soluble forms of Toll-like receptor (TLR)2 capable of modulating TLR2 signaling are present in human plasma and breast milk. J. Immunol. 171, 6680–6689. [DOI] [PubMed] [Google Scholar]
- 90.Iwami K. I., Matsuguchi T., Masuda A., Kikuchi T., Musikacharoen T., Yoshikai Y. (2000) Cutting edge: naturally occurring soluble form of mouse Toll-like receptor 4 inhibits lipopolysaccharide signaling. J. Immunol. 165, 6682–6686. [DOI] [PubMed] [Google Scholar]
- 91.Dulay A. T., Buhimschi C. S., Zhao G., Oliver E. A., Mbele A., Jing S., Buhimschi I. A. (2009) Soluble TLR2 is present in human amniotic fluid and modulates the intraamniotic inflammatory response to infection. J. Immunol. 182, 7244–7253. [DOI] [PubMed] [Google Scholar]
- 92.Zunt S. L., Burton L. V., Goldblatt L. I., Dobbins E. E., Srinivasan M. (2009) Soluble forms of Toll-like receptor 4 are present in human saliva and modulate tumour necrosis factor-alpha secretion by macrophage-like cells. Clin. Exp. Immunol. 156, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raby A. C., Le Bouder E., Colmont C., Davies J., Richards P., Coles B., George C. H., Jones S. A., Brennan P., Topley N., Labéta M. O. (2009) Soluble TLR2 reduces inflammation without compromising bacterial clearance by disrupting TLR2 triggering. J. Immunol. 183, 506–517. [DOI] [PubMed] [Google Scholar]
- 94.Chockalingam A., Cameron J. L., Brooks J. C., Leifer C. A. (2011) Negative regulation of signaling by a soluble form of Toll-like receptor 9. Eur. J. Immunol. 41, 2176–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee S., Kang D., Ra E. A., Lee T. A., Ploegh H. L., Park B. (2014) Negative self-regulation of TLR9 signaling by its N-terminal proteolytic cleavage product. J. Immunol. 193, 3726–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mitsuzawa H., Nishitani C., Hyakushima N., Shimizu T., Sano H., Matsushima N., Fukase K., Kuroki Y. (2006) Recombinant soluble forms of extracellular TLR4 domain and MD-2 inhibit lipopolysaccharide binding on cell surface and dampen lipopolysaccharide-induced pulmonary inflammation in mice. J. Immunol. 177, 8133–8139. [DOI] [PubMed] [Google Scholar]
- 97.Langjahr P., Díaz-Jiménez D., De la Fuente M., Rubio E., Golenbock D., Bronfman F. C., Quera R., González M. J., Hermoso M. A. (2014) Metalloproteinase-dependent TLR2 ectodomain shedding is involved in soluble Toll-like receptor 2 (sTLR2) production. PLoS One 9, e104624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ueland T., Espevik T., Kjekshus J., Gullestad L., Omland T., Squire I. B., Frøland S. S., Mollnes T. E., Dickstein K., Aukrust P. (2006) Mannose binding lectin and soluble Toll-like receptor 2 in heart failure following acute myocardial infarction. J. Card. Fail. 12, 659–663. [DOI] [PubMed] [Google Scholar]
- 99.Heggelund L., Flo T., Berg K., Lien E., Mollnes T. E., Ueland T., Aukrust P., Espevik T., Frøland S. S. (2004) Soluble Toll-like receptor 2 in HIV infection: association with disease progression. AIDS 18, 2437–2439. [PubMed] [Google Scholar]
- 100.De Zoete M. R., Bouwman L. I., Keestra A. M., van Putten J. P. (2011) Cleavage and activation of a Toll-like receptor by microbial proteases. Proc. Natl. Acad. Sci. USA 108, 4968–4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brooks J. C., Sun W., Chiosis G., Leifer C. A. (2012) Heat shock protein gp96 regulates Toll-like receptor 9 proteolytic processing and conformational stability. Biochem. Biophys. Res. Commun. 421, 780–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim T. W., Hong S., Talukder A. H., Pascual V., Liu Y. J. (2016) Grancalcin (GCA) modulates Toll-like receptor 9 (TLR9)-mediated signaling through its direct interaction with TLR9. Eur. J. Immunol. 46, 712–724. [DOI] [PubMed] [Google Scholar]
- 103.Jiang Z., Georgel P., Du X., Shamel L., Sovath S., Mudd S., Huber M., Kalis C., Keck S., Galanos C., Freudenberg M., Beutler B. (2005) CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6, 565–570. [DOI] [PubMed] [Google Scholar]
- 104.Lee H. K., Dunzendorfer S., Soldau K., Tobias P. S. (2006) Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity 24, 153–163. [DOI] [PubMed] [Google Scholar]
- 105.Baumann C. L., Aspalter I. M., Sharif O., Pichlmair A., Blüml S., Grebien F., Bruckner M., Pasierbek P., Aumayr K., Planyavsky M., Bennett K. L., Colinge J., Knapp S., Superti-Furga G. (2010) CD14 is a coreceptor of Toll-like receptors 7 and 9. J. Exp. Med. 207, 2689–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jersmann H. P. A. (2005) Time to abandon dogma: CD14 is expressed by non-myeloid lineage cells. Immunol. Cell Biol. 83, 462–467. [DOI] [PubMed] [Google Scholar]
- 107.Kennedy M. N., Mullen G. E., Leifer C. A., Lee C., Mazzoni A., Dileepan K. N., Segal D. M. (2004) A complex of soluble MD-2 and lipopolysaccharide serves as an activating ligand for Toll-like receptor 4. J. Biol. Chem. 279, 34698–34704. [DOI] [PubMed] [Google Scholar]
- 108.Yang Z., Breider M. A., Carroll R. C., Miller M. S., Bochsler P. N. (1996) Soluble CD14 and lipopolysaccharide-binding protein from bovine serum enable bacterial lipopolysaccharide-mediated cytotoxicity and activation of bovine vascular endothelial cells in vitro. J. Leukoc. Biol. 59, 241–247. [DOI] [PubMed] [Google Scholar]
- 109.Wald D., Qin J., Zhao Z., Qian Y., Naramura M., Tian L., Towne J., Sims J. E., Stark G. R., Li X. (2003) SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat. Immunol. 4, 920–927. [DOI] [PubMed] [Google Scholar]
- 110.Xiao H., Gulen M. F., Qin J., Yao J., Bulek K., Kish D., Altuntas C. Z., Wald D., Ma C., Zhou H., Tuohy V. K., Fairchild R. L., de la Motte C., Cua D., Vallance B. A., Li X. (2007) The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26, 461–475. [DOI] [PubMed] [Google Scholar]
- 111.Rose W. A. II, Sakamoto K., Leifer C. A. (2012) TLR9 is important for protection against intestinal damage and for intestinal repair. Sci. Rep. 2, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rose W. A. II, Sakamoto K., Leifer C. A. (2012) Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol. 13, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Divanovic S., Trompette A., Atabani S. F., Madan R., Golenbock D. T., Visintin A., Finberg R. W., Tarakhovsky A., Vogel S. N., Belkaid Y., Kurt-Jones E. A., Karp C. L. (2005) Negative regulation of Toll-like receptor 4 signaling by the Toll-like receptor homolog RP105. Nat. Immunol. 6, 571–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu B., Zhang N., Liu Z., Fu Y., Feng S., Wang S., Cao Y., Li D., Liang D., Li F., Song X., Yang Z. (2013) RP105 involved in activation of mouse macrophages via TLR2 and TLR4 signaling. Mol. Cell. Biochem. 378, 183–193. [DOI] [PubMed] [Google Scholar]
- 115.Nagai Y., Kobayashi T., Motoi Y., Ishiguro K., Akashi S., Saitoh S., Kusumoto Y., Kaisho T., Akira S., Matsumoto M., Takatsu K., Miyake K. (2005) The radioprotective 105/MD-1 complex links TLR2 and TLR4/MD-2 in antibody response to microbial membranes. J. Immunol. 174, 7043–7049. [DOI] [PubMed] [Google Scholar]
- 116.Blumenthal A., Kobayashi T., Pierini L. M., Banaei N., Ernst J. D., Miyake K., Ehrt S. (2009) RP105 facilitates macrophage activation by Mycobacterium tuberculosis lipoproteins. Cell Host Microbe 5, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ferrao R., Li J., Bergamin E., Wu H. (2012) Structural insights into the assembly of large oligomeric signalosomes in the Toll-like receptor-interleukin-1 receptor superfamily. Sci. Signal. 5, re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ohnishi T., Muroi M., Tanamoto K. (2001) N-Linked glycosylations at Asn(26) and Asn(114) of human MD-2 are required for Toll-like receptor 4-mediated activation of NF-kappaB by lipopolysaccharide. J. Immunol. 167, 3354–3359. [DOI] [PubMed] [Google Scholar]
- 119.Weber A. N., Morse M. A., Gay N. J. (2004) Four N-linked glycosylation sites in human Toll-like receptor 2 cooperate to direct efficient biosynthesis and secretion. J. Biol. Chem. 279, 34589–34594. [DOI] [PubMed] [Google Scholar]
- 120.Feng C., Stamatos N. M., Dragan A. I., Medvedev A., Whitford M., Zhang L., Song C., Rallabhandi P., Cole L., Nhu Q. M., Vogel S. N., Geddes C. D., Cross A. S. (2012) Sialyl residues modulate LPS-mediated signaling through the Toll-like receptor 4 complex. PLoS One 7, e32359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amith S. R., Jayanth P., Franchuk S., Finlay T., Seyrantepe V., Beyaert R., Pshezhetsky A. V., Szewczuk M. R. (2010) Neu1 desialylation of sialyl alpha-2,3-linked beta-galactosyl residues of Toll-like receptor 4 is essential for receptor activation and cellular signaling. Cell. Signal. 22, 314–324. [DOI] [PubMed] [Google Scholar]
- 122.Abdulkhalek S., Amith S. R., Franchuk S. L., Jayanth P., Guo M., Finlay T., Gilmour A., Guzzo C., Gee K., Beyaert R., Szewczuk M. R. (2011) Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for Toll-like receptor activation and cellular signaling. J. Biol. Chem. 286, 36532–36549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sun J., Duffy K. E., Ranjith-Kumar C. T., Xiong J., Lamb R. J., Santos J., Masarapu H., Cunningham M., Holzenburg A., Sarisky R. T., Mbow M. L., Kao C. (2006) Structural and functional analyses of the human Toll-like receptor 3. Role of glycosylation. J. Biol. Chem. 281, 11144–11151. [DOI] [PubMed] [Google Scholar]
- 124.Iavarone C., Ramsauer K., Kubarenko A. V., Debasitis J. C., Leykin I., Weber A. N., Siggs O. M., Beutler B., Zhang P., Otten G., D’Oro U., Valiante N. M., Mbow M. L., Visintin A. (2011) A point mutation in the amino terminus of TLR7 abolishes signaling without affecting ligand binding. J. Immunol. 186, 4213–4222. [DOI] [PubMed] [Google Scholar]
- 125.Heaton S. M., Borg N. A., Dixit V. M. (2016) Ubiquitin in the activation and attenuation of innate antiviral immunity. J. Exp. Med. 213, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Coornaert B., Carpentier I., Beyaert R. (2009) A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 284, 8217–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carpenter S., O’Neill L. A. (2009) Recent insights into the structure of Toll-like receptors and post-translational modifications of their associated signalling proteins. Biochem. J. 422, 1–10. [DOI] [PubMed] [Google Scholar]
- 128.Chuang T. H., Ulevitch R. J. (2004) Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 5, 495–502. [DOI] [PubMed] [Google Scholar]
- 129.Boone D. L., Turer E. E., Lee E. G., Ahmad R. C., Wheeler M. T., Tsui C., Hurley P., Chien M., Chai S., Hitotsumatsu O., McNally E., Pickart C., Ma A. (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5, 1052–1060. [DOI] [PubMed] [Google Scholar]
- 130.Lee E. G., Boone D. L., Chai S., Libby S. L., Chien M., Lodolce J. P., Ma A. (2000) Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science 289, 2350–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wertz I. E., O’Rourke K. M., Zhou H., Eby M., Aravind L., Seshagiri S., Wu P., Wiesmann C., Baker R., Boone D. L., Ma A., Koonin E. V., Dixit V. M. (2004) De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature 430, 694–699. [DOI] [PubMed] [Google Scholar]
- 132.Guedes R. P., Csizmadia E., Moll H. P., Ma A., Ferran C., da Silva C. G. (2014) A20 deficiency causes spontaneous neuroinflammation in mice. J. Neuroinflammation 11, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xuan N. T., Wang X., Nishanth G., Waisman A., Borucki K., Isermann B., Naumann M., Deckert M., Schlüter D. (2015) A20 expression in dendritic cells protects mice from LPS-induced mortality. Eur. J. Immunol. 45, 818–828. [DOI] [PubMed] [Google Scholar]
- 134.Kool M., van Loo G., Waelput W., De Prijck S., Muskens F., Sze M., van Praet J., Branco-Madeira F., Janssens S., Reizis B., Elewaut D., Beyaert R., Hammad H., Lambrecht B. N. (2011) The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35, 82–96. [DOI] [PubMed] [Google Scholar]
- 135.Chang M., Jin W., Sun S. C. (2009) Peli1 facilitates TRIF-dependent Toll-like receptor signaling and proinflammatory cytokine production. Nat. Immunol. 10, 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiao Y., Jin J., Chang M., Chang J. H., Hu H., Zhou X., Brittain G. C., Stansberg C., Torkildsen Ø., Wang X., Brink R., Cheng X., Sun S. C. (2013) Peli1 promotes microglia-mediated CNS inflammation by regulating Traf3 degradation. Nat. Med. 19, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ni J., Liu J., Leng R. X., Pan H. F., Ye D. Q. (2016) Genetic polymorphism (rs329498) in the Pellino-1 gene as possible predisposal factor for systemic lupus erythematosus in a Chinese population. Immunol. Invest. 45, 181–190. [DOI] [PubMed] [Google Scholar]
- 138.Musone S. L., Taylor K. E., Lu T. T., Nititham J., Ferreira R. C., Ortmann W., Shifrin N., Petri M. A., Kamboh M. I., Manzi S., Seldin M. F., Gregersen P. K., Behrens T. W., Ma A., Kwok P. Y., Criswell L. A. (2008) Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat. Genet. 40, 1062–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elsby L. M., Orozco G., Denton J., Worthington J., Ray D. W., Donn R. P. (2010) Functional evaluation of TNFAIP3 (A20) in rheumatoid arthritis. Clin. Exp. Rheumatol. 28, 708–714. [PMC free article] [PubMed] [Google Scholar]
- 140.Yang S., Wang B., Humphries F., Jackson R., Healy M. E., Bergin R., Aviello G., Hall B., McNamara D., Darby T., Quinlan A., Shanahan F., Melgar S., Fallon P. G., Moynagh P. N. (2013) Pellino3 ubiquitinates RIP2 and mediates Nod2-induced signaling and protective effects in colitis. Nat. Immunol. 14, 927–936. [DOI] [PubMed] [Google Scholar]
- 141.Arbibe L., Mira J. P., Teusch N., Kline L., Guha M., Mackman N., Godowski P. J., Ulevitch R. J., Knaus U. G. (2000) Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat. Immunol. 1, 533–540. [DOI] [PubMed] [Google Scholar]
- 142.Xiong Y., Song C., Snyder G. A., Sundberg E. J., Medvedev A. E. (2012) R753Q polymorphism inhibits Toll-like receptor (TLR) 2 tyrosine phosphorylation, dimerization with TLR6, and recruitment of myeloid differentiation primary response protein 88. J. Biol. Chem. 287, 38327–38337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Medvedev A. E., Piao W., Shoenfelt J., Rhee S. H., Chen H., Basu S., Wahl L. M., Fenton M. J., Vogel S. N. (2007) Role of TLR4 tyrosine phosphorylation in signal transduction and endotoxin tolerance. J. Biol. Chem. 282, 16042–16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarkar S. N., Peters K. L., Elco C. P., Sakamoto S., Pal S., Sen G. C. (2004) Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 11, 1060–1067. [DOI] [PubMed] [Google Scholar]
- 145.Sarkar S. N., Smith H. L., Rowe T. M., Sen G. C. (2003) Double-stranded RNA signaling by Toll-like receptor 3 requires specific tyrosine residues in its cytoplasmic domain. J. Biol. Chem. 278, 4393–4396. [DOI] [PubMed] [Google Scholar]
- 146.Lee K. G., Xu S., Kang Z. H., Huo J., Huang M., Liu D., Takeuchi O., Akira S., Lam K. P. (2012) Bruton’s tyrosine kinase phosphorylates Toll-like receptor 3 to initiate antiviral response. Proc. Natl. Acad. Sci. USA 109, 5791–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamashita M., Chattopadhyay S., Fensterl V., Saikia P., Wetzel J. L., Sen G. C. (2012) Epidermal growth factor receptor is essential for Toll-like receptor 3 signaling. Sci. Signal. 5, ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarkar S. N., Elco C. P., Peters K. L., Chattopadhyay S., Sen G. C. (2007) Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-kappa B activation. J. Biol. Chem. 282, 3423–3427. [DOI] [PubMed] [Google Scholar]
- 149.Jefferies C. A., Doyle S., Brunner C., Dunne A., Brint E., Wietek C., Walch E., Wirth T., O’Neill L. A. (2003) Bruton’s tyrosine kinase is a Toll/interleukin-1 receptor domain-binding protein that participates in nuclear factor kappaB activation by Toll-like receptor 4. J. Biol. Chem. 278, 26258–26264. [DOI] [PubMed] [Google Scholar]
- 150.Chen L. Y., Zuraw B. L., Zhao M., Liu F. T., Huang S., Pan Z. K. (2003) Involvement of protein tyrosine kinase in Toll-like receptor 4-mediated NF-kappa B activation in human peripheral blood monocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 284, L607–L613. [DOI] [PubMed] [Google Scholar]
- 151.Xiong Y., Murphy M., Manavalan T. T., Pattabiraman G., Qiu F., Chang H. H., Ho I. C., Medvedev A. E. (2016) Endotoxin tolerance inhibits Lyn and c-Src phosphorylation and association with Toll-like receptor 4 but increases expression and activity of protein phosphatases. J. Innate Immun. 8, 171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin Y. C., Huang D. Y., Chu C. L., Lin Y. L., Lin W. W. (2013) The tyrosine kinase Syk differentially regulates Toll-like receptor signaling downstream of the adaptor molecules TRAF6 and TRAF3. Sci. Signal. 6, ra71. [DOI] [PubMed] [Google Scholar]
- 153.Ivison S. M., Khan M. A., Graham N. R., Bernales C. Q., Kaleem A., Tirling C. O., Cherkasov A., Steiner T. S. (2007) A phosphorylation site in the Toll-like receptor 5 TIR domain is required for inflammatory signalling in response to flagellin. Biochem. Biophys. Res. Commun. 352, 936–941. [DOI] [PubMed] [Google Scholar]
- 154.Rajagopal R., Waller A. S., Mendoza J. D., Wightman P. D. (2008) The covalent modification and regulation of TLR8 in HEK-293 cells stimulated with imidazoquinoline agonists. Biochem. J. 409, 275–287. [DOI] [PubMed] [Google Scholar]
- 155.Lee K. G., Xu S., Wong E. T., Tergaonkar V., Lam K. P. (2008) Bruton’s tyrosine kinase separately regulates NFkappaB p65RelA activation and cytokine interleukin (IL)-10/IL-12 production in TLR9-stimulated B cells. J. Biol. Chem. 283, 11189–11198. [DOI] [PubMed] [Google Scholar]
- 156.Doyle S. L., Jefferies C. A., Feighery C., O’Neill L. A. (2007) Signaling by Toll-like receptors 8 and 9 requires Bruton’s tyrosine kinase. J. Biol. Chem. 282, 36953–36960. [DOI] [PubMed] [Google Scholar]
- 157.Sanjuan M. A., Rao N., Lai K. T., Gu Y., Sun S., Fuchs A., Fung-Leung W. P., Colonna M., Karlsson L. (2006) CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol. 172, 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hasan M., Gruber E., Cameron J., Leifer C. A. (2016) TLR9 stability and signaling are regulated by phosphorylation and cell stress. J. Leukoc. Biol [Epub ahead of print].. jlb.2A0815-337R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ogus A. C., Yoldas B., Ozdemir T., Uguz A., Olcen S., Keser I., Coskun M., Cilli A., Yegin O. (2004) The Arg753GLn polymorphism of the human Toll-like receptor 2 gene in tuberculosis disease. Eur. Respir. J. 23, 219–223. [DOI] [PubMed] [Google Scholar]
- 160.Ben-Ali M., Barbouche M. R., Bousnina S., Chabbou A., Dellagi K. (2004) Toll-like receptor 2 Arg677Trp polymorphism is associated with susceptibility to tuberculosis in Tunisian patients. Clin. Diagn. Lab. Immunol. 11, 625–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu H., Yang L. (2015) Arg753Gln polymorphisms in Toll-like receptor 2 gene are associated with tuberculosis risk: a meta-analysis. Med. Sci. Monit. 21, 2196–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dalgic N., Tekin D., Kayaalti Z., Soylemezoglu T., Cakir E., Kilic B., Kutlubay B., Sancar M., Odabasi M. (2011) Arg753Gln polymorphism of the human Toll-like receptor 2 gene from infection to disease in pediatric tuberculosis. Hum. Immunol. 72, 440–445. [DOI] [PubMed] [Google Scholar]
- 163.Kagan J. C., Medzhitov R. (2006) Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell 125, 943–955. [DOI] [PubMed] [Google Scholar]
- 164.Lin S. C., Lo Y. C., Wu H. (2010) Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 465, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. (2003) Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 197, 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Palsson-McDermott E. M., Doyle S. L., McGettrick A. F., Hardy M., Husebye H., Banahan K., Gong M., Golenbock D., Espevik T., O’Neill L. A. (2009) TAG, a splice variant of the adaptor TRAM, negatively regulates the adaptor MyD88-independent TLR4 pathway. Nat. Immunol. 10, 579–586. [DOI] [PubMed] [Google Scholar]
- 167.Carty M., Goodbody R., Schröder M., Stack J., Moynagh P. N., Bowie A. G. (2006) The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7, 1074–1081. [DOI] [PubMed] [Google Scholar]
- 168.Carlsson E., Ding J. L., Byrne B. (2016) SARM modulates MyD88-mediated TLR activation through BB-loop dependent TIR-TIR interactions. Biochim. Biophys. Acta 1863, 244–253. [DOI] [PubMed] [Google Scholar]
- 169.Li X. (2008) IRAK4 in TLR/IL-1R signaling: possible clinical applications. Eur. J. Immunol. 38, 614–618. [DOI] [PubMed] [Google Scholar]
- 170.Kobayashi K., Hernandez L. D., Galán J. E., Janeway C. A. Jr.,Medzhitov R., Flavell R. A. (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110, 191–202. [DOI] [PubMed] [Google Scholar]
- 171.Rao N., Nguyen S., Ngo K., Fung-Leung W. P. (2005) A novel splice variant of interleukin-1 receptor (IL-1R)-associated kinase 1 plays a negative regulatory role in Toll/IL-1R-induced inflammatory signaling. Mol. Cell. Biol. 25, 6521–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou H., Yu M., Fukuda K., Im J., Yao P., Cui W., Bulek K., Zepp J., Wan Y., Kim T. W., Yin W., Ma V., Thomas J., Gu J., Wang J. A., DiCorleto P. E., Fox P. L., Qin J., Li X. (2013) IRAK-M mediates Toll-like receptor/IL-1R-induced NFκB activation and cytokine production. EMBO J. 32, 583–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Piao W., Song C., Chen H., Wahl L. M., Fitzgerald K. A., O’Neill L. A., Medvedev A. E. (2008) Tyrosine phosphorylation of MyD88 adapter-like (Mal) is critical for signal transduction and blocked in endotoxin tolerance. J. Biol. Chem. 283, 3109–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gray P., Dunne A., Brikos C., Jefferies C. A., Doyle S. L., O’Neill L. A. (2006) MyD88 adapter-like (Mal) is phosphorylated by Bruton’s tyrosine kinase during TLR2 and TLR4 signal transduction. J. Biol. Chem. 281, 10489–10495. [DOI] [PubMed] [Google Scholar]
- 175.Kubo-Murai M., Hazeki K., Sukenobu N., Yoshikawa K., Nigorikawa K., Inoue K., Yamamoto T., Matsumoto M., Seya T., Inoue N., Hazeki O. (2007) Protein kinase Cdelta binds TIRAP/Mal to participate in TLR signaling. Mol. Immunol. 44, 2257–2264. [DOI] [PubMed] [Google Scholar]
- 176.Paracha R. Z., Ali A., Ahmad J., Hussain R., Niazi U., Muhammad S. A. (2014) Structural evaluation of BTK and PKCδ mediated phosphorylation of MAL at positions Tyr86 and Tyr106. Comput. Biol. Chem. 51, 22–35. [DOI] [PubMed] [Google Scholar]
- 177.Mansell A., Smith R., Doyle S. L., Gray P., Fenner J. E., Crack P. J., Nicholson S. E., Hilton D. J., O’Neill L. A., Hertzog P. J. (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat. Immunol. 7, 148–155. [DOI] [PubMed] [Google Scholar]
- 178.Xi C. X., Xiong F., Zhou Z., Mei L., Xiong W. C. (2010) PYK2 interacts with MyD88 and regulates MyD88-mediated NF-kappaB activation in macrophages. J. Leukoc. Biol. 87, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Semaan N., Alsaleh G., Gottenberg J. E., Wachsmann D., Sibilia J. (2008) Etk/BMX, a Btk family tyrosine kinase, and Mal contribute to the cross-talk between MyD88 and FAK pathways. J. Immunol. 180, 3485–3491. [DOI] [PubMed] [Google Scholar]
- 180.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y. T., Grishin N. V., Chen Z. J. (2015) Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630. [DOI] [PubMed] [Google Scholar]
- 181.Jiao H., Zhang Y., Yan Z., Wang Z. G., Liu G., Minshall R. D., Malik A. B., Hu G. (2013) Caveolin-1 Tyr14 phosphorylation induces interaction with TLR4 in endothelial cells and mediates MyD88-dependent signaling and sepsis-induced lung inflammation. J. Immunol. 191, 6191–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lee Y. S., Park J. S., Kim J. H., Jung S. M., Lee J. Y., Kim S. J., Park S. H. (2011) Smad6-specific recruitment of Smurf E3 ligases mediates TGF-β1-induced degradation of MyD88 in TLR4 signalling. Nat. Commun. 2, 460. [DOI] [PubMed] [Google Scholar]
- 183.Lee B. C., Miyata M., Lim J. H., Li J. D. (2016) Deubiquitinase CYLD acts as a negative regulator for bacterium NTHi-induced inflammation by suppressing K63-linked ubiquitination of MyD88. Proc. Natl. Acad. Sci. USA 113, E165–E171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xue Q., Zhou Z., Lei X., Liu X., He B., Wang J., Hung T. (2012) TRIM38 negatively regulates TLR3-mediated IFN-β signaling by targeting TRIF for degradation. PLoS One 7, e46825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Medvedev A. E., Murphy M., Zhou H., Li X. (2015) E3 ubiquitin ligases Pellinos as regulators of pattern recognition receptor signaling and immune responses. Immunol. Rev. 266, 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Moynagh P. N. (2014) The roles of Pellino E3 ubiquitin ligases in immunity. Nat. Rev. Immunol. 14, 122–131. [DOI] [PubMed] [Google Scholar]
- 187.Humphries F., Moynagh P. N. (2015) Molecular and physiological roles of Pellino E3 ubiquitin ligases in immunity. Immunol. Rev. 266, 93–108. [DOI] [PubMed] [Google Scholar]
- 188.Ordureau A., Smith H., Windheim M., Peggie M., Carrick E., Morrice N., Cohen P. (2008) The IRAK-catalysed activation of the E3 ligase function of Pellino isoforms induces the Lys63-linked polyubiquitination of IRAK1. Biochem. J. 409, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Smith H., Liu X. Y., Dai L., Goh E. T., Chan A. T., Xi J., Seh C. C., Qureshi I. A., Lescar J., Ruedl C., Gourlay R., Morton S., Hough J., McIver E. G., Cohen P., Cheung P. C. (2011) The role of TBK1 and IKKε in the expression and activation of Pellino 1. Biochem. J. 434, 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang Z., Zamanian-Daryoush M., Nie H., Silva A. M., Williams B. R., Li X. (2003) Poly(I-C)-induced Toll-like receptor 3 (TLR3)-mediated activation of NFkappa B and MAP kinase is through an interleukin-1 receptor-associated kinase (IRAK)-independent pathway employing the signaling components TLR3-TRAF6-TAK1-TAB2-PKR. J. Biol. Chem. 278, 16713–16719. [DOI] [PubMed] [Google Scholar]
- 191.Murphy M., Xiong Y., Pattabiraman G., Qiu F., Medvedev A. E. (2015) Pellino-1 positively regulates Toll-like receptor (TLR) 2 and TLR4 signaling and is suppressed upon Iinduction of endotoxin tolerance. J. Biol. Chem. 290, 19218–19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jensen L. E., Whitehead A. S. (2003) Pellino2 activates the mitogen activated protein kinase pathway. FEBS Lett. 545, 199–202. [DOI] [PubMed] [Google Scholar]
- 193.Strelow A., Kollewe C., Wesche H. (2003) Characterization of Pellino2, a substrate of IRAK1 and IRAK4. FEBS Lett. 547, 157–161. [DOI] [PubMed] [Google Scholar]
- 194.Murphy M. B., Xiong Y., Pattabiraman G., Manavalan T. T., Qiu F., Medvedev A. E. (2015) Pellino-3 promotes endotoxin tolerance and acts as a negative regulator of TLR2 and TLR4 signaling. J. Leukoc. Biol. 98, 963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Siednienko J., Jackson R., Mellett M., Delagic N., Yang S., Wang B., Tang L. S., Callanan J. J., Mahon B. P., Moynagh P. N. (2012) Pellino3 targets the IRF7 pathway and facilitates autoregulation of TLR3- and viral-induced expression of type I interferons. Nat. Immunol. 13, 1055–1062. [DOI] [PubMed] [Google Scholar]
- 196.Tzieply N., Kuhn A. M., Morbitzer D., Namgaladze D., Heeg A., Schaefer L., von Knethen A., Jensen L. E., Brüne B. (2012) OxLDL inhibits LPS-induced IFNβ expression by Pellino3- and IRAK1/4-dependent modification of TANK. Cell. Signal. 24, 1141–1149. [DOI] [PubMed] [Google Scholar]
- 197.Kawagoe T., Takeuchi O., Takabatake Y., Kato H., Isaka Y., Tsujimura T., Akira S. (2009) TANK is a negative regulator of Toll-like receptor signaling and is critical for the prevention of autoimmune nephritis. Nat. Immunol. 10, 965–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xiong H., Li H., Kong H. J., Chen Y., Zhao J., Xiong S., Huang B., Gu H., Mayer L., Ozato K., Unkeless J. C. (2005) Ubiquitin-dependent degradation of interferon regulatory factor-8 mediated by Cbl down-regulates interleukin-12 expression. J. Biol. Chem. 280, 23531–23539. [DOI] [PubMed] [Google Scholar]
- 199.Fearns C., Pan Q., Mathison J. C., Chuang T. H. (2006) Triad3A regulates ubiquitination and proteasomal degradation of RIP1 following disruption of Hsp90 binding. J. Biol. Chem. 281, 34592–34600. [DOI] [PubMed] [Google Scholar]
- 200.Stacey K. B., Breen E., Jefferies C. A. (2012) Tyrosine phosphorylation of the E3 ubiquitin ligase TRIM21 positively regulates interaction with IRF3 and hence TRIM21 activity. PLoS One 7, e34041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Allen J. L., Flick L. M., Divanovic S., Jackson S. W., Bram R., Rawlings D. J., Finkelman F. D., Karp C. L. (2012) Cutting edge: regulation of TLR4-driven B cell proliferation by RP105 is not B cell autonomous. J. Immunol. 188, 2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gringhuis S. I., den Dunnen J., Litjens M., van Het Hof B., van Kooyk Y., Geijtenbeek T. B. (2007) C-Type lectin DC-SIGN modulates Toll-like receptor signaling via Raf-1 kinase-dependent acetylation of transcription factor NF-kappaB. Immunity 26, 605–616. [DOI] [PubMed] [Google Scholar]
- 203.Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517. [DOI] [PubMed] [Google Scholar]
- 204.Suzuki-Inoue K., Fuller G. L., García A., Eble J. A., Pöhlmann S., Inoue O., Gartner T. K., Hughan S. C., Pearce A. C., Laing G. D., Theakston R. D., Schweighoffer E., Zitzmann N., Morita T., Tybulewicz V. L., Ozaki Y., Watson S. P. (2006) A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood 107, 542–549. [DOI] [PubMed] [Google Scholar]
- 205.Rothlin C. V., Ghosh S., Zuniga E. I., Oldstone M. B., Lemke G. (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131, 1124–1136. [DOI] [PubMed] [Google Scholar]
- 206.Chu C. L., Yu Y. L., Shen K. Y., Lowell C. A., Lanier L. L., Hamerman J. A. (2008) Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur. J. Immunol. 38, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hamerman J. A., Tchao N. K., Lowell C. A., Lanier L. L. (2005) Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat. Immunol. 6, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Peng Q., Long C. L., Malhotra S., Humphrey M. B. (2013) A physical interaction between the adaptor proteins DOK3 and DAP12 is required to inhibit lipopolysaccharide signaling in macrophages. Sci. Signal. 6, ra72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Androulidaki A., Iliopoulos D., Arranz A., Doxaki C., Schworer S., Zacharioudaki V., Margioris A. N., Tsichlis P. N., Tsatsanis C. (2009) The kinase Akt1 controls macrophage response to lipopolysaccharide by regulating microRNAs. Immunity 31, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Benakanakere M. R., Li Q., Eskan M. A., Singh A. V., Zhao J., Galicia J. C., Stathopoulou P., Knudsen T. B., Kinane D. F. (2009) Modulation of TLR2 protein expression by miR-105 in human oral keratinocytes. J. Biol. Chem. 284, 23107–23115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Chen X. M., Splinter P. L., O’Hara S. P., LaRusso N. F. (2007) A cellular micro-RNA, let-7i, regulates Toll-like receptor 4 expression and contributes to cholangiocyte immune responses against Cryptosporidium parvum infection. J. Biol. Chem. 282, 28929–28938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006) NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 103, 12481–12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Carpenter S., Aiello D., Atianand M. K., Ricci E. P., Gandhi P., Hall L. L., Byron M., Monks B., Henry-Bezy M., Lawrence J. B., O’Neill L. A., Moore M. J., Caffrey D. R., Fitzgerald K. A. (2013) A long noncoding RNA mediates both activation and repression of immune response genes. Science 341, 789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]