Human neutrophil hydrogen peroxide and hypochlorous acid production is reduced by S. aureus SaeR/S-regulated factors.
Keywords: innate immunity, bacteria, host-pathogen interactions, two-component systems
Abstract
Neutrophils are the first line of defense after a pathogen has breached the epithelial barriers, and unimpaired neutrophil functions are essential to clear infections. Staphylococcus aureus is a prevalent human pathogen that is able to withstand neutrophil killing, yet the mechanisms used by S. aureus to inhibit neutrophil clearance remain incompletely defined. The production of reactive oxygen species (ROS) is a vital neutrophil antimicrobial mechanism. Herein, we test the hypothesis that S. aureus uses the SaeR/S two-component gene regulatory system to produce virulence factors that reduce neutrophil ROS production. With the use of ROS probes, the temporal and overall production of neutrophil ROS was assessed during exposure to the clinically relevant S. aureus USA300 (strain LAC) and its isogenic mutant LACΔsaeR/S. Our results demonstrated that SaeR/S-regulated factors do not inhibit neutrophil superoxide (O2−) production. However, subsequent neutrophil ROS production was significantly reduced during exposure to LAC compared with LACΔsaeR/S. In addition, neutrophil H2O2 production was reduced significantly by SaeR/S-regulated factors by a mechanism independent of catalase. Consequently, the reduction in neutrophil H2O2 resulted in decreased production of the highly antimicrobial agent hypochlorous acid/hypochlorite anion (HOCl/−OCl). These findings suggest a new evasion strategy used by S. aureus to diminish a vital neutrophil antimicrobial mechanism.
Introduction
PMNs (leukocytes or neutrophils) are the most abundant WBC in the human body and the first line of defense during bacterial infection [1]. Following migration to the site of infection and phagocytosis, neutrophils expose pathogens to an abundance of microbicidal components, including cationic peptides, proteases, and potent ROS [2, 3]. Assembly and activation of the NADPH oxidase system result in the production of O2− from molecular O2, followed by dismutation to H2O2 and to the formation of the highly bactericidal agent HOCl/−OCl, catalyzed by the enzyme MPO [4–6].The production of neutrophil ROS is highly effective at killing many pathogens, including the Gram-positive pathogen S. aureus [7, 8]. Microbicidal capacity of ROS against S. aureus is exemplified further by the observed increase in susceptibility to infections in individuals with genetic defects in any of the 5 structural components of the NADPH oxidase complex, resulting in chronic granulomatous disease [9, 10]. Collectively, neutrophil microbicidal systems are very efficient at killing ingested bacteria and limiting inflammation.
Despite the neutrophil’s capacity to contain most bacterial pathogens, its antimicrobial mechanisms are not fully effective in killing S. aureus, and bacterial survival following neutrophil phagocytosis has been proposed as a virulence strategy used by this bacterium [11, 12]. This is best exemplified by the epidemic of CA-MRSA that began in the late 1990s [13]. CA-MRSA can cause uncomplicated skin and soft-tissue infections, as well as invasive, life-threatening illnesses in otherwise healthy individuals [13–15], and its emergence has fostered research investigating the ability of S. aureus to survive after neutrophil phagocytosis.
Survival of S. aureus following neutrophil phagocytosis is dependent on the concerted effort of multiple virulence factors [11, 12, 16–18]. The S. aureus SaeR/S two-component system regulates virulence genes essential for evasion of neutrophil killing [12, 19, 20]. In brief, the sae locus consists of 4 open-reading frames (saePQRS). SaeS and saeR code for the two-component module of the SaeR/S system, where SaeS is the histidine kinase, and SaeR is the cognate response regulator. SaeR/S-regulated genes are up-regulated in response to neutrophil-derived components, including human α-defensin-1 and H2O2 [19, 21, 22]. Studies using isogenic saeR/S deletion mutants have shown that neutrophil lysis is reduced, and S. aureus has significantly reduced survival following neutrophil phagocytosis in the absence of SaeR/S [11, 12]. SaeR also controls the expression of secreted virulence factors, including those that contribute to neutrophil lysis, such as leukocidin G/H (lukG/H also known as lukA/B), γ hemolysins (hlgA, B, C), and Panton-Valentine leukocidin (lukF/S-PV) [12, 23]. Additionally, SaeR-regulated factors influence neutrophil cell fate contributing to pathogen survival [22].
In this study, we investigate further the role of S. aureus SaeR/S in modulating neutrophil function by examining its influence on ROS production. Our results demonstrate that SaeR/S-regulated factors decrease neutrophil-derived H2O2 and HOCl production by a mechanism independent of catalase activity.
MATERIALS AND METHODS
Bacterial strains and culture
WT S. aureus pulsed-field gel electrophoresis-type USA300 (strain LAC) [24] and its previously generated isogenic saeR/S mutant strain (LACΔsaeR/S) [23] were grown in tryptic soy broth containing 0.5% glucose and harvested at midexponential growth, as described previously [11, 22, 23].
Neutrophil and ROS assays
Human neutrophils were isolated from heparinized venous blood of healthy volunteer donors in accordance with a protocol approved by the Institutional Review Board for Human Subjects at Montana State University. Human neutrophils were isolated as described previously [11, 12]. For all ROS assays, 1 × 106 neutrophils (loaded with various probes, described below) were exposed to 1 × 107 bacteria (10:1 bacteria:PMN ratio) in 96-well serum-coated plates in duplicate. Neutrophils were also exposed to LAC HK (5 min at 99°C), 500 ng/ml PMA, or RPMI, and phagocytosis was synchronized [11]. All measurements were done using a SpectraMax Paradigm Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Luminol.
Neutrophil-derived ROS detection by the oxidation of cell-permeable luminol, resulting in chemiluminescence, was measured as described previously [25]. Neutrophils were stained with 100 μM luminol for 15 min in the dark at 4°C. Chemiluminescence was measured in 1 min intervals. The final concentrations of SOD and catalase were 50 and 2000 U/ml, respectively.
Isoluminol.
Neutrophil-derived O2− production by the oxidation of cell-impermeable isoluminol, resulting in chemiluminescence, was measured as described previously [25]. Neutrophils were stained with 100 μM isoluminol for 15 min in the dark at 4°C. Chemiluminescence was measured in 1 min intervals.
Amplex Red.
Neutrophil-derived extracellular H2O2 production was measured using the Amplex Red Hydrogen Peroxide/Peroxidase Kit (Thermo Fisher Scientific, Waltham, MA, USA), following the manufacturer’s instructions. Amplex Red oxidation was measured in 1 min intervals with 535/595 nm excitation/emission wavelengths.
Secreted catalase.
The Amplex Red Catalase Assay Kit (Thermo Fisher Scientific) was used following the manufacturer’s suggested protocol. Supernatants from PMN and/or bacterial incubations were collected following 90 min exposure at 37°C and sterile filtered with 0.22 μm syringe filters.
R19-S.
Neutrophil-derived HOCl production was measured using R19-S (FutureChem, Seoul, South Korea) [26, 27]. Oxidation of R19-S was measured in 1 min intervals with 485/535 nm excitation/emission wavelengths. Alternatively, neutrophil intracellular HOCl production at 90 min was measured by flow cytometry with a BD FACSCalibur (BD Biosciences, San Jose, CA, USA) using 488/530 nm emission/excitation wavelengths.
Neutrophil phagocytosis assay
Phagocytosis of S. aureus by human neutrophils was determined with fluorescence microscopy, as described previously [11]. FITC-labeled bacteria were added (10:1 bacteria:neutrophil ratio), and phagocytosis was synchronized as above. To counterstain uningested bacteria, samples were stained with anti-FITC conjugated to Alexa Fluor 594 (Thermo Fisher Scientific), and mounted coverslips were evaluated using fluorescence microscopy. The number of S. aureus bound and/or ingested was evaluated in 25 or 50 neutrophils per experiment from separate fields of view, and percent phagocytosis was calculated as (number of ingested bacteria per cell/total number of PMN-associated bacteria per cell, bound or ingested) × 100.
HOCl killing
A mixture of HOCl/−OCl was generated by mixing 10 ml commercially available Clorox, 5 ml DPBS, and 160 μl 36.5–38.0% hydrochloric acid. After overnight incubation in the dark, the concentration of HOCl/−OCl was calculated using Beer’s Law [28]. The average pH of the solution was 7.51 ± 0.09, similar to the acid dissociation constant for HOCl/−OCl (pKa = 7.44) [28, 29]. Bacteria (1 × 107), resuspended in DPBS, were mixed with HOCl/−OCl, diluted in DPBS to desired working concentrations and incubated at 37°C for 30 min. Bacteria were enumerated following overnight incubation at 37°C. Bacterial survival was calculated relative to bacterial concentration following exposure to DPBS only.
Statistical procedures
Statistical analyses were performed using GraphPad Prism version 6.0a (GraphPad Software, La Jolla, CA, USA) with t tests and ANOVA as indicated, and error bars represent the sem.
RESULTS AND DISCUSSION
SaeR/S-regulated factors decrease human neutrophil ROS production
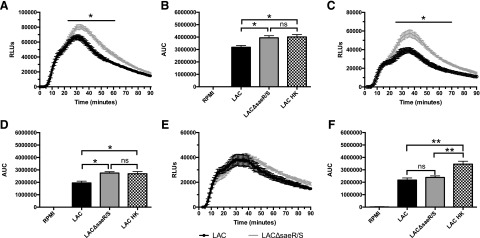
Previous studies have shown that human neutrophils fail to kill S. aureus completely after phagocytosis, and SaeR/S-regulated factors are at least partially responsible for reduced bactericidal activity [11, 12]. However, the mechanisms affected by SaeR/S-regulated factors leading to reduced neutrophil staphylococcal killing have yet to be elucidated fully. To this end, neutrophil ROS production in response to WT S. aureus strain LAC and its isogenic ΔsaeR/S mutant strain (LACΔsaeR/S) was analyzed using probes to measure different ROS. First, intracellular neutrophil ROS production was measured with luminol. Whereas there were no significant differences in the time to maximum neutrophil ROS production, total neutrophil ROS production was reduced significantly in response to the S. aureus LAC compared with LACΔsaeR/S (Fig. 1A and B). To determine if extracellular ROS contributed to abundance of intracellular ROS, neutrophils were exposed to LAC or LACΔsaeR/S, including SOD or catalase. As expected and in congruence with published findings, the addition of exogenous SOD reduced total neutrophil ROS production in response to LAC and LACΔsaeR/S (Fig. 1C) [30, 31]. In the presence of SOD, significant differences remained in total neutrophil intracellular ROS production during exposure to LAC and LACΔsaeR/S (Fig. 1D). The addition of exogenous catalase also reduced total neutrophil intracellular ROS production in response to LAC and LACΔsaeR/S compared with untreated neutrophils exposed to bacteria (Fig. 1E). However, neutrophils produced similar amounts of intracellular ROS in response to LAC and LACΔsaeR/S in the presence of exogenous catalase (Fig. 1F). Collectively, these results suggest that S. aureus SaeR/S-regulated factors reduce intracellular ROS production. Additionally, neutrophils exposed to LAC HK produced similar amounts of intracellular ROS as those exposed to LACΔsaeR/S (Fig. 1B and D), confirming that reduction of neutrophil ROS by S. aureus is an active process that requires a viable organism and SaeR/S. Importantly, the neutralization of extracellular H2O2 with exogenous catalase eliminated differences in intracellular neutrophil ROS production in response to LAC and LACΔsaeR/S. These results suggest that S. aureus SaeR/S-regulated factors reduce the production of neutrophil-derived H2O2, leading to a reduction in overall ROS production. Of note, neutrophil propidium iodide uptake was not significantly different between neutrophils exposed to LAC or LACΔsaeR/S after 90 min, confirming that differences in ROS production were not a result of differences in neutrophil membrane damage (data not shown). As differences in uptake of LAC and LACΔsaeR/S could influence ROS abundance, we assessed neutrophil phagocytosis using fluorescence microscopy. There were no differences in neutrophil ingestion of WT LAC compared with the LACΔsaeR/S mutant (Supplemental Fig. 1), which is in agreement with previous findings [12].
Figure 1. SaeR/S-regulated factors decrease intracellular ROS.
Human PMNs were preloaded with luminol, as described in Materials and Methods, and exposed to LAC, LACΔsaeR/S, or RPMI, and chemiluminescence was measured. (A) Time-dependent neutrophil intracellular ROS production following exposure to S. aureus LAC or LACΔsaeR/S. (B) Total relative neutrophil ROS production determined by calculating the area under the curve (AUC) from A. (C) Time-dependent neutrophil intracellular ROS production following exposure to S. aureus LAC or LACΔsaeR/S in the presence of exogenous SOD. (D) Total relative neutrophil ROS production determined by calculating the area under the curve from C. (E) Time-dependent neutrophil intracellular ROS production following exposure to S. aureus LAC or LACΔsaeR/S in the presence of exogenous catalase. (F) Total relative neutrophil ROS production determined by calculating the area under the curve from E. Data represent 5 separate experiments, using 5 different neutrophil donors; *P ≤ 0.05, **P ≤ 0.01, as determined by two-way ANOVA (A, C, and E) and one-way ANOVA (B, D, and F). RLUs, Relative luminescence units.
SaeR/S-regulated factors reduce the production of neutrophil-derived H2O2
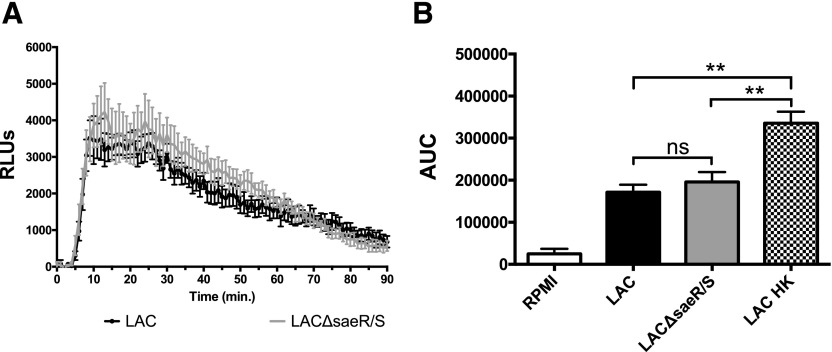
Luminol measures overall intracellular ROS production. As our results showed that extracellular ROS played a significant role in enhancing intracellular neutrophil ROS production (Fig. 1A–F) and suggested that SaeR/S-regulated factors reduce the production of neutrophil-derived H2O2, we used isoluminol and Amplex Red probes to measure specifically O2− and H2O2, respectively. Cell-impermeable isoluminol was used to measure neutrophil extracellular O2− production in response to LAC and LACΔsaeR/S. We hypothesized that neutrophil O2− production would not be reduced by SaeR/S-regulated factors, as significant differences remained in overall neutrophil intracellular ROS production during exposure to LAC and LACΔsaeR/S in the presence of exogenous SOD (Fig. 1C and D). The neutrophil O2− burst occurred within 10 min after recording luminescence (Fig. 2A). Consistent with results shown in Fig. 1C and D, there were no significant differences in neutrophil O2− production during exposure to LAC or LACΔsaeR/S (Fig. 2B). This suggests that activation and assembly of the NADPH oxidase complex are not affected by SaeR/S-regulated factors. Exposure to LAC HK did result in significantly increased O2− production compared with LAC and LACΔsaeR/S. This is expected, as S. aureus produces SOD to neutralize O2− via sodA and sodM, but these genes are not regulated by SaeR/S [12, 23, 32, 33].
Figure 2. Human neutrophil extracellular O2− production following exposure to WT S. aureus LAC and LACΔsaeR/S using isoluminol.
(A) Time-dependent neutrophil extracellular O2− production following exposure to S. aureus LAC or LACΔsaeR/S. (B) Total relative neutrophil O2− production determined by calculating the area under the curve from A. Data represent 4 separate experiments, using 4 different neutrophil donors; **P ≤ 0.01, as determined by two-way ANOVA (A) and one-way ANOVA (B).
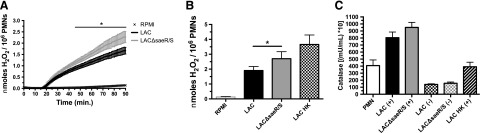
As the measurement of neutrophil intracellular ROS with luminol in the presence of catalase suggested that SaeR/S-regulated factors reduce production of extracellular H2O2 (Fig. 1C and D), we used the H2O2-specific probe Amplex Red to measure neutrophil extracellular H2O2 production in response to S. aureus LAC and LACΔsaeR/S. Detectable neutrophil-derived H2O2 was observed within 20 min of exposure to LAC and LACΔsaeR/S (Fig. 3A). Significant differences in extracellular H2O2 production between neutrophils exposed to LAC and LACΔsaeR/S were observed, starting at 43 min. In addition, there were significant increases in molar amounts of H2O2 produced by neutrophils in response to LACΔsaeR/S versus LAC at the end of the 90 min assay (Fig. 3B). Differences in extracellular H2O2 production by neutrophils confirmed results showing overall reduction in ROS—and no significant differences between neutrophils exposed to LAC and LACΔsaeR/S—when the cell-impermeable catalase was present (Fig. 1E and F). Importantly, there were no differences in the secreted catalase that could degrade extracellular H2O2 (Fig. 3C), confirming that SaeR/S does not regulate catalase following neutrophil phagocytosis.
Figure 3. Human neutrophil extracellular H2O2 production is significantly reduced by SaeR/S-regulated S. aureus factors.
(A) Time-dependent neutrophil extracellular H2O2 production was measured using Amplex Red, following exposure S. aureus LAC or LACΔsaeR/S or RPMI as a control. (B) Neutrophil-derived extracellular H2O2 production calculated from an H2O2 standard curve. (C) Secreted catalase following S. aureus exposure to human neutrophils. Bacteria exposed (+) or not exposed (−) to neutrophils. Data represent 4 separate experiments (A and C) and 5 separate experiments (B), with *P ≤ 0.05, as determined by two-way ANOVA (A) and paired t test (B).
SaeR/S-regulated factors reduce the production of neutrophil-derived HOCl
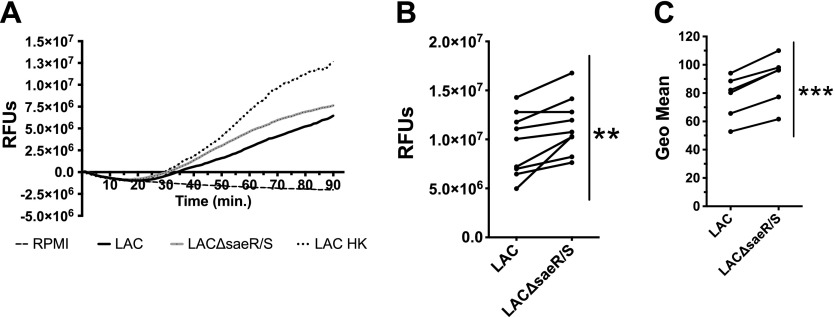
HOCl is present in neutrophil phagosomes and is highly bactericidal. H2O2 is a precursor to the production of MPO-catalyzed HOCl. Therefore, we measured intracellular HOCl production using the cell-permeable R19-S probe to assess how SaeR/S-regulated factors affect HOCl production (Fig. 4A). Consistent with our results showing that SaeR/S reduced H2O2 production, SaeR/S-regulated factors significantly decreased neutrophil HOCl production (Fig. 4B and C). Production of neutrophil H2O2 and HOCl was detected at ∼20 min, with the maximum H2O2 burst occurring earlier than the HOCl burst (Supplemental Fig. 2).
Figure 4. Human neutrophil intracellular hypochlorite production is reduced significantly by SaeR/S-regulated S. aureus factors.
HOCl production was measured in human neutrophils using R19-S, following exposure to WT S. aureus LAC or LACΔsaeR/S. (A) Representative plot of time-dependent neutrophil intracellular HOCl production following exposure to S. aureus LAC or LACΔsaeR/S or LAC HK or RPMI as a control. (B) Relative neutrophil-derived HOCl production at the end of the 90 min assay from A. (C) Relative neutrophil-derived intracellular HOCl measured by flow cytometry. Data represent 9 separate experiments (B) and 6 separate experiments (C); **P ≤ 0.01, ***P ≤ 0.001, as determined by paired t test (B and C). RFUs, Relative fluorescence units.
The formation of H2O2 from O2− can occur spontaneously or is catalyzed by SOD or MPO [34, 35]. Based on our observations, we propose that unidentified SaeR/S-regulated factor(s) interfere with the enzymatic reactions catalyzed by MPO to produce H2O2 and HOCl. Inasmuch as there are no differences in secreted catalase between neutrophils exposed to S. aureus LAC and LACΔsaeR/S (Fig. 3C), we propose that the significant decrease in H2O2 produced by neutrophils exposed to LAC compared with LACΔsaeR/S is a result of SaeR/S-regulated factors inhibiting the SOD activity of MPO to produce H2O2. In addition, SaeR/S-regulated factors may interfere with the chlorination activity of MPO, resulting in decreased production of HOCl. Ongoing studies are determining if decreased HOCl production is a direct result of lower H2O2 production or if SaeR/S-regulated factors directly interfere with both the dismutase and chlorination activity of MPO.
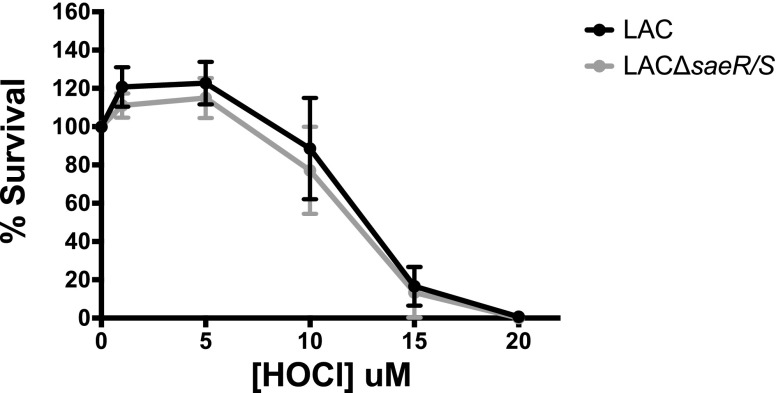
WT S. aureus LAC and LACΔsaeR/S are equally susceptible to reagent HOCl
S. aureus SaeR/S-regulated factors significantly decreased neutrophil-derived HOCl production in response to WT LAC versus LACΔsaeR/S (Fig. 4). To determine if the WT and mutant were differentially susceptible to HOCl, we exposed LAC and LACΔsaeR/S to varied concentrations of HOCl. As shown in Fig. 5, LAC and LACΔsaeR/S were equally susceptible to HOCl exposure. HOCl concentrations below 5 μM did not kill S. aureus. However, there was a precipitous decrease in S. aureus survival following exposure between 5 and 15 μM HOCl. These data are in agreement with previously published findings [36]. Future studies will investigate the physiologically relevant question as to whether reduction in ROS makes WT S. aureus more resistant to neutrophil killing reflective of clinical syndromes, demonstrating the importance of ROS in controlling S. aureus [9, 10]. It is possible that a SaeR/S-mediated reduction in ROS makes the ROS amount insufficient to kill S. aureus, as in vitro studies have demonstrated a fine line between ROS amounts that are effective versus amounts that the pathogen can tolerate (Fig. 5 and ref. [21]).
Figure 5. S. aureus LAC and LACΔsaeR/S are equally susceptible to killing by HOCl.
Bacteria (1 × 107) were exposed to different concentrations of HOCl for 30 min at 37°C. Subsequently, bacteria were serially diluted and plated on trypticase soy agar plates, and CFUs were enumerated following overnight incubation at 37°C. Data represent 5 separate experiments.
The importance of detoxifying ROS is demonstrated by the many mechanisms used by S. aureus that include scavenging and neutralizing ROS, such as O2− and H2O2, with SODs (SodA and SodM) and catalase (KatA), respectively [37, 38]. In addition, the iron-regulated surface-determinant proteins IsdA and IsdB have been implicated in increasing S. aureus resistance to killing by H2O2 [21], as well as methionine sulfoxide reductases to reduce oxidized methionine residues following oxidative stress that increase S. aureus resistance to ROS [39]. Unlike previously described mechanisms to neutralize ROS, our data suggest that S. aureus can directly inhibit H2O2 and HOCl production, resulting in increased S. aureus survival. Future studies will focus on identifying specific SaeR/S-regulated factors that inhibit neutrophil ROS production.
AUTHORSHIP
F.E.G. contributed to project design and experimental procedures, analyzed data, provided the figure presentation, and wrote the manuscript. C.B.A., J.A., N.W.M.d.J., K.B.P., and J.v.S. contributed to project design and experimental procedures. J.M.V. provided oversight and contributed to project design, data analysis, figure presentation, and manuscript writing.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (Grants NIH-R01A1090046, NIH-PAR98-072, and NIH-RR020185 for a fellowship award to F.E.G.), as well as funds from the Montana University System Research Initiative (51040-MUSRI2015-03) and Montana State University Agriculture Experiment Station and an equipment grant from Murdoch Charitable Trust.
Glossary
- CA-MRSA
community-associated methicillin-resistant Staphylococcus aureus
- DPBS
Dulbecco’s PBS
- HOCl/−OCl
hypochlorous acid/hypochlorite anion
- LAC HK
heat-killed LAC
- MPO
myeloperoxidase
- O2−
superoxide
- PMN
polymorphonuclear leukocyte
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors have declared that there are no conflicts of interest.
REFERENCES
- 1.Amulic B., Cazalet C., Hayes G. L., Metzler K. D., Zychlinsky A. (2012) Neutrophil function: from mechanisms to disease. Annu. Rev. Immunol. 30, 459–489. [DOI] [PubMed] [Google Scholar]
- 2.Nordenfelt P., Tapper H. (2011) Phagosome dynamics during phagocytosis by neutrophils. J. Leukoc. Biol. 90, 271–284. [DOI] [PubMed] [Google Scholar]
- 3.Hurst J. K. (2012) What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 53, 508–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeLeo F. R., Quinn M. T. (1996) Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J. Leukoc. Biol. 60, 677–691. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo F. R., Allen L. A., Apicella M., Nauseef W. M. (1999) NADPH oxidase activation and assembly during phagocytosis. J. Immunol. 163, 6732–6740. [PubMed] [Google Scholar]
- 6.Winterbourn C. C., Kettle A. J. (2013) Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 18, 642–660. [DOI] [PubMed] [Google Scholar]
- 7.Hampton M. B., Winterbourn C. C. (1995) Modification of neutrophil oxidant production with diphenyleneiodonium and its effect on bacterial killing. Free Radic. Biol. Med. 18, 633–639. [DOI] [PubMed] [Google Scholar]
- 8.Hampton M. B., Kettle A. J., Winterbourn C. C. (1996) Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 64, 3512–3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lekstrom-Himes J. A., Gallin J. I. (2000) Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 343, 1703–1714. [DOI] [PubMed] [Google Scholar]
- 10.Roos D., de Boer M. (2014) Molecular diagnosis of chronic granulomatous disease. Clin. Exp. Immunol. 175, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Saïd-Salim B., Porcella S. F., Long R. D., Dorward D. W., Gardner D. J., Kreiswirth B. N., Musser J. M., DeLeo F. R. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919. [DOI] [PubMed] [Google Scholar]
- 12.Voyich J. M., Vuong C., DeWald M., Nygaard T. K., Kocianova S., Griffith S., Jones J., Iverson C., Sturdevant D. E., Braughton K. R., Whitney A. R., Otto M., DeLeo F. R. (2009) The SaeR/S gene regulatory system is essential for innate immune evasion by Staphylococcus aureus. J. Infect. Dis. 199, 1698–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLeo F. R., Chambers H. F. (2009) Reemergence of antibiotic-resistant Staphylococcus aureus in the genomics era. J. Clin. Invest. 119, 2464–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller L. G., Perdreau-Remington F., Rieg G., Mehdi S., Perlroth J., Bayer A. S., Tang A. W., Phung T. O., Spellberg B. (2005) Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N. Engl. J. Med. 352, 1445–1453. [DOI] [PubMed] [Google Scholar]
- 15.Kreisel K. M., Stine O. C., Johnson J. K., Perencevich E. N., Shardell M. D., Lesse A. J., Gordin F. M., Climo M. W., Roghmann M.-C. (2011) USA300 methicillin-resistant Staphylococcus aureus bacteremia and the risk of severe sepsis: is USA300 methicillin-resistant Staphylococcus aureus associated with more severe infections? Diagn. Microbiol. Infect. Dis. 70, 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genestier A. L., Michallet M. C., Prévost G., Bellot G., Chalabreysse L., Peyrol S., Thivolet F., Etienne J., Lina G., Vallette F. M., Vandenesch F., Genestier L. (2005) Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115, 3117–3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bubeck Wardenburg J., Bae T., Otto M., Deleo F. R., Schneewind O. (2007) Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 13, 1405–1406. [DOI] [PubMed] [Google Scholar]
- 18.Wang R., Braughton K. R., Kretschmer D., Bach T.-H. L., Queck S. Y., Li M., Kennedy A. D., Dorward D. W., Klebanoff S. J., Peschel A., DeLeo F. R., Otto M. (2007) Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13, 1510–1514. [DOI] [PubMed] [Google Scholar]
- 19.Flack C. E., Zurek O. W., Meishery D. D., Pallister K. B., Malone C. L., Horswill A. R., Voyich J. M. (2014) Differential regulation of staphylococcal virulence by the sensor kinase SaeS in response to neutrophil-derived stimuli. Proc. Natl. Acad. Sci. USA 111, E2037–E2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zurek O. W., Pallister K. B., Voyich J. M. (2015) Staphylococcus aureus inhibits neutrophil-derived IL-8 to promote cell death. J. Infect. Dis. 212, 934–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palazzolo-Ballance A. M., Reniere M. L., Braughton K. R., Sturdevant D. E., Otto M., Kreiswirth B. N., Skaar E. P., DeLeo F. R. (2008) Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J. Immunol. 180, 500–509. [DOI] [PubMed] [Google Scholar]
- 22.Zurek O. W., Nygaard T. K., Watkins R. L., Pallister K. B., Torres V. J., Horswill A. R., Voyich J. M. (2014) The role of innate immunity in promoting SaeR/S-mediated virulence in Staphylococcus aureus. J. Innate Immun. 6, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nygaard T. K., Pallister K. B., Ruzevich P., Griffith S., Vuong C., Voyich J. M. (2010) SaeR binds a consensus sequence within virulence gene promoters to advance USA300 pathogenesis. J. Infect. Dis. 201, 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diep B. A., Gill S. R., Chang R. F., Phan T. H., Chen J. H., Davidson M. G., Lin F., Lin J., Carleton H. A., Mongodin E. F., Sensabaugh G. F., Perdreau-Remington F. (2006) Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367, 731–739. [DOI] [PubMed] [Google Scholar]
- 25.Bylund J., Björnsdottir H., Sundqvist M., Karlsson A., Dahlgren C. (2014) Measurement of respiratory burst products, released or retained, during activation of professional phagocytes. Methods Mol. Biol. 1124, 321–338. [DOI] [PubMed] [Google Scholar]
- 26.Chen X., Lee K.-A., Ha E.-M., Lee K. M., Seo Y. Y., Choi H. K., Kim H. N., Kim M. J., Cho C.-S., Lee S. Y., Lee W.-J., Yoon J. (2011) A specific and sensitive method for detection of hypochlorous acid for the imaging of microbe-induced HOCl production. Chem. Commun. (Camb.) 47, 4373–4375. [DOI] [PubMed] [Google Scholar]
- 27.Ng H. P., Zhou Y., Song K., Hodges C. A., Drumm M. L., Wang G. (2014) Neutrophil-mediated phagocytic host defense defect in myeloid Cftr-inactivated mice. PLoS One 9, e106813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris J. C. (1966) The acid ionization constant of HOCl from 5 to 35°. J. Phys. Chem. 70, 3798–3805. [Google Scholar]
- 29.Kettle A. J., Albrett A. M., Chapman A. L., Dickerhof N., Forbes L. V., Khalilova I., Turner R. (2014) Measuring chlorine bleach in biology and medicine. Biochim. Biophys. Acta 1840, 781–793. [DOI] [PubMed] [Google Scholar]
- 30.Ohno Y., Gallin J. I. (1985) Diffusion of extracellular hydrogen peroxide into intracellular compartments of human neutrophils. Studies utilizing the inactivation of myeloperoxidase by hydrogen peroxide and azide. J. Biol. Chem. 260, 8438–8446. [PubMed] [Google Scholar]
- 31.Gerber C. E., Bruchelt G., Falk U. B., Kimpfler A., Hauschild O., Kuçi S., Bächi T., Niethammer D., Schubert R. (2001) Reconstitution of bactericidal activity in chronic granulomatous disease cells by glucose-oxidase-containing liposomes. Blood 98, 3097–3105. [DOI] [PubMed] [Google Scholar]
- 32.Rogasch K., Rühmling V., Pané-Farré J., Höper D., Weinberg C., Fuchs S., Schmudde M., Bröker B. M., Wolz C., Hecker M., Engelmann S. (2006) Influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J. Bacteriol. 188, 7742–7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun F., Li C., Jeong D., Sohn C., He C., Bae T. (2010) In the Staphylococcus aureus two-component system sae, the response regulator SaeR binds to a direct repeat sequence and DNA binding requires phosphorylation by the sensor kinase SaeS. J. Bacteriol. 192, 2111–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCord J. M., Fridovich I. (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055. [PubMed] [Google Scholar]
- 35.Kettle A. J., Anderson R. F., Hampton M. B., Winterbourn C. C. (2007) Reactions of superoxide with myeloperoxidase. Biochemistry 46, 4888–4897. [DOI] [PubMed] [Google Scholar]
- 36.Chapman A. L. P., Hampton M. B., Senthilmohan R., Winterbourn C. C., Kettle A. J. (2002) Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 277, 9757–9762. [DOI] [PubMed] [Google Scholar]
- 37.Horsburgh M. J., Clements M. O., Crossley H., Ingham E., Foster S. J. (2001) PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect. Immun. 69, 3744–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karavolos M. H., Horsburgh M. J., Ingham E., Foster S. J. (2003) Role and regulation of the superoxide dismutases of Staphylococcus aureus. Microbiology 149, 2749–2758. [DOI] [PubMed] [Google Scholar]
- 39.Singh V. K., Vaish M., Johansson T. R., Baum K. R., Ring R. P., Singh S., Shukla S. K., Moskovitz J. (2015) Significance of four methionine sulfoxide reductases in Staphylococcus aureus. PLoS One 10, e0117594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.