Targeting CXCL1, but not IL6, reduced inflammation, protected retinal function, and minimized disease pathogenesis, resulting in an improved outcome for B. cereus intraocular infection.
Keywords: eye endophthalmitis, Bacillus, cytokine, chemokine, mouse
Abstract
During intraocular bacterial infections, the primary innate responders are neutrophils, which may cause bystander damage to the retina or perturb the clarity of the visual axis. We hypothesized that cytokine IL-6 and chemokine CXCL1 contributed to rapid neutrophil recruitment during Bacillus cereus endophthalmitis, a severe form of intraocular infection that is characterized by explosive inflammation and retinal damage that often leads to rapid vision loss. To test this hypothesis, we compared endophthalmitis pathogenesis in C57BL/6J, IL-6−/−, and CXCL1−/− mice. Bacterial growth in eyes of CXCL1−/−, IL-6−/−, and C67BL/6J mice was similar. Retinal function retention was greater in eyes of IL-6−/− and CXCL1−/− mice compared with that of C57BL/6J, despite these eyes having similar bacterial burdens. Neutrophil influx into eyes of CXCL1−/− mice was reduced to a greater degree compared with that of eyes of IL6−/− mice. Histology confirmed significantly less inflammation in eyes of CXCL1−/− mice, but similar degrees of inflammation in IL6−/− and C57BL/6J eyes. Because inflammation was reduced in eyes of infected CXCL1−/− mice, we tested the efficacy of anti-CXCL1 in B. cereus endophthalmitis. Retinal function was retained to a greater degree and there was less overall inflammation in eyes treated with anti-CXCL1, which suggested that anti-CXCL1 may have therapeutic efficacy in limiting inflammation during B. cereus endophthalmitis. Taken together, our results indicate that absence of IL-6 did not affect overall pathogenesis of endophthalmitis. In contrast, absence of CXCL1, in CXCL1−/− mice or after anti-CXCL1 treatment, led to an improved clinical outcome. Our findings suggest a potential benefit in targeting CXCL1 to control inflammation during B. cereus and perhaps other types of intraocular infections.
Introduction
Bacterial infections in the posterior segment of the eye—endophthalmitis—can cause severe inflammation and significant vision loss in a short period of time. Bacterial entry into the posterior segment can occur after a surgical procedure (postoperative), after trauma caused by a penetrating foreign object (post-traumatic), or by metastasis of bacteria into the eye from a distant infection site (endogenous). Within the eye, immune privileged sites, such as the anterior chamber, vitreous cavity, and subretinal space, are devoid of vasculature to maintain the clarity of the visual axis that is required for proper vision [1]; however, during endophthalmitis, immune privilege is compromised as bacteria replicate in the posterior segment and immune cells infiltrate the area, which perturbs the visual axis. Severity of endophthalmitis typically ranges from a mild inflammation that resolves with treatment to a rapidly evolving infection and inflammation that is refractory to treatment.
Bacillus cereus endophthalmitis is an example of the latter case and is one of the most devastating forms of this disease. B. cereus is a rod-shaped, motile, β-hemolytic, Gram-positive bacterium that is commonly associated with self-limiting gastrointestinal infections, but has also been linked with such nongastrointestinal infections as meningitis, pneumonia, wound infections, endocarditis, bacteremia, and septicemia [2–15]. Some of these infections were the result of B. cereus contamination of implant devices, alcohol prep pads, airflow sensors, hospital linens, and indwelling catheters [16–20]. B. cereus typically infects the eye after a traumatic injury but can also contaminate eye after ocular surgery or as a consequence of bacteremia [21–28]. Symptoms of B. cereus endophthalmitis include significant pain, swollen eyelids, a hypopyon (neutrophils in the anterior chamber), a corneal ring abscess, fever, and decreased visual acuity [21, 22, 29]. Intraocular infection with B. cereus results in disruption of retinal architecture, immune cell infiltration into the vitreous, and vision loss within 2–3 d [21, 30]. Vision loss is thought to result from a combination of toxin activity that destroys the retina, and neutrophil influx, which may cause bystander damage to the retina or perturb the clarity of visual axis.
As stated above, the eye is an immune privileged site in which several mechanisms work together to protect the visual axis from destructive inflammation. We and others have reported that the major immune cells that infiltrate during experimental bacterial endophthalmitis after an innate immune response are primarily PMNs [30–32]. During infection, TLRs recognize microbial ligands, which results in an acute and sometimes rapidly developing inflammation that overcomes ocular immune privilege. In experimental B. cereus endophthalmitis, TLR2 and TLR4, but not TLR5, were essential for rapid intraocular inflammation during infection [33–35]. Similarly, MyD88 and TRIF, the innate immune adaptors through which these TLRs signal, were essential for rapid intraocular inflammation and disease pathogenesis [35]. Intraocular inflammation in transgenic mice that are deficient in TLR2, TLR4, MyD88, or TRIF was significantly attenuated, which indicates an important role for these receptors and adaptors in B. cereus endophthalmitis [35].
The downstream effect of TLR-mediated immune responses in experimental bacterial endophthalmitis is secretion of proinflammatory cytokines and chemokines that recruit neutrophils into the eye. The following proinflammatory mediators have been detected in experimental mouse models of bacterial endophthalmitis: TNF-α, CXCL1, IL-6, IL-1β, MIP1α, and MIP2 [30, 33–38]. In general, ocular concentrations of proinflammatory mediators increased in parallel with neutrophil infiltration during the course of intraocular infection. We previously reported that during B. cereus endophthalmitis in TNF-α−/− mice, inflammation was significantly reduced, but bacterial replication was greater and retinal function loss was significant compared with infections in wild-type mice [39]. Other proinflammatory cytokines and chemokines, such as CXCL1 and IL-6, compensated for the absence of TNF-α, recruiting neutrophils into the eye and contributing to inflammation and overall pathogenesis of endophthalmitis [39]. In experimental B. cereus endophthalmitis, CXCL1 and IL-6 secretion correlated with increasing neutrophil infiltration and were also dependent on TLR2 and TLR4 and their adaptors, as the concentrations of these cytokines were significantly reduced in infected eyes of TLR2−/−, TLR4−/−, MyD88−/−, and TRIF−/− mice [33, 35]. We therefore hypothesized that CXCL1 and IL-6 contributed to early and rapid neutrophil infiltration and overall pathogenesis of B. cereus endophthalmitis. Our results demonstrate that absence of IL-6 delayed overall pathogenesis of disease, but not to a clinically sufficient degree; however, the absence of CXCL1 significantly attenuated disease, as did treating infected eyes with anti-CXCL1, which indicates that CXCL1 may be a viable anti-inflammatory target for treatment of B. cereus and perhaps other types of endophthalmitis.
MATERIALS AND METHODS
Ethics statement
The experiments described below involved the use of mice. All animal procedures were conducted according to guidelines and recommendations from the Guide for the Care and Use of Laboratory Animals, the University of Oklahoma Health Sciences Center Institutional Animal Care and Use Committee, and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. These studies were approved under protocols 13-086 and 14-012.
Murine B. cereus endophthalmitis
C57BL/6J mice were purchased from commercially available stock colonies (The Jackson Laboratory, Bar Harbor, ME, USA). Generation of CXCL1−/− mice on the C57BL/6J background has been described [40]. Breeding pairs of IL-6−/− mice (B6.129S2-Il6tm1Kopf/J) were also purchased from The Jackson Laboratory. CXCL1−/− and IL-6−/− mice were bred on the C57BL/6J background. All breeders were maintained under barrier facility conditions on a 12-h on/off light cycle. Vendor-supplied or weaned animals were accustomed to housing under biosafety level 2 conditions and were cohoused for at least 2 wk to equilibrate their microbiota. Mice were used in experiments at 8–10 wk of age.
Each mouse was anesthetized with a combination of ketamine (85 mg/kg body weight; Ketathesia; Henry Schein Animal Health, Dublin, OH, USA) and xylazine (14 mg/kg body weight; AnaSed; Akorn, Decatur, IL, USA). Experimental B. cereus endophthalmitis was induced in mouse eyes by injecting 0.5 µl brain heart infusion media that contained ∼100 CFU B. cereus American Type Culture Collection strain 14579 into the midvitreous, as previously described [30, 33–35]. The contralateral eye served as the uninjected control.
Intraocular growth quantitation
Intraocular bacterial growth in whole eyes of C57BL/6J, CXCL1−/−, and IL6−/− mice was quantified as previously described [30, 33–35, 39]. Whole eyes were removed from euthanized mice at 4, 8, and 12 h postinfection. Eyes were homogenized in PBS with sterile 1-mm glass beads (BioSpec Products, Bartlesville, OK, USA). Homogenates were plated and bacterial numbers were estimated by track dilution method. Values represent means ± sem for n ≥ 6 eyes per time point. At least 3 independent experiments were performed.
ERG
ERG was used to analyze retinal function, as previously described [30, 33–35]. Scotopic ERGs were performed at 8 and 12 h postinfection (Espion E2; Diagnosys, Lowell, MA, USA). After infection and before ERG, mice were dark-adapted for at least 6 h. After dark-adaptation, mice were anesthetized and pupils were dilated with topical phenylephrine (Akorn). Retinal function was recorded with gold-wire electrodes, which were placed on each cornea, and a reference electrode. The retinal response was stimulated by a transient flash of white light (1,200 cd·s/m2), which resulted in electrical responses in the form of graded potentials evoked in each layer of the retina. The average A- and B-wave amplitudes after each of 5 flashes were calculated. A-wave provides a direct measurement of photoreceptor activity, whereas B-wave represents the combined activity of Muller cells, bipolar cells, and second-order neurons. A- and B-wave amplitudes were measured from initiation of light flash to trough of the A-wave, from trough of the A-wave to peak of the B-wave, and were recorded for infected and contralateral uninfected eyes in the same animal. The percentage of retinal function retained in the infected eye was compared with uninfected left eye controls as: 100 – {[1 – (experimental A- or B-wave amplitude/control A- or B-wave amplitude)] × 100}. Values represent means ± sem for n ≥ 6 eyes per time point. At least 3 independent experiments were performed.
Histology
Uninfected eyes were harvested from each mouse strain or infection group at 4, 8, and 12 h postinfection. Harvested eyes were incubated in buffered zinc formalin or Davison’s fixative for 24 h at room temperature [30, 33–35, 39]. Eyes were then transferred to 70% ethanol, embedded in paraffin, sectioned, and stained with H&E. Images are representative of at least 3 eyes per time point from at least 3 independent experiments.
MPO expression
MPO is the major enzyme in azurophilic granules of PMNs. PMN influx into the eye was estimated by measuring the concentration of MPO in whole-eye homogenates at 0, 4, 8, and 12 h postinfection by mouse MPO ELISA (Hycult Biotech, Plymouth Meeting, PA, USA), as previously described [33–35, 41]. The lower limit of detection for this assay was 1.5 ng/ml. Values represent means ± sem for n ≥ 5 eyes per group per time point. At least 3 independent experiments were performed.
Proinflammatory mediators
Concentrations of proinflammatory mediators in infected eyes were measured by using ELISAs as previously described [30, 33–35, 39, 41]. At the time points noted above, infected eyes were harvested and homogenized in PBS that contained a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN, USA). Concentrations of TNF-α, IL-1β, IL-6, and CXCL1 in these homogenates were estimated by using respective mouse ELISA kits (Quantikine; R&D Systems, Minneapolis, MN, USA). Lower limits of detection for these ELISAs were: TNF-α, 14 pg/ml; IL-1β, 16 pg/ml; IL-6, 4 pg/ml; and CXCL1, 30 pg/ml. Values represent means ± sem for n ≥ 5 eyes per time point. At least 2 independent experiments were performed.
Neutralization of CXCL1
Anti-CXCL1 monoclonal antibody (125 ng/0.5 μl anti-KC/CXCL1 IgG2A MAB 453, clone 48415; R&D Systems) was intravitreally injected with 100 CFU B. cereus [42]. The isotype control antibody (0.5 μg nonspecific control IgG2A/0.5 μl MAB 006, clone 54447l; R&D Systems) was intravitreally injected with 100 CFU B. cereus. Untreated mice were intravitreally injected with 100 CFU B. cereus alone. All eyes were injected once. Bacterial growth, retinal function, and intraocular inflammation were assessed at 12 h postinfection as described above. Values represent means ± sem for n ≥ 7 eyes per time point. At least 3 independent experiments were performed.
Statistics
Data represented are arithmetic means ± sem of all samples in the same experimental group in replicate experiments unless otherwise specified. A value of P < 0.05 was considered statistically significant. Mann-Whitney rank-sum test was used to compare unpaired experimental groups to determine the statistical significance for all assays [35]. All statistical analyses were performed by using Prism 6.05 (GraphPad Software, La Jolla, CA, USA).
Supplemental material
Supplemental Figures include magnified histology sections of optic nerve head regions (×20) and retinas (×40) of eyes of C57BL/6J, IL-6−/−, and CXCL1−/− mice infected with B. cereus at 12 h postinfection (Supplemental Fig. 1) and B. cereus–infected eyes of C57BL/6J mice that were treated with IgG2A isotype antibody, anti-CXCL1 antibody, or were left untreated (Supplemental Fig. 2).
RESULTS
Endophthalmitis in the absence of IL-6 or CXCL1
Intraocular bacterial growth was not affected by absence of IL-6 or CXCL1.
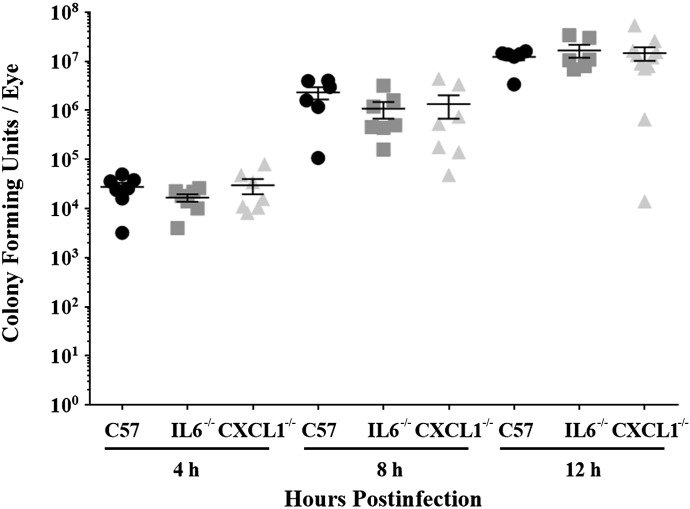
Intraocular bacterial growth in CXCL1−/− and IL-6−/− mice was compared with that of C57BL/6J mice after infection with B. cereus (Fig. 1). The starting inoculum for all groups was 102 ± 10 CFU. Intraocular B. cereus grew rapidly during 12 h in eyes of C57BL/6J, IL-6−/−, and CXCL1−/− mice. Intraocular B. cereus numbers in infected eyes of IL-6−/− mice were similar to that of infected eyes of C57BL/6J mice at all experimental time points (P ≥ 0.163). B. cereus intraocular numbers in eyes of CXCL1−/− and C57BL/6J mice were also similar at all experimental time points (P ≥ 0.362). These results indicated that the absence of CXCL1 or IL-6 did not hinder the intraocular growth of B. cereus.
Figure 1. Intraocular B. cereus growth is not affected by absence of IL-6 or CXCL1.
Eyes of IL-6−/−, CXCL1−/−, and C57BL/6J mice were infected with 100 CFU B. cereus. Eyes were harvested and B. cereus were quantified. No significant differences in B. cereus burden were observed among mouse strains (P ≥ 0.163). Values represent means ± sem of n ≥ 6 eyes at each time point with at least 3 independent experiments.
Retinal function loss during endophthalmitis was delayed in the absence of IL-6 or CXCL1.
Retinal function in B. cereus–infected eyes of C57BL/6J, IL6−/−, and CXCL1−/− mice was compared by electroretinography (Fig. 2). In infected eyes of C57BL/6J mice, both A- and B-wave responses declined rapidly by 8 h postinfection. In infected eyes of IL-6−/− mice, retained A- and B-wave responses were significantly greater than that of infected eyes of C57BL/6J mice at 8 h postinfection (P ≤ 0.007) and at 12 h postinfection (P ≤ 0.0256). Similarly, in infected eyes of CXCL1−/− mice, A- and B-wave responses were significantly greater than that of infected eyes of C57BL/6J mice at 8 h postinfection (P ≤ 0.0001) and at 12 h postinfection (P ≤ 0.001). Although the retained A- and B-wave responses in infected eyes of CXCL1−/− mice were greater than that of infected eyes of IL-6−/− mice at 8 h postinfection, these differences were not significant (P ≥ 0.1128). At 12 h postinfection, retained A- and B-wave responses in eyes of CXCL1−/− and IL-6−/− mice were similar (P ≥ 0.8009). Taken together, these results suggested that there was a delay in loss of retinal function in the absence of CXCL1 or IL-6, despite the presence of similar numbers of B. cereus in the eyes of all infection groups.
Figure 2. Retinal function loss was delayed in the absence of IL-6 or CXCL1.
Eyes of IL-6−/−, CXCL1−/−, and C57BL/6J mice were infected with 100 CFU B. cereus. Retinal function was assessed by ERG at 8 and 12 h postinfection. In infected eyes of IL-6−/− and CXCL1−/− mice, retained A- and B-wave responses were significantly greater than retained A- and B-wave responses in infected eyes of C57BL/6J mice. Values represent means ± sem of n ≥ 6 eyes each time point with at least 3 independent experiments. *P ≤ 0.0256
Retinal damage and inflammation during endophthalmitis was delayed in the absence of CXCL1, but not in the absence of IL-6.
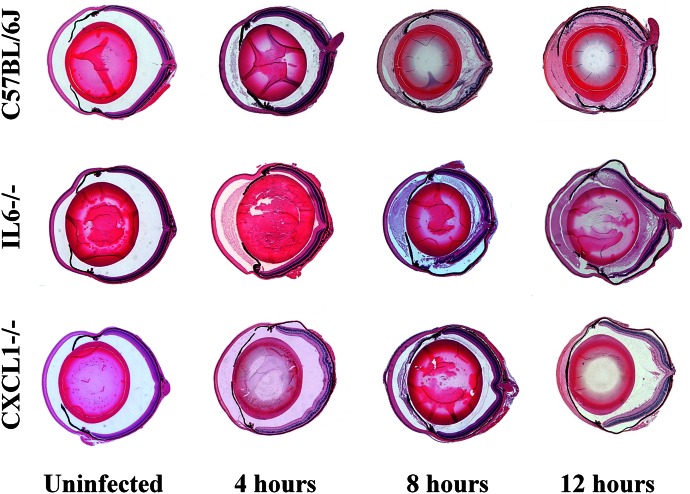
Histologic sections of uninfected and infected eyes of C57BL/6J, IL-6−/−, and CXCL1−/− mice are depicted in Fig. 3 and in Supplemental Figs. 1 and 2. Uninfected eyes of IL-6−/− and CXCL1−/− mice appeared architecturally and morphologically similar to eyes of C57BL/6J mice, with no overt differences in corneal or retinal cellular morphology.
Figure 3. Retinal damage and intraocular inflammation were delayed in the absence of CXCL1, but not in the absence of IL-6.
Eyes of IL-6−/−, CXCL1−/−, and C57BL/6J mice were infected with 100 CFU B. cereus. Uninfected and infected globes were harvested at 4, 8, and 12 h postinfection and processed for H&E staining. Uninfected C57BL/6J, IL-6−/− and CXCL1−/− had no inflammation and were architecturally and morphologically similar. At 4 h postinfection, there was fibrin in the anterior chamber and few inflammatory cells in the posterior segment in infected all eyes. At 8 and 12 h postinfection, infected eyes of C57BL/6J and IL-6−/− mice were similarly inflamed, with inflammatory cells in the posterior segment, dissolution of retinal layers, and retinal detachments. In contrast, infected eyes of CXCL1−/− mice had minimal inflammation and intact retinal layers at the same time points. Sections are representative of 3 eyes per time point with at least 3 independent experiments. Original magnification, ×10.
At 4 h postinfection, there was fibrin in the anterior chamber and few inflammatory cells in the posterior segment in infected eyes of C57BL/6J and IL-6−/− mice. At 8 and 12 h postinfection, infected eyes of C57BL/6J and IL-6−/− mice had similar pathologic changes, including increased inflammatory cell infiltrate in the posterior segment, dissolution of retinal layers, and retinal detachments. Overall, the evolving intraocular inflammation and damage to retinal architecture in infected eyes of IL-6−/− and C57BL/6J mice during B. cereus endophthalmitis was similar.
Neutrophil infiltration into the posterior segment was minimal in infected eyes of both CXCL1−/− and C57BL/6J mice at 4 h postinfection. In contrast to the severe pathology observed in infected eyes of C57BL/6J and IL-6−/− mice, there was less inflammation and retinal layers were intact in infected eyes of CXCL1−/− mice at 8 h postinfection. Minimal inflammatory cell infiltration and intact retinas remained in infected eyes of CXCL1−/− mice at 12 h postinfection. Overall, there was less damage to retinal architecture and limited neutrophil influx in infected eyes of CXCL1−/− mice compared with that of infected eyes of C57BL/6J and IL-6−/− mice during the course of experimental B. cereus endophthalmitis (Fig. 3 and Supplemental Fig. 1 and 2).
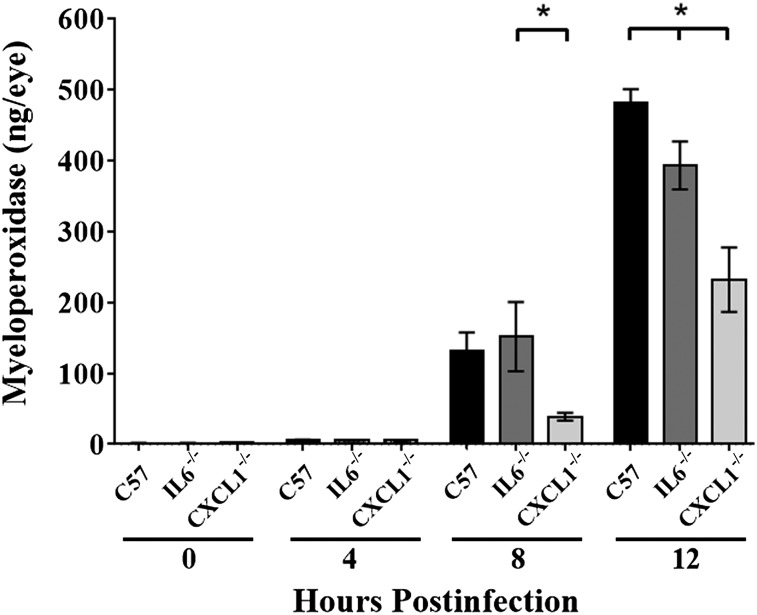
MPO concentrations were reduced to a greater degree in the absence of CXCL1 during endophthalmitis.
MPO concentrations represented a quantitative comparison of neutrophil influx in B. cereus–infected eyes (Fig. 4). MPO concentrations increased steadily in infected eyes of C57BL/6J mice, with a 22-fold increase in MPO concentrations from 4 to 8 h postinfection and a 2.5-fold increase from 8 to 12 h postinfection. Similarly, in infected eyes of IL-6−/− mice, MPO concentrations increased steadily, with a 27-fold increase in MPO concentrations from 4 to 8 h postinfection and a 1.7-fold increase from 8 to 12 h postinfection. MPO concentrations in infected eyes of IL-6−/− mice were similar to that of eyes of C57BL/6J mice at 4 and 8 h postinfection (P ≥ 0.68). However, at 12 h postinfection, there was significantly less MPO in infected eyes of IL-6−/− mice compared with that of infected eyes of C57BL/6J mice, with 11% less MPO activity in eyes of IL6−/− vs. C57BL/6J mice (P = 0.028).
Figure 4. MPO concentrations were reduced to a greater degree in the absence of CXCL1.
Eyes of IL-6−/−, CXCL1−/−, and C57BL/6J (C57) mice were infected with 100 CFU B. cereus. PMN infiltration in mouse eyes was estimated by quantifying MPO levels by using ELISA. MPO concentrations were similar in eyes from all groups at 4 h postinfection (P ≥ 0.38). MPO concentrations in infected eyes of IL-6−/− mice were similar at 8 h postinfection (P = 0.69) but were less than in eyes of C57BL/6J mice (P = 0.03) at 12 h postinfection. MPO concentrations in infected eyes of CXCL1−/− mice were less than that in infected eyes of C57BL/6J mice at 12 h postinfection (P ≤ 0.0001) and less than that in infected eyes of IL-6−/− mice at 8 and 12 h postinfection (P ≤ 0.009). Values represent means ± sem for n ≥ 5 eyes per group per time point with at least 3 independent experiments. *P ≤ 0.03.
MPO concentrations in infected eyes of CXCL1−/− mice were similar to that of infected eyes of C57BL/6J mice at 4 h postinfection (P = 0.37). At 8 h postinfection, MPO concentrations in infected eyes of CXCL1−/− mice were 74% less than that of infected eyes of IL-6−/− mice (P = 0.03) and 70% less than that of infected eyes of C57BL6J mice (P = 0.07). At 12 h postinfection, there was significantly less MPO in infected eyes of CXCL1−/− mice: 43% less than that of infected eyes of IL-6−/− mice (P = 0.008) and 50% less than that of infected eyes of C57BL/6J mice (P ≤ 0.0001). In infected eyes of CXCL1−/− mice, MPO concentrations increased only 7-fold from 4 to 8 h postinfection and only 5-fold from 8 to 12 h postinfection. These results demonstrated a significant delay in neutrophil influx in infected eyes of CXCL1−/− mice. Overall, these data suggest that absence of CXCL1 or IL-6 delays neutrophil influx into infected eyes, but the absence of CXCL1 impacted neutrophil influx to a much greater extent than did absence of IL-6.
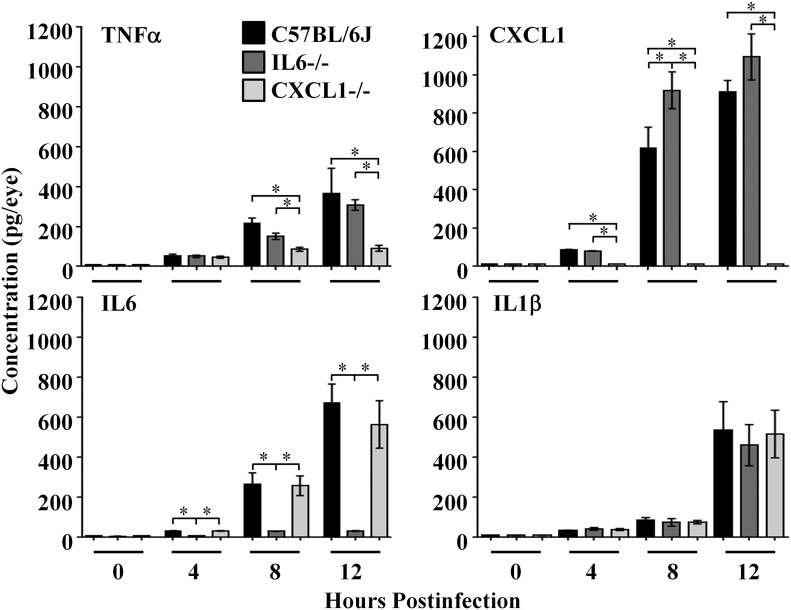
Absence of CXCL1 or IL-6 resulted in different proinflammatory mediator profiles during B. cereus endophthalmitis.
Concentrations of TNF-α, IL-6, IL-1β, and CXCL1 were measured by ELISA in B. cereus–infected eyes, as summarized in Fig. 5. As we reported previously [30, 33–35], concentrations of ocular TNF-α, CXCL1, and IL-6 increased from 4 to 12 h postinfection, whereras ocular concentrations of IL-1β increased from 8 to 12 h postinfection.
Figure 5. Absence of CXCL1 or IL-6 resulted in different proinflammatory mediator profiles during B. cereus endophthalmitis.
Eyes of IL-6−/−, CXCL1−/−, and C57BL/6J mice were infected with 100 CFU B. cereus. Concentrations of TNF-α, IL-6, IL-1β, and CXCL1 were measured by using ELISA. Concentrations of IL-1β were similar in eyes of all mouse strains at all time points tested (P ≥ 0.17). Concentrations of TNF-α in infected eyes of C57BL/6J mice were similar to those in infected eyes of IL-6−/− mice at 8 and 12 h postinfection (P ≥ 0.14) but greater than that in infected eyes of CXCL1−/− mice at the same time points (P ≤ 0.004). CXCL1 concentrations in infected eyes of IL-6−/− mice were greater than that in infected eyes of C57BL/6J mice at 8 and 12 h postinfection, but the difference was only significant at 8 h postinfection (P = 0.03). IL-6 concentrations in infected eyes of CXCL1−/− mice were similar to that in eyes of C57BL/6J mice at all time points (P ≥ 0.57). Concentrations of IL-1β were similar in eyes of all mouse strains at all time points tested (P ≥ 0.17). Values represent means ± sem for n ≥ 5 eyes per group per time point with at least 3 independent experiments. *P ≤ 0.03.
In the absence of IL-6, concentrations of TNF-α were similar to that of infected eyes of C57BL/6J mice throughout the course of infection (P ≥ 0.1). IL-1β concentrations were also similar in infected eyes of IL6−/− and C57BL/6J mice throughout the course of infection (P ≥ 0.4); however, CXCL1 concentrations were significantly greater in infected eyes of IL-6−/− mice than in infected eyes of C57BL/6J mice at 8 h postinfection (P = 0.03). Although there were greater CXCL1 concentrations in infected eyes of IL-6−/− mice than in infected eyes of C57BL/6J mice at 12 h postinfection, this difference was not significant (P = 0.27). In eyes of IL-6−/− mice, concentrations of IL-6 were at background levels, as expected.
In the absence of CXCL1, a different pattern of proinflammatory mediator synthesis emerged. In infected eyes of CXCL1−/− mice, concentrations of TNF-α were similar to those of infected eyes of C57BL/6J mice at 4 h postinfection (P = 0.89); however, at 8 and 12 h postinfection, TNF-α concentrations were significantly less in infected eyes of CXCL1−/− mice than in infected eyes of C57BL/6J mice (P ≤ 0.004). Concentrations of IL-6 were similar in infected eyes of CXCL1−/− and C57BL/6J mice throughout the course of infection (P ≥ 0.5). IL-1β concentrations were also similar in infected eyes of CXCL1−/− and C57BL/6J mice throughout the course of infection (P ≥ 0.1). In eyes of CXCL1−/− mice, CXCL1 concentrations were at background levels, as expected.
Taken together, these results indicated that intraocular inflammation was delayed to a greater degree in infected eyes of CXCL1−/− mice than in infected eyes of IL-6−/− mice during B. cereus endophthalmitis. Our results suggest that CXCL1 and IL-6 are important proinflammatory mediators that influence neutrophil influx into the eye during endophthalmitis; however, loss of IL-6 was compensated for by other proinflammatory mediators, which led to inflammation similar to that observed in wild-type mouse eyes. In contrast, the absence of CXCL1 greatly impacted overall course of inflammation by reducing neutrophil influx, retinal damage, and TNF-α concentrations during infection, which resulted in a more positive infection outcome.
Anti-CXCL1 arrests intraocular inflammation and protects retinal function
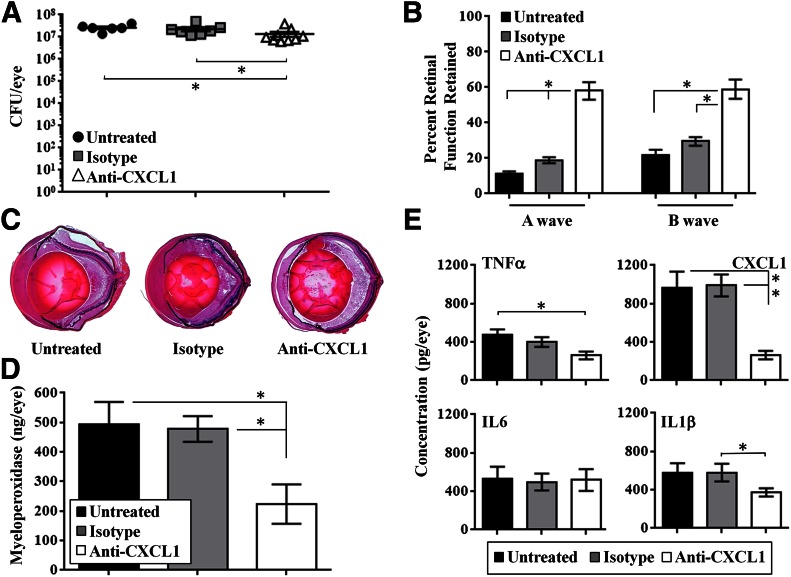
Because the absence of CXCL1 significantly reduced inflammation and prevented rapid retinal function loss in eyes that were infected with B. cereus, we tested whether CXCL1 neutralization would limit inflammation during infection. Mice were randomly divided into 3 experimental groups: C57BL/6J mice injected with B. cereus alone, C57BL/6J mice injected with B. cereus and isotype IgG2A, or C57BL/6J mice injected with B. cereus and anti-CXCL1 antibody. The starting inoculum for all groups was 101 ± 12 CFU. There were fewer B. cereus in eyes treated with anti-CXCL1 at 12 h postinfection compared with the isotype-treated and untreated eyes (P = 0.011), but the reduction was only 2-fold compared with those groups (Fig. 6A).
Figure 6. Treatment with anti-CXCL1 antibody arrests intraocular inflammation and protects retinal function.
C57BL/6J mice were injected with 100 CFU B. cereus alone (Untreated), B. cereus and 0.5 μg nonspecific isotype IgG2A (Isotype), or B. cereus and 125 ng/0.5 μl anti-CXCL1 antibody (Anti-CXCL1). All eyes were analyzed at 12 h postinfection. (A) B. cereus in the anti-CXCL1 treatment group were significantly less than in the isotype-treated and untreated groups (*P ≤ 0.011), but reduction was only 2-fold. (B) Retained A- and B-wave function was greater in anti-CXCL1 treated eyes than in untreated or isotype-treated eyes (*P ≤ 0.002). (C) Histologic sections showed that eyes of untreated and isotype-treated mice were similar. Eyes in these groups were highly inflamed and retinal architecture was lost. In contrast, there was less inflammation and intact retinal layers in infected eyes treated with anti-CXCL1 antibody. Original magnification, ×10. (D) MPO concentrations in infected eyes of mice treated with anti-CXCL1 antibody were significantly less than in untreated or isotype-treated eyes (*P ≤ 0.01). (E) Concentrations of all proinflammatory mediators in untreated and isotype-treated eyes were similar (P ≥ 0.05). Concentrations of TNF-α and CXCL1 were greater in untreated eyes than in anti-CXCL1 eyes (P ≤ 0.01), whereas concentrations of IL-6 and IL-1β were similar (P ≥ 0.11). Values represent means ± sem for n ≥ 5 eyes per group per time point with at least 3 independent experiments. *P ≤ 0.05.
Eyes from untreated, infected mice retained A-wave responses of ∼11%, whereas infected eyes from mice treated with isotype antibody retained A-wave responses of ∼18% at 12 h postinfection (P = 0.002). In contrast, infected eyes from mice treated with anti-CXCL1 antibody retained ∼58% A-wave function at 12 h postinfection, which was significantly greater than that of untreated and isotype-treated eyes (P ≤ 0.0001). Retained B-wave responses in untreated or isotype-treated, infected eyes were ∼20 or 29%, respectively, at 12 h postinfection (P = 0.056). In contrast, retained B-wave responses in mice treated with anti-CXCL1 monoclonal antibody were ∼59% and were significantly greater than that of untreated or isotype-treated eyes (P < 0.0001; Fig. 6B).
Pathologic changes in eyes of untreated and isotype-treated mice were similar at 12 h postinfection. In these groups, all eyes were highly inflamed with disrupted retinal layers and retinal detachments, as described above. Anterior segments of eyes in these groups were filled with fibrin, and posterior segments were filled with infiltrating inflammatory cells. In contrast, inflammation and pathologic changes in infected eyes treated with anti-CXCL1 antibody were not as severe compared with other groups. Retinal layers in infected eyes treated with anti-CXCL1 antibody were still intact at 12 h postinfection and there was less inflammation in these eyes compared with the other groups (Fig. 6C).
MPO concentrations in infected eyes of untreated and isotype-treated mice were similar at 12 h postinfection (P = 0.66). In contrast, infected eyes of mice treated with anti-CXCL1 antibody had significantly less MPO concentrations (P ≤ 0.01), which suggested that there was less neutrophil influx into these eyes in the presence of anti-CXCL1 antibody (Fig. 6D).
Concentrations of IL-6 were similar in untreated, isotype-treated, and anti-CXCL1–treated groups (P ≥ 0.57), similar to that observed in eyes of C57BL/6J, IL-6−/−, and CXCL1−/− mice. Concentrations of IL-1β were similar in eyes of untreated and isotype-treated mice (P = 0.94) and similar in eyes of untreated and anti-CXCL1–treated mice (P = 0.11). TNF-α concentrations were similar in eyes of untreated and isotype-treated mice at 12 h postinfection (P = 0.26), but were significantly less in eyes treated with anti-CXCL1 antibody compared with untreated eyes (P = 0.01). These results were similar to the lower TNF-α concentrations observed in CXCL1−/− mice. CXCL1 concentrations in eyes treated with anti-CXCL1 antibody were significantly less than in untreated or isotype-treated eyes (P ≤ 0.0012), though not completely absent as in CXCL1−/− mice (Fig. 6E).
Taken together, these results demonstrated that intraocular inflammation during B. cereus endophthalmitis could be limited with anti-CXCL1 treatment in our model, which suggests that CXCL1 may be a viable anti-inflammatory therapeutic target for this disease.
DISCUSSION
Bacterial endophthalmitis is a severe ocular infection that can result in rapid loss of vision, despite antibiotic and anti-inflammatory treatment. It has been suggested that the intraocular immune response to contaminating pathogens, in part, is responsible for retinal damage and loss of vision during endophthalmitis [43, 44]. Immune cells are recruited into the eye, potentially causing irreversible damage to retinal cells and impeding formation of a clear image. Because of the potential danger to vision, inflammatory responses in the eye are tightly controlled. In the case of avirulent organisms, such as S. epidermidis, bacterial clearance by an effective but not overtly robust inflammatory response results in maintenance of visual clarity [37]. In the case of virulent pathogens, such as B. cereus, effective bacterial clearance can occur when the inoculum is sparse; however, if a large enough inoculum contaminates the posterior segment, bacterial clearance mechanisms are ineffective, the inflammatory response is overwhelmed, and the infection progresses. Although B. cereus causes the most rapidly blinding form of endophthalmitis, other pathogens—Staphylococcus aureus, Streptococcus pneumoniae, viridans streptococci, Enterococcus spp.—can also cause sight-threatening endophthalmitis. Current therapies for B. cereus and other severe forms of endophthalmitis include intravitreal, topical, and systemic antibiotics [45–50], which can adequately sterilize the eye if administered early during infection. Use of corticosteroids for anti-inflammatory therapy in endophthalmitis is common, but their efficacy in this disease is controversial [51–56]. Identifying pathways that can be effectively targeted to reduce intraocular inflammation is critical in protecting the eye during intraocular infection.
Previous studies from our lab and others reported the role of TLR-mediated pathways in the ocular inflammatory response during bacterial endophthalmitis [33–36, 57–59]. In acute inflammation, such as that during endophthalmitis, activation of TLR pathways results in NF-κB activation, which leads to up-regulation of proinflammatory mediators that recruit neutrophils into the eye in an effort to clear infection. Up-regulation of proinflammatory mediators in the eye during the course of infection has been reported in several models of experimental bacterial endophthalmitis [30–38]. In a rat model of S. aureus endophthalmitis, TNF-α, IL-1β, and CINC (rat homolog of IL-8) were detected in the vitreous at 6 h postinfection and contributed to blood–retinal barrier permeability and neutrophil recruitment [38]. Proinflammatory mediator expression correlated with clinical inflammatory signs, including anterior chamber inflammatory cells and fibrin, posterior synechiae (adhesion of the iris to the lens), and vitreous exudate. In B. cereus endophthalmitis, cytokines TNF-α, IL-6, and CXCL1 were detected as early as 4 h postinfection, which correlated with early neutrophil influx and fibrin accumulation in the anterior chamber [30, 33–35]. These studies demonstrated that initialization of proinflammatory mediator synthesis was closely associated with neutrophil influx and subsequent retinal function decline, which suggested the importance of these mediators as targets in controlling intraocular inflammation and overall pathogenesis of disease. Moreover, because acute inflammation is a common finding in bacterial endophthalmitis, targets that drive inflammation in B. cereus endophthalmitis may be the same or similar in slower-developing forms of endophthalmitis.
Two important cytokines found to be up-regulated during experimental B. cereus endophthalmitis were IL-6 and CXCL1, and, thus, these were our potential targets of interest in limiting inflammation during B. cereus endophthalmitis. IL-6 is a cytokine that has been classified as both a pro- and an anti-inflammatory. IL-6 plays a key role in inflammation as the primary inducer of C-reactive protein, fibrinogen, and serum amyloid A protein, and is also involved in the regulation of metabolic, regenerative, and neural processes [60, 61]. IL-6 has been investigated as a target in regulation of a number of inflammatory diseases. Tocilizumab, a humanized monoclonal antibody for IL-6R, has been successfully employed for treatment of inflammatory autoimmune diseases [62–66]. In an experimental autoimmune uveitis model, absence of IL-6 or treatment with recombinant anti–IL-6R antibody ameliorated disease by inhibiting Th17 expansion and inflammatory cytokine synthesis [67]. Our present study indicated a significant delay in retinal function loss and neutrophil influx during experimental B. cereus endophthalmitis in IL-6−/− mice. Although neutrophil influx, as measured by MPO, in eyes of IL-6−/− mice was statistically less than that of eyes of C57BL/6J mice, MPO concentrations were only 11% less, and histologic sections demonstrated that infected eyes of IL-6−/− mice were as inflamed and retinas were as damaged as infected eyes of C57BL/6J mice. Proinflammatory cytokines TNF--α and IL1β were also similar in infected eyes of IL-6−/− and C57BL/6J mice, which suggested that absence of IL-6 did not impact these cytokines’ production. In contrast, CXCL1 concentrations were greater in infected eyes of IL-6−/− mice than in infected eyes of C57BL/6J mice. Neutrophil influx into infected eyes of IL-6−/− mice could have been a result of compensation of recruitment by CXCL1 in the absence of IL-6; however, because absence of IL-6 did not limit inflammation and pathogenesis to a clinically relevant extent in our model, we did not pursue neutralization studies with this cytokine.
CXCL1 belongs to the CXC family of chemokines [68]. The gene that encodes murine CXCL1 is homologous to human GRO-α and codes for secretory CXCL1 that is mainly induced by LPS in macrophages and vascular cells [69]. CXCL1 is a functional homolog of human IL-8 and its major function is recruitment and mobilization of neutrophils to the site of infection [70–72]. CXCL1 inhibitors have been reported as potential antiangiogenic factors in colorectal cancer models [73]. CXCL1 has been reported as important for neutrophil recruitment in models of invasive pulmonary aspergillosis [74], LPS-induced lung inflammation [75, 76], Klebsiella pneumoniae lung infection [77], and in a model of dextran sodium sulfate–induced colitis [78]. In the eye, in a model of experimental adenoviral keratitis, loss of CXCL1 affected neutrophil chemotaxis; however, overall clinical signs of keratitis in adenovirus-infected eyes of CXCL1−/− mice were similar to that of infected eyes of wild-type mice, which suggested that corneal opacity depended, to some degree, on alterations in corneal stroma in addition to leukocyte infiltration [79]. In the present study, we showed that in B. cereus–infected CXCL1−/− mice, there was a significant delay in retinal function loss relative to that of infected wild-type mice, but a greater delay in neutrophil influx compared with that observed in infected IL-6−/− mice. B. cereus–infected eyes of CXCL1−/− mice had low MPO concentrations, very few neutrophils in the vitreous, and minimal retinal damage. Ocular IL-6 and IL-1β levels were not changed in the absence of CXCL1, but TNF-α concentrations were reduced, which suggested an effect of CXCL1 absence on TNF-α. TNF-α has been shown to induce CXCL1-mediated recruitment of neutrophils both in vitro and in vivo through JNK- and MAPK-mediated signaling pathways [80–85]. CXCL1 receptor (CXCR2) inhibitor has also been shown to block TNF-α in an ischemia-reperfusion model [86]; however, a direct effect of CXCL1 on the synthesis of TNF-α has not yet been reported. In the present study, CXCL1 depletion resulted in a positive functional outcome and limited retinal damage similar to that observed with B. cereus–infected TLR2−/−, TLR4−/−, MyD88−/− and TRIF−/− mice [33–35].
We previously reported that in the absence of TNF-α, other proinflammatory cytokines and chemokines facilitated recruitment of PMN during experimental B. cereus endophthalmitis [39]. In that study, CXCL1 was detected in greater concentrations than in infected eyes of C57BL/6J mice, potentially compensating for absence of TNF-α [39]. Neutrophil recruitment in the absence of TNF-α was delayed, which led to greater bacterial growth and more severe disease. In the current study, CXCL1 concentrations were greater in eyes of IL-6−/− mice than in eyes of C57BL/6J mice, so neutrophil recruitment may not have been delayed enough to allow greater bacterial replication, and, thus, B. cereus replication in eyes of IL-6−/− and C57BL/6J mice were similar. Taken together, our results in B. cereus–infected TNF-α−/−, IL-6−/−, and CXCL1−/− mice suggest that depletion of an individual proinflammatory mediator may be compensated by synthesis of other mediators, but the level of compensation in recruiting neutrophils to the site of infection depends on which mediators are present in its absence. In the present study, impact of the absence of IL-6 in delaying retinal function loss and neutrophil influx was not as significant as was the absence of CXCL1 in improving the clinical outcome of experimental B. cereus endophthalmitis.
Because intraocular inflammation in CXCL1−/− mice was greatly minimized, we tested the therapeutic efficacy of neutralizing CXCL1 in the B. cereus endophthalmitis model. Although there was a 2-fold reduction in bacterial burden at 12 h postinfection in eyes treated with anti-CXCL1 antibody compared with infected untreated and isotype-treated eyes, there was still in excess of 6 million bacteria in these eyes. Therefore, reduction in bacterial burden would likely not account for the significant reduction in intraocular inflammation in anti-CXCL1–treated eyes. Anti-CXCL1 treatment resulted in significantly reduced MPO concentrations and neutrophil influx as well as limited retinal damage and greater retained retinal function. Whether the protection of the retina in anti-CXCL1–treated eyes was a direct result of a lack of inflammation remains an open question; however, on the basis of previous studies, the presence of 106 viable B. cereus in the eye should have caused significant retinal function loss [35–38], and there was an approximate 40% reduction in retinal function in eyes treated with anti-CXCL1 antibody. Similar to the reduction in TNF-α concentrations observed in infected eyes of CXCL1−/− mice, we observed a reduction in TNF-α concentrations in anti-CXCL1–treated eyes. CXCL1 concentrations in anti-CXCL1–treated eyes were not reduced to baseline levels observed in CXCL1−/− mice. Nevertheless, these results indicated that application of anti-CXCL1 at the initiation of B. cereus endophthalmitis successfully reduced inflammation and protected retinal function. In recurrent herpes stromal keratitis [42] and LPS-induced keratitis [86] models, anti-CXCL1 treatment reduced neutrophil recruitment and improved disease outcome. In clinical cases and experimental models of bacterial endophthalmitis, corticosteroids are commonly used but are not always effective [51–56]. Successful treatment of bacterial endophthalmitis, especially that caused by B. cereus, depends on timely therapeutic intervention. Although direct neutrophil depletion would seem to be an effective way to reduce inflammation, depletion of neutrophils early in the course of experimental S. aureus endophthalmitis severely hampered bacterial clearance, which resulted in more severe disease [32]. Reducing neutrophil recruitment by depleting proinflammatory mediators that recruit them may be a viable alternative in allowing a neutrophil threshold to be reached at a slower rate. In this case, an adequate number of neutrophils would be available for bacterial clearance, but the neutrophil burden would not be so great as to damage the retina or perturb clarity of the visual axis.
In summary, we demonstrated that an absence of CXCL1, either by genetic knockout or neutralizing antibody, resulted in a better clinical outcome for a severe form of bacterial endophthalmitis. Neutralization of CXCL1 holds promise in significantly reducing inflammation during B. cereus endophthalmitis; therefore, it is reasonable to hypothesize that this strategy would successfully reduce inflammation in endophthalmitis that is caused by other bacterial pathogens. Overall, our results set forth a novel therapeutic strategy of targeting CXCL1 in the eye as an anti-inflammation strategy that can be tested as an adjunct with antibiotic treatment for B. cereus and other types of endophthalmitis.
AUTHORSHIP
S.M.P and M.C.C. conceived and designed the experiments. S.M.P., C.B.R., and R.A.A. performed the experiments. S.M.P. and M.C.C. analyzed the data. G.C.F. and S.A.L. contributed breeding pairs. M.C.C. contributed reagents/materials/analysis tools. S.M.P. and M.C.C. wrote the paper.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) Grants R01EY024140 and R01EY012985 (to M.C.C.). Work in S.A.L.’s laboratory is supported by NIH Grants P01DK072201 and R01CA161373. M.C.C.’s research is supported in part by NIH Grants R21EY022466 (to M.C.C.) and CORE Grant P30EY027125 (Robert E. Anderson, Oklahoma University Health Sciences Center), and an unrestricted grant to the Dean A. McGee Eye Institute from Research to Prevent Blindness. The authors thank Dr. James Chodosh (Massachusetts Eye and Ear Infirmary, Boston, MA, USA) for providing the original CXCL1 breeding pair with Dr. Lira’s permission and Dr. Phillip Coburn [University of Oklahoma Health Sciences Center (OUHSC), Oklahoma City, OK, USA] for invaluable discussions and comments. The authors thank Nanette Wheatley, Dr. Feng Li, and Mark Dittmar (OUHSC Live Animal Imaging Core) for their invaluable technical assistance, and Excalibur Pathology (Moore, OK, USA) and the OUHSC Cellular Imaging Core for histology expertise.
Glossary
- ERG
electroretinography
- MPO
myeloperoxidase
- PMN
polymorphonuclear neutrophil
- TRIF
TIR domain–containing adapter inducing IFN-β
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
DISCLOSURES
The authors declare no conflicts of nterest.
REFERENCES
- 1.Streilein J. W. (2003) Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat. Rev. Immunol. 3, 879–889. [DOI] [PubMed] [Google Scholar]
- 2.Uchino Y., Iriyama N., Matsumoto K., Hirabayashi Y., Miura K., Kurita D., Kobayashi Y., Yagi M., Kodaira H., Hojo A., Kobayashi S., Hatta Y., Takeuchi J. (2012) A case series of Bacillus cereus septicemia in patients with hematological disease. Intern. Med. 51, 2733–2738. [DOI] [PubMed] [Google Scholar]
- 3.Arnaout M. K., Tamburro R. F., Bodner S. M., Sandlund J. T., Rivera G. K., Pui C. H., Ribeiro R. C. (1999) Bacillus cereus causing fulminant sepsis and hemolysis in two patients with acute leukemia. J. Pediatr. Hematol. Oncol. 21, 431–435. [DOI] [PubMed] [Google Scholar]
- 4.Frankard J., Li R., Taccone F., Struelens M. J., Jacobs F., Kentos A. (2004) Bacillus cereus pneumonia in a patient with acute lymphoblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 23, 725–728. [DOI] [PubMed] [Google Scholar]
- 5.Berner R., Heinen F., Pelz K., van Velthoven V., Sauer M., Korinthenberg R. (1997) Ventricular shunt infection and meningitis due to Bacillus cereus. Neuropediatrics 28, 333–334. [DOI] [PubMed] [Google Scholar]
- 6.Tuazon C. U., Murray H. W., Levy C., Solny M. N., Curtin J. A., Sheagren J. N. (1979) Serious infections from Bacillus sp. JAMA 241, 1137–1140. [PubMed] [Google Scholar]
- 7.Drobniewski F. A. (1993) Bacillus cereus and related species. Clin. Microbiol. Rev. 6, 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ihde D. C., Armstrong D. (1973) Clinical spectrum of infection due to Bacillus species. Am. J. Med. 55, 839–845. [DOI] [PubMed] [Google Scholar]
- 9.Colpin G. G. D., Guiot H. F. L., Simonis R. F. A., Zwaan F. E. (1981) Bacillus cereus meningitis in a patient under gnotobiotic care. Lancet 2, 694–695. [DOI] [PubMed] [Google Scholar]
- 10.Funada H., Uotani C., Machi T., Matsuda T., Nonomura A. (1988) Bacillus cereus bacteremia in an adult with acute leukemia. Jpn. J. Clin. Oncol. 18, 69–74. [DOI] [PubMed] [Google Scholar]
- 11.Jenson H. B., Levy S. R., Duncan C., Mcintosh S. (1989) Treatment of multiple brain abscesses caused by Bacillus cereus. Pediatr. Infect. Dis. J. 8, 795–798. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida H., Moriyama Y., Tatekawa T., Tominaga N., Teshima H., Hiraoka A., Masaoka T., Yoshinaga T. (1993) Two cases of acute myelogenous leukemia with Bacillus cereus bacteremia resulting in fatal intracranial hemorrhage [in Japanese]. Rinsho Ketsueki 34, 1568–1572. [PubMed] [Google Scholar]
- 13.Gaur A. H., Patrick C. C., McCullers J. A., Flynn P. M., Pearson T. A., Razzouk B. I., Thompson S. J., Shenep J. L. (2001) Bacillus cereus bacteremia and meningitis in immunocompromised children. Clin. Infect. Dis. 32, 1456–1462. [DOI] [PubMed] [Google Scholar]
- 14.Marley E. F., Saini N. K., Venkatraman C., Orenstein J. M. (1995) Fatal Bacillus cereus meningoencephalitis in an adult with acute myelogenous leukemia. South. Med. J. 88, 969–972. [DOI] [PubMed] [Google Scholar]
- 15.Steen M. K., Bruno-Murtha L. A., Chaux G., Lazar H., Bernard S., Sulis C. (1992) Bacillus cereus endocarditis: report of a case and review. Clin. Infect. Dis. 14, 945–946. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC) (2011) Notes from the field: contamination of alcohol prep pads with Bacillus cereus group and Bacillus species--Colorado, 2010. MMWR Morb. Mortal. Wkly. Rep. 60, 347. [PubMed] [Google Scholar]
- 17.Dolan S. A., Littlehorn C., Glodé M. P., Dowell E., Xavier K., Nyquist A. C., Todd J. K. (2012) Association of Bacillus cereus infection with contaminated alcohol prep pads. Infect. Control Hosp. Epidemiol. 33, 666–671. [DOI] [PubMed] [Google Scholar]
- 18.Sasahara T., Hayashi S., Morisawa Y., Sakihama T., Yoshimura A., Hirai Y. (2011) Bacillus cereus bacteremia outbreak due to contaminated hospital linens. Eur. J. Clin. Microbiol. Infect. Dis. 30, 219–226. [DOI] [PubMed] [Google Scholar]
- 19.Kato K., Matsumura Y., Yamamoto M., Nagao M., Ito Y., Takakura S., Ichiyama S. (2014) Seasonal trend and clinical presentation of Bacillus cereus bloodstream infection: association with summer and indwelling catheter. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1371–1379. [DOI] [PubMed] [Google Scholar]
- 20.Turabelidze G., Gee J. E., Hoffmaster A. R., Manian F., Butler C., Byrd D., Schildknecht S., Hauser L. C., Duncan M., Ferrett R., Evans D., Talley C. (2013) Contaminated ventilator air flow sensor linked to Bacillus cereus colonization of newborns. Emerg. Infect. Dis. 19, 781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David D. B., Kirkby G. R., Noble B. A. (1994) Bacillus cereus endophthalmitis. Br. J. Ophthalmol. 78, 577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey R. T. Jr., Tauber W. B. (1987) Posttraumatic endophthalmitis: the emerging role of Bacillus cereus infection. Rev. Infect. Dis. 9, 110–123. [DOI] [PubMed] [Google Scholar]
- 23.Chan W. M., Liu D. T., Chan C. K., Chong K. K., Lam D. S. (2003) Infective endophthalmitis caused by Bacillus cereus after cataract extraction surgery. Clin. Infect. Dis. 37, e31–e34. [DOI] [PubMed] [Google Scholar]
- 24.Roy M., Chen J. C., Miller M., Boyaner D., Kasner O., Edelstein E. (1997) Epidemic Bacillus endophthalmitis after cataract surgery I: acute presentation and outcome. Ophthalmology 104, 1768–1772. [DOI] [PubMed] [Google Scholar]
- 25.Chen J. C., Roy M. (2000) Epidemic Bacillus endophthalmitis after cataract surgery II: chronic and recurrent presentation and outcome. Ophthalmology 107, 1038–1041. [DOI] [PubMed] [Google Scholar]
- 26.Cowan C. L. Jr., Madden W. M., Hatem G. F., Merritt J. C. (1987) Endogenous Bacillus cereus panophthalmitis. Ann. Ophthalmol. 19, 65–68. [PubMed] [Google Scholar]
- 27.Masi R. J. (1978) Endogenous endophthalmitis associated with Bacillus cereus bacteremia in a cocaine addict. Ann. Ophthalmol. 10, 1367–1370. [PubMed] [Google Scholar]
- 28.Bouza E., Grant S., Jordan C., Yook R. H., Sulit H. L. (1979) Bacillus cereus endogenous panophthalmitis. Arch. Ophthalmol. 97, 498–499. [DOI] [PubMed] [Google Scholar]
- 29.O’Day D. M., Smith R. S., Gregg C. R., Turnbull P. C., Head W. S., Ives J. A., Ho P. C. (1981) The problem of bacillus species infection with special emphasis on the virulence of Bacillus cereus. Ophthalmology 88, 833–838. [DOI] [PubMed] [Google Scholar]
- 30.Ramadan R. T., Ramirez R., Novosad B. D., Callegan M. C. (2006) Acute inflammation and loss of retinal architecture and function during experimental Bacillus endophthalmitis. Curr. Eye Res. 31, 955–965. [DOI] [PubMed] [Google Scholar]
- 31.Ravindranath R. M., Mondino B. J., Adamu S. A., Pitchekian-Halabi H., Hasan S. A., Glasgow B. J. (1995) Immunopathologic features of Staphylococcus aureus endophthalmitis in the rat. Invest. Ophthalmol. Vis. Sci. 36, 2482–2491. [PubMed] [Google Scholar]
- 32.Giese M. J., Rayner S. A., Fardin B., Sumner H. L., Rozengurt N., Mondino B. J., Gordon L. K. (2003) Mitigation of neutrophil infiltration in a rat model of early Staphylococcus aureus endophthalmitis. Invest. Ophthalmol. Vis. Sci. 44, 3077–3082. [DOI] [PubMed] [Google Scholar]
- 33.Novosad B. D., Astley R. A., Callegan M. C. (2011) Role of Toll-like receptor (TLR) 2 in experimental Bacillus cereus endophthalmitis. PLoS One 6, e28619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkunan S. M., Astley R., Callegan M. C. (2014) Role of TLR5 and flagella in Bacillus intraocular infection. PLoS One 9, e100543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkunan S. M., Randall C. B., Coburn P. S., Astley R. A., Staats R. L., Callegan M. C. (2015) Unexpected roles for Toll-like receptor 4 and TRIF in intraocular infection with gram-positive bacteria. Infect. Immun. 83, 3926–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar A., Singh C. N., Glybina I. V., Mahmoud T. H., Yu F. S. (2010) Toll-like receptor 2 ligand-induced protection against bacterial endophthalmitis. J. Infect. Dis. 201, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petropoulos I. K., Vantzou C. V., Lamari F. N., Karamanos N. K., Anastassiou E. D., Pharmakakis N. M. (2006) Expression of TNF-α, IL-1β, and IFN-γ in Staphylococcus epidermidis slime-positive experimental endophthalmitis is closely related to clinical inflammatory scores. Graefes Arch. Clin. Exp. Ophthalmol. 244, 1322–1328. [DOI] [PubMed] [Google Scholar]
- 38.Giese M. J., Sumner H. L., Berliner J. A., Mondino B. J. (1998) Cytokine expression in a rat model of Staphylococcus aureus endophthalmitis. Invest. Ophthalmol. Vis. Sci. 39, 2785–2790. [PubMed] [Google Scholar]
- 39.Ramadan R. T., Moyer A. L., Callegan M. C. (2008) A role for tumor necrosis factor-alpha in experimental Bacillus cereus endophthalmitis pathogenesis. Invest. Ophthalmol. Vis. Sci. 49, 4482–4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boisvert W. A., Rose D. M., Johnson K. A., Fuentes M. E., Lira S. A., Curtiss L. K., Terkeltaub R. A. (2006) Up-regulated expression of the CXCR2 ligand KC/GRO-α in atherosclerotic lesions plays a central role in macrophage accumulation and lesion progression. Am. J. Pathol. 168, 1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunt J. J., Wang J. T., Callegan M. C. (2011) Contribution of mucoviscosity-associated gene A (magA) to virulence in experimental Klebsiella pneumoniae endophthalmitis. Invest. Ophthalmol. Vis. Sci. 52, 6860–6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West D. M., Del Rosso C. R., Yin X. T., Stuart P. M. (2014) CXCL1 but not IL-6 is required for recurrent herpetic stromal keratitis. J. Immunol. 192, 1762–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callegan M. C., Engelbert M., Parke D. W. II, Jett B. D., Gilmore M. S. (2002) Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 15, 111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Callegan M. C., Kane S. T., Cochran D. C., Gilmore M. S. (2002) Molecular mechanisms of Bacillus endophthalmitis pathogenesis. DNA Cell Biol. 21, 367–373. [DOI] [PubMed] [Google Scholar]
- 45.Wiskur B. J., Robinson M. L., Farrand A. J., Novosad B. D., Callegan M. C. (2008) Toward improving therapeutic regimens for Bacillus endophthalmitis. Invest. Ophthalmol. Vis. Sci. 49, 1480–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callegan M. C., Novosad B. D., Ramadan R. T., Wiskur B., Moyer A. L. (2009) Rate of bacterial eradication by ophthalmic solutions of fourth-generation fluoroquinolones. Adv. Ther. 26, 447–454. [DOI] [PubMed] [Google Scholar]
- 47.Benz M. S., Scott I. U., Flynn H. W. Jr., Unonius N., Miller D. (2004) Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases. Am. J. Ophthalmol. 137, 38–42. [DOI] [PubMed] [Google Scholar]
- 48.Recchia F. M., Busbee B. G., Pearlman R. B., Carvalho-Recchia C. A., Ho A. C. (2005) Changing trends in the microbiologic aspects of postcataract endophthalmitis. Arch. Ophthalmol. 123, 341–346. [DOI] [PubMed] [Google Scholar]
- 49.Callegan M. C., Cochran D. C., Kane S. T., Ramadan R. T., Chodosh J., McLean C., Stroman D. W. (2006) Virulence factor profiles and antimicrobial susceptibilities of ocular Bacillus isolates. Curr. Eye Res. 31, 693–702. [DOI] [PubMed] [Google Scholar]
- 50.Hariprasad S. M., Mieler W. F., Holz E. R. (2003) Vitreous and aqueous penetration of orally administered gatifloxacin in humans. Arch. Ophthalmol. 121, 345–350. [DOI] [PubMed] [Google Scholar]
- 51.Das T., Jalali S., Gothwal V. K., Sharma S., Naduvilath T. J. (1999) Intravitreal dexamethasone in exogenous bacterial endophthalmitis: results of a prospective randomised study. Br. J. Ophthalmol. 83, 1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gan I. M., Ugahary L. C., van Dissel J. T., Feron E., Peperkamp E., Veckeneer M., Mulder P. G., Platenkamp G. J., van Meurs J. C. (2005) Intravitreal dexamethasone as adjuvant in the treatment of postoperative endophthalmitis: a prospective randomized trial. Graefes Arch. Clin. Exp. Ophthalmol. 243, 1200–1205. [DOI] [PubMed] [Google Scholar]
- 53.Shah G. K., Stein J. D., Sharma S., Sivalingam A., Benson W. E., Regillo C. D., Brown G. C., Tasman W. (2000) Visual outcomes following the use of intravitreal steroids in the treatment of postoperative endophthalmitis. Ophthalmology 107, 486–489. [DOI] [PubMed] [Google Scholar]
- 54.Meredith T. A., Aguilar H. E., Drews C., Sawant A., Gardner S., Wilson L. A., Grossniklaus H. E. (1996) Intraocular dexamethasone produces a harmful effect on treatment of experimental Staphylococcus aureus endophthalmitis. Trans. Am. Ophthalmol. Soc. 94, 241–252, discussion 252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu S. M., Way T., Rodrigues M., Steidl S. M. (2000) Effects of intravitreal corticosteroids in the treatment of Bacillus cereus endophthalmitis. Arch. Ophthalmol. 118, 803–806. [DOI] [PubMed] [Google Scholar]
- 56.Pollack J. S., Beecher D. J., Pulido J. S., Lee Wong A. C. (2004) Failure of intravitreal dexamethasone to diminish inflammation or retinal toxicity in an experimental model of Bacillus cereus endophthalmitis. Curr. Eye Res. 29, 253–259. [DOI] [PubMed] [Google Scholar]
- 57.Kochan T. J., Blair J., Kumar A. (2011) Modulation of Toll-like receptor signaling in microglia by Tlr2, And Tlr4 ligands and their relevance to bacterial endophthalmitis. Invest. Ophthalmol. Vis. Sci. 52, 2958. [Google Scholar]
- 58.Hunt J. J., Astley R., Wheatley N., Wang J. T., Callegan M. C. (2014) TLR4 contributes to the host response to Klebsiella intraocular infection. Curr. Eye Res. 39, 790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gregory-Ksander, M.S., Crane, M., Vincent W. J., TLR2, but not NOD2, is required for clearance of bacteria during S. aureus induced endophthalmitis. Invest. Ophthalmol. Vis. Sci.52, 5864.
- 60.Kishimoto T. (2006) Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res. Ther. 8, S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kishimoto T. (2005) IL-6: from laboratory to bedside. Clin. Rev. Allergy Immunol. 28, 177–186. [DOI] [PubMed] [Google Scholar]
- 62.Mima T., Nishimoto N. (2009) Clinical value of blocking IL-6 receptor. Curr. Opin. Rheumatol. 21, 224–230. [DOI] [PubMed] [Google Scholar]
- 63.Murakami M., Nishimoto N. (2011) The value of blocking IL-6 outside of rheumatoid arthritis: current perspective. Curr. Opin. Rheumatol. 23, 273–277. [DOI] [PubMed] [Google Scholar]
- 64.Nishimoto N., Kanakura Y., Aozasa K., Johkoh T., Nakamura M., Nakano S., Nakano N., Ikeda Y., Sasaki T., Nishioka K., Hara M., Taguchi H., Kimura Y., Kato Y., Asaoku H., Kumagai S., Kodama F., Nakahara H., Hagihara K., Yoshizaki K., Kishimoto T. (2005) Humanized anti-interleukin-6 receptor antibody treatment of multicentric Castleman disease. Blood 106, 2627–2632. [DOI] [PubMed] [Google Scholar]
- 65.Nishimoto N., Kishimoto T. (2006) Interleukin 6: from bench to bedside. Nat. Clin. Pract. Rheumatol. 2, 619–626. [DOI] [PubMed] [Google Scholar]
- 66.Rossi J. F., Lu Z. Y., Jourdan M., Klein B. (2015) Interleukin-6 as a therapeutic target. Clin. Cancer Res. 21, 1248–1257. [DOI] [PubMed] [Google Scholar]
- 67.Yoshimura T., Sonoda K.-H., Ohguro N., Ohsugi Y., Ishibashi T., Cua D. J., Kobayashi T., Yoshida H., Yoshimura A. (2009) Involvement of Th17 cells and the effect of anti-IL-6 therapy in autoimmune uveitis. Rheumatology (Oxford) 48, 347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomson A. W. (1994) Cytokines and their receptors as potential therapeutic targets. In: The Cytokine Handbook, 2nd ed, Elsevier, Maryland Heights, MO, USA, 525–566. [Google Scholar]
- 69.Lira S. A., Zalamea P., Heinrich J. N., Fuentes M. E., Carrasco D., Lewin A. C., Barton D. S., Durham S., Bravo R. (1994) Expression of the chemokine N51/KC in the thymus and epidermis of transgenic mice results in marked infiltration of a single class of inflammatory cells. J. Exp. Med. 180, 2039–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baggiolini M., Walz A., Kunkel S. L. (1989) Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J. Clin. Invest. 84, 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rollins B. J. (1997) Chemokines. Blood 90, 909–928. [PubMed] [Google Scholar]
- 72.Bacon K. B., Oppenheim J. J. (1998) Chemokines in disease models and pathogenesis. Cytokine Growth Factor Rev. 9, 167–173. [DOI] [PubMed] [Google Scholar]
- 73.Wang D., Wang H., Brown J., Daikoku T., Ning W., Shi Q., Richmond A., Strieter R., Dey S. K., DuBois R. N. (2006) CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 203, 941–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mehrad B., Wiekowski M., Morrison B. E., Chen S.-C., Coronel E. C., Manfra D. J., Lira S. A. (2002) Transient lung-specific expression of the chemokine KC improves outcome in invasive aspergillosis. Am. J. Respir. Crit. Care Med. 166, 1263–1268. [DOI] [PubMed] [Google Scholar]
- 75.Frevert C. W., Huang S., Danaee H., Paulauskis J. D., Kobzik L. (1995) Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J. Immunol. 154, 335–344. [PubMed] [Google Scholar]
- 76.Bozic C. R., Kolakowski L. F. Jr., Gerard N. P., Garcia-Rodriguez C., von Uexkull-Guldenband C., Conklyn M. J., Breslow R., Showell H. J., Gerard C. (1995) Expression and biologic characterization of the murine chemokine KC. J. Immunol. 154, 6048–6057. [PubMed] [Google Scholar]
- 77.Cai S., Batra S., Lira S. A., Kolls J. K., Jeyaseelan S. (2010) CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. J. Immunol. 185, 6214–6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shea-Donohue T., Thomas K., Cody M. J., Aiping Zhao, Detolla L. J., Kopydlowski K. M., Fukata M., Lira S. A., Vogel S. N. (2008) Mice deficient in the CXCR2 ligand, CXCL1 (KC/GRO-α), exhibit increased susceptibility to dextran sodium sulfate (DSS)-induced colitis. Innate Immun. 14, 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chintakuntlawar A. V., Chodosh J. (2009) Chemokine CXCL1/KC and its receptor CXCR2 are responsible for neutrophil chemotaxis in adenoviral keratitis. J. Interferon Cytokine Res. 29, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lo H. M., Lai T. H., Li C. H., Wu W. B. (2014) TNF-α induces CXCL1 chemokine expression and release in human vascular endothelial cells in vitro via two distinct signaling pathways. Acta Pharmacol. Sin. 35, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hao Q., Wang L., Tang H. (2009) Vascular endothelial growth factor induces protein kinase D-dependent production of proinflammatory cytokines in endothelial cells. Am. J. Physiol. Cell Physiol. 296, C821–C827. [DOI] [PubMed] [Google Scholar]
- 82.Shieh J. M., Tsai Y. J., Tsou C. J., Wu W. B. (2014) CXCL1 regulation in human pulmonary epithelial cells by tumor necrosis factor. Cell. Physiol. Biochem. 34, 1373–1384. [DOI] [PubMed] [Google Scholar]
- 83.Anisowicz A., Messineo M., Lee S. W., Sager R. (1991) An NF-kappa B-like transcription factor mediates IL-1/TNF-alpha induction of gro in human fibroblasts. J. Immunol. 147, 520–527. [PubMed] [Google Scholar]
- 84.Tessier P. A., Naccache P. H., Clark-Lewis I., Gladue R. P., Neote K. S., McColl S. R. (1997) Chemokine networks in vivo: involvement of C-X-C and C-C chemokines in neutrophil extravasation in vivo in response to TNF-alpha. J. Immunol. 159, 3595–3602. [PubMed] [Google Scholar]
- 85.Vieira S. M., Lemos H. P., Grespan R., Napimoga M. H., Dal-Secco D., Freitas A., Cunha T. M., Verri W. A. Jr., Souza-Junior D. A., Jamur M. C., Fernandes K. S., Oliver C., Silva J. S., Teixeira M. M., Cunha F. Q. (2009) A crucial role for TNF-α in mediating neutrophil influx induced by endogenously generated or exogenous chemokines, KC/CXCL1 and LIX/CXCL5. Br. J. Pharmacol. 158, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Souza D. G., Bertini R., Vieira A. T., Cunha F. Q., Poole S., Allegretti M., Colotta F., Teixeira M. M. (2004) Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. Br. J. Pharmacol. 143, 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lin M., Carlson E., Diaconu E., Pearlman E. (2007) CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J. Leukoc. Biol. 81, 786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]