Inhibition of mTOR kinase alters macrophage metabolism, and is protective against lipid toxicity in primary macrophages independent of autophagy.
Keywords: lysosome, metabolism, diabetes, TLRs, TFEB
Abstract
Macrophage dysfunction in obesity and diabetes is associated with persistent inflammation and poor wound healing responses. Relevant to these phenotypes, we have previously shown that macrophage activation in a high-fat environment results in cell death via a mechanism that involves lysosome damage. While searching for signaling pathways that were required for this response, we discovered that mTOR inhibitors, torin and rapamycin, were protective against lipotoxic cell death in primary peritoneal macrophages. The protective effect of mTOR inhibition was also confirmed by using genetic loss-of-function approaches. Given the importance of mTOR in regulation of autophagy we hypothesized that this pathway would be important in protection from cell death. We first demonstrated that autophagy was disrupted in response to palmitate and LPS as a consequence of impaired lysosome function. Conversely, the mTOR inhibitor, torin, increased macrophage autophagy and protected against lysosome damage; however, the beneficial effects of torin persisted in autophagy-deficient cells. Inhibition of mTOR also triggered nuclear localization of TFEB, a transcription factor that regulates lysosome biogenesis and function, but the rescue phenotype did not require the presence of TFEB. Instead, we demonstrated that mTOR inhibition reduces mitochondrial oxidative metabolism and attenuates the negative effects of palmitate on LPS-induced mitochondrial respiration. These results suggest that inhibition of mTOR is protective against lipotoxicity via an autophagy-independent mechanism that involves relieving mitochondrial substrate overload. On the basis of these findings, we suggest that therapies to reduce macrophage mTOR activation may protect against dysfunctional inflammation in states of overnutrition, such as diabetes.
Introduction
The prevalence of obesity and diabetes continues to increase at a staggering rate in the Western world. Impaired responses to tissue injury and infection are characteristic of these diseases, which leads to significant morbidity and mortality [1–3]. Increasing evidence suggests that macrophage dysfunction in diabetes is an important contributor to these phenotypes [4, 5]; however, mechanisms that underlie macrophage pathology in diabetes are not well understood.
Diabetes is associated with excess circulating nutrients, including glucose and FAs, which likely contribute to complications of this disease [6, 7]. When FA levels exceed the storage capacity of adipose tissue, ectopic lipid deposition occurs in peripheral tissues, including macrophages [8]. Diabetic macrophages have increased FA content and dietary SFA palmitate, in particular, is abundant [9]. These observations led us to explore how palmitate and other SFAs impact macrophage response to inflammatory stimuli. To investigate this question, we focused on the macrophage activation program downstream of TLR4, as this molecule is robustly expressed by macrophages and plays a key role in the response to bacterial and host-derived danger signals [10, 11]. Although it has been reported that SFAs can also be ligands for TLR4, more recent evidence calls this into question [12, 13].
In previous studies, we have shown that activation of lipid-loaded macrophages with the TLR4 ligand LPS produces several lipotoxic phenotypes, including ceramide accumulation, inflammasome activation, and cell death [14–16]. Of interest, cell death and inflammasome activation occur via a pathway that requires lysosome damage. Surprisingly, upstream signaling involved in macrophage cell death is independent of canonical TLR signaling molecules, including MyD88, JNK, ERK, p38 MAPK, and NF-κB. Instead, activation of the alternate TLR4 adaptor, TIR (Toll/IL-1R) domain-containing adapter inducing IFN-β (TRIF), independent of interferon, is required for toxic crosstalk with dietary SFAs [14].
In this study, we discovered that mTOR is activated downstream of TLR4 and its inhibition reduces lipotoxic macrophage cell death. The central role of mTOR in regulation of autophagy led us to investigate whether this process was responsible for the cytoprotective effect of mTOR inhibitors. We found that the combination of LPS and palmitate impaired autophagic flux at the level of the lysosome. Conversely, treatment with the mTOR inhibitor, torin, induced autophagy and reduced lysosome pathology; however, beneficial effects of mTOR inhibition occurred independently of autophagy or autophagy/lysosome transcription factor TFEB. Instead, we provide evidence that mTOR loss of function alters macrophage cell metabolism by decreasing the imbalance between metabolite influx and mitochondrial oxidative capacity, a response that may be protective against lipid stress.
MATERIALS AND METHODS
Reagents
Torin was purchased from R&D Systems (Minneapolis, MN, USA); bafilomycin A from Enzo Life Sciences (Farmingdale, NY, USA); and etomoxir, UK5099, BPTES, α-tubulin antibody, α-actin antibody, and α-lamininB antibody from Sigma-Aldrich (St. Louis, MO, USA). S6 kinase inhibitor and rapamycin were from EMD Millipore (Billerica, MA, USA). Phospho- and total S6 Kinase, AKT S473, and S6 antibodies were from Cell Signaling Technology (Danvers, MA, USA). α-LC3 antibody was purchased from Novus Biologicals (NB100-2220; Littleton, CO, USA); α-TFEB antibody from MyBioSsource (MBS120432; San Diego, CA, USA); and α-p62 antibody from Abcam (ab56416; Cambridge, MA USA). Lysotracker red was from Thermo Fisher Scientific (Waltham, MA, USA). CD107a (LAMP1)-PE antibody conjugate was from Ebioscience (San Diego, CA, USA). Ultrapure Escherichia coli LPS was from Invivogen (San Diego, CA, USA). Thioglycollate was from Difco-BD (Franklin Lakes, NJ, USA). FAs were from Nu-Chek Prep (Waterville, MN, USA). Ultrapure BSA was from Lampire (Ottsville, PA, USA) and was tested for TLR ligand contamination before use by treating primary macrophages and assaying for TNF-α release.
Cell culture
Peritoneal macrophages were isolated from C57BL/6 or the indicated knockout mice 4 d after i.p. injection of 3.85% thioglycollate and plated at a density of 1 × 106 cells/ml in DMEM that contained 10% inactivated fetal serum, 50 U/ml penicillin G sodium, and 50 U/ml streptomycin sulfate. Stimulations were performed on the day after harvest. For flow cytometry experiments, peritoneal cells were cultured on low adherence plates (Greiner Bio-One, Monroe, NC, USA) to facilitate cell harvest. Cells were removed from low adherence plates by washing with PBS followed by 10 min with Cell Stripper (Thermo Fisher Scientific), and then 10 min with DTA/trypsin (Sigma-Aldrich). Growth medium was supplemented with palmitate or stearate complexed to BSA at a 2:1 molar ratio as described previously [14]. BSA-supplemented media was used as control. For cell stimulations, PBS or LPS (50 ng/ml) were added to BSA or free FA-containing media.
Mice
WT C57BL/6 mice were bred in our mouse facility. ATG5flox × LysM-Cre, ATG16Lflox × LysM-Cre, and GFP-LC3 mice were from Skip Virgin (Washington University, St. Louis, MO, USA). mTOR and raptor floxed mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and bred to LysM-Cre mice. All lines were in the C57BL/6 background. Mice were maintained in a pathogen-free facility on a standard chow diet ad libitum (6% fat). All animal experiments were conducted in strict accordance with U.S. National Institutes of Health guidelines for humane treatment of animals and were reviewed by the Animal Studies Committee of Washington University School of Medicine.
RNA isolation and quantitative RT-PCR
Total cellular RNA was isolated by using Qiagen RNeasy columns (Valencia, CA, USA) and reverse transcribed by using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA). Real-time quantitative RT-PCR was performed by using SYBR green reagent (Applied Biosystems) on an ABI 7500 fast thermocycler. Relative gene expression was determined by using the δ-δ CT method normalized to 36B4 expression. Mouse primers sequences were as follows (all 5′–3′): 36B4: forward: ATC CCT GAC GCA CCG CCG TGA, reverse: TGC ATC TGC TTG GAG CCC ACG TT; LC3: forward: CGT CCT GGA CAA GAC CAA GT, reverse: ATT GCT GTC CCG AAT GTC TC; p62: forward: GCT GCC CTA TAC CCA CAT CT, reverse: CGC CTT CAT CCG AGA AAC; GFP: forward: ATC ATG GCC GAC AAG CAG AAG AAC, reverse: GTA CAG CTC GTC CAT GCC GAG AGT.
siRNA transfection
pMACs were seeded at 7.5 × 105 cells/ml and allowed to adhere to plates for 4 h. After adhesion, cells were washed and transfected with 5 nM of control or TFEB siRNA SMART pool oligos (Thermo Fisher Scientific) by using DeliverX transfection reagent according to manufacturer instructions (Afftmetrix, Santa Clara, CA, USA). After transfection, cells were incubated for 72 h at 37°C followed by protein isolation to assess TFEB expression or stimulation with palm-LPS.
Cytosolic and nuclear extract preparation
Macrophages were plated in 5-cm dishes for a total of 5 × 106 cells. After stimulations, cells were washed with ice-cold PBS followed by addition of 200 μl cytosolic lysis buffer (0.5% Trition X-100, 50 mM Tris HCL, pH 8, 138 mM NaCl, 10% glycerol, 5 mM EDTA in distilled water). The cell slurry was then placed on ice for 15 min followed by centrifugation at 200 g for 10 min. Supernatant was saved as cytosolic fraction. The nuclear pellet was then washed with cytosolic lysis buffer for 5 min on ice and repelleted. To isolate nuclear protein, the pellet was incubated with 80 ml of nuclear lysis buffer (cytosolic lysis buffer + 0.5% SDS) and sonicated. Protein levels were assessed via Western blotting.
Western blotting
Total cellular protein was isolated by lysing cells in 150 mM NaCl, 10 mM Tris (pH 8), 1% Triton X-100, and 1× protease complete and phosphatase inhibitors (Thermo Fisher Scientific). Subsequently, 25 μg of protein (40 μg for TFEB detection) from each sample was separated on a TGX gradient gel (4–20%; Bio-Rad, Hercules, CA, USA) and transferred to a nitrocellulose membrane. For blots of phospho-S6, S6K, and AKT, transfer was for 1 h on ice. For LC3 and TFEB blots, proteins were transferred overnight in the cold room at 140 mAmp constant current.
After stimulation of 5 × 105 cells, supernatants and cell lysates were collected at 20 h and LDH release was quantified by using the CytoTox 96 nonradioactive cytotoxicity assay (Promega, Madison, WI, USA), per manufacturer instructions, with a Tecan Infinite M200 plate reader.
p62 immunofluorescence
Macrophages were grown on glass coverslips at a density of 5 × 105 cells/ml and stimulated as indicated. After washing, cells were fixed in 4% paraformaldehyde for 15 min and permeablized with 0.1% NP-40 in PBS for 10 min.
Lysosome imaging
After the indicated stimulations, cells were stained with 500 nM lysotracker red in tissue culture media for 15 min at 37°C. After staining, cells were washed 3 times with PBS, harvested as described in the Cell culture section above, and analyzed by flow cytometry. For LAMP1 surface staining, pMACs were stimulated for 24 h in presence or absence of torin, and cells were removed from plate as described above. Cells were pelleted, incubated with Fc block, and then stained with CD107a-PE antibody at a 1:200 dilution for 30 min on ice in the dark. After staining, cells were washed in PBS + 1% FBS and analyzed by flow cytometry.
Metabolism assays
Cells were plated into 96 well Seahorse Bioscience plates (North Billerica, MA, USA) at a density of 75,000 cells/well and stimulated as indicated in the text. After stimulation, cells were washed and placed in XF media (nonbuffered RPMI 1640 that contained 25 mM glucose, 2 mM l-glutamine, and 1 mM sodium pyruvate) with 10% FCS. OCR and ECAR were measured at baseline and after addition of the following drugs: for the mitochondrial stress test - 1.5 μM flurorcarbonyl cynade phenylhydrazon, and 100 nM rotenone + 1 μM antimycin A (all from Sigma-Aldrich) and for the substrate use experiments UK5099 (10 μM), etomoxir (50 μM), BPTES (10 μM), and 100 nM rotenone + 1 μM antimycin A (all from Sigma-Aldrich). Measurements were taken by using a 96-well Extracellular Flux Analyzer (Seahorse Bioscience). For calculation of mitochondrial OCR, the residual oxygen consumption present after rotenone/antimycin injection was subtracted from the total OCR.
RESULTS
mTOR inhibitors attenuate lipotoxic cell death in macrophages
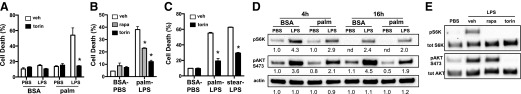
We have previously shown that activation of TLR4 in macrophages that are exposed to SFAs, such as palmitate, undergo a form of cell death that is dependent on TRIF and lysosome dysfunction [14]. However, the mechanisms linking inflammatory signaling to lysosome pathology remain unclear. To investigate signaling pathways that alter lipotoxic cell death in macrophages, we screened several chemical inhibitors of inflammatory signaling pathways for protection against macrophage death in response to palmitate and LPS. Of interest, of compounds tested, only rapamycin and torin reduced cell death. These findings were subsequently confirmed using annexin-PI staining (Fig. 1A and B). Torin also protected from cell death induced by the SFA, stearate, which indicated that this phenomenon was not unique to palmitate (Fig. 1C)
Figure 1. mTOR inhibitors protect macrophages from lipotoxic cell death.
(A) pMACs were treated with 250 μM palmitate (palm) or BSA ± 50 ng/ml LPS in the presence of vehicle (veh; white bars) or 100 nM torin (black bars), and cell death was assessed by annexin-PI flow cytometry. (B) pMACs were incubated with BSA-PBS or palm-LPS in the presence of veh (white bars), 100 nM rapamycin (rapa; gray bars) or 100 nM torin (black bars), and cell death was determined by 30 h via annexin-PI flow cytometry. (C) Macrophages were stimulated with palm-LPS or stearate (stear; 100 μM)-LPS in combination with veh or torin, and cell death was quantified by annexin-PI flow cytometry. (D) Kinetic analysis of S6K and AKT phosphorylation by Western blotting under the indicated conditions. (E) pMACs were stimulated with PBS or LPS for 4 h in presence of veh, rapa, or torin, and S6K and AKT phosphorylation was assessed by Western blotting. Densitometric quantification of the bands is shown beneath the blot. nd, not determined. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for veh vs. inhibitor.
Both rapamycin and torin inhibit mTOR, which suggested that pathways regulated by this kinase may modulate macrophage cell death. mTOR can exist in 2 distinct complexes, known as mTORC1 and mTORC2, which are associated with adaptor proteins, raptor and rictor, respectively [17]. To evaluate activation of this kinase in response to LPS and palmitate we determined the phosphorylation status of the mTORC1 substrate S6K and mTORC2 substrate AKT (S473) after macrophage activation. LPS treatment led to sustained phosphorylation of S6K and AKT, whereas palmitate alone had little effect on either pathway. The combination of palmitate with LPS modestly decreased S6K phosphorylation and substantially blunted the levels of phospho-AKT compared with LPS alone (Fig. 1D). As expected, both torin and rapamycin prevented phosphorylation of the mTORC1 substrate S6 kinase. Rapamycin is a relatively selective mTORC1 inhibitor and torin inhibits both mTOR signaling complexes. Consistent with this, AKT phosphorylation was affected by torin, but not rapamycin (Fig. 1E).
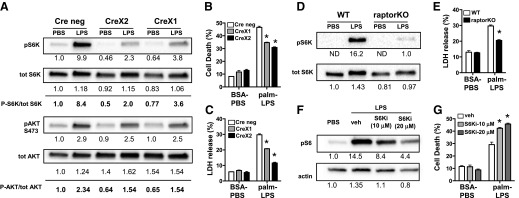
mTOR knockdown in primary macrophages reduces lipid-induced cell death
To further evaluate the role of mTOR in macrophage cell death after palmitate and LPS activation, we isolated primary macrophages from mTOR floxed mice crossed to animals that contained the lysM-Cre transgene. In the LysM-Cre model, excision of the floxed allele with 1 copy of Cre can be incomplete; therefore, we compared phenotypes between floxed mice with no Cre (Cre neg) with those that contained 1 or 2 copies of Cre, referred to as CreX1 or CreX2. As can be seen in Fig. 2, phosphorylation of the mTORC1 substrate S6K was reduced by 57 and 76% in CreX1 and CreX2 macrophages, respectively. In contrast, phosphorylation of the mTORC2 substrate AKT was reduced by ∼35% in both Cre-expressing cells. After stimulation with control or palm-LPS, both lines of Cre-expressing macrophages were less susceptible to death as measured by annexin-PI or LDH release (Fig. 2B and C). Protection from cell death was greatest in macrophages that contained 2 copies of Cre. To assess whether loss of mTORC1 was critical for this phenotype, we compared macrophages from LysM-Cre mice with LysM-Cre crossed to raptor floxed mice, in which mTORC1 signaling is selectively disrupted. LPS-induced phosphorylation of mTORC1 substrate S6K was suppressed in raptor KO macrophages, and cell death was also reduced in these cells after palm-LPS treatment (Fig. 2D and E). Together this data confirms a role for mTOR in macrophage cell death in response to lipid inflammatory stimuli and suggests that mTORC1 signaling is more important for this phenotype.
Figure 2. Genetic mTOR loss of function attenuates lipotoxic cell death in primary macrophages.
(A) pMACS from mTOR flx/flx mice with no Cre (Cre neg), 1 copy LysM-Cre (CreX1), or 2 copies of LysM-Cre (CreX2) were stimulated with PBS or LPS (50 ng/ml) for 4 h, and phosphorylation of S6K and AKT was analyzed by Western blotting. Densitometric quantification of bands is shown beneath the blot. (B and C) mTOR flx/flx pMACs with no Cre (white bars), 1 copy of LysM-Cre (gray bars), or 2 copies of LysM-Cre (black bars) were stimulated with BSA-PBS or palm-LPS, and cell death was quantified by annexin-PI flow cytometry at 30 h (B) or LDH release at 24 h (C). (D) Macrophages from control or raptor floxed mice crossed with LysM-Cre (raptorKO) were stimulated LPS for 4 h, and S6K phosphorylation was assessed by Western blotting. (E) WT or raptorKO macrophages were stimulated with control of palm-LPS, and cell death was determined by LDH release. (F and G) pMACs were treated with vehicle (veh), 10 μM S6K inhibitor (S6Ki; light gray bars), or 20 μM S6Ki (dark gray bars) in the presence of LPS (F) or palm-LPS (G), and S6 phosphoylation or cell death was assessed by Western blotting or annexin-PI flow cytometry, respectively. Densitometric quantification of the bands is shown beneath the blots. ND, not determined. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for WT vs. mTOR KO lines.
S6K is an important substrate of mTORC1. To assess the role of this enzyme in lipotoxic cell death, we treated macrophages with a pharmacologic inhibitor of S6K. Inhibition of S6K did not rescue, but, in fact, increased cell death in response to palm-LPS. This occurred at doses of the inhibitor that significantly reduced phosphorylation of S6K substrate S6, which indicates that the protective effect of mTORC1 loss of function occurs independent of S6K (Fig. 2F and G).
Lipid stress leads to dysfunctional autophagy
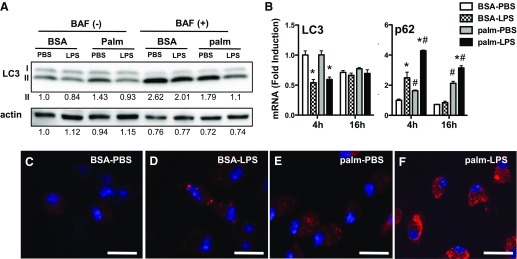
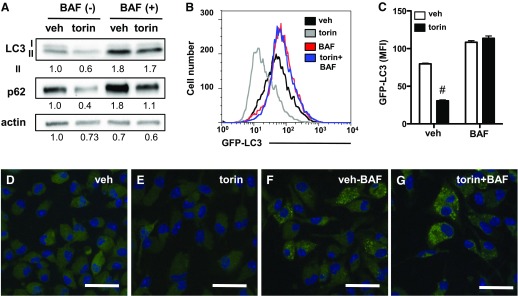
Autophagy is a cellular pathway that is tightly regulated by mTOR and that can be protective against many forms of cell stress [18]. To understand the interplay between autophagy and lipid overload in primary macrophages, we used several approaches to dissect the impact of LPS and palmitate on autophagic flux in pMACs. First, we evaluated protein levels of LC3-I and -II after stimulation with LPS, palmitate, or combination. Bafilomycin was included for the last 2 h of stimulation in a duplicate set of samples to assess flux to the lysosome. In the absence of bafilomycin, there were only subtle differences in LC3 levels in activated macrophages, with LPS-treated groups having slightly less LC3-II (Fig. 3A). Addition of bafilomycin to prevent lysosomal degradation significantly increased levels of LC3-II in most groups, which was consistent with ongoing autophagic flux. The modest decrease in LC3-II that was observed with LPS was still apparent after bafilomycin treatment, which suggests that this observation was not a consequence of increased rates of autophagy, but rather of less total LC3 protein. Of note, the level of LC3-II protein in cells that were treated with palm-LPS only modestly increased with addition of bafilomycin, which suggests that lysosomal degradation was already impaired in these cells. The total amount of LC3 protein was also significantly decreased in macrophages that were treated with palm-LPS. In line with reduced levels of LC3 protein in LPS-treated groups, LC3 gene expression was also decreased by LPS. (Fig. 3B).
Figure 3. LPS activation in the presence of palmitate impairs autophagic flux.
(A) pMACs were stimulated as indicated for 16 h + 2 h vehicle vs. 50 nM bafilomycin (BAF) to assess autophagic flux. LC3 expression was analyzed by Western blotting. (B) Macrophages were stimulated as denoted and, expression of LC3 and p62 mRNA was assessed by using quantitative RT-PCR at indicated time points. (C–F) pMACs were stimulated with BSA-PBS (C), BSA-LPS (D), palm-PBS (E), or palm-LPS (F), and p62 protein was analyzed by immunofluorescence microscopy. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for PBS vs. LPS; #P < 0.05 for BSA vs. palm. Scale bar, 25 μm.
The chaperone protein p62 plays an important role in shuttling ubiquinated proteins and macromolecules to autophagosomes for lysosomal degradation [19]. When autophagic flux increases, the rate of p62 degradation in the lysosome also increases. The opposite is true if autophagy is decreased and/or lysosomes are dysfunctional, which leads to p62 accumulation. Expression of p62 protein is also regulated at the transcriptional level, which can confound the interpretation of autophagic flux assays. When pMACs were treated with LPS, p62 mRNA was rapidly but transiently induced, and p62 aggregates could be observed in the cytoplasm (Fig. 3B and D). In contrast, palmitate elicited a delayed increase in p62 mRNA and was associated with a more diffuse appearance of p62 protein in the cytoplasm (Fig. 3E). The combination of palmitate and LPS synergistically induced p62 mRNA and led to a massive accumulation of p62 protein that was diffuse throughout the cytosol (Fig. 3F). Thus, the combination of palmitate and LPS results in profound accumulation of p62, likely involving transcriptional up-regulation and impaired lysosomal degradation.
Impaired lysosome function in lipid-stressed macrophages
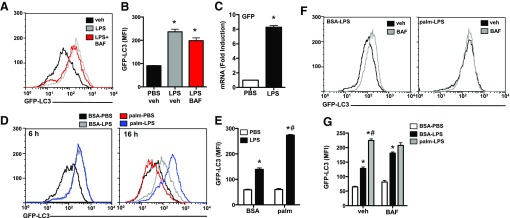
Data from LC3 and p62 analyses suggested that the combination of LPS and lipid stress altered autophagic flux, mainly at the level of the lysosome; however, transcriptional changes in both LC3 and p62 complicated this interpretation. As an alternate strategy to evaluate autophagic flux, we used mice that contained the GFP-LC3 transgene. Treatment of GFP-LC3 pMACs with LPS led to a large increase in GFP fluorescence that was not enhanced with bafilomycin, which suggested that LC3 protein was increasing but was not being delivered to lysosome at a high rate (Fig. 4A and B). This finding argued that either expression of GFP-LC3 was increasing or its degradation was decreasing in response to LPS. To investigate these possibilities, we quantified GFP mRNA expression by quantitative RT-PCR and discovered that LPS significantly increased GFP mRNA levels in primary macrophages (Fig. 4C). Despite this limitation, we reasoned that this system could still be used to determine the effects of palmitate on LC3 clearance in LPS-treated macrophages. As shown in Fig. 4, initial induction of GFP-LC3 was similar between LPS- and palm-LPS–treated macrophages at 6 h; however, by 16 h, there was significantly more GFP-LC3 in the palm-LPS condition (Fig. 4D and E). Palmitate by itself did not affect GFP-LC3 signal. To determine whether the increased GFP fluorescence observed under palm-LPS conditions was related to impaired lysosomal degradation of GFP-LC3, we treated macrophages with LPS or palm-LPS with or without bafilomycin. Inhibition of lysosome function increased GFP fluorescence in LPS-treated macrophages, but had no effect in palm-LPS–treated cells (Fig. 4F and G). In fact, in the presence of bafilomycin, LPS- and palm-LPS–treated cells were almost indistinguishable (Fig. 4G). Together with LC3 protein flux analysis, these data suggest that neither palmitate nor LPS significantly modulates autophagic flux independently, but in combination, these stimuli disrupt autophagy by inducing lysosome dysfunction.
Figure 4. Impaired lysosomal clearance of GFP-LC3 in lipid stressed macrophages.
(A and B) GFP-LC3 macrophages were stimulated PBS, LPS, or LPS with bafilomycin (BAF) for 6 h, and GFP fluorescence was quantified by flow cytometry. A representative histogram (A) and bar graph of triplicate samples (B) are shown. (C) GFP-LC3 pMACs were treated with PBS (white bar) or LPS (black bar) for 6 h, and GFP mRNA expression was assessed by quantitative RT-PCR. (D and E) GFP-LC3 pMACs were stimulated as indicated for 6 or 16 h, and LC3-GFP expression was assessed via flow cytometry. Representative histograms (D) or bar graph of triplicate samples at 16 h (E) are shown. (F and G) GFP-LC3 macrophages were treated with BSA-LPS or palm-LPS ± BAF for 16 h, and GFP fluorescence was quantified by flow cytometry. Representative histograms (F) or bar graph of triplicate samples (G) are shown. veh, vehicle. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for PBS vs. LPS; #P < 0.05 for BSA vs. palm.
mTOR inhibition increases autophagic flux in primary macrophages
When activated, mTOR strongly suppresses autophagy. As such, mTOR inhibitors are typically strong activators of autophagy. To determine the effect of mTOR inhibitor torin on autophagy in primary macrophages, cells were treated with vehicle or torin with or without bafilomycin, and LC3 and p62 protein levels were assessed. Consistent with induction of autophagy, LC3 decreased with torin and this was blocked by coincubation with bafilomycin (Fig. 5A). Moreover, p62 protein decreased with torin and its expression levels were largely recovered with inhibition of lysosomal degradation (Fig. 5A). Similar results were obtained by using macrophages from GFP-LC3 mice to quantify the effects of torin on autophagy. In these cells, torin treatment decreased GFP fluorescence and this was prevented by bafilomycin, which indicated that decrease in signal was related to lysosomal degradation of LC3-GFP (Fig. 5B and C). Moreover, immunofluorescence studies revealed increased LC3 puncta in torin-treated cells when lysosomal degradation was inhibited with bafilomycin (Fig. 5D–G). Thus, torin treatment induces autophagy in primary macrophages.
Figure 5. Inhibition of mTOR with torin stimulates autophagy in primary macrophages.
(A) pMACs were treated with vehicle (veh) or torin ± bafilomycin (BAF), and LC3 and p62 protein expression was assessed by Western blotting. (B and C) GFP-LC3 macrophages were treated with veh or torin ± BAF for 6 h, followed by GFP quantification by flow cytometry. A representative histogram (B) and bar graph of triplicates samples (C) are shown. (D–G) Immunofluorescence microscopy of GFP-LC3 in pMACs treated with the indicated stimuli. Densitometric quantification of bands is shown beneath the blots. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. #P < 0.05 for veh vs. torin. Scale bar, 50 μm.
Reduced cell death with mTOR inhibition is independent of autophagy
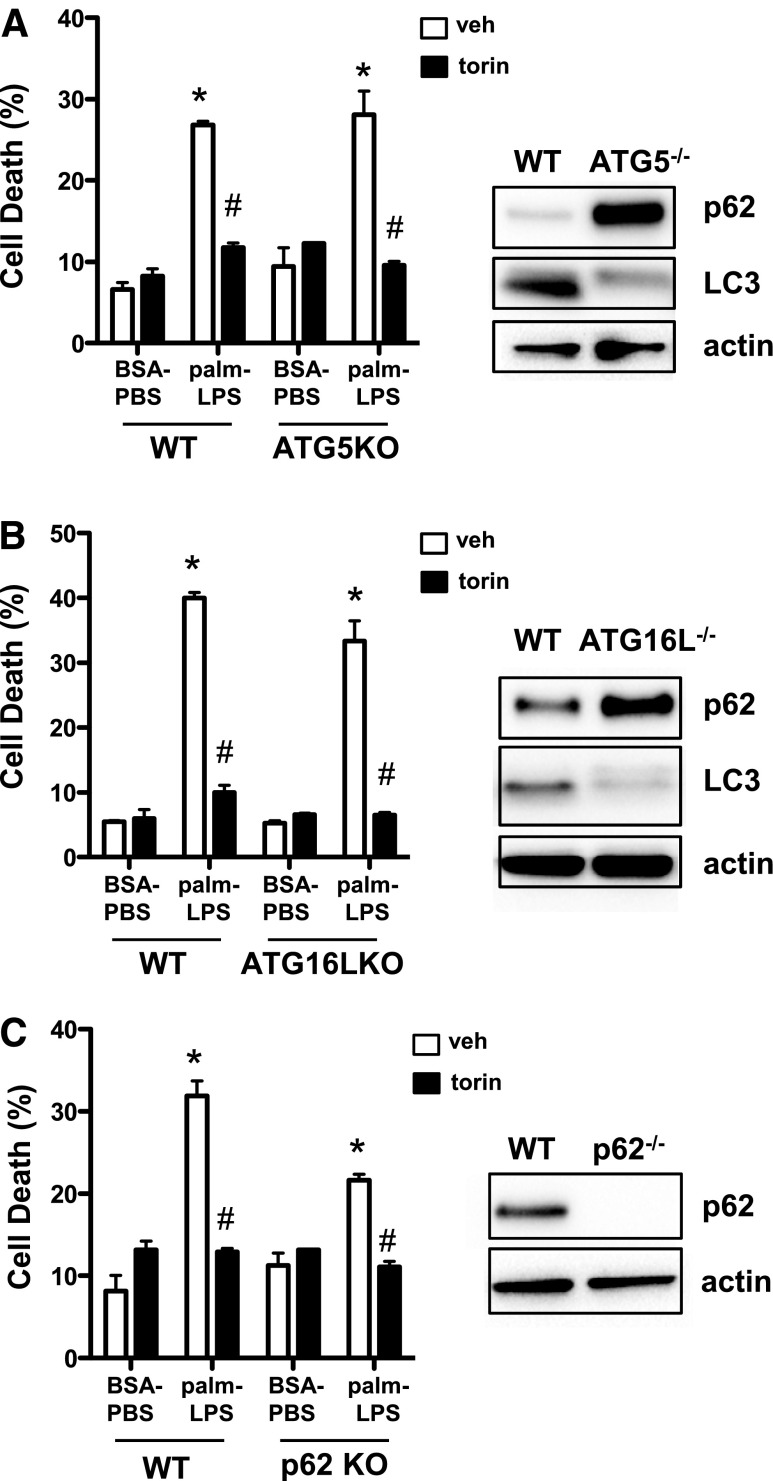
On the basis of the above findings, we hypothesized that torin might rescue lipotoxic cell death in macrophages by up-regulating autophagy and detoxifying the cell to prevent lysosome damage. To determine whether autophagy was required for the protective effect of torin on cell death, we used macrophages that were deficient in autophagy proteins ATG5, ATG16L, and p62. The degree of cell death in response to palm-LPS was similar in WT compared with ATG5 and ATG16L KO cells and was slightly reduced in cells that lacked p62 (Fig. 6A–C). However, torin treatment rescued cell death in all autophagy-deficient cells, despite immunoblot evidence of impaired autophagy, which suggests that mTOR inhibition is protective via an autophagy-independent pathway.
Figure 6. Rescue of lipotoxic cell death by mTOR inhibition occurs independent of autophagy.
(A) WT and ATG5KO pMACs were treated with BSA-PBS or palm-LPS ± torin, and cell death was assessed by annexin-PI flow cytometry. (B) WT and ATG16LKO pMACs were treated with BSA-PBS or palm-LPS ± torin, and cell death was assessed by annexin-PI flow cytometry. (C) WT and p62 KO pMACs were treated with BSA-PBS or palm-LPS ± torin, and cell death was assessed by annexin-PI flow cytometry. For each KO mouse, total protein was isolated and analyzed for p62 ± LC3 by Western blotting. veh, vehicle. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for BSA-PBS vs. palm-LPS; #P < 0.05 for veh vs. torin.
TFEB accumulates in the nucleus with mTOR inhibition but is not required for protection from cell death
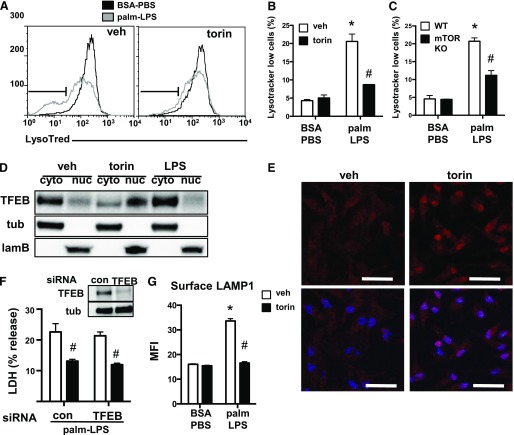
We have previously shown that lysosome dysfunction is involved in cell death that is triggered by lipid inflammatory stimuli [14]. To determine the impact of mTOR inhibition on lysosome damage, we performed lysotracker red staining on torin-treated or mTOR KO macrophages stimulated with palm-LPS. When incubated with vehicle alone, palm-LPS caused a significant downshift in macrophage lysotracker red staining and this response was almost completely prevented by torin (Fig. 7A and B). Similar protection was seen in mTOR-deficient macrophages (Fig. 7C). The fact that loss of mTOR was protective against lysosome dysfunction led us to investigate the role of TFEB. Phosphorylation of TFEB by mTORC1 prevents this transcription factor from moving to the nucleus and renders it inactive [20]. Thus, mTOR inhibition would be predicted to increase nuclear TFEB and enhance expression of genes that are involved in lysosome biogenesis and/or lysosome exocytosis. In light of our prior data that indicate that TFEB overexpression can attenuate cell death in response to palm-LPS, we hypothesized that mTOR inhibition could protect against cell death by activating TFEB [14].
Figure 7. TFEB is not required for the protective effect of torin on lipotoxic macrophage cell death.
(A) pMACs were stimulated with BSA-PBS or palm-LPS in the presence (right) or absence (left) of torin, and lysotracker red staining was assessed by flow cytometry. Lysotracker red low cell population is defined by the gate shown in black. (B and C) Bar graph quantification lysotracker red low staining cells from WT macrophages treated with vehicle (veh) or torin (B) or macrophages from mTOR flx/flx Cre negative vs. mTOR flx/flx LysM-Cre double-positive (mTOR KO) mice (C). (D) pMACs were treated with veh, torin, or LPS for 2 h, after which protein was isolated and cytosolic (cyto) vs. nuclear (nuc) fractionation was performed. TFEB distribution and fractionation controls [tubulin (tub) for cytosol, laminin B (lamB) for nucleus] were assessed by Western blotting. (E) Cells were treated with veh or torin for 2 h, after which they were stained with an antibody to TFEB (red) and imaged by fluorescence microscopy. Hoechst nuclear counterstaining (blue) is overlaid on TFEB staining in lower panels. (F) pMACs were transfected with control (con) or TFEB-specific siRNA pools and stimulated with palm-LPS ± torin. Degree of protein knockdown is shown in the figure inset. (G) The effect of torin on lysosome exocytosis was assessed by staining surface LAMP1 by using a fluorescently conjugated antibody on nonpermeablized cells, followed by flow cytometric quantification of mean fluorescence intensity (MFI). Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for BSA-PBS vs. palm-LPS; #P < 0.05 for veh vs. torin. Scale bar, 75 μm.
To test this hypothesis, we first determined whether torin treatment induced TFEB nuclear localization in macrophages. pMACs were treated with vehicle, torin, or LPS, and cellular localization of TFEB was assessed by protein fractionation and immunofluorescence. We found that TFEB was predominantly cytoplasmic at baseline, but moved to the nucleus upon treatment with torin (Fig. 7D). LPS treatment by itself further increased cytosolic TFEB, which was consistent with heightened mTORC1 activation. Nuclear translocation of TFEB was also visualized by immunofluorescence imaging of endogenous TFEB in macrophages that were exposed to torin (Fig. 7E). To determine whether this response was responsible for the protective effect of mTOR inhibiters against lipotoxic cell death, we performed siRNA knockdown of TFEB. Treatment with siRNA oligos against TFEB reduced protein levels by >75% compared with control siRNA; however, torin treatment still reduced cell death after stimulation with palm-LPS (Fig. 7F). Therefore, rescue of cell death by torin is independent of TFEB activation.
Lysosome exocytosis is often up-regulated in response to lysosome damage to facilitate clearance of cellular waste [21]. In fact, augmenting lysosome exocytosis can be protective in lysosomal storage diseases [22]. To determine the effect of mTOR inhibition on lysosome exocytosis, we quantified surface expression of the lysosome membrane protein LAMP1 by using flow cytometry. In vehicle-treated cells, surface LAMP1 expression increased with palm-LPS treatment, likely as an adaptive response to lysosome stress. In contrast, surface LAMP1 did not increase in torin-treated macrophages that were treated with palm-LPS (Fig. 7G). Thus, torin-mediated protection does not occur by enhancing lysosome exocytosis, but, rather, seems to act upstream of the lysosomal stress response.
mTOR inhibition alters macrophage metabolism
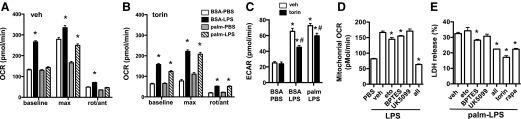
Activation of both mTOR complexes is also known to modulate cellular metabolism [23, 24]. To assess the impact of mTOR inhibition on macrophage metabolism, we treated primary macrophages with palmitate or LPS alone or in combination with or without torin, and measured mitochondrial oxidative function (OCR) and glycolysis by using a Seahorse Bioscience metabolic flux analyzer. At baseline and with maximal respiration, OCR was increased by LPS. Palmitate treatment by itself had very little effect on respiration; however, palmitate significantly blunted the LPS-induced OCR response. In contrast, cells treated with torin had decreased OCR at baseline compared with vehicle-treated cells. LPS still increased OCR in torin-treated macrophages, but the absolute magnitude remained less than that observed for vehicle-treated cells. Of interest, torin attenuated the suppressive effect of palmitate on LPS-induced increases in OCR. Similar profiles were observed at maximal respiration (Fig. 8A and B). We also assessed glycolytic rates by measuring the ECAR, a surrogate for glycolysis. LPS treatment significantly increased glycolysis, whether or not palmitate was present. At baseline, torin did not affect ECAR; however, in LPS- and palm-LPS–treated macrophages, torin reduced ECAR by ∼28 and 18%, respectively (Fig. 8C).
Figure 8. mTOR inhibition modulates macrophage mitochondrial metabolism.
(A and B) OCR was assessed via Seahorse Bioscience flux analyzer in macrophages stimulated as indicated for 16 h in presence of vehicle (veh; A) or torin (B) at baseline and after injection of flurorcarbonyl cynade phenylhydrazon (max), or rotenone/antimycin (rot/ant). (C) pMACS were stimulated as indicated in the presence of veh or torin for 16 h, and glycolytic rate was estimated by ECAR using a Seahorse Bioscience metabolic flux analyzer. (D) pMACs were stimulated with PBS or LPS, and mitochondrial OCR (total OCR − nonmitochondrial OCR) was measured at baseline and after injection of indicated metabolic inhibitors [UK5099 (pyruvate); etomoxir (eto; FA oxidation); BPTES (glutamine)]. (E) Macrophages were treated with palm-LPS in the presence of veh or the indicated metabolic inhibitors, and cell death was assessed by LDH release. rapa, rapamycin. Bar graphs report means ± se for a minimum of 3 experiments, each performed in triplicate. *P < 0.05 for BSA-PBS vs. palm-LPS or veh vs. inhibitor; #P < 0.05 for veh vs. torin.
To investigate whether suppression of mitochondrial metabolism could be related to the protective effects of mTOR inhibition in macrophages, we assessed substrate use in LPS-activated macrophages. To perform this analysis, we measured OCR in macrophages under baseline and LPS-stimulated conditions, then injected inhibitors of the 3 main mitochondrial respiratory substrates, glucose (UK5099), FAs (etomoxir), and glutamine (BPTES), alone or in combination. Macrophages were stimulated with LPS for 16 h, then drugs were injected on the Seahorse Bioscience machine to assess their immediate effects on mitochondrial metabolism. Both etomoxir and BPTES significantly reduced mitochondrial respiration, but the magnitude of the effect was modest. UK5099 by itself had no significant effect on OCR (Fig. 8D). Of interest, combining all 3 drugs decreased oxygen consumption by 62%, which was similar to the reduction observed with torin treatment (Fig. 8D). We next investigated the impact of these metabolic inhibitors on palm-LPS–induced macrophage cell death. For the individual compounds, only BPTES reduced cell death and its effect was modest; however, the combination of metabolic inhibitors significantly reduced cell death, and this response was similar to what was seen with mTOR inhibitors (Fig. 8E). Thus, reducing mitochondrial substrate flux protects against lipid-induced macrophage cell damage.
DISCUSSION
A growing body of evidence implicates lipid-induced macrophage dysfunction in development of diabetes complications after tissue injury or infection [4, 5, 14]; however, the molecular basis of macrophage toxicity remains unclear. In response to tissue damage, TLR agonists promote macrophage activation and inflammation [25–27]. This is followed by a resolution phase during which macrophages release anti-inflammatory molecules and proangiogenic factors. Diabetes is associated with increased circulating FAs, which leads to lipid accumulation in nonadipose cells, including macrophages. In prior studies, we have shown that macrophage activation by TLR4 agonists in an SFA-rich nutrient microenvironment promotes macrophage cell death via a pathway that involves lysosome damage and dysfunction [14, 15, 28]. To dissect the signaling pathways upstream of macrophage cell death, we performed a small-scale chemical screen of signaling inhibitors. By using this approach, we discovered that mTOR inhibitors, rapamycin and torin, attenuated macrophage lipotoxicity, which suggested a pathologic role for mTOR in the response to lipid stress.
mTOR kinase exists as 2 protein complexes, known as mTORC1 and mTORC2, each of which have distinct substrates [29]. The canonical targets of mTORC1 and mTORC2 are S6K and AKT, respectively. On one hand, torin is an ATP-competitive inhibitor of the mTOR catalytic site and, thus, inhibits both mTORC1 and mTORC2 [30]. Rapamycin, on the other hand, interacts with the mTOR kinase indirectly through FKBP12, which leads to selective inhibition of mTORC1; however, prolonged rapamycin exposure has been shown to also disrupt mTORC2 signaling in some cell types [31]. After treatment with LPS, both mTORC1 and mTORC2 are activated in pMACs. Given that both torin and rapamycin reduce lipotoxic macrophage cell death, we hypothesized that mTORC1 inhibition was more likely to mediate protection. Further support for this came from our genetic studies in which the extent of mTORC1 signaling inhibition directly correlated with protection. This was not observed for loss of mTORC2 signaling. Moreover, we also confirmed these findings by using raptor KO mice, in which mTORC1 signaling is selectively disrupted.
Although mTORC1 has many functions, it is a well-described regulator of autophagy. Autophagy is a highly conserved process that facilitates cell survival in times of nutrient deprivation and also plays a role in cell quality control through removal of dysfunctional proteins and organelles [32, 33]. In this study, we provide evidence that autophagy is disrupted at the level of the lysosome in macrophages that are exposed to palmitate and LPS by using Western blot analysis of autophagy markers and GFP-LC3 flux assays. Of interest, neither LPS nor palmitate altered autophagy in pMACs on their own. Relevant to the field, we also discovered that LPS treatment increases the level of GFP-LC3 mRNA in mice that harbor this transgene. This is likely to occur by TLR4-mediated activation of the CMV promoter that drives transgene expression. The observation that LPS and palmitate block autophagy at the step of lysosome degradation is consistent with our prior data that demonstrate that these stimuli induce lysosome damage [14, 16].
We hypothesized that inhibition of mTOR might activate autophagy and prevent accumulation of damaged proteins and organelles and thereby mitigate lysosome damage. In line with this predication, treatment with torin led to increased autophagic flux in primary macrophages; however, mTOR inhibitors still attenuated cell death, even in macrophages that were deficient in autophagy proteins ATG16L or ATG5, which are essential for autophagy. Thus, the protective effect of torin on macrophage cell death occurred independent of autophagy.
Another candidate protective molecule that can be activated as a consequence of mTOR inhibition is the transcription factor, TFEB. TFEB regulates the expression of genes that are involved in lysosome biogenesis, lysosome function, and autophagy [34]. Moreover, TFEB overexpression can reduce cell damage in the setting of lysosomal storage diseases or lipid stress [22, 35–37]. TFEB is phosphorylated mTORC1 and this event prevents TFEB from entering the nucleus and inducing transcription [38]. In this study, we demonstrated that torin treatment induces TFEB translocation to the nucleus; however, siRNA knockdown of TFEB failed to prevent the rescue of cell death by torin. Whether other members of the TFEB family are able to compensate for the loss of TFEB is not known, but this may be an area of future investigation. It also remains a possibility that torin could have lysosome-dependent, TFEB-independent effects that protect macrophages from cell death. One such mechanism is lysosome exocytosis, which is known to be protective in other lysosomal storage diseases [22]; however, lysosome exocytosis was not increased, but, in fact, was reduced in response to torin treatment, which suggests that mTOR inhibition protects at a point upstream of lysosome damage.
mTOR signaling is known to modulate cellular lipid metabolism and mitochondrial function [23, 39]. To investigate the influence of mTOR inhibition on macrophage glucose and mitochondrial metabolism, we performed Seahorse Bioscience metabolism assays coupled with inhibitor studies. Macrophage activation leads to a well-described increase in glycolysis, and consistent with this, we observed a significant increase in ECAR in pMACs that were stimulated with LPS. In line with prior studies that implicated mTORC1 activation in the induction of glycolysis, torin reduced LPS-stimulated glycolysis by ∼30% [40]. We also observed that LPS dramatically increased mitochondrial OCR in macrophages, and this response was significantly reduced by mTOR inhibition. Thus, as in other systems, mTOR signaling seems to augment mitochondrial oxidative capacity in macrophages [41–43]. It is important to note that these results are distinct from prior metabolic studies performed with bone marrow–derived macrophages in which LPS treatment inhibits mitochondrial respiration [44]; however, data from other human and mouse tissue macrophage systems have shown that LPS can enhance mitochondrial respiratory function [44, 45]. The reason for these discrepancies is not known, but may reflect differences in NO or ROS production, both of which can suppress mitochondrial oxidative function [46].
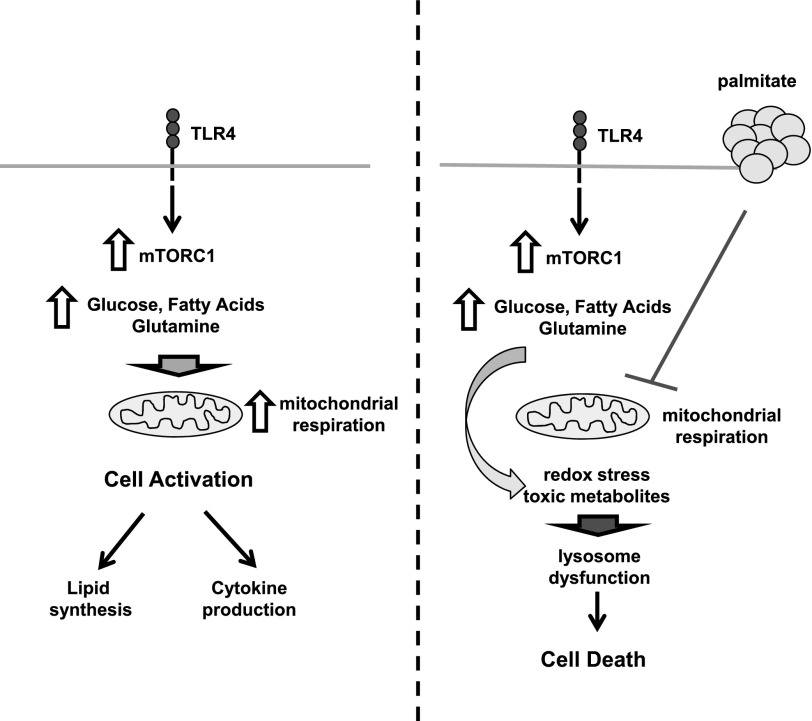
Our metabolic studies also revealed that palmitate suppresses the increase in mitochondrial OCR induced by LPS, but did not affect the enhanced glycolytic flux. This observation raised the possibility that excess SFAs, in combination with the enhanced flux of other oxidative substrates induced by LPS, could impair mitochondrial function. When this occurs with continued influx of metabolic substrates, the net effect would be metabolic gridlock in a manner analogous to a car accident on the highway during rush hour (Fig. 9). Such a situation could lead to accumulation of metabolic intermediates that produce lysosome damage directly or indirectly. Torin reversed the suppressive action of palmitate on mitochondrial metabolism and seems to restore balance of metabolic substrate influx with downstream mitochondrial use. Thus, turning down the rate of total cellular metabolism and mitochondrial respiration via mTORC1 inhibition may be protective against the toxicity of lipids in activated macrophages.
Figure 9. Model of mTOR signaling in macrophage lipotoxicity.
In response to TLR4 stimulation, mTOR is activated, which leads to enhanced mitochondrial metabolism of multiple substrates. This is an important event for cell activation that leads to increased cytokine production and lipid synthesis. When this occurs in the presence of excess SFAs, such as palmitate, mTORC1 activation still occurs, but downstream mitochondrial use of energetic substrates is suppressed, likely leading to accumulation of toxic metabolites and/or disrupted cell signaling. This imbalance of substrate entry and mitochondrial use could drive lysosome pathology and macrophage cell death.
Relevant to this concept, several recent studies of lipid-induced insulin resistance and diabetes suggest that toxicity from lipid excess occurs as a consequence of overloading mitochondria with energetic substrates [47]. In this situation, generation of acetyl-CoA exceeds the energetic demands and capacity of mitochondrial oxidative phosphorylation. When this occurs, excess ROS can be generated and/or metabolic intermediates accumulate [48]. Our previous studies excluded a role for ROS in pMACs, which suggests that other metabolic intermediates are likely responsible for toxicity [14]. Human and animal studies have also revealed a synergy between high-fat diet, branched-chain amino acid catabolism, and mTOR activation in the development of diabetes and its complications [49, 50].
On the basis of these data, we directly tested whether substrate overload might influence macrophage lipotoxicity by using compounds that block metabolism of the 3 primary fuels that feed mitochondrial oxidation, namely, glucose, FA, and glutamine. Although the individual blockade of these substrates did not significantly protect against palm-LPS, the combined inhibition of all 3 pathways significantly reduced cell death. In line with these results, we saw only a mild decrease in mitochondrial OCR after individual inhibition of glucose, glutamine, or FA metabolism; however, blockade of all 3 substrate pathways substantially decreased mitochondrial OCR. Taken together, these findings suggest that mTOR inhibition in LPS-activated, lipid-overloaded macrophages may prevent mitochondrial substrate overload via suppression of substrate flux into the mitochondria. As a consequence, accumulation of toxic metabolic intermediates would be diminished. Metabolomic studies are underway to identify specific molecules that accumulate in lipid-stressed macrophages and to determine their relationship to lysosome damage.
In this article, we provide evidence that inhibition of mTOR can protect inflammatory macrophages from the toxic effects of lipid excess by preventing lysosome damage. These findings fit into a growing body of literature that suggests that mTOR signaling in the context of excess lipids leads to accelerated development of obesity associated complications, including insulin resistance and atherosclerosis [49, 51]. Further research in vitro and in vivo will be necessary to more completely define the links between altered cellular metabolism and lysosome dysfunction in macrophages and to determine its relevance to human disease.
AUTHORSHIP
L.H. and K.J.W. conducted experiments. A.D. conceived of and interpreted experiments. J.D.S. conceived of experiments, interpreted experiments, and wrote the manuscript.
ACKNOWLEDGMENTS
This work was funded in part by a U.S. National Institutes of Health Grant KO8-HL09837305 (to J.D.S.). The authors thank Skip Virgin for the generous provision of ATG5KO, ATG16LKO, p62 KO, and LC3-GFP mice.
Glossary
- BPTES
bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethylsulfide
- ECAR
extracellular acidification rate
- FA
fatty acid
- KO
knockout
- LDH
lactate dehydrogenase
- LysM
lysozyme M
- mTOR
mechanistic target of rapamycin
- OCR
oxygen consumption rate
- palm-LPS
palmitate LPS
- pMAC
thioglycollate-elicited peritoneal macrophage
- ROS
reactive oxygen species
- siRNA
small interfering RNA
- SFA
saturated fatty acid
- TFEB
transcription factor EB
- UK5099
2-cyano-3-(1-phenyl-1H-indol-3-yl)-2-propenoic acid
- WT
wild type
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- 1.Jeffcoate W. J., Harding K. G. (2003) Diabetic foot ulcers. Lancet 361, 1545–1551. [DOI] [PubMed] [Google Scholar]
- 2.Joshi N., Caputo G. M., Weitekamp M. R., Karchmer A. W. (1999) Infections in patients with diabetes mellitus. N. Engl. J. Med. 341, 1906–1912. [DOI] [PubMed] [Google Scholar]
- 3.Muller L. M., Gorter K. J., Hak E., Goudzwaard W. L., Schellevis F. G., Hoepelman A. I., Rutten G. E. (2005) Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 41, 281–288. [DOI] [PubMed] [Google Scholar]
- 4.Khanna S., Biswas S., Shang Y., Collard E., Azad A., Kauh C., Bhasker V., Gordillo G. M., Sen C. K., Roy S. (2010) Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS One 5, e9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mirza R., Koh T. J. (2011) Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 56, 256–264. [DOI] [PubMed] [Google Scholar]
- 6.Hallgren B., Stenhagen S., Svanborg A., Svennerholm L. (1960) Gas chromatographic analysis of the fatty acid composition of the plasma lipids in normal and diabetic subjects. J. Clin. Invest. 39, 1424–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaffer J. E. (2003) Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 14, 281–287. [DOI] [PubMed] [Google Scholar]
- 8.Brookheart R. T., Michel C. I., Schaffer J. E. (2009) As a matter of fat. Cell Metab. 10, 9–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong B. X., Kyle R. A., Croft K. D., Quinn C. M., Jessup W., Yeap B. B. (2011) Modulation of macrophage fatty acid content and composition by exposure to dyslipidemic serum in vitro. Lipids 46, 371–380. [DOI] [PubMed] [Google Scholar]
- 10.Li W., Sama A. E., Wang H. (2006) Role of HMGB1 in cardiovascular diseases. Curr. Opin. Pharmacol. 6, 130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takeda K., Akira S. (2004) TLR signaling pathways. Semin. Immunol. 16, 3–9. [DOI] [PubMed] [Google Scholar]
- 12.Lee J. Y., Zhao L., Youn H. S., Weatherill A. R., Tapping R., Feng L., Lee W. H., Fitzgerald K. A., Hwang D. H. (2004) Saturated fatty acid activates but polyunsaturated fatty acid inhibits Toll-like receptor 2 dimerized with Toll-like receptor 6 or 1. J. Biol. Chem. 279, 16971–16979. [DOI] [PubMed] [Google Scholar]
- 13.Erridge C., Samani N. J. (2009) Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arterioscler. Thromb. Vasc. Biol. 29, 1944–1949. [DOI] [PubMed] [Google Scholar]
- 14.Schilling J. D., Machkovech H. M., He L., Diwan A., Schaffer J. E. (2013) TLR4 activation under lipotoxic conditions leads to synergistic macrophage cell death through a TRIF-dependent pathway. J. Immunol. 190, 1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schilling J. D., Machkovech H. M., He L., Sidhu R., Fujiwara H., Weber K., Ory D. S., Schaffer J. E. (2013) Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem. 288, 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber K., Schilling J. D. (2014) Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J. Biol. Chem. 289, 9158–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laplante M., Sabatini D. M. (2012) mTOR signaling in growth control and disease. Cell 149, 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y. C., Guan K. L. (2015) mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 125, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogov V., Dötsch V., Johansen T., Kirkin V. (2014) Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 53, 167–178. [DOI] [PubMed] [Google Scholar]
- 20.Settembre C., Fraldi A., Medina D. L., Ballabio A. (2013) Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat. Rev. Mol. Cell Biol. 14, 283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballabio A., Gieselmann V. (2009) Lysosomal disorders: from storage to cellular damage. Biochim. Biophys. Acta 1793, 684–696. [DOI] [PubMed] [Google Scholar]
- 22.Medina D. L., Fraldi A., Bouche V., Annunziata F., Mansueto G., Spampanato C., Puri C., Pignata A., Martina J. A., Sardiello M., Palmieri M., Polishchuk R., Puertollano R., Ballabio A. (2011) Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell 21, 421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamming D. W., Sabatini D. M. (2013) A Central role for mTOR in lipid homeostasis. Cell Metab. 18, 465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morita M., Gravel S. P., Hulea L., Larsson O., Pollak M., St-Pierre J., Topisirovic I. (2015) mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 14, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson U., Tracey K. J. (2011) HMGB1 is a therapeutic target for sterile inflammation and infection. Annu. Rev. Immunol. 29, 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L., Guo S., Ranzer M. J., DiPietro L. A. (2013) Toll-like receptor 4 has an essential role in early skin wound healing. J. Invest. Dermatol. 133, 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arslan F., Keogh B., McGuirk P., Parker A. E. (2010) TLR2 and TLR4 in ischemia reperfusion injury. Mediators Inflamm. 2010, 704202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weber K., Schilling J. D. (2014) Distinct lysosome phenotypes influence inflammatory function in peritoneal and bone marrow-derived macrophages. Int. J. Inflamm. 2014, 154936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laplante M., Sabatini D. M. (2012) mTOR Signaling. Cold Spring Harb. Perspect. Biol. 4, a011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Q., Kang S. A., Thoreen C. C., Hur W., Wang J., Chang J. W., Markhard A., Zhang J., Sim T., Sabatini D. M., Gray N. S. (2012) Development of ATP-competitive mTOR inhibitors. Methods Mol. Biol. 821, 447–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sarbassov D. D., Ali S. M., Sengupta S., Sheen J. H., Hsu P. P., Bagley A. F., Markhard A. L., Sabatini D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168. [DOI] [PubMed] [Google Scholar]
- 32.Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi A. M., Ryter S. W., Levine B. (2013) Autophagy in human health and disease. N. Engl. J. Med. 368, 651–662. [DOI] [PubMed] [Google Scholar]
- 34.Settembre C., Ballabio A. (2011) TFEB regulates autophagy: an integrated coordination of cellular degradation and recycling processes. Autophagy 7, 1379–1381. [DOI] [PubMed] [Google Scholar]
- 35.Spampanato C., Feeney E., Li L., Cardone M., Lim J. A., Annunziata F., Zare H., Polishchuk R., Puertollano R., Parenti G., Ballabio A., Raben N. (2013) Transcription factor EB (TFEB) is a new therapeutic target for Pompe disease. EMBO Mol. Med. 5, 691–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pastore N., Ballabio A., Brunetti-Pierri N. (2013) Autophagy master regulator TFEB induces clearance of toxic SERPINA1/α-1-antitrypsin polymers. Autophagy 9, 1094–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emanuel R., Sergin I., Bhattacharya S., Turner J. N., Epelman S., Settembre C., Diwan A., Ballabio A., Razani B. (2014) Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae. Arterioscler. Thromb. Vasc. Biol. 34, 1942–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roczniak-Ferguson A., Petit C. S., Froehlich F., Qian S., Ky J., Angarola B., Walther T. C., Ferguson S. M. (2012) The transcription factor TFEB links mTORC1 signaling to transcriptional control of lysosome homeostasis. Sci. Signal. 5, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jewell J. L., Guan K. L. (2013) Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 38, 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moon J. S., Hisata S., Park M. A., DeNicola G. M., Ryter S. W., Nakahira K., Choi A. M. (2015) mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Reports 12, 102–115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Morita M., Gravel S. P., Chénard V., Sikström K., Zheng L., Alain T., Gandin V., Avizonis D., Arguello M., Zakaria C., McLaughlan S., Nouet Y., Pause A., Pollak M., Gottlieb E., Larsson O., St-Pierre J., Topisirovic I., Sonenberg N. (2013) mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 18, 698–711. [DOI] [PubMed] [Google Scholar]
- 42.Ramanathan A., Schreiber S. L. (2009) Direct control of mitochondrial function by mTOR. Proc. Natl. Acad. Sci. USA 106, 22229–22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schieke S. M., Phillips D., McCoy J. P. Jr., Aponte A. M., Shen R. F., Balaban R. S., Finkel T. (2006) The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 281, 27643–27652. [DOI] [PubMed] [Google Scholar]
- 44.Tannahill G. M., Curtis A. M., Adamik J., Palsson-McDermott E. M., McGettrick A. F., Goel G., Frezza C., Bernard N. J., Kelly B., Foley N. H., Zheng L., Gardet A., Tong Z., Jany S. S., Corr S. C., Haneklaus M., Caffrey B. E., Pierce K., Walmsley S., Beasley F. C., Cummins E., Nizet V., Whyte M., Taylor C. T., Lin H., Masters S. L., Gottlieb E., Kelly V. P., Clish C., Auron P. E., Xavier R. J., O’Neill L. A. (2013) Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature 496, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu T. F., Vachharajani V., Millet P., Bharadwaj M. S., Molina A. J., McCall C. E. (2015) Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J. Biol. Chem. 290, 396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Everts B., Amiel E., van der Windt G. J., Freitas T. C., Chott R., Yarasheski K. E., Pearce E. L., Pearce E. J. (2012) Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood 120, 1422–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muoio D. M. (2014) Metabolic inflexibility: when mitochondrial indecision leads to metabolic gridlock. Cell 159, 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koves T. R., Ussher J. R., Noland R. C., Slentz D., Mosedale M., Ilkayeva O., Bain J., Stevens R., Dyck J. R., Newgard C. B., Lopaschuk G. D., Muoio D. M. (2008) Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 7, 45–56. [DOI] [PubMed] [Google Scholar]
- 49.Newgard C. B., An J., Bain J. R., Muehlbauer M. J., Stevens R. D., Lien L. F., Haqq A. M., Shah S. H., Arlotto M., Slentz C. A., Rochon J., Gallup D., Ilkayeva O., Wenner B. R., Yancy W. S. Jr., Eisenson H., Musante G., Surwit R. S., Millington D. S., Butler M. D., Svetkey L. P. (2009) A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 9, 311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhattacharya S., Granger C. B., Craig D., Haynes C., Bain J., Stevens R. D., Hauser E. R., Newgard C. B., Kraus W. E., Newby L. K., Shah S. H. (2014) Validation of the association between a branched chain amino acid metabolite profile and extremes of coronary artery disease in patients referred for cardiac catheterization. Atherosclerosis 232, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ai D., Jiang H., Westerterp M., Murphy A. J., Wang M., Ganda A., Abramowicz S., Welch C., Almazan F., Zhu Y., Miller Y. I., Tall A. R. (2014) Disruption of mammalian target of rapamycin complex 1 in macrophages decreases chemokine gene expression and atherosclerosis. Circ. Res. 114, 1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]