Review of autophagy’s role in cytoplasmic homeostasis, innate and adaptive immune processes, inflammation, and the latest advances toward understanding these processes.
Keywords: ATG, TRIM, IRGM, tuberculosis, Crohn's disease
Abstract
Autophagy is a fundamental biologic process that fulfills general and specialized roles in cytoplasmic homeostasis. The cell-autonomous antimicrobial functions of autophagy have been established in the macrophage. These cells and other leukocytes continue to be the cells of choice in studying autophagy in immunity and inflammation. This review uses several model examples that will be of interest to leukocyte and cell biologists alike. Furthermore, it comprehensively covers the subsystems in autophagy as they apply to all mammalian cells and incorporates the recent progress in our understanding of how these modules come together—a topic that should be of interest to all readers.
Introduction
Autophagy is a ubiquitous eukaryotic intracellular homeostatic process affecting all cell types in multicellular organisms [1], including mammals, where it was first observed morphologically [2–4]. This review will cover the mechanisms of autophagy as a pathway in mammalian cells, with the emphasis on aspects underlying the role of autophagy in innate immunity [5]. To make it immediately relevant for researchers working in the field of leukocyte biology, we introduce below the topic using an example of the role of autophagy, with both its promise and current controversies, in control of Mycobacterium tuberculosis, a microbe parasitizing the macrophage. Of note, the examination of how autophagy intersects with bacteria is of relevance for fundamental principles of autophagy. This is best appreciated from parallels that exist [6, 7] between autophagy of mitochondria, which are organelles of endosymbiotic bacterial origin and are one of the earliest recognized targets of autophagy [2], and autophagy of intracellular bacteria [8].
MACROPHAGES AND AUTOPHAGIC CONTROL OF M. tuberculosis
Our present intense interest in autophagy has its roots in the antecedent studies with leukocytes conducted in late 1990s [9] on the mechanism of M. tuberculosis phagosome maturation block in infected macrophages [10–12]. That prior work led to one major conclusion—that there was something amiss with the production and dynamics of PI3P on mycobacterial phagosomes, which in turn, arrested membrane trafficking and precluded maturation and progression to phagolysosomes, where M. tuberculosis could presumably be eliminated [9, 12]. At that time, we wondered whether there was any naturally occurring cellular process that could be co-opted to help with PI3P production on intracellular membranes. One of the prominent candidates considered was the process of autophagy, which is exquisitely dependent on PI3P generation, necessary for the massive membrane remodeling involved in autophagosome formation and their maturation into autolysosomes [13, 14]. Thus, we used physiologic, immunologic, and pharmacological inducers of autophagy, such as starvation, IFN-γ, and rapamycin, and found that these maneuvers enabled maturation of M. tuberculosis phagosomes into compartments with lysosomal properties [15]. Moreover, induction of autophagy endowed maturing phagosomes with robust mycobactericidal properties [15], with several candidate effector molecules and processes underlying this phenomenon [16–18]. These ex vivo studies with cultured macrophages have led to validations in murine models of tuberculosis with a role in both controlling bacteria but also, perhaps more importantly, in suppressing damaging inflammation dominated by prolonged IL-1 signaling, extended Th17 response, and excessive neutrophilic infiltration [19–23].
The above chronological recap of autophagy studies in the context of tuberculosis shows just one line of investigation concerning the role of autophagy in antimicrobial defense. In the context of numerous other infectious agents, including other bacteria such as Salmonella [7, 24, 25], streptococci [26], and viruses [27], autophagy has been shown to play a significant role and has been reviewed extensively [7, 26, 28]. It is also important to point out that many microbes have well-recognized adaptations to counter autophagy [28–30]. Possibly, a best molecularly defined example of the interference of intracellular bacteria with autophagy is the injection of a protease RavZ that enzymatically incapacitates lipidation of mAtg8 factors [31] described here in the later sections dealing with the autophagy subsystems. The existence of countermeasures in microbes directed at interference [28] or even exploitation of autophagy by certain microbes [32] further underscores the significance of autophagy as an innate defense mechanism with which pathogens have to contend.
AUTOPHAGIC CONTROL OF M. tuberculosis AND RECENT CONTROVERSIES
The prolonged neutrophilic response in autophagy-deficient animals and its role in pathology in mouse models of tuberculosis, first reported by Castillo et al. [19] and Watson et al. [20], have been confirmed in a recent study [23]. The latter study provides an in-depth, invaluable follow-up and raises additional important questions [23] to be subjects of future studies. First, this study reported data that the neutrophil phenotype may be independent of autophagy [23]. Nevertheless, ex vivo studies by others (Deretic and coworkers [19]) have demonstrated that excessive IL-1 activation by autophagy-deficient macrophages leads to Th17 polarization as a likely contributor to neutrophil-associated effects in vivo. This is in keeping with reports by numerous groups regarding the excess IL-1 activation secondary to loss of autophagy function observed both in vitro and in vivo in various models of inflammatory disease [33–35].
Furthermore, Kimmey et al. [23] reported data, and others [36] interpreted them as an indication that autophagy, as a pathway, may not matter for control of M. tuberculosis. Such proposals call for further investigations. Of relevance is that the above studies covered only the early stages of M. tuberculosis infection in a mouse model, whereas the modeling of tuberculosis infection in mice (that inherently control M. tuberculosis far better than humans) requires observations well beyond the early 80 d covered in the study in question [23]. As a contemporary illustration of this issue, one may want to consider the recently reported negative findings with cGAS when M. tuberculosis infection was monitored for only 100 d [37], whereas positive (albeit surprising data) on cGAS and control of M. tuberculosis have been reported in a simultaneously published study extending past the first 100 d of infection [38]. It should also be understood that the claims made as autophagy being insufficient to control M. tuberculosis [23, 36] may be limited to basal autophagy. In this context, it is relevant to recall that the initial studies showing the role of autophagy in defense against M. tuberculosis were based entirely on induced autophagy and not on its basal levels [15]. Furthermore, the inability of basal autophagy to suppress M. tuberculosis is potentially explained by the ability of M. tuberculosis to suppress innate levels of autophagy [39–45]. Most recent studies extend the repertoire of anti-autophagic mechanisms possessed by M. tuberculosis, showing that infection with the tubercle bacillus reprograms autophagy via microRNA-33 induction [46]. These considerations bring us back full circle where we started, underscoring the need for pharmacological intervention to induce autophagy [15]. This is important from a translational standpoint if the therapeutic potential of autophagy is to be realized in control of tuberculosis; such efforts are underway [47, 48].
AUTOPHAGY PATHWAY
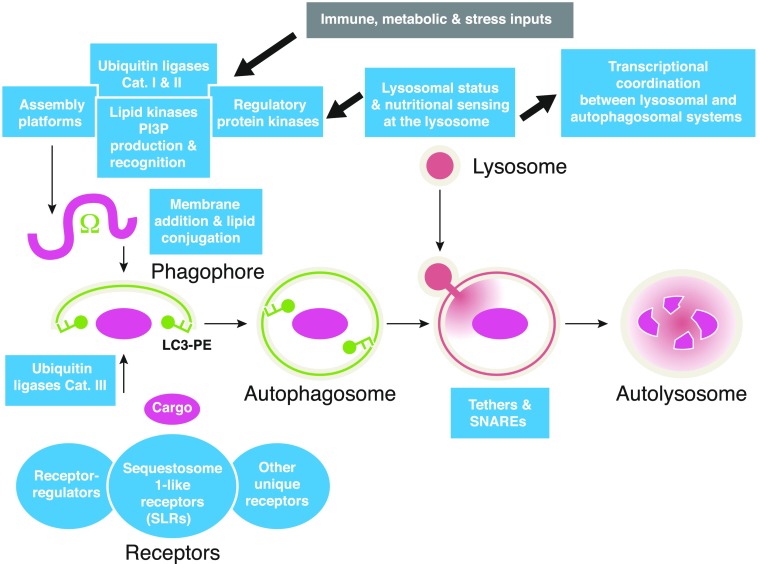
The role of autophagy in cellular physiology exceeds its immune functions. It maintains energy and nutrient homeostasis, carries out organellar repair or elimination when needed, and is among the key intracellular quality control processes of the eukaryotic cell [7, 49–51]. The sensu stricto form of autophagy (the only type covered herein and also known as “macroautophagy”) is a defined pathway dependent on conserved ATG proteins [52]. During autophagy, the cytoplasmic cargo is typically sequestered into specialized endomembranous organelles, termed autophagosomes, distinguished by a “popular” marker called LC3, which is 1 of the 6 mAtg8 homologs [53]. These organelles fuse with lysosomal compartments to form autolysosomes, where the captured material is degraded or otherwise processed [52], although functionally different terminations of the pathway are possible, such as secretion [54–56], reviewed recently elsewhere [57]. We cover here only the canonical autophagosomal pathway, terminating with autolysosomal organelles and cargo degradation. The core autophagy machinery in mammalian cells has several seemingly independent subsystems (Table 1), dissected in sections below. These subsystems are interconnected via specific molecular interactions into a unifying apparatus (Fig. 1).
TABLE 1.
Autophagy subsystems
| Subsystem | ATG and other components | Function | Notes | References |
|---|---|---|---|---|
| Regulatory Ser/Thr protein kinases and phosphatases | ULK1, AMPK, MK2, mTOR, calcineurin | Upstream kinases regulating activation of the VPS34-Beclin 1-ATG14L system; phosphatases regulating TFEB | ULK1 (mAtg1 ortholog) is the first autophagy pathway-dedicated protein kinase; mTOR and AMPK provide nutrient and energy inputs; MK2 transduces stress signals; mTOR phosphorylates TFEB; and calcineurin dephosphorylates it to transcriptionally activate the autophagy-lysosomal system. | [89–93, 102] |
| Lipid kinases and PI3P production | VPS34, Beclin 1, ATG14L | Localized PI3P generation leading to autophagosomal membrane biogenesis | ATG14L also connects with autolysosomal fusion via syntaxin 17. | [14, 83–85, 88] |
| PI3P recognition and recruitment of other ATG factors | WIPI2, DFCP1 | Connecting spatially PI3P production with building of autophagosomal membrane | WIPI2 binds both PI3P and ATG16L1, thus connecting PI3P production with mAtg8 conjugation. DFCP is a marker. | [14, 95] |
| Autophagosomal membrane identity and formation | mAtg8s: LC3A, LC3B, LC3C, GABARAP, GABARAPL1, GABARAPL2 | Roles in autophagic membrane biogenesis, closure, and cargo capture | LC3B used as autophagosome marker; GABARAPs scaffolding other ATG factors and cargo receptors | [76–79, 81, 132, 133, 141] |
| Membrane addition | ATG9 | In yeast, cooperation with Atg1 complex in pre-autophagosomal structure formation | Addition of new membrane to nascent phagosomes | [162–165] |
| “E3 ligase-like” system for mATG8 lipidation | ATG3, ATG4, ATG5, ATG7, ATG10, ATG12, ATG16L1 | Conjugation system for lipidation of mATG8 | ATG16L1 plays a central role in connecting mAtg8 lipidation with PI3P production through binding to WIPI2 and also connects with ULK1 via FIP200. | [94–98] |
| Autophagosome-lysosome fusion | Syntaxin 17, ATG14L | Control and execution of autophagosome-lysosome fusion | SNARE-mediated fusion between autophagosomes and lysosomes (syntaxin 17-SNAP29-VAMP8); ATG14L regulates syntaxin 17. | [87, 88] |
| SLRs | NDP52, TAXBP1, NBR1, optineurin, TOLLIP | Receptors recognizing tags (ubiquitin or galectin) on cargo | Binding to ubiquitin or galectin on cargo and to LC3 on autophagic membrane | [24, 125–128, 130] |
| Receptor-regulators | TRIM5, TRIM20, TRIM21 | Precision autophagy | Coupling of cargo recognition with recruitment and activation of ULK1, Beclin 1, and other ATGs | [132, 133, 141] |
| Other receptors | FUNDC1, Bcl-2-L-13, Nix, BNIP3, SMURF1, FAM134B, NCOA4 | Unique receptors thus far implicated in autophagy of different substrates | Receptors not falling into above categories (SLRs, receptor-regulators) | [59, 124, 135–140] |
| Assembly platforms | Exo84, IRGM | Coalescence of autophagy factors coupled with stimuli or cargo recognition | Exocyst, TRIMosome | [108, 132, 133, 142] |
| IRGM platform | ||||
| Ubiquitin ligases category I | AMBRA1 | Regulatory (promoting autophagy) | ULK1, Beclin 1, IRGM | [105–108] |
| TNFR-associated factor 6 | ||||
| Ubiquitin ligases category II | Cullin3-KLHL20, Nedd4 | Regulatory (inhibiting autophagy) | De-stabilization of positive regulators (VPS34, Beclin 1, ULK1, ATG14L, AMBRA, ATG4B, DEPTOR) and stabilization of negative regulators (Bcl-2) | [109–117] |
| RNF216 | ||||
| ZBTB16-Cullin3-Roc1 | ||||
| RNF2, DDB1/Cullin4 | ||||
| RNF5 | ||||
| SCF, Cullin5 | ||||
| Parkin | ||||
| Ubiquitin ligases category III | Parkin, LRSAM1, SMURF1 | Targeting (placing ubiquitin tags on autophagic cargo) | Mitophagy, xenophagy | [21, 118–124] |
| Coordination between autophagosomal and lysosomal systems via transcriptional control | TFEB, MiT/TEF | TFEB translocates from the cytoplasm to the nucleus to coactivate autophagy and lysosomal expression | TFEB and other MiT/TEF couple mTOR-nutrition and calcineurin-Ca2+ sensory systems on lysosomes with expression of autophagosomal/lysosomal systems | [100–104] |
DFCP1, Double FYVE domain-containing protein 1.
Figure 1. Autophagy pathway with its regulatory and execution subsystems.
Depicted are the simplified conventional views of canonical macroautophagy organelles and pathway with different subsystems detailed in Table 1. Ω, Omegasome, a PI3P-positive structure on the ER believed to provide a cradle for formation of phagophores positive for mAtg8 orthologs (e.g., LC3-PE, also known as LC3-II). Phagophores enwrap the cargo, enlarge, and close to form double-membrane autophagosomes that then fuse with lysosomes to form autolysosomes, where the captured cargo is degraded. Cargo is brought into the phagophores via receptors that recognize cargo through binding to tags, such as ubiquitin, placed on the cargo by E3 ubiquitin ligases (category III; see Table 1). Protein kinases, lipid kinases, and assembly platforms (detailed in Table 1) drive the formation of autophagosomes. These are regulated by E3 ubiquitin ligases that either stabilize (category I) or destabilize (category II) them. The entire system is regulated by signals that can be immunologic, nutritional, differentiation in nature, or from various stressors. Lysosome is not just a passive contributor of lysosomal contents but also works as a sensory organelle that relays the status to the nucleus, whereby autophagosomal and lysosomal biogenesis are coordinated at the transcriptional level.
Among many cytoplasmic targets of autophagy are mitochondria (the process being termed “mitophagy” [7], protein aggregates (“aggrephagy”) [58], ER (“ER-phagy”) [59], lipid droplets (“lipophagy”) [60], lysosomes (“lysophagy”) [61], and invading microbes (“xenophagy”) [29] (Fig. 1). The relationships between autophagosomes and membranous compartments, such as ER, mitochondria, and lipid droplets/neutral lipid stores, are complex, and whereas autophagy consumes these as targets through lysosomal degradation, these organelles also contribute membranes or lipids as sources for formation and growth of newly formed autophagosomes [49, 62–65].
Autophagy is active in all eukaryotic cells, and in mammals, it can play both nutritional and cytoplasmic quality control roles, which is of relevance for function and survival of nearly all cells. Although autophagy has been considered on and off a type of programmed cell death, it is now primarily appreciated as a programmed cell survival [66], with some potential crossover connections with cell death [67, 68]. With such broad fundamental roles, autophagy affects both the functionality and pathology, exerted through numerous cell types, including leukocytes. This is reflected in its role in cancer, where it is proposed to prevent tumorigenesis [69], but also sustains growth of established tumors [70], myodegeneration, and muscle wasting. Autophagy can also be associated with cancer-related cachexia [71]; loss of muscle stem cell-regenerative capacity in aging [72]; metabolic diseases (including type II diabetes [73] that may include role of autophagy in myeloid cells [74]); neurodegeneration [51]; inflammatory disorders, such as Crohn’s disease; and numerous other pathologic states [75].
AUTOPHAGY CORE SUBSYSTEMS
The 6 mAtg8 homologs (LC3A, -B, and -C, GABARAP, and GABARAPL1 and -2), are C-terminally lipidated with PE, a process facilitated by the ATG5–ATG12/ATG16L1 E3 ligase-like system. The lipid modification enables the membrane association of mAtg8 [76]. Different mAtg8s may play distinct roles in autophagosomal membrane biogenesis [77]. Moreover, mAtg8s also bind and scaffold other ATG factors, including ULK1 [78, 79] (one of several mAtg1 paralogs [80]) and autophagy cargo receptors [81].
As already introduced, PI3P production is key for autophagy [13, 14], albeit another monophosphoinositide (PI5P) has recently been suggested to play a role, especially during glucose starvation [82]. The production of PI3P is accomplished through the action of the PI3K VPS34 complex containing Beclin 1 [83] and ATG14L [84]. ATG14L confers membrane curvature preferences upon VPS34, compatible with autophagosomal formation [85, 86]. ATG14L also interacts with a key SNARE, syntaxin 17, of significance for autophagosome formation and maturation [65, 87, 88]. The production of PI3P occurs on membrane precursors yielding nascent autophagosomes [14].
The Ser/Thr kinase ULK1 phosphorylates and activates Beclin 1 [89], along with other kinases, such as MK2 [90] and AMPK [91]. The nutritional and possibly other signals are transmitted via AMPK, which positively regulates ULK1 [92], and via mTOR, which phosphorylates ULK1 on inactivating regulatory sites [92, 93].
The linchpins connecting the above systems are WIPI2 and ATG16L1. WIPI2 recognizes PI3P (the product of the human VPS34-Beclin 1-ATG14L complex) on membranes, whereas it also interacts with ATG16L1, a key component of the mAtg8/LC3 lipid conjugation system [94], thus spatially connecting LC3 conjugation with PI3P production [95]. ATG16L1 is also a binding partner for FIP200, a component of the ULK1 complex [95–98], ensuring that all core subsystems are coming together (Table 1 and Fig. 1).
Final steps of autophagy include fusion of autophagosomes with lysosomes to form autolysosomes [99]. Autophagy is transcriptionally coordinated with lysosomal biogenesis via TFEB and other MiT/TEF factors [100–104]. At the membrane-trafficking level, among other processes, syntaxin-17 [87], in cooperation with ATG14L [88], authorizes fusion between autophagosomes and lysosomes, leading to formation of autolysosomes, where the cargo is degraded (Table 1 and Fig. 1).
UBIQUITIN LIGASES AND AUTOPHAGY
Autophagy interacts with the ubiquitin system. These overlaps can be classified into 3 major categories (Table 1): 1) category I refers to ubiquitination as a positive regulator (often through stabilization) of autophagy factors. Ubiquitination can stabilize Beclin 1 [105, 106], ULK1 [107], and IRGM [108] to promote autophagy. 2) Category II, by far the most numerous, refers to E3 ubiquitin ligases and ubiquitination, acting to down-regulate autophagy through proteasomal degradation of Beclin 1 [109–111], ULK1 [111], ATG14L [111, 112], ATG4B [113], and AMBRA [114, 115], stabilizing Bcl-2 [116], a negative regulator of Beclin 1, or destabilizing the negative regulator of mTOR DEPTOR [114, 117]. 3) Category III refers to cargo targeting [50]. This includes the well-established case of the Pink 1–Parkin system in mitophagy. Parkin is the E3 ligase, which is activated by Pink 1-dependent phosphorylation of the ubiquitin-like domain within Parkin and ubiquitin, conjugated to targets [118–122]. Parkin has also been implicated in M. tuberculosis control by autophagy [20, 21]. Two additional E3 ligases, LRSAM1 in autophagy of Salmonella [123] and SMURF1 in autophagy of the Sindbis virus capsid [124], have been reported.
SELECTIVE AUTOPHAGY: SLRs, TRIMs, AND OTHER UNIQUE RECEPTORS
Autophagy can be bulk or selective. During the latter, selective form of autophagy, the cargo to be captured by the autophagosomes is modified with tags, such as galectins [7, 125], ubiquitin (category III described above) [50], or phosphorylated ubiquitin [120]. These tags can then be recognized by SLRs [28, 81]. The founding member of SLRs is sequestosome 1 (commonly referred to as p62) [126]. SLRs include NDP52, TAXBP1, NBR1, and optineurin [24, 125–129]. SLRs bind both to mAtg8s through their LC3-interacting motifs and to ubiquitin via a variety of ubiquitin-binding domains (e.g., ubiquitin binding in ABIN and NEMO domain, ubiquitin-associated domain, ubiquitin-binding zinc finger) [50]. TOLLIP, the most recently described SLR, binds to ubiquitin and LC3 [130].
There are close to 80 TRIMs in humans and at least one subset of them acts as selective autophagy receptors. The TRIM protein family [131] members contain N-terminal RING domain, B-box domains, a coiled-coil domain, and a C-terminal domain, such as SPRY, involved in binding to autophagic cargo [132, 133]. TRIM5, TRIM20, and TRIM21 bind mAtg8s [132, 133], while recognizing cargo via their SPRY (TRIM5, TRIM21) or Pyrin (TRIM20) domains. TRIM5 recognizes viral capsids within the retroviral core and delivers the capsid protein p24 (CA) for degradation [134]. TRIM20 is a selective autophagy receptor for the inflammasome components, procaspase 1, NLRP1, and NLRP [132]. TRIM21 is a selective autophagy receptor for the dimerized (activated) form of IFN regulator factor 3, a transcriptional activator of the type I IFN response [132].
Unique autophagy receptors, not belonging to SLRs or TRIMs, also exist. In addition to SLRs [129], several unique autophagy receptors work in mitophagy: FUNDC1 [135], Bcl-2-L-13 [136], Nix [137], BNIP3 [138] and SMURF1 [124]. ER-phagy is carried out by FAM134B [59]. NCOA4 is a receptor for autophagy of ferritin [139, 140].
PLATFORMS FOR AUTOPHAGY APPARATUS ASSEMBLY
The TRIM proteins, in addition to their function as autophagic receptors, also function as platforms for assembly of autophagy factors, including Beclin 1, ULK1, and mAtg8s [132, 133]. Their double duty as regulators and receptors has lead to the concept of "precision autophagy" [132, 141], whereby the same entity, e.g., TRIM, which recognizes autophagic substrate, also assembles autophagy machinery. This type of autophagy occurs only at the location where it is needed by coupling cargo recognition with a localized activation of autophagy apparatus.
Other proteins or protein complexes have been implicated in assembly of autophagy machinery in mammalian cells. For example, exocyst, a multicomponent complex, may scaffold starvation-induced autophagy [142], whereas IRGM (see below for more details) acts as a platform for assembly of ULK1, Beclin 1, and ATG16L1 in response to the presence of microbial products [108].
COUPLING BETWEEN INNATE IMMUNITY RECEPTORS AND AUTOPHAGY: THE NOD2-IRGM-ATG16L1 EXAMPLE
Autophagy responds to endogenous danger-associated molecular patterns and microbial products commonly referred to as PAMPs [5]. Autophagy triggered by these agonists can eliminate microbes through a process termed xenophagy or can control inflammation and other immune processes [5, 27]. Connections among PRRs, TLRs, cGAS, receptor-interacting serine/threonine–protein kinase 2, NLRs, etc., have been established and reviewed [5, 27, 28]. Among the more recently delineated systems explaining how recognition of PAMPs via PRRs results in activation of autophagy is the NOD2-IRGM-ATG16L1 complex [108]. Polymorphisms in the NOD2, ATG16L1, and IRGM genes all confer risk for Crohn’s disease [143–146], whereas polymorphisms in IRGM are associated with a risk for active tuberculosis [147, 148].
It has been known that ATG16L1 and NOD2 interact [149, 150], placing these 2 of the Crohn’s disease genetic risk factors together, but until recently, IRGM was not firmly linked to these factors. A complicating factor in understanding the exact function of IRGM has been that it is a distinctly human gene [151], with orthologs present only in African great apes and active alleles absent in the ancestral lineages [151]. The mouse has 21 IRG genes, as opposed to the distantly related single IRGM gene in humans, encoding a Ras-sized, 21 kDa protein. However, the prevailing past view of the murine IRGs (which all encode 40 kDa proteins) is that they have predominantly nonautophagy functions [152]. Nevertheless, this remains to be examined further, as mutant mice with inactivated Irgm1 (the presumed closest mouse homolog of human IRGM [151, 153]) also show an inflammatory bowel disease phenotype, along with the ATG16L1 hypomorph [154] or knockout mice [33], as well as the NOD2 mutant mice [155]. However, the latest studies indicate that murine Irgm1 [156] causes mitochondrial fission, similarly to human IRGM [157, 158], and that as in the case of IRGM controlling autophagy [157], Irgm1 affects autolysosomal processing [159].
As mentioned in the subsection on autophagy platforms, IRGM interacts with key autophagy regulators, ULK1, Beclin 1, ATG14L, and ATG16L1 [108]. Colocalization in cells between IRGM and ATG16L1 upon NOD2 activation on M. tuberculosis-harboring phagosomes has been observed by independent studies [160], in keeping with the previously reported NOD2–ATG16L1 colocalization in control of microbially stimulated autophagy [149, 150]. Furthermore, IRGM interacts with NOD2 and physically links NOD2 and other microbial sensors (such as NOD1 and a subset of TLRs) with the core autophagic apparatus assembled on IRGM as a platform [108]. These findings clarify how IRGM works. Nevertheless, as a cautionary note, ATG16L1 has been reported to have a genetically separable autophagy-independent function in suppressing inflammation [161], and thus, the above factors likely play multifactorial roles.
CONCLUSIONS
Autophagy is significant, both as a fundamental intracellular homeostatic pathway and as an antimicrobial and anti-inflammatory process of particular significance for leukocyte function. Recent advances have revealed how core autophagy factors become organized in subsystems and how these subsystems come together into a fully functional unit. Furthermore, we now know how these parts are coupled with immune signaling. The number of autophagy receptors and platforms for assembly of the autophagic apparatus in mammalian cells is growing and suggests versatility while increasingly emphasizing selectivity. The function of these systems in leukocytes deserves continuing in-depth attention and may provide unique opportunities for physiologic, immunologic, and pharmacological intervention in infectious and inflammatory diseases.
AUTHORSHIP
V.D. conceived of the review, analyzed the literature, wrote the text, and created the table and figure.
ACKNOWLEDGMENTS
The work in the V.D. laboratory is supported by U.S. National Institutes of Health Grants AI042999, AI111935, and UH2AI122313. The author thanks all past and present trainees and collaborators.
Glossary
- AMBRA1
autophagy and coiled-coil, moesin-like B-cell lymphoma 2-interacting protein regulator 1
- AMPK
AMP-activated protein kinase
- ATG
autophagy-related protein
- Bcl-2
B-cell lymphoma 2
- Bcl-2-L-13
Bcl2-like 13
- Beclin 1
coiled-coil, moesin-like B-cell lymphoma 2-interacting protein
- BNIP3
B-cell lymphoma 2 and adenovirus E1B 19 kDa-interacting protein 3
- cGAS
cyclic GMP-AMP synthase
- DEPTOR
domain containing mammalian target of rapamycin-interacting protein
- ER
endoplasmic reticulum
- FAM134B
family with sequence similarity 134 member B
- FIP200
focal adhesion kinase family interacting protein of 200 kDa
- FUNDC1
FUN14 domain-containing 1
- GABARAP
GABA receptor-associated protein
- GABARAPL1/2
GABA receptor-associated protein-like 1/2
- IRGM
immunity- related GTPase M
- LC3
microtubule-associated protein 1A/1B-light chain 3
- LRSAM1
leucine-rich repeat and sterile alpha motif containing 1
- mAtg8
mammalian autophagy-related protein 8 protein (microtubule-associated protein 1A/1B-light chain 3A, -B, -C, GABA receptor-associated protein, -like 1, -like 2)
- MiT/TEF
microphthalmia/transcription factor E family
- MK2
MAPK-activated protein kinase 2
- mTOR
mammalian target of rapamycin
- NBR1
neighbor of BRCA1 gene 1
- NCOA4
nuclear receptor coactivator 4
- NDP52
nuclear dot protein 52 kDa
- Nix/BNIP3L
BCL2 and adenovirus E1B 19-kDa interacting protein 3-like
- NLRP
nucleotide-binding oligomerization domain-containing protein-like receptor protein
- NOD
nucleotide-binding oligomerization domain-containing protein
- PAMP
pathogen-associated molecular pattern
- PE
phosphatidylethanolamine
- PI3P
phosphatidylinositol 3-phosphate
- Pink 1
phosphatase and tensin homolog-induced kinase 1
- PRR
pattern recognition receptor
- SLR
sequestosome 1/p62-like receptor
- SMURF1
drosophila mothers against decapentaplegic protein-ubiquitination-related factor 1
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- SPRY
Spla and the ryanodine receptor domain
- TAX1BP
T cell leukemia virus type I-binding protein
- TFEB
transcription factor EB
- TOLLIP
Toll-interacting protein
- TRIM
tripartite motif family of proteins
- ULK
Unc-51-like autophagy activating kinase
- VPS34
vacuolar protein sorting 34
- WIPI2
WD repeat domain phosphoinositide-interacting protein 2
DISCLOSURES
The author declares no conflicts of interest.
REFERENCES
- 1.Mizushima N., Komatsu M. (2011) Autophagy: renovation of cells and tissues. Cell 147, 728–741. [DOI] [PubMed] [Google Scholar]
- 2.Ashford T. P., Porter K. R. (1962) Cytoplasmic components in hepatic cell lysosomes. J. Cell Biol. 12, 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deter R. L., De Duve C. (1967) Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J. Cell Biol. 33, 437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deter R. L., Baudhuin P., De Duve C. (1967) Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J. Cell Biol. 35, C11–C16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deretic V., Kimura T., Timmins G., Moseley P., Chauhan S., Mandell M. (2015) Immunologic manifestations of autophagy. J. Clin. Invest. 125, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deretic V. (2010) Autophagy of intracellular microbes and mitochondria: two sides of the same coin? F1000 Biol. Rep. 2, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randow F., Youle R. J. (2014) Self and nonself: how autophagy targets mitochondria and bacteria. Cell Host Microbe 15, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deretic V. (2010) Autophagy in infection. Curr. Opin. Cell Biol. 22, 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vergne I., Chua J., Deretic V. (2003) Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198, 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong J. A., Hart P. D. A. (1971) Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J. Exp. Med. 134, 713–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sturgill-Koszycki S., Schlesinger P. H., Chakraborty P., Haddix P. L., Collins H. L., Fok A. K., Allen R. D., Gluck S. L., Heuser J., Russell D. G. (1994) Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263, 678–681. [DOI] [PubMed] [Google Scholar]
- 12.Vergne I., Chua J., Singh S. B., Deretic V. (2004) Cell biology of Mycobacterium tuberculosis phagosome. Annu. Rev. Cell Dev. Biol. 20, 367–394. [DOI] [PubMed] [Google Scholar]
- 13.Petiot A., Ogier-Denis E., Blommaart E. F., Meijer A. J., Codogno P. (2000) Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J. Biol. Chem. 275, 992–998. [DOI] [PubMed] [Google Scholar]
- 14.Axe E. L., Walker S. A., Manifava M., Chandra P., Roderick H. L., Habermann A., Griffiths G., Ktistakis N. T. (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 182, 685–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutierrez M. G., Master S. S., Singh S. B., Taylor G. A., Colombo M. I., Deretic V. (2004) Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell 119, 753–766. [DOI] [PubMed] [Google Scholar]
- 16.Ponpuak M., Davis A. S., Roberts E. A., Delgado M. A., Dinkins C., Zhao Z., Virgin H. W. IV, Kyei G. B., Johansen T., Vergne I., Deretic V. (2010) Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity 32, 329–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alonso S., Pethe K., Russell D. G., Purdy G. E. (2007) Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc. Natl. Acad. Sci. USA 104, 6031–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim B. H., Shenoy A. R., Kumar P., Das R., Tiwari S., MacMicking J. D. (2011) A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332, 717–721. [DOI] [PubMed] [Google Scholar]
- 19.Castillo E. F., Dekonenko A., Arko-Mensah J., Mandell M. A., Dupont N., Jiang S., Delgado-Vargas M., Timmins G. S., Bhattacharya D., Yang H., Hutt J., Lyons C. R., Dobos K. M., Deretic V. (2012) Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc. Natl. Acad. Sci. USA 109, E3168–E3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson R. O., Manzanillo P. S., Cox J. S. (2012) Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150, 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manzanillo P. S., Ayres J. S., Watson R. O., Collins A. C., Souza G., Rae C. S., Schneider D. S., Nakamura K., Shiloh M. U., Cox J. S. (2013) The ubiquitin ligase Parkin mediates resistance to intracellular pathogens. Nature 501, 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bonilla D. L., Bhattacharya A., Sha Y., Xu Y., Xiang Q., Kan A., Jagannath C., Komatsu M., Eissa N. T. (2013) Autophagy regulates phagocytosis by modulating the expression of scavenger receptors. Immunity 39, 537–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmey J. M., Huynh J. P., Weiss L. A., Park S., Kambal A., Debnath J., Virgin H. W., Stallings C. L. (2015) Unique role for ATG5 in neutrophil-mediated immunopathology during M. tuberculosis infection. Nature 528, 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., Richter B., Korac J., Waidmann O., Choudhary C., Dötsch V., Bumann D., Dikic I. (2011) Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perrin A. J., Jiang X., Birmingham C. L., So N. S., Brumell J. H. (2004) Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr. Biol. 14, 806–811. [DOI] [PubMed] [Google Scholar]
- 26.Shibutani S. T., Saitoh T., Nowag H., Münz C., Yoshimori T. (2015) Autophagy and autophagy-related proteins in the immune system. Nat. Immunol. 16, 1014–1024. [DOI] [PubMed] [Google Scholar]
- 27.Levine B., Mizushima N., Virgin H. W. (2011) Autophagy in immunity and inflammation. Nature 469, 323–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deretic V., Saitoh T., Akira S. (2013) Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 13, 722–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deretic V., Levine B. (2009) Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5, 527–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Virgin H. W., Levine B. (2009) Autophagy genes in immunity. Nat. Immunol. 10, 461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Choy A., Dancourt J., Mugo B., O’Connor T. J., Isberg R. R., Melia T. J., Roy C. R. (2012) The Legionella effector RavZ inhibits host autophagy through irreversible Atg8 deconjugation. Science 338, 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niu H., Xiong Q., Yamamoto A., Hayashi-Nishino M., Rikihisa Y. (2012) Autophagosomes induced by a bacterial Beclin 1 binding protein facilitate obligatory intracellular infection. Proc. Natl. Acad. Sci. USA 109, 20800–20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saitoh T., Fujita N., Jang M. H., Uematsu S., Yang B. G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., Akira S. (2008) Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456, 264–268. [DOI] [PubMed] [Google Scholar]
- 34.Zhou R., Yazdi A. S., Menu P., Tschopp J. (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225. [DOI] [PubMed] [Google Scholar]
- 35.Nakahira K., Haspel J. A., Rathinam V. A., Lee S. J., Dolinay T., Lam H. C., Englert J. A., Rabinovitch M., Cernadas M., Kim H. P., Fitzgerald K. A., Ryter S. W., Choi A. M. (2011) Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Behar S. M., Baehrecke E. H. (2015) Tuberculosis: autophagy is not the answer. Nature 528, 482–483. [DOI] [PubMed] [Google Scholar]
- 37.Watson R. O., Bell S. L., MacDuff D. A., Kimmey J. M., Diner E. J., Olivas J., Vance R. E., Stallings C. L., Virgin H. W., Cox J. S. (2015) The cytosolic sensor cGAS detects Mycobacterium tuberculosis DNA to induce type I interferons and activate autophagy. Cell Host Microbe 17, 819–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins A. C., Cai H., Li T., Franco L. H., Li X. D., Nair V. R., Scharn C. R., Stamm C. E., Levine B., Chen Z. J., Shiloh M. U. (2015) Cyclic GMP-AMP synthase is an innate immune DNA sensor for Mycobacterium tuberculosis. Cell Host Microbe 17, 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zullo A. J., Lee S. (2012) Mycobacterial induction of autophagy varies by species and occurs independently of mammalian target of rapamycin inhibition. J. Biol. Chem. 287, 12668–12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim K. H., An D. R., Song J., Yoon J. Y., Kim H. S., Yoon H. J., Im H. N., Kim J., Kim do J., Lee S. J., Kim K. H., Lee H. M., Kim H. J., Jo E. K., Lee J. Y., Suh S. W. (2012) Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP-7. Proc. Natl. Acad. Sci. USA 109, 7729–7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin D. M., Jeon B. Y., Lee H. M., Jin H. S., Yuk J. M., Song C. H., Lee S. H., Lee Z. W., Cho S. N., Kim J. M., Friedman R. L., Jo E. K. (2010) Mycobacterium tuberculosis eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 6, e1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganaie A. A., Lella R. K., Solanki R., Sharma C. (2011) Thermostable hexameric form of Eis (Rv2416c) protein of M. tuberculosis plays an important role for enhanced intracellular survival within macrophages. PLoS One 6, e27590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shui W., Petzold C. J., Redding A., Liu J., Pitcher A., Sheu L., Hsieh T. Y., Keasling J. D., Bertozzi C. R. (2011) Organelle membrane proteomics reveals differential influence of mycobacterial lipoglycans on macrophage phagosome maturation and autophagosome accumulation. J. Proteome Res. 10, 339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romagnoli A., Etna M. P., Giacomini E., Pardini M., Remoli M. E., Corazzari M., Falasca L., Goletti D., Gafa V., Simeone R., Delogu G., Piacentini M., Brosch R., Fimia G. M., Coccia E. M. (2012) ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy 8, 1357–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang L., Zhang H., Zhao Y., Mao F., Wu J., Bai B., Xu Z., Jiang Y., Shi C. (2012) Effects of Mycobacterium tuberculosis ESAT-6/CFP-10 fusion protein on the autophagy function of mouse macrophages. DNA Cell Biol. 31, 171–179. [DOI] [PubMed] [Google Scholar]
- 46.Ouimet M., Koster S., Sakowski E., Ramkhelawon B., van Solingen C., Oldebeken S., Karunakaran D., Portal-Celhay C., Sheedy F. J., Ray T. D., Cecchini K., Zamore P. D., Rayner K. J., Marcel Y. L., Philips J. A., Moore K. J. (2016) Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 17, 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiebler M., Brown K., Hegyi K., Newton S. M., Renna M., Hepburn L., Klapholz C., Coulter S., Obregón-Henao A., Henao Tamayo M., Basaraba R., Kampmann B., Henry K. M., Burgon J., Renshaw S. A., Fleming A., Kay R. R., Anderson K. E., Hawkins P. T., Ordway D. J., Rubinsztein D. C., Floto R. A. (2014) Functional drug screening reveals anticonvulsants as enhancers of mTOR-independent autophagic killing of Mycobacterium tuberculosis through inositol depletion. EMBO Mol. Med. 7, 127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chauhan S., Ahmed Z., Bradfute S. B., Arko-Mensah J., Mandell M. A., Won Choi S., Kimura T., Blanchet F., Waller A., Mudd M. H., Jiang S., Sklar L., Timmins G. S., Maphis N., Bhaskar K., Piguet V., Deretic V. (2015) Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential. Nat. Commun. 6, 8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sica V., Galluzzi L., Bravo-San Pedro J. M., Izzo V., Maiuri M. C., Kroemer G. (2015) Organelle-specific initiation of autophagy. Mol. Cell 59, 522–539. [DOI] [PubMed] [Google Scholar]
- 50.Khaminets A., Behl C., Dikic I. (2016) Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 26, 6–16. [DOI] [PubMed] [Google Scholar]
- 51.Rubinsztein D. C., Bento C. F., Deretic V. (2015) Therapeutic targeting of autophagy in neurodegenerative and infectious diseases. J. Exp. Med. 212, 979–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mizushima N., Yoshimori T., Ohsumi Y. (2011) The role of Atg proteins in autophagosome formation. Annu. Rev. Cell Dev. Biol. 27, 107–132. [DOI] [PubMed] [Google Scholar]
- 53.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manjithaya R., Anjard C., Loomis W. F., Subramani S. (2010) Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J. Cell Biol. 188, 537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dupont N., Jiang S., Pilli M., Ornatowski W., Bhattacharya D., Deretic V. (2011) Autophagy-based unconventional secretory pathway for extracellular delivery of IL-1β. EMBO J. 30, 4701–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang M., Kenny S. J., Ge L., Xu K., Schekman R. (2015) Translocation of interleukin-1β into a vesicle intermediate in autophagy-mediated secretion. eLife 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ponpuak M., Mandell M. A., Kimura T., Chauhan S., Cleyrat C., Deretic V. (2015) Secretory autophagy. Curr. Opin. Cell Biol. 35, 106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johansen T., Lamark T. (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7, 279–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khaminets A., Heinrich T., Mari M., Grumati P., Huebner A. K., Akutsu M., Liebmann L., Stolz A., Nietzsche S., Koch N., Mauthe M., Katona I., Qualmann B., Weis J., Reggiori F., Kurth I., Hübner C. A., Dikic I. (2015) Regulation of endoplasmic reticulum turnover by selective autophagy. Nature 522, 354–358. [DOI] [PubMed] [Google Scholar]
- 60.Kaushik S., Cuervo A. M. (2015) Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 17, 759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maejima I., Takahashi A., Omori H., Kimura T., Takabatake Y., Saitoh T., Yamamoto A., Hamasaki M., Noda T., Isaka Y., Yoshimori T. (2013) Autophagy sequesters damaged lysosomes to control lysosomal biogenesis and kidney injury. EMBO J. 32, 2336–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tooze S. A., Yoshimori T. (2010) The origin of the autophagosomal membrane. Nat. Cell Biol. 12, 831–835. [DOI] [PubMed] [Google Scholar]
- 63.Dupont N., Chauhan S., Arko-Mensah J., Castillo E. F., Masedunskas A., Weigert R., Robenek H., Proikas-Cezanne T., Deretic V. (2014) Neutral lipid stores and lipase PNPLA5 contribute to autophagosome biogenesis. Curr. Biol. 24, 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shpilka T., Welter E., Borovsky N., Amar N., Mari M., Reggiori F., Elazar Z. (2015) Lipid droplets and their component triglycerides and steryl esters regulate autophagosome biogenesis. EMBO J. 34, 2117–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamasaki M., Furuta N., Matsuda A., Nezu A., Yamamoto A., Fujita N., Oomori H., Noda T., Haraguchi T., Hiraoka Y., Amano A., Yoshimori T. (2013) Autophagosomes form at ER-mitochondria contact sites. Nature 495, 389–393. [DOI] [PubMed] [Google Scholar]
- 66.Kroemer G., Levine B. (2008) Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 9, 1004–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubinstein A. D., Eisenstein M., Ber Y., Bialik S., Kimchi A. (2011) The autophagy protein Atg12 associates with antiapoptotic Bcl-2 family members to promote mitochondrial apoptosis. Mol. Cell 44, 698–709. [DOI] [PubMed] [Google Scholar]
- 68.Liu Y., Shoji-Kawata S., Sumpter R. M. Jr.,Wei Y., Ginet V., Zhang L., Posner B., Tran K. A., Green D. R., Xavier R. J., Shaw S. Y., Clarke P. G., Puyal J., Levine B. (2013) Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. USA 110, 20364–20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galluzzi L., Pietrocola F., Bravo-San Pedro J. M., Amaravadi R. K., Baehrecke E. H., Cecconi F., Codogno P., Debnath J., Gewirtz D. A., Karantza V., Kimmelman A., Kumar S., Levine B., Maiuri M. C., Martin S. J., Penninger J., Piacentini M., Rubinsztein D. C., Simon H. U., Simonsen A., Thorburn A. M., Velasco G., Ryan K. M., Kroemer G. (2015) Autophagy in malignant transformation and cancer progression. EMBO J. 34, 856–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.White E. (2015) The role for autophagy in cancer. J. Clin. Invest. 125, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sandri M. (2016) Protein breakdown in cancer cachexia. Semin. Cell Dev. Biol. 54, 11–19. [DOI] [PubMed] [Google Scholar]
- 72.García-Prat L., Martínez-Vicente M., Perdiguero E., Ortet L., Rodríguez-Ubreva J., Rebollo E., Ruiz-Bonilla V., Gutarra S., Ballestar E., Serrano A. L., Sandri M., Muñoz-Cánoves P. (2016) Autophagy maintains stemness by preventing senescence. Nature 529, 37–42. [DOI] [PubMed] [Google Scholar]
- 73.Rivera J. F., Costes S., Gurlo T., Glabe C. G., Butler P. C. (2014) Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. J. Clin. Invest. 124, 3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang Y. H., Cho M. H., Kim J. Y., Kwon M. S., Peak J. J., Kang S. W., Yoon S. Y., Song Y. (2016) Impaired macrophage autophagy induces systemic insulin resistance in obesity. Oncotarget [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murrow L., Debnath J. (2013) Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu. Rev. Pathol. 8, 105–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kabeya Y., Mizushima N., Yamamoto A., Oshitani-Okamoto S., Ohsumi Y., Yoshimori T. (2004) LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J. Cell Sci. 117, 2805–2812. [DOI] [PubMed] [Google Scholar]
- 77.Weidberg H., Shvets E., Shpilka T., Shimron F., Shinder V., Elazar Z. (2010) LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 29, 1792–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alemu E. A., Lamark T., Torgersen K. M., Birgisdottir A. B., Larsen K. B., Jain A., Olsvik H., Øvervatn A., Kirkin V., Johansen T. (2012) ATG8 family proteins act as scaffolds for assembly of the ULK complex: sequence requirements for LC3-interacting region (LIR) motifs. J. Biol. Chem. 287, 39275–39290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joachim J., Jefferies H. B., Razi M., Frith D., Snijders A. P., Chakravarty P., Judith D., Tooze S. A. (2015) Activation of ULK kinase and autophagy by GABARAP trafficking from the centrosome is regulated by WAC and GM130. Mol. Cell 60, 899–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan E. Y., Kir S., Tooze S. A. (2007) siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 282, 25464–25474. [DOI] [PubMed] [Google Scholar]
- 81.Birgisdottir A. B., Lamark T., Johansen T. (2013) The LIR motif—crucial for selective autophagy. J. Cell Sci. 126, 3237–3247. [DOI] [PubMed] [Google Scholar]
- 82.Vicinanza M., Korolchuk V. I., Ashkenazi A., Puri C., Menzies F. M., Clarke J. H., Rubinsztein D. C. (2015) PI(5)P regulates autophagosome biogenesis. Mol. Cell 57, 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liang X. H., Jackson S., Seaman M., Brown K., Kempkes B., Hibshoosh H., Levine B. (1999) Induction of autophagy and inhibition of tumorigenesis by Beclin 1. Nature 402, 672–676. [DOI] [PubMed] [Google Scholar]
- 84.Sun Q., Fan W., Chen K., Ding X., Chen S., Zhong Q. (2008) Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 105, 19211–19216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baskaran S., Carlson L. A., Stjepanovic G., Young L. N., Kim J., Grob P., Stanley R. E., Nogales E., Hurley J. H. (2014) Architecture and dynamics of the autophagic phosphatidylinositol 3-kinase complex. eLife 3, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rostislavleva K., Soler N., Ohashi Y., Zhang L., Pardon E., Burke J. E., Masson G. R., Johnson C., Steyaert J., Ktistakis N. T., Williams R. L. (2015) Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science 350, aac7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Itakura E., Kishi-Itakura C., Mizushima N. (2012) The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269. [DOI] [PubMed] [Google Scholar]
- 88.Diao J., Liu R., Rong Y., Zhao M., Zhang J., Lai Y., Zhou Q., Wilz L. M., Li J., Vivona S., Pfuetzner R. A., Brunger A. T., Zhong Q. (2015) ATG14 promotes membrane tethering and fusion of autophagosomes to endolysosomes. Nature 520, 563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russell R. C., Tian Y., Yuan H., Park H. W., Chang Y. Y., Kim J., Kim H., Neufeld T. P., Dillin A., Guan K. L. (2013) ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat. Cell Biol. 15, 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei Y., An Z., Zou Z., Sumpter R., Su M., Zang X., Sinha S., Gaestel M., Levine B. (2015) The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. eLife 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim J., Kim Y. C., Fang C., Russell R. C., Kim J. H., Fan W., Liu R., Zhong Q., Guan K. L. (2013) Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152, 290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim J., Kundu M., Viollet B., Guan K. L. (2011) AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 13, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Egan D. F., Shackelford D. B., Mihaylova M. M., Gelino S., Kohnz R. A., Mair W., Vasquez D. S., Joshi A., Gwinn D. M., Taylor R., Asara J. M., Fitzpatrick J., Dillin A., Viollet B., Kundu M., Hansen M., Shaw R. J. (2011) Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fujita N., Itoh T., Omori H., Fukuda M., Noda T., Yoshimori T. (2008) The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 19, 2092–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dooley H. C., Razi M., Polson H. E., Girardin S. E., Wilson M. I., Tooze S. A. (2014) WIPI2 links LC3 conjugation with PI3P, autophagosome formation, and pathogen clearance by recruiting Atg12-5-16L1. Mol. Cell 55, 238–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fujita N., Morita E., Itoh T., Tanaka A., Nakaoka M., Osada Y., Umemoto T., Saitoh T., Nakatogawa H., Kobayashi S., Haraguchi T., Guan J. L., Iwai K., Tokunaga F., Saito K., Ishibashi K., Akira S., Fukuda M., Noda T., Yoshimori T. (2013) Recruitment of the autophagic machinery to endosomes during infection is mediated by ubiquitin. J. Cell Biol. 203, 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishimura T., Kaizuka T., Cadwell K., Sahani M. H., Saitoh T., Akira S., Virgin H. W., Mizushima N. (2013) FIP200 regulates targeting of Atg16L1 to the isolation membrane. EMBO Rep. 14, 284–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gammoh N., Florey O., Overholtzer M., Jiang X. (2013) Interaction between FIP200 and ATG16L1 distinguishes ULK1 complex-dependent and -independent autophagy. Nat. Struct. Mol. Biol. 20, 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Klionsky D. J., Eskelinen E. L., Deretic V. (2014) Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes... wait, I’m confused. Autophagy 10, 549–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Settembre C., Di Malta C., Polito V. A., Garcia Arencibia M., Vetrini F., Erdin S., Erdin S. U., Huynh T., Medina D., Colella P., Sardiello M., Rubinsztein D. C., Ballabio A. (2011) TFEB links autophagy to lysosomal biogenesis. Science 332, 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Settembre C., Zoncu R., Medina D. L., Vetrini F., Erdin S., Erdin S., Huynh T., Ferron M., Karsenty G., Vellard M. C., Facchinetti V., Sabatini D. M., Ballabio A. (2012) A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J. 31, 1095–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medina D. L., Di Paola S., Peluso I., Armani A., De Stefani D., Venditti R., Montefusco S., Scotto-Rosato A., Prezioso C., Forrester A., Settembre C., Wang W., Gao Q., Xu H., Sandri M., Rizzuto R., De Matteis M. A., Ballabio A. (2015) Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 17, 288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perera R. M., Stoykova S., Nicolay B. N., Ross K. N., Fitamant J., Boukhali M., Lengrand J., Deshpande V., Selig M. K., Ferrone C. R., Settleman J., Stephanopoulos G., Dyson N. J., Zoncu R., Ramaswamy S., Haas W., Bardeesy N. (2015) Transcriptional control of autophagy-lysosome function drives pancreatic cancer metabolism. Nature 524, 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nezich C. L., Wang C., Fogel A. I., Youle R. J. (2015) MiT/TFE transcription factors are activated during mitophagy downstream of Parkin and Atg5. J. Cell Biol. 210, 435–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shi C. S., Kehrl J. H. (2010) TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 3, ra42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xia P., Wang S., Du Y., Zhao Z., Shi L., Sun L., Huang G., Ye B., Li C., Dai Z., Hou N., Cheng X., Sun Q., Li L., Yang X., Fan Z. (2013) WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. EMBO J. 32, 2685–2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nazio F., Strappazzon F., Antonioli M., Bielli P., Cianfanelli V., Bordi M., Gretzmeier C., Dengjel J., Piacentini M., Fimia G. M., Cecconi F. (2013) mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat. Cell Biol. 15, 406–416. [DOI] [PubMed] [Google Scholar]
- 108.Chauhan S., Mandell M. A., Deretic V. (2015) IRGM governs the core autophagy machinery to conduct antimicrobial defense. Mol. Cell 58, 507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Platta H. W., Abrahamsen H., Thoresen S. B., Stenmark H. (2012) Nedd4-dependent lysine-11-linked polyubiquitination of the tumour suppressor Beclin 1. Biochem. J. 441, 399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu C., Feng K., Zhao X., Huang S., Cheng Y., Qian L., Wang Y., Sun H., Jin M., Chuang T. H., Zhang Y. (2014) Regulation of autophagy by E3 ubiquitin ligase RNF216 through BECN1 ubiquitination. Autophagy 10, 2239–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu C. C., Lin Y. C., Chen Y. H., Chen C. M., Pang L. Y., Chen H. A., Wu P. R., Lin M. Y., Jiang S. T., Tsai T. F., Chen R. H. (2016) Cul3-KLHL20 ubiquitin ligase governs the turnover of ULK1 and VPS34 complexes to control autophagy termination. Mol. Cell 61, 84–97. [DOI] [PubMed] [Google Scholar]
- 112.Zhang T., Dong K., Liang W., Xu D., Xia H., Geng J., Najafov A., Liu M., Li Y., Han X., Xiao J., Jin Z., Peng T., Gao Y., Cai Y., Qi C., Zhang Q., Sun A., Lipinski M., Zhu H., Xiong Y., Pandolfi P. P., Li H., Yu Q., Yuan J. (2015) G-Protein-coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of Atg14L. eLife 4, e06734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuang E., Okumura C. Y., Sheffy-Levin S., Varsano T., Shu V. C., Qi J., Niesman I. R., Yang H. J., López-Otín C., Yang W. Y., Reed J. C., Broday L., Nizet V., Ronai Z. A. (2012) Regulation of ATG4B stability by RNF5 limits basal levels of autophagy and influences susceptibility to bacterial infection. PLoS Genet. 8, e1003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Antonioli M., Albiero F., Nazio F., Vescovo T., Perdomo A. B., Corazzari M., Marsella C., Piselli P., Gretzmeier C., Dengjel J., Cecconi F., Piacentini M., Fimia G. M. (2014) AMBRA1 interplay with cullin E3 ubiquitin ligases regulates autophagy dynamics. Dev. Cell 31, 734–746. [DOI] [PubMed] [Google Scholar]
- 115.Xia P., Wang S., Huang G., Du Y., Zhu P., Li M., Fan Z. (2014) RNF2 is recruited by WASH to ubiquitinate AMBRA1 leading to downregulation of autophagy. Cell Res. 24, 943–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen D., Gao F., Li B., Wang H., Xu Y., Zhu C., Wang G. (2010) Parkin mono-ubiquitinates Bcl-2 and regulates autophagy. J. Biol. Chem. 285, 38214–38223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Y., Xiong X., Sun Y. (2011) DEPTOR, an mTOR inhibitor, is a physiological substrate of SCF(βTrCP) E3 ubiquitin ligase and regulates survival and autophagy. Mol. Cell 44, 304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kondapalli C., Kazlauskaite A., Zhang N., Woodroof H. I., Campbell D. G., Gourlay R., Burchell L., Walden H., Macartney T. J., Deak M., Knebel A., Alessi D. R., Muqit M. M. (2012) PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2, 120080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cunningham C. N., Baughman J. M., Phu L., Tea J. S., Yu C., Coons M., Kirkpatrick D. S., Bingol B., Corn J. E. (2015) USP30 and Parkin homeostatically regulate atypical ubiquitin chains on mitochondria. Nat. Cell Biol. 17, 160–169. [DOI] [PubMed] [Google Scholar]
- 120.Koyano F., Okatsu K., Kosako H., Tamura Y., Go E., Kimura M., Kimura Y., Tsuchiya H., Yoshihara H., Hirokawa T., Endo T., Fon E. A., Trempe J. F., Saeki Y., Tanaka K., Matsuda N. (2014) Ubiquitin is phosphorylated by PINK1 to activate Parkin. Nature 510, 162–166. [DOI] [PubMed] [Google Scholar]
- 121.Kane L. A., Lazarou M., Fogel A. I., Li Y., Yamano K., Sarraf S. A., Banerjee S., Youle R. J. (2014) PINK1 phosphorylates ubiquitin to activate Parkin E3 ubiquitin ligase activity. J. Cell Biol. 205, 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wauer T., Simicek M., Schubert A., Komander D. (2015) Mechanism of phospho-ubiquitin-induced PARKIN activation. Nature 524, 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huett A., Heath R. J., Begun J., Sassi S. O., Baxt L. A., Vyas J. M., Goldberg M. B., Xavier R. J. (2012) The LRR and RING domain protein LRSAM1 is an E3 ligase crucial for ubiquitin-dependent autophagy of intracellular Salmonella typhimurium. Cell Host Microbe 12, 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Orvedahl A., Sumpter R. Jr.,Xiao G., Ng A., Zou Z., Tang Y., Narimatsu M., Gilpin C., Sun Q., Roth M., Forst C. V., Wrana J. L., Zhang Y. E., Luby-Phelps K., Xavier R. J., Xie Y., Levine B. (2011) Image-based genome-wide siRNA screen identifies selective autophagy factors. Nature 480, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thurston T. L., Wandel M. P., von Muhlinen N., Foeglein A., Randow F. (2012) Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482, 414–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Overvatn A., Stenmark H., Johansen T. (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kirkin V., Lamark T., Sou Y. S., Bjørkøy G., Nunn J. L., Bruun J. A., Shvets E., McEwan D. G., Clausen T. H., Wild P., Bilusic I., Theurillat J. P., Øvervatn A., Ishii T., Elazar Z., Komatsu M., Dikic I., Johansen T. (2009) A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol. Cell 33, 505–516. [DOI] [PubMed] [Google Scholar]
- 128.Newman A. C., Scholefield C. L., Kemp A. J., Newman M., McIver E. G., Kamal A., Wilkinson S. (2012) TBK1 kinase addiction in lung cancer cells is mediated via autophagy of Tax1bp1/Ndp52 and non-canonical NF-κB signalling. PLoS One 7, e50672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lazarou M., Sliter D. A., Kane L. A., Sarraf S. A., Wang C., Burman J. L., Sideris D. P., Fogel A. I., Youle R. J. (2015) The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature 524, 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lu K., Psakhye I., Jentsch S. (2014) Autophagic clearance of polyQ proteins mediated by ubiquitin-Atg8 adaptors of the conserved CUET protein family. Cell 158, 549–563. [DOI] [PubMed] [Google Scholar]
- 131.Reymond A., Meroni G., Fantozzi A., Merla G., Cairo S., Luzi L., Riganelli D., Zanaria E., Messali S., Cainarca S., Guffanti A., Minucci S., Pelicci P. G., Ballabio A. (2001) The tripartite motif family identifies cell compartments. EMBO J. 20, 2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kimura T., Jain A., Choi S. W., Mandell M. A., Schroder K., Johansen T., Deretic V. (2015) TRIM-mediated precision autophagy targets cytoplasmic regulators of innate immunity. J. Cell Biol. 210, 973–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mandell M. A., Jain A., Arko-Mensah J., Chauhan S., Kimura T., Dinkins C., Silvestri G., Münch J., Kirchhoff F., Simonsen A., Wei Y., Levine B., Johansen T., Deretic V. (2014) TRIM proteins regulate autophagy and can target autophagic substrates by direct recognition. Dev. Cell 30, 394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mandell M. A., Kimura T., Jain A., Johansen T., Deretic V. (2014) TRIM proteins regulate autophagy: TRIM5 is a selective autophagy receptor mediating HIV-1 restriction. Autophagy 10, 2387–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu L., Feng D., Chen G., Chen M., Zheng Q., Song P., Ma Q., Zhu C., Wang R., Qi W., Huang L., Xue P., Li B., Wang X., Jin H., Wang J., Yang F., Liu P., Zhu Y., Sui S., Chen Q. (2012) Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat. Cell Biol. 14, 177–185. [DOI] [PubMed] [Google Scholar]
- 136.Murakawa T., Yamaguchi O., Hashimoto A., Hikoso S., Takeda T., Oka T., Yasui H., Ueda H., Akazawa Y., Nakayama H., Taneike M., Misaka T., Omiya S., Shah A. M., Yamamoto A., Nishida K., Ohsumi Y., Okamoto K., Sakata Y., Otsu K. (2015) Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat. Commun. 6, 7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sandoval H., Thiagarajan P., Dasgupta S. K., Schumacher A., Prchal J. T., Chen M., Wang J. (2008) Essential role for Nix in autophagic maturation of erythroid cells. Nature 454, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang H., Bosch-Marce M., Shimoda L. A., Tan Y. S., Baek J. H., Wesley J. B., Gonzalez F. J., Semenza G. L. (2008) Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J. Biol. Chem. 283, 10892–10903. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 139.Mancias J. D., Wang X., Gygi S. P., Harper J. W., Kimmelman A. C. (2014) Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 509, 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dowdle W. E., Nyfeler B., Nagel J., Elling R. A., Liu S., Triantafellow E., Menon S., Wang Z., Honda A., Pardee G., Cantwell J., Luu C., Cornella-Taracido I., Harrington E., Fekkes P., Lei H., Fang Q., Digan M. E., Burdick D., Powers A. F., Helliwell S. B., D’Aquin S., Bastien J., Wang H., Wiederschain D., Kuerth J., Bergman P., Schwalb D., Thomas J., Ugwonali S., Harbinski F., Tallarico J., Wilson C. J., Myer V. E., Porter J. A., Bussiere D. E., Finan P. M., Labow M. A., Mao X., Hamann L. G., Manning B. D., Valdez R. A., Nicholson T., Schirle M., Knapp M. S., Keaney E. P., Murphy L. O. (2014) Selective VPS34 inhibitor blocks autophagy and uncovers a role for NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat. Cell Biol. 16, 1069–1079. [DOI] [PubMed] [Google Scholar]
- 141.Kimura T., Mandell M., Deretic V. (2016) Precision autophagy directed by receptor regulators—emerging examples within the TRIM family. J. Cell Sci. 129, 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bodemann B. O., Orvedahl A., Cheng T., Ram R. R., Ou Y. H., Formstecher E., Maiti M., Hazelett C. C., Wauson E. M., Balakireva M., Camonis J. H., Yeaman C., Levine B., White M. A. (2011) RalB and the exocyst mediate the cellular starvation response by direct activation of autophagosome assembly. Cell 144, 253–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ogura Y., Bonen D. K., Inohara N., Nicolae D. L., Chen F. F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R. H., Achkar J. P., Brant S. R., Bayless T. M., Kirschner B. S., Hanauer S. B., Nuñez G., Cho J. H. (2001) A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606. [DOI] [PubMed] [Google Scholar]
- 144.Hugot J. P., Chamaillard M., Zouali H., Lesage S., Cézard J. P., Belaiche J., Almer S., Tysk C., O’Morain C. A., Gassull M., Binder V., Finkel Y., Cortot A., Modigliani R., Laurent-Puig P., Gower-Rousseau C., Macry J., Colombel J. F., Sahbatou M., Thomas G. (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411, 599–603. [DOI] [PubMed] [Google Scholar]
- 145.Wellcome Trust Case Control Consortium (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447, 661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wellcome Trust Case Control Consortium (2010) Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature 464, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Intemann C. D., Thye T., Niemann S., Browne E. N., Amanua Chinbuah M., Enimil A., Gyapong J., Osei I., Owusu-Dabo E., Helm S., Rüsch-Gerdes S., Horstmann R. D., Meyer C. G. (2009) Autophagy gene variant IRGM -261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog. 5, e1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Song J. H., Kim S. Y., Chung K. S., Moon C. M., Kim S. W., Kim E. Y., Jung J. Y., Park M. S., Kim Y. S., Kim S. K., Chang J., Shin D. J., Kang Y. A. (2014) Association between genetic variants in the IRGM gene and tuberculosis in a Korean population. Infection 42, 655–660. [DOI] [PubMed] [Google Scholar]
- 149.Travassos L. H., Carneiro L. A., Ramjeet M., Hussey S., Kim Y. G., Magalhães J. G., Yuan L., Soares F., Chea E., Le Bourhis L., Boneca I. G., Allaoui A., Jones N. L., Nuñez G., Girardin S. E., Philpott D. J. (2010) Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62. [DOI] [PubMed] [Google Scholar]
- 150.Cooney R., Baker J., Brain O., Danis B., Pichulik T., Allan P., Ferguson D. J., Campbell B. J., Jewell D., Simmons A. (2010) NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16, 90–97. [DOI] [PubMed] [Google Scholar]
- 151.Bekpen C., Marques-Bonet T., Alkan C., Antonacci F., Leogrande M. B., Ventura M., Kidd J. M., Siswara P., Howard J. C., Eichler E. E. (2009) Death and resurrection of the human IRGM gene. PLoS Genet. 5, e1000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi J., Park S., Biering S. B., Selleck E., Liu C. Y., Zhang X., Fujita N., Saitoh T., Akira S., Yoshimori T., Sibley L. D., Hwang S., Virgin H. W. (2014) The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity 40, 924–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bekpen C., Hunn J. P., Rohde C., Parvanova I., Guethlein L., Dunn D. M., Glowalla E., Leptin M., Howard J. C. (2005) The interferon-inducible p47 (IRG) GTPases in vertebrates: loss of the cell autonomous resistance mechanism in the human lineage. Genome Biol. 6, R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cadwell K., Liu J. Y., Brown S. L., Miyoshi H., Loh J., Lennerz J. K., Kishi C., Kc W., Carrero J. A., Hunt S., Stone C. D., Brunt E. M., Xavier R. J., Sleckman B. P., Li E., Mizushima N., Stappenbeck T. S., Virgin H. W. IV (2008) A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Caruso R., Warner N., Inohara N., Núñez G. (2014) NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41, 898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Henry S. C., Schmidt E. A., Fessler M. B., Taylor G. A. (2014) Palmitoylation of the immunity related GTPase, Irgm1: impact on membrane localization and ability to promote mitochondrial fission. PLoS One 9, e95021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh S. B., Davis A. S., Taylor G. A., Deretic V. (2006) Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313, 1438–1441. [DOI] [PubMed] [Google Scholar]
- 158.Singh S. B., Ornatowski W., Vergne I., Naylor J., Delgado M., Roberts E., Ponpuak M., Master S., Pilli M., White E., Komatsu M., Deretic V. (2010) Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nat. Cell Biol. 12, 1154–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Maric-Biresev J., Hunn J. P., Krut O., Helms J. B., Martens S., Howard J. C. (2016) Loss of the interferon-γ-inducible regulatory immunity-related GTPase (IRG), Irgm1, causes activation of effector IRG proteins on lysosomes, damaging lysosomal function and predicting the dramatic susceptibility of Irgm1-deficient mice to infection. BMC Biol. 14, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Juárez E., Carranza C., Hernández-Sánchez F., León-Contreras J. C., Hernández-Pando R., Escobedo D., Torres M., Sada E. (2012) NOD2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur. J. Immunol. 42, 880–889. [DOI] [PubMed] [Google Scholar]
- 161.Sorbara M. T., Ellison L. K., Ramjeet M., Travassos L. H., Jones N. L., Girardin S. E., Philpott D. J. (2013) The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner. Immunity 39, 858–873. [DOI] [PubMed] [Google Scholar]
- 162.Rao Y., Perna M. G., Hofmann B., Beier V., Wollert T. (2016) The Atg1-kinase complex tethers Atg9-vesicles to initiate autophagy. Nat. Commun. 7, 10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lamb C. A., Nühlen S., Judith D., Frith D., Snijders A. P., Behrends C., Tooze S. A. (2016) TBC1D14 regulates autophagy via the TRAPP complex and ATG9 traffic. EMBO J. 35, 281–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Suzuki S. W., Yamamoto H., Oikawa Y., Kondo-Kakuta C., Kimura Y., Hirano H., Ohsumi Y. (2015) Atg13 HORMA domain recruits Atg9 vesicles during autophagosome formation. Proc. Natl. Acad. Sci. USA 112, 3350–3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reggiori F., Tooze S. A. (2012) Autophagy regulation through Atg9 traffic. J. Cell Biol. 198, 151–153. [DOI] [PMC free article] [PubMed] [Google Scholar]