Abstract
Human gammaherpesviruses are associated with lymphomas and other malignancies. Murine gammaherpesvirus 68 (MHV-68) infection of mice has emerged as a model for understanding gammaherpesvirus pathogenesis in vivo. In contrast to human gammaherpesviruses, MHV-68 replicates in permissive cell lines in a robust manner, presenting an efficient model to study the basic mechanisms for DNA replication and recombination processes. In addition, MHV-68 also infects a broad range of cells of different tissue types and from different host species, and the viral genome persists as an episome in infected cells. These features make MHV-68 an attractive system on which to build gene delivery vectors. We have therefore undertaken a study to identify the cis elements required for MHV-68 genome replication and packaging. Here we report that an 8.4-kb MHV-68 genomic fragment between ORF66 and ORF73 conferred on the plasmid the ability to replicate; replication required the presence of either de novo viral infection or viral reactivation from latency. We further mapped the origin of lytic replication (oriLyt) to a 1.25-kb region. Moreover, we demonstrated that the terminal repeat of the viral genome is sufficient for packaging of the replicated oriLyt plasmid into mature viral particles. Functional identification of the MHV-68 oriLyt and packaging signal has laid a foundation for investigating the mechanisms controlling gammaherpesvirus DNA replication during the viral lytic phase and will also serve as a base on which to design gene delivery vectors.
Murine gammaherpesvirus 68 (MHV-68), like its two human counterparts, Epstein-Barr virus (EBV) and human herpesvirus 8 (HHV-8; also called Kaposi's sarcoma-associated herpesvirus), belongs to the gammaherpesvirus subfamily (12, 25, 47). Originally isolated from wild murid rodents, MHV-68 also infects laboratory strains of mice. Following intranasal inoculation, MHV-68 initiates productive infection in the lung and then establishes transient latent infection in B lymphocytes, macrophages, dendritic cells, and epithelial cells (16, 39, 43, 50) as well as long-term latency in a subset of B cells (44, 51). When administered orally, MHV-68 can survive the harsh gastrointestinal environment to establish persistent infection in intestinal epithelial cells (31). The MHV-68 genome has been completely sequenced and is closely related to EBV and HHV-8 at the molecular level (47). As such, MHV-68 infection of mice has emerged as a model for studying gammaherpesvirus-host interactions as well as pathobiology (36-38).
Herpesviruses have two phases in their life cycle, latency and lytic replication. During the lytic cycle, DNA replication proteins assemble at an origin of lytic replication (oriLyt) and initiate the replication process, resulting in “head-to-tail” concatemers (although more complicated replicative intermediates have also been reported) (21, 28, 34). Sequences present at the genomic termini become linked and provide the substrate for cleavage and packaging of unit-length viral genomes (10, 11, 32, 41, 46). Besides the cis-acting cleavage-packaging signal, there also seems to be a length requirement for packaging, i.e., DNA molecules whose size is close to that of the wild-type virus are preferentially encapsidated (8, 49).
The strategies used to maintain the viral genome during latency, however, are different for different herpesviruses. For the neurotropic herpes simplex virus type 1, the host cells are postmitotic, and the viral genomes persist as long as the host cells survive (34). In contrast, gammaherpesviruses very often establish latency in mitotic cells, such as lymphocytes and epithelial cells, as well as nonmitotic cells, such as macrophages (16, 39, 43, 50). Moreover, the viral genomes remain as episomes (45). Therefore, these gammaherpesviruses have evolved a strategy not only to replicate their genomes in synchrony with the host cell chromosomes but also to partition the viral genomes into daughter cells. In EBV, two viral elements are required for this function, a cis element, oriP (54, 55), and a trans-acting factor that is encoded by EBV nuclear antigen 1 (EBNA-1) (6, 33). In HHV-8, the cis-acting terminal repeat sequence and latency-associated nuclear antigen protein are required (6, 7). The mechanism by which EBNA-1 and oriP replicate and partition the DNA on which oriP resides has been well characterized (17, 23, 40). The fact that EBNA-1 and oriP function in the absence of other EBV genes also makes the duo a popular tool for research and gene delivery applications. When incorporated into recombinant vectors, the pair allows investigation of the effect of chromatin structure on gene expression (epigenetics) as well as stably expressing foreign genes without disrupting host chromosomes.
Two types of herpesvirus vectors can be engineered, recombinant viruses and amplicons (9, 13, 18, 26). For a recombinant virus-based vector, one or several viral genes are deleted from the viral genome (which encodes approximately 100 proteins) to make the virus replication deficient. The vector can be propagated when the products of the deleted genes are provided in trans by a complementing cell line or by plasmids or cosmids (13, 26). In contrast, an amplicon vector includes only the minimal cis elements required for viral DNA replication and processing, namely, oriLyt and the packaging signal; therefore, it can be viewed as a highly defective, high-capacity plasmid-based vector. As such, propagation of an amplicon requires a large number of viral genomes to be provided in trans by a suitable helper virus or a helper virus genome (9, 18).
To understand the biology of MHV-68 and also to facilitate the development of MHV-68-based amplicon vectors, it is critical to define the cis sequences required for viral DNA replication and packaging. However, no work has been conducted to address this issue with MHV-68. In this study, we identified an MHV-68 oriLyt with replication assays and further mapped the minimal oriLyt through deletion analysis. Moreover, we demonstrated that plasmids containing both the MHV-68 oriLyt and terminal repeat sequences were successfully packaged into mature viral particles.
(Some of our preliminary results were presented at the Fifth International Workshop on KSHV/HHV-8 and Related Agents held 7 to 11 August 2002 at Kloster Irsee, Germany.)
MATERIALS AND METHODS
Cell culture and viruses.
All cells were cultured at 37°C in the presence of 5% CO2. 293T cells and BHK cells were grown and maintained in Dulbecco's modified Eagle's medium (Cellgro) containing 10% fetal bovine serum (Gemini) and antibiotics (50 U of penicillin and 50 μg of streptomycin per ml). S11E, a subclone of a murine B-cell lymphoma cell line latently infected by MHV-68 (45), was propagated in RPMI 1640 medium (Cellgro) containing 10% fetal bovine serum (HyClone) and antibiotics (50 U of penicillin and 50 μg of streptomycin per ml).
MHV-68 was originally obtained from the American Type Culture Collection (VR1465) and propagated by infecting BHK-21 cells at a multiplicity of infection of 0.01 to 0.05 PFU/cell. Viral titers were measured by plaque assays, as described previously (53).
Plasmid construction.
MHV-68 virion DNA was digested with HindIII to obtain the 8.4-kb D fragment from the MHV-68 genome, which was then cloned into the pBluescript-KS vector to produce pMO in two orientations, + and −. pMO(−) was then digested with BamHI to generate a 4-kb MHV-68 genomic fragment that contains the GC-rich repeats and 1.9 kb upstream of the 4-kb MHV-68 genomic fragment. The 2.4-kb fragment remained on the vector, and the plasmid was blunt ended and self-ligated to form pMOΔ5. The 4-kb GC-rich MHV-68 fragment was cloned back into BamHI-digested pMO(−) to generate the 6.4-kb pMOΔ2. The 4-kb GC-rich MHV-68 fragment was also cloned into pBluescript-KS vector to produce pMOΔ3. pMO(+) was digested with BamHI to generate the 4-kb GC-rich MHV-68 fragment and 2.4 kb downstream of the 4-kb fragment. The 4-kb GC-rich MHV-68 fragment was cloned back into the BamHI-digested pMO(+) to generate the 6-kb pMOΔ1. The 1.9-kb fragment upstream of the 4-kb GC-rich fragment remained on the vector, and the plasmid was blunt ended and self-ligated to form pMOΔ4.
To generate 5′ deletion constructs across the GC-rich repeat region, we used an Erase-a-Base kit (Promega). The 8.4-kb MHV-68 fragment was cloned into the HindIII site of pGEM7Zf(+) in two orientations, + and −. Deletion constructs were made from the − plasmid with AatII as the exonuclease III-resistant (3′ overhang) site and XhoI as the exonuclease III-sensitive (5′ overhang) site from which exonuclease III digests the 5′ end of the MHV-68 fragment. Aliquots were taken at 30-s intervals from the reaction mixture, blunt ended, ligated, and screened for the desired deletions: pMOΔ6 (5.9 kb), pMOΔ7 (5.1 kb), pMOΔ8 (4.8 kb), pMOΔ9 (4 kb), pMOΔ10 (3.8 kb), pMOΔ11 (3.3 kb), and pMOΔ12 (2.8 kb). To generate 3′ deletion constructs, pMOΔ11 was digested with HindIII plus PstI, BglII, EcoRI, and SacII, blunt ended, and self-ligated to generate pMOΔ13 (2.4 kb), pMOΔ14 (2.0 kb), pMOΔ15 (1.5 kb), and pMOΔ16 (1.25 kb), respectively.
MHV-68 virion DNA was digested with NotI, and a 1.2-kb fragment corresponding to the terminal repeat was isolated. One copy and two tandem copies of the terminal repeats were cloned into the NotI site on pMOΔ2 to derive pMOΔ2-1TR and pMOΔ2-2TR, respectively.
Replication assays and packaging assay.
To test the oriLyt constructs during de novo infection, plasmids were transfected into 293T cells in six-well plates with Lipofectamine Plus (Invitrogen) in accordance with the manufacturer's protocols. A total of 1.5 μg of DNA that included 0.2 pmol of the oriLyt plasmid and salmon sperm DNA (as filler) was transfected into each well. At 24 h posttransfection, the cells were infected with wild-type MHV-68 at a multiplicity of infection of 0.1 to provide the trans factors required for viral DNA replication. At 72 h postinfection, when >95% of the cells showed cytopathic effect, either total cellular DNA or extrachromosomal (Hirt) DNA was harvested from the cells and prepared for Southern blot analysis.
To test oriLyt during reactivation, the following mixtures of DNA were introduced into 107 S11E cells via electroporation (Bio-Rad; 960 μF, 240 V): 5 μg of pFlag/MRTA (53) or pFlag-CMV-2 (Kodak); 5 μg of pMOΔ2, 2.9 μg of pMOΔ5, 3.1 μg of pMOΔ12, 2.2 μg of pMOΔ16, or 1.6 μg of pKS (corresponding to 0.8 pmol of each plasmid) plus various amount of salmon sperm filler DNA to make the total amount of transfected DNA 10 μg. At 24 h posttransfection, total cellular DNA was harvested and prepared for Southern blot analysis.
To test packaging of plasmid DNA into virions, 293T cells were transfected with test plasmids and infected by MHV-68, and the supernatant was collected 4 days postinfection, when >95% of the cells showed cytopathic effect. Viral particles were collected by high-speed centrifugation at 17,000 rpm at 4°C for 2 h, washed with and resuspended in phosphate-buffered saline, mixed with an equal volume of 2% agarose, and cast into blocks. Agarose-embedded virions were lysed in situ by incubation in pulsed-field gel electrophoresis lysis buffer at 37°C for 24 h, with one buffer change, and five rinses for 15 min each in 1× Tris-EDTA at 50°C, as described previously (11). Blocks were stored in Tris-EDTA at 4°C prior to analysis. For pulsed-field gel electrophoresis, one-eighth of a block was sealed into each well of a 0.8% agarose gel, and electrophoresis was performed in 0.5× TBE (Tris-borate-EDTA) at ≈5 V/cm overnight with buffer circulation. Power inversion was controlled by a PPI-200 programmable power inverter (MJ Research; program 3). Gels were then processed for Southern blot analysis.
DNA preparation and Southern blot analysis.
Extrachromosomal DNA was prepared according to the Hirt procedure (20). Total cellular DNA was extracted according to standard procedures. Half of each extrachromosomal DNA sample or one-twelfth of each total cellular DNA sample was digested overnight with DpnI and a unique restriction enzyme. The unique restriction enzymes used were as follows: XhoI for pMOΔ1 to -Δ5, pMO, and pKS; HindIII for pMOΔ6 to -Δ12, pMOΔ2-1TR, pMOΔ2-2TR, and pGEM; and NdeI for pMOΔ13 to -Δ16.
For Southern blot analysis, the digested samples and molecular size markers were run on a 0.8% agarose gel in 1× TBE overnight. The agarose gel was treated with 0.2 M HCl, followed by denaturation and neutralization. The separated DNAs were transferred onto a Hybond-N+ membrane (Amersham Pharmacia Biotech) with a vacuum blotter (Bio-Rad) and UV crosslinked. The blot was prehybridized at 65°C and hybridized overnight with a radiolabeled probe. The blot was then washed, exposed to a phosphorimage screen, and analyzed with a Storm machine (Molecular Dynamics).
RESULTS AND DISCUSSION
Identification of an MHV-68 oriLyt in a replication assay.
Based on sequence analysis of MHV-68 and other gammaherpesviruses (19, 30, 47, 56), we predicted that an MHV-68 genomic fragment between ORF67 and ORF72 would contain an oriLyt (Fig. 1). Upon examination of restriction sites on the viral genome, we chose to digest MHV-68 virion DNA with HindIII and cloned the 8.4-kb D fragment (47) into a pBluescript-KS (pKS) vector. The resulting plasmids, pMO(+) and pMO(−) (+ and − indicate the relative orientation of the insert to the vector backbone), were tested in a replication assay. Plasmids were transfected into 293T cells, followed by MHV-68 de novo infection. Cells were harvested, and DNAs were extracted (20) and digested by DpnI (which cuts only unreplicated plasmid) and XhoI (which cuts at a unique XhoI site in both the input and replicated plasmids). The digests were analyzed by Southern blotting, with radiolabeled pKS as a probe (Fig. 2A).
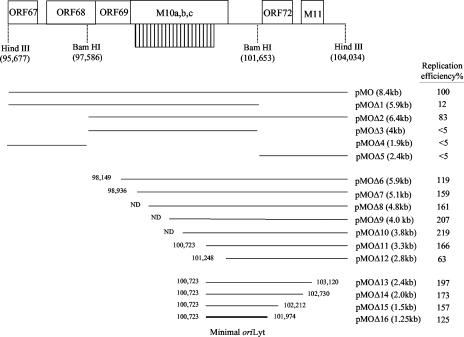
FIG. 1.
Diagram of the plasmid constructs used in this study to identify the minimal MHV-68 oriLyt. A schematic of the 8.4-kb HindIII-D fragment of the MHV-68 genome is shown on top. Open reading frames, the 100-bp repeat region (striped box), and restriction enzyme sites used in cloning are indicated. Numbers correspond to nucleotide positions on the MHV-68 genome (47). The full-length oriLyt plasmids pMO(+) and pMO(−) span ORFs 67, 68, 69, M10, 72, and M11. Deletion constructs were generated as described in Materials and Methods, with the length of each insert indicated in parentheses. The relative replication efficiencies of the constructs, determined from at least three independent experiments, are summarized on the right, with the normalized replication efficiency of pMO set at 100%. The bold line indicates the minimal oriLyt region mapped in this study. ND, not determined precisely due to the GC-rich nature of the 100-bp repeat; the sizes of these plasmids were estimated by restriction enzyme digestion and agarose gel analysis.
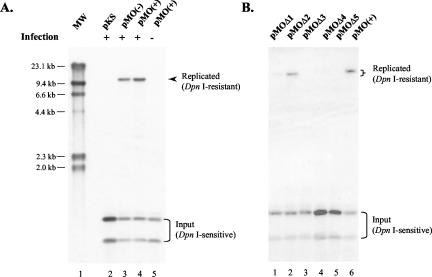
FIG. 2.
Identification of an MHV-68 oriLyt with a replication assay. (A) The 8.4-kb HindIII-D fragment contains a functional oriLyt. pKS, pMO(+), or pMO(−) was transfected into 293T cells, followed by infection with wild-type MHV-68. DNA was harvested and treated with DpnI to digest input plasmid and also with XhoI, which cuts once in each copy of the oriLyt plasmid. The samples were then run on an agarose gel and analyzed by Southern blotting with a radiolabeled pKS probe. HindIII-digested λ DNA served as molecular size markers. (B) Initial analysis of the 8.4-kb oriLyt region. Deletion plasmids pMOΔ1 to -Δ5 were generated and tested in the replication assays as described in A. Arrowhead and upper bracket, DpnI-resistant replicated DNA; lower bracket, DpnI-sensitive input DNA.
Both pMO(+) and pMO(−) replicated successfully in infected cells, as demonstrated by the11.3-kb bands that were resistant to DpnI digestion (Fig. 2A, lanes 3 and 4, arrowhead). In contrast, the pKS vector failed to replicate (Fig. 2A, lane 2). Furthermore, pMO did not replicate in uninfected cells (Fig. 2A, lane 5), indicating that replication required trans factors expressed during viral lytic infection. Taken together, these data demonstrated that the 8.4-kb fragment contains a functional oriLyt which conferred on the plasmid the ability to replicate in the presence of viral trans factors.
Analysis of the previously identified oriLyt of other gammaherpesviruses showed that the minimal oriLyt usually resides next to or includes a GC-rich repeat region (19, 30, 56). For instance, the minimal oriLyt of rhesus macaque rhadinovirus contains an AT-rich region and downstream GC-rich repeats (30), whereas the minimal oriLyt of bovine herpesvirus 4 is positioned downstream of a GC-rich repeat stretch and comprises a second repeat stretch with a predicted hairpin-loop structure (56).
The 8.4-kb viral sequences in pMO contain 21 copies of a 100-bp repeat that is highly GC-rich (86%). Therefore, as a first step to mapping the minimal oriLyt, we constructed deletion plasmids pMOΔ1 to -Δ5, which carry various regions 5′ of, 3′ of, or including the 100-bp repeat (Fig. 1). These plasmids, together with pMO(+), were transfected into 293T cells and analyzed in the replication assay. Among these five deletion plasmids, pMOΔ2 replicated most efficiently, producing a 9.4-kb band that was resistant to DpnI digestion (Fig. 2B, lane 2). Replication of pMOΔ1 was severely impaired (Fig. 2B, lane 1) compared to that of pMO (Fig. 2B, lane 6). The remaining three plasmids, pMOΔ3 to -Δ5, did not replicate (Fig. 2B, lanes 3 to 5). The results from this initial deletion analysis suggested that the minimal oriLyt may reside in the 6.4-kb subfragment contained in pMOΔ2.
Mapping a minimal MHV-68 oriLyt.
To further map the minimal oriLyt, we took a systematic approach. We first made a series of 5′ nested deletion constructs based on pMOΔ2, pMOΔ6 to -Δ12 (Fig. 1), and tested them experimentally. DNAs were extracted from transfected 293T cells, digested with appropriate enzymes, and analyzed by Southern blotting. All the constructs replicated and gave rise to DpnI-resistant bands of appropriate sizes (Fig. 3A, upper bracket). Among them, pMOΔ6 to -Δ11 replicated efficiently (Fig. 3A, lanes 2 to 7), compared to pMOΔ2 (Fig. 3A, lane 1). Although pMOΔ12 (Fig. 3A, lane 8) replicated less efficiently than pMOΔ6 to -Δ11, it fared better than pMOΔ1 (Fig. 2B, lane 1), as judged by the replication efficiency (defined by the ratio of the intensity of the DpnI-resistant band to that of the DpnI-sensitive band in the same sample). This result suggested that sequences 3′ of the 100-bp repeat are important for the function of oriLyt.
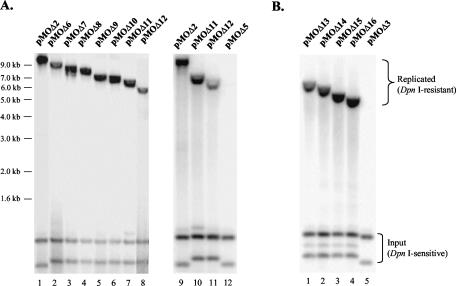
FIG. 3.
Mapping the minimal MHV-68 oriLyt. The 5′ deletion plasmids pMOΔ6 to -Δ12, pMOΔ2, and pMOΔ5 (A) or 3′ deletion plasmids pMOΔ13 to -Δ16 and pMOΔ3 (B) were tested in replication assays. DNA was extracted from each sample, digested, and analyzed by Southern blotting with radiolabeled pKS as a probe. Lower bracket, DpnI-sensitive input DNA. Upper bracket, DpnI-resistant replicated DNA.
We next repeated the replication assay to directly compare plasmids pMOΔ2, pMOΔ11, and pMOΔ12 to pMOΔ5. The oriLyt-containing sequences on these plasmids have the same 3′ end but differ in their 5′ end positions relative to the viral genome (Fig. 1). As shown in Fig. 3A, pMOΔ2 and pMOΔ11 (lanes 9 and 10) replicated more efficiently than pMOΔ12 (lane 11), whereas pMOΔ5 failed to replicate (lane 12), corroborating the previous results (Fig. 2B, lane 5). Therefore, the viral sequences present in pMOΔ12 but missing in pMOΔ5 (corresponding to the region between nucleotides 101248 and 101653 on the MHV-68 genome) are an essential part of MHV-68 oriLyt.
To determine the 3′ boundary of the minimal oriLyt, we generated a series of 3′ deletion constructs, pMOΔ13 to -Δ16, based on pMOΔ11 (Fig. 1). When tested together with pMOΔ3, all four plasmids showed efficient replication (Fig. 3B, lanes 1 to 4), in contrast to pMOΔ3, which reproducibly failed to do so (Fig. 3B, lane 5; Fig. 2B, lane 3). Two conclusions can be drawn from these data.:first, the viral sequence 3′ of the region contained in pMOΔ16 (corresponding to nucleotides 101974 to 104034 on the viral genome) is not required for oriLyt function; second, the region 3′ of the corresponding sequence in pMOΔ3 that is contained in pMOΔ16 (corresponding to nucleotides 101653 to 101974 on the viral genome) is essential to confer oriLyt function.
Identified MHV-68 oriLyt replicates during viral reactivation.
The oriLyt of gammaherpesviruses have been identified through replication assays driven by either de novo infection (e.g., rhesus macaque rhadinovirus) (30, 56) or reactivation of latent viruses (e.g., EBV, bovine herpesvirus 4, and HHV-8) (5, 19, 24). However, no studies to test and compare oriLyt in both de novo infection and viral reactivation have been reported. The availability of both permissive cell lines for MHV-68 de novo infection and a latently infected B-cell line (S11E) (45) makes it possible to examine this question in the MHV-68 system.
In this study, we took advantage of the robust replication of MHV-68 during de novo infection to identify its oriLyt. To test whether the identified oriLyt also functions during viral reactivation, we cotransfected pMOΔ2 and an RTA expression plasmid pFlag/MRTA (53) or the vector pFlag-CMV-2 into S11E cells. As controls, we also cotransfected pKS with either pFlag/MRTA or pFlag-CMV-2. Ectopic expression of the “master switch” protein RTA activated MHV-68 lytic replication in S11E cells and provided trans factors for pMOΔ2 to replicate (Fig. 4A, lane 3). In the absence of viral lytic proteins, pMOΔ2 failed to replicate (Fig. 4A, lane 4). As expected, pKS, which lacks the cis element for oriLyt function, did not replicate (Fig. 4A, lanes 1 and 2). These results demonstrated that the identified MHV-68 oriLyt functions in both de novo infection and viral reactivation and that viral latent gene products do not support the replication of oriLyt.
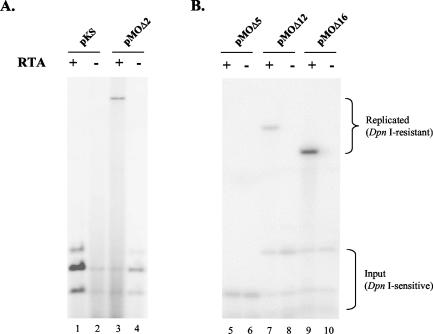
FIG. 4.
Function of MHV-68 oriLyt during viral reactivation. (A) pMOΔ2 or pKS and an RTA-expressing plasmid, pFlag/MRTA, or pFlag-CMV-2, were cotransfected by electroporation into S11E cells, a murine B-cell line harboring latent MHV-68. At 24 h posttransfection, DNA was prepared from each sample and probed with radiolabeled pKS for Southern blot analysis. Expression of MHV-68 RTA induced latent MHV-68 in S11E cells to reactivate and thus the oriLyt plasmid pMOΔ2 to replicate. (B) The oriLyt deletion constructs pMOΔ5, pMOΔ12, and pMOΔ16 were tested in S11E cells as described for A. Lower bracket, DpnI-sensitive input DNA. Upper bracket, DpnI-resistant replicated DNA.
To examine whether the oriLyt deletion constructs exhibit similar replication efficiencies during reactivation as in de novo infection, we next tested pMOΔ12, pMOΔ16, and pMOΔ5 in S11E cells. pMOΔ12 and pMOΔ16 are the smallest 5′ and 3′ deletion constructs, respectively, that replicated in de novo-infected 293T cells; pMOΔ5, on the other hand, failed to replicate. As shown in Fig. 4B, in the absence of viral lytic replication, none of the plasmids replicated (lanes 6, 8, and 10). However, when RTA was expressed, replication of pMOΔ12 and pMOΔ16 occurred, as indicated by the DpnI-resistant band (lanes 7 and 9). Consistent with previous data in 293T cells (Fig. 2B, lane 5, and Fig. 3A, lane 12), no replication of pMOΔ5 was observed (Fig. 4B, lane 5). The relative replication efficiencies of these plasmids during viral reactivation in S11E cells were also very similar to those during de novo infection of 293T cells (Fig. 1).
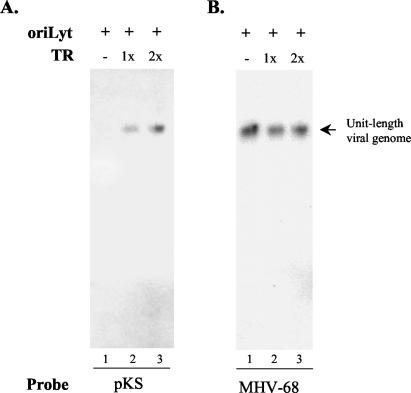
Terminal repeat sequences of MHV-68 contain a functional packaging signal.
The packaging signal of herpesviruses is usually contained in the termini of the viral genomes (10, 11, 41, 46). We therefore cloned one or two copies of the 1.2-kb MHV-68 terminal repeat into the oriLyt plasmid pMOΔ2. The constructs, pMOΔ2-1TR and pMOΔ2-2TR, were individually introduced into 293T cells and tested in a packaging assay. As a control, pMOΔ2, which lacks the terminal repeat, was also included in the assay. Extracellular virions were purified through centrifugation from supernatants, embedded in agarose plugs, lysed in situ, and analyzed by pulsed-field gel electrophoresis and Southern blotting.
Two probes were used sequentially. A probe specific for pKS detected a signal only in samples from pMOΔ2-1TR or pMOΔ2-2TR transfection (Fig. 5A, lanes 2 and 3), but not in the sample from the pMOΔ2 transfection (Fig. 5A, lane 1), indicating that the terminal sequence is sufficient for packaging of the plasmid (amplicon) sequences into mature virions. The blot was then stripped and probed again with a sequence specific for wild-type virus (this sequence is absent from pMOΔ2-based plasmids). Not surprisingly, a fragment corresponding to wild-type virus (helper virus) genomes was detected in all three samples (Fig. 5B, lanes 1 to 3). The size of the fragment detected by the sequence specific for wild-type virus was the same as or very close to that detected by pKS (Fig. 5, arrow). As the sizes for the plasmids pMOΔ2-1TR, pMOΔ2-2TR, and wild-type MHV-68 genome are 10.6, 11.8, and ≈130 kb, respectively, this result suggested that ≈11 to 12 copies of the plasmid are packaged as a concatemer into each viral particle.
FIG. 5.
Terminal repeat of MHV-68 contains a functional packaging signal. One and two copies of the MHV-68 terminal repeat were cloned into pMOΔ2 to generate pMOΔ2-1TR and pMOΔ2-2TR, respectively. pMOΔ2, pMOΔ2-1TR, or pMOΔ2-2TR was transfected into 293T cells. At 24 h posttransfection, the samples were infected with MHV-68. At 72 h postinfection, viral particles were harvested from the supernatant, embedded in agarose, lysed in situ, and analyzed by pulsed-field gel electrophoresis and Southern blotting. The membrane was first probed with radiolabeled pKS to reveal the packaged amplicon DNA (A), stripped, and reprobed with a radiolabeled DNA sequence corresponding to the ORF57 promoter on the viral genome (which is absent from the plasmids) to identify the wild-type viral genome being packaged (B).
Furthermore, we examined the ratio of packaged plasmid DNA (as indicated by the intensity of the individual radiolabeled band in Fig. 5A, lane 2 or 3) to packaged wild-type viral DNA (as indicated by the intensity of the corresponding band in Fig. 5B, lane 2 or 3) in each sample. The data showed that pMOΔ2-2TR was packaged at an average 50% higher efficiency than pMOΔ2-1TR, consistent with observations from other herpesviruses that there are usually multiple copies of the terminal repeat at the viral genomic termini (4, 21, 22, 28, 34), which may provide a higher packaging efficiency.
Comparison of lytic DNA replication of MHV-68 and HHV-8.
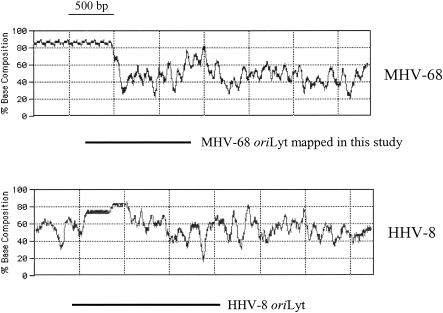
The oriLyt regions of HHV-8 have recently been identified (5, 24). The 1.7-kb oriLyt sequences on the right end of the genome comprise a GC-rich repeat sequence and a downstream AT-rich region. We mapped the MHV-68 oriLyt to a shorter region of 1.25 kb. Comparison of the corresponding regions containing oriLyt from the right end of the MHV-68 and HHV-8 genomes showed that they have a similar arrangement, with a GC-rich region at the 5′ end and a relatively AT-rich region at the 3′ end (Fig. 6). Our deletion analysis also suggested that, within the 1.25-kb MHV-68 minimal oriLyt sequences (nucleotides 100723 to 101974), the region corresponding to nucleotides 101248 to 101974 is essential, whereas the region 5′ of it may serve as an auxiliary element to enhance replication efficiency (compare pMOΔ11 and pMOΔ12, Fig. 3A).
FIG. 6.
Comparison of oriLyt regions from MHV-68 and HHV-8. The G+C content of the corresponding regions from MHV-68 and HHV-8 is shown. Horizontal lines indicate the relative position of the minimal oriLyt mapped in this study compared to that of HHV-8 oriLyt (5, 24). Window size, 500 bp.
In addition to the oriLyt present towards the right end of the HHV-8 genome, a second oriLyt has also been identified towards the left end of the viral genome (5, 24). These two oriLyt elements share an almost identical 1.15-kb sequence (>90% homology) and a 600-bp downstream GC-rich repeat sequence in opposite directions. Our sequence analysis showed that such an extended, almost duplicated copy of the 1.25-kb sequence that we mapped (nucleotides 100723 to 101974) does not exist in MHV-68. Therefore, if MHV-68 contains a second copy of oriLyt towards the left end of the viral genome, like HHV-8, the functionality of the putative oriLyt-containing region needs to be tested experimentally. This can be conducted by cloning it into a heterologous plasmid and testing whether it confers on the plasmid the ability to replicate in the presence of appropriate replication proteins. Furthermore, over the last few years, a number of herpesvirus genomes have been successfully cloned into a bacterial artificial chromosome (BAC) (1, 27). Generation and manipulation of mutant viruses can now be efficiently performed in Escherichia coli with prokaryotic recombination. The 1.25-kb oriLyt that we mapped in this study and/or the putative second oriLyt may also be knocked out from the MHV-68 genome with the BAC system. Inhibition of replication in permissive mammalian cells for these mutant BAC plasmids should further confirm the function of oriLyt.
On the other hand, there is one important difference between MHV-68 and HHV-8 or EBV in terms of trans factors required for mediating viral lytic DNA replication. For EBV, seven viral proteins are required, including single-stranded-DNA-binding protein, DNA polymerase, helicase, primase, helicase-associated factor, polymerase processivity factor, and the transcriptional activator Zebra (also called Zta), which also serves as an origin-binding protein (14, 15). In HHV-8, functional homologues of six of these proteins have been identified (35, 52). In addition, the K8 (also called KbZip) protein, which shares similarity with EBV Zebra, has recently been shown to carry out the origin-binding protein function (24). In MHV-68, genes encoding homologues of six of these proteins have also been identified. They are ORF6, ORF9, ORF40, ORF44, ORF56, and ORF59, respectively (47). However, despite extensive research, no Zebra or K8 homologue has been identified, suggesting that there may be unique aspects to MHV-68 lytic DNA replication.
There are two possible scenarios. MHV-68 encodes a protein different from Zebra or K8 that serves the origin-binding function. Alternatively, although less likely, MHV-68 may have evolved a strategy to replicate its DNA during the lytic phase without requiring a viral origin-binding protein. Defining the cis sequences required for viral genome replication and maturation as reported here makes possible future studies to distinguish between these scenarios and reveal unique aspects of MHV-68 DNA replication. Collectively, these studies will provide insights into the biology of MHV-68. As MHV-68 is becoming an increasingly important experimental system, understanding of its replication elements is also essential for further development of this system.
The fact that six of seven proteins involved in viral DNA replication during the lytic phase were identified in MHV-68 suggests that MHV-68 also shares with HHV-8 and EBV basic mechanisms for lytic DNA replication. Unlike its counterparts from other gammaherpesviruses whose replication is very inefficient (5, 19, 24, 30, 56), the replication of MHV-68 oriLyt in a cell culture system is robust, judged by the ratio of replicated plasmid DNA to input plasmid DNA in the replication assays in our studies. Therefore, MHV-68 also represents an excellent model system to investigate the general mechanisms governing gammaherpesviral DNA replication and packaging during the viral lytic phase.
Potential of MHV-68-based vectors for gene delivery.
Originally isolated from wild murid rodents, MHV-68 is capable of infecting laboratory strains of mice without adverse pathogenic effect (29, 42). This mirrors EBV infection in healthy people, which usually results in life-long viral persistence without clinical symptoms (21). During the course of studying MHV-68, we noticed its many attractive features for development as a gene delivery vector. First, MHV-68 infects cells in vitro with high efficiency and replicates in permissive cell lines in a robust manner. Viral titers are in the range of 108 PFU/ml (without concentration), 3 to 4 logs higher than that obtained with EBV or HHV-8 and considerably higher than those obtained with retroviruses and lentiviruses. Second, in nonpermissive cell types, MHV-68 maintains its genome as an episome in multiple copies during latency (45), most likely utilizing a strategy similar to that of EBV involving EBNA-1 and oriP. The persistence of the viral genome as an episome independent of host chromosomes avoids the possible risk of insertional mutagenesis that is generally associated with several other viral vectors.
Third, three distinct regions of MHV-68 genome are transcriptionally active in latently infected mice (48), allowing persistent expression of transgenes. This is in contrast to herpes simplex virus type 1-based vectors, in which foreign gene expression is often shut down (9, 13, 18, 26). Fourth, humans have no preexisting immunity to MHV-68; therefore, packaged MHV-68 amplicon particles will not be neutralized by existing immune responses. Furthermore, the particle-to-infectivity ratio for MHV-68 is less than 10 (Y. Shu and R. Sun, unpublished data), allowing efficient infection with fewer viral particles. This property decreases the immunogenicity caused by virion components when introduced into a host, in contrast to adenovirus- or retrovirus-based delivery vectors. Lastly, MHV-68 not only infects a broad range of cells of different tissue types in vivo, including epithelial cells from the oral and nasal cavities, lung and gastrointestinal tracts, B cells, macrophages and dendritic cells in the spleen, peritoneal extrude cells and liver cells, but also establishes latency (and thereby potentially allows persistent foreign gene expression) in B cells, dendritic cells, macrophages, and epithelial cells (16, 39, 43, 50). This is again in contrast to herpes simplex virus type 1, which only establishes latency in terminally differentially neuronal cells (9, 13, 18, 26). In vitro, MHV-68 infects and replicates in a broad range of cell types from both humans and mice, including cell lines, primary macrophages, and stem cells (unpublished observation). Therefore, MHV-68 has the potential to deliver foreign genes into different host tissues.
Because an amplicon vector contains only the oriLyt and packaging signal, the minimal cis elements required for viral DNA replication and processing, propagation of an amplicon requires many viral proteins to be provided in trans by a suitable helper virus or a helper virus genome (9, 18). Construction of a helper virus has traditionally been carried out via homologous recombination in a permissive cell line, an inefficient and time-consuming process. With the recent availability of herpesvirus genomes cloned into a BAC (1, 27), viral particles containing the desired mutation can be reconstituted by transfection of BAC plasmids into eukaryotic cells. We and other groups have independently cloned the MHV-68 genome into a BAC. The MHV-68/BAC virus, when introduced into mice, replicated similarly to the wild-type virus (2, 3; Wu et al., unpublished data). Therefore, it is quite feasible to generate suitable MHV-68 helper viruses (e.g., those with the terminal repeats deleted from the viral genome and therefore incapable of being packaged themselves) for our MHV-68 amplicons. Furthermore, viral genes essential for MHV-68 replication in vitro are currently being mapped in our laboratory (Song et al., unpublished data). Genes dispensable for MHV-68 replication in vitro may play roles in viral replication in vivo and are likely associated with viral pathogenesis. The genome-mapping information generated will facilitate the design of minimal helper viruses to enhance the safety of the amplicon vectors in the event of recombination between helper viruses and amplicons during propagation of amplicon viral particles.
Many fundamental issues related to gene therapy are best addressed experimentally in mouse models. The fact that MHV-68 infects both mouse and human cells suggests that MHV-68-based gene delivery vectors can be studied extensively in the mouse model system before being tested in human clinical trials in the future. Taken together, the features illustrated here make MHV-68 an appealing system on which to build gene delivery vectors. Identification of the MHV-68 oriLyt and packaging signal has laid a solid foundation for designing amplicon-type gene delivery vectors based on MHV-68.
Acknowledgments
We thank members of the Sun laboratory for helpful discussion.
This work was supported by NIH grants CA91791, CA83525, and DE14153 and support from the Stop Cancer Foundation and Jonsson Cancer Center Foundation (R.S.). H.D. was a Lymphoma Research Foundation Fellow.
REFERENCES
- 1.Adler, H., M. Messerle, and U. H. Koszinowski. 2003. Cloning of herpesviral genomes as bacterial artificial chromosomes. Rev. Med. Virol. 13:111-121. [DOI] [PubMed] [Google Scholar]
- 2.Adler, H., M. Messerle, and U. H. Koszinowski. 2001. Virus reconstituted from infectious bacterial artificial chromosome (BAC)-cloned murine gammaherpesvirus 68 acquires wild-type properties in vivo only after excision of BAC vector sequences. J. Virol. 75:5692-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler, H., M. Messerle, M. Wagner, and U. H. Koszinowski. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 74:6964-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht, J. C., J. Nicholas, D. Biller, K. R. Cameron, B. Biesinger, C. Newman, S. Wittmann, M. A. Craxton, H. Coleman, B. Fleckenstein, et al. 1992. Primary structure of the herpesvirus saimiri genome. J. Virol. 66:5047-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AuCoin, D. P., K. S. Colletti, Y. Xu, S. A. Cei, and G. S. Pari. 2002. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains two functional lytic origins of DNA replication. J. Virol. 76:7890-7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballestas, M. E., P. A. Chatis, and K. M. Kaye. 1999. Efficient persistence of extrachromosomal KSHV DNA mediated by latency-associated nuclear antigen. Science 284:641-644. [DOI] [PubMed] [Google Scholar]
- 7.Ballestas, M. E., and K. M. Kaye. 2001. Kaposi's sarcoma-associated herpesvirus latency-associated nuclear antigen 1 mediates episome persistence through cis-acting terminal repeat (TR) sequence and specifically binds TR DNA. J. Virol. 75:3250-3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloss, T. A., and B. Sugden. 1994. Optimal lengths for DNAs encapsidated by Epstein-Barr virus. J. Virol. 68:8217-8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burton, E. A., Q. Bai, W. F. Goins, and J. C. Glorioso. 2002. Replication-defective genomic herpes simplex vectors: design and production. Curr. Opin. Biotechnol. 13:424-428. [DOI] [PubMed] [Google Scholar]
- 10.Deiss, L. P., and N. Frenkel. 1986. Herpes simplex virus amplicon: cleavage of concatemeric DNA is linked to packaging and involves amplification of the terminally reiterated a sequence. J. Virol. 57:933-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng, H., and S. Dewhurst. 1998. Functional identification and analysis of cis-acting sequences which mediate genome cleavage and packaging in human herpesvirus 6. J. Virol. 72:320-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Efstathiou, S., Y. M. Ho, S. Hall, C. J. Styles, S. D. Scott, and U. A. Gompels. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 71:1365-1372. [DOI] [PubMed] [Google Scholar]
- 13.Fink, D. J., N. A. DeLuca, W. F. Goins, and J. C. Glorioso. 1996. Gene transfer to neurons using herpes simplex virus-based vectors. Annu. Rev. Neurosci. 19:265-287. [DOI] [PubMed] [Google Scholar]
- 14.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of Epstein-Barr virus oriLyt: lack of a dedicated virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 69:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1992. trans-acting requirements for replication of Epstein-Barr virus ori-Lyt. J. Virol. 66:5030-5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 17.Frappier, L., and M. O'Donnell. 1991. Epstein-Barr nuclear antigen 1 mediates a DNA loop within the latent replication origin of Epstein-Barr virus. Proc. Natl. Acad. Sci. USA 88:10875-10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frenkel, N., O. Singer, and A. D. Kwong. 1994. Minireview: the herpes simplex virus amplicon-a versatile defective virus vector. Gene Ther. 1(Suppl.) 1:S40-S46. [PubMed] [Google Scholar]
- 19.Hammerschmidt, W., and B. Sugden. 1988. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell 55:427-433. [DOI] [PubMed] [Google Scholar]
- 20.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 21.Kieff, E., and A. B. Rickinson. 2001. Epstein-Barr virus and its replication, p. 2511-2627. In D. M. Knipe and P. M. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, Pa. [Google Scholar]
- 22.Lagunoff, M., and D. Ganem. 1997. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology 236:147-154. [DOI] [PubMed] [Google Scholar]
- 23.Leight, E. R., and B. Sugden. 2000. EBNA-1: a protein pivotal to latent infection by Epstein-Barr virus. Rev. Med. Virol. 10:83-100. [DOI] [PubMed] [Google Scholar]
- 24.Lin, C. L., H. Li, Y. Wang, F. X. Zhu, S. Kudchodkar, and Y. Yuan. 2003. Kaposi's sarcoma-associated herpesvirus lytic origin (ori-Lyt)-dependent DNA replication: identification of the ori-Lyt and association of K8 bZip protein with the origin. J. Virol. 77:5578-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackett, M., J. P. Stewart, S. de V Pepper, M. Chee, S. Efstathiou, A. A. Nash, and J. R. Arrand. 1997. Genetic content and preliminary transcriptional analysis of a representative region of murine gammaherpesvirus 68. J. Gen. Virol. 78:1425-1433. [DOI] [PubMed] [Google Scholar]
- 26.Maguir-Zeis, K. A., W. J. Bowers, and H. J. Federoff. 2001. HSV vector-mediated gene delivery to the central nervous system. Curr. Opin. Mol. Ther. 3:482-490. [PubMed] [Google Scholar]
- 27.Messerle, M., G. Hahn, W. Brune, and U. H. Koszinowski. 2000. Cytomegalovirus bacterial artificial chromosomes: a new herpesvirus vector approach. Adv. Virus Res. 55:463-478. [DOI] [PubMed] [Google Scholar]
- 28.Mocarski, E. S. J., and C. T. Courcelle. 2001. Cytomegaloviruses and their replication, p. 2629-2673. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 29.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine gamma-herpesvirus infection. Phil. Trans. R. Soc. London B Biol. Sci. 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pari, G. S., D. AuCoin, K. Colletti, S. A. Cei, V. Kirchoff, and S. W. Wong. 2001. Identification of the rhesus macaque rhadinovirus lytic origin of DNA replication. J. Virol. 75:11401-11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peacock, J. W., and K. L. Bost. 2000. Infection of intestinal epithelial cells and development of systemic disease following gastric instillation of murine gammaherpesvirus-68. J. Gen. Virol. 81:421-429. [DOI] [PubMed] [Google Scholar]
- 32.Raab-Traub, N., and K. Flynn. 1986. The structure of the termini of the Epstein-Barr virus as a marker of clonal cellular proliferation. Cell 47:883-889. [DOI] [PubMed] [Google Scholar]
- 33.Rawlins, D. R., G. Milman, S. D. Hayward, and G. S. Hayward. 1985. Sequence-specific DNA binding of the Epstein-Barr virus nuclear antigen (EBNA-1) to clustered sites in the plasmid maintenance region. Cell 42:859-868. [DOI] [PubMed] [Google Scholar]
- 34.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 35.Russo, J. J., R. A. Bohenzky, M. C. Chien, J. Chen, M. Yan, D. Maddalena, J. P. Parry, D. Peruzzi, I. S. Edelman, Y. Chang, and P. S. Moore. 1996. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc. Natl. Acad. Sci. USA 93:14862-14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6:276-282. [DOI] [PubMed] [Google Scholar]
- 37.Speck, S. H., and H. W. Virgin. 1999. Host and viral genetics of chronic infection: a mouse model of gamma-herpesvirus pathogenesis. Curr. Opin. Microbiol. 2:403-409. [DOI] [PubMed] [Google Scholar]
- 38.Stevenson, P. G., and P. C. Doherty. 1998. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 72:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su, W., T. Middleton, B. Sugden, and H. Echols. 1991. DNA looping between the origin of replication of Epstein-Barr virus and its enhancer site: stabilization of an origin complex with Epstein-Barr nuclear antigen 1. Proc. Natl. Acad. Sci. USA 88:10870-10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun, R., T. A. Spain, S. F. Lin, and G. Miller. 1997. Sp1 binds to the precise locus of end processing within the terminal repeats of Epstein-Barr virus DNA. J. Virol. 71:6136-6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sunil-Chandra, N. P., S. Efstathiou, J. Arno, and A. A. Nash. 1992. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J. Gen. Virol. 73:2347-2356. [DOI] [PubMed] [Google Scholar]
- 43.Sunil-Chandra, N. P., S. Efstathiou, and A. A. Nash. 1992. Murine gammaherpesvirus 68 establishes a latent infection in mouse B lymphocytes in vivo. J. Gen. Virol. 73:3275-3279. [DOI] [PubMed] [Google Scholar]
- 44.Tibbetts, S. A., J. Loh, V. Van Berkel, J. S. McClellan, M. A. Jacoby, S. B. Kapadia, S. H. Speck, and H. W. Virgin. 2003. Establishment and maintenance of gammaherpesvirus latency are independent of infective dose and route of infection. J. Virol. 77:7696-7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Usherwood, E. J., J. P. Stewart, and A. A. Nash. 1996. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. J. Virol. 70:6516-6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Varmuza, S. L., and J. R. Smiley. 1985. Signals for site-specific cleavage of HSV DNA: maturation involves two separate cleavage events at sites distal to the recognition sequences. Cell 41:793-802. [DOI] [PubMed] [Google Scholar]
- 47.Virgin, H. W., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virgin, H. W., R. M. Presti, X. Y. Li, C. Liu, and S. H. Speck. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 73:2321-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlazny, D. A., A. Kwong, and N. Frenkel. 1982. Site-specific cleavage/packaging of herpes simplex virus DNA and the selective maturation of nucleocapsids containing full-length viral DNA. Proc. Natl. Acad. Sci. USA 79:1423-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weck, K. E., S. S. Kim, H. I. Virgin, and S. H. Speck. 1999. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J. Virol. 73:3273-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willer, D. O., and S. H. Speck. 2003. Long-term latent murine Gammaherpesvirus 68 infection is preferentially found within the surface immunoglobulin D-negative subset of splenic B cells in vivo. J. Virol. 77:8310-8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu, F. Y., J. H. Ahn, D. J. Alcendor, W. J. Jang, J. Xiao, S. D. Hayward, and G. S. Hayward. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J. Virol. 75:1487-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu, T. T., E. J. Usherwood, J. P. Stewart, A. A. Nash, and R. Sun. 2000. Rta of murine gammaherpesvirus 68 reactivates the complete lytic cycle from latency. J. Virol. 74:3659-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yates, J., N. Warren, D. Reisman, and B. Sugden. 1984. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc. Natl. Acad. Sci. USA 81:3806-3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yates, J. L., N. Warren, and B. Sugden. 1985. Stable replication of plasmids derived from Epstein-Barr virus in various mammalian cells. Nature 313:812-815. [DOI] [PubMed] [Google Scholar]
- 56.Zimmermann, W., H. Broll, B. Ehlers, H. J. Buhk, A. Rosenthal, and M. Goltz. 2001. Genome sequence of bovine herpesvirus 4, a bovine rhadinovirus, and identification of an origin of DNA replication. J. Virol. 75:1186-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]