Introduction
The implementation of optogenetic tools to manipulate neuronal activity with light is transforming our ability to investigate neural systems. By expressing light-sensitive proteins, or opsins, in genetically defined neuronal populations, optogenetic approaches permit new experimental questions that span from the specific properties of defined synaptic connections to their roles in complex behaviors [41,57,106,121]. In this review we focus on opsins for manipulating neuronal activity and discuss their biophysical properties, delivery strategies, and how these techniques have been adapted to unravel somatosensory circuits.
Opsin Features
Light-gated ion flux was first identified in extremophile bacteria [114,115], but three decades passed until this phenomenon was exploited to activate mammalian neurons with light using channelrhodopsin-2 (ChR2) [22,87,110,111]. The majority of opsins used for fast optical control are light-gated ion channels and pumps isolated from diverse microorganisms, and these proteins exhibit a vast array of biophysical properties and light sensitivities [46,162] (Fig. 1A,B). Excitatory opsins, like ChR2, are cation selective [110,111], and gate inward photocurrents that depolarize neurons when illuminated by a variety of light wavelengths [6,22,166] (Fig. 1A,D). The most widely used inhibitory opsins are pumps that mediate chloride influx [54,56,167] or proton efflux [34,83] to generate outward photocurrents and rapidly silence neuronal firing (Fig. 1B,E).
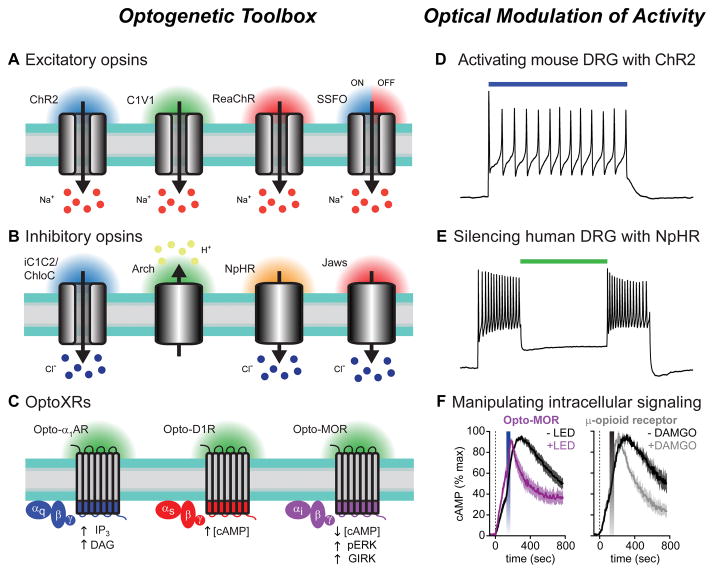
Figure 1. Optogenetic toolbox and functional properties.
(A–C) Opsins are illustrated with their respective wavelengths of light activation. Arrows indicate direction of ion flux or effect on intracellular signaling pathways. (A) Excitatory opsins like ChR2 (far left) are nonspecific cation channels that depolarize neurons when stimulated with blue light. Interest in separately controlling different cell-types led to the development of opsins with shifted spectral properties, including the green light activatable C1V1 chimera (center left), and a red-light activatable ChR2 variant, ReaChR (center right). Stable step-function opsins (SSFOs) gate long-lasting photocurrents after brief pulses of blue light that can be rapidly terminated by red light (far right). Sodium ions flowing down their electrochemical gradient are depicted as the predominant charge carrier; however, these channels are also permeable to protons, potassium, and calcium ions.
(B) iC1C2 and ChloC (far left) are channelrhodopsin variants engineered to function as inhibitory chloride-conducting channels. These opsins silence neuronal activity when illuminated with blue light. Archaerhodopsin (Arch, center left) is a green-light activated proton pump that generates proton efflux to hyperpolarize neurons. Halorhodopsins, like NpHR (center right) and Jaws (far right), are chloride pumps activated by yellow and red light, respectively. Owing to the greater tissue penetration of red-shifted light, Jaws can silence activity in vivo in combination with non-invasive transcranial illumination with red light.
(C) Cartoon depicting optoXR strategy for optogenetic control of G-protein coupled receptor (GPCR) signaling. These chimeric proteins consist of the extracellular and transmembrane domains of light-sensitive rhodopsins with the intracellular regions of a GPCR of interest. Shown are Gq-coupled α1 adrenergic receptors (left, blue), Gs-coupled D1 dopamine receptors (middle, red) and Gi-coupled μ-opioid receptors (right, purple). The intracellular signaling molecules engaged by optical stimulation with blue-green light are shown below each receptor.
(D) Voltage trace showing action potential firing in ChR2-expressing mouse DRG neurons during illumination with blue light (indicated by the colored bar). Modified from reference [122] with permission.
(E) Using green light to silence human DRG neurons expressing the inhibitory chloride pump NpHR. Primary cultures of human sensory neurons were infected with Herpes simplex virus carrying the inhibitory opsin NpHR. Voltage trace showing sustained action potential firing during depolarizing current injections, which was inhibited when neurons were illuminated with green light (colored bar).
(F) Summary graphs showing Gi-mediated inhibition of forskolin-induced cAMP production in cells expressing the light sensitive μ-opioid receptor chimera opto-MOR after blue LED stimulation (left graph, blue bar). The kinetics of cAMP inhibition were indistinguishable from cells expressing the wild-type μ-opioid receptor stimulated with the agonist DAMGO (right graph, gray bar). Modified from reference [137] with permission.
Currently available opsins are the result of genome screening [34,53,78,149,156,165] and molecular engineering strategies [36,55,79,89,101,104,126,127,150,153] to expand the optogenetic toolbox for diverse applications. These approaches have generated faster excitatory variants for reliable high-speed manipulations [15,60,145], red-shifted opsins for improved light penetration [35,88], and bistable step-function mutations to trigger long-lasting changes in activity [9,16]. Recently, using the crystal structure of channelrhodopsin [74], rationale-based protein engineering strategies transformed excitatory opsins into chloride channels for prolonged optical inhibition [13,14,155,157].
In addition to rapid control of ion flux, molecular engineering strategies have developed optical approaches for manipulating intracellular signaling cascades of cell-surface G-protein coupled receptors (GPCRs). Endowing light sensitivity to GPCRs was achieved by splicing the intracellular loops of GPCRs of interest to the extracellular and transmembrane domains of the light-sensitive GPCR rhodopsin [3,75]. This chimeric approach has generated photo-activatable adrenergic [3,75,138], serotonin [116], μ-opioid [10,137], dopamine [59], adenosine [86], and metabotropic glutamate receptors [159]. Different optoXRs can couple to their endogenous G-protein-mediated intracellular signaling cascades to modulate second messenger systems (Fig. 1C). Of potential clinical relevance, an optically activated μ-opioid receptor was recently demonstrated to engage the same signaling cascades as native receptors, including Gi-mediated inhibition of cAMP (Fig. 1C,F), and Gβ/γ activation of inwardly rectifying potassium channels [137]. Triggering GPCR activation with light offers spatiotemporal precision currently impossible with traditional pharmacological agents or chemogenetic manipulations [142]. By mimicking the signaling systems of endogenous receptors in a physiologically relevant manner, optoXRs may be advantageous for studying the roles of these important therapeutic targets in precisely defined regions of the body.
Targeting Opsin Expression in vivo
Two primary strategies have been employed to deliver opsins into the nervous system: viral vectors and opsin-expressing transgenic mice [66,93,163,164]. Expression specificity with viral transgene delivery can be generated by natural tropism [17,164], incorporation of endogenous promoters [1,166], or recombinase-dependent expression [23,112] (Fig. 2A–D). The first two approaches have limitations with cell-type specificity and variable or non-specific transgene expression, potentially leading to problems in experimental interpretation. The most common viral expression method relies on Cre/LoxP-mediated recombination and conditional expression of transgenes delivered by adeno-associated viruses (AAVs) [7,28,134,139] (Fig. 2A). These viruses must be injected into transgenic animals where Cre-recombinase expression is restricted to genetically-defined cell types [51,52,61,144] (Fig. 2B). Crossing Cre driver mice with genetically encoded opsin lines can enable specific photo-manipulation of molecularly-defined cell-types [96,97]. This strategy is advantageous for investigating large neuronal populations; however, targeting of pathway-specific projections with viral strategies is lost (Fig. 2C,D). While Cre driver lines grant genetic access to molecularly-defined cell-types, they may still comprise heterogeneous populations with distinct functions. New strategies employing intersectional genetic techniques, which utilizes multiple recombinase steps to enable genetic specificity [44,95], offers tremendous potential for investigating neuronal subpopulations [21,45,69,117,129,131,132] (Fig. 2D). By targeting different recombinase enzymes to distinct, but partially overlapping cellular populations, transgene expression can be more precisely refined. Opsins are now being implemented with these new genetic approaches to investigate neural circuits [47,95,132].
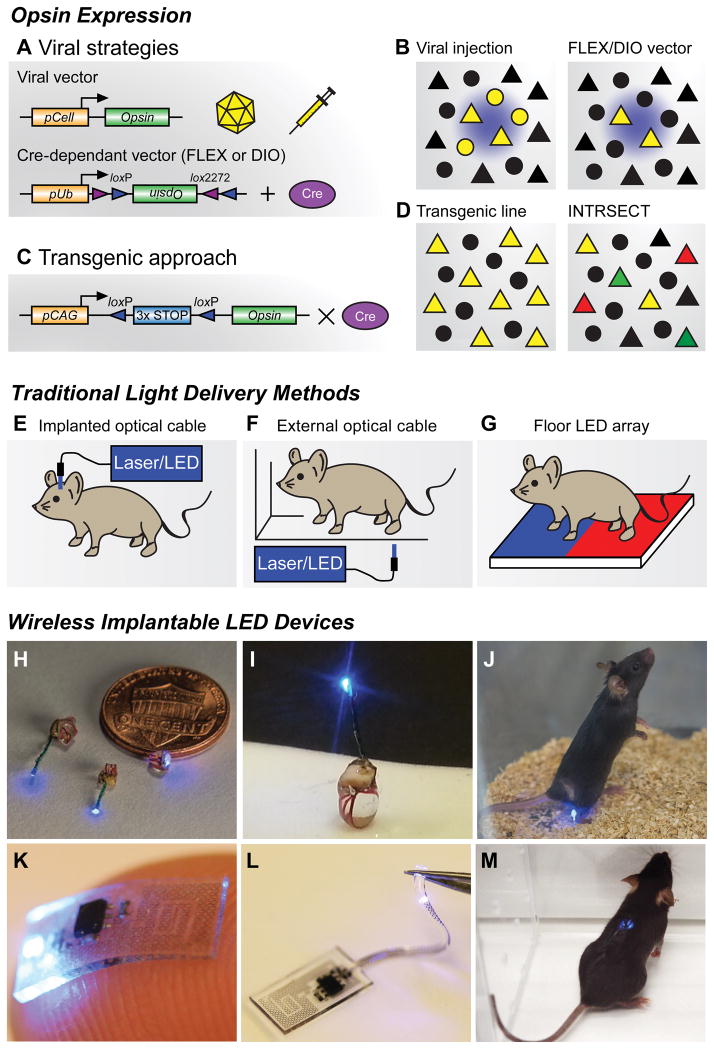
Figure 2. Opsin expression strategies and light delivery approaches.
(A) Viral vector strategies for delivering opsins in vivo. Transgene delivery most commonly uses injections of adeno-associated viral (AAV) vectors, though Herpes simplex virus and lentivirus can also be used. Specificity can be achieved by using a promoter of interest to restrict expression to specific cell types (pCell, top), however this approach can lead to weak or non-specific opsin expression. Cre-recombinase dependent vectors using flip-excision (FLEX) or double inverted orientation (DIO) targeting approaches (bottom). An inverted opsin gene (inactive) is flanked with two non-homologous recombination sites (loxP and lox2272). In the presence of Cre recombinase, the opsin is excised and flipped into a functional orientation. Because specificity is generated through tissue-specific expression of Cre-recombinase, strong ubiquitous promoters (pUb) can be used; however, these viruses must be injected in Cre-expressing transgenic animals (Cre database - http://www.gensat.org/cre.jsp).
(B) Cartoon illustrating focal transgene expression (yellow) from a non-Cre-dependent construct in a population of cells near the injection site (left). Similar example illustrating viral delivery of a FLEX/DIO vector into a transgenic animal with Cre-recombinase, depicted here with triangles (right).
(C) Transgenic approach for expressing opsins in vivo. Opsin constructs can be introduced into genetic loci to generate transgenic animals for conditional expression. In this approach, opsin genes are preceded by multiple stop codons to prevent expression. These stop codons are flanked by loxP sites, which are excised in the presence of Cre recombinase, generating strong cell-type and region-specific expression.
(D) Crossing transgenic opsin lines with Cre driver mice can grant optogenetic access to large neuronal populations (left). Further genetic refinement can be achieved using intersectional genetic approaches like INTRSECT (right). Here cellular specificity is controlled by using multiple recombination events with different enzymes. One group of genetically-defined cells is depicted in green, while a separate population is red. Yellow triangles represent the targeted subpopulation of cells that exhibit genetic features of both of groups.
(E) Different brain regions can be anatomically targeted by implanting optical fibers into cannulas affixed to the skull. Light is frequently delivered through a laser or LED light source; however, mice must be tethered to optical cables during behavioral experiments.
(F) Optical fibers can be used to illuminate external tissues with light from a laser or LED light source. While animals are untethered, access to deeper tissues is not possible, and uniform light delivery can be problematic.
(G) Opsins expressed in peripheral tissues of the paw can be stimulated by LED arrays placed in the floor of behavioral chambers, allowing for untethered movement during experiments. This approach can easily incorporate different stimulation wavelengths.
(H,I) Wireless implantable LED devices for stimulating superficial areas in the brain, spinal cord, and peripheral tissues. Reproduced from reference [68] with permission.
(J) Image of a freely moving animal with a LED device implanted in the hindpaw. Wireless activation is achieved via radio frequency waves generated by a resonant cavity below the chamber. This couples electromagnetic energy to the mouse, which is harvested by the implant. Reproduced from reference [68] with permission.
(K,L) Flexible wireless μLED devices designed to directly interface with the sciatic nerve (K), or be threaded into the epidural space of the spinal column to spinal cord (L) for optogenetic stimulation. Reproduced from reference [122] with permission.
(M) Image of a freely moving mouse with a flexible device implanted in the spinal column. Wireless activation is achieved by an external radio frequency antenna that directly powers the μLED device. Reproduced from reference [122] with permission.
Illuminating Pain Circuits in the Brain
Within two years of demonstrating optical control of cultured neurons with ChR2 [22], optogenetic approaches were applied in vivo, utilizing surgically implanted optical fibers in cannulas affixed to the skull [1,5]. This approach has been used to interrogate a variety of neural circuits in the brain [94,140,141,146,147] and has recently been applied to studying components of the pain neuraxis [29].
Initial studies utilizing optogenetics to understand higher-order nociceptive processing, targeted brain circuits involved in regulating the sensory and affective aspects of pain. In corticolimbic networks, sensory information from the basolateral amygdala (BLA) is routed to the medial prefrontal cortex (mPFC) [113]. Viral expression of ChR2 in ascending BLA projections to the mPFC revealed input-specific connectivity. Synaptic efficacy depended on both the laminar location and postsynaptic target of mPFC neurons in a region-specific manner, highlighting the complexities of these circuits [33]. In rodent models of chronic pain, selective photostimulation of BLA inputs onto pyramidal neurons in the mPFC revealed increased feed-forward inhibition by local GABAergic neurons [77]. Similarly, projections from the mPFC returning processed nociceptive information to the limbic system [72,113], are also suppressed following peripheral nerve injury by elevated interneuron activity [168]. This suggests that persistent pain states can lead to wide-spread dysfunction in the inhibitory tone of this circuit. Enhancing the activity of parvalbumin+ GABAergic interneurons in the mPFC, using optogenetic stimulation in transgenic mice, exacerbated both sensory and emotional pain behaviors in rodent models of chronic pain. In contrast, optogenetic silencing of this same neuronal population blunted these responses [168]. Increased polysynaptic inhibition from inputs arising in the BLA, was attributed to reduced mGlu5-mediated endocannabinoid signaling at these synapses [77]. Human brain imaging experiments have revealed enhanced activity in the PFC of patients with chronic pain [8], which may reflect the increased excitability of local interneurons observed in rodents. Alternatively, elevated activity in human PFC may represent a long-term maladaptive response to the acute suppression of these projections by local interneurons, possibly through synapse-specific alterations in endocannabinoid signaling.
A major target of these cortical projections is the central nucleus of the amygdala (CeA), which plays a key role in regulating the emotional components of sensory stimuli [103]. In the CeA, pain processing and plasticity are lateralized [30,31,71], and selective optogenetic stimulation of ChR2 in the right CeA induced visceral hyperalgesia in response to bladder distension [38]. Nociceptive information is also relayed to the CeA via projections from the lateral parabrachial nucleus (LPB) in the brainstem. Viral expression of ChR2 in projections from the LPB revealed direct monosynaptic glutamatergic inputs onto CeA neurons, which are increased by inflammatory pain [143]. In the nucleus accumbens (NAc), cortical inputs converge and are integrated with signals from midbrain dopaminergic neurons. Optogenetic stimulation of cortical projections to NAc exhibited anti-nociceptive effects and alleviated negative affective behaviors in a model of neuropathic pain [82]. At the synaptic level, persistent pain produced input-specific changes in synaptic connectivity to indirect pathway spiny projection neurons (iSPNs) of the NAc shell and increased their excitability [130]. Dampened excitability of iSPNs alleviated tactile allodynia, while enhancing their activity increased mechanical hypersensitivity [130]. Persistent pain can lead to numerous synapse-specific and network-wide changes in activity that influences the processing of nociceptive information. These initial optogenetic investigations of corticolimbic connections are a small snapshot of the central circuits involved in pain processing. Additional cell- and region-specific manipulations of these projections are needed to develop a clearer picture of how these connections are altered during the transition from acute to chronic pain.
Monoaminergic neurons are powerful modulators of nociceptive information, however their broad-reaching projections to both pro- and anti-nociceptive cell types has made their distinct roles in pain processing difficult to pin down [105,107,119]. Noradrenergic (NA) neurons in the locus coeruleus (LC) project throughout the brain and spinal cord [58,91,92] and are thought to be predominantly anti-nociceptive [124,125]. However, optogenetic stimulation of LC NA neurons caused either pro- or anti-nociceptive behavioral responses to thermal pain stimuli [63]. Subsequent anatomical investigation of viral expression and fiber optic placement revealed that pro-nociceptive behavior resulted from neurons located dorsally within the LC, while ventral NA neurons were anti-nociceptive [63]. The rostral ventral medulla (RVM) contains another family of neuromodulatory neurons in the brainstem that send descending serotonergic projections to the spinal cord [11,48,81]. However, serotonin has both excitatory and inhibitory effects in the dorsal horn, and electrical stimulation of the RVM produced both pro- and anti-nociceptive behaviors [25,48,100,160,170]. These disparate observations have made it difficult to establish precise roles for these neurons in nociceptive processing. By targeting the serotonergic system in transgenic mice with ChR2 restricted to tryptophan hydroxylase-2+ neurons [169], optical stimulation of the RVM produced robust hypersensitivity [24]. Repeated stimulation led to hypersensitivity that lasted over two weeks, suggesting that these connections are plastic [24]. Whether discrete subpopulations of serotonergic neurons engage distinct nociceptive behaviors can now be addressed with refined genetic targeting approaches and optical stimulation strategies [4,44].
Optical Investigation of Peripheral Pain Systems
Optogenetic experiments venturing outside the brain have focused on the transmission of nociceptive information from sensory neurons of dorsal root ganglia (DRG) to the spinal cord. Despite initial difficulties in delivering light to peripheral structures, optogenetic stimulation was rapidly implemented for studying these systems in vitro [26]. The first transgenic mouse to express ChR2 in the peripheral nervous system, targeted Mrgprd+ polymodal nociceptive neurons [151,171]. Importantly, opsins were efficiently trafficked to both peripheral nerve endings in skin and central terminals in spinal cord. Photostimulation of these molecularly defined terminals revealed that Mrgprd+ neurons form synaptic connections with all know classes of spinal cord neurons in lamina II [151]. A similar approach was also used to dissect contributions of opioid and GABAB receptors in presynaptic modulation of synaptic transmission onto spinal neurons by distinct subtypes of primary afferents. Activation of presynaptic δ-opioid receptors did not affect synaptic responses, in contrast to the μ-opioid receptor agonist DAMGO, which preferentially inhibited C-fibers innervating lamina I over lamina II [65]. Presynaptic GABABR activation depressed transmission from all fiber types, demonstrating clear input-specific modulation of sensory transmission [43,65]. Optogenetic approaches to studying synaptic specificity are not restricted to nociceptive afferents. Selective expression of ChR2 in GABAergic interneurons in the spinal cord revealed a critical role for presynaptic inhibition of proprioceptive axons to execute smooth movements [49]. These studies highlight the unique advantages to utilizing optical approaches in delineating neural circuit connectivity [133].
Optogenetic targeting of different types of primary afferent fibers may also be useful in dissecting their contributions to pain behavior. Illumination of the hindpaw of transgenic mice expressing ChR2 in Nav1.8+ neurons caused robust nociceptive responses and place aversion, both of which could be blocked with analgesics [40]. Real-time place aversion is also seen by selectively stimulating ChR2-expressing nociceptors in TrpV1Cre mice [108,122]. Conversely, pain behaviors are attenuated in transgenic mice expressing the inhibitory opsin archaerhodopsin [39]. Optical manipulations of pain behaviors have also been achieved in nontransgenic animals using AAV vectors to transduce peripheral fibers [68]. More relevant clinically, viral delivery of the inhibitory opsins halorodopsin [68] or archaerhodopsin [19,85] to peripheral sensory neurons enabled light-dependent blunting of behavioral responses to thermal and mechanical stimulation [68,85]. Optical inhibition also reversed mechanical and thermal hypersensitivity in models of neuropathic pain [19,68], suggesting a possible therapeutic option for using optogenetics in treating chronic pain. These manipulations of somatosensory signaling are not limited to neurons that comprise these circuits. Illumination of the epidermis in transgenic mice expressing ChR2 or inhibitory opsins in Merkel cells and keratinocytes, bidirectionally influenced the activity of innervating sensory neurons [12,99]. While these approaches have great potential to dissect somatosensory circuits, behavioral studies have largely been restricted to tethered fiber optic implantation in the brain or non-targeted illumination of peripheral tissues (Fig. 2E,F).
Novel Approaches to Light Delivery
Recent engineering advancements have generated breakthroughs in light delivery solutions. In the brain, chronic fiber optic implants are straightforward and effective [2,5,123,152] (Fig. 2E). However, these approaches require tethered operation that can hamper behavior experiments and limit chronic stimulation paradigms. Advances in optoelectronic interfaces led to untethered LED devices that can be secured to the skull and powered wirelessly [154] or by battery [67]. Further refinement of this approach has miniaturized designs and replaced optical fibers with microscale-LEDs that can be injected into the brain [76,102,158], permitting focal control of illumination through independent LEDs [4,76,158]. These engineering advances allow for more precise stimulation, particularly for anatomically distinct subpopulations of neurons.
In contrast to optogenetic manipulation in brain, approaches to photostimulation of sensory fibers and spinal circuits have been extremely limited until recently. Initial approaches relied on illumination of the hindpaw in restrained animals [73] or the exposed sciatic nerve in anesthetized transgenic mice [90]. Illumination via fiber optic cables permitted basic reflexive assays of thermal and mechanical pain, but required simultaneous illumination of the hindpaw during stimulation [40,68,85] (Fig. 2F). The aversive nature of peripheral optogenetic manipulations was cleverly explored using behavioral chambers with multi-colored illumination through the floor (Fig. 2G). This consisted of a blue light “stimulation” zone and an orange/red “neutral” area. When placed in these chambers, ChR2-expressing mice exhibited aversion to the blue light zone [40,68]. This approach limits photostimulation to cutaneous fibers in the paw, and does not allow manipulation of sensory afferents projecting to other areas of the body, or nociceptive circuits in spinal cord. Additionally, illumination of these chambers may trigger off-target behavioral effects, particularly as they relate to stress, anxiety, and affective components of pain [64,84,118,119]. Potential solutions to these confounds have recently been developed, using implantable light-delivery interfaces.
The first implantable light delivery approach in peripheral tissues consisted on an “optical cuff” surrounding the sciatic nerve. Light was delivered through an optical cable tethered to the skull and tunneled subcutaneously, where it was reflected by an encapsulated aluminum sheet [148]. Illumination of ChR2-expressing motor neurons elicited activity from those innervated leg muscles. To access opsin-expressing neurons and sensory afferents in the spinal cord, fiber optic cables were threaded into the epidural space of the spinal column, allowing for activation of either ChR2 or archaerhodopsin [20]. Extending these approaches, two recent reports have demonstrated fully implantable and wireless devices to stimulate peripheral sensory axons and neurons in the spinal cord in freely moving mice [109,122] (Fig. 2H–M). One version of these implantable devices can be constructed using standard equipment, and their energy harvesting properties allow reliable activation throughout the body (Fig. 2H–J). Wireless functionality was provided by a resonant chamber that coupled electromagnetic energy from lattice in the floor directly to the mouse, which was harvested by the device [109,161]. Arenas were designed to accommodate these chambers; however their current dimensions may limit their application with existing behavioral equipment [109]. Though broadly applicable to implantation near a wide variety of biological structures, device rigidity may preclude access to deeper brain and spinal cord neurons.
Another untethered, implantable approach for light delivery consisted of a flexible device with stretchable miniaturized antennas to harvest energy through capacitive coupling (Fig. 2K–M). This permitted direct LED activation by radio frequency transmission, operation throughout 3-dimensional spaces, and implementation with most existing behavioral equipment [122]. Both of these wireless, implantable designs effectively demonstrated behavioral aversion from wireless activation of nociceptive neurons in peripheral tissues and in the spinal cord, greatly expanding experimental flexibility for investigating these circuits in vivo. Instructions for fabricating these wireless devices by independent laboratories are available [102,109,122]; however, continued development and technology refinements should lead to more widespread availability. Future implementation of these and other multimodal devices [27,70], will help to develop a more complete picture of somatosensory processing in freely moving animals.
Important Experimental Considerations
Implementing optogenetic techniques grants unprecedented access to neurons and the circuits they comprise. However, as with any technology, limitations exist [62]. While temporal control of activity is a primary strength of optogenetics, many studies report single frequency trains of stimulation. Ideally, frequencies should be tuned around normal physiological firing rates, and “dose-response” data can be very informative [32]. Synchronous neuronal stimulation may also generate non-physiological phase-locked firing patterns, in addition to possibly activating plasticity mechanisms. This could lead to inaccurate interpretations of their function within a network or the unintended engagement of downstream targets [62,120].
The locus of photostimulation is also an important consideration. Activation of ChR2 in axon terminals directly gates presynaptic calcium channels, potentially affecting normal transmitter release [135]. Sustained illumination of archaerhodopsin-expressing presynaptic terminals for silencing experiments, directly stimulated Ca2+ influx and spontaneous vesicle release [98]. Ionic equilibrium potentials must also be considered when designing optogenetic experiments. Activation of the chloride pump NpHR shifted the GABAAR reversal potential, resulting in excitatory GABA-mediated currents and rebound firing [128]. This has important behavioral consequences for studying chronic pain conditions that alter chloride gradients, resulting in excitation rather than inhibition [37,42]. Careful experimental planning can mitigate these known confounds. However, other limitations are bound to exist, and caution should be exercised when interpreting optogenetic data.
Clinical Applications
The enormous potential in using light to control signaling of defined cell-types throughout the nervous system not only provides researchers with precise tools to dissect these neural networks, but could potentially open doors to novel approaches in treating human diseases. Phase I/II clinical trials are now underway using intraocular injections of AAV vectors to express ChR2 in the retina to restore sight to blind patients suffering from retinitis pigmentosa (ClinicalTrials.gov). In the somatosensory system, peripheral sensory fibers represent an obvious target for optogenetic therapies in treating chronic pain, where dampening excitability with inhibitory opsins should provide analgesia. Viral vectors have already been used for peripheral delivery of transgenes to patients in clinical trials [50]. We have confirmed functional expression of these tools in human sensory neurons, taking advantage of the natural tropism of herpes simplex viral (HSV) vectors (Fig. 1E). A major hurdle for implementing optogenetics in different brain regions will be targeting the appropriate neuronal populations in humans [136]. Across different neural circuits, simple ON/OFF control may not be desirable or effective. The vast majority of clinically available drugs target various GPCRs, raising the possibility that manipulating endogenous signaling pathways with optoXRs may have therapeutic potential. For example, activation of optically sensitive μ-opioid receptors could be restricted to desired targets, like spinal pain processing circuits, while avoiding reward centers in the brain that are activated by opioid medications [137].
Light delivery strategies will likely require custom implementations to stimulate the targeted region of the nervous system. Due to the improved tissue penetration by longer wavelengths of light, red-shifted opsins have permitted transcranial stimulation [35]. Further spectral refinements could permit less invasive methods for light delivery. In combination with these strategies, advances in wireless device technology may offer potential for future optogenetic clinical trials [18,80].
Conclusion
The implementation of optogenetics offers remarkable potential for understanding complex circuits underlying sensory processing. The development of non-invasive tools to probe neural circuits, coupled with engineering advancements to visualize and manipulate their functions, are rapidly advancing this front. Future breakthroughs may enable highly selective therapeutic interventions, and will continue to transform our ability to interrogate complex physiological systems.
Acknowledgments
We would like to thank the entire Gereau lab, particularly Vijay Samineni, Aaron Mickle, and Jordan McCall, for insightful discussions and comments on this manuscript. We also thank Ada Poon for sharing unpublished images of wireless devices. This work was supported by a NIH Transformative Research Award (R01 NS081707 to RWG), a SPARC grant (U18 EB021793 to RWG), and T32 training grants (GM007067 to MYP and GM108539 to BAC).
Footnotes
Conflict of interest statement: RWG is a co-founder and stockholder of Neurolux Systems. The other authors do not have a conflict of interest.
References
- 1.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamantidis AR, Zhang F, de Lecea L, Deisseroth K. Establishing a fiber-optic-based optical neural interface. Cold Spring Harb Protoc. 2014;2014:839–44. doi: 10.1101/pdb.prot083337. [DOI] [PubMed] [Google Scholar]
- 3.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 4.Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, Pyo C-O, Park S, II, Marcinkiewcz CM, Crowley NA, Krashes MJ, Lowell BB, Kash TL, Rogers JA, Bruchas MR. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87:1063–77. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aravanis AM, Wang L-P, Zhang F, Meltzer La, Mogri MZ, Schneider MB, Deisseroth K. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J Neural Eng. 2007;4:S143–S156. doi: 10.1088/1741-2560/4/3/S02. [DOI] [PubMed] [Google Scholar]
- 6.Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–18. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–60. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bamann C, Gueta R, Kleinlogel S, Nagel G, Bamberg E. Structural guidance of the photocycle of channelrhodopsin-2 by an interhelical hydrogen bond. Biochemistry. 2010;49:267–278. doi: 10.1021/bi901634p. [DOI] [PubMed] [Google Scholar]
- 10.Barish Pa, Xu Y, Li J, Sun J, Jarajapu YPR, Ogle WO. Design and functional evaluation of an optically active m-opioid receptor. Eur J Pharmacol. 2013;705:42–48. doi: 10.1016/j.ejphar.2013.01.065. [DOI] [PubMed] [Google Scholar]
- 11.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–38. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 12.Baumbauer KM, DeBerry JJ, Adelman PC, Miller RH, Hachisuka J, Lee KH, Ross SE, Koerber HR, Davis BM, Albers KM. Keratinocytes can modulate and directly initiate nociceptive responses. Elife. 2015;4:1–14. doi: 10.7554/eLife.09674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berndt A, Lee SY, Ramakrishnan C, Deisseroth K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science. 2014;344:420–4. doi: 10.1126/science.1252367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berndt A, Lee SY, Wietek J, Ramakrishnan C, Steinberg EE, Rashid AJ, Kim H, Park S, Santoro A, Frankland PW, Iyer SM, Pak S, Ährlund-Richter S, Delp SL, Malenka RC, Josselyn SA, Carlén M, Hegemann P, Deisseroth K. Structural foundations of optogenetics: determinants of channelrhodopsin ion selectivity. Proc Natl Acad Sci U S A. 2016;113:822–9. doi: 10.1073/pnas.1523341113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berndt A, Schoenenberger P, Mattis J, Tye KM, Deisseroth K, Hegemann P, Oertner TG. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc Natl Acad Sci U S A. 2011;108:7595–7600. doi: 10.1073/pnas.1017210108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berndt A, Yizhar O, Gunaydin LA, Hegemann P, Deisseroth K. Bi-stable neural state switches. Nat Neurosci. 2009;12:229–234. doi: 10.1038/nn.2247. [DOI] [PubMed] [Google Scholar]
- 17.Betley JN, Sternson SM. Adeno-associated viral vectors for mapping, monitoring, and manipulating neural circuits. Hum Gene Ther. 2011;22:669–77. doi: 10.1089/hum.2010.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, Pasricha P, Weber D, Ludwig K, Famm K. Bioelectronic medicines: a research roadmap. Nat Rev Drug Discov. 2014;13:399–400. doi: 10.1038/nrd4351. [DOI] [PubMed] [Google Scholar]
- 19.Boada MD, Martin TJ, Peters CM, Hayashida K, Harris MH, Houle TT, Boyden ES, Eisenach JC, Ririe DG. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain. 2014;155:2646–2655. doi: 10.1016/j.pain.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonin RP, Wang F, Desrochers-Couture M, Ga Secka A, Boulanger M-E, Côté DC, De Koninck Y. Epidural optogenetics for controlled analgesia. Mol Pain. 2016;12:1–11. doi: 10.1177/1744806916629051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourane S, Duan B, Koch SC, Dalet A, Britz O, Garcia-Campmany L, Kim E, Cheng L, Ghosh A, Ma Q, Goulding M. Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science. 2015;350:550–554. doi: 10.1126/science.aac8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 23.Branda CS, Dymecki SM. Talking about a revolution: The impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004;6:7–28. doi: 10.1016/s1534-5807(03)00399-x. [DOI] [PubMed] [Google Scholar]
- 24.Cai Y-Q, Wang W, Hou Y-Y, Pan ZZ. Optogenetic activation of brainstem serotonergic neurons induces persistent pain sensitization. Mol Pain. 2014;10:70. doi: 10.1186/1744-8069-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calejesan AA, Ch’Ang MHC, Zhuo M. Spinal serotonergic receptors mediate facilitation of a nociceptive reflex by subcutaneous formalin injection into the hindpaw in rats. Brain Res. 1998;798:46–54. doi: 10.1016/s0006-8993(98)00394-1. [DOI] [PubMed] [Google Scholar]
- 26.Campagnola L, Wang H, Zylka MJ. Fiber-coupled light-emitting diode for localized photostimulation of neurons expressing channelrhodopsin-2. J Neurosci Methods. 2008;169:27–33. doi: 10.1016/j.jneumeth.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Canales A, Jia X, Froriep UP, Koppes RA, Tringides CM, Selvidge J, Lu C, Hou C, Wei L, Fink Y, Anikeeva P. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol. 2015;33:277–284. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 28.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L, Moore CI. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carr FB, Zachariou V. Nociception and pain: lessons from optogenetics. Front Behav Neurosci. 2014;8:69. doi: 10.3389/fnbeh.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrasquillo Y, Gereau RW., IV Activation of the extracellular signal-regulated kinase in the amygdala modulates pain perception. J Neurosci. 2007;27:1543–51. doi: 10.1523/JNEUROSCI.3536-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carrasquillo Y, Gereau RW., IV Hemispheric lateralization of a molecular signal for pain modulation in the amygdala. Mol Pain. 2008;4:24. doi: 10.1186/1744-8069-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–1533. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheriyan J, Kaushik MK, Ferreira AN, Sheets PL. Specific targeting of the basolateral amygdala to projectionally defined pyramidal neurons in prelimbic and infralimbic cortex. eNeuro. 2016:3. doi: 10.1523/ENEURO.0002-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger Ma, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chuong AS, Miri ML, Busskamp V, Matthews GAC, Acker LC, Sørensen AT, Young A, Klapoetke NC, Henninger MA, Kodandaramaiah SB, Ogawa M, Ramanlal SB, Bandler RC, Allen BD, Forest CR, Chow BY, Han X, Lin Y, Tye KM, Roska B, Cardin JA, Boyden ES. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat Neurosci. 2014;17:1123–9. doi: 10.1038/nn.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosentino C, Alberio L, Gazzarrini S, Aquila M, Romano E, Cermenati S, Zuccolini P, Petersen J, Beltrame M, Van Etten JL, Christie JM, Thiel G, Moroni A. Engineering of a light-gated potassium channel. Science. 2015;348:707–10. doi: 10.1126/science.aaa2787. [DOI] [PubMed] [Google Scholar]
- 37.Coull JAM, Boudreau D, Bachand K, Prescott SA, Nault F, Sík A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 38.Crock LW, Kolber BJ, Morgan CD, Sadler KE, Vogt SK, Bruchas MR, Gereau RW., IV Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J Neurosci. 2012;32:14217–26. doi: 10.1523/JNEUROSCI.1473-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daou I, Beaudry H, Ase AR, Wieskopf JS, Ribeiro-da-Silva A, Mogil JS, Séguéla P. Optogenetic Silencing of Nav1. 8-Positive Afferents Alleviates Inflammatory and Neuropathic Pain. eNeuro. 2016;3:1–12. doi: 10.1523/ENEURO.0140-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daou I, Tuttle AH, Longo G, Wieskopf JS, Bonin RP, Ase AR, Wood JN, De Koninck Y, Ribeiro-da-Silva A, Mogil JS, Séguéla P. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J Neurosci. 2013;33:18631–40. doi: 10.1523/JNEUROSCI.2424-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deisseroth K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat Neurosci. 2015;18:1213–1225. doi: 10.1038/nn.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyon N, Vinay L, Prescott SA, De Koninck Y. Chloride regulation: a dynamic equilibrium crucial for synaptic inhibition. Neuron. 2016;89:1157–72. doi: 10.1016/j.neuron.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Draxler P, Honsek SD, Forsthuber L, Hadschieff V, Sandkühler J. VGluT3+ primary afferents play distinct roles in mechanical and cold hypersensitivity depending on pain etiology. J Neurosci. 2014;34:12015–28. doi: 10.1523/JNEUROSCI.2157-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dymecki SM, Kim JC. Molecular neuroanatomy’s “Three Gs”: a primer. Neuron. 2007;54:17–34. doi: 10.1016/j.neuron.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farago AF, Awatramani RB, Dymecki SM. Assembly of the brainstem cochlear nuclear complex is revealed by intersectional and subtractive genetic fate maps. Neuron. 2006;50:205–18. doi: 10.1016/j.neuron.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Fenno L, Yizhar O, Deisseroth K. The development and application of optogenetics. Annu Rev Neurosci. 2011;34:389–412. doi: 10.1146/annurev-neuro-061010-113817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fenno LE, Mattis J, Ramakrishnan C, Hyun M, Lee SY, He M, Tucciarone J, Selimbeyoglu A, Berndt A, Grosenick L, Zalocusky KA, Bernstein H, Swanson H, Perry C, Diester I, Boyce FM, Bass CE, Neve R, Huang ZJ, Deisseroth K. Targeting cells with single vectors using multiple-feature Boolean logic. Nat Methods. 2014;11:763–72. doi: 10.1038/nmeth.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fields HL. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–75. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 49.Fink AJP, Croce KR, Huang ZJ, Abbott LF, Jessell TM, Azim E. Presynaptic inhibition of spinal sensory feedback ensures smooth movement. Nature. 2014;509:43–8. doi: 10.1038/nature13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fink DJ, Wechuck J, Mata M, Glorioso JC, Goss J, Krisky D, Wolfe D. Gene therapy for pain: Results of a phase I clinical trial. Ann Neurol. 2011;70:207–212. doi: 10.1002/ana.22446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC Cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–83. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–23. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Govorunova EG, Sineshchekov OA, Janz R, Liu X, Spudich JL. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349:647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–65. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grosenick L, Marshel JH, Deisseroth K. Closed-loop and activity-guided optogenetic control. Neuron. 2015;86:106–139. doi: 10.1016/j.neuron.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- 59.Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, Lammel S, Mirzabekov JJ, Airan RD, Zalocusky KA, Tye KM, Anikeeva P, Malenka RC, Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157:1535–51. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gunaydin LA, Yizhar O, Berndt A, Sohal VS, Deisseroth K, Hegemann P. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 61.Harris JA, Hirokawa KE, Sorensen SA, Gu H, Mills M, Ng LL, Bohn P, Mortrud M, Ouellette B, Kidney J, Smith KA, Dang C, Sunkin S, Bernard A, Oh SW, Madisen L, Zeng H. Anatomical characterization of Cre driver mice for neural circuit mapping and manipulation. Front Neural Circuits. 2014;8:1–16. doi: 10.3389/fncir.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hausser M. Optogenetics: the age of light. Nat Methods. 2014;11:1012–1014. doi: 10.1038/nmeth.3111. [DOI] [PubMed] [Google Scholar]
- 63.Hickey L, Li Y, Fyson SJ, Watson TC, Perrins R, Hewinson J, Teschemacher AG, Furue H, Lumb BM, Pickering AE. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J Neurosci. 2014;34:4148–60. doi: 10.1523/JNEUROSCI.4835-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 65.Honsek SD, Seal RP, Sandkühler J. Presynaptic inhibition of optogenetically identified VGluT3+ sensory fibres by opioids and baclofen. Pain. 2015;156:243–251. doi: 10.1097/01.j.pain.0000460304.63948.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang ZJ, Zeng H. Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- 67.Iwai Y, Honda S, Ozeki H, Hashimoto M, Hirase H. Technical note A simple head-mountable LED device for chronic stimulation of optogenetic molecules in freely moving mice. Neurosci Res. 2011;70:124–127. doi: 10.1016/j.neures.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 68.Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol. 2014;32:274–8. doi: 10.1038/nbt.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jensen P, Farago AF, Awatramani RB, Scott MM, Deneris ES, Dymecki SM. Redefining the serotonergic system by genetic lineage. Nat Neurosci. 2008;11:417–419. doi: 10.1038/nn2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeong J, McCall JG, Shin G, Zhang Y, Al-Hasani R, Kim M, Li S, Sim JY, Jang K-I, Shi Y, Hong DY, Liu Y, Schmitz GP, Xia L, He Z, Gamble P, Ray WZ, Huang Y, Bruchas MR, Rogers JA. Wireless optofluidic systems for programmable in vivo pharmacology and optogenetics. Cell. 2015;162:662–74. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ji G, Neugebauer V. Hemispheric lateralization of pain processing by amygdala neurons. J Neurophysiol. 2009;102:2253–64. doi: 10.1152/jn.00166.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ji G, Neugebauer V. Modulation of medial prefrontal cortical activity using in vivo recordings and optogenetics. Mol Brain. 2012;5:36. doi: 10.1186/1756-6606-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ji ZG, Ito S, Honjoh T, Ohta H, Ishizuka T, Fukazawa Y, Yawo H. Light-evoked somatosensory perception of transgenic rats that express channelrhodopsin-2 in dorsal root ganglion cells. PLoS One. 2012;7:1–10. doi: 10.1371/journal.pone.0032699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kato HE, Zhang F, Yizhar O, Ramakrishnan C, Nishizawa T, Hirata K, Ito J, Aita Y, Tsukazaki T, Hayashi S, Hegemann P, Maturana AD, Ishitani R, Deisseroth K, Nureki O. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482:369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J-M, Hwa J, Garriga P, Reeves PJ, RajBhandary UL, Khorana HG. Light-driven activation of b2-adrenergic receptor signaling by a chimeric rhodopsin containing the b2-adrenergic receptor cytoplasmic loops. Biochemistry. 2005;44:2284–92. doi: 10.1021/bi048328i. [DOI] [PubMed] [Google Scholar]
- 76.Kim T, McCall JG, Jung YH, Huang X, Siuda ER, Li Y, Song J, Song YM, Pao HA, Kim R-HR-H, Lu C, Lee SD, Song I-S, Shin G, Al-Hasani R, Kim S, Tan MP, Huang Y, Omenetto FG, Rogers JA, Bruchas MR. Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science. 2013;340:211–216. doi: 10.1126/science.1232437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiritoshi T, Ji G, Neugebauer V. Rescue of impaired mGluR5-driven endocannabinoid signaling restores prefrontal cortical output to inhibit pain in arthritic rats. J Neurosci. 2016;36:837–50. doi: 10.1523/JNEUROSCI.4047-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, Wang J, Xie Y, Yan Z, Zhang Y, Chow BY, Surek B, Melkonian M, Jayaraman V, Constantine-Paton M, Wong GK-S, Boyden ES. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat Neurosci. 2011;14:513–518. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- 80.Krook-Magnuson E, Gelinas JN, Soltesz I, Buzsáki G. Neuroelectronics and biooptics: closed-loop technologies in neurological disorders. JAMA Neurol. 2015;72:823–9. doi: 10.1001/jamaneurol.2015.0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lau BK, Vaughan CW. Descending modulation of pain: the GABA disinhibition hypothesis of analgesia. Curr Opin Neurobiol. 2014;29:159–64. doi: 10.1016/j.conb.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 82.Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. 2015;35:5247–5259. doi: 10.1523/JNEUROSCI.3494-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee S-H, Kwan AC, Zhang S, Phoumthipphavong V, Flannery JG, Masmanidis SC, Taniguchi H, Huang ZJ, Zhang F, Boyden ES, Deisseroth K, Dan Y. Activation of specific interneurons improves V1 feature selectivity and visual perception. Nature. 2012;488:379–383. doi: 10.1038/nature11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–5. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- 85.Li B, Yang X, Qian F, Tang M, Ma C, Chiang L-Y. A novel analgesic approach to optogenetically and specifically inhibit pain transmission using TRPV1 promoter. Brain Res. 2015;1609:12–20. doi: 10.1016/j.brainres.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Li P, Rial D, Canas PM, Yoo J, Li W, Zhou X, Wang Y, van Westen GJP, Payen M, Augusto E, Gonçalves N, Tomé AR, Li Z, Wu Z, Hou X, Zhou Y, PIJzerman A, Boyden ES, Cunha RA, Qu J, Chen J. Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry. 2015;20:1–11. doi: 10.1038/mp.2015.43. [DOI] [PubMed] [Google Scholar]
- 87.Li X, Gutierrez DV, Hanson MG, Han J, Mark MD, Chiel H, Hegemann P, Landmesser LT, Herlitze S. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lin JY, Knutsen PM, Muller A, Kleinfeld D, Tsien RY. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci. 2013;16:1499–508. doi: 10.1038/nn.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lin JY, Lin MZ, Steinbach P, Tsien RY. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J. 2009;96:1803–1814. doi: 10.1016/j.bpj.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liske H, Towne C, Anikeeva P, Zhao S, Feng G, Deisseroth K, Delp S. Optical inhibition of motor nerve and muscle activity in vivo. Muscle Nerve. 2013;47:916–21. doi: 10.1002/mus.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: Topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- 92.Loughlin SE, Foote SL, Grzanna R. Efferent projections of nucleus locus coeruleus: morphologic subpopulations have different efferent targets. Neuroscience. 1986;18:307–19. doi: 10.1016/0306-4522(86)90156-9. [DOI] [PubMed] [Google Scholar]
- 93.Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57:634–60. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lüscher C, Pascoli V, Creed M. Optogenetic dissection of neural circuitry: from synaptic causalities to blue prints for novel treatments of behavioral diseases. Curr Opin Neurobiol. 2015;35:95–100. doi: 10.1016/j.conb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Madisen L, Garner AR, Shimaoka D, Chuong AS, Klapoetke NC, Li L, van der Bourg A, Niino Y, Egolf L, Monetti C, Gu H, Mills M, Cheng A, Tasic B, Nguyen TN, Sunkin SM, Benucci A, Nagy A, Miyawaki A, Helmchen F, Empson RM, Knöpfel T, Boyden ES, Reid RC, Carandini M, Zeng H. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron. 2015;85:942–58. doi: 10.1016/j.neuron.2015.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Madisen L, Mao T, Koch H, Zhuo J, Berenyi A, Fujisawa S, Hsu Y-Wa, Garcia AJ, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mahn M, Prigge M, Ron S, Levy R, Yizhar O. Biophysical constraints of optogenetic inhibition at presynaptic terminals. Nat Neurosci. 2016:1–5. doi: 10.1038/nn.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz Sa, Firozi P, Woo S-H, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–21. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–77. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- 101.Mattis J, Tye KM, Ferenczi EA, Ramakrishnan C, O’Shea DJ, Prakash R, Gunaydin LA, Hyun M, Fenno LE, Gradinaru V, Yizhar O, Deisseroth K. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat Methods. 2012;9:159–72. doi: 10.1038/nmeth.1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McCall JG, Kim T, Shin G, Huang X, Jung YH, Al-Hasani R, Omenetto FG, Bruchas MR, Rogers JA. Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat Protoc. 2013;8:2413–2428. doi: 10.1038/nprot.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDonald AJ. Cortical pathways to the mammalian amygdala. Prog Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- 104.McIsaac RS, Bedbrook CN, Arnold FH. Recent advances in engineering microbial rhodopsins for optogenetics. Curr Opin Struct Biol. 2015;33:8–15. doi: 10.1016/j.sbi.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Micó JA, Ardid D, Berrocoso E, Eschalier A. Antidepressants and pain. Trends Pharmacol Sci. 2006;27:348–54. doi: 10.1016/j.tips.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 106.Miesenböck G. The optogenetic catechism. Science. 2009;326:395–9. doi: 10.1126/science.1174520. [DOI] [PubMed] [Google Scholar]
- 107.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 108.Mishra SK, Tisel SM, Orestes P, Bhangoo SK, Hoon MA. TRPV1-lineage neurons are required for thermal sensation. EMBO J. 2011;30:582–593. doi: 10.1038/emboj.2010.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Montgomery KL, Yeh AJ, Ho JS, Tsao V, Mohan Iyer S, Grosenick L, Ferenczi EA, Tanabe Y, Deisseroth K, Delp SL, Poon ASY. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nat Methods. 2015;12:969–974. doi: 10.1038/nmeth.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nagel G. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 111.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nagy A. Cre recombinase: The universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 113.Neugebauer V. Amygdala pain mechanisms. Handb Exp Pharmacol. 2015;227:261–84. doi: 10.1007/978-3-662-46450-2_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oesterhelt D, Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973;70:2853–7. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233:149–52. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 116.Oh E, Maejima T, Liu C, Deneris E, Herlitze S. Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem. 2010;285:30825–36. doi: 10.1074/jbc.M110.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Okaty BW, Freret ME, Rood BD, Brust RD, Hennessy ML, DeBairos D, Kim JC, Cook MN, Dymecki SM. Multi-scale molecular deconstruction of the serotonin neuron system. Neuron. 2015;88:774–91. doi: 10.1016/j.neuron.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Olsen CM, Winder DG. Stimulus dynamics increase the self-administration of compound visual and auditory stimuli. Neurosci Lett. 2012;511:8–11. doi: 10.1016/j.neulet.2011.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ossipov MH, Dussor GO, Porreca F. Central modulation of pain. J Clin Invest. 2010;120:3779–3787. doi: 10.1172/JCI43766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Otchy TM, Wolff SBE, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SMH, Ölveczky BP. Acute off-target effects of neural circuit manipulations. Nature. 2015;528:358–63. doi: 10.1038/nature16442. [DOI] [PubMed] [Google Scholar]
- 121.Packer AM, Roska B, Häusser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–15. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park S, II, Brenner DS, Shin G, Morgan CD, Copits BA, Chung HU, Pullen MY, Noh KN, Davidson S, Oh SJ, Yoon J, Jang K-I, Samineni VK, Norman M, Grajales-Reyes JG, Vogt SK, Sundaram SS, Wilson KM, Ha JS, Xu R, Pan T, Kim T, Huang Y, Montana MC, Golden JP, Bruchas MR, Gereau RW, Rogers JA. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nat Biotechnol. 2015;33:1280–1286. doi: 10.1038/nbt.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pashaie R, Anikeeva P, Lee JH, Prakash R, Yizhar O, Prigge M, Chander D, Richner TJ, Williams J. Optogenetic brain interfaces. IEEE Rev Biomed Eng. 2014;7:3–30. doi: 10.1109/RBME.2013.2294796. [DOI] [PubMed] [Google Scholar]
- 124.Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Pertovaara A. The noradrenergic pain regulation system_: A potential target for pain therapy. Eur J Pharmacol. 2013;716:2–7. doi: 10.1016/j.ejphar.2013.01.067. [DOI] [PubMed] [Google Scholar]
- 126.Prakash R, Yizhar O, Grewe B, Ramakrishnan C, Wang N, Goshen I, Packer AM, Peterka DS, Yuste R, Schnitzer MJ, Deisseroth K. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat Methods. 2012;9:1171–1179. doi: 10.1038/nmeth.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prigge M, Schneider F, Tsunoda SP, Shilyansky C, Wietek J, Deisseroth K, Hegemann P. Color-tuned channelrhodopsins for multiwavelength optogenetics. J Biol Chem. 2012;287:31804–31812. doi: 10.1074/jbc.M112.391185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neurosci. 2012;15:1102–1104. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–42. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ren W, Centeno MV, Berger S, Wu Y, Na X, Liu X, Kondapalli J, Apkarian AV, Martina M, Surmeier DJ. The indirect pathway of the nucleus accumbens shell amplifies neuropathic pain. Nat Neurosci. 2016;19:220–2. doi: 10.1038/nn.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–23. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ruffault P-L, D’Autréaux F, Hayes JA, Nomaksteinsky M, Autran S, Fujiyama T, Hoshino M, Hägglund M, Kiehn O, Brunet J-F, Fortin G, Goridis C. The retrotrapezoid nucleus neurons expressing Atoh1 and Phox2b are essential for the respiratory response to CO2. Elife. 2015;4:1–25. doi: 10.7554/eLife.07051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Scanziani M, Häusser M. Electrophysiology in the age of light. Nature. 2009;461:930–939. doi: 10.1038/nature08540. [DOI] [PubMed] [Google Scholar]
- 134.Schnütgen F, Doerflinger N, Calléja C, Wendling O, Chambon P, Ghyselinck NB. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat Biotechnol. 2003;21:562–565. doi: 10.1038/nbt811. [DOI] [PubMed] [Google Scholar]
- 135.Schoenenberger P, Schärer Y-PZ, Oertner TG. Channelrhodopsin as a tool to investigate synaptic transmission and plasticity. Exp Physiol. 2011;96:34–39. doi: 10.1113/expphysiol.2009.051219. [DOI] [PubMed] [Google Scholar]
- 136.Simonato M, Bennett J, Boulis NM, Castro MG, Fink DJ, Goins WF, Gray SJ, Lowenstein PR, Vandenberghe LH, Wilson TJ, Wolfe JH, Glorioso JC. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9:277–91. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ, Funderburk SC, McCall JG, Gereau RW, IV, Bruchas MR. Spatiotemporal control of opioid signaling and behavior. Neuron. 2015;86:923–35. doi: 10.1016/j.neuron.2015.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Siuda ER, McCall JG, Al-Hasani R, Shin G, Il Park S, Schmidt MJ, Anderson SL, Planer WJ, Rogers JA, Bruchas MR. Optodynamic simulation of β-adrenergic receptor signalling. Nat Commun. 2015;6:8480. doi: 10.1038/ncomms9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC. Illuminating circuitry relevant to psychiatric disorders with optogenetics. Curr Opin Neurobiol. 2015;30:9–16. doi: 10.1016/j.conb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sternson SM, Atasoy D, Betley JN, Henry FE, Xu S. An emerging technology framework for the neurobiology of appetite. Cell Metab. 2016;23:234–53. doi: 10.1016/j.cmet.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 142.Sternson SM, Roth BL. Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 143.Sugimura YK, Takahashi Y, Watabe AM, Kato F. Synaptic and network consequences of monosynaptic nociceptive inputs of parabrachial nucleus origin in the central amygdala. J Neurophysiol. 2016;81 doi: 10.1152/jn.00946.2015. jn.00946.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Kvitsani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tee BC-K, Chortos A, Berndt A, Nguyen AK, Tom A, McGuire A, Lin ZC, Tien K, Bae W-G, Wang H, Mei P, Chou H-H, Cui B, Deisseroth K, Ng TN, Bao Z. A skin-inspired organic digital mechanoreceptor. Science. 2015;350:313–316. doi: 10.1126/science.aaa9306. [DOI] [PubMed] [Google Scholar]
- 146.Tonegawa S, Liu X, Ramirez S, Redondo R. Memory engram cells have come of age. Neuron. 2015;87:918–931. doi: 10.1016/j.neuron.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 147.Tovote P, Fadok JP, Lüthi A. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 148.Towne C, Montgomery KL, Iyer SM, Deisseroth K, Delp SL. Optogenetic control of targeted peripheral axons in freely moving animals. PLoS One. 2013;8:e72691. doi: 10.1371/journal.pone.0072691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vogt A, Guo Y, Tsunoda SP, Kateriya S, Elstner M, Hegemann P. Conversion of a light-driven proton pump into a light-gated ion channel. Sci Rep. 2015;5:16450. doi: 10.1038/srep16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang H, Sugiyama Y, Hikima T, Sugano E, Tomita H, Takahashi T, Ishizuka T, Yawo H. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from Chlamydomonas. J Biol Chem. 2009;284:5685–5696. doi: 10.1074/jbc.M807632200. [DOI] [PubMed] [Google Scholar]
- 151.Wang H, Zylka MJ. Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons. J Neurosci. 2009;29:13202–13209. doi: 10.1523/JNEUROSCI.3248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Warden MR, Cardin JA, Deisseroth K. Optical neural interfaces. Annu Rev Biomed Eng. 2014;16:103–29. doi: 10.1146/annurev-bioeng-071813-104733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wen L, Wang H, Tanimoto S, Egawa R, Matsuzaka Y, Mushiake H, Ishizuka T, Yawo H. Opto-current-clamp actuation of cortical neurons using a strategically designed channelrhodopsin. PLoS One. 2010:5. doi: 10.1371/journal.pone.0012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wentz CT, Bernstein JG, Monahan P, Guerra A, Rodriguez A, Boyden ES. A wirelessly powered and controlled device for optical neural control of freely-behaving animals. J Neural Eng. 2011;8:046021. doi: 10.1088/1741-2560/8/4/046021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wietek J, Beltramo R, Scanziani M, Hegemann P, Oertner TG, Simon Wiegert J. An improved chloride-conducting channelrhodopsin for light-induced inhibition of neuronal activity in vivo. Sci Rep. 2015;5:14807. doi: 10.1038/srep14807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wietek J, Broser M, Krause BS, Hegemann P. Identification of a natural green light absorbing chloride conducting channelrhodopsin from Proteomonas sulcata. J Biol Chem. 2016;291:4121–7. doi: 10.1074/jbc.M115.699637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wietek J, Wiegert JS, Adeishvili N, Schneider F, Watanabe H, Tsunoda SP, Vogt A, Elstner M, Oertner TG, Hegemann P. Conversion of channelrhodopsin into a light-gated chloride channel. Science. 2014;344:409–12. doi: 10.1126/science.1249375. [DOI] [PubMed] [Google Scholar]
- 158.Wu F, Stark E, Ku P-C, Wise KD, Buzsáki G, Yoon E. Monolithically Integrated μLEDs on Silicon Neural Probes for High-Resolution Optogenetic Studies in Behaving Animals. Neuron. 2015;88:1136–48. doi: 10.1016/j.neuron.2015.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.van Wyk M, Pielecka-Fortuna J, Löwel S, Kleinlogel S. Restoring the ON switch in blind retinas: opto-mGluR6, a next-generation, cell-tailored optogenetic tool. PLoS Biol. 2015;13:e1002143. doi: 10.1371/journal.pbio.1002143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yaksh TL, Wilson PR. Spinal serotonin terminal system mediates antinociception. J Pharmacol Exp Ther. 1979;208:446–53. [PubMed] [Google Scholar]
- 161.Yeh AJ, Ho JS, Tanabe Y, Neofytou E, Beygui RE, Poon ASY. Wirelessly powering miniature implants for optogenetic stimulation. Appl Phys Lett. 2013;103:163701. [Google Scholar]
- 162.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 163.Zeng H, Madisen L. Optogenetics: tools for controlling and monitoring neuronal activity. Vol. 196. Elsevier B.V: 2012. Mouse transgenic approaches in optogenetics; pp. 193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhang F, Prigge M, Beyrière F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang F, Wang L, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 167.Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep. 2015;12:752–9. doi: 10.1016/j.celrep.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 169.Zhao S, Ting JT, Atallah HE, Qiu L, Tan J, Gloss B, Augustine GJ, Deisseroth K, Luo M, Graybiel AM, Feng G. Cell type–specific channelrhodopsin-2 transgenic mice for optogenetic dissection of neural circuitry function. Nat Methods. 2011;8:745–752. doi: 10.1038/nmeth.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhuo M, Gebhart GF. Spinal serotonin receptors mediate descending facilitation of a nociceptive reflex from the nuclei reticularis gigantocellularis and gigantocellularis pars alpha in the rat. Brain Res. 1991;550:35–48. doi: 10.1016/0006-8993(91)90402-h. [DOI] [PubMed] [Google Scholar]
- 171.Zylka MJ, Rice FL, Anderson DJ. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron. 2005;45:17–25. doi: 10.1016/j.neuron.2004.12.015. [DOI] [PubMed] [Google Scholar]