Abstract
Estrogen-induced cholestasis occurs in many women who are susceptible due to pregnancy or hormone replacement therapy for postmenopausal syndrome. 17α-Ethinylestradiol (EE), as a synthetic estrogen, has been widely used to study the underlying mechanisms of estrogen-induced cholestasis. Recent studies have also reported that liver kinase B1 (LKB1)-mediated activation of AMP-activated protein kinase (AMPK) plays a critical role in the regulation of canalicular network formation. However, the role of AMPK in EE-induced cholestasis remains to be determined. In this study, the effects of EE (1–100 μM) on AMPK activation and the expression of farnesoid X receptor (FXR) and hepatic bile acid transporters were examined in vitro using 3D-cultured rat primary hepatocytes and in vivo using rat cholestasis models. We also used specific chemical agonist and antagonist of AMPK, AMPK subunit-specific antibodies and lentiviral shRNAs for AMPKα1 and AMPKα2 to delineate the role of AMPK in EE-induced cholestasis and potential cellular mechanisms. We found that EE-induced phosphorylation of AMPKα1 via extracellular-signal-regulated kinases (ERK1/2)-LKB1-mediated signaling pathways and subsequent nuclear translocation accounted for the down-regulation of FXR and bile acid transporters and disruption of bile acid homeostasis. Inhibition of AMPK activation using an AMPK antagonist Compound C (2 μM) or down-regulation of AMPKα1 using gene-specific shRNA attenuated EE-induced cholestasis both in vitro and in vivo. In conclusion, these results revealed that activation of cAMP-ERK-LKB1-AMPKα1 signaling pathway plays a critical role in EE-mediated dysregulation of the expression of FXR and bile acid transporters. AMPKα1 may represent an important therapeutic target for estrogen-induced cholestasis.
Keywords: AMPKα1, FXR, Bile acid transporters, Estrogen, cholestasis
Introduction
Estrogen is a potent promoter in the pathogenesis of intrahepatic cholestasis (Abu-Hayyeh et al. 2013). Estrogen-induced cholestasis represents one of the most common liver diseases in women who are pregnant, undergoing hormone replacement therapy or taking oral contraceptives. The therapeutic agents for treating estrogen-induced cholestatic liver injury are limited due to lack of understanding of disease pathogenesis and progression (Williamson and Geenes 2014). 17α-ethinylestradiol (EE) is a synthetic estrogen derivative and has been widely used in many oral contraceptives and hormone replacement therapy. Clinical studies have shown that EE significantly reduced the clearance of sulfobromophthalein and increased serum bile acid levels (Barth et al. 2003; Segovia and Oberhauser 1981). In experimental animals, EE reduced the bile flow and impaired tight junction integrity (Rahner et al. 1996). It also has been reported that EE markedly increased expression of multidrug resistance-associated protein 3 (Mrp3) and reduced expression of Mrp2, bile salt export pump (Bsep) and Na+-dependent taurocholate cotransporter (Ntcp) in hepatocytes (Henriquez-Hernandez et al. 2007; Ruiz et al. 2013). However, the major cellular mechanisms and signaling pathways involved in EE-induced cholestasis remain to be fully identified.
Bile acid homeostasis is tightly controlled by a group of nuclear receptors (NRs), such as farnesoid X receptor α (FXR), peroxisome proliferator-activated receptors (PPARs) and pregnane X receptor (PXR). Dysregulation of the expression and activation of NRs has been associated with the pathogenesis and progression of cholestasis. FXR, the first NR identified as a bile acid receptor, plays an important role in regulating the synthesis and transport of bile acids via the activation of the small heterodimer partner (SHP) (Li and Chiang 2013). The expression or transcriptional activity of FXR was repressed in more than 90% of patients with cholestasis (Van Mil et al. 2007), which was consistent with findings in EE-induced cholestasis animal models. Furthermore, FXR agonist GW4064 has been demonstrated to have a protective effect against cholestasis in rat models (Liu et al. 2003).
AMP-activated protein kinase (AMPK), which consists of three heterogeneous subunits (α, β and γ), is a master regulator of energy metabolism (Hardie and Ashford 2014). Several recent studies indicate that AMPK also plays a role in regulating the canalicular polarization in epithelial cells (Lien et al. 2014; Zhang et al. 2006). A recent study reported that taurocholate (TCA)-induced activation of the cAMP-Epac-MEK-LKB1-AMPK signaling pathway plays a critical role in stimulating canalicular network formation in normal hepatocyte development (Fu et al. 2011). Yet, under cholestatic conditions, activation of AMPK induced phosphorylation of FXR, inhibited FXR transcriptional activity and subsequently down-regulated the expression of FXR-targeted genes in hepatocytes (Lien et al. 2014). However, the role of AMPK in EE-induced cholestasis has not been identified.
In the current study, we explored the role of AMPK in regulating bile acid homeostasis in EE-induced cholestasis. Our findings indicated that EE-induced activation and nuclear translocation of AMPKα1 via the cAMP-ERK1/2-LKB1 signaling pathway contributed to EE-mediated inhibition of FXR and bile acid receptors by down-regulating their expression. AMPKα1 represents a key player in EE-induced cholestasis.
Methods and Materials
Detailed materials and general methods used in this study are provided in the Electronic Supplementary Materials.
Animal studies
Male Sprague-Dawley (SD) rats (male, 200–250 g) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China) and housed in a temperature-controlled room (22°C ± 2°C) with a humidity of 40% ± 10%, and 12-hour light/12-hour dark cycle. Rats were fed ad libitum, with free access to water. Rats (n=6/group) were randomly divided into three groups, 1) control; 2) EE (10 mg/kg); 3) EE (10 mg/kg) + CC (10 mg/kg). Rats were intraperitoneally injected with CC (Group 3) or the same volume of vehicle solution (Group 1 and 2) for 1 hour, followed by subcutaneous injection with vehicle control solution (80% propylene glycol in saline) or EE. After 24-hour, rats were sacrificed. Blood and liver tissues were harvested for analysis. For the bile flow studies, animals were under anesthesia by intraperitoneal injection with urethane and maintained in monitored temperature at 37 °C. During the bile accumulation experiment, the bile duct was cannulated with a polyethylene tube, stabilized for 10-minute and collected into pre-tared tubes for 2-hour.
All experiments and procedures involving rats were approved by the Institutional Animal Care and Use Committee of China Pharmaceutical University and were conducted in accord with all applicable regulations.
Isolation and sandwich culture of rat primary hepatocyte
Rat primary hepatocytes were isolated by a two-step collagenase perfusion method and cultured in a collagen-sandwich configuration as reported previously (Liu et al. 1999).
Quantitative RT-PCR
Total RNA was isolated and quantitative real-time RT-PCR (qPCR) was performed as described previously (Wu et al. 2010). All primers used were listed in Online Resource list 1.
Western blot analysis
Western blot analysis of total, nuclear or cytosolic proteins from rat primary hepatocytes or rat livers was carried out as described previously (Wu et al. 2010).
Lentiviral shRNA for AMPKα1 and AMPKα2, and lentiviral overexpression for FXR
The lentiviral vectors containing the stem loop sequences of short hairpin RNA (shRNA) specifically targeting rat AMPKα1, AMPKα2, or lentiviral vectors containing full-length cDNA of rat FXR for over-expression were designed and produced by Genepharma (Shanghai, China). Rat primary hepatocytes were exposed to lentiviruses at a multiplicity of infection (MOI) of 20 for 30-hour with 8 μg/mL polybrene. After 30-hour transduction, hepatocytes were treated for further experiments.
Statistical Analysis
All of the experiments were repeated at least three times, and the results are expressed as mean ± S.E. One-way ANOVA was employed to compare the differences between multiple groups by GraphPad Prism 5. P-values of ≤0.05 were considered statistically significant.
Results
EE activates AMPK via ERK-LKB1 pathway in rat primary hepatocytes
Recent studies suggested a controversial role of AMPK in regulating canalicular formation and hepatic transporter expression under different physiological and pathological conditions (Fu et al. 2011; Lien et al. 2014). Activation of AMPK via the cAMP-ERK-LKB1 pathway is critical to bile acid-induced hepatocyte polarization under normal physiological conditions. However, under cholestatic conditions, activation of AMPK inhibits the transcriptional activity of FXR (Lien et al. 2014). It has been documented that administration of high-dose estrogen induces intrahepatic cholestasis (Crocenzi et al. 2001). To investigate the effect of AMPK in EE-induced cholestasis, the in vitro sandwich-cultured primary rat hepatocytes, which are capable of forming the bile canalicular network, were used.
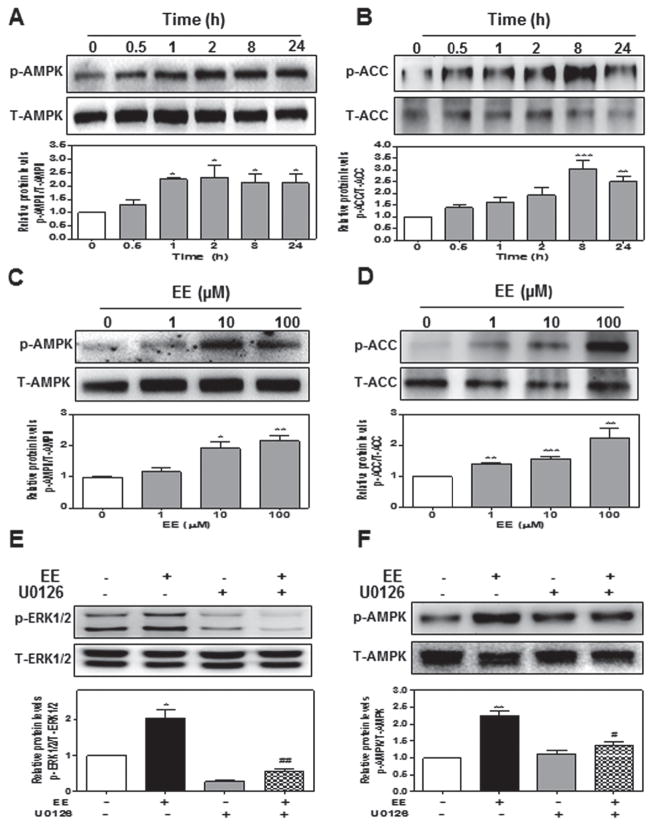
First, we examined whether EE had any effect on AMPK activation in hepatocytes. The sandwich-cultured primary rat hepatocytes were treated with 10 μM of EE for different time periods (0, 0.5, 1, 2, 8, or 24-hour) or different concentrations of EE (0, 1, 10, 100 μM) for 2-hour. The protein levels of phosphorylated AMPK (p-AMPK), total AMPK (T-AMPK), phosphorylated acetyl coenzyme carboxylase (p-ACC, the major target gene of AMPK), and total ACC (T-ACC) were determined by Western blot analysis. As shown in Fig. 1a, b, EE significantly induced activation of AMPK after 1-hour treatment and peaked at 2-hour. The p-ACC level was also markedly increased by EE at 8-hour. EE-induced phosphorylation of AMPK and ACC was also dose-dependent (Fig. 1c, d). To further identify the up-stream signaling pathway involved in EE-induced activation of AMPK, we examined the effect of EE on ERK1/2 activation. As shown in Online Resource Fig. 1a and b, EE rapidly and dose-dependently induced ERK1/2 activation with the highest induction at 1-hour. EE-induced AMPK activation was completely blocked by U0126 (10 μM), a chemical inhibitor of ERK1/2 (Fig. 1e, f). Previous studies have shown that increased intracellular cAMP levels and LKB1-activation were closely linked to ERK-induced AMPK activation (Homolya et al. 2014). We further examined whether EE had any effect on intracellular cAMP production and LKB1 activation in hepatocytes. As shown in Online Resource Fig. 1c–e, EE not only dose-dependently increased intracellular cAMP levels, but also rapidly and dose-dependently induced LKB1 activation.
Fig. 1. EE activates AMPK via ERK1/2 in rat primary hepatocytes.
Rat primary hepatocytes were a, b treated with EE (10 μM) for different time periods or c, d different concentrations (0 to 100 μM) for 2-hour. e Cells were pretreated with ERK1/2 inhibitor U0126 (10 μM) for 1 hour, and then treated with EE (10 μM) for 1 hour e or 2-hour f. Representative Western blot and densitometry of phosphorylated AMPK, total AMPK, p-ACC, T-ACC, p-ERK1/2 and T-ERK1/2. *P<0.05, **P<0.01, ***P<0.001 vs. control group
It has been reported that cAMP-induced activation of PKA is involved in regulation of canalicular trafficking in hepatocytes (Homolya et al. 2014). In addition, estrogen receptor (ER)β-mediated activation of Ca2+/Calmodulin dependent protein kinase kinase β (CaMKKβ) also activated AMPK in human endothelial cells (Yang and Wang 2015). In order to determine whether PKA and CaMKKβ are also involved in EE-induced AMPK activation in hepatocytes, specific chemical inhibitors of CaMKKβ and PKA, STO-609 and H89, were used. As shown in Online Resource Fig. 2a, STO-609 had no effect on AMPK activation. Although EE induced activation of PKA, which was effectively blocked by H89, H89 couldn’t block EE-induced AMPK activation (Online Resource Fig. 2b and c). AMPK is an intracellular energy sensor that is primarily regulated by intracellular AMP, as well as ADP (Gowans and Hardie 2014). However, in rat primary hepatocytes, EE had no effect on intracellular ADP/ATP ratio (Online Resource Fig. 2d). These results indicated that ERK1/2-LKB1 signaling pathways, but not PKA, CaMKKβ and ADP, play a critical role in EE-induced AMPK activation.
In addition, we examined the effect of EE on cell viability and hepatic functional enzymes. EE had no significant effect on cell viability and the enzyme activities of AST, ALT and LDH up to 100 μM (Online Resource Fig. 3a, b). However, the ALP activity and total cellular bile acid levels were dose-dependently increased after 24-hour treatment, indicating the development of cholestasis (Online Resource Fig. 3c and d). Furthermore, the mRNA and protein levels of bile acid transporters, Bsep, Mrp2, Ntcp and Oatp2 were significantly suppressed by EE in a dose-dependent manner (Online Resource Fig. 4).
Activation of AMPK is critical for EE-Induced cholestasis in rat primary hepatocytes
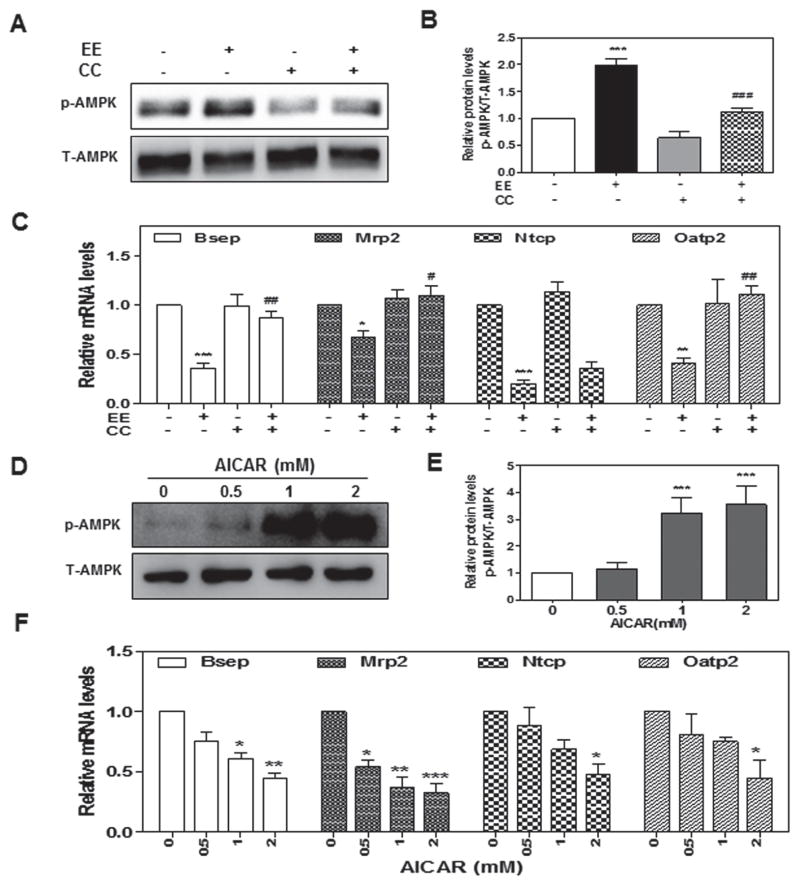
To further define the role of AMPK activation in EE-induced cholestasis, we first examined whether inhibition of AMPK activation using a chemical inhibitor of AMPK, Compound C (CC), had any effect on EE-induced cholestasis. As shown in Fig. 2a–c, pretreatment of rat primary hepatocytes with CC (2 μM) for 1-hour not only inhibited EE-induced AMPK activation, but also completely attenuated EE-induced down-regulation of Bsep, Mrp2, and Oatp2, but not Ntcp. We further examined whether activation of AMPK with AICAR, an analog of AMP, had a similar effect to EE on bile acid transporters. In agreement with our hypothesis, AICAR markedly activated AMPK at concentrations of 1 and 2 mM, which was accompanied by the suppression of mRNA expression of hepatic bile acid transporters (Fig. 2d–f). These results indicated AMPK activation is responsible for EE-induced suppression of hepatocyte bile acid transporters.
Fig. 2. The role of AMPK activation in EE-induced hepatocyte cholestasis.
Rat primary hepatocytes were pretreated with an AMPK inhibitor, Compound C (CC, 2 μM), for 1-hour and then treated with EE (10 μM) for 2- or 24-hour, or treated with AICAR (0 to 2 μM) for 2 or 24-hour. a, b, d, e Representative immunoblots against p-AMPK and T-AMPK are shown. c, f The relative mRNA levels were determined by real-time RT-PCR and normalized using GAPDH as an internal control. ***P<0.001 vs. control group. ###P<0.001 vs. EE group
EE suppresses FXR via AMPK activation in rat primary hepatocytes
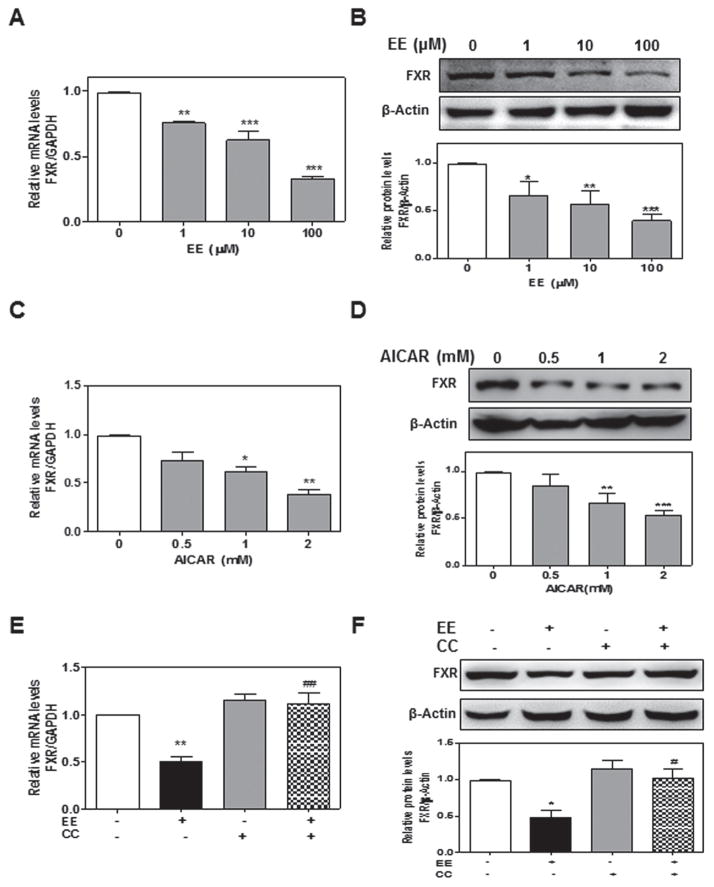
FXR is one of the most important NRs involved in the regulation of bile acid homeostasis in the liver. Suppression of FXR expression or inhibition of FXR activation contributes to estrogen-induced cholestasis (Abu-Hayyeh et al. 2013; Gonzalez-Sanchez et al. 2015). In order to identify the molecular mechanism by which EE-induced AMPK activation promotes cholestasis, we examined the effect of EE-induced activation of AMPK on FXR expression in rat primary hepatocytes. As shown in Fig. 3a, b, EE does-dependently inhibited FXR expression at both mRNA and protein levels. Similar results were obtained with AICAR (Fig. 3c, d). Furthermore, EE-induced suppression of FXR expression was attenuated by inhibition of AMPK activation with CC (Fig. 3e, f). Since EE-induced ERK1/2 activation is required for AMPK activation, we further examined whether inhibition of ERK1/2 activation had any effect on FXR expression. As shown in Online Resource Fig. 5, EE-induced suppression of FXR expression was also blocked by U0126.
Fig. 3. Effect of EE-induced AMPK activation on FXR expression in rat primary hepatocytes.
a, b Rat primary hepatocytes were treated with EE (0 to 100 μM) for 24-hour. c, d Cells were treated with AICAR (0 to 2 mM) for 24-hour. e, f Cells were pretreated with CC (2 μM) for 1 hour and then treated with EE (10 μM) for 2-hour. a, c, e The relative mRNA levels of FXR were normalized using GAPDH as an internal control. b, d, f Representative immunoblots against FXR and β-actin are shown. *P<0.05, **P<0.01,***P<0.001 vs. control group. #P<0.05, ##P<0.01 vs EE group
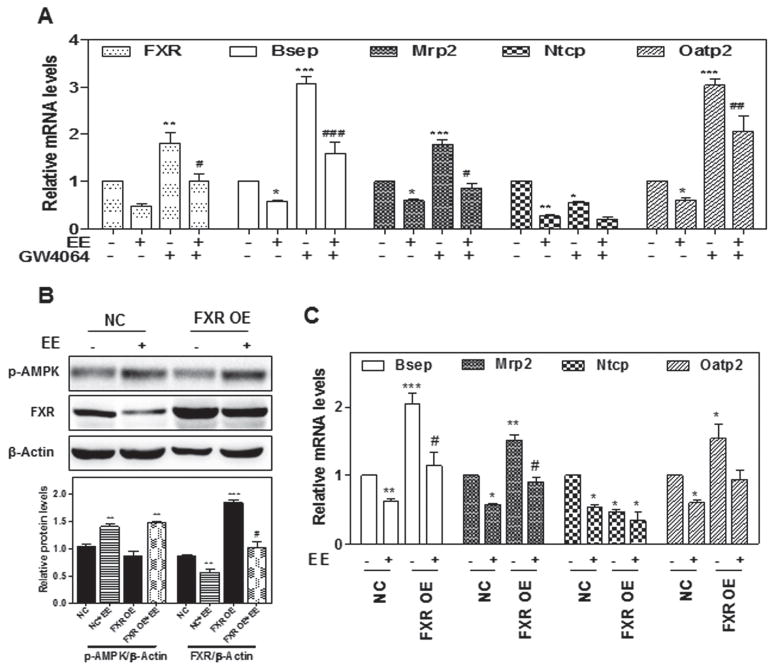
Based on the observations that EE induced the suppression of FXR, we investigated whether the activation of FXR could protect hepatocytes from EE-induced cholestatic injury. Notably, a synthetic FXR agonist, GW4064 (10 μM), significantly inhibited EE-induced down-regulation of Bsep, Mrp2, and Oatp2, but not Ntcp (Fig. 4a). Furthermore, overexpression of FXR using lentivirus expression system significantly reversed EE-induced down-regulation of Bsep, Mrp2, and Oatp2, but not Ntcp (Fig. 4b, c). These findings indicated that EE impairs bile acid homeostasis via activation of the ERK1/2-LKB1-AMPK signaling pathway, which further suppresses FXR expression in hepatocytes.
Fig. 4. The role of FXR in EE-induced cholestasis.
a Rat primary hepatocytes were treated with EE (10 μM) in the presence or absence of the FXR agonist GW4064 (10 μM) for 24-hour. b, c Rat primary hepatocytes were transduced with FXR overexpression lentivirus or control lentivirus for 24-hour, then treated with EE (10 μM) for 2 or 24-hour. b Representative immunoblots against p-AMPK and FXR are shown and normalized with β-Actin. a, c The relative mRNA levels of relative genes were determined by qPCR and normalized using GAPDH as an internal control. *P<0.05, **P<0.01,***P<0.001 vs. control group. #P<0.05 vs. EE group
Previous studies have shown that EE activated intracellular adenylyl cyclase (AC) via G protein-coupled receptor, GRP30, in human breast cancer cells (Pang et al. 2008). G15, a specific antagonist of GRP30, also significantly inhibited EE-induced AMPK activation, but only partially reversed EE-induced inhibition of FXR and bile acid transporters in rat primary hepatocytes (Online Resource Fig. 6). Similarly, the nuclear Estrogen receptor (ER) antagonist ICI182,780 also partially prevented EE-induced down-regulation of bile acid transporters, but had no effect on EE-induced AMPK activation and FXR suppression (Online Resource Fig. 7).
EE-induced AMPK activation contributes to cholestasis in rat models
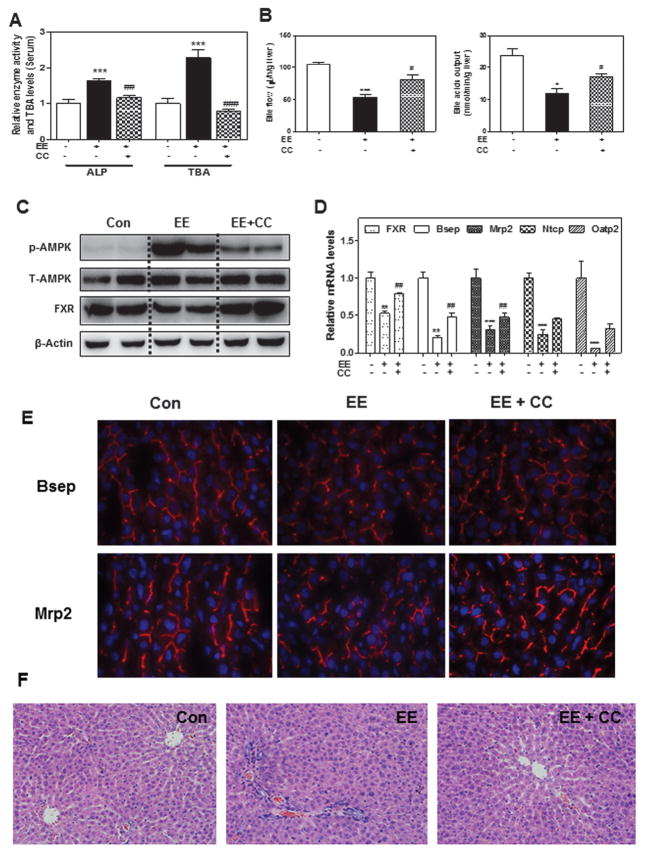
EE-induced cholestatic rat model has been widely used to study the cellular/molecular mechanisms of estrogen-induced cholestasis (Henriquez-Hernandez et al. 2007). In order to determine whether our findings in rat primary hepatocytes could be translated into in vivo animal models, EE-induced rat models were used. Adult SD rats (male) were subcutaneously injected with EE at a dose of 10 mg/kg for 1 or 5 days. Total serum bile acid levels (TBA), liver functional enzyme activities (ALP, AST and ALT) and mRNA levels of hepatic transporters were measured. The results indicated that EE induced significant cholestasis even after treatment for one day (Data not shown). The TBA levels in the liver and serum as well as serum ALP levels were similar after one day or five days’ treatment of EE. Therefore, a one day EE-treated rat model was used in the following studies. To further verify the role of AMPK activation in EE-induced cholestasis in vivo, SD rats were intraperitoneally injected (ip) with the AMPK inhibitor CC (10 mg/kg) for 1-hour before subcutaneous administration of EE. After 24-hour, the animals were sacrificed. As shown in Fig. 5a and Online Resource Fig. 8a, the serum ALP activity and TBA levels in serum and liver were significantly increased by EE treatment. Similarly, EE significantly decreased bile acid flow accompanied with remarkably decreased levels of bile acid output (Fig. 5b) and phospholipid in bile (Online Resource Fig. 8b). These effects were markedly diminished by CC treatment. Western blot analysis further indicated that CC significantly inhibited the EE-induced phosphorylation of AMPK and the suppression of FXR expression (Fig. 5c and Online Resource Fig. 8c). Similar to our findings in rat primary hepatocytes, CC also inhibited EE-induced suppression of FXR and bile acid transporters at the mRNA levels (Fig. 5d). To investigate the effect of CC on the EE-induced impairment of bile acid transporters in vivo, we analyzed frozen liver sections by immunofluorescent staining for Bsep and Mrp2. As shown in Fig. 5e and Online Resource Fig. 8d, EE markedly reduced protein expression of Bsep and Mrp2 as well as the canalicular-localization of these two proteins, indicating the impairment of bile acid transporters, which was alleviated by CC. However, in rats only treated with CC, the expression and canalicular-localization of Bsep and Mrp2 were similar to those in control animals (data not shown). In addition, EE-induced inflammatory cell infiltration was prevented by CC treatment as indicated by H&E staining (Fig. 5f). These findings supported our hypothesis that AMPK activation plays a critical role in EE-induced cholestasis.
Fig. 5. Protective effect of Compound C on EE-induced cholestasis in rat model.
a The serum ALP activity and TBA, b Bile acid flow rates and biliary TBA levels in rats. c Representative immunoblots against p-AMPK, T-AMPK, FXR and β-Actin in liver lysates are shown. d The relative mRNA levels of relative genes were determined by real-time RT-PCR and normalized using GAPDH as an internal control. e Frozen liver sections of the Bsep and Mrp2expression. f Representative images of rat liver sections by HE staining. *P<0.05, **P<0.01, ***P<0.001 vs. control group, n=6; #P<0.05, ##P<0.01, ###P<0.001vs. EE group, n=6
EE induces phosphorylation and nuclear translocation of AMPKα1 rather than AMPKα2
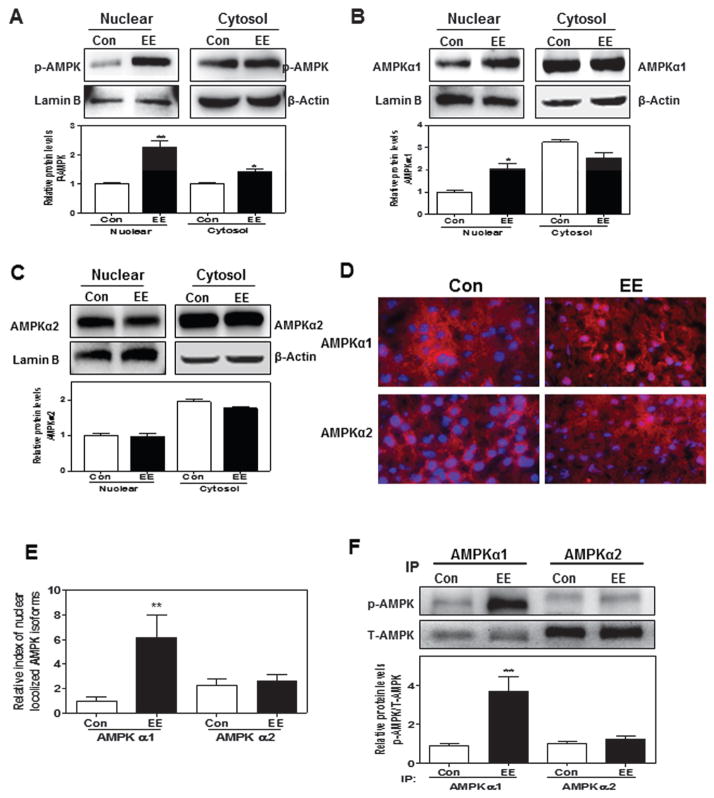
Previous studies have shown that the nuclear translocation of phosphorylated AMPK is important for its biological functions. We first examined whether EE-induced increase of p-AMPK was correlated to its nuclear translocation. As shown in Fig. 6a, EE significantly induced the accumulation of p-AMPK in the nucleus after a 4-hour treatment in rat primary hepatocytes. It has been reported that the α subunit of AMPK contains the catalytic domain and determines its kinase activity. Two AMPKα isoforms (α1 and α2) have been identified. The expression and activation of AMPKα1 and AMPKα2 are differentially regulated under different pathophysiological conditions (Novikova et al. 2015). In the liver, both AMPKα1 and AMPKα2 are expressed at similar levels. To further determine whether EE-induced AMPK nuclear translocation is isoform specific, we measured the protein levels for each isoform both in nuclear and cytosolic fractions. As shown in Fig. 6b–c, EE specifically induced nuclear translocation of AMPKα1 but not AMPKα2. To further confirm these observations, we used the EE-induced cholestasis rat model to first measure the mRNA levels for both isoforms. The results indicated that the mRNA level of AMPKα1 was slightly higher than that of AMPKα2 in the liver (Data not shown). Immunofluorescence staining of rat liver sections showed that EE significantly induced AMPKα1 nuclear accumulation, but not AMPKα2 (Fig. 6d, e). Immunoprecipitation with isoform-specific antibodies further confirmed that EE specifically induced isoform-specific phosphorylation and nuclear translocation of AMPKα1 in cholestatic rat livers (Fig. 6f). Taken together, these findings suggested that AMPKα1, but not AMPKα2 may be the key player in EE-induced cholestasis.
Fig. 6. Effect of EE on sub-cellular distribution of AMPKα1 and AMPKα2 subunits.
a, b, c Primary rat hepatocytes were treated with EE (10 μM) for 4-hour. Representative immunoblots against p-AMPK, AMPKα1, AMPKα2, lamin B and β-Actin are shown and normalized with lamin B or β-Actin. d, e Co-localization of AMPKα1 and AMPKα2 (both red) with nuclear (blue) in rat liver after EE treatment for 1 day. f The protein levels of phosphorylated AMPKα1 and AMPKα2 were detected by immunoprecipitation using indicated antibodies, following Western blot analysis against p-AMPK and T-AMPK, and normalized with T-AMPK. **P<0.01 vs. control group, n=6
AMPKα1 knockdown attenuates the EE-Induced disruption of bile acid hemostasis
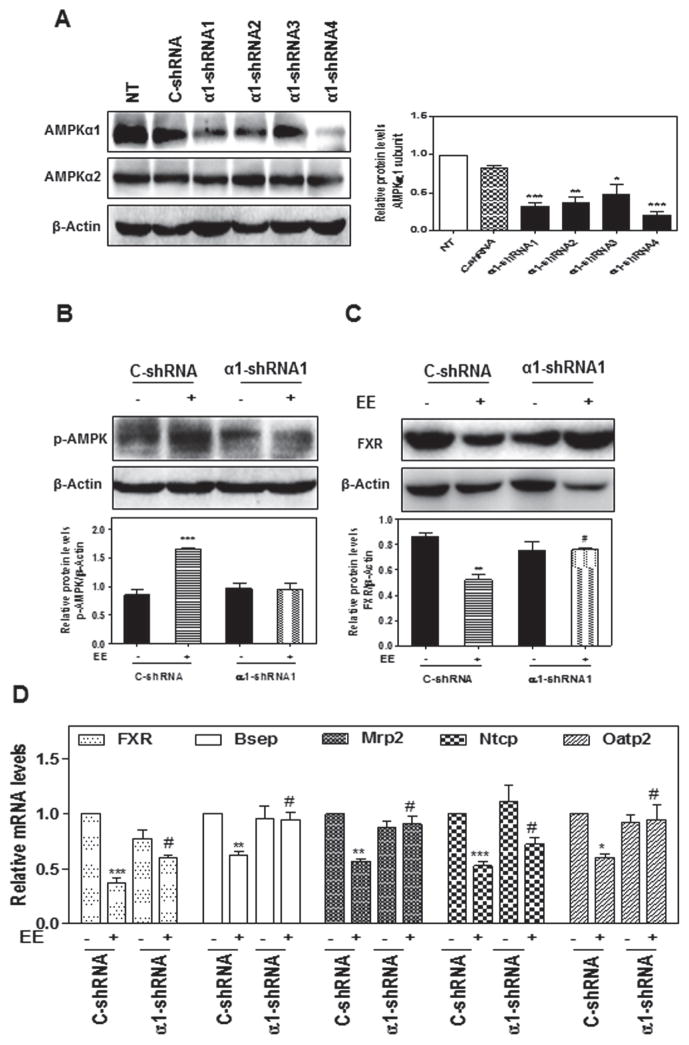
To further define the crucial role of AMPKα1 in EE-induced cholestasis, specific lentiviral shRNAs were constructed to knockdown the expression of AMPKα1 in rat primary hepatocytes. The constructed AMPKα1 shRNA1 was able to efficiently down-regulate the expression of AMPKα1, but had no effect on AMPKα2 in rat primary hepatocytes (Fig. 7a). EE-induced increase of p-AMPK and decrease of FXR expression were completely blocked by AMPKα1 shRNA1 (Fig. 7b–c). Furthermore, EE-induced suppression of FXR and hepatic transporters were also significantly reversed by down-regulation of AMPKα1 expression (Fig. 7d). In contrast, knockdown of AMPKα2 expression with AMPKα2 shRNA had no effect on EE-induced AMPK activation and suppression of FXR and hepatic transporters (Online Resource Fig. 9). These findings further demonstrated that activation of AMPKα1 is responsible for EE-induced cholestasis via down-regulating FXR expression, which further inhibits bile acid transporter expression.
Fig. 7. Effect of AMPKα1 knock down on EE-induced cholestasis in rat primary hepatocytes.
a Rat primary hepatocytes were transduced with lentivirus shRNA targeting AMPKα1 (α1-shRNA 1–4) or negative control lentivirus (C-shRNA) for 24-hour. NT: no treatment. b, c, d Rat primary hepatocytes were transduced with lentivirus α1-shRNA or C-shRNA for 24-hour. b Cells were then treated with EE for 2-houror c 24-hour. Representative immunoblots are shown. d The relative mRNA levels of relative geneswere determined by qPCR and normalized using GAPDH as an internal control. *P<0.05, ** P<0.01 and *** P<0.001 vs. control group; #P<0.05 vs. EE group
Discussion
Maintenance of hepatocyte bile flow and highly specialized canalicular transporters is crucial to bile acid homeostasis. In cholestatic liver diseases, dysregulation of bile acid transporters responsible for bile acid uptake or efflux are often associated with limitation of bile excretion and disease progression. In the present study, we have identified for the first time that AMPKα1 is an important regulator of the nuclear bile acid sensor FXR and bile acid transporters in EE-induced cholestasis. In both in vitro sandwich-cultured rat primary hepatocytes and in vivo cholestatic rats, EE significantly induced the activation and nuclear translocation of AMPKα1, but not AMPKα2. Furthermore, down-regulation of AMPKα1 expression with gene-specific shRNA or inhibition of its activation with specific chemical inhibitor, CC, significantly reduced EE-induced inhibition of FXR expression at both mRNA and protein levels and prevented EE-induced impairment of bile acid homeostasis. Although activation of FXR had no effect on AMPK phosphorylation, overexpression of FXR or activation of FXR with the chemical agonist, GW4064, significantly inhibited EE-induced cholestatic injury.
FXR is a central controller in regulating bile acid enterohepatic circulation. Activation of FXR by bile acids rapidly induces SHP expression in hepatocytes, which in turn leads to transcriptional repression of key genes involved in the synthesis, transport and metabolism of bile acids and lipids (Gonzalez-Sanchez et al. 2015). AMPK is an important regulator of cellular energy homeostasis in response to changes in the intracellular ADP/ATP ratio, the most sensitive indicator of energy status (Novikova et al. 2015). Regulation of AMPK activation is mainly implemented by kinase-mediated phosphorylation of its catalytic α-subunit. Three kinases have been identified so far to be responsible for AMPK activation, LKB1, CaMKKβ, and TGF-β-activated kinase 1 (Novikova et al. 2015). Contribution of each upstream kinase to AMPK activation may vary in different tissues and under different physiological and pathological conditions. In vitro studies in rodent primary hepatocytes and in vivo mouse models demonstrated that LKB1-mediated activation of AMPK is required for bile acid-induced canalicular network formation (Homolya et al. 2014). However, a recent study identified that AMPK subunits directly bind to FXR and phosphorylate the serine 250 residue of FXR, which prevents the loading of coactivator complexes and results in the inhibition of FXR activation (Lien et al. 2014). This study indicated that activation of hepatic AMPK selectively repressed FXR-mediated transcription in hepatocytes under the conditions of increased haptic bile acids, such as (pre)cholestatic conditions.
Estrogen-induced cholestasis is one of the most common liver diseases, especially in pregnant women where it is known as intrahepatic cholestasis of pregnancy (ICP). The most common clinical symptoms of ICP include pruritus and jaundice, which are often associated with increased rates of adverse fetal outcomes (Williamson and Geenes 2014). In ICP patients, hepatic bile acid accumulation associated with dysregulation of bile excretion is correlated with abnormally high estrogen levels (Abu-Hayyeh et al. 2013). Consistent with the findings from other groups, we found that EE impaired bile acid homeostasis through the down-regulation of specific transporters as well as the suppression of FXR expression in both sandwich-cultured primary rat hepatocytes and a rat cholestasis model. Our current studies further identified that cAMP-ERK1/2-LKB1-induced activation of the AMPKα1 isoform and subsequent nuclear translocation are responsible for EE-mediated cholestasis. It has been reported that AMPKα1 is the most common catalytic subunit of AMPK in the liver (Stapleton et al. 1996). Although previous studies reported that FXR activation resulted in AMPK activation (Noh et al. 2011) or induced LKB1 expression (Lee et al. 2012). In this current study, EE primarily activated AMPKα1, not AMPKα2. Knockdown of AMPKα1 with specific shRNA or FXR overexpression inhibited EE-induced down-regulation of FXR and hepatic transporters, suggesting AMPK acting as upstream regulator of FXR, but not the reverse. Interestingly, it also has been demonstrated that aberrant activation of AMPKα1 in the nuclei of striatal cells represents a new toxic pathway in Huntington’s disease, a neurodegenerative disorder (Ju et al. 2011).
Among the bile acid transporters regarded as FXR targets, the regulation of Bsep by FXR has been well characterized. Although previous studies have shown that EE down-regulates protein levels of Mrp2, without significant effect on mRNA levels (Trauner et al. 1997), recent studies indicate that modulation of FXR activity through pharmacological activation and genetic deletion affects the expression of Mrp2 at both mRNA and protein levels. Consistent with our findings, these studies suggest that Mrp2 may be a potential target of FXR (Meng et al. 2015). Additionally, it also has been reported that, at the early time point of EE injection for 24h, hepatocyte nuclear factor 1α (HNF1α) was induced significantly, which might contribute to sustained down-regulation of Ntcp and Oatp2 expression(Geier et al. 2003). However, the expression of Oatp2 and Ntcp were not significantly up-regulated by pretreatment with CC or over expression of FXR, which indicates other mechanisms might be involved in EE-mediated Oatp2 and Ntcp expression. Interestingly, several studies also showed that a FXR-bound agonist caused repression of HNF4α and, concordantly, Oatp2 (Jung et al. 2007). HNF4α also has been shown to directly transactivate the Ntcp promoter (Geier et al. 2008) and decreased HNF4α nuclear binding activity to the HNF1α promoter repressed Ntcp (Jung and Kullak-Ublick 2003). It is noteworthy that phosphorylation of AMPK induces the down-regulation of FXR expression at the transcriptional level. It also has been demonstrated that AMPK directly phosphorylated HNF4α and induced the inhibition of its transcriptional activity (Chang et al. 2016). Furthermore, there is complicated cross-talk between HNF4α and HNF1α via a direct protein-protein interaction (Eeckhoute et al. 2004), which inhibits the transactivity of HNF1α on the FXR promoter and subsequently decreases FXR transcription (Liu et al. 2012). Further study is needed to delineate the exact role of HNF4α and HNF1α in the EE-induced bile acid transporter decrease and AMPK-mediated FXR inhibition.
Recent studies have shown that estrogen also can activate a transmembrane-bound G protein-coupled receptor 30 (GRP30)(Xu et al. 2009). Recent work from Zucchetti, et al. demonstrated that activation of the GPR30-AC-PKA signaling pathway is a key factor in estradiol-17β-D-glucuronide (E17G)-induced cholestasis (Zucchetti et al. 2014). We also found EE significantly increased cAMP levels in hepatocytes, which may occur through activation of GRP30. Our studies also showed inhibition of GRP30 activation with G15 blocking EE-induced AMPK activation (Online Resource Fig. 6). However, chemical inhibitors of PKA (H89) and CaMMKβ (STO-609) failed to inhibit EE-induced activation of AMPK (Online Resource Fig. 2). It has been reported that activation of the ER suppressed the transcriptional activity of FXR by direct interaction (Song et al. 2014). The resistance of EE-induced cholestasis in ER-null mice supported this potential mechanism (Yamamoto et al. 2006). However, our results showed that EE decreased the expression of FXR via activation of AMPK. Although the nuclear ER antagonist ICI182,780 partially prevented EE-induced down-regulation of bile acid transporters, it had no effect on EE-induced AMPK activation and FXR suppression (Online Resource Fig. 7). This evidence strongly suggests that, in addition to ER-induced inhibition of FXR activity, AMPK-dependent down-regulation of FXR also plays a critical role in EE-induced cholestasis. Over the last decade, a number of studies have shown that bile acid-induced activation of bile acid receptor TGR5 induces an increase of intracellular cAMP in many different types of cells (Stepanov et al. 2013). However, TGR5 is hardly detected in hepatocytes. Our previous studies also identified that a conjugated bile acid, TCA, induces activation of ERK1/2 via sphingosine-1 phosphate receptor 2 (S1PR2) in hepatocytes (Studer et al. 2012). Considering the bile acid accumulation including TCA during a cholestasis period, we investigated the role TCA plays in EE-induced AMPK activation. Our results showed that TCA increased the EE-induced phosphorylation of AMPK, which was blocked by chemical antagonist of S1PR2, JTE-013 (Data not shown). Whether S1PR2 is also involved in EE-induced cholestasis has not been fully investigated and is our ongoing project.
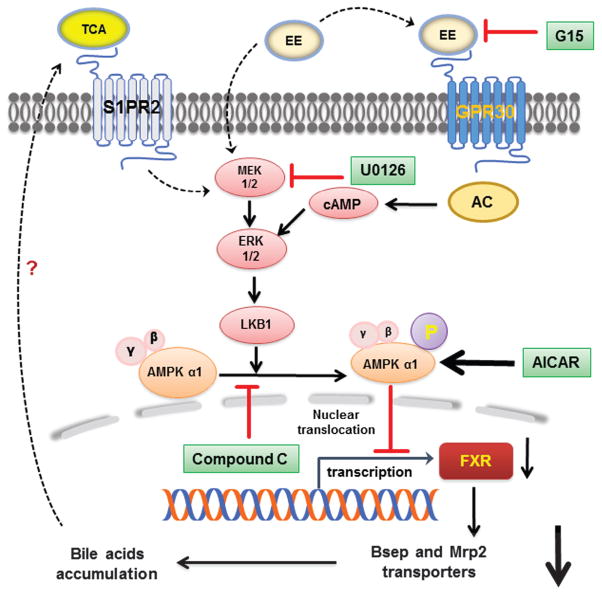
In summary, our results demonstrated that ERK1/2-LKB1-dependent AMPKα1 activation and nuclear translocation are responsible for the EE-induced suppression of FXR expression and subsequently impairment of bile acid homeostasis via down-regulation of bile acid transporters (Fig. 8). The inhibition of AMPK or activation of FXR markedly reduced EE-induced cholestasis. Based on our previous studies, EE-induced accumulation of TCA may further activate S1PR2 and induce ERK1/2 activation. Overall, the current study provides novel insight into the pathogenesis of EE-induced cholestasis. Targeting AMPKα1 may represent a potential therapeutic target for ICP and other hormone-induced hepatotoxicity.
Fig. 8. Proposed model of EE-induced impairment of bile acid homeostasis.
Possible mechanisms underlying AMPKα1 activation via cAMP-ERK1/2-LKB1 signaling pathway in EE-induc ed cholestasis.
Supplementary Material
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants (81320108029 to LZ; 81573514 to ZJ; 81070245, 81270489 to HZ); Specific Fund for Public Interest Research of Traditional Chinese Medicine, Ministry of Finance (201507004-002 to LZ), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD); National Institutes of Heath Grant R01 DK-057543-11 (to PBH and HZ), VA Merit Awards (to HZ; I01BX001390); VCU Massey Cancer Pilot grant (A35362 to HZ).
List of Abbreviations
- ICP
intrahepatic cholestasis of pregnancy
- EE
17α-ethinylestradiol
- Bsep
bile salt export pump
- Mrp2
multidrug resistance-associated protein 2
- Ntcp
Na+-dependent taurocholate cotransporter
- Oatp2
organic anion transporters 2
- FXR
farnesoid X receptor
- AMPK
AMP-activated protein kinase
- LKB1
liver kinase B1
- ERK1/2
extracellular-signal-regulated kinases
- ER
estrogen receptor
- TCA
taurocholate
- GPR30
G protein-coupled receptor 30
- CC
compound C
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
Footnotes
Contributions: LX, PBH, ZJ, LZ and HZ conceived the original ideas, designed the study, analyzed the data and wrote the manuscript; LX, RL, LL, XC, LS, TW carried out the experiments and data analysis. ZJ and LZ contributed equally.
Conflict of interest: The authors declare no competing financial interest.
References
- Abu-Hayyeh S, Papacleovoulou G, Lovgren-Sandblom A, et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57(2):716–726. doi: 10.1002/hep.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth A, Klinger G, Rost M. Influence of ethinyloestradiol propanolsulphonate on serum bile acids in healthy volunteers. Experimental and Toxicologic Pathology. 2003;54(5):381–386. doi: 10.1078/0940-2993-00274. [DOI] [PubMed] [Google Scholar]
- Chang HR, Nam S, Kook MC, et al. HNF4alpha is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut. 2016;65(1):19–32. doi: 10.1136/gutjnl-2014-307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocenzi FA, Sanchez Pozzi EJ, Pellegrino JM, et al. Beneficial effects of silymarin on estrogen-induced cholestasis in the rat: a study in vivo and in isolated hepatocyte couplets. Hepatology. 2001;34(2):329–339. doi: 10.1053/jhep.2001.26520. [DOI] [PubMed] [Google Scholar]
- Eeckhoute J, Formstecher P, Laine B. Hepatocyte nuclear factor 4alpha enhances the hepatocyte nuclear factor 1alpha-mediated activation of transcription. Nucleic acids research. 2004;32(8):2586–2593. doi: 10.1093/nar/gkh581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Wakabayashi Y, Lippincott-Schwartz J, Arias IM. Bile acid stimulates hepatocyte polarization through a cAMP-Epac-MEK-LKB1-AMPK pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(4):1403–1408. doi: 10.1073/pnas.1018376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier A, Dietrich CG, Gerloff T, et al. Regulation of basolateral organic anion transporters in ethinylestradiol-induced cholestasis in the rat. Biochimica et biophysica acta. 2003;1609(1):87–94. doi: 10.1016/s0005-2736(02)00657-0. [DOI] [PubMed] [Google Scholar]
- Geier A, Martin IV, Dietrich CG, et al. Hepatocyte nuclear factor-4alpha is a central transactivator of the mouse Ntcp gene. American journal of physiology Gastrointestinal and liver physiology. 2008;295(2):226–233. doi: 10.1152/ajpgi.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Sanchez E, Firrincieli D, Housset C, Chignard N. Nuclear receptors in acute and chronic cholestasis. Digestive diseases. 2015;33(3):357–366. doi: 10.1159/000371688. [DOI] [PubMed] [Google Scholar]
- Gowans GJ, Hardie DG. AMPK: a cellular energy sensor primarily regulated by AMP. Biochemical Society transactions. 2014;42(1):71–75. doi: 10.1042/BST20130244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ashford ML. AMPK: regulating energy balance at the cellular and whole body levels. Physiology. 2014;29(2):99–107. doi: 10.1152/physiol.00050.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez-Hernandez LA, Flores-Morales A, Santana-Farre R, et al. Role of pituitary hormones on 17alpha-ethinylestradiol-induced cholestasis in rat. The Journal of pharmacology and experimental therapeutics. 2007;320(2):695–705. doi: 10.1124/jpet.106.113209. [DOI] [PubMed] [Google Scholar]
- Homolya L, Fu D, Sengupta P, et al. LKB1/AMPK and PKA control ABCB11 trafficking and polarization in hepatocytes. PloS one. 2014;9(3):e91921. doi: 10.1371/journal.pone.0091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju TC, Chen HM, Lin JT, et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. The Journal of cell biology. 2011;194(2):209–227. doi: 10.1083/jcb.201105010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D, Elferink MG, Stellaard F, Groothuis GM. Analysis of bile acid-induced regulation of FXR target genes in human liver slices. Liver international : official journal of the International Association for the Study of the Liver. 2007;27(1):137–144. doi: 10.1111/j.1478-3231.2006.01393.x. [DOI] [PubMed] [Google Scholar]
- Jung D, Kullak-Ublick GA. Hepatocyte nuclear factor 1 alpha: a key mediator of the effect of bile acids on gene expression. Hepatology. 2003;37(3):622–631. doi: 10.1053/jhep.2003.50100. [DOI] [PubMed] [Google Scholar]
- Lee CG, Kim YW, Kim EH, et al. Farnesoid X receptor protects hepatocytes from injury by repressing miR-199a-3p, which increases levels of LKB1. Gastroenterology. 2012;142(5):1206–1217. doi: 10.1053/j.gastro.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chiang JY. Nuclear receptors in bile acid metabolism. Drug metabolism reviews. 2013;45(1):145–155. doi: 10.3109/03602532.2012.740048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien F, Berthier A, Bouchaert E, et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. The Journal of clinical investigation. 2014;124(3):1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Meng Z, Lou G, et al. Hepatocarcinogenesis in FXR−/− mice mimics human HCC progression that operates through HNF1alpha regulation of FXR expression. Molecular endocrinology. 2012;26(5):775–785. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, LeCluyse EL, Brouwer KR, et al. Biliary excretion in primary rat hepatocytes cultured in a collagen-sandwich configuration. The American journal of physiology. 1999;277(1 Pt 1):12–21. doi: 10.1152/ajpgi.1999.277.1.G12. [DOI] [PubMed] [Google Scholar]
- Liu Y, Binz J, Numerick MJ, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. The Journal of clinical investigation. 2003;112(11):1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q, Chen X, Wang C, et al. Protective Effects of Alisol B 23-Acetate Via Farnesoid X Receptor-Mediated Regulation of Transporters and Enzymes in Estrogen-Induced Cholestatic Liver Injury in Mice. Pharmaceutical research. 2015;32(11):3688–3698. doi: 10.1007/s11095-015-1727-x. [DOI] [PubMed] [Google Scholar]
- Noh K, Kim YM, Kim YW, Kim SG. Farnesoid X receptor activation by chenodeoxycholic acid induces detoxifying enzymes through AMP-activated protein kinase and extracellular signal-regulated kinase 1/2-mediated phosphorylation of CCAAT/enhancer binding protein beta. Drug metabolism and disposition: the biological fate of chemicals. 2011;39(8):1451–1459. doi: 10.1124/dmd.111.038414. [DOI] [PubMed] [Google Scholar]
- Novikova DS, Garabadzhiu AV, Melino G, Barlev NA, Tribulovich VG. AMP-activated protein kinase: structure, function, and role in pathological processes. Biochemistry Biokhimiia. 2015;80(2):127–144. doi: 10.1134/S0006297915020017. [DOI] [PubMed] [Google Scholar]
- Pang Y, Dong J, Thomas P. Estrogen signaling characteristics of Atlantic croaker G protein-coupled receptor 30 (GPR30) and evidence it is involved in maintenance of oocyte meiotic arrest. Endocrinology. 2008;149(7):3410–3426. doi: 10.1210/en.2007-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahner C, Stieger B, Landmann L. Structure-function correlation of tight junctional impairment after intrahepatic and extrahepatic cholestasis in rat liver. Gastroenterology. 1996;110(5):1564–1578. doi: 10.1053/gast.1996.v110.pm8613064. [DOI] [PubMed] [Google Scholar]
- Ruiz ML, Rigalli JP, Arias A, et al. Induction of hepatic multidrug resistance-associated protein 3 by ethynylestradiol is independent of cholestasis and mediated by estrogen receptor. Drug metabolism and disposition: the biological fate of chemicals. 2013;41(2):275–280. doi: 10.1124/dmd.112.047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia N, Oberhauser E. Sulfobromophthalein clearance tests before and after ethinyl estradiol administration, in women and men with familial history of intrahepatic cholestasis of pregnancy. Gastroenterology. 1981;81:226–231. [PubMed] [Google Scholar]
- Song X, Vasilenko A, Chen Y, et al. Transcriptional dynamics of bile salt export pump during pregnancy: mechanisms and implications in intrahepatic cholestasis of pregnancy. Hepatology. 2014;60(6):1993–2007. doi: 10.1002/hep.27171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, et al. Mammalian AMP-activated Protein Kinase Subfamily. Journal of Biological Chemistry. 1996;271(2):611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. Journal of receptor and signal transduction research. 2013;33(4):213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- Studer E, Zhou X, Zhao R, et al. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55(1):267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauner M, Arrese M, Soroka CJ, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113(1):255–264. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- Van Mil SW, Milona A, Dixon PH, et al. Functional variants of the central bile acid sensor FXR identified in intrahepatic cholestasis of pregnancy. Gastroenterology. 2007;133(2):507–516. doi: 10.1053/j.gastro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Williamson C, Geenes V. Intrahepatic Cholestasis of Pregnancy. Obstetrics & Gynecology. 2014;124(1):120–133. doi: 10.1097/AOG.0000000000000346. [DOI] [PubMed] [Google Scholar]
- Wu X, Sun L, Zha W, et al. HIV protease inhibitors induce endoplasmic reticulum stress and disrupt barrier integrity in intestinal epithelial cells. Gastroenterology. 2010;138(1):197–209. doi: 10.1053/j.gastro.2009.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, et al. Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience. 2009;158(4):1599–1607. doi: 10.1016/j.neuroscience.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess HA, et al. Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. The Journal of biological chemistry. 2006;281(24):16625–16631. doi: 10.1074/jbc.M602723200. [DOI] [PubMed] [Google Scholar]
- Yang S, Wang J. Estrogen Activates AMP-Activated Protein Kinase in Human Endothelial Cells via ERbeta/Ca/Calmodulin-Dependent Protein Kinase Kinase beta Pathway. Cell biochemistry and biophysics. 2015;72(3):701–707. doi: 10.1007/s12013-015-0521-z. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(46):17272–17277. doi: 10.1073/pnas.0608531103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchetti AE, Barosso IR, Boaglio AC, et al. G-protein-coupled receptor 30/adenylyl cyclase/protein kinase A pathway is involved in estradiol 17ss-D-glucuronide-induced cholestasis. Hepatology. 2014;59(3):1016–1029. doi: 10.1002/hep.26752. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.