Introduction
Drug abuse and overdose has become a national epidemic over the last twenty years. Nearly 500,000 people in the United States have died of drug overdose from 2000–2014, and it is the leading cause of injury death. The rate of prescription drug overdose deaths has been driven by a dramatic increase in the number of fatalities attributed to opioid analgesics; in 2014, 28,647 drug overdose deaths involved opioids[13].
In response to this epidemic, governmental agencies and professional organizations have implemented programs designed to limit inappropriate opioid prescriptions. These programs include prescription drug monitoring programs, legislation regulating pain clinics, and clinical guidelines for opioid therapy[10]. Because patients with mood disorders are at higher risk of opioid abuse[14] and overdose[4], most clinical guidelines recommend screening for mood disorders prior to initiating opioid therapy and recommend referral to appropriate services when necessary[12].
Despite these guidelines, patients with mood disorders continue to use opioids more often than other populations[5]. This finding is partially explained by the high frequency of comorbid pain conditions[1]. However, even among populations with pain, patients with mood disorders tend to have a higher prevalence of opioid use[8,14]. Whether this phenomenon reflects a higher prevalence of new opioid prescriptions or a greater predilection for longer-term opioid use is not clear. A few studies suggest that patients with mood disorders may take opioids for longer periods of time[5,8,14]. These prior studies have been limited to select patient populations and have not been able to control for certain clinical factors, such as the type of pain condition and clinical disability.
In this context, we examined the complex relationship between mood disorders, pain conditions, and opioid use in a nationally representative sample. We also measured the association between mood disorders, new opioid use, and the transition to longer-term opioid use. Understanding the mechanisms behind opioid use in this high-risk population will help clinicians more effectively treat pain during the opioid epidemic.
Methods
Data Source
We used the nationally representative Medical Expenditure Panel Surveys household component (MEPS-HC) to characterize the relationship between mood disorders, pain conditions, and opioid use. MEPS-HC provides nationally representative estimates of health care expenditure along with data on respondents’ health, demographics, socioeconomic status, employment, and access to healthcare. The panel-design survey includes five interviews over two calendar years. The MEPS Medical Provider Component (MEPS-MPC) collects data from clinics, pharmacies, and other providers regarding the self-reported medical events and prescriptions reported in the MEPS-HC. This information supplements and verifies the MEPS-HC data, although the MEPS-MPC dataset is not immediately available for public use.
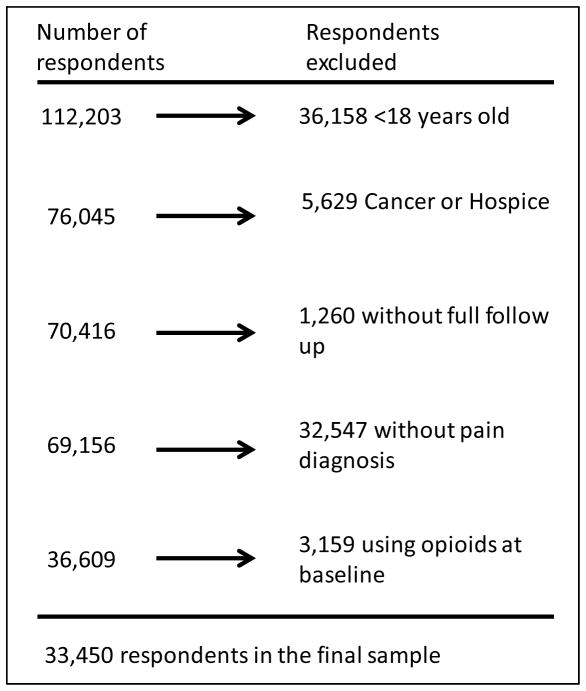
We used survey data from seven consecutive MEPS-HC panels, enrolling patients from 2005 to 2011. Adults were included in our sample population if they were 18 years or older on January 1st of their first year in MEPS-HC, reported no opioid use during the first survey period, had full two year follow up, and reported at least one acute or chronic pain condition (see below). Adults with any cancer diagnosis or hospice care were excluded from the sample (Figure 1).
Figure 1.
Inclusion and exclusion criteria for final analytic sample
Identification of key measures
Mood disorders
Medical conditions self-reported during MEPS were professionally coded to fully specified ICD-9 codes. The ICD-9 codes were aggregated into clinically meaningful categories using AHRQ Clinical Classification Software (CCS) diagnosis codes[9]. We identified CCS codes 657 and 651, corresponding to mood and anxiety disorders, reported at least once during the first calendar year of MEPS. Please see Appendix A for complete ICD-9 codes associated with each clinical classification code.
Pain conditions, comorbidity, and functional status
We identified pain conditions using the MEPS-HC medical condition file after linking CCS codes to opioid prescriptions in the MEPS-HC prescription medication file. We then reviewed the list of conditions most frequently associated with opioid use, and used clinical judgment to classify these conditions as a likely acute pain conditions or potentially chronic pain conditions. The likely acute conditions included fractures, tooth and jaw pain, kidney stones, traumatic injuries, and sprains. The potentially chronic conditions included back pain, chronic joint pain, connective tissue disorders, and headache/migraine. Because of the general nature of CCS codes, not all conditions linked to opioid use could be classified as likely acute or potentially chronic pain conditions. Please see appendix C for the listing of all CCS codes associated with all opioid prescriptions from 2005–2012 and their classification.
We calculated Charlson comorbidity scores for each patient according the method described by D’Hoore[7]. Baseline disability was evaluated with the Mental Component Score (MCS) and Physical Component Score (PCS) of the Short Form 12 (SF-12) survey administered during the second MEPS interview. The SF-12 question evaluating the degree pain limited the individual’s work in the prior 4 weeks was also analyzed separately, and we recorded whether the patient used non-opioid analgesics during the study period.
Opioid Use
Respondents reported all prescription medication purchases during each survey period. These prescriptions were categorized using Multum Lexicon therapeutic class codes[6]. We identified opioid prescriptions using sub-class codes corresponding to opioid analgesics and opioid analgesic combinations, after excluding methadone due to its use in treating substance use disorders. We identified new opioid prescriptions linked to the above pain conditions during the second through fifth survey periods. Among patients who started new opioid therapy, we defined the transition to longer-term opioid use as receiving 3 or more opioid prescriptions during consecutive survey periods.
Other key measures
We also considered age, sex, self-reported race, BMI (calculated from self-reported height and weight), annual household income, insurance, access to usual care provider, region of country, and highest level of education as reported during the first year of MEPS.
Statistical Analysis
We used Rao-Scott chi-square tests to compare proportions of respondents with and without mood disorders who initiated opioid therapy for any likely acute condition or any potentially chronic condition, and for each individual condition. Among the respondents who initiated new opioid therapy, we also compared the proportions that transitioned to longer-term opioid therapy. Data was suppressed when any cell size was <10.
Then, we developed logistic regression models to control for socio-demographic and baseline clinical disability. We adjusted for age, race, sex, education, BMI, PCS, MCS, the number of potentially chronic pain conditions reported by each individual, the degree to which pain limited daily function, the availability of a usual care provider, and use of non-opioid analgesics. All analyses were performed on SAS 9.3, and accounted for the complex survey design of MEPS. This study was granted exempt status by the institutional review board at Beth Israel Deaconess Medical Center.
Results
Of the 112,203 people enrolled in MEPS from 2005 to 2011, 33,450 met our inclusion criteria (Figure 1). Of these, 6,276 (weighted estimate 18.8%) reported a mood disorder. Table 1 shows demographic and clinical characteristics of the respondents at baseline. Adults with mood disorders were more likely to be female, and were disproportionately white. They also had lower income, were more likely to be publically insured, and had greater access to usual care providers. There were no significant differences in age or education level. Adults with mood disorders also had higher BMI, higher Charlson comorbidity scores, greater physical and mental disability, and were more likely to use non-opioid analgesics.
Table 1.
Weighted respondent characteristics, by presence of mood disorder
| Total | Mood disorder | No mood disorder | ||
|---|---|---|---|---|
|
|
||||
| n=33450 | n=6276 | n=27174 | p | |
| Age, mean (St Dev) | 48.0 (17.0) | 48.4 (15.9) | 47.9 (17.2) | 0.06 |
| Sex, % | <0.01 | |||
| Female | 54.4% | 67.8% | 51.2% | |
| Race, % | <0.01 | |||
| White | 71.7% | 78.5% | 70.1% | |
| Black | 10.9% | 7.3% | 11.7% | |
| Hispanic | 11.5% | 9.8% | 11.9% | |
| Other | 6.0% | 4.5% | 6.4% | |
| BMI, mean (St Dev) | 28.19 (6.34) | 29.31 (7.23) | 27.92 (6.08) | <0.01 |
| Region, % | <0.01 | |||
| Northeast | 18.5% | 17.5% | 18.8% | |
| Midwest | 23.2% | 25.7% | 22.6% | |
| South | 34.9% | 32.5% | 35.5% | |
| West | 23.3% | 24.3% | 23.1% | |
| Poverty category | <0.01 | |||
| Low income | 29.3% | 35.2% | 27.8% | |
| Medium income | 31.3% | 30.5% | 31.5% | |
| High income | 39.4% | 34.3% | 40.6% | |
| Highest degree, % | 0.13 | |||
| Less than high school | 12.7% | 11.7% | 13.0% | |
| High school or GED | 50.1% | 50.9% | 49.9% | |
| College | 27.6% | 27.8% | 27.6% | |
| Masters or doctorate | 9.6% | 9.6% | 9.5% | |
| Insurance Coverage, % | <0.01 | |||
| Any private | 70.6% | 67.2% | 71.4% | |
| Public only | 16.3% | 21.6% | 15.1% | |
| Uninsured | 13.1% | 11.2% | 13.5% | |
| Have usual care provider, % | <0.01 | |||
| Yes | 79.6% | 87.3% | 77.8% | |
| Use of non-opioid analgesics, % | <0.01 | |||
| Yes | 29.7% | 35.4% | 28.4% | |
| Charlson comorbidity index, % | ||||
| 0 | 77.7% | 69.2% | 79.7% | <0.01 |
| 1–2 | 18.3% | 23.6% | 17.0% | |
| >2 | 4.1% | 7.2% | 3.3% | |
| How much does pain limit normal work, % | <0.01 | |||
| None to little | 73.4% | 62.0% | 76.2% | |
| Moderate to extremely | 26.6% | 38.0% | 23.8% | |
| PCS, mean (St Dev) | 48.3 (10.6) | 46.2 (11.8) | 48.9 (10.2) | <0.01 |
| MCS, mean (St Dev) | 50.1 (9.8) | 43.0 (11.6) | 51.9 (8.6) | <0.01 |
| Acute pain conditions, % | 51.5% | 52.4% | 51.3% | 0.22 |
| Fracture | 7.1% | 7.8% | 6.9% | 0.04 |
| Tooth and jaw pain | 11.0% | 12.1% | 10.7% | <0.01 |
| Kidney Stone | 2.4% | 2.5% | 2.4% | 0.62 |
| Injury | 26.0% | 27.8% | 25.6% | <0.01 |
| Sprains | 14.4% | 14.7% | 14.4% | 0.60 |
| Chronic pain conditions, % | 74.3% | 82.5% | 72.3% | <0.01 |
| Back pain | 24.6% | 29.7% | 23.4% | <0.01 |
| Chronic joint pain | 41.9% | 50.1% | 40.0% | <0.01 |
| Connective tissue disorder | 21.6% | 28.1% | 20.0% | <0.01 |
| Headache | 13.8% | 18.6% | 12.6% | <0.01 |
| Number of acute conditions, % | <0.01 | |||
| 0 | 48.5% | 47.6% | 48.7% | |
| 1 | 43.0% | 41.3% | 43.4% | |
| 2 | 7.6% | 9.7% | 7.1% | |
| 3 | 0.9% | 1.3% | 0.8% | |
| 4 | 0.0% | 0.1% | 0.0% | |
| Number of chronic conditions, % | <0.01 | |||
| 0 | 25.7% | 17.5% | 27.7% | |
| 1 | 51.5% | 48.3% | 52.2% | |
| 2 | 18.3% | 25.4% | 16.6% | |
| 3 | 4.1% | 7.8% | 3.3% | |
| 4 | 0.3% | 1.0% | 0.2% | |
Overall, 16,475 respondents reported a likely acute pain condition. Of these, 2,995 (17.6%, weighted) started new opioid therapy for that condition and 209 (1.2%, weighted) continued on to longer-term opioid therapy. Additionally, 25,100 respondents reported a potentially chronic pain condition. Of these, 2,545 (9.7%, weighted) started new opioid therapy for that condition and 610 (2.4%, weighted) continued on to longer-term opioid therapy. Adults with mood disorders were more likely to reports potentially chronic pain conditions, as well as certain acute pain conditions, such as fracture, tooth and jaw pain, and traumatic injury (Table 1).
Table 2 shows the percentage of adults who started new opioid therapy linked to likely acute or potentially chronic pain conditions stratified by the presence of a mood disorder. Among all adults who reported any likely acute pain condition, a significantly higher proportion of those with a mood disorder started opioids for that condition compared to those without a mood disorder (19.3% vs 17.2%, p=0.01). There were no significant differences of opioid initiation for each individual acute pain condition. Similarly, among adults who reported a potentially chronic pain condition, a higher proportion of those with a mood disorder started opioids compared to those without a mood disorder (11.5% vs 9.2%, p<0.01). There were also higher rates of new opioid use among adults with mood disorders specifically for back pain and chronic joint pain.
Table 2.
Individuals reporting new opioid use during two year follow up
| Mood Disorder | No Mood Disorder | ||||
|---|---|---|---|---|---|
|
| |||||
| n | Weighted %* | n | Weighted %* | p | |
| For acute pain conditions | 631 | 19.3% | 2364 | 17.2% | 0.01 |
| Any fracture | 144 | 29.9% | 491 | 28.5% | 0.62 |
| Tooth and jaw disorders | 157 | 21.3% | 639 | 20.8% | 0.80 |
| Kidney stones | 32 | 24.7% | 188 | 31.0% | 0.22 |
| Traumatic injury | 203 | 11.5% | 730 | 10.7% | 0.42 |
| Sprains | 134 | 14.3% | 458 | 12.0% | 0.11 |
| For chronic pain conditions | 680 | 11.5% | 1865 | 9.2% | <0.01 |
| Back pain | 302 | 14.5% | 745 | 11.7% | 0.01 |
| Chronic joint pain | 304 | 8.5% | 773 | 6.7% | <0.01 |
| Connective tissue disorders | 160 | 8.0% | 423 | 7.2% | 0.27 |
| Headache and migraine | 80 | 5.5% | 157 | 4.4% | 0.19 |
Weighted percent of individuals with each condition who reported new opioid use linked to the condition
Table 3 shows the percentage of new opioid users who transitioned to longer-term opioid therapy stratified by the presence of a mood disorder. Among adults who started new opioid therapy for any likely acute pain condition, a higher proportion of those with a mood disorder received ≥3 prescriptions in consecutive survey periods compared to those without a mood disorder (11.7% vs 5.3%, p<0.01). Similarly, among adults who started new opioid therapy for a potentially chronic pain condition, a higher percentage of those with a mood disorder transitioned to longer term therapy compared to those without a mood disorder (36.8% vs 19.9%, p<0.01). There were also significant differences in the transition to longer-term opioid use for most specific conditions, aside from traumatic injuries and headache/migraine.
Table 3.
New opioid users who transition to longer-term opioid use
| Mood Disorder | No Mood Disorder | ||||
|---|---|---|---|---|---|
|
| |||||
| n | Weighted %* | n | Weighted %* | p | |
| For acute pain conditions | 72 | 11.7% | 137 | 5.3% | <0.01 |
| Any fracture | 19 | 13.6% | 34 | 6.1% | 0.02 |
| Tooth and jaw disorders | 13 | 7.6% | 18 | 3.0% | 0.03 |
| Kidney stones | <10 | <10 | |||
| Traumatic injury | 16 | 8.0% | 53 | 6.1% | 0.42 |
| Sprains | 21 | 17.8% | 27 | 5.7% | <0.01 |
| For chronic pain conditions | 244 | 36.8% | 366 | 19.9% | <0.01 |
| Back pain | 122 | 41.4% | 150 | 19.2% | <0.01 |
| Chronic joint pain | 108 | 36.2% | 176 | 22.7% | <0.01 |
| Connective tissue disorders | 42 | 26.1% | 60 | 16.0% | 0.05 |
| Headache and migraine | 20 | 27.7% | 20 | 16.6% | 0.14 |
Weighted percent of patients who received ≥3 prescriptions for the condition in consecutive survey periods among new opioid users for the given condition
Table 4 shows the association between having a mood disorder and new or longer-term opioid use, before and after adjusting for basic patient characteristics (Model 1) and with additional adjustment for clinical disability (Model 2). Mood disorders were associated with new opioid use for acute and chronic pain after initial adjustment for basic patient characteristics. However, mood disorders were no longer associated with new opioid use after further adjustment for SF-12 PCS and MCS score, degree of pain, the number of chronic pain conditions, and use of non-opioid analgesics. In contrast, there was a strong association between mood disorders and the transition to longer-term opioid therapy for acute and chronic pain conditions after both initial and full adjustment.
Table 4.
Odds ratios for the association of mood disorders with new opioid use and transition to longer term opioid use
| Unadjusted | Model 1* | Model 2† | |||
|---|---|---|---|---|---|
|
| |||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |||
| New Opioid Use | |||||
| Acute pain conditions | 1.15 (1.03, 1.29) | 1.16 (1.03, 1.30) | 1.05 (0.92, 1.20) | ||
| Chronic pain conditions | 1.28 (1.15, 1.42) | 1.21 (1.08, 1.35) | 0.91 (0.80, 1.03) | ||
| Transition to longer-term opioid use | |||||
| Acute pain conditions | 2.54 (1.77, 3.65) | 2.35 (1.63, 3.38) | 1.77 (1.15, 2.72) | ||
| Chronic pain conditions | 2.53 (1.94, 3.30) | 2.65 (1.97, 3.55) | 1.95 (1.42, 2.68) | ||
Model 1 includes age, sex, race, education, access to usual care provider, and BMI
Model 2 includes age, sex, race, education, access to usual care provider, BMI, PCS score, MCS score, degree to which pain limited daily function, number of chronic pain conditions, and use of non-opioid analgesics
Discussion
Our study demonstrates that adults with mood disorders are more likely to start new opioid therapy and to continue opioid therapy longer-term for both acute and chronic pain conditions. The increased rate of opioid initiation appears to be driven by a combination of socio-demographic and clinical disability. However, adults with mood disorders were substantially more likely to continue opioid therapy longer-term independent of these factors.
The association between mood disorders and pain has been long established[1] and is complicated; pain can cause depression and depression can cause pain. However, among patients with pain conditions, those with mood or anxiety disorders continue to have higher prevalent opioid use. Therefore, prescription opioid use among patients with mood disorders must be driven by the rate of new opioid use and/or the duration of opioid therapy. Recent studies have shown that patients with mood disorders take opioid for longer periods of time[8,14]. One study also found higher rates of incident long-term opioid use among patients with depression[5]. Our unadjusted findings confirm these prior studies using a nationally representative data set.
These prior studies, however, were not able to account for important variables, such as socio-demographics and clinical disability, which could explain the higher incident and longer-term opioid use. We were able to demonstrate that clinical disability may account for the higher rate of opioid initiation. However, the higher predilection for patients with mood disorders to transition to longer-term opioid use appears to be independent of both socio-demographics and baseline clinical disability.
It is important that we fully understand the causes of high opioid use among adults with mood disorders, because they are at higher risk for opioid abuse, misuse, and other adverse events[14],[4],[2]. In fact, up to 25–30% of long term opioid users who have anxiety or major depressive disorder qualify for DSM-V moderate to severe opioid use disorder[3]. Additionally, the benefits of long-term opioid therapy in this population remains unclear; most trials of opioid efficacy excluded subjects with psychiatric disorders[15]. The phenomenon that results when a population at high risk for opioid adverse events preferentially receives opioids, despite known risks and unclear benefits, has been dubbed “adverse selection”[15].
Our study may give some insight into this adverse selection. Von Korff[11] has introduced the idea of ‘de facto long term opioid therapy’, defined as the real-world opioid prescription pattern that results in extended use of opioids for chronic pain without explicitly pre-planning such a course. Such practice evolves through patient self-selection in the absence of a clear treatment plan. We do not know if longer-term opioid use in our study was pre-planned, but the idea that patients with mood disorders would preferentially self-select into de facto long term opioid therapy would explain our findings that they are more likely to transition to longer-term therapy independent of clinical disability.
Our study reiterates the importance of setting clear goals when initiating opioids, especially for patients with known mood disorders. Future work evaluating initial treatment plans, attitudes toward pain, and expectations of opioid therapy among patients with mood disorders will help further clarify the relationship between mood disorders and the transition to longer-term opioid use. Additionally, we must provide effective alternative pain treatment to patients with chronic pain and mood disorders. Better access to mental health care, behavioral interventions (such as cognitive behavioral therapy) and alternative pain treatments such as yoga or acupuncture are attractive options to limit opioid burden in this high-risk population.
Our study is not without limitations. Due to significant baseline differences, it is inherently difficult to compare adults with mood disorders to those without mood disorders. As a result, there may be residual confounding in our definition of clinical disability. Additionally, MEPS-HC was not designed to capture pain syndromes; therefore, there is likely some misclassification of likely acute or potentially chronic pain conditions. Also, we only accounted for baseline disability, as we could not reliably evaluate changes in functional status after opioid initiation. Future studies may evaluate response to opioid therapy as a mediator in the transition to longer-term opioid use. Finally, because we could not calculate morphine-milligram equivalents, total number of days supplied per opioid episode, or the exact number of days for each opioid use episode, we defined longer-term opioid therapy as ≥3 prescriptions in consecutive survey periods, rather than more commonly used definitions of chronic opioid therapy in other studies[11].
Despite these limitations, we found that U.S. adults with mood disorders disproportionately use opioids for a variety of pain conditions. After accounting for socio-demographics and clinical disability, this appears to reflect a tendency for adults with mood disorders to transition to longer-term opioid use rather than a higher probability of initiating opioids. This transition is an attractive target for clinicians to reduce potentially inappropriate opioid prescriptions in this high-risk population, and future work should clarify its underlying etiology.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the assistance of Ms. Sarah Chiodi in statistical programming. Dr. Halbert received funding support from an Institutional National Research Service Award from T32HP12706, the Ryoichi Sasakawa Fellowship Fund, and by the Division of General Medicine and Primary Care at Beth Israel Deaconess Medical Center. Dr. Wee is supported by NIH midcareer mentorship award (K24DK087932). This work was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR0011002) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Conflicts of interest:
The authors have no financial conflicts of interest to disclose.
Contributor Information
Brian Halbert, Email: bhalbert@bidmc.harvard.edu.
Roger Davis, Email: rdavis@bidmc.harvard.edu.
Christina C. Wee, Email: cwee@bidmc.harvard.edu.
References
- 1.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–45. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 2.Becker WC, Sullivan LE, Tetrault JM, Desai Ra, Fiellin Da. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Boscarino JA, Hoffman SN, Han JJ. Opioid-use disorder among patients on long-term opioid therapy: impact of final DSM-5 diagnostic criteria on prevalence and correlates. Subst Abuse Rehabil. 2015;6:83–91. doi: 10.2147/SAR.S85667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braden JB, Russo J, Fan M, Edlund MJ, Martin BC, DeVries A, Sullivan MD. Emergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170:1425–32. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braden JB, Sullivan MD, Ray GT, Saunders K, Merrill J, Silverberg MJ, Rutter CM, Weisner C, Banta-Green C, Campbell C, Von Korff M. Trends in long-term opioid therapy for noncancer pain among persons with a history of depression. Gen Hosp Psychiatry. 2009;31:564–70. doi: 10.1016/j.genhosppsych.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerner Multum. [Accessed 4 Jun 205AD];Lexicon Plus. 2014 Available: http://www.multum.com/lexicon.html.
- 7.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49:1429–33. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 8.Edlund MJ, Martin BC, Devries A, Fan M-Y, Braden JB, Sullivan MD. Trends in use of opioids for chronic noncancer pain among individuals with mental health and substance use disorders: the TROUP study. Clin J Pain. 2010;26:1–8. doi: 10.1097/AJP.0b013e3181b99f35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS) 2014. [Google Scholar]
- 10.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Von Korff M, Saunders K, Thomas Ray G, Boudreau D, Campbell C, Merrill J, Sullivan MD, Rutter CM, Silverberg MJ, Banta-Green C, Weisner C. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24:521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nuckols TK, Anderson L, Popescu I, Diamant AL, Doyle B, Di Capua P, Chou R. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160:38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in Drug and Opioid Overdose Deaths - United States, 2000–2014. Morb Mortal Wkly Rep. 2016;64:1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 14.Seal KH, Shi Y, Cohen G, Cohen BE, Maguen S, Krebs EE, Neylan TC. Association of mental health disorders with prescription opioids and high-risk opioid use in US veterans of Iraq and Afghanistan. JAMA. 2012;307:940–7. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain. 2013;154(Suppl):S94–100. doi: 10.1016/j.pain.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.