Abstract
Purpose
Symptoms of urinary irritation, urgency, frequency, and obstruction, known as lower urinary tract symptoms (LUTS), are common in urological practice. However, little is known about the etiology or pathogenesis of LUTS, especially the relative contributions of genetic and environmental factors to development of these symptoms. We used a classical twin study design to examine the relative contribution of genetic and environmental factors to the occurrence of LUTS in middle-aged men.
Materials and Methods
Twins were members of the Vietnam Era Twin (VET) Registry. We used a mail survey to collect lower urinary tract symptoms (LUTS) using the International Prostate Symptom Score (I-PSS) instrument. Twin correlations and biometric modeling was used to determine the relative genetic and environmental contributions to variance in I-PSS total score and individual items.
Results
Participants were 1,002 monozygotic and 580 dizygotic middle-aged male twin pairs (mean age = 50.2 years; S.D. = 3.0 years). Nearly 25% of the sample had an I-PSS score > 8, indicating at least moderate LUTS. The heritability of the total I-PSS was 37% (95% CI = 32-42%). Heritability estimates ranged from 21% for nocturia to 40% for straining, with moderate heritability (34–36%) for urinary frequency and urgency.
Conclusions
Genetic factors provide a moderate contribution (20–40%) to LUTS in middle-aged men, suggesting that environmental factors may also contribute substantially to LUTS. Future research is needed to define specific genetic and environmental mechanisms that underlie the development of these symptoms and conditions associated with LUTS.
Keywords: Lower Urinary Tract Symptoms, heritable quantitative traits, Twins
Conditions such as urinary incontinence, overactive bladder, benign prostatic hyperplasia, interstitial cystitis/bladder pain syndrome, and chronic prostatitis/chronic pelvic pain syndrome are prevalent, comorbid, disabling, and without clear etiology.1 Non-specific symptoms of irritation, urgency, frequency, and obstruction known as lower urinary tract symptoms (LUTS) are common features of many of these conditions. LUTS are associated with increased rates of depression, sleep problems, sexual dysfunction, lower work productivity, and decreased quality of life.2, 3 Prevalence of severe LUTS increases with age,4, 5 such that 30–40% of men report symptoms by 80 years of age.2, 6 Because of the aging US population, it is projected that over 21 million men will have LUTS by 2025.7 Given their high prevalence and impact on daily functioning, LUTS present a significant public health and economic burden.8
Despite their prevalence and significance, little is known about the etiology of LUTS.2, 6 Classical twin studies represent a powerful approach to identify potential mechanisms underlying clinical syndromes and symptoms. Twin studies have provided insights into the epidemiology, etiology, pathophysiology, and natural history of conditions as diverse as multiple sclerosis, cardiovascular disease, schizophrenia, and type II diabetes.9 Because monozygotic (MZ) twins share all of their genes while dizygotic (DZ) twins share, on average, half of their genes, twin correlations for LUTS in MZ and DZ twins provide insight into the relative importance of genetic (i.e., heritability) and non-genetic environmental factors.
Few twin studies have examined heritability of urinary symptoms, likely because these types of studies require access to large registries of twin pairs. One study using data from female twins in the Swedish Twin Registry found a moderate genetic influence for susceptibility to urinary incontinence and nocturia (i.e., 34% heritability), but showed that shared environmental factors seem more important for predisposition to overactive bladder.10 Another female twin study from the Swedish Twin Registry estimated heritability of stress urinary incontinence and pelvic organ prolapse as 41% and 43%, respectively.11 A female twin study from the Danish Twin Registry estimated the heritability of urge incontinence at 42% among middle-aged women and 49% among women 70 years and older.12 Two studies on benign prostatic disease in male twins reported moderate heritability of symptoms but were limited by small sample size and neither used biometric modeling.13, 14 One previous study examined concordance rates of total LUTS scores in male twins but did not evaluate individual symptom heritability through direct biometric modeling.15
The current study used biometric modeling techniques with data from the large Vietnam Era Twin (VET) Registry to evaluate heritability of recent LUTS in middle-aged men. We used this large and genetically informative twin sample to test the hypothesis that additive genetic factors (i.e., heritability) provide an important contribution to the occurrence of LUTS in men.
Materials & Methods
Participants
The VET Registry was established by the U.S. Department of Veterans Affairs in the 1980’s to evaluate the long-term consequences of service in Vietnam. The Registry consists of nearly 7,500 male-male twin pairs born between the years 1939 and 1957 who both served in the military during 1965–1975.16 The VET Registry is one of the largest US twin registries with participants in all 50 states. Initial contact with the twins occurred in 1987 when basic demographic information, health assessment, and zygosity evaluation occurred.
In 1999, a male health survey was mailed to 10,762 active VET Registry members (4,636 pairs). With two mailed follow-ups, a response rate of 49.8% (5,361 individuals; 1,621 pairs) was achieved. Consistent with other twin studies, the response rate was marginally higher among MZ (51.2%) than DZ twins (49.1%). Pairs with complete LUTS information from both twins were included in the present study, leading to an analytic sample of 1,582 pairs (1,002 MZ and 580 DZ). Survey procedures were approved by the Hines VA Cooperative Studies Program Coordinating Center Human Subjects Committee and the use of data for this study was approved by Institutional Review Boards at VA Puget Sound Healthcare System (where the VET Registry is now situated), the University of California, San Diego, and the VA San Diego Healthcare System.
Zygosity assessment
Zygosity was assigned based on childhood similarity questions using a multi-step process. This assignment was validated against DNA assessment in a subset of twin pairs.17
Measures
Self-reported socio-demographic factors assessed included age, race, ethnicity, and marital status. Participants completed the International Prostate Symptom Score (I-PSS), a validated self-report measure of LUTS that assesses seven common symptoms associated with urinary dysfunction during the past month.18 Incomplete emptying, frequency, intermittency, urgency, weak stream, and straining are assessed on a 0- to-5 scale, ranging from, “not at all,” to, “almost always.” Nocturia is assessed by the number of times a person had to get up in a typical night, with a scale ranging from 0 to 5. These seven I-PSS items are identical to questions appearing on the American Urological Association Symptom Index and were used in the current analyses. Total scores range from 0-to-35 (i.e., asymptomatic to very symptomatic) and are classified as mild (< 8), moderate (8–19), or severe (20–35).
Analyses
We first calculated means and standard deviations (SD) or percentages for study variables for the entire sample and for individuals above and below an I-PSS total score of 8 (e.g., mild versus moderate-or-severe LUTS), to consider if any of the socio-demographic factors should be used as covariates in further analyses. The interrelationships of the individual I-PSS items were examined using polychoric correlations. Biometrical genetic twin data analysis first estimated the within pair correlations for each I-PSS items and the total I-PSS in MZ and DZ pairs. Greater similarity in MZ than DZ twin correlations suggests genetic influence. Using structural equation modeling we then estimated additive genetic (A), shared environmental (C), and non-shared environmental (E) variance components for the I-PSS items and total score (Figure 1). ACE estimates and 95% confidence intervals (CI) were obtained by fitting structural equation models to the raw data. Overall model fit was evaluated using the chi-square (χ2) goodness-of-fit statistic with non-significant χ2 values suggesting adequate fit. To obtain a parsimonious solution, nested models (AE and CE) were fit to the data then compared to the full model (ACE). Non-significant χ2 difference suggests that the reduced model could be accepted without a substantial loss of fit to the data. Akaike’s Information Criterion (AIC; χ2 − 2df19), a measure that balances goodness-of-fit relative to model parsimony, was used to further evaluate model fit (model fit information is available as an on-line supplemental table). Models were fit to the raw data using full information maximum-likelihood in OpenMx, a structural equation modeling program.20
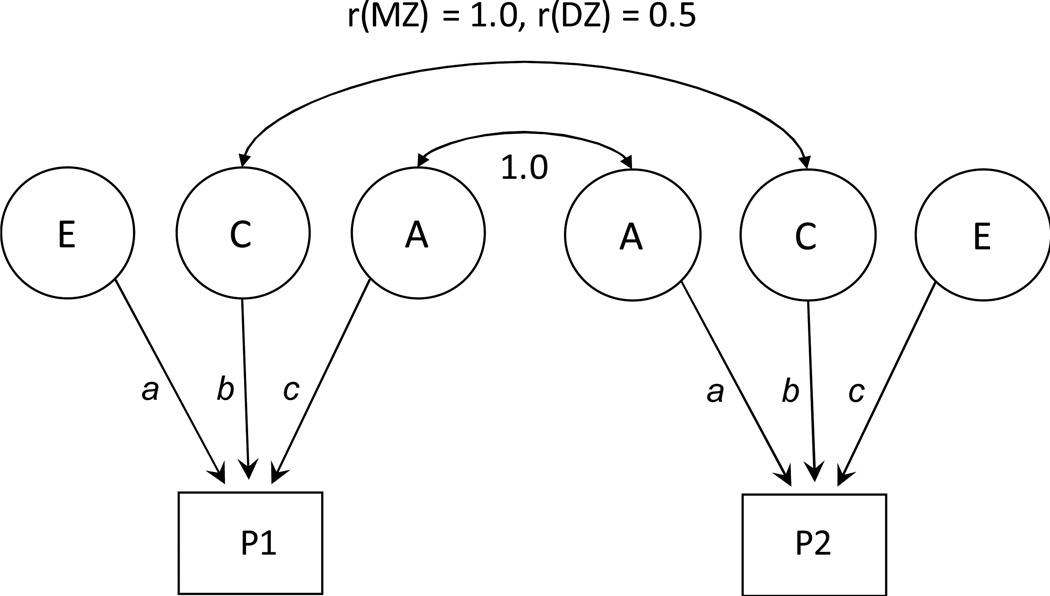
Figure 1.
Path diagram of the classic twin design. The twin model decomposes phenotypic variance into genetic and environmental components in monozygotic (MZ) and dizygotic (DZ) twins. P1 represents the phenotype for the first twin and P2 the phenotype for the second twin. Circles represent latent factors hypothesized to account for individual differences: A= additive genetic influences, C= common, or shared, environmental influences, E = non-shared, or unique, environmental influences. Curved, double-headed arrows represent the correlation between twins.
Results
Table 1 presents socio-demographic findings for all twins and by I-PSS status (< 8 or ≥8). The 3,164 men were middle-aged (mean = 50.2 ± 3.0 years of age, range = 42 – 60). The majority (71.5%) had some college education, were white (94.3%), and married or widowed (80.2%). Overall, 777 participants (24.6%) scored ≥ 8 on the I-PSS. Twins with I-PSS scores above and below 8 were similar in age, education, race, ethnicity, or marital status.
Table 1.
Socio-demographics for all twins and by I-PSS status
| All Twins (N=3,164) |
Twins w/ IPSS < 8 (n=2,387) |
Twins w/ IPSS ≥ 8 (n=777) |
|
|---|---|---|---|
| Age, M ± SD | 50.2 ± 3.0 | 50.2 ± 3.0 | 50.3 ± 3.0 |
| Education, % | |||
| < High School | 1.8 | 1.8 | 2.0 |
| HS Grad | 26.7 | 26.9 | 26.0 |
| Some College | 42.3 | 42.0 | 43.3 |
| College + | 29.2 | 29.3 | 28.8 |
| Race, % | |||
| White | 94.3 | 94.1 | 94.6 |
| Non-white | 5.7 | 5.9 | 5.4 |
| Ethnicity, % | |||
| Hispanic | 2.7 | 2.8 | 2.6 |
| Non-Hispanic | 97.3 | 97.2 | 97.4 |
| Marital Status, % | |||
| Married/Widowed | 80.2 | 80.5 | 79.2 |
| Not Married | 19.8 | 19.5 | 20.8 |
I-PSS = International Prostate Symptom Score.
Table 2 shows the proportion of twins reporting each I-PSS symptom at least half of the time within the past month. Urinary frequency was the most common symptom (18%) and straining was the least common (3.5%). Nocturia was reported by 15.2% as occurring three or more times per night within the preceding month.
Table 2.
Proportion of twins with I-PSS symptoms present at least half the time during the past month
| I-PSS Symptom | N | % |
|---|---|---|
| Incomplete emptying | 215 | 6.8 |
| Frequency | 570 | 18.0 |
| Intermittency | 263 | 8.3 |
| Urgency | 213 | 6.7 |
| Weak stream | 257 | 8.1 |
| Straining | 110 | 3.5 |
| Nocturia | 479 | 15.2 |
| I-PSS score ≥ 8 | 777 | 24.6 |
I-PSS = International Prostate Symptom Score.
Table 3 provides the polychoric correlations among the I-PSS symptoms. There were strong correlations between the logically related symptoms of weak stream, straining, and intermittency (r = 0.75 to 0.70, ps <.001). There were weaker but significant correlations between nocturia and other I-PSS symptoms (r = 0.29 to 0.39, ps < .001).
Table 3.
Phenotypic correlations of I-PSS symptoms
| I-PSS Symptom* | Incomplete Emptying |
Frequency | Intermittency | Urgency | Weak Stream |
Straining |
|---|---|---|---|---|---|---|
| Frequency | 0.63 | |||||
| Intermittency | 0.69 | 0.59 | ||||
| Urgency | 0.56 | 0.62 | 0.55 | |||
| Weak stream | 0.67 | 0.56 | 0.72 | 0.56 | ||
| Straining | 0.65 | 0.52 | 0.70 | 0.52 | 0.75 | |
| Nocturia | 0.34 | 0.39 | 0.33 | 0.31 | 0.29 | 0.31 |
I-PSS = International Prostate Symptom Score. All correlations are polychoric.
All p’s < .001
Table 4 presents the results of MZ and DZ twin correlations and the best-fitting most parsimonious biometrical genetic models. Within-pair MZ twin correlations for each of the individual symptoms and the I-PSS total score were twice as large as those for DZ twins, suggesting genetic influence. Biometrical genetic models showed the strongest relative additive genetic contribution (i.e., heritability) in the symptoms of straining (40%) and intermittency (39%). Moderate heritability was found for symptoms of urgency (36%), weak stream (36%), frequency (34%), and incomplete emptying (33%). Nocturia (21%) had the lowest heritability estimate. The remaining variance for all symptoms could be attributed to non-shared environmental influences and measurement error (E). Analyses for the total I-PSS score showed a similar pattern with moderate heritability (37%). None of the best-fitting models included shared environmental influences (C).
Table 4.
Twin correlations for I-PSS symptoms and total score, and estimates of genetic and environmental variance components
| Correlations | Standardized Variance Components (95% CI) | ||||
|---|---|---|---|---|---|
| I-PSS Symptom | MZ | DZ | A | C | E |
| Incomplete emptying | .33 | .16 | .33 (.25, .41) | - | .67 (.59, .75) |
| Frequency | .34 | .17 | .34 (.28, .40) | - | .66 (.61, .72) |
| Intermittency | .39 | .21 | .39 (.32, .46) | - | .61 (.54, .69) |
| Urgency | .36 | .18 | .36 (.29, .44) | - | .64 (.56, .71) |
| Weak stream | .36 | .18 | .36 (.28, .44) | - | .64 (.56, .72) |
| Straining | .39 | .25 | .40 (.30, .49) | - | .60 (.51, .70) |
| Nocturia | .21 | .10 | .21 (.14, .27) | - | .79 (.73, .86) |
| I-PSS Total | .37 | .16 | .37 (.32, .42) | - | .63 (.58, .68) |
Results presented for best-fitting model for each variable. CI = Confidence Interval; MZ= monozygotic; DZ= Dizygotic; A = additive genetic influences; C = shared environmental influences; E = non-shared environmental influences; I-PSS = International Prostate Symptom Score; Correlations are polychoric for symptoms, and Pearson for I-PSS total.
Discussion
This is the first twin study using biometric genetic analyses to examine heritability of LUTS in men. Classical twin studies have proven valuable in estimating the importance of genetic and environmental influences on complex traits and diseases, comorbidity among conditions, and the interaction between genes and environment across the life course. Only classical twin studies with biometric model fitting approaches can provide simultaneous estimates of the relative contribution of genes and environment to a phenotype.
Prevalence rates and correlations among LUTS symptoms in our twins closely resemble reports from other community-based samples.4, 6, 21 We found that 24.6% of the middle-aged men in our sample reported recent moderate or severe LUTS based on the I-PSS cutoff of ≥8, which is nearly identical to rates found in men of the same age group in the Boston Area Community Health (BACH) survey.4 These findings suggest that our community-based twin sample was similar to the population-based sample of the BACH survey and support the validity of our findings.
More importantly, we found a moderate and significant contribution of genetic factors to phenotypic variance of recent LUTS in men, supporting our hypothesis. Estimates for these symptoms were considered moderate compared to the heritability of conditions with known pathophysiology such as type I diabetes (88%),22 but similar to other complex symptom-based conditions that lack unequivocal physical examination or laboratory findings like chronic pelvic pain (41%)23 and benign prostatic disease (39 – 49%).13, 14 The strongest genetic effects were shown in straining, intermittency, frequency, urgency, and weak stream, with the lowest genetic effect in nocturia. This variation in heritability combined with the range of correlations between symptoms suggests that LUTS are related yet distinct symptoms, perhaps with distinct etiologies. That is, genetic and environmental factors may directly influence individual symptom differently. Given the increasing prevalence of LUTS with age, heritability also may change over the life course with symptom variability becoming more genetically-influenced with age. Further research should focus on identifying shared and distinct risk factors as well as the moderating effects of age for each symptom.
Our findings are largely consistent with the handful of studies on the heritability of urinary problems in men and women,10–13, 24 but differ from one previous study that estimated a genetic contribution of 72% based on concordance rates of total I-PSS scores in male twins.15 Aside from methodological differences, one possibility that could account for our differing estimates could be the age of the twin sample. The previous study estimated heritability of LUTS in men 70 years or older, and may have been able to capture the potentially moderating effects of age. Future longitudinal studies are necessary to be able to evaluate this potential genetic amplification.
We found the lowest heritability for nocturia, whereas the only twin study that examined nocturia in women found that symptom to have the greatest heritability (48%) amongst symptoms they examined.10 It is likely that men and women with urological symptoms differ on many characteristics. Previous studies were limited to one sex or to have used different assessment instruments, making direct comparisons difficult. Thus, the discrepancy between our findings and the previous study might reflect actual differences between sexes, in population selection, or assessment methods. The Multidisciplinary Approach to the study of Pelvic Pain (MAPP) Research Network25 is one of very few large studies including large numbers of men and women assessed using similar instruments and clinical approaches. Findings from MAPP Network and other similar studies can shed light on potential sex differences.
The etiology of LUTS is likely multifactorial and complex. Although this study does not identify specific genetic and environmental factors that contribute to LUTS, it does provide evidence for moderate genetic and large non-shared environmental contributions to LUTS in men. There is some evidence that environmental factors such as diet are associated with urinary health. For example, diets low in fat and red meat and high in vitamin C, protein and vegetables have been linked to lower benign prostatic hyperplasia and LUTS symptoms.26, 27 Obesity and high-energy intake have been associated with increased urinary symptoms, with insulin and sex hormone metabolism potentially playing roles in these relationships.28 Given the moderate heritability and substantial environmental influences, future research should focus on identifying specific environmental risk factors that contribute to the onset, severity, and course of LUTS. Additional studies can examine gene × environment, epigenetic, or other biological mechanisms underlying the symptom development and severity.
Our findings also are relevant to potential mechanisms underlying urological conditions with unknown etiology. For example, urinary frequency and urgency are key symptoms of interstitial cystitis/bladder pain syndrome (IC/BPS) in both men and women. There are no twin or family studies of IC/BPS in men, but 2 recent studies in women found moderate genetic contribution to IC/BPS.29, 30 Future research should examine both genetic and environmental contributions to IC/BPS and other urological conditions with LUTS in men and women, to develop prevention and treatment strategies for these disabling conditions.
This study has important limitations. First, our sample was predominantly white, so findings may not be relevant to other race and ethnicity groups. Second, the LUTS assessment was based on a one-time self-report measure and provides only a snapshot in time. Given the transient nature of LUTS and potential use of treatment at the time of our assessment, it is possible that the rates of LUTS in our sample are conservative. Alternately, these rates were consistent with other studies of men in their 50’s.4 Examining the contribution of genes and environment to LUTS over time would be important given potential changes in symptom severity and potential increase with age. Third, we did not have access to data to examine genetic and environmental overlap of LUTS with diabetes, prostate cancer, and other conditions that are known to co-occur with LUTS. Finally, we were unable to directly evaluate sex differences in the genetic and environmental contribution to LUTS. Future research with large samples of both male and female twins would be important to determine potential differences in the genetic and environmental mechanisms of LUTS.
Conclusions
We identified a moderate contribution of genetic factors to the phenotypic variance of recent LUTS. Environmental factors remain critical to the conceptualization and treatment of LUTS. Understanding the heritability of these symptoms is important to elucidate the underlying factors contributing to urinary conditions with unclear or overlapping etiology. Future research should examine specific biological mechanisms and environmental factors that contribute to the development of LUTS.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health MAPP Research Network grant U01 DK082325. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry: without their contribution this research would not have been possible. The views expressed in this paper are those of the authors and do not reflect the official policy or position of the National Institute of Diabetes and Digestive and Kidney Diseases, Department of Veterans Affairs, the United States Government, or any of the institutions with which the authors are affiliated.
Abbreviations in order of appearance
- LUTS
Lower urinary tract symptoms
- I-PSS
International Prostate Symptom Score
- MZ
Monozygotic
- DZ
Dizygotic (DZ)
- VET
Vietnam Era Twin Registry
- A
Additive genetic
- C
Shared environmental
- E
Non-shared environmental (E)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have any conflicts of interest to declare.
References
- 1.McNaughton Collins M, Pontari MA, O'Leary MP, et al. Quality of Life Is Impaired in Men with Chronic Prostatitis the Chronic Prostatitis Collaborative Research Network*. Journal of General Internal Medicine. 2001;16:656. doi: 10.1111/j.1525-1497.2001.01223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stranne J, Damber J-E, Fall M, et al. One-third of the Swedish male population over 50 years of age suffers from lower urinary tract symptoms. Scandinavian Journal of Urology and Nephrology. 2009;43:199. doi: 10.1080/00365590902833747. [DOI] [PubMed] [Google Scholar]
- 3.Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well - being in men and women: results from the EPIC study. BJU Int. 2008;101:1388. doi: 10.1111/j.1464-410X.2008.07601.x. [DOI] [PubMed] [Google Scholar]
- 4.Kupelian V, Wei JT, O’Leary MP, et al. Prevalence of lower urinary tract symptoms and effect on quality of life in a racially and ethnically diverse random sample: the Boston Area Community Health (BACH) Survey. Archives of Internal Medicine. 2006;166:2381. doi: 10.1001/archinte.166.21.2381. [DOI] [PubMed] [Google Scholar]
- 5.Malmsten UG, Molander U, Peeker R, et al. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: a longitudinal population-based survey in men aged 45–103 years. European Urology. 2010;58:149. doi: 10.1016/j.eururo.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409. doi: 10.1046/j.1464-410x.2003.04369.x. [DOI] [PubMed] [Google Scholar]
- 7.Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int. 2007;100:820. doi: 10.1111/j.1464-410X.2007.07018.x. [DOI] [PubMed] [Google Scholar]
- 8.Hu T-W, Wagner TH, Bentkover JD, et al. Estimated economic costs of overactive bladder in the United States. Urology. 2003;61:1123. doi: 10.1016/s0090-4295(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 9.Van Dongen J, Slagboom PE, Draisma HH, et al. The continuing value of twin studies in the omics era. Nature Reviews Genetics. 2012;13:640. doi: 10.1038/nrg3243. [DOI] [PubMed] [Google Scholar]
- 10.Wennberg A-L, Altman D, Lundholm C, et al. Genetic influences are important for most but not all lower urinary tract symptoms: a population-based survey in a cohort of adult Swedish twins. European Urology. 2011;59:1032. doi: 10.1016/j.eururo.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altman D, Forsman M, Falconer C, et al. Genetic influence on stress urinary incontinence and pelvic organ prolapse. European Urology. 2008;54:918. doi: 10.1016/j.eururo.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Rohr G, Kragstrup J, Gaist D, et al. Genetic and environmental influences on urinary incontinence: a Danish population - based twin study of middle - aged and elderly women. Acta Obstetricia et Gynecologica Scandinavica. 2004;83:978. doi: 10.1111/j.0001-6349.2004.00635.x. [DOI] [PubMed] [Google Scholar]
- 13.Meikle AW, Bansal A, Murray DK, et al. Heritability of the symptoms of benign prostatic hyperplasia and the roles of age and zonal prostate volumes in twins. Urology. 1999;53:701. doi: 10.1016/s0090-4295(98)00569-x. [DOI] [PubMed] [Google Scholar]
- 14.Martin AW, Page WE, Lee BR, et al. Concordance rates for benign prostatic disease among twins suggest hereditary influence. Urology. 1994;44:646. doi: 10.1016/s0090-4295(94)80197-5. [DOI] [PubMed] [Google Scholar]
- 15.Rohrmann S, Fallin MD, Page WF, et al. Concordance rates and modifiable risk factors for lower urinary tract symptoms in twins. Epidemiology. 2006;17:419. doi: 10.1097/01.ede.0000219723.14476.28. [DOI] [PubMed] [Google Scholar]
- 16.Eisen S, Neuman R, Goldberg J, et al. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clinical Genetics. 1989;35:423. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 17.Forsberg CW, Goldberg J, Sporleder J, et al. Determining zygosity in the Vietnam era twin registry: an update. Twin Research and Human Genetics. 2010;13:461. doi: 10.1375/twin.13.5.461. [DOI] [PubMed] [Google Scholar]
- 18.Barry MJ, Fowler FJ, Jr, O'Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. The Journal of Urology. 1992;148:1549. doi: 10.1016/s0022-5347(17)36966-5. [DOI] [PubMed] [Google Scholar]
- 19.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317. [Google Scholar]
- 20.Boker S, Neale M, Maes H, et al. OpenMx: an open source extended structural equation modeling framework. Psychometrika. 2011;76:306. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glina S, Santana AW, Azank F, et al. Lower urinary tract symptoms and erectile dysfunction are highly prevalent in ageing men. BJU Int. 2006;97:763. doi: 10.1111/j.1464-410X.2005.06008.x. [DOI] [PubMed] [Google Scholar]
- 22.Hyttinen V, Kaprio J, Kinnunen L, et al. Genetic Liability of Type 1 Diabetes and the Onset Age Among 22,650 Young Finnish Twin Pairs A Nationwide Follow-Up Study. Diabetes. 2003;52:1052. doi: 10.2337/diabetes.52.4.1052. [DOI] [PubMed] [Google Scholar]
- 23.Zondervan KT, Cardon LR, Kennedy SH, et al. Multivariate genetic analysis of chronic pelvic pain and associated phenotypes. Behavior Genetics. 2005;35:177. doi: 10.1007/s10519-004-1017-6. [DOI] [PubMed] [Google Scholar]
- 24.Dietz H, Hansell N, Grace M, et al. Bladder neck mobility is a heritable trait. BJOG: An International Journal of Obstetrics & Gynaecology. 2005;112:334. doi: 10.1111/j.1471-0528.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Landis JR, Williams DA, Lucia MS, et al. The MAPP research network: design, patient characterization and operations. BMC Urology. 2014;14:1. doi: 10.1186/1471-2490-14-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dallosso HM, Matthews RJ, McGrother CW, et al. The association of diet and other lifestyle factors with the onset of overactive bladder: a longitudinal study in men. Public Health Nutrition. 2004;7:885. doi: 10.1079/phn2004627. [DOI] [PubMed] [Google Scholar]
- 27.Rohrmann S, Giovannucci E, Willett WC, et al. Fruit and vegetable consumption, intake of micronutrients, and benign prostatic hyperplasia in US men. The American Journal of Clinical Nutrition. 2007;85:523. doi: 10.1093/ajcn/85.2.523. [DOI] [PubMed] [Google Scholar]
- 28.Mondul AM, Giovannucci E, Platz EA. A prospective study of obesity, and the incidence and progression of lower urinary tract symptoms. The Journal of Urology. 2014;191:715. doi: 10.1016/j.juro.2013.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman D, Lundholm C, Milsom I, et al. The genetic and environmental contribution to the occurrence of bladder pain syndrome: an empirical approach in a nationwide population sample. European Urology. 2011;59:280. doi: 10.1016/j.eururo.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tunitsky E, Barber M, Jeppson P, et al. Bladder pain syndrome/interstitial cystitis in twin sisters. The Journal of Urology. 2012;187:148. doi: 10.1016/j.juro.2011.09.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.