Abstract
Purpose
The aim of this study was to quantify the effect of propranolol on hemodynamic parameters assessed using the PiCCO system in burned children.
Methods
We analyzed hemodynamic data from patients who were randomized to receive either propranolol (4 mg/kg/day) or placebo (control), which was initiated as a prospective randomized controlled trial. Endpoints were cardiac index (CI), percent predicted heart rate (%HR), mean arterial pressure (MAP), percent predicted stroke volume (%SV), rate pressure product (RPP), cardiac work (CW), systemic vascular resistance index (SVRI), extravascular lung water index (EVLWI), arterial blood gases, events of lactic acidosis, and mortality. Mixed multiple linear regressions were applied, and a 95% level of confidence was assumed.
Results
One hundred twenty-one burned children (control: N=62, propranolol: N=59) were analyzed. Groups were comparable in demographics, EVLWI, SVRI, %SV, arterial blood gases, Denver 2 post injury organ failure score, incidence of lactic acidosis, or mortality. Percent predicted HR, MAP, CI, CW, and RPP were significantly reduced in the propranolol-treated group (p<0.01).
Conclusions
Propranolol significantly reduces cardiogenic stress by reducing CI and MAP in children with severe burn injury. Although, peripheral oxygen delivery, events of lactic acidosis, and organ dysfunction was not higher in propranolol treated patients.
Keywords: cardiac output, cardiac index, burns, transpulmonary thermodilution, hypermetabolism
Introduction
Elevated circulatory variables, such as cardiac index (CI), cardiac work (CW), heart rate (HR), and blood pressure, are hallmarks of the ongoing hyperdynamic changes observed following severe burns (1–3) and persist for up to 2 years post burn (2). Williams et al.(4) used non-invasive transthoracic echocardiography to show that propranolol, a non-selective β1-, β2-adrenergic receptor antagonist, can reduce CW and cardiac oxygen consumption in a dose-dependent manner following severe burn injury.
Appropriate hemodynamic monitoring in critically ill patients is crucial to guide fluid resuscitation, reduce morbidity, and decrease mortality (5). Nevertheless, the right choice of the hemodynamic monitoring tool is still controversial in the critical care field (6). In the past, we have used transpulmonary thermodilution, which has been shown to be a reliable tool for determining hemodynamic changes in critically ill patients (7) as well as in severely burned children (3,8,9). Beyond providing general cardiac output measures, transpulmonary thermodilution enables collection of a large number of variables including extravascular lung water index (EVLWI) and systemic vascular resistance index (SVRI), both of which may aid in post-burn fluid resuscitation.
EVLWI does not correlate with radiographically visible pulmonary edema in critically ill children (10). In adult burn patients, EVLWI has been described as a predictor for sepsis (11). Additionally, propranolol is capable of reducing the hyperdynamic state of burned children (4), but the effect on the EVLWI remains unknown.
In pediatric burns, SVRI increases during the acute burn shock and later continuously decreases with time (3). The diameter of peripheral vessels is a key factor influencing SVRI and is mainly controlled via α1-adrenoceptors on vascular smooth muscle cells. In 1983, it was shown that, in adult patients with heart disease, intravenous administration of a bolus of propranolol significantly increases the SVRI, as measured with a pulmonary artery catheter and a thermodilution technique (12). These findings were confirmed in another trial with 11 healthy normotensive male subjects (13). This elevation in SVRI is postulated to be β2-adrenoceptor-mediated (12,14). Hence, propranolol may affect SVRI in severely burned children. However, the effect of propranolol on SVRI, when given during the acute hospitalization of severely burned children, has not been described. Here we tested the hypothesis that, in severely burned children, acute administration of propranolol reduces the hyperdynamic response to burns without adversely affecting SVRI, EVLWI, or peripheral oxygen delivery.
Patients and Methods
Study Design and Patients
We analyzed transpulmonary thermodilution measurements obtained using the PiCCO system (Pulse Index Continuous Cardiac Output, Pulsion Medical Systems, Munich, Germany) from severely burned children who were admitted to our institution between 2005 and 2015. This study has been approved by the Institutional Review Board at the University of Texas Medical Branch (Galveston, TX; protocol #15-0074). Inclusion criteria were defined as follows: age between 6 months and 18 years, thermal burns, received PiCCO measurements during the acute hospitalization, and received either propranolol (4 mg/kg/day), administered as part of a separately conducted prospective randomized controlled trial (protocol #04-157) or no acute study drug. Patients were excluded from the study if they had any prior history of cardiopulmonary illness (e.g., aortic or pulmonic stenosis, ventricular or atrial septal defect, or atrioventricular canal defect).
As part of our standard-of-care protocol, we determined weight at admission and calculated all indexed values derived from percent total body surface area (TBSA) burned and individual body surface area (BSA). Analgesia, sedation, and mechanical ventilation were administered according to our institutional guidelines. In each thermally injured patient, we performed early total burn wound excision of the burn eschar and skin grafting between 48 and 72 hours after admission. At weekly intervals, additional skin grafting procedures with autograft or allograft were performed until all wounds were covered.
In addition to hemodynamic measurements, we analyzed the following patient characteristics: age, sex, percent TBSA burnt, percent TBSA with third-degree (full-thickness) burns, length of hospital stay, days to admission, time to first PiCCO measurement, time between surgeries, number of surgical interventions during acute hospitalization, type of burn, and mortality.
Hemodynamic measurements
At admission, central venous lines (inferior or superior vena cava) was placed in all patients. In addition, if indicated by the attending physician based on the severity of the burn, patients received an arterial line (femoral artery) for continuous arterial blood pressure monitoring. Beyond that, when indicated by the attending physician, a Pulsiocath 3- or 4-French thermistor-tipped catheter (Pulsion Medical Systems, Munich, Germany) was used to collect hemodynamic PiCCO measurements. The PiCCO catheter placement was in some cases limited due to its size, thus only patients aging 2 years and up underwent hemodynamic monitoring with the PiCCO system. CI (ml/min/m2), percent predicted stroke volume (%SV, %), CW (J), EVLWI (ml/kg), and SVRI (dynes-sec/cm-5/m2) were measured as previously described (3). HR (1/min), mean arterial pressure (MAP, mmHg), and systolic blood pressure (SBP, mmHg) were recorded directly via hardware at each point of PiCCO measurement. Rate pressure product (RPP, mmHg/min) was calculated as HR × SBP for each time point. Percent predicted stroke volume and percent predicted heart rate (%HR) were derived from age-predicted values for children (15).
Organ failure score and blood gases
To determine multiple organ dysfunction syndrome, we calculated the daily Denver 2 score according to the criteria defined by the National Institute of General Medical Sciences “Glue Grant” (16). This Denver 2 score is the sum of four component scores of pulmonary, renal, hepatic and cardiac function (with values ranging from 0 to 3 for each organ). A sum score greater than or equal to 3 is defined as multiple organ dysfunction syndrome. Blood samples were collected from either a subclavian central venous catheter or a femoral arterial line where appropriate. Arterial and venous partial oxygen pressure (PaO2 and PvO2, mmHg), arterial pH, and lactate serum levels (mmol/L) were obtained and recorded as daily median values. In addition to using the time between surgeries as a marker for peripheral perfusion and wound healing, we used PaO2-PvO2 gradient as a marker for peripheral oxygen consumption and lactic acidosis as a marker for peripheral perfusion. Lactic acidosis was defined as a combination of a pH<7.35 and a lactate level >4 (mmol/L).
Data analysis
Statistical analyses were performed using R statistical software (R Core Team 2015, version 3.2.1, Vienna, Austria). Demographic data were summarized by treatment group. Differences between groups were assessed by Welch's two-sample t-test or by Chi-square test, where appropriate. For each outcome (CI, CW, EVLWI, MAP, arterial pH, PaO2, PvO2, RPP, SVRI, %HR, %SV, lactate levels, and Denver 2 score, as daily median values), mixed multiple linear regression was used to model the relation between the outcome and treatment group (control versus propranolol) while adjusting for effects due to potentially prognostic covariates such as age at burn, burn size, and days post burn, while blocking on subject. We log-transformed EVLWI, CW, PaO2, and PvO2 to improve approximations of normality prior to analysis, with results inverted for presentation. These models were also compared by likelihood-ratio tests with models that included an interaction between treatment and time; however, inclusion of the interaction term failed to improve the models. The analysis focused on the 28 days following burn injury, and all figures show the regression model estimate over time post burn with 95% shaded confidence intervals. Regression estimates are based upon holding constant the control treatment group and median values of other covariates. A 95% level of confidence was assumed.
Results
There were no significant differences between the groups regarding age, sex, burn size, time between burn and admission, length of hospital stay, mortality, or type of burn (Table 1). Hemodynamic values of both groups were comparable at 24 h post admission (Table 2), and no adverse events were reported in the 59 patients who received propranolol as well as hemodynamic measurements with the PiCCO system.
Table 1.
Patient characteristics.
| Characteristica | Propranolol (N=59) |
Control (N=62) |
p value |
|---|---|---|---|
| Age at burn, years | 10 ± 6 | 10 ± 6 | 0.99 |
| Sex: female:male, N | 23:36 | 21:41 | 0.56 |
| Mortality, N (%) | 4 (7%) | 5 (8%) | 1.00 |
| TBSAb burn, % | 58 ± 16 | 59 ± 18 | 0.57 |
| TBSAb full thickness burn, % | 45 ± 22 | 44 ± 25 | 0.80 |
| Burn to admit, days | 3 ± 7 | 3 ± 4 | 0.77 |
| Length of stay survivors, days | 41 ± 26 | 44 ± 38 | 0.28 |
| Length of stay non-survivors, days | 39 ± 26 | 42 ± 39 | 0.64 |
| Number of acute surgeries | 8 ± 4 | 7 ± 5 | 0.49 |
| Time between acute surgeries, days | 6 ± 1 | 6 ± 2 | 0.67 |
| Year admitted, year | 2011 ± 3 | 2011 ± 3 | 0.78 |
| Type of burn | 0.67 | ||
| Flame | 47 (80%) | 46 (74%) | |
| Scald | 8 (14%) | 9 (15%) | |
| Electrical | 4 (7%) | 7 (11%) |
Data presented as mean ± standard deviation or count.
TBSA: total body surface area.
Table 2.
Hemodynamic assessments at 24 h post admission.
| Hemodynamic measurementa | Propranolol | Control | p value |
|---|---|---|---|
| Cardiac index, ml/min/m2 | 5.5 ± 1.4 | 5.8 ± 1.3 | 0.52 |
| Heart rate, bpm | 142 ± 23 | 149 ± 15 | 0.84 |
| Mean arterial pressure, mmHg | 79 ± 12 | 76 ± 13 | 0.80 |
| Rate pressure product, mmHg/min | 16544 ± 3714 | 16915 ± 3149 | 0.93 |
| Cardiac work, J | 503 ± 331 | 525 ± 250 | 0.22 |
| Stroke volume, ml | 46 ± 21 | 46 ± 24 | 0.55 |
| EVLWIb, ml/kg | 7.8 ± 3.1 | 8.8 ± 3.4 | 0.53 |
| SVRIc, dynes-sec/cm-5/m2 | 1101 ± 295 | 958 ± 315 | 0.79 |
Data presented as mean ± standard deviation.
EVLWI: extravascular lung water index
SVRI: systemic vascular resistance index
Administration of propranolol significantly reduced CI by 0.67 l/min/m (p<0.01, Figure 2a). In both groups, CI significantly increased throughout the first 4 weeks post burn (both p<0.01). In the propranolol treated group, %HR was reduced significantly by an average of 16% (p<0.01, Figure 2b). With time post burn, %HR decreased significantly in both groups (both p<0.01). Propranolol significantly reduced MAP by an average of 4 mmHg (p=0.01, Figure 2c), however MAP did not change in either group with time post burn (p=0.35). Additionally, both RPP and CW were significantly lower in the propranolol-treated group (p<0.03, Figures 2d and 2e) and also decreased with time post burn in both groups (both p<0.01). Percent predicted SV increased with time post burn (p=0.01, Figure 2f), whereas propranolol administration had no impact on percent predicted SV values (p=0.46). A younger age at burn was associated with significantly decreased EVLWI (p<0.01, Figure 3a). With time post burn, EVLWI significantly increased in both groups (both p<0.01). In addition, propranolol had no significant affect on EVLWI (p=0.11). The average SVRI was significantly higher in the propranolol-treated group for up to 2 weeks post burn (p<0.05), but the overall difference during the 4 weeks post burn period was not significant (p=0.07, Figure 3b).
Figure 2.
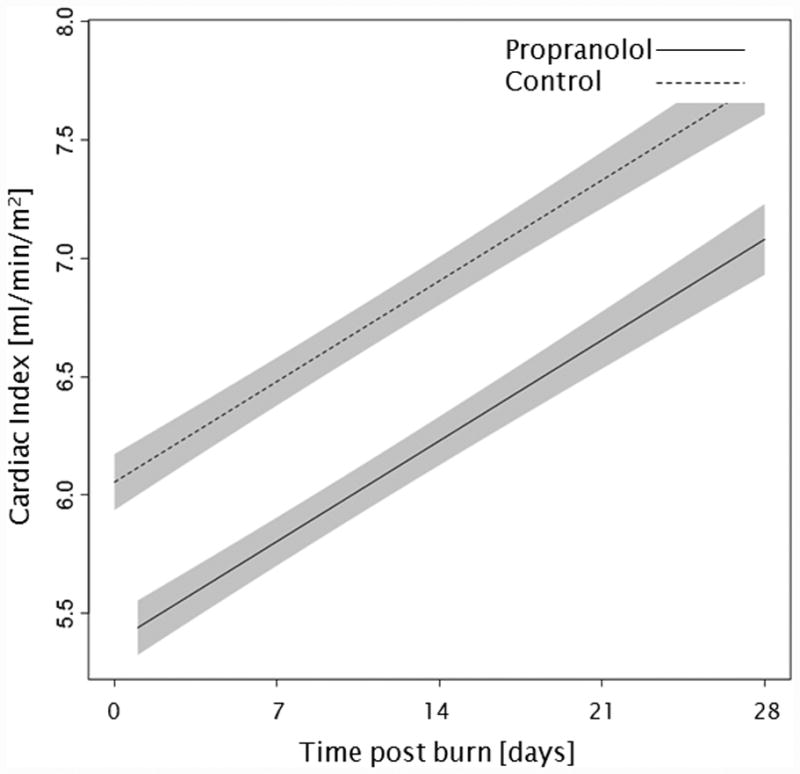
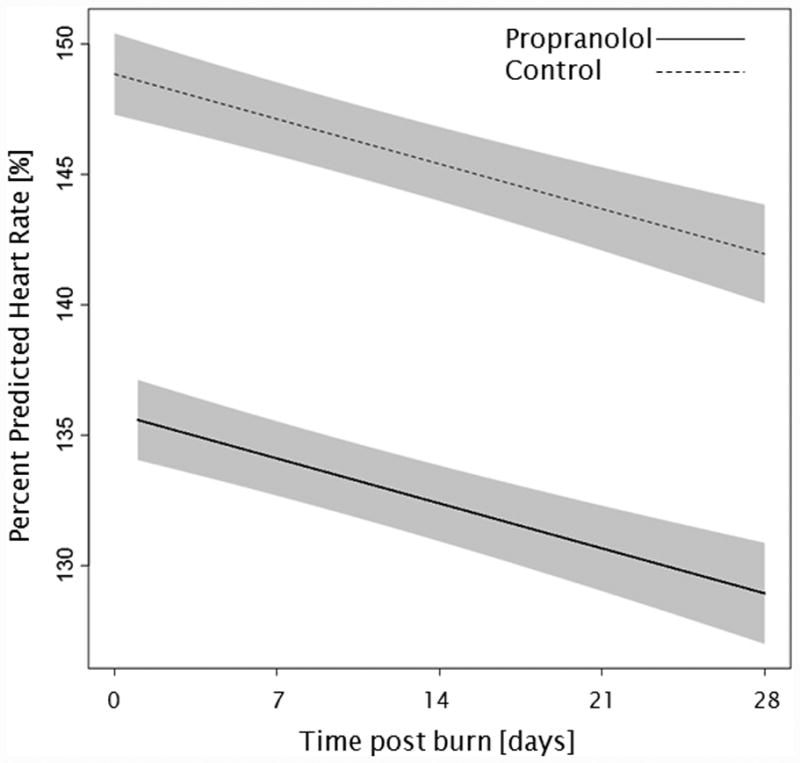
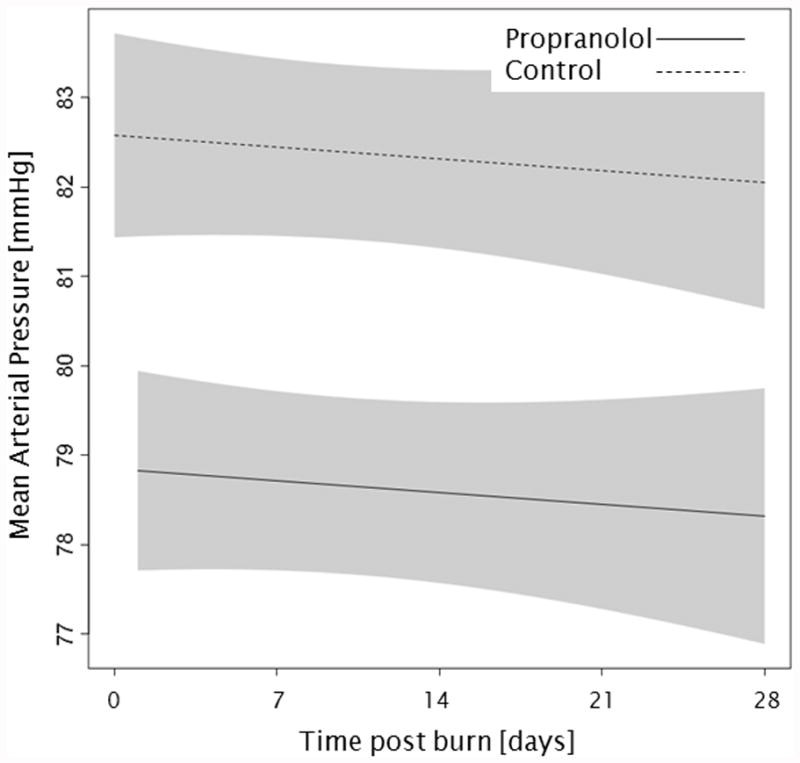
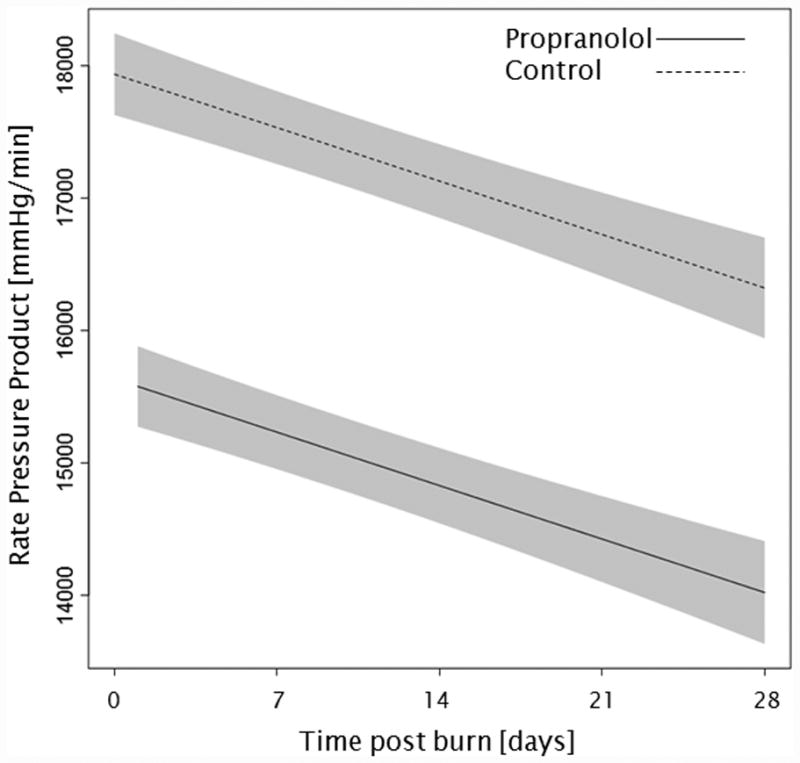
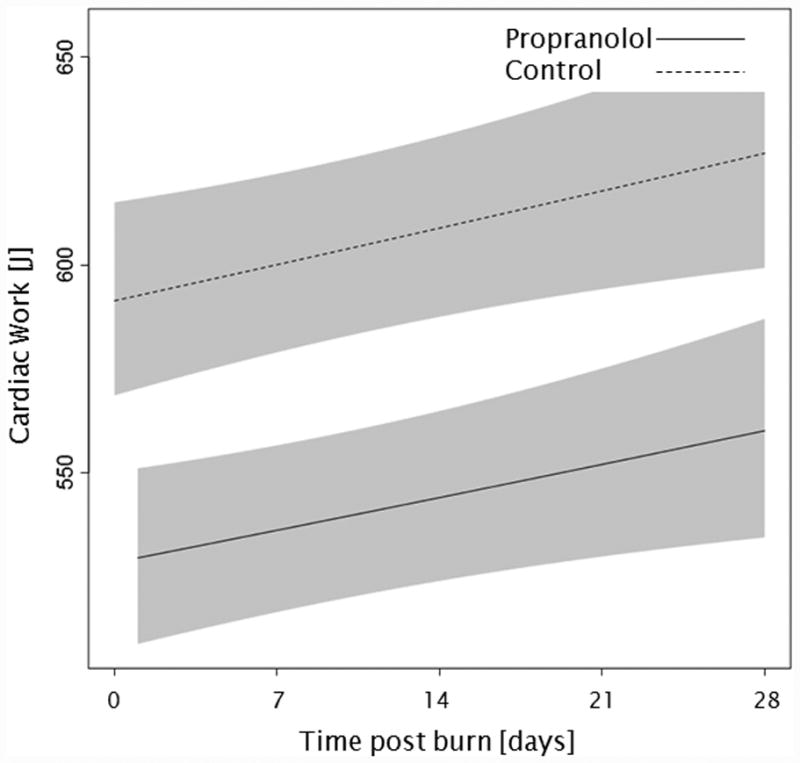
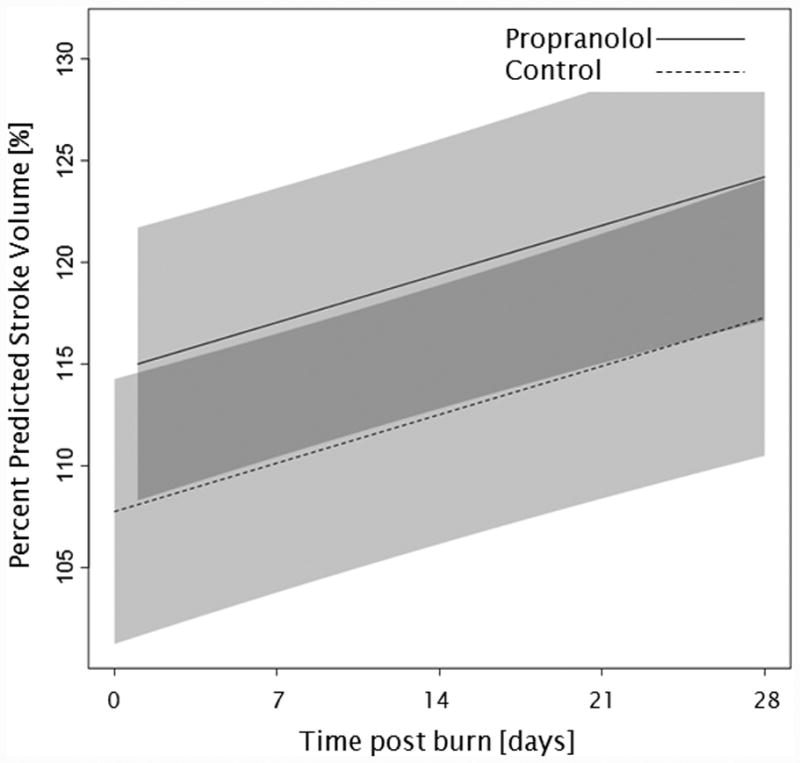
Trends for (A) cardiac index, (B) percent predicted heartrate, (C) mean arterial pressure, (D) rate pressure product, (E) cardiac work, and (F) percent predicted stroke volume over 28 days post burn. Lines show the regression model estimate over time post burn with 95% shaded confidence intervals.
Figure 3.
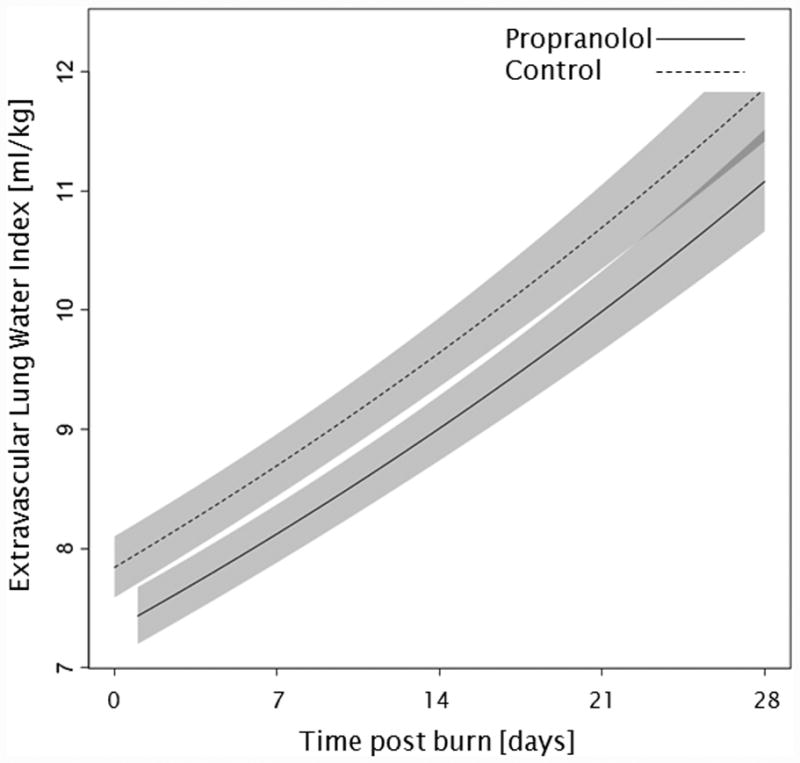
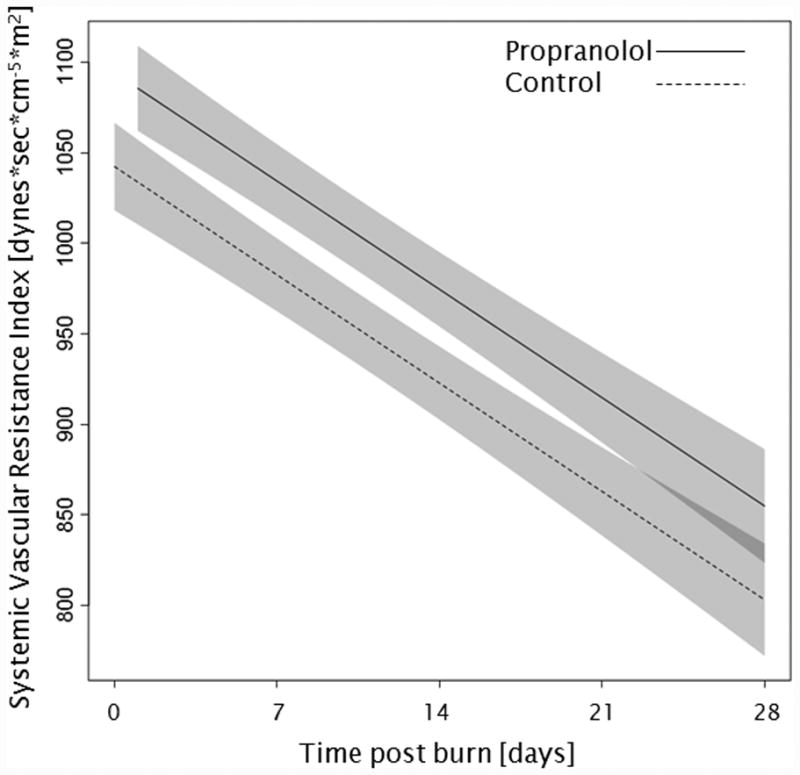
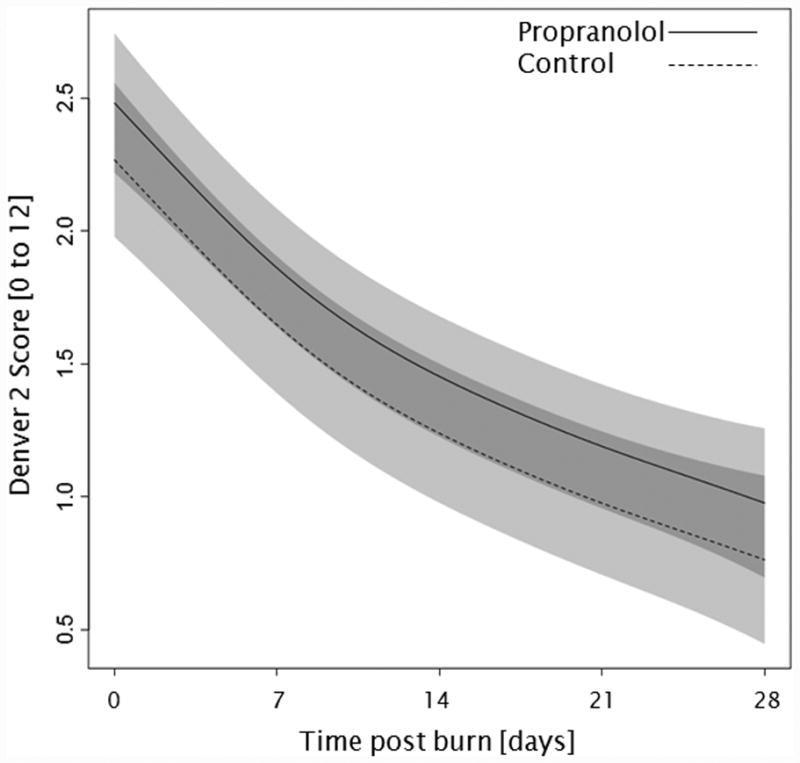
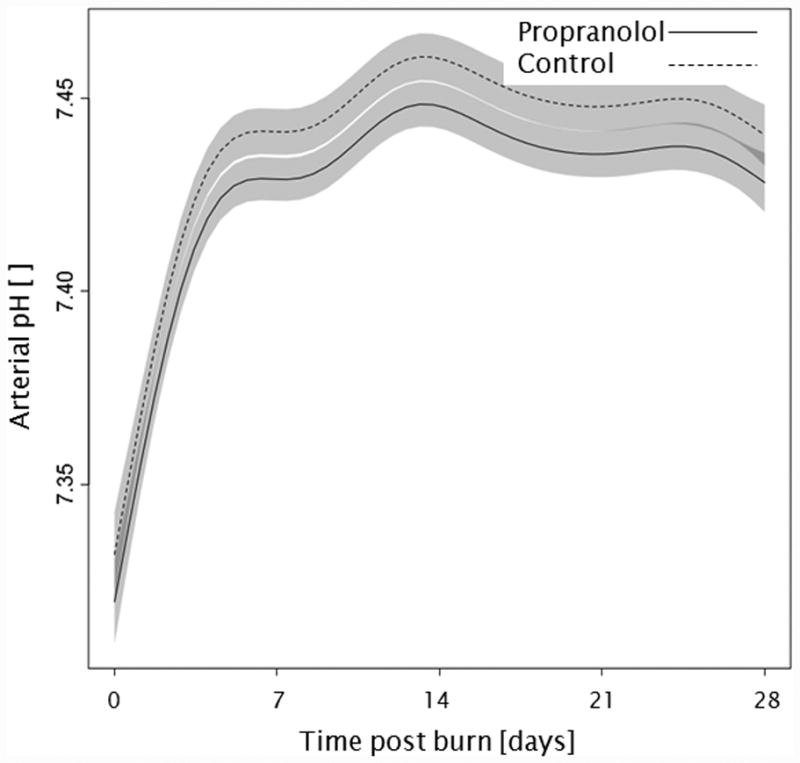
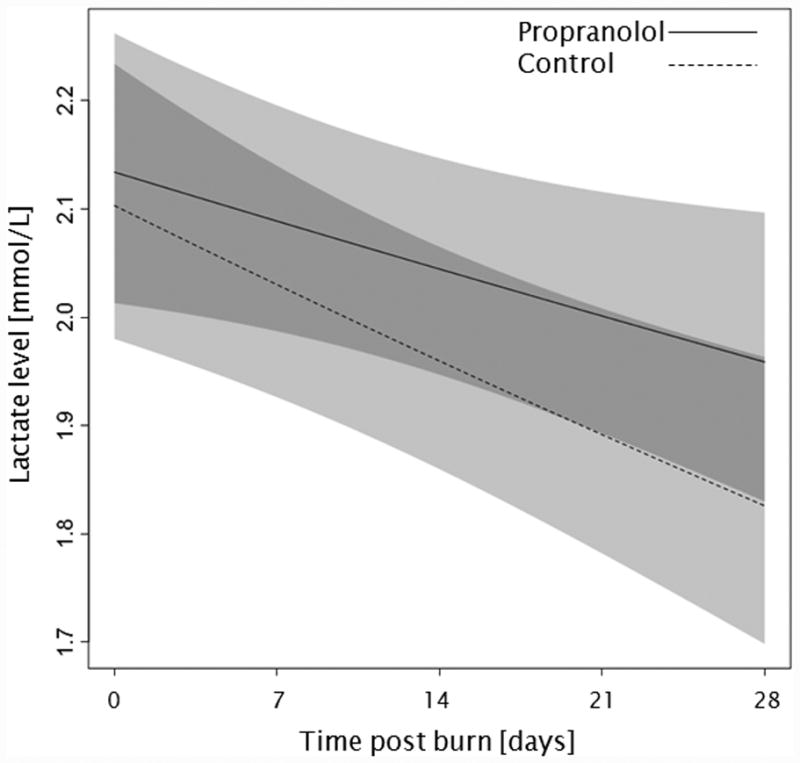
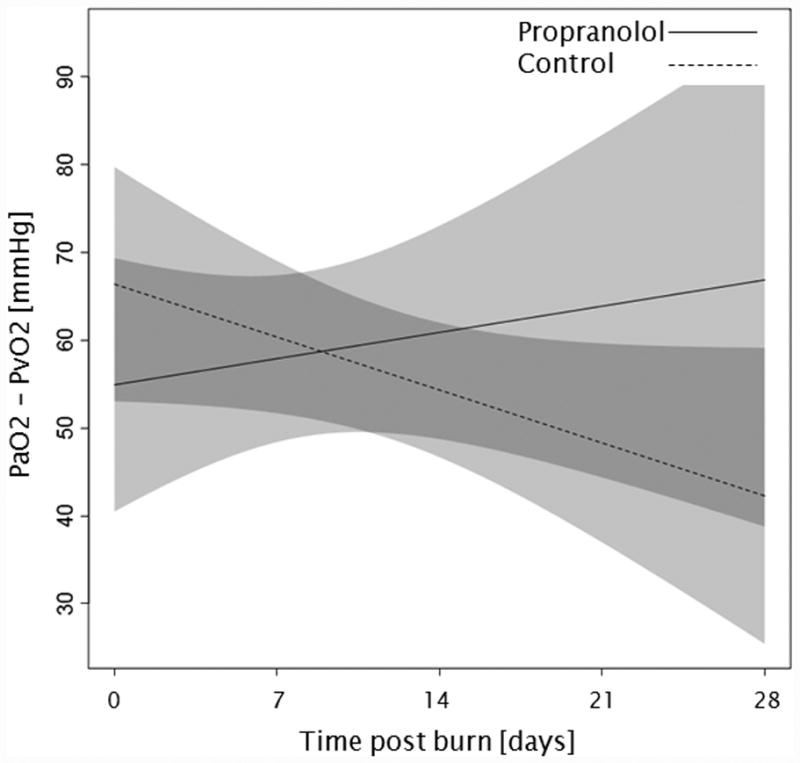
Trends for (A) extravascular lung water index, (B) systemic vascular resistance index, (C) Denver 2 score, (D) arterial pH, (E) lactate levels, and (F) PaO2-PvO2 over 28 days post burn. Lines show the regression model estimate over time post burn with 95% shaded confidence intervals.
Administration of propranolol had no significant affect on the overall Denver 2 score (p=0.52, Figure 3c). Additionally, Denver 2 score significantly decreased in both treatment groups throughout the patients' stay (p<0.01). Arterial blood pH significantly changed in both groups with days post burn (p<0.01), but propranolol had no significant affect on arterial pH (p=0.10, Figure 3d). One hundred and nine patients, 59 in the propranolol group and 50 in the control group, had at least one arterial pH and one lactate level measurement within the first 28 days post burn. There were no significant differences in of the incidence of lactic acidosis (p=1.00, Table 3) or lactate levels (p=0.86, Figure 3e) between groups during the analyzed period. Peripheral oxygen consumption measured as PaO2-PvO2 did not change with time post burn (p=0.43, Figure 3f), and both groups were comparable (p=0.56).
Table 3.
Lactic acidosis episodes during the first 28 days post burn.
| Lactic acidosis | Propranolol | Control | p value |
|---|---|---|---|
| Patients with lactic acidosis, n (%) | 4 (7%) | 4 (8%) | 1.00 |
| Duration of lactic acidosis, daysa | 0.2 ± 0.7 | 0.1 ± 0.3 | 0.88 |
| Days where lactic acidosis was assessed, daysa | 16.0 ± 8.7 | 16.9 ± 8.1 | 0.67 |
Data presented as mean ± standard deviation.
Discussion
Ongoing clinical trials focus on the reduction of cardiac stress associated with the hypermetabolic and hyperdynamic circulatory response to severe burns (covering more than one-third of TBSA) with anti-adrenergic drugs (2,17). However, no study has investigated the effect of propranolol on hemodynamic measurements assessed with transpulmonary thermodilution. Specifically, the effect of propranolol on EVLWI and SVRI is not described in the burn literature. The current study confirms the effects of propranolol on cardiac stress, documented with the PiCCO system. In contrast to a previously published study from our group (4) that showed that propranolol decreased cardiac output but had no effect on CI, we showed for the first time that propranolol significantly decreased CI and MAP throughout the acute hospitalization. Peripheral perfusion and incidence of lactic acidosis were not increased in the propranolol-treated group, and thus, the reduction of CI was not associated with any adverse events. Propranolol administration did not increase or decrease EVLWI or SVRI as compared to control.
Vast increases in serum catecholamine levels in response to severe burn injury lead to a hypermetabolic and hyperdynamic circulatory response. Four decades ago, a study in normotensive men showed that RPP is an index of myocardial oxygen consumption (15). In 1989, Minifee et al. (18) showed that propranolol can significantly reduce stress on cardiac muscle by reducing RPP. Thus, we hypothesized that propranolol attenuates hyperdynamic circulatory response following severe burn injury. In the current analysis, we were able to show that propranolol diminishes RPP as a marker for myocardial oxygen consumption. By reducing MAP and HR, propranolol also reduced CW. These effects have previously been shown to be dose-dependent in severely burned children through the use of transthoracic echocardiography (4). While echocardiography may not be readily available to continuously monitor effectiveness of propranolol treatment, the PiCCO system can be seen as a more feasible alternative.
The aforementioned increase in cardiac output and perfusion as response to large burns starts within the first week post burn and can remain elevated for up to 2 years post burn (3,19). At the same time, adequate peripheral perfusion is crucial to prevent organ failure and promote burn wound healing. In 1991, the effects of propranolol on CI have been previously described in a small cohort of adult burn patients (20). It is clear that any administered drug should not decrease CI below age-predicted values. In the current analysis, we were able to show at the first time in a large cohort of severely burned children that propranolol reduces the increased CI as response to burn by 0.67 l/min/m2, a decrease of approximately 10%. This reduction in the hyperdynamic cardiac response brings CI closer to norm values, which are around 4.5 l/min/m2 in children (21). Arterial pH levels, PaO2-PvO2 assessments, as well as events of lactic acidosis were comparable in both groups throughout the studied period, and thus, we can conclude that the reduction of CI had no significant effect on peripheral perfusion. In addition, both groups underwent comparable amounts of surgical interventions during acute hospitalization and had comparable time periods between operations; both measures can be seen as markers of wound healing and morbidity.
Propranolol had no significant effect on the Denver 2 post injury organ failure score as well as mortality in the cohort studied. However, the mortality rates observed here (7 to 8% in both groups) were high when compared to rates in the pediatric burn literature. We believe this to be due to the fact the studied patients had an average burn size of around 60% TBSA and burn size is still a predictor for mortality in burns (22).
Case reports from the 1990s described that propranolol caused pulmonary edema by having unopposed effects on the α adrenergic receptor which caused an elevation of afterload (23,24). In our current study, there was no significant effect of propranolol on EVLWI, which can be used as a marker for pulmonary edema. The correlation among age, weight, and EVLWI (3,10) was confirmed in our analysis, with older age at the time of the burn being significantly correlated with a higher EVLWI. Furthermore, we were able to show for the first time that propranolol has no direct effect on EVLWI in severely burned children during acute hospitalization. Interestingly, EVLWI was low during the early post-burn period and increased significantly with time in each group. In 2010, Bognar et al. (11) suggested that an EVLWI of more than 9 ml/kg is an predictor for sepsis in burns. We further postulate that the association between weight and EVLWI strongly biases EVLWI as predictor for sepsis in burned children. In pediatric burns, age-predicted and/or weight-predicted norm values for EVLWI need to be defined to further establish predictors for sepsis. An association between mortality and EVLWI was seen by Eisenberg et al.(25) and Branski et al.(3), whereas other studies in adults showed no correlation between mortality and EVLWI in burns (26). Owing to the relatively rare occurrence of death, we did not perform subgroup-analysis for EVLWI in patients who deceased during acute hospitalization.
A second limitation of this study is the observation time frame of 28 days, which is only one week longer than the time studied by Branski et al. (3). It would be of interest to know if EVLWI decreases with further time during acute hospitalization and reaches age-predicted values. This PiCCO assessment was conducted in this short period because the arterial lines, which are necessary to operate the system, are removed as soon as hemodynamic stabilization is reached.
Little is known about SVRI in pediatric burn patients. As previously shown, SVRI decreases continuously with time post burn (3). Our current study is the first to show that this trend lasts for up to 28 days post burn. Nevertheless, the difference between groups was not significant when compared over the entire 4 week period (p=0.07). In general, a high SVRI could be associated with hypovolemia or sepsis; it could also be related to vasoconstrictors such as circulating serum catecholamines. Burns are associated with a high concentration of circulating levels of the catecholamines epinephrine and norepinephrine (27). Epinephrine has been shown to be responsible for β2-adrenoceptor-mediated vasodilation in an animal study (14). Thus, we postulate that propranolol, a non-selective β1-, β 2-adrenoceptor antagonist, is able to inhibit the epinephrine-induced vasodilatation and cause vasoconstriction, resulting in an increased SVRI in pediatric burn patients. The ability of propranolol to alter SVRI has been described in non-burned patients (12). However, further studies with a larger number of patients are warranted to determine the effects of propranolol on β2-adrenoceptor-mediated vasodilation in burns. These findings call for close observation of the cardiovascular effects of propranolol when used for the treatment of the hyperdynamic and hypermetabolic response in children during the acute phase after severe burns.
Cardiogenic stress, which could lead to cardiac failure during the acute stay, in adult burn patients is a common cause for death amongst adult burn patients. The long-lasting impact on the cardiovascular system in adults due to the with burns associated hyperdynamic response and increased length of stay have been recently described by Duke et al.(28). Propranolol could play an important role in the reduction of long-term cardiovascular morbidities following severe burns in adults. Prospective randomized controlled trials are currently ongoing to evaluate safety and efficacy of propranolol in adults with burns.
Conclusions
Propranolol reduces cardiogenic stress in children with severe burn injury but does not adversely affect peripheral perfusion, wound healing, organ dysfunction and mortality. Further studies are warranted to investigate the possibility of propranolol-associated inhibition of β2-adrenoceptor-mediated vasodilation in our pediatric burn patients.
Supplementary Material
Figure 1.
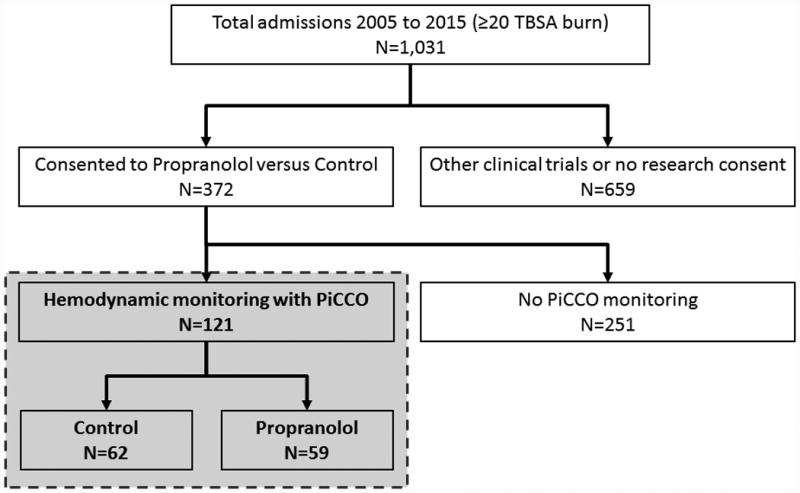
Flow diagram.
Acknowledgments
This study was supported by the National Institutes of Health (P50 GM060338, UL1TR000071, T32 GM008256, RO1-GM056687, RO1-112936, R01-HD049471), the Shriners Hospitals for Children (71006, 71008, 71009, 80100, 84080, 84291), and Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a Clinical and Translational Science Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Footnotes
Authors' Contributions: All authors made substantial contributions to the conception or design of the work (CCF, DNH, LKB, LCW, PW) or to the acquisition, analysis, and interpretation of data (AAF, CCF, CDV, CRA, DNH, GH, LKB, LPK, PW, RPC) as well as drafting the manuscript or revising it critically for important intellectual content (all authors).
Competing Interests: The authors declare that they have no competing interests. Parts of this research were presented in abstract form at the 46th Annual Congress of the Society of Critical Care Medicine in 2016 in Orlando, Florida.
References
- 1.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008 Sep;248(3):387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeschke MG, Herndon DN. Burns in children: standard and new treatments. Lancet. 2014 Mar 29;383(9923):1168–78. doi: 10.1016/S0140-6736(13)61093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Branski LK, Herndon DN, Byrd JF, Kinsky MP, Lee JO, Fagan SP, et al. Transpulmonary thermodilution for hemodynamic measurements in severely burned children. Crit Care. 2011;15(2):R118. doi: 10.1186/cc10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2011 Feb;149(2):231–9. doi: 10.1016/j.surg.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pruitt BA, Mason ADJ, Moncrief JA. Hemodynamic changes in the early post-burn period: The influence of fluid administration and of a vasodilator (hydralazine) J Trauma. 1971;1:36–46. doi: 10.1097/00005373-197101000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ramsingh D, Alexander B, Cannesson M. Clinical review: Does it matter which hemodynamic monitoring system is used? Crit Care. 2013;17(2):208. doi: 10.1186/cc11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Proulx F, Lemson J, Choker G, Tibby SM. Hemodynamic monitoring by transpulmonary thermodilution and pulse contour analysis in critically ill children. Pediatr Crit Care Med. 2011 Jul;12(4):459–66. doi: 10.1097/PCC.0b013e3182070959. [DOI] [PubMed] [Google Scholar]
- 8.Sánchez M, García-de-Lorenzo A, Herrero E, Lopez T, Galvan B, Asensio MJ, et al. A protocol for resuscitation of severe burn patients guided by transpulmonary thermodilution and lactate levels: a 3-year prospective cohort study. Crit Care. 2013;17(4):R176. doi: 10.1186/cc12855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wurzer P, Branski LK, Jeschke MG, Ali A, Kinsky MP, Bohanon FJ, et al. Transpulmonary Thermodilution Versus Transthoracic Echocardiography for Cardiac Output Measurements in Severely Burned Children. Shock. 2016 Apr 4; doi: 10.1097/SHK.0000000000000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lemson J, van Die LE, Hemelaar AE, van der Hoeven JG. Extravascular lung water index measurement in critically ill children does not correlate with a chest x-ray score of pulmonary edema. Crit Care. 2010;14(3):R105. doi: 10.1186/cc9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bognar Z, Foldi V, Rezman B, Bogar L, Csontos C. Extravascular lung water index as a sign of developing sepsis in burns. Burns. 2010 Dec;36(8):1263–70. doi: 10.1016/j.burns.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Silke B, Nelson GIC, Ahuja RC, Okoli RC, Taylor SH. Comparative haemodynamic dose-response effects of intravenous propranolol and pindolol in patients with coronary heart disease. Eur J Clin Pharmacol. 1983 Aug;25(2):157–65. doi: 10.1007/BF00543785. [DOI] [PubMed] [Google Scholar]
- 13.Jacobsen TN, Converse RL, Victor RG. Contrasting effects of propranolol on sympathetic nerve activity and vascular resistance during orthostatic stress. Circulation. 1992 Mar;85(3):1072–6. doi: 10.1161/01.cir.85.3.1072. [DOI] [PubMed] [Google Scholar]
- 14.Moreira-Rodrigues M, Graça AL, Ferreira M, Afonso J, Serrão P, Morato M, et al. Attenuated aortic vasodilation and sympathetic prejunctional facilitation in epinephrine-deficient mice: selective impairment of β2-adrenoceptor responses. J Pharmacol Exp Ther. 2014 Nov;351(2):243–9. doi: 10.1124/jpet.114.217281. [DOI] [PubMed] [Google Scholar]
- 15.Hazinski M. Manual of Pediatric Critical Care. Mosby, Inc; 1999. Cardiovascular Disorders; pp. 84–288. [Google Scholar]
- 16.Glue Grant. Denver 2 Score [Internet] Inflammation and Host Response to Injury Glue Grant: How the Denver Score is Computed. Available from: https://www.gluegrant.org/PDFs/Denver_score.pdf.
- 17.Rojas Y, Finnerty CC, Radhakrishnan RS, Herndon DN. Burns: an update on current pharmacotherapy. Expert Opin Pharmacother. 2012 Dec;13(17):2485–94. doi: 10.1517/14656566.2012.738195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minifee PK, Barrow RE, Abston S, Desai M, Herndon DN. Improved myocardial oxygen utilization following propranolol infusion in adolescents with postburn hypermetabolism. J Pediatr Surg. 1989 Aug;24(8):806, 810. doi: 10.1016/s0022-3468(89)80541-x. 811. [DOI] [PubMed] [Google Scholar]
- 19.Williams FN, Herndon DN, Suman OE, Lee JO, Norbury WB, Branski LK, et al. CHANGES IN CARDIAC PHYSIOLOGY AFTER SEVERE BURN INJURY. J Burn Care Res. 2011;32(2):269–74. doi: 10.1097/BCR.0b013e31820aafcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gore DC, Honeycutt D, Jahoor F, Barrow RE, Wolfe RR, Herndon DN. Propranolol diminishes extremity blood flow in burned patients. Ann Surg. 1991 Jun;213(6):568–74. doi: 10.1097/00000658-199106000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cattermole GN, Leung PYM, Mak PSK, Chan SSW, Graham CA, Rainer TH. The normal ranges of cardiovascular parameters in children measured using the Ultrasonic Cardiac Output Monitor. Crit Care Med. 2010 Sep;38(9):1875–81. doi: 10.1097/CCM.0b013e3181e8adee. [DOI] [PubMed] [Google Scholar]
- 22.Taylor SL, Lawless M, Curri T, Sen S, Greenhalgh DG, Palmieri TL. Predicting Mortality from Burn Injuries: The need for age-group specific models. Burns. 2014 Sep;40(6):1106–15. doi: 10.1016/j.burns.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pogson GW, Sharma J, Crouch TT. Pulmonary edema with low-dose propranolol in pheochromocytoma. South Med J. 1980 Jun;73(6):795–6. doi: 10.1097/00007611-198006000-00036. [DOI] [PubMed] [Google Scholar]
- 24.Sloand EM, Thompson BT. Propranolol-induced pulmonary edema and shock in a patient with pheochromocytoma. Arch Intern Med. 1984 Jan;144(1):173–4. [PubMed] [Google Scholar]
- 25.Eisenberg PR, Hansbrough JR, Anderson D, Schuster DP. A prospective study of lung water measurements during patient management in an intensive care unit. Am Rev Respir Dis. 1987 Sep;136(3):662–8. doi: 10.1164/ajrccm/136.3.662. [DOI] [PubMed] [Google Scholar]
- 26.Küntscher MV, Blome-Eberwein S, Pelzer M, Erdmann D, Germann G. Transcardiopulmonary vs pulmonary arterial thermodilution methods for hemodynamic monitoring of burned patients. J Burn Care Rehabil. 2002 Feb;23(1):21–6. doi: 10.1097/00004630-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Wilmore DW, Long JM, Mason AD, Skreen RW, Pruitt BA. Catecholamines: Mediator of the Hypermetabolic Response to Thermal Injury. Ann Surg. 1974 Oct;180(4):653–68. doi: 10.1097/00000658-197410000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Understanding the long-term impacts of burn on the cardiovascular system. Burns. 2016 Mar;42(2):366–74. doi: 10.1016/j.burns.2015.08.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


