Abstract
Microorganisms growing in a biofilm state are very resilient in the face of treatment by many antimicrobial agents. Biofilm infections are a significant problem in chronic and long-term infections, including those colonizing medical devices and implants. Anti-biofilm peptides represent a very promising approach to treat biofilm-related infections and have an extraordinary ability to interfere with various stages of the biofilm growth mode. Anti-biofilm peptides possess promising broad-spectrum activity in killing both Gram-positive and Gram-negative bacteria in biofilms, show strong synergy with conventional antibiotics, and act by targeting a universal stringent stress response. Understanding downstream processes at the molecular level will help to develop and design peptides with increased activity. Anti-biofilm peptides represent a novel, exciting approach to treating recalcitrant bacterial infections.
Keywords: biofilm, synergy, c-di-GMP, ppGpp, stringent response
Introduction
Biofilms are multicellular, three-dimensional aggregates that form on surfaces in both nature and the clinic. They are difficult to treat since biofilms are adaptively resistant to antibiotics (up to 1000-fold) as compared to their free-swimming, planktonic counterparts [1]. Biofilms can form on a variety of tissues and implanted devices, and are implicated in diverse diseases such as cystic fibrosis, wounds, otitis media, pneumonia, and osteomyelitis [2]. Bacterial aggregates that form on medical implants, such as catheters, valves, stents and shunts are difficult to remove except by surgery [2]. The annual cost to the U.S. health care system is on the order of billions [3]. Therefore new therapeutic options are urgently needed. The treatment of biofilm-related infections is very challenging and scientific attention has recently turned to developing agents with specific anti-biofilm activity [4,5]. In particular, this review will focus on anti-biofilm peptides, their activity in combination with other antimicrobial agents, and their mechanism of action.
Antimicrobial peptides with potential to fight biofilm-related infections
Antimicrobial peptides (AMPs), characterized here as peptides with activity vs. planktonic bacteria, possess broad-spectrum antibiotic activity against most bacterial pathogens. They are a subset of the host defence peptides, named due to their frequent anti-infective immunomodulatory activity, and are an important part of human innate immunity [6]. Importantly, AMPs do not necessarily affect biofilms. For example, numerous peptides have been developed over the past few years, but comparatively few show anti-biofilm activity below their minimal inhibitory concentration (MIC). A few examples of recently described peptides with anti-biofilm properties are shown in Table 1 and described below.
Table 1.
Recent studies on peptides with specific anti-biofilm activity.
| Peptide | Minimal Inhibitory Concentration | Active biofilm concentration | Active against | Reference |
|---|---|---|---|---|
| AS10 | 50 μMa | 0.22 μMb | C. albicans | [16] |
| KT2 and RT2 | 5 – 18 μM | 1 μMc | E. coli | [13] |
| SB056 and derivatives | 10 – >40 μM | 5–20 μMd | S. epidermidis, P. aeruginosa | [14] |
| Cyclic lipopetide 3 | 22 –55 μM | 4 μMc | S. aureus, P. aeruginosa | [15] |
| LL-37 and derivatives | 32 μg/ml | 1–16 μg/mld | S. aureus, E. coli | [9,31] |
| (IDR-)1018 | 8 – 128 μg/ml | 2–8 μg/mlb | A. baumannii, E. coli, K. pneumoniae, P. aeruginosa, S. enterica, S. aureus | [10] |
| DJK-5 | 1.6 – 16 μg/ml | 0.8 – 4 μg/mlb | as for IDR-1018 | [11] |
| DJK-6 | 4 – 16 μg/ml | 0.5 – 8 μg/mlb | as for IDR-1018 | [11] |
Minimal fungicidal concentration
Minimal biofilm inhibitory concentration
Active concentration in flow cells
Concentrations showing biofilm inhibition
The human cathelicidin peptide LL-37 has very weak AMP (planktonic antibiotic) activity under physiological conditions [7]. A breakthrough was achieved when it was demonstrated that LL-37 inhibited biofilm formation at concentrations 16-fold below its MIC against planktonic bacteria [8]. LL-37 was subsequently shown to possess anti-biofilm activity against urinary tract isolates of Staphylococcus aureus and Escherichia coli at 1/32 to 1/2 MIC [9]. Recently, synthetic cathelicidin-derived anti-biofilm peptides (such as innate defense regulator 1018, DJK-5, and DJK-6) were developed, which exhibited broad-spectrum activity against multidrug resistant organisms [10,11].
Aside from cathelicidins, novel discoveries also draw from the diversity of AMPs found in nearly all domains of life [12]. For example, Anunthawan et al. demonstrated that the two tryptophan-rich cationic antimicrobial peptides KT2 and RT2 showed anti-biofilm activity at sub-MIC levels against the multidrug-resistant, enterohemorrhagic E. coli O157:H7 strain and were able to prevent biofilm formation and eradicate mature biofilms at a concentration of 1 μM [13]. Both peptides interacted with and bound to negatively-charged LPS molecules to enable self-promoted uptake (without forming pores or aggregates) across the outer membrane and subsequently interacted with cytoplasmic membrane phospholipids [13].
Two classes of peptides with unusual structure were also recently developed. The first, SB056, a semi-synthetic peptide with a dendrimeric (dimeric) scaffold was active against planktonic E. coli and S. aureus and showed anti-biofilm activity against Staphylococcus epidermidis and Pseudomonas aeruginosa at concentrations half or less the MICs [14]. Remarkably, an optimized linear form (with enhanced amphiphilic profile) of SB056 as well as the dimeric dendrimer were even more active against S. epidermidis biofilms. These peptides showed strong affinity for bacterial membranes and the authors postulated that the distribution of hydrophobic and charged residues within the peptide sequence play a role in peptide-lipid interaction [14]. Secondly, Bionda et al. [15] used a positional-scanning combinatorial method to screen a cyclic lipopeptide library (peptides derived from fusaricidin/LI-F) against multidrug resistant pathogens. The lead peptide from this study showed activity against all ESKAPE pathogens at 110 μM. Intriguingly, at much lower concentrations (22–28 μM), antibacterial activity was observed against Enterococcus faecium, S. aureus, Acinetobacter baumannii, and P. aeurginosa. Furthermore, at a concentration as low as 4.4 μM, the lead peptide inhibited biofilm formation and eradicated mature biofilms of both P. aeruginosa and S. aureus. A screen revealed that improved potency depended on hydrophobic as well as positively charged amino acids. These examples highlight the importance of studying and understanding peptide scaffolds, structural order of amino acids within a sequence, peptide activity, and interaction with bacterial membranes.
Peptides can also be active against fungal biofilms. De Brucker et al. [16] showed that the cathelicidin-derived peptide AS10 had specific anti-biofilm activity at a concentration of only 0.22 μM (~1 μg/ml) against fungal Candida albicans biofilms. This concentration was more than 200-fold less than that needed to inhibit planktonic growth. AS10 also inhibited biofilm formation in a mixed C. albicans and S. epidermidis population and was active against Gram-negative pathogens including E. coli, P. aeruginosa, and Porphyromonas gingivalis.
Peptides enhance the activity of other antimicrobial agents
In the past few years, many peptides were identified that show strong action against microbial biofilms. Recent studies have also demonstrated that peptides can be used in conjunction with antibiotics, antifungals, or other antimicrobial compounds, which leads to enhanced activity (i.e. synergistic effects) [16–18]. Lowering antibiotic concentrations helps to reduce expenses, toxic side effects, and the spread of antimicrobial resistance. Synergy with peptides can also enhance the activity of antibiotics against multidrug resistant strains [17]. This is highly relevant because biofilm-related infections often result in chronic diseases that fail to be eradicated by antibiotics alone [4].
The synthetic peptides IDR-1018, DJK5, and DJK6 acted synergistically against several Gram-negative pathogens with one or more of the major conventional antibiotics ceftazidime, ciprofloxacin, imipenem and tobramycin [11,19], lowering their effective concentrations up to 64-fold. IDR-1018 also showed synergy with the antiseptic agent chlorhexidine against multispecies oral biofilm [20] and DJK-6 enhanced the activity of the carbapenem imipenem against plasmid-mediated carbapenemase-producing Klebsiella pneumoniae [17], highlighting how peptides can be used to repurpose antibiotics.
Not only do peptides show synergy with antibiotics, they also enhance the activity of antifungal drugs. The combination of the lipopeptide bacillomycin D and the antifungal drug amphotericin B was strongly synergistic against C. albicans biofilms [18]. Moreover, peptide AS10 was able to act synergistically with the antifungal drugs caspofungin and amphotericin B against C. albicans biofilms [16]. The concentration of antifungal required to eradicate a biofilm was reduced 5- to 8-fold in the presence of 0.39–1.56 μM AS10 [16].
In addition to their synergy with antimicrobial agents, peptides can also be used to extend the spectrum of antibiotics. Recently, Mishra et al. [21] conjugated CRAMP (murine cathelicidin) to vancomycin and found that the resultant compound was active against both Gram-positive and Gram-negative species. The activity of the conjugate was strongly improved compared to an equimolar mixture of CRAMP and vancomycin, demonstrating the benefit of covalent linkage. The authors postulated that CRAMP helped translocate vancomycin into the periplasm of Gram-negative bacteria, showing that peptides can help to repurpose and extend the spectrum of antibiotics.
Controlling biofilm infections: Interference with small signaling molecules
Although much attention has been given to AMPs and the broad-spectrum activity of their anti-biofilm counterparts against various bacterial biofilms, the treatment of chronic infections is still very challenging. Critically, the above-described disparity between antibiotic (vs. planktonic cells) and anti-biofilm activity (see also [22]) indicates clear mechanistic differences. Therefore, novel approaches that show how anti-biofilm peptides work on a molecular level in terms of preventing biofilm formation and/or eradicating pre-existing biofilms is the next step in anti-biofilm peptide research.
Intracellular signaling systems are ubiquitous in that the regulatory mechanisms are found in many Gram-positive and Gram-negative pathogens. In this context, nucleotide signaling is an important mechanism that allows microorganisms to control several key processes required for bacterial colonization and adaptation (including quorum sensing), host-microbe interaction [23], and biofilm formation [24]. Second messenger nucleotides include guanosine tetraphosphate (ppGpp), cyclic adenosine/guanosine monophosphate (cAMP, cGMP), and cyclic di-adenosine/di-guanosine (c-di-AMP, c-di-GMP) [4].
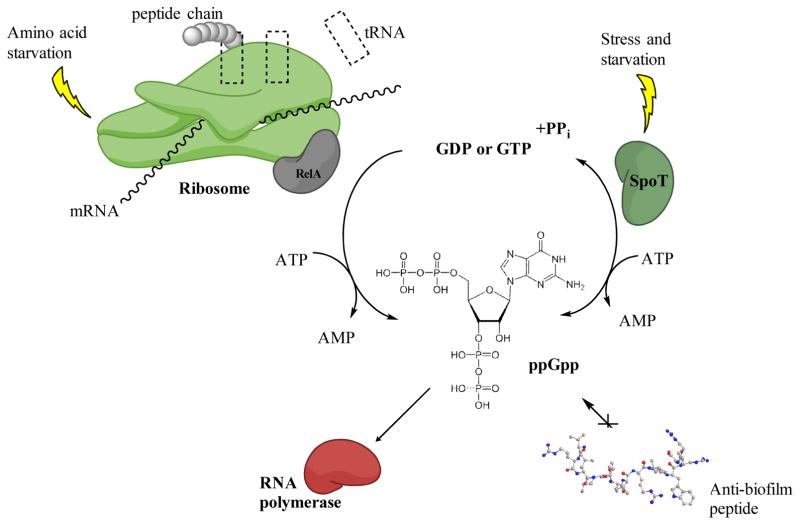
Microorganisms must cope with stressors encountered in their environment. The stringent response is mediated by rapid accumulation of pppGpp that is quickly processed to the second messenger ppGpp. Synthesis of these molecules occurs through the global stress response regulator enzymes RelA and SpoT in most Gram-negative bacteria, while a single bifunctional enzyme Rsh is present in Gram-positive bacteria. Amino acid starvation triggers the production of the cellular alarmone ppGpp through RelA. RelA binds to the ribosome, which is blocked by uncharged tRNA molecules, hence catalyzing the synthesis of ppGpp (Figure 1) [25]. Conversely, SpoT promotes ppGpp synthesis under phosphorus, fatty acid or iron starvation [26]. SpoT is additionally a bifunctional enzyme that is able to hydrolyze ppGpp. Rapidly accumulating intracellular ppGpp triggers a switch from cell growth to survival [25]. Recent studies have demonstrated that ppGpp signaling plays a pivotal role in antibiotic resistance, virulence, and biofilm formation. Stringent response signaling acts through several mechanisms such as interaction with RNA polymerase (thereby affecting transcription or influencing the binding of the sigma factor) [27], interaction with other proteins involved in translation, replication and RNA turnover, crosstalk with other second messengers such as c-di-GMP, and by regulation of cellular processes (such as cell-to-cell communication) [26].
Figure 1. The stringent response.
In response to amino acid starvation (uncharged tRNA molecules bind to the ribosome) RelA binds to the ribosome and triggers the production of cellular alarmones ppGpp. Under other stresses such as iron starvation, SpoT triggers the production of ppGpp. ppGpp signalling molecules bind to the RNA polymerase thereby affecting transcription. Rapid accumulation of ppGpp in the cell cause a switch from cell growth to survival. Anti-biofilm peptides are able to block intracellular accumulation of ppGpp. Figure modified from [32].
Recently, de la Fuente-Nunez et al. [10,11] reported that the anti-biofilm peptides IDR-1018, DJK-5, and DJK-6 are able to bind to and trigger degradation of ppGpp, thus preventing intracellular accumulation of this second messenger and thereby preventing biofilm formation in multiple Gram-negative and Gram-positive pathogens. These results show promise that anti-biofilm peptides can be used as broad-spectrum biofilm inhibitors. Of further interest, the stringent stress response also controls the development of so-called persisters that are able to avoid the action of antibiotics [28].
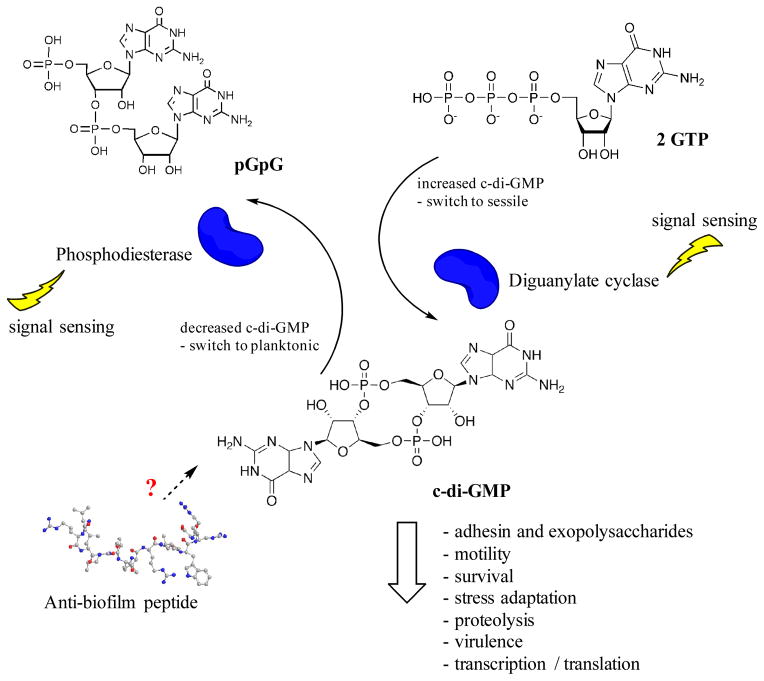
Second messenger c-di-GMP has emerged as an important universal bacterial second messenger molecule that regulates lifestyle changes in many bacteria. As such it is recognized to play a role in the establishment of multicellular communities (i.e. switch from motile to sessile state), biofilm dispersal, motility, adaptations from the virulent state of acute infections to less virulent (but more resilient) states of chronic infections, cell differentiation and other processes [4,23,24]. Interfering with c-di-GMP signaling pathways potentially constitutes a novel approach of controlling biofilm formation and dispersal, especially since these molecules are absent in mammalian organisms [24].
Biofilm-related infections are often associated with elevated c-di-GMP levels in bacterial pathogens [29], which show reduced motility and increased expression of extracellular matrix components (such as exopolysaccharides, pili formation, adhesins, extracellular DNA) [24,27]. Low intracellular c-di-GMP levels promote dispersal from biofilms and increase bacterial motility [24]. Microorganisms respond to various environmental stimuli by quickly adjusting intracellular c-di-GMP concentrations through the enzymes diguanylate cyclase (catalyzes the formation of c-di-GMP from two GTP) and phosphodiesterase (degrades c-di-GMP into pGpG) (Figure 2) [23,24,27]. It has been shown in P. aeruginosa that cells with low c-di-GMP levels are more resistant to the lipopeptide antibiotic colistin, indicating that c-di-GMP signaling also plays an important role in antimicrobial peptide resistance [30]. However, many downstream processes and molecular mechanisms of this regulation involving c-di-GMP have yet to be identified.
Figure 2. Cyclic-di-GMP levels are controlled by the enzymes diguanylate cyclase (increasing) and phosphodiesterase (decreasing).
Thereby, they regulate the switch from planktonic growth to sessile and vice versa. High c-di-GMP levels stimulate biofilm formation and other factors such as stress adaptation, virulence, etc. The role of anti-biofilm peptides in the c-di-GMP pathway has yet to be identified.
Concluding remarks and future directions
Currently, biofilm-related infections are one of the most recalcitrant diseases that make treatment with conventional antibiotics a major challenge in clinics. Understanding the nature of microbial biofilms will help combat biofilm infections. Consequently, research towards the development of novel therapeutic strategies is urgently needed and anti-biofilm peptides appear to be a very promising approach. Single antibiotic administration is often inadequate to overcome bacterial invaders, high antibiotic concentrations are toxic, and multiple antibiotic resistant strains are emerging. Co-administration of antibiotics with anti-biofilm peptides offers a novel strategy that will enhance human health and medicine. Anti-biofilm peptides that can kill multiple species in biofilms or inhibit developed biofilms are promising since they allow administration of lower antibiotic concentrations and therefore present a hopeful alternative treatment with conventional antibiotics. Anti-biofilm peptides interfere with second messenger molecules that control global signaling pathways in both Gram-positive and Gram-negative bacteria, indicative of their broad-spectrum activity. Interrupting complex regulatory systems without killing bacteria should help to circumvent the emergence of drug resistant populations through synergy with existing antibiotics. Future directions will lead to understanding the downstream processes of anti-biofilm peptides and this will help to optimize peptides and enable them to be developed as antibiotic adjuvants as well as stand-alone anti-biofilm therapies.
Highlights.
To date no antibiotics have been specifically developed for biofilm infections
Anti-biofilm peptides directly address chronic multi-resistant bacterial infections
They demonstrate broad spectrum activity
They uniquely target the stringent stress response required for biofilm growth
They are synergistic with common antibiotics even against antibiotic resistant bacteria
Acknowledgments
Our peptide research has been generously supported by grants from the Canadian Institutes for Health Research (CIHR) (funding reference number MOP-123477) and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R33AI098701. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. DP received a Feodor Lynen postdoctoral fellowship from the Alexander von Humboldt Foundation. SRC received studentships from CIHR (GSD-146221), while REWH holds a Canada Research Chair.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jefferson KK. What drives bacteria to produce a biofilm? FEMS Microbiol Lett. 2004;236:163–173. doi: 10.1016/j.femsle.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 3.Neethirajan S, Clond MA, Vogt A. Medical biofilms-nanotechnology approaches. J Biomed Nanotechnol. 2014;10:2806–2827. doi: 10.1166/jbn.2014.1892. [DOI] [PubMed] [Google Scholar]
- 4.Wu H, Moser C, Wang HZ, Hoiby N, Song ZJ. Strategies for combating bacterial biofilm infections. Int J Oral Sci. 2015;7:1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor PK, Yeung AT, Hancock REW. Antibiotic resistance in Pseudomonas aeruginosa biofilms: towards the development of novel anti-biofilm therapies. J Biotechnol. 2014;191:121–130. doi: 10.1016/j.jbiotec.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Hancock REW, Haney EF, Gill EE. The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol. 2016 doi: 10.1038/nri.2016.29. advance online publication. [DOI] [PubMed] [Google Scholar]
- 7.Mansour SC, Pena OM, Hancock REW. Host defense peptides: front-line immunomodulators. Trends Immunol. 2014;35:443–450. doi: 10.1016/j.it.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 8*.Overhage J, Campisano A, Bains M, Torfs EC, Rehm BH, Hancock REW. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect Immun. 2008;76:4176–4182. doi: 10.1128/IAI.00318-08. This paper showed that a human peptides traditionally labelled as an antimicrobial peptide, but having very weak activity under physiological conditions is able to inhibit biofilm formation at one sixteenth the MIC, alerting researchers to the likelihood that antimcrobial and anti-biofilm activity were independent properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aka ST. Killing efficacy and anti-biofilm activity of synthetic human cationic antimicrobial peptide cathelicidin hCAP-18/LL37 against urinary tract pathogens. J Microbiol Infect Dis. 2015;5:15–20. [Google Scholar]
- 10**.de la Fuente-Nunez C, Reffuveille F, Haney EF, Straus SK, Hancock REW. Broad-spectrum anti-biofilm peptide that targets a cellular stress response. PLoS Pathog. 2014;10:e1004152. doi: 10.1371/journal.ppat.1004152. A breakthrough study that showed that synthetic peptide 1018 has potent broad-spectrum anti-biofilm activity and acts by targeting a cellular stress response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Fuente-Nunez C, Reffuveille F, Mansour SC, Reckseidler-Zenteno SL, Hernandez D, Brackman G, Coenye T, Hancock REW. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem Biol. 2015;22:196–205. doi: 10.1016/j.chembiol.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz J, Ortiz C, Guzman F, Fernandez-Lafuente R, Torres R. Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr Med Chem. 2014;21:2299–2321. doi: 10.2174/0929867321666140217110155. [DOI] [PubMed] [Google Scholar]
- 13.Anunthawan T, de la Fuente-Nunez C, Hancock REW, Klaynongsruang S. Cationic amphipathic peptides KT2 and RT2 are taken up into bacterial cells and kill planktonic and biofilm bacteria. Biochim Biophys Acta. 2015;1848:1352–1358. doi: 10.1016/j.bbamem.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 14.Batoni G, Casu M, Giuliani A, Luca V, Maisetta G, Mangoni ML, Manzo G, Pintus M, Pirri G, Rinaldi AC, et al. Rational modification of a dendrimeric peptide with antimicrobial activity: consequences on membrane-binding and biological properties. Amino Acids. 2016;48:887–900. doi: 10.1007/s00726-015-2136-5. [DOI] [PubMed] [Google Scholar]
- 15.Bionda N, Fleeman RM, de la Fuente-Nunez C, Rodriguez MC, Reffuveille F, Shaw LN, Pastar I, Davis SC, Hancock REW, Cudic P. Identification of novel cyclic lipopeptides from a positional scanning combinatorial library with enhanced antibacterial and antibiofilm activities. Eur J Med Chem. 2016;108:354–363. doi: 10.1016/j.ejmech.2015.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Brucker K, Delattin N, Robijns S, Steenackers H, Verstraeten N, Landuyt B, Luyten W, Schoofs L, Dovgan B, Frohlich M, et al. Derivatives of the mouse cathelicidin-related antimicrobial peptide (CRAMP) inhibit fungal and bacterial biofilm formation. Antimicrob Agents Chemother. 2014;58:5395–5404. doi: 10.1128/AAC.03045-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribeiro SM, de la Fuente-Nunez C, Baquir B, Faria-Junior C, Franco OL, Hancock REW. Antibiofilm peptides increase the susceptibility of carbapenemase-producing Klebsiella pneumoniae clinical isolates to beta-lactam antibiotics. Antimicrob Agents Chemother. 2015;59:3906–3912. doi: 10.1128/AAC.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabbene O, Azaiez S, Di Grazia A, Karkouch I, Ben Slimene I, Elkahoui S, Alfeddy MN, Casciaro B, Luca V, Limam F, et al. Bacillomycin D and its combination with amphotericin B: promising antifungal compounds with powerful antibiofilm activity and wound-healing potency. J Appl Microbiol. 2016;120:289–300. doi: 10.1111/jam.13030. [DOI] [PubMed] [Google Scholar]
- 19.Reffuveille F, de la Fuente-Nunez C, Mansour S, Hancock REW. A broad-spectrum antibiofilm peptide enhances antibiotic action against bacterial biofilms. Antimicrob Agents Chemother. 2014;58:5363–5371. doi: 10.1128/AAC.03163-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, de la Fuente-Nunez C, Shen Y, Haapasalo M, Hancock REW. Treatment of oral multispecies biofilms by an anti-biofilm peptide. PLoS One. 2015;10:e0132512. doi: 10.1371/journal.pone.0132512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21*.Mishra NM, Briers Y, Lamberigts C, Steenackers H, Robijns S, Landuyt B, Vanderleyden J, Schoofs L, Lavigne R, Luyten W, et al. Evaluation of the antibacterial and antibiofilm activities of novel CRAMP-vancomycin conjugates with diverse linkers. Org Biomol Chem. 2015;13:7477–7486. doi: 10.1039/c5ob00830a. An interesting article where the authors show for the first time a vancomycin-CRAMP conjugate leads to broad-spectrum activity. [DOI] [PubMed] [Google Scholar]
- 22.de la Fuente-Nunez C, Korolik V, Bains M, Nguyen U, Breidenstein EB, Horsman S, Lewenza S, Burrows L, Hancock REW. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother. 2012;56:2696–2704. doi: 10.1128/AAC.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23**.Gao J, Tao J, Liang W, Jiang Z. Cyclic (di)nucleotides: the common language shared by microbe and host. Curr Opin Microbiol. 2016;30:79–87. doi: 10.1016/j.mib.2015.12.005. An excellent review on the host-pathogen crosstalk mediated by c-di-nucleotides. [DOI] [PubMed] [Google Scholar]
- 24*.Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77:1–52. doi: 10.1128/MMBR.00043-12. A comprehensive overview of c-di-GMP-dependent signaling pathways with emphasis on common trends and interesting illustration of various examples. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jimenez PN, Koch G, Thompson JA, Xavier KB, Cool RH, Quax WJ. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romling U, Balsalobre C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J Intern Med. 2012;272:541–561. doi: 10.1111/joim.12004. [DOI] [PubMed] [Google Scholar]
- 27.Chua SL, Sivakumar K, Rybtke M, Yuan M, Andersen JB, Nielsen TE, Givskov M, Tolker-Nielsen T, Cao B, Kjelleberg S, et al. C-di-GMP regulates Pseudomonas aeruginosa stress response to tellurite during both planktonic and biofilm modes of growth. Sci Rep. 2015;5:10052. doi: 10.1038/srep10052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maisonneuve E, Castro-Camargo M, Gerdes K. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell. 2013;154:1140–1150. doi: 10.1016/j.cell.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 29.Tamayo R. The characterization of a cyclic-di-GMP (c-Di-GMP) pathway leads to a new tool for studying c-Di-GMP metabolic genes. J Bacteriol. 2013;195:4779–4781. doi: 10.1128/JB.00925-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chua SL, Tan SY, Rybtke MT, Chen Y, Rice SA, Kjelleberg S, Tolker-Nielsen T, Yang L, Givskov M. Bis-(3′–5′)-cyclic dimeric GMP regulates antimicrobial peptide resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57:2066–2075. doi: 10.1128/AAC.02499-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra B, Golla RM, Lau K, Lushnikova T, Wang G. Anti-Staphylococcal biofilm effects of human cathelicidin peptides. ACS Med Chem Lett. 2016;7:117–121. doi: 10.1021/acsmedchemlett.5b00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pletzer D, Hancock REW. Anti-biofilm peptides: Potential as broad-spectrum agents. J Bacteriol. 2016 doi: 10.1128/JB.00017-16. [DOI] [PMC free article] [PubMed] [Google Scholar]