Abstract
Depression in later life is a severe public health problem, associated with higher rates of mortality, suicide, and dementia. Effectiveness of treatment is limited by the failure to deconstruct the heterogeneity of the illness and because diagnostic criteria, pathophysiological models, and treatment algorithms for depression are primarily based on studies of younger adults even though symptoms of the illness and physiology of the patient change with age. Thus, understanding how aging interacts with depressive illness may elucidate endophenotypes of late life depression with different clinical manifestations and underlying mechanisms that can then be targeted with more personalized approaches to treatment. This paper proposes a model for the critical confluence between depression and frailty, a high-risk morbidity and mortality syndrome of later life. This model hypothesizes that characteristics of frailty in adults with late life depression represent the clinical manifestation of greater biological aging and their presence in the context of a depressive illness exposes elders to deleterious trajectories. Potential common biological substrates that may result in the manifestation of the depressed frail phenotype including mitochondrial functioning, dopaminergic neurotransmission, and inflammatory processes and implications for the assessment and treatment of adults with late life depression are discussed. As society continues to live longer, the preservation of the quality of these added years becomes paramount, and the combined impact of depression and frailty on the preservation of this quality warrants the attention of clinical researchers and physicians.
Keywords: depression, frailty, inflammation, dopamine, mitochondria
By the year 2030, there will be 65 million Americans over the age of 65, and older adults will represent 22% of the population. In the same time period, the number of Americans 85 and older will more than double. (www.cdc.gov/nchs/data/hus/hus10.pdf) Although people are living longer, the quality of these years is impacted by the increased prevalence of illness. Indeed, 25% of individuals over the age of 65 are considered in fair or poor health, so identifying the syndromes and diseases that result in the greatest decrements in the quality of these latter years is of the utmost importance for individuals, families, and society as a whole.
One of the most prevalent, disabling, and lethal disorders of late life is depression, as over 15% of community dwelling older adults report clinically significant depressive symptoms.1 Adults with late life depression (LLD) tend to respond poorly to available treatments and thus incur massive personal, social, and economic cost.2,3 Depression in later life is associated with increased risk falls, incident disability, and mortality.4,5 Part of the reason for these treatment failures in older adults may be the fact that diagnostic criteria, pathophysiological models and treatment trials of depression are primarily based on studies of younger adults, even though the symptoms and underlying physiology change with age.6–9 Thus, understanding how aging interacts with depressive illness in later life may elucidate different endophenotypes of LLD with different clinical manifestations and underlying mechanisms that can then be targeted with more personalized approaches to treatment.
Researchers have identified a constellation of clinical characteristics that include weakness, low activity level, slowed gait speed, fatigue, and unintentional weight loss that together define the syndrome of frailty, a physiological state of increased vulnerability associated with increased morbidity and mortality risk in later life. These declines across multiple physiologic systems10 develop incrementally, with weakness and slowness emerging early in the process and weight loss and fatigue representing a final pathway towards frailty.11 Frailty characteristics are prevalent in community dwelling elders, more often observed in women and in individuals who are socially isolated or who live in socioeconomically disadvantaged communities.12 Frailty is associated with falls, hospitalizations, disability, and death.10 Thus, as with depressive illness, the presence of frailty in later life predicts dire outcomes.
It should be noted that there are two prevalent definitions and models of frailty. The one described above (the Fried phenotypic model) defines frailty as a biologic syndrome of decreased reserve and resistance to stressors. Although associated with comorbidity and disability, the syndrome of frailty is separable from these constructs. This syndrome can be operationalized via specific clinical characteristics that represent dysfunction in multiple pathophysiological systems. By assessing these characteristics, researchers and clinicians can identify the underlying mechanism associated with these deficits, and target these mechanisms to improve the clinical trajectory for these frail individuals. The second model defines frailty as an accumulation of deficits, and as such a frailty index can be calculated based on the occurrence and severity of 70 different deficits.13 These deficits include medical diseases, deficits in activities of daily living, and physical signs based on clinical and neurological examinations. This model considers frailty in absolute and relative terms, and allows for the easy quantification of vulnerability to adverse outcomes (i.e. mortality, institutionalization, health services usage) in older individuals. Although both models have their merit, the Fried phenotypic model provides specificity both in terms of clinical presentation and mechanistic identification. As such, it is more applicable to a personalized medicine approach with our patients.
The critical confluence of depression and frailty was pointed out over a decade ago14 with the suggestion of a need for a multidisciplinary approach to the study of LLD. In particular, the overlap between LLD and frailty was noted, with symptoms common to both LLD (psychomotor slowing, weight loss, decreased activities, low energy) and frailty (low energy, decreased leisure activities, decreased walking speed, weight loss). Unfortunately, the study of LLD has remained the bailiwick of psychiatry and geropsychology, while the study of frailty has remained the focus of geriatricians and epidemiologists. Only recently have researchers begun to focus on the combined depressed frail phenotype as a high risk, vulnerable population of elders (Figure 1). The purpose of this paper is not to provide a standard review of the relationship between depression and frailty which has been done previously,15,16 but rather to propose a model for the depressed frail phenotype that elucidates the nature of the association and the potential biological pathways that explain this association, thereby setting a research agenda focused on the assessment and treatment of this high risk phenotype.
Figure 1. The Cycle of the Depressed Frail Phenotype.
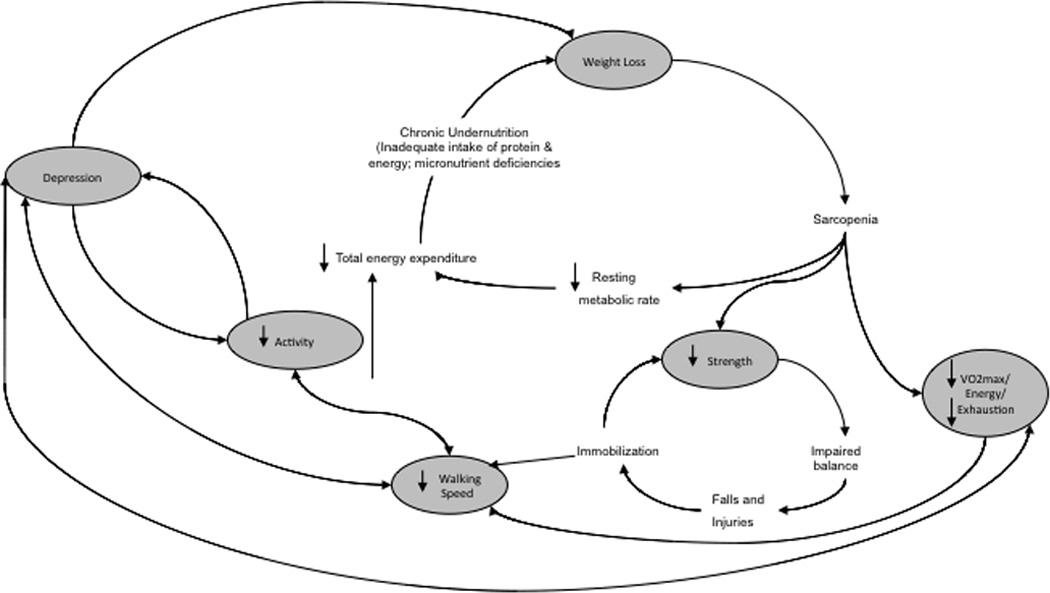
Note. Of note is the phenomenological overlap in symptoms/characteristics, most notably low activity levels, low energy, weight loss, and psychomotor slowing btween depression and frailty. Original frailty cycle adapted for the inclusion of depression; original frailty cycle published in Fried et al. 2001 in the J Gerontol A Biol Sci Med Sci.
The Relationship Between Frailty and Depression
There are many possible ways to model the relationship between frailty and depression (Figure 2 A–C). As stated by Katz in 2004,14 “…it is possible to make a case for each of these conditions as a cause, consequence, or comorbidity of the other. It is also possible to argue for their congruence.” Determining which model of the relationship between frailty and depression is most supported by the data has both etiological and treatment implications.
Figure 2. Conceptual Models of the Depressed Frail Phenotype.
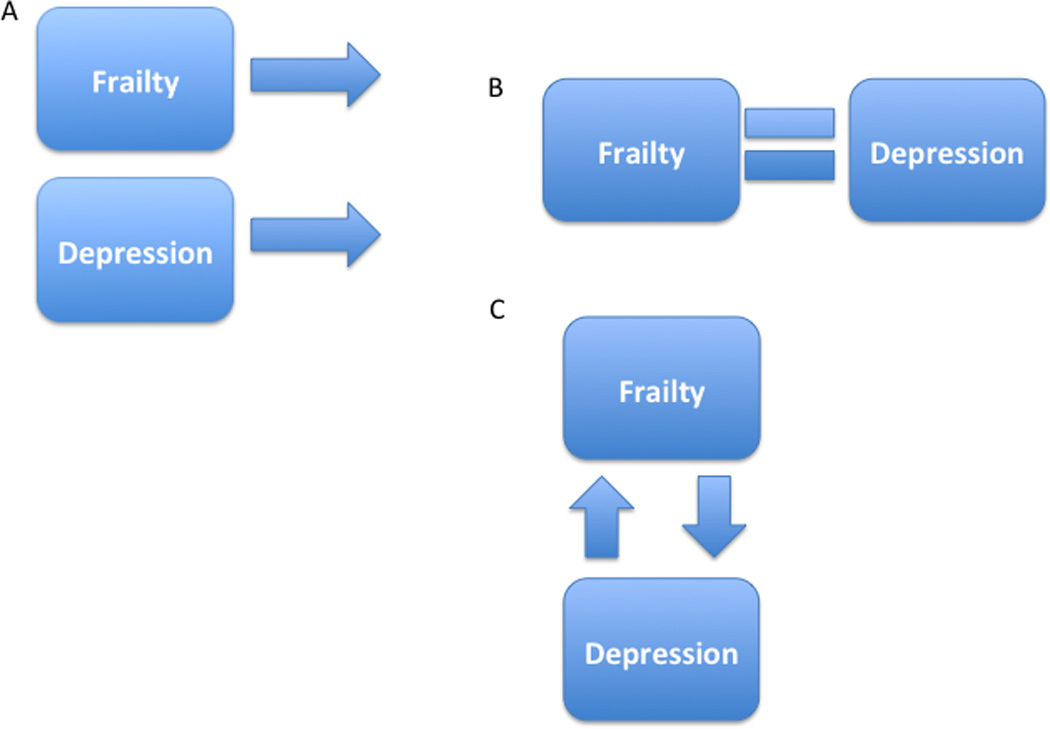
Note. The different conceptual models for the association between depression and frailty in later life are presented. Model A depicts the two conditions as unrelated to one another, but yet frequently coexisting given their increased prevalence in the elderly; Model B depicts frailty and depression as different manifestations of the same disorder as opposed to separable entities; Model C depicts frailty and depression as related and distinct constructs that are each a risk factor for the development of the other.
First, the two conditions may be unrelated to each other but frequently coexist because they both are more prevalent in the elderly, similar to cataracts and arthritis for instance (Figure 2A). If two conditions were simply serendipitously comorbid, appropriate treatment plans would consider each separately. The frailty characteristics would be treated with the nutritional counseling and/or exercise programs17,18 that have been shown to be successful in improving the physical abilities of frail or functionally limited older individuals. The depressive illness would be treated with antidepressant medications,19 psychotherapy (specifically cognitive behavioral therapy, interpersonal therapy, or problem solving therapy, all of which have been shown to be effective for the treatment of depression in late life),20 or, if very severe, electroconvulsive therapy (ECT).
Another possible model for the relationship between frailty and depression is that they are not two separate disorders but rather different manifestations of the same disorder (Figure 2B).21 For example, depression is common in individuals with neurodegenerative diseases, and there is now evidence to suggest that depression is an early symptom of one particular neurodegenerative disease, Huntington’s disease, rather than an independent diagnosis.22 This model appears possible given the preponderance of cross-sectional studies that highlight the co-occurrence or association between depression and frailty. Yet recently, researchers applying latent variable analytic methods to data from the Baltimore Epidemiologic Catchment Area Study23 concluded that late life depression and frailty although highly interrelated constructs are indeed distinct: Almost 70% of individuals classified as moderately depressed and 100% of individuals classified as severely depressed were considered frail. Although frailty and depression appear statistically distinct,24 clinicians may question the meaningfulness of this distinction given the level of association between frailty and depression. In fact, depressed elders have greater levels of impairment compared to non-depressed elders across each frailty characteristic, and, depending on the frailty characteristic present, a different clinical course:6 The presence of slow gait or fatigue in the context of depression is associated with substantially higher rates of mortality in later life. Thus, not only is frailty more prevalent in depressed elders but also this comorbidity is associated with substantially worse clinical trajectories.
Given that frailty and depression are highly related and distinct constructs,6,23,24 each may be a risk factor for the development of the other (Figure 2C). If so the ordering of presentation of the phenotype and the specific characteristics of frailty that denote increased risk may identify different underlying endophenotypes of LLD with different etiologies that have treatment implications. If the depressive disorder precedes the characteristics of frailty, for example one could hypothesize that a depressed older adult becomes increasingly sedentary and socially isolated, thereby resulting in increased weakness, decreased energy, and slowed gait as well as the perpetuation of the affective symptoms of depressive illness including sadness, anhedonia, and helplessness. Thus the successful treatment of the depression itself may result in increased behavioral and social activation thereby increasing physical and social activity levels, improving muscle tone and strength in one’s lower extremities, improving the elder’s overall energy levels and thus reducing frailty.25 Similarly, one could also imagine that the increased physical limitations or decreased independence accompanying specific characteristics of frailty would lead to a depressive disorder as a consequence of decreased social and physical engagement. In fact, recent findings support the bi-directionality of the depression-frailty relationship.26 Slow gait speed27 has been shown to be a risk factor for and a consequence of depression in later life. Using the longitudinal data from the Health Aging and Body Composition Study and controlling for demographic characteristics, medical comorbidity, inflammatory status, cognition, and body mass index, elevated depressive symptoms predict subsequent slow gait and slow gait predicts subsequent elevations in depression symptoms. Furthermore, the association between mortality and the trajectories of slow gait depend on the trajectories of depression.7 Similarly, slow gait and fatigue in the context of depression are associated with higher mortality6 and increased cardiovascular risk in later life.28 These studies are consistent with the conclusion that the relationship between frailty and depression is neither serendipitous nor represents different manifestations of the same disorder; rather there is a clinically relevant and bidirectional association between characteristics of frailty and depression in later life.
Biological Substrates of the Depressed Frail Phenotype
Given the most likely bidirectional relationship between depression and frailty in later life and the increased mortality risk associated with the depressed frail phenotype, the identification of common biological substrates of the phenotype has potential implications for treatment of LLD. Over the last fifteen years, research has identified physiological changes associated with depressive disorders that are consistent with increased biological aging (defined as systemic aging beyond that which is expected for one’s chronological age).29–32 The proliferation of mitochondrial DNA damage or dysfunction, mitochondrial dysfunction associated with oxidative stress both in the brain and the periphery, and increased proinflammatory cytokines such as tumor necrosis factor alpha (TNFα) or Interleukin 6 (IL6) are associated with a cycle resulting in cellular senescence and apoptosis,33 major depression and other psychiatric illnesses,34,35 and decreased physical activities, slowing and mobility deficits, and decrements in energy capacity in older adults.36–39 Furthermore, depression in late life is associated with greater decrements in physical functioning. It appears to be this association, specifically in those elders with depression and either slow gait or fatigue, which results in increased morbidity and mortality risk. Thus we hypothesize that the characteristics of frailty in adults with LLD represent the clinical manifestation of overall biological aging and their presence in the context of a depressive illness identifies a subset of elders at risk for deleterious trajectories.6,7
The identification of a common biological pathway that explains the interaction between characteristics of frailty and LLD is critical, as this biological pathway may be the target for the development of more personalized treatment interventions. For instance, fatigue becomes more prevalent with advanced age and in the context of LLD is associated with increased mortality. In younger adults with depression however fatigue presents as “mental fatigue”, conceptualized as the result of dysfunction in the reward-network and thus applying this model into later life ignores the physiological changes with age that may account for the increased prevalence of fatigue.40 In fact, in older adults with depression the presentation of fatigue is more somatic (“physical fatigue”),41,42 and these differences may have implications for treatment; patients’ mental fatigue but not physical fatigue improves with antidepressant treatment in patients with chronic fatigue syndrome.43 What follows are candidate pathways that may result in the manifestation of the depressed frail phenotype and specifically the two characteristics of frailty that in the context of LLD incur increased mortality: slow gait and fatigue (Figure 3).
Figure 3. Biological substrates of the Depressed Frail Phenotype.
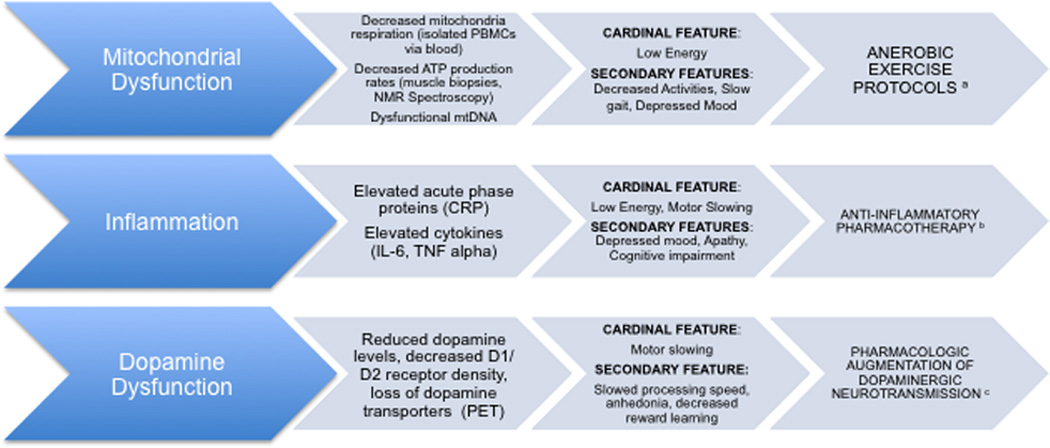
Note. The biological substrates of the Depressed Frail Phenotype are outlined above with their biological and clinical signatures and potential personalized approach to treatment. aAerobic exercise has been shown to improve muscle oxidative capacity and improved skeletal muscle mitochondrial content in older adults; bThe anti-inflammatory medication infliximab was shown to be a more effective antidepressant than placebo in treatment resistant adults with elevated CRP; cDopamine augmentation has been shown to improve cognition and motor and processing speed in both healthy adults and patients with Parkinson’s. PBMC, peripheral blood mononuclear cells; ATP, adenosine triphosphate, NMR, nuclear magnetic resonance; CRP, C-reactive protein; IL-6, Interleukin 6; TNF alpha, Tumor necrosis factor alpha; PET, positron emission tomography
Mitochondrial function
Mitochondria44 are responsible for the majority of energy production in the body, specifically from oxidative phosphorylation. Changes in mitochondrial function are apparent with age, including decreased energy conversion of O2 uptake into adenosine triphosphate (ATP) generation, and reduced mitochondrial respiratory chain activity.32,45 Decreased mitochondrial capacity (ATPmax) and respiration46 are associated with reduced activity levels and mobility37 and greater fatigability in later life.47 Mitochondrial dysfunction has also been identified in numerous neuroimmune and neurodegenerative diseases including multiple sclerosis and Parkinson’s disease, as well as depression.35,48–50 Muscle biopsies in younger adults with depression have shown decreased ATP production,51 and adults with depression had significantly impaired mitochondrial respiration in peripheral blood mononuclear cells compared with healthy controls, most strongly correlating with the symptom of fatigue.35 The deleterious cycle between diminished activity, mobility, and energy levels resembles the clinical presentation of some adults with LLD as well as those with the frailty syndrome.11,52 Mitochondrial bioenergetics may represent a common biological pathway that explains the interactive effect between depressive illness and specific characteristics of frailty on morbidity and mortality.
Dopamine
There is evidence that dopamine dysfunction plays a particularly important role in both cognitive and motor slowing. Researchers have observed53 a decrease in D2 receptor availability in both the caudate and the putamen in older adults. This decrease is associated with decreased motor speed (as assessed by finger tapping) and worsening in frontal functioning (as assessed by both the Stroop Color-Word task and the Wisconsin Card Sorting). Striatal dopamine levels in the elderly are only 40% of those in young adults,54 and D1/D2 receptor density as well as dopamine transporter expression decrease across the lifespan at a rate of approximately 10% per decade.39,55,56 Hickie and colleagues57 reported that decreased cerebral blood flow in the caudate nucleus in depressed adults was associated with psychomotor slowing on a choice reaction time task. Others have extended the association between decreased dopamine functioning in the basal ganglia and slowing beyond processing speed or finger tapping assessments to slow gait.56 Thus dopaminergic neurotransmission declines with age across multiple biomarkers (e.g., neurotransmitter levels, receptor density, DAT expression). It has been associated with both cognitive and motor slowing, and appears to underlie manifestations of the depressed frail phenotype including slowness and fatigue.
Inflammation
One shared biological characteristic between frailty and depression is a chronic inflammatory state. Low levels of pro-inflammatory markers such as IL-6 and TNFα are associated with low risk of frailty, while high levels of an acute phase protein such as C-reactive protein (CRP) are associated with greater risk of frailty in late life.58 Likewise, high levels of IL-6, TNFα and CRP are associated with major depressive disorders and treatment resistance to some antidepressant medications.9,59 Chronic inflammation has been associated with low physical activity, diabetes mellitus, metabolic syndrome, and smoking, all risk factors associated with both frailty and vascular depression in late life. The presence of these comorbid factors may result in elevated levels of markers of inflammation in adults in the depressed frail phenotype (or vice versa), thereby setting in motion a cycle of physical decline resulting in frailty, depressive illness, medical comorbidity, and a chronic inflammatory state. A chronic inflammatory state can indirectly result in deficits in specific characteristics of frailty including low energy, slowing, and weakness. Animal models have demonstrated that pro-inflammatory cytokines increase muscle protein degradation,60 and elevated inflammatory cytokines are associated with decreased muscle mass and strength in the elderly.31,61 Inflammatory processes also negatively affect the CNS, specifically dopaminergic function in the basal ganglia, which may result in depressive affect, fatigue, and cognitive and motor slowing.38 Similarly, increased reactive oxygen species production that results in mitochondrial dysfunction can result in or be a consequence of elevations in proinflammatory cytokines such IL6 and TNFα.49,62 Thus the inflammaging phenomenon can increase risk for each of the biological markers outlined.
Clinical Implications of the Depressed Frail Phenotype
The intersection between frailty and LLD has important implications for clinicians and researchers. Clinically, comprehensive assessment results in the formulation of more accurate treatment planning and can inform clinicians about the long-term trajectory of the patient. A comprehensive assessment, whether conducted by a psychiatrist, a neurologist, or a geriatrician, should include 1) the information necessary to make a DSM V diagnosis of a mood disorder and a measurement of severity, 2) the testing of cognitive function so that the specific cognitive deficit(s) experienced by a patient (episodic memory function, processing speed deficits, executive dysfunction) can be identified and the patient’s cognitive function properly classified (executive dysfunction, mild cognitive impairment, amnestic mild cognitive impairment), and 3) a review of vascular risk factors that let the clinician estimate the probability that the patient has ischemic cerebrovascular disease even in the absence of neurological findings and therefore may indicate the need for an MRI of the brain.
Given the data presented in this paper and the hypothesized model, an assessment of the characteristics of frailty should be added to this comprehensive strategy (Table 1). To include the assessment of frailty into the evaluation of the late life patient requires an assessment of gait speed, grip strength, level of fatigue, weight loss, and activity levels that can be accomplished in less than 10 minutes and requires very little extra measurement tools, with a dynamometer to assess grip strength the most elaborate device required for adequate assessment. Although walking speeds of .80 m/s is the criteria consistent with the frailty literature,63 a walking speed of less than 1 m/s has been shown to identify older adults at greater risk for both home-based and motor-based functional impairment and to denote elders at risk for poor outcome.7,64 An 18-foot space within a physicians office or hallway (measuring walking speed over 15 feet with space provided for initial acceleration) can provide a clinician with a measure of gait speed. If the assessment of grip strength via dynamometer is too cumbersome, the use of a measure such as the Short Physical Performance Battery (SPPB) with its domains of balance and chair stands will identify patients with musculature difficulties. The SPPB adequately identifies those individuals at risk for poor outcome, and a .5-point decrease in total SPPB score over time identifies those at increased risk for incident disability and falls.18,65 Similarly, the original frailty criteria used two items from the CES-D to identify those individuals with fatigue. Given the limited nature of these two items and the inherent confound of using items on a depression screen to identify significant fatigue, using other measures of fatigue and fatigability41,42,47,66 may provide physicians with a more useful assessment of the patients’ energy capacity, specifically distinguishing physical fatigue from an amotivational state. A brief new measure, the Pittsburgh Fatigability Scale,42 takes no more than five minutes to complete and provides the clinician with information on the patient’s energy capacity to complete everyday tasks. The presence of fatigue, motor slowing or decreased strength (cut-offs can be found in the original operationalization of the syndrome of frail)10 may indicate a physical therapy consult, implementation of behavioral activation strategies, or an exercise intervention that improves energy and lower extremity strength.
Table 1.
Assessment recommendations of frailty characteristics in older adults with depression.
| Clinical characteristics | Assessment measure | Notes |
|---|---|---|
| Overall physical function | Short Physical Performance Battery (SPPB) |
A performance measure of gait speed, balance, and lower extremity strength sensitive to meaningful change. |
| Gait speed | 18’ of straight walk way (such as a hallway or office corridor) |
Measure walking speed over 15’, with extras space used to eliminate acceleration and deceleration effects; speeds ≤ 1 m/s and especially .8 m/s denote frailty level deficits. |
| Strength | Handgrip strength measured by JAMAR dynamometer (setting II is recommended) |
The average score over typically three trials with an individual’s dominate hand (unless the dominant hand is weakened) with brief breaks in between is recommended, with handgrip strengths of ≤ 30 kg of force for men and ≤ 18 for women denoting frailty level deficits. |
| Activity Levels | 18-item Minnesota Leisure Time Physical Activity Questionnaire. |
Calculates kcal expended per week for each patient. Levels ≤ 383 kcal/week for men and 270 kcal/week for women denote frailty level deficits, but activity levels ≤ the recommended 1000 kcal/per week may denote individuals at risk for poor health outcome. |
| Fatigue/Fatigability | Pittsburgh Fatigability Scale | A 20-item self-report measure of physical (10-items) and mental (10- items) fatigability shown to be associated with worse physical performance and lower fitness. |
| Multidimensional Fatigue Inventory | A 20-item multidimensional scale validated in cancer-related research and subsequently in the general population shown to predict poor outcome. Its assesses five dimensions of fatigue including general fatigue, mental fatigue, physical fatigue, reduced activity, and reduced motivation |
|
| Weight Loss | Probe for significant weight loss over last calendar year |
Unintentional loss of 10 lbs or 5% of the previous year’s body weight denotes frailty level deficits. |
Note. Assessment recommendations for clinicians to capture characteristics of frailty or characteristics similar to those identified as part of the frailty phenotype. Recommended assessments of fatigue differ from those originally recommended by Fried and colleagues to avoid the phenomenological overlap between depression and the original fatigue assessments for frailty (two energy items taken from the Center for Epidemiologic Studies-Depression Scale). Frailty cut-scores are derived from original operationalization of the frailty phenotype, averaging across different body sizes for men and women.
Abbreviations: m/s = meters per second; kg = kilograms; kcal = kilocalories; lbs = pounds.
The rating of depression and the length of the antidepressant trial may also be impacted by assessing and understanding the presence of frailty in depressed elders. Given the overlap between symptoms of LLD (psychomotor slowing, weight loss, decreased activities, low energy) and frailty (low energy, decreased leisure activities, decreased walking speed, weight loss), one could extrapolate that focusing on more affective symptoms may better aid in the identification of depression in this population.67 This could potentially avert the over diagnosis of depression in a nondepressed, frail population. Such a decision, however, would be hypothetical and could result in potentially negative consequences given that LLD can commonly present without the classic sadness symptom.68 Research should first examine the relationship between the prevalence of depressive symptoms in a nonfrail, intermediate frail, and frail population before such recommendations can be made. Relatedly, there are no studies examining the effect of antidepressant medication treatment on characteristics of frailty such as gait speed, fatigue, and weight loss. These somatic symptoms may not show improvements within the time frame of a standard eight-week antidepressant trial, and as such may require longer, extended trials to note these somatic improvements. This hypothesis however would need to be tested, as there is currently no data to support such a recommendation.
For researchers, the consideration of frailty both impacts the inclusion/exclusion criteria of our studies and measures clinical characteristics that identify differing underlying pathophysiologies to target with novel interventions. Depression studies should no longer exclude frail patients nor should depressed patients be excluded from studies on frailty; to do so excludes those patients at greatest risk. The identification of the pathophysiology that underlies the depressed frail phenotype can lead to the development of targeted interventions aimed at altering the clinical course of this high-risk phenotype. For example, if mitochondrial dysfunction is associated with mobility deficits and fatigue in LLD, targeting the mitochondrial dysfunction may alter the deleterious trajectories of these patients. Aerobic exercise interventions have resulted in improved muscle oxidative capacity and up-regulated mitochondrial genes,22,23,30–32 and improved skeletal muscle mitochondrial content in sedentary older adults,69 and are effective for improving physical functioning in frail elders.17,18 Studies on the effectiveness of exercise as an antidepressant strategy in adults with LLD, however, report mixed results.70–72 This may be due to the heterogeneity of LLD. The antidepressant effect of exercise protocols may be more consistently evident if it is used targeting those adults with LLD and characteristics of frailty such as fatigue or mobility deficits for which exercise has proven beneficial, and may provide an understanding of the mechanism aerobic exercise targets to provide an antidepressant effect. Similarly, anti-inflammatory medications may provide a greater antidepressant effect in older adults when applied to those with comorbid characteristics of frailty. For instance, Infliximab was shown to improve depressive symptoms in depressed adults with elevated baseline levels of CRP.9 TNF is a viable cytokine to target as it has been associated with depressive symptoms,9 and TNF antagonists have been used to treat major depression in Crohn’s disease and reduce fatigue in cancer patients.73,74 Similarly, pharmacologic augmentation of dopaminergic neurotransmission may be beneficial to ameliorating slowing and depressive symptoms in adults within the depressed frail phenotype, specifically in the slowed, depressed subset of elders with LLD.75,76
Conclusions
In conclusion, this paper has identified the depressed frail phenotype as a high-risk mortality profile or phenotype in later life and proposed a model that this phenotype represents the clinical manifestation of greater biological aging. Potential common biological substrates that may result in the manifestation of the depressed frail phenotype, and implications for the assessment and treatment of adults with LLD are discussed. Gallo and colleagues seminal work68 on the identification of a “depression without sadness” in late life highlighted the heterogeneity of LLD. Treatment of LLD has had limited success in part due to this heterogeneity. As society continues to live longer, the preservation of the quality of these added years becomes paramount, and the combined impact of depression and frailty on the preservation of this quality warrants the attention of clinical researchers and physicians. In order to treat LLD more successfully, a more nuanced and comprehensive approach to assessment is needed to identify high-risk endophenotypes of LLD who respond poorly to standard antidepressant treatment approaches. In identifying these endophenotypes, the mechanism by which they develop can then be targeted to tailor treatment to improve outcome (i.e. personalized medicine). If the goal of the National Institute of Mental Health is to understand the mechanism of illness to personalize treatment implementation to improve response or to find new interventions to treat the illness in those who do not respond, then the manner in which we assess and conceptualize our patient population needs to transcend the restrictions inherent in current psychiatric concepts of diagnosis.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health (grant number K23 MH099097, PI Brown; T32 MH20004, PI Roose).
Dr. Rutherford reports receiving honoraria from Pfizer in the past year. This sponsor had no role in this current manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Drs. Brown, Yaffe, and Roose, Ms. Tandler, Ray, Chung, and Pott have nothing to disclose.
References
- 1.Meeks TW, Vahia IV, Lavretsky H, Kulkarni G, Jeste DV. A tune in "a minor" can "b major": a review of epidemiology, illness course, and public health implications of subthreshold depression in older adults. J Affect Disord. 2011 Mar;129(1–3):126–142. doi: 10.1016/j.jad.2010.09.015. S0165-0327(10)00583-5 [pii]10.1016/j.jad.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavretsky H, Lesser IM, Wohl M, Miller BL, Mehringer CM. Clinical and neuroradiologic features associated with chronicity in late-life depression. Am J Geriatr Psychiatry. 1999 Fall;7(4):309–316. [PubMed] [Google Scholar]
- 3.Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, Sackeim HA. Response inhibition predicts poor antidepressant treatment response in very old depressed patients. Am J Geriatr Psychiatry. 2007 Jul;15(7):553–563. doi: 10.1097/JGP.0b013e3180302513. 15/7/553 [pii]10.1097/JGP.0b013e3180302513. [DOI] [PubMed] [Google Scholar]
- 4.Kerse N, Flicker L, Pfaff JJ, et al. Falls, depression and antidepressants in later life: a large primary care appraisal. PLoS One. 2008;3(6):e2423. doi: 10.1371/journal.pone.0002423. 10.1371/journal.pone.0002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolinsky FD, Callahan CM, Fitzgerald JF, Johnson RJ. Changes in functional status and the risks of subsequent nursing home placement and death. J Gerontol. 1993 May;48(3):S94–S101. [PubMed] [Google Scholar]
- 6.Brown PJ, Roose SP, Fieo R, et al. Frailty and depression in older adults: a high-risk clinical population. Am J Geriatr Psychiatry. 2014 Nov;22(11):1083–1095. doi: 10.1016/j.jagp.2013.04.010. 10.1016/j.jagp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown PJ, Roose SP, Zhang J, et al. Inflammation, Depression, and Slow Gait: A High Mortality Phenotype in Later Life. J Gerontol A Biol Sci Med Sci. 2015 Sep 20; doi: 10.1093/gerona/glv156. 10.1093/gerona/glv156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003 Sep 1;54(5):566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- 9.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013 Jan;70(1):31–41. doi: 10.1001/2013.jamapsychiatry.4. 1356541 [pii]10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 11.Xue QL, Bandeen-Roche K, Varadhan R, Zhou J, Fried LP. Initial manifestations of frailty criteria and the development of frailty phenotype in the Women's Health and Aging Study II. J Gerontol A Biol Sci Med Sci. 2008 Sep;63(9):984–990. doi: 10.1093/gerona/63.9.984. 63/9/984 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004 Dec;59(12):1310–1317. doi: 10.1093/gerona/59.12.1310. [DOI] [PubMed] [Google Scholar]
- 13.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci. 2007 Jul;62(7):738–743. doi: 10.1093/gerona/62.7.738. [DOI] [PubMed] [Google Scholar]
- 14.Katz IR. Depression and frailty: the need for multidisciplinary research. Am J Geriatr Psychiatry. 2004 Jan-Feb;12(1):1–6. [PubMed] [Google Scholar]
- 15.Buigues C, Padilla-Sanchez C, Garrido JF, Navarro-Martinez R, Ruiz-Ros V, Cauli O. The relationship between depression and frailty syndrome: a systematic review. Aging Ment Health. 2015;19(9):762–772. doi: 10.1080/13607863.2014.967174. 10.1080/13607863.2014.967174. [DOI] [PubMed] [Google Scholar]
- 16.Mezuk B, Edwards L, Lohman M, Choi M, Lapane K. Depression and frailty in later life: a synthetic review. Int J Geriatr Psychiatry. 2011 Oct 7; doi: 10.1002/gps.2807. 10.1002/gps.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002 Dec;50(12):1921–1928. doi: 10.1046/j.1532-5415.2002.50601.x. jgs50601 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006 Nov;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. 61/11/1157 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Nelson JC, Delucchi K, Schneider LS. Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry. 2008 Jul;16(7):558–567. doi: 10.1097/JGP.0b013e3181693288. 10.1097/JGP.0b013e318169328816/7/558 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Huang AX, Delucchi K, Dunn LB, Nelson JC. A Systematic Review and Meta-analysis of Psychotherapy for Late-Life Depression. Am J Geriatr Psychiatry. 2014 Apr 23; doi: 10.1016/j.jagp.2014.04.003. 10.1016/j.jagp.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krogias C, Strassburger K, Eyding J, et al. Depression in patients with Huntington disease correlates with alterations of the brain stem raphe depicted by transcranial sonography. J Psychiatry Neurosci. 2011 May;36(3):187–194. doi: 10.1503/jpn.100067. 10.1503/jpn.100067 [pii] 10.1503/jpn.100067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mezuk B, Lohman M, Dumenci L, Lapane KL. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am J Geriatr Psychiatry. 2012 May 16; doi: 10.1016/j.jagp.2012.12.019. 10.1097/JGP.0b013e31824afd4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohman M, Dumenci L, Mezuk B. Depression and Frailty in Late Life: Evidence for a Common Vulnerability. J Gerontol B Psychol Sci Soc Sci. 2015 Jan 23; doi: 10.1093/geronb/gbu180. 10.1093/geronb/gbu180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lakey SL, LaCroix AZ, Gray SL, et al. Antidepressant use, depressive symptoms, and incident frailty in women aged 65 and older from the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2012 May;60(5):854–861. doi: 10.1111/j.1532-5415.2012.03940.x. 10.1111/j.1532-5415.2012.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders JB, Bremmer MA, Deeg DJ, Beekman AT. Do depressive symptoms and gait speed impairment predict each other's incidence? A 16-year prospective study in the community. J Am Geriatr Soc. 2012 Sep;60(9):1673–1680. doi: 10.1111/j.1532-5415.2012.04114.x. 10.1111/j.1532-5415.2012.04114.x. [DOI] [PubMed] [Google Scholar]
- 27.Demakakos P, Cooper R, Hamer M, de Oliveira C, Hardy R, Breeze E. The bidirectional association between depressive symptoms and gait speed: evidence from the English Longitudinal Study of Ageing (ELSA) PLoS One. 2013;8(7):e68632. doi: 10.1371/journal.pone.0068632. 10.1371/journal.pone.0068632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hajjar I, Yang F, Sorond F, et al. A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci. 2009 Sep;64(9):994–1001. doi: 10.1093/gerona/glp075. 10.1093/gerona/glp075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000 Aug;29(3–4):222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 30.Epel ES, Lithgow GJ. Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci. 2014 Jun;69(Suppl 1):S10–S16. doi: 10.1093/gerona/glu055. 10.1093/gerona/glu055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franceschi C, Capri M, Monti D, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007 Jan;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Tosato M, Zamboni V, Ferrini A, Cesari M. The aging process and potential interventions to extend life expectancy. Clin Interv Aging. 2007;2(3):401–412. [PMC free article] [PubMed] [Google Scholar]
- 33.Lindqvist D, Epel ES, Mellon SH, et al. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neuroscience and biobehavioral reviews. 2015 Aug;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai N, Chang S, Li Y, et al. Molecular signatures of major depression. Current biology : CB. 2015 May 4;25(9):1146–1156. doi: 10.1016/j.cub.2015.03.008. 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karabatsiakis A, Bock C, Salinas-Manrique J, et al. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Translational psychiatry. 2014;4:e397. doi: 10.1038/tp.2014.44. 10.1038/tp.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander NB, Taffet GE, Horne FM, et al. Bedside-to-Bench conference: research agenda for idiopathic fatigue and aging. J Am Geriatr Soc. 2010 May;58(5):967–975. doi: 10.1111/j.1532-5415.2010.02811.x. 10.1111/j.1532-5415.2010.02811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013 Apr;68(4):447–455. doi: 10.1093/gerona/gls196. 10.1093/gerona/gls196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Frontiers in neuroendocrinology. 2012 Aug;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Dyck CH, Avery RA, MacAvoy MG, et al. Striatal dopamine transporters correlate with simple reaction time in elderly subjects. Neurobiol Aging. 2008 Aug;29(8):1237–1246. doi: 10.1016/j.neurobiolaging.2007.02.012. 10.1016/j.neurobiolaging.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wise RA. Neurobiology of addiction. Current opinion in neurobiology. 1996 Apr;6(2):243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- 41.Eldadah BA. Fatigue and fatigability in older adults. PM & R : the journal of injury, function, and rehabilitation. 2010 May;2(5):406–413. doi: 10.1016/j.pmrj.2010.03.022. 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Glynn NW, Santanasto AJ, Simonsick EM, et al. The Pittsburgh Fatigability scale for older adults: development and validation. J Am Geriatr Soc. 2015 Jan;63(1):130–135. doi: 10.1111/jgs.13191. 10.1111/jgs.13191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnold LM, Blom TJ, Welge JA, Mariutto E, Heller A. A Randomized, Placebo-Controlled, Double-Blinded Trial of Duloxetine in the Treatment of General Fatigue in Patients With Chronic Fatigue Syndrome. Psychosomatics. 2014 Dec 16; doi: 10.1016/j.psym.2014.12.003. 10.1016/j.psym.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 44.Harman D. Origin and evolution of the free radical theory of aging: a brief personal history, 1954–2009. Biogerontology. 2009 Dec;10(6):773–781. doi: 10.1007/s10522-009-9234-2. 10.1007/s10522-009-9234-2. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Freire M, de Cabo R, Bernier M, et al. Reconsidering the Role of Mitochondria in Aging. J Gerontol A Biol Sci Med Sci. 2015 Nov;70(11):1334–1342. doi: 10.1093/gerona/glv070. 10.1093/gerona/glv070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJ. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. J Gerontol A Biol Sci Med Sci. 2015 Nov;70(11):1394–1399. doi: 10.1093/gerona/glu096. 10.1093/gerona/glu096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santanasto AJ, Glynn NW, Jubrias SA, et al. Skeletal Muscle Mitochondrial Function and Fatigability in Older Adults. J Gerontol A Biol Sci Med Sci. 2014 Aug 28; doi: 10.1093/gerona/glu134. 10.1093/gerona/glu134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amorini AM, Nociti V, Petzold A, et al. Serum lactate as a novel potential biomarker in multiple sclerosis. Biochim Biophys Acta. 2014 Jul;1842(7):1137–1143. doi: 10.1016/j.bbadis.2014.04.005. 10.1016/j.bbadis.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Morris G, Berk M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC medicine. 2015;13:68. doi: 10.1186/s12916-015-0310-y. 10.1186/s12916-015-0310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rango M, Bonifati C, Bresolin N. Parkinson's disease and brain mitochondrial dysfunction: a functional phosphorus magnetic resonance spectroscopy study. J Cereb Blood Flow Metab. 2006 Feb;26(2):283–290. doi: 10.1038/sj.jcbfm.9600192. 10.1038/sj.jcbfm.9600192. [DOI] [PubMed] [Google Scholar]
- 51.Gardner A, Johansson A, Wibom R, et al. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J Affect Disord. 2003 Sep;76(1–3):55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 52.Bogner HR, Morales KH, Reynolds CF, 3rd, Cary MS, Bruce ML. Course of Depression and Mortality Among Older Primary Care Patients. Am J Geriatr Psychiatry. 2011 Oct 12; doi: 10.1097/JGP.0b013e3182331104. 10.1097/JGP.0b013e3182331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998 Mar;155(3):344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 54.Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum. J Neurochem. 2003 Nov;87(3):574–585. doi: 10.1046/j.1471-4159.2003.02017.x. [DOI] [PubMed] [Google Scholar]
- 55.Troiano AR, Schulzer M, de la Fuente-Fernandez R, et al. Dopamine transporter PET in normal aging: dopamine transporter decline and its possible role in preservation of motor function. Synapse. 2010 Feb;64(2):146–151. doi: 10.1002/syn.20708. 10.1002/syn.20708. [DOI] [PubMed] [Google Scholar]
- 56.Volkow ND, Chang L, Wang GJ, et al. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001 Mar;158(3):377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 57.Hickie IB, Naismith SL, Ward PB, et al. Psychomotor slowing in older patients with major depression: Relationships with blood flow in the caudate nucleus and white matter lesions. Psychiatry Res. 2007 Aug 15;155(3):211–220. doi: 10.1016/j.pscychresns.2007.01.006. 10.1016/j.pscychresns.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 58.Walston JD, Matteini AM, Nievergelt C, et al. Inflammation and stress-related candidate genes, plasma interleukin-6 levels, and longevity in older adults. Exp Gerontol. 2009 May;44(5):350–355. doi: 10.1016/j.exger.2009.02.004. S0531-5565(09)00026-6 [pii] 10.1016/j.exger.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine Targets in the Brain: Impact on Neurotransmitters and Neurocircuits. Depress Anxiety. 2013 Mar 6; doi: 10.1002/da.22084. 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. Journal of applied physiology. 2005 Mar;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 61.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002 May;57(5):M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 62.Figueiredo-Pereira ME, Rockwell P, Schmidt-Glenewinkel T, Serrano P. Neuroinflammation and J2 prostaglandins: linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Frontiers in molecular neuroscience. 2014;7:104. doi: 10.3389/fnmol.2014.00104. 10.3389/fnmol.2014.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 64.Verghese J, Wang C, Holtzer R. Relationship of clinic-based gait speed measurement to limitations in community-based activities in older adults. Arch Phys Med Rehabil. 2011 May;92(5):844–846. doi: 10.1016/j.apmr.2010.12.030. 10.1016/j.apmr.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 66.Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014 Feb;62(2):347–351. doi: 10.1111/jgs.12638. 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cavanaugh S, Clark DC, Gibbons RD. Diagnosing depression in the hospitalized medically ill. Psychosomatics. 1983 Sep;24(9):809–815. doi: 10.1016/S0033-3182(83)73151-8. 10.1016/S0033-3182(83)73151-8. [DOI] [PubMed] [Google Scholar]
- 68.Gallo JJ, Anthony JC, Muthen BO. Age differences in the symptoms of depression: a latent trait analysis. J Gerontol. 1994 Nov;49(6):P251–P264. doi: 10.1093/geronj/49.6.p251. [DOI] [PubMed] [Google Scholar]
- 69.Broskey NT, Greggio C, Boss A, et al. Skeletal muscle mitochondria in the elderly: effects of physical fitness and exercise training. The Journal of clinical endocrinology and metabolism. 2014 May;99(5):1852–1861. doi: 10.1210/jc.2013-3983. 10.1210/jc.2013-3983. [DOI] [PubMed] [Google Scholar]
- 70.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999 Oct 25;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 71.Forsman AK, Schierenbeck I, Wahlbeck K. Psychosocial interventions for the prevention of depression in older adults: systematic review and meta-analysis. J Aging Health. 2011 Apr;23(3):387–416. doi: 10.1177/0898264310378041. 0898264310378041 [pii]10.1177/0898264310378041. [DOI] [PubMed] [Google Scholar]
- 72.Matthews MM, Hsu FC, Walkup MP, Barry LC, Patel KV, Blair SN. Depressive symptoms and physical performance in the lifestyle interventions and independence for elders pilot study. J Am Geriatr Soc. 2011 Mar;59(3):495–500. doi: 10.1111/j.1532-5415.2011.03319.x. 10.1111/j.1532-5415.2011.03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Persoons P, Vermeire S, Demyttenaere K, et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn's disease treatment with infliximab. Aliment Pharmacol Ther. 2005 Jul 15;22(2):101–110. doi: 10.1111/j.1365-2036.2005.02535.x. 10.1111/j.1365-2036.2005.02535.x. [DOI] [PubMed] [Google Scholar]
- 74.Monk JP, Phillips G, Waite R, et al. Assessment of tumor necrosis factor alpha blockade as an intervention to improve tolerability of dose-intensive chemotherapy in cancer patients. J Clin Oncol. 2006 Apr 20;24(12):1852–1859. doi: 10.1200/JCO.2005.04.2838. 10.1200/JCO.2005.04.2838. [DOI] [PubMed] [Google Scholar]
- 75.Floel A, Garraux G, Xu B, et al. Levodopa increases memory encoding and dopamine release in the striatum in the elderly. Neurobiol Aging. 2008 Feb;29(2):267–279. doi: 10.1016/j.neurobiolaging.2006.10.009. 10.1016/j.neurobiolaging.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lavretsky H, Reinlieb M, St Cyr N, Siddarth P, Ercoli LM, Senturk D. Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 2015 Jun;172(6):561–569. doi: 10.1176/appi.ajp.2014.14070889. 10.1176/appi.ajp.2014.14070889. [DOI] [PMC free article] [PubMed] [Google Scholar]


