Abstract
Background & Aims
Despite recent attention to differences in access to livers for transplantation, research has focused on patients already on the waitlist. We analyzed data from a large administrative database that represents the entire US population, as well as state Medicaid data, to identify factors associated with differences in access to waitlists for liver transplantation.
Methods
We performed a retrospective cohort study of transplant-eligible patients with end-stage liver disease using the HealthCore Integrated Research Database (2006–2014; n=16,824) and Medicaid data from 5 states (2002–2009; CA, FL, NY, OH, and PA; n=67,706). Transplant-eligible patients had decompensated cirrhosis, hepatocellular carcinoma (HCC), and/or liver synthetic dysfunction, based on validated ICD-9-based algorithms and data from laboratory studies. Placement on the waitlist was determined through linkage with the Organ Procurement and Transplantation Network database.
Results
In an unadjusted analysis of the HealthCore database, we found that 29% of patients with HCC were placed on the 2 year waitlist (95% CI, 25.4%–33.0%) compared to 11.9% of patients with stage 4 cirrhosis (ascites) (95% CI, 11.0%–12.9%) and 12.6% patients with stage 5 cirrhosis (ascites and variceal bleeding) (95% CI, 9.4%–15.2%). Among patients with each stage of cirrhosis, those with HCC were significantly more likely to be placed on the waitlist; adjusted sub-hazard ratios ranged from 1.7 (for patients with stage 5 cirrhosis and HCC vs those without HCC) to 5.8 (for patients with stage 1 cirrhosis with HCC those without HCC). Medicaid beneficiaries with HCC were also more likely to be placed on the transplant waitlist, compared to patients with decompensated cirrhosis, with a sub-hazard ratio of 2.34 (95% CI, 2.20–2.49). Local organ supply and waitlist-level demand were not associated with placement on the waitlist.
Conclusions
In an analysis of US healthcare databases, we found patients with HCC to be more likely to be placed on liver transplant waitlists than patients with decompensated cirrhosis. Previously reported reductions in access to transplant care for waitlisted patients with decompensated cirrhosis underestimate the magnitude of this difference.
Keywords: disparities, liver cancer, waitlist, UNOS
INTRODUCTION
In the US, the underlying principles for prioritizing patients are waitlisted for an organ transplant differs for each organ. Liver transplant (LT) uses an urgency-based ‘sickest-first’ model. Yet transplant centers are monitored and evaluated by post-transplant outcomes, thus transplant physicians must take into consideration potentially opposing goals: transplanting the sickest patients versus having high post-transplant survival.
This balancing act of transplanting the sickest first, maximizing post-transplant survival, and optimizing use of a scarce resource occurs in an environment where differences in access to LT care has received frequent attention. From the standpoint of waitlist prioritization (allocation), there have been efforts to revise policies that award Model for End-Stage Liver Disease (MELD) exception points for patients with hepatocellular carcinoma (HCC) to normalize their substantially higher transplant rates. Concurrently, geographic differences in waitlist mortality and transplant rates have been the impetus for redistricting efforts to revise organ distribution.1 However the evidence-base supporting these allocation and distribution inequalities is based on data from patients on the waitlist, and does not account for the broader population of patients with end-stage liver disease (ESLD) who could benefit from a transplant. As a result, it is unknown whether such waitlist differences may in fact be artifacts of variable waitlisting practices based on complications of liver disease. Conversely, it may also be that there are differences in access to LT care that are underestimated by waitlist data, insofar as disparities in access to the waitlist, coupled with differential transplant rates for certain conditions, lead to greater differences in LT care than are appreciated using only waitlist data.2
There are limited data evaluating access to LT from the perspective of access to the waitlist using data from a population-based cohort, rather than only including those who have already successfully navigated the medical system to gain access to the waitlist.2 Thus the aims of this study were to use data from two large, nationally representative administrative databases to evaluate factors associated with waitlisting among a geographically diverse sample of patients with ESLD.
METHODS
This was a retrospective cohort study using two nationally representative databases: HealthCore Integrated Research Database (HealthCore) from 2006–2014; and 5-state Medicaid (California, Florida, New York, Ohio, and Pennsylvania; includes 40% of all Medicaid beneficiaries from 2002–2009.3 HealthCore is a wholly owned subsidiary of Anthem, Inc. serving members in all 50 states. It is is a nationally-representative dataset of commercially insured patients, with longitudinal medical and pharmacy claims on 25 million patients.4,5 The study period for inclusion in HealthCore was 1/1/06-6/30/14. Medicaid patients were included because they represent 15% of LT recipients, and allowed us to verify our findings in a distinct population. The Medicaid population included all adult Medicaid beneficiaries from the five aforementioned states with an incident diagnosis of ESLD between 2/27/02-9/2/09.
Study sample and inclusion criteria
This study sample has been previously described.2 The primary inclusion criterion was the presence of clinical criteria meriting evaluation for waitlisting (decompensated cirrhosis, HCC, and/or hepatocellular dysfunction—collectively referred to as ESLD). These are the three main indications for LT per the American Association for the Study of Liver Diseases (AASLD).6,7
ESLD was defined using algorithms based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes that have been validated to have positive predictive values of >85%.8–12 All patients first required a diagnosis of cirrhosis: ≥1 inpatient or ≥2 outpatient ICD-9-CM codes for cirrhosis (571.2, 571.5).8–12 Decompensated cirrhosis was then defined by having ≥1 inpatient or ≥2 outpatient ICD-9-CM codes for a complication of portal hypertension (ascites, bleeding esophageal varices, ascites, and/or spontaneous bacterial peritonitis) occurring after the diagnosis of cirrhosis. HCC required a diagnosis of cirrhosis and ≥1 inpatient or ≥2 outpatient ICD-9-CM code for HCC (ICD-9-CM code: 155.0). In the subset of cirrhotic patients without HCC or a complication of portal hypertension, we used laboratory criteria to define hepatocellular dysfunction (calculated MELD score ≥15 and/or a total serum bilirubin ≥3mg/dL; only available for n=3,499, 20.8% of the HealthCore cohort; available lab data was based on capitation to a specific lab and not any particular demographic). The age cutoff for inclusion was 18–75 years at ESLD diagnosis—patients <18 years receive organs using different allocation criteria, while patients older than 75 represent only 0.1% of new waitlist additions during the study period.
Patients were excluded if they had an extra-hepatic malignancy, excluding non-melanoma skin cancer, diagnosed in the 365 days prior to the ESLD index date.13 Exclusion of active malignancies is consistent with AASLD guidelines that recommends “adequate” tumor-free survival prior to listing.14 Extra-hepatic malignancies were identified using validated ICD-9-based algorithms.13 The 365-day incident cancer-free period excluded patients with active cancers while including those with previously cured early-stage cancers.7 Secondary analyses excluded all patients with any extra-hepatic malignancy (excluding non-melanoma skin cancer) prior to the ESLD index date.
Study outcomes
The primary outcome was waitlisting for LT. Ascertainment of this outcome was achieved by linking the HealthCore and Medicaid datasets with data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, wait-listed candidates, and transplant recipients in the US, submitted by the members of OPTN. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor. Linkages were based on patient name, date of birth, and social security number. For HealthCore and Medicaid patients, waitlisting was assessed through March 5, 2015 and September 30, 2013, respectively.
The separate linkages of HealthCore and OPTN/UNOS data, and Medicaid and OPTN/UNOS data were performed only after obtaining regulatory approval from the University of Pennsylvania Institutional Review Board, HRSA, HealthCore, and the Centers for Medicare & Medicaid Services (CMS). All data linkages followed security protocols specified by HRSA, CMA, and HealthCore. The HealthCore-OPTN/UNOS linkage was performed by a programmer at HealthCore who had access to identifiers at HealthCore, and an identified OPTN/UNOS STAR file that was sent to HealthCore via a password-protected CD from UNOS. After performing the linkage, all direct patient health identifiers were removed, and the dataset was sent via a password-protected CD to Dr. Goldberg. An identified Medicaid dataset was housed on a secure, password-protected server at Penn, and the linkage was performed by a programmer in the Biostatistics and Analysis Center at the University of Pennsylvania who received an identified OPTN/UNOS STAR file via password-protected CD from UNOS. Both identified OPTN/UNOS STAR files were destroyed after the data linkages. Review of OPTN/UNOS codes of primary payer upon waitlisting correlated with the HealthCore and Medicaid linkages suggesting accuracy of the database merges.
Death was considered a competing risk to waitlisting (and LT in secondary analyses), rather than a censor, because death influences (in this case, nullifies) the probability that a patient will be waitlisted, and fitting death as a censoring event could bias the results.15,16
Study covariates
The HealthCore dataset contained longitudinal data on all outpatient and inpatient visits during the study period. Decompensated cirrhosis was defined using the ‘stages of cirrhosis’, a validated predictor of death related to liver disease: no ascites or varices (stage 1); non-bleeding varices (stage 2); bleeding varices (stage 3); ascites with or without non-bleeding varices (stage 4); and ascites and bleeding varices (stage 5).17 Patients with evidence of hepatocellular dysfunction, in the absence of complications of portal hypertension, were categorized as Stage 1 cirrhosis. HCC was also a time-varying covariate).
In Medicaid we only had access to data on baseline complications of liver disease (via ICD-9-CM codes), rather than longitudinal data like HealthCore, thus patients were categorized by the initial manifestation of ESLD (decompensated cirrhosis or HCC).
Statistical analysis
We fit competing risk Cox regression models with the outcome as waitlisting, and death the competing risk. Separate models were fit for HealthCore and Medicaid. We tested for an interaction between cirrhosis stage and HCC given that the decision to waitlist a patient with HCC may differentially be impacted by cirrhosis stage. Etiology of liver disease was categorized based on ICD-9-CM diagnoses during the index period (Supplementary Table 1). Demographic covariates included: gender; age at diagnosis of ESLD; medical co-morbidities using the modified Charlson co-morbidity index18 (HealthCore only); race/ethnicity (only available in Medicaid); and state of residence (Medicaid only due to HealthCore data use restrictions). We used a robust variance estimator to account for patient clustering within OPTN/UNOS region in analyses of HealthCore patients.
We evaluated additional covariates related to geographic access to care, aggregating data into donor service areas (DSAs): number of LT centers, organ supply (transplanted livers), organ demand (waitlisted patients), mean number of gastroenterologists per DSA19, and rural/urban status (modified rural urban commuting area (mRUCA) code20). Lastly, for HealthCore patients, distance to the closest LT center was modeled as a continuous and categorical variable, from the centroid of the zip code of residence to the closest LT center.
We conducted a sensitivity analysis in HealthCore patients with laboratory data to calculate the MELD score (bilirubin, creatinine, and INR) resulted on the same day, in addition to all HCC patients (with or without lab data). Cirrhosis stage and MELD score cutoff ≥15 were time-varying covariates. These models were fit including all HCC patients, independent of having lab data available, and secondarily excluded HCC patients without lab data to assess the interaction between waitlisting, HCC, and achieving a MELD score ≥15.
We fit competing risk models in HealthCore that modeled transplantation, rather than waitlisting, as the outcome. We conducted a sensitivity analysis of patients with an incident diagnosis of ESLD between 2006–2009 in HealthCore or Medicaid in order to restrict analyses in the two populations to overlapping time periods.
The study was approved by the Institutional Review Board at the University of Pennsylvania. Stata 14.0 (StataCorp, College Station, TX) was used for analyses.
RESULTS
There were 16,904,409 unique adult patients aged 18–75 years registered in the researchable HealthCore population from 2006–2014, of which 16,824 (0.10%) had transplant-eligible ESLD. Of the 40,290,278 unique adult patients aged 18–75 years enrolled in Medicaid in the five states from 2002–2009, 67,706 (0.17%) had ESLD. There were significant differences in all measured demographic and clinical characteristics of waitlisted versus non-waitlisted patients in both cohorts (Tables 1 and 2). Waitlisted patients in HealthCore were older than waitlisted Medicaid patients (median: 55.9, IQR: 50.4–61.3 versus 51.0, IQR: 45.0–56.0; p<0.001), and more likely to be male (68.0% versus 61.3%; p<0.001).
Table 1.
Baseline characteristics of HealthCore patients with ESLD by waitlisting status*
| Variable | Waitlisted N=1,779 |
Not waitlisted N=15,045 |
|---|---|---|
| Patient demographics | ||
| Age, median (IQR) | 55.9 (50.4–61.3) | 59.8 (52.5–67.5) |
| Male gender, N (%) | 1,210 (11.5) | 9,305 (88.5) |
| Female gender, N (%) | 569 (9.0) | 5,740 (91.0) |
| Patient clinical characteristics* | ||
| Etiology of liver disease | ||
| HCV only | 423 (16.2) | 2,189 (83.8) |
| HCV + EtOH | 563 (22.2) | 1,970 (77.8) |
| EtOH only | 437 (6.4) | 6,361 (93.6) |
| HBV | 36 (13.2) | 237 (86.8) |
| PSC | 68 (24.4) | 211 (75.6) |
| PBC | 46 (12.1) | 33 (87.9) |
| Autoimmune hepatitis | 22 (14.6) | 129 (85.4) |
| Possible NASH | 63 (3.4) | 1,802 (96.6 |
| All other etiologies | 121 (6.3) | 1,812 (93.7) |
| Geographic variables‡ | ||
| Gastroenterologists/DSA† | 385 (191–677) | 318 (191–677) |
| Liver transplant centers/DSA | 2 (2–5) | 2 (2–5) |
| Distance to closest liver transplant center in miles, median (IQR) | 27.1 (12.2–60.5) | 31.5 (12.8–71.5) |
| Modified rural/urban classification | ||
| Metropolitan, >1 million in county and largest city | 515 (12.4) | 3,649 (87.6) |
| Metropolitan, >1 million in county and <1 million in largest city | 569 (10.7) | 4,733 (89.3) |
| Metropolitan, 250,000–1,000,000 in county | 331 (9.7) | 3,081 (90.3) |
| Metropolitan, <250,000 in county | 113 (9.3) | 1,099 (90.7) |
| Rural | 251 (9.2) | 2,483 (90.8) |
p<0.05 for all comparisons of waitlisted vs non-waitlisted patients
Table 2.
Baseline characteristics of Medicaid patients with ESLD by waitlisting status*
| Variable | Waitlisted N=6,403 |
Not waitlisted N=61,303 |
|---|---|---|
| Patient demographics | ||
| Age, median (IQR) | 51 (45–56) | 53 (47–61) |
| Male gender, N (%) | 3,922 (9.0) | 39,569 (91.0) |
| Female gender, N (%) | 2,481 (10.3) | 21,729 (89.8) |
| Race/ethnicity | ||
| White, non-Hispanic | 2,847 (8.4) | 31,027 (91.6) |
| Black, non-Hispanic | 531 (5.8) | 8,658 (94.2) |
| Hispanic only specified | 1,796 (13.0) | 12,007 (87.0) |
| Hispanic + other race | 446 (12.3) | 3,195 (87.8) |
| Asian or Pacific Islander | 314 (17.3) | 1,497 (82.7) |
| Native Hawaiian or Pacific Islander | 186 (11.8) | 1,388 (88.2) |
| American Indian/Alaskan native | 55 (9.2) | 541 (90.8) |
| >1 race specified | 15 (10.1) | 133 (89.9) |
| Unknown | 213 (6.9) | 2,857 (93.1) |
| Patient clinical characteristics* | ||
| Etiology of liver disease | ||
| HCV | 3,419 (13.0) | 22,921 (87.0) |
| EtOH only | 1,251 (5.4) | 21,995 (94.6) |
| HBV | 441 (13.8) | 2,764 (86.2) |
| PSC | 36 (23.1) | 120 (76.9) |
| PBC | 242 (21.4) | 889 (78.6) |
| Other/missing | 1,014 (7.4) | 12,614 (92.6) |
| Indication for transplant waitlisting | ||
| Decompensated cirrhosis | 4,954 (8.2) | 55,448 (91.8) |
| HCC | 1,449 (19.8) | 5,855 (80.2) |
| Geographic variables‡ | ||
| Gastroenterologists/DSA† | 571 (216–592) | 447 (200–592) |
| Liver transplant centers/DSA | 3 (2–6) | 3 (2–6) |
p<0.05 for all comparisons of waitlisted vs non-waitlisted patients
Unadjusted waitlisting rates in HealthCore based on stage of liver disease
There were significantly higher unadjusted waitlisting rates in patients with incident HCC compared to patients with any stage of cirrhosis (p<0.001). The 2-year cumulative waitlisting rates from the time of diagnosis was 32.6% (95% CI: 29.44–36.2%) in patients with HCC (measured from incident data of HCC diagnosis, independent of cirrhosis stage), compared to 14.1% (95% CI: 13.0–15.3%) and 15.8% (95% CI: 10.8–22.7%) in non-HCC patients with stages 4 and 5 cirrhosis at baseline, respectively.
Association between cirrhosis stage and hepatocellular carcinoma on waitlisting
HealthCore
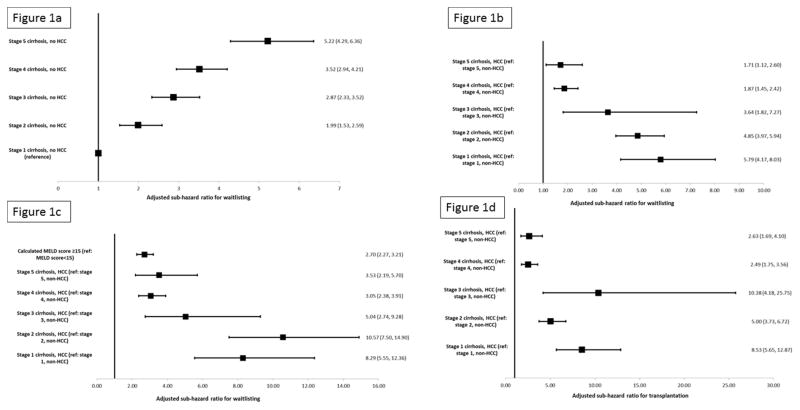
There was a significant interaction between cirrhosis stage and HCC (p<0.001) in multivariable competing risk models. Increasing cirrhosis stage in non-HCC patients was associated with significantly increased waitlisting (Figure 1a). However, at every cirrhosis stage, patients with HCC were significantly more likely to be waitlisted compared to non-HCC patients with a similar stage of cirrhosis (Figure 1b). This waitlisting advantage was most pronounced in patients with stages 1–3 of cirrhosis, but was seen even among patients with the most advanced stages of cirrhosis (stages 4 and 5; Figure 1b). These HCC vs non-HCC differences were even more pronounced when considering the outcome of transplantation, which accounts for differences in waitlisting and LT once waitlisted (Figure 1c). Inclusion of year of ESLD diagnosis did not significantly alter the primary findings of the association between cirrhosis stage and HCC on waitlisting.
Figure 1.
- Figure 1a: Adjusted sub-hazard ratios for waitlisting for in HealthCore patients based on time-varying cirrhosis stage in non-HCC patients
- Figure 1b: Adjusted sub-hazard ratios for waitlisting for HealthCore patients adjusted for time-varying cirrhosis stage and hepatocellular carcinoma
-
Figure 1c: Adjusted sub-hazard ratios for waitlisting for HealthCore patients adjusted for time-varying cirrhosis stage, hepatocellular carcinoma, and calculated MELD score*
-
i*Footnote: Sample restricted to all patients with hepatocellular carcinoma and patients with decompensated cirrhosis and laboratory data to calculate the MELD score
-
i
- Figure 1d: Adjusted sub-hazard ratios for liver transplantation for HealthCore patients adjusted for time-varying cirrhosis stage and hepatocellular carcinoma
In patients with decompensated cirrhosis and laboratory MELD data (n= 3,062, 18.2% of entire cohort) or HCC (with or without laboratory data), the highest waitlisting rates were in patients with HCC. In multivariable models, there was significantly increased risk of waitlisting, adjusting for cirrhosis stage, for patients with a calculated MELD score ≥15 or HCC. The risk of waitlisting for HCC was higher than MELD score ≥15, with significantly increased subhazards for waitlisting for patients with HCC at every cirrhosis stage (Figure 1c). Furthermore, in models restricted only to patients with laboratory data (with or without HCC), the risks of waitlisting for HCC patients did not differ based on MELD score ≥15 vs <15. As a result, when stratified by MELD score, the increased risks of waitlisting for patients with HCC compared to non-HCC patients was less pronounced at MELD scores ≥15. The primary results of increased waitlisting by cirrhosis stage and HCC were unchanged with exclusion of patients whose only inclusion criterion for ESLD was laboratory evidence of liver synthetic dysfunction.
Medicaid
Patients whose initial manifestation of ESLD was HCC were more than 2.5 times as likely to be placed on a LT waitlist compared to patients who initial complication of ESLD was decompensated cirrhosis (sub-hazard ratio (SHR): 2.34, 95% CI: 2.20–2.49; Table 4).
Table 4.
Multivariable competing risk model evaluating risk factors for waitlisting in Medicaid population*
| Variable | Sub-hazard ratio | P-value |
|---|---|---|
| Patient demographics and clinical characteristics | ||
| Hepatocellular carcinoma (ref: decompensated cirrhosis) | 2.34 (2.20–2.49) | <0.001 |
| Age at end-stage liver disease† | 0.96 (0.96–0.97) | <0.001 |
| Female gender | 1.25 (1.19–1.32) | <0.001 |
| Race/ethnicity | <0.001 | |
| White | Reference | |
| Black, non-Hispanic | 0.63 (0.57–0.69) | |
| Hispanic only | 1.54 (1.45–1.64) | |
| Hispanic + other race | 1.33 (1.19–1.49) | |
| Asian or Pacific Islander | 1.80 (1.59–2.03) | |
| American Indian/Alaskan native | 1.01 (0.78–1.32) | |
| Native Hawaiian or Pacific Islander | 1.21 (1.05–1.40) | |
| Etiology of liver disease‡ | <0.001 | |
| Hepatitis C | Reference | |
| Alcohol-induced liver disease | 0.45 (0.42–0.47) | |
| Hepatitis B | 0.93 (0.84–1.03) | |
| Primary sclerosing cholangitis | 1.63 (1.43–1.86) | |
| Primary biliary cirrhosis | 1.76 (1.29–2.40) | |
| Geographic variables | ||
| Gastroenterologists in DSA** | 0.96 (0.94–0.98) | <0.001 |
| Number of LT centers in DSA | 1.02 (0.99–1.05) | 0.16 |
| State | <0.001 | |
| California | Reference | |
| Florida | 0.66 (0.60–0.72) | |
| New York | 1.15 (1.04–1.26) | |
| Ohio | 0.69 (0.62–0.77) | |
| Pennsylvania | 1.24 (1.11–1.38) | |
Abbreviations: DSA=donor service area; LT=liver transplant
Model also included covariates for etiology of liver disease (Figures 2c) and initial manifestation of ESLD (decompensated cirrhosis or HCC; see text). Covariates for liver supply and demand in a patient’s donor service area, and rural/urban status were not included in final models as they were not significant in multivariable models (p>0.1 for each covariate). Data on the Charlson co-morbidity index not available in the Medicaid dataset analyzed for this study
Per 1-year increase in age at entry into cohort
Only a single diagnosis was available in Medicaid data
Reported as sub-hazard of waitlisting for every 1 unit increase in 100 board-certified gastroenterologists in the donor service area where a patient resides.
Other factors associated with waitlisting for liver transplantation
Several other factors were associated with waitlisting in both HealthCore and Medicaid. In both cohorts, primary sclerosing cholangitis was associated with increased waitlisting, while increasing age and alcohol-induced liver disease were inversely associated with waitlisting (Tables 3 and 4). In Medicaid, for which race/ethnicity data were available, Asian or Pacific Islander race, or Hispanic ethnicity (compared to white, non-Hispanics) was associated with increased waitlisting, while black race was associated with decreased waitlisting (Table 4). Although the magnitude of the differences in waitlisting based on race/ethnicity was not uniform across the 5 Medicaid states, the direction of the differences were uniform in all 5 states: compared to white patients, in all 5 states, black patients were significantly less likely to be waitlisted, while Hispanic and Asian patients were significantly more likely to be waitlist (p<0.05 for all within state, among-race comparisons).
Table 3.
Other variables significantly associated with waitlisting and transplantation in HealthCore in competing risk Cox regression models*
| Variable | Waitlisting as outcome | Transplantation as outcome | ||
|---|---|---|---|---|
|
| ||||
| Sub-hazard ratio | P-value | Sub-hazard ratio | P-value | |
| Patient factors | ||||
| Age at end-stage liver disease† | 0.97 (0.96–0.98) | <0.001 | 0.97 (0.96–0.97) | <0.001 |
| Female gender | 0.88 (0.81–0.95) | <0.001 | 0.75 (0.67–0.85) | <0.001 |
| Baseline Charlson co-morbidity index score‡ | <0.001 | <0.001 | ||
| 0 | Reference | Reference | ||
| 1 | 0.86 (0.73–1.02) | 0.85 (0.67–1.08) | ||
| 2 | 0.84 (0.68–1.04) | 0.88 (0.62–1.26) | ||
| 3 | 1.37 (1.15–1.63) | 0.88 (0.62–1.26) | ||
| 4 | 1.21 (1.01–1.44) | 1.42 (1.05–1.92) | ||
| 5 | 1.19 (0.95–1.48) | 1.33 (1.19–1.49) | ||
| 6 | 1.37 (1.18–1.59) | 1.11 (0.81–1.53) | ||
| ≥7 | 0.74 (0.56–0.98) | 0.92 (0.60–1.40) | ||
| Etiology of liver disease | <0.001 | <0.001 | ||
| Hepatitis C only | Reference | Reference | ||
| Hepatitis C + alcohol-induced liver disease | 1.06 (0.86–1.30) | 1.08 (0.89–1.29) | ||
| Alcohol-induced liver disease only | 0.39 (0.32–0.48) | 0.42 (0.35–0.53) | ||
| Hepatitis B | 0.65 (0.36–1.18) | 0.86 (0.53–1.40) | ||
| Primary sclerosing cholangitis | 1.30 (1.04–1.63) | 1.31 (0.84–2.05) | ||
| Primary biliary cirrhosis | 0.83 (0.60–1.14) | 0.88 (0.67–1.15) | ||
| Autoimmune hepatitis | 1.03 (0.59–1.82) | 1.54 (0.72–3.29) | ||
| Possible NASH | 0.26 (0.22–0.32) | 0.27 (0.20–0.36) | ||
| Other etiologies | 0.51 (0.39–0.68) | 0.48 (0.35–0.66) | ||
| Geographic factors | ||||
| Gastroenterologists in DSA** | 1.00 (0.99–1.00) | 0.13 | 1.001 (1.00–1.001) | 0.005 |
| Number of LT centers in DSA | 0.95 (0.91–0.98) | 0.005 | 0.91 (0.86–0.97) | 0.003 |
| Median liver supply in DSA** | 1.00 (0.99–1.01) | 0.16 | 1.01 (1.00–1.01) | 0.001 |
| Median waitlist liver demand in DSA‡‡ | 1.00 (0.99–1.02) | 0.70 | 0.97 (0.96–0.99) | |
| Distance to closest LT center | <0.001 | 0.66 | ||
| 0–20.0 miles | Reference | Reference | ||
| 20.1–40.0 miles | 1.05 (0.90–1.22) | 1.02 (0.80–1.30) | ||
| 40.1–70.0 miles | 0.90 (0.76–1.05) | 0.96 (0.77–1.19) | ||
| 70.1–150.0 miles | 0.85 (0.75–0.96) | 0.86 (0.65–1.14) | ||
| >150.0 miles | 0.84 (0.65–1.09) | 0.83 (0.61–1.13) | ||
Abbreviations: DSA=donor service area; LT=liver transplant
Model also included covariates for etiology of liver disease (Figures 2a), time-varying cirrhosis stage and presence of HCC (Figure 2b), and distance from patient’s home to closest LT center (Figure 2c).
Per 1-year increase in age at entry into cohort
Modified Charlson co-morbidity index, excluding points awarded for liver disease
Reported as sub-hazard of waitlisting for every increase in 100 board-certified gastroenterologists in the donor service area where a patient resides.
Per increase in one deceased-liver donor per-million population in the patient’s respective donor service area, measured as the median monthly liver supply since diagnosis of end-stage liver disease for a given patient
Per increase in 50 waitlisted paGents at all liver transplant centers in the patient’s respective donor service area, measured as the median monthly demand since diagnosis of end-stage liver disease for a given patient
The sensitivity analysis of factors associated with waitlisting in HealthCore and Medicaid in patients with incident diagnoses of ESLD between 2006 and 2009 were similar to the primary analyses, with the magnitude and direction of the subhazard for waitlisting for patients with HCC being unchanged in both patient cohorts.
Transplantation rates based on complications of cirrhosis and HCC status
HealthCore
In multivariable models, patients with HCC were significantly more likely to be waitlisted at every cirrhosis stage (Figure 1d). Although this risk was attenuated at the highest cirrhosis stages, patients with HCC and stage 4 or 5 cirrhosis had a 2.5 times increased risk of transplantation compared to non-HCC patients with a similar cirrhosis stage.
Medicaid
The unadjusted 1-, 3-, and 5-year transplant rates were significantly higher for patients with HCC compared to those with decompensated cirrhosis: 6.4% (95% CI: 5.7–7.2%), 17.3% (16.0–18.8%), and 24.2% (22.5–26.1%) in patients with HCC, respectively, compared to 2.3% (2.1–2.4%), 5.1% (4.8–5.4%), and 7.1% (6.8–7.5%) in Medicaid patients with decompensated cirrhosis.
DISCUSSION
This analysis of greater than 80,000 patients with ESLD from two nationally representative databases highlights several factors associated with access to the LT waitlist. These data are distinct from previous studies evaluating access to LT care in that we utilized a population-based cohort to provide new insights into differential access to the waitlist based on diagnoses, medical co-morbidities, demographics, and geography.
We consistently demonstrate significantly higher rates of waitlisting for patients with HCC, independent of cirrhosis stage and other comorbidities. From the perspective of the larger ESLD population, the higher transplant rates for patients with HCC are even greater than appreciated from waitlist data alone because the higher waitlisting rates for patients with HCC compound higher transplant rates once waitlisted. These data are important when viewed from the standpoint of how to best ration the scarce resource of donor livers. Unlike patients with decompensated cirrhosis for which LT is the only cure, patients with HCC have other curative options. The overall survival of patients with HCC undergoing hepatic resection and/or radiofrequency ablation is not different than LT, although recurrence rates are substantially higher. Post-transplant survival for patients with HCC is similar to other indications (i.e., decompensated cirrhosis from HCV and alcoholic liver disease).21 However, because current loco-regional treatments (i.e., trans-arterial chemo-embolization) can substantially slow the growth of HCC, recent data suggest that LT confers little-to-no net survival benefit over a 5-year time horizon in the 80% of transplant recipients with HCC and preserved liver synthetic function.22
These data do not allow us to determine specifically why patients with HCC are more likely to be waitlisted. Although it may be that they are deemed more clinically stable, have fewer medical co-morbidities, and are less frail, this does not translate into superior post-transplant outcomes.22 More likely is the waitlist prioritization awarded to patients with HCC within Milan criteria who receive MELD exception points leading to preferential transplantation.21,23 Our hypothesis that waitlist priority drives these waitlist differences is strengthened by the finding that calculated MELD score ≥15 was associated with increased waitlisting, and that the increased risk of waitlisting with a MELD score of ≥15 was only for patients with decompensated cirrhosis. These findings can trace back to 2005, when allocation rules restricted access to livers in a patient’s donor service area if the patient has a MELD score <15 until the organ is first offered to all patients in the OPTN/UNOS region with a MELD score ≥15. As a result, this MELD score cutoff has been viewed as the threshold to waitlist a patient, because of waitlist prioritization and probability of being transplanted, rather than survival benefit or benefit to the patient of being waitlisted. In fact, studies have demonstrated a survival benefit with LT for patients with MELD scores <15 when viewed over a time horizon beyond one year24 (the MELD 15 cutoff to define survival benefit was based only on benefit over a one-year time horizon 25). Thus many patients with a MELD score <15 without HCC who were not waitlisted could have benefited from a transplant, and in many cases, to a larger degree than those whose only indication for transplant was HCC. Given that many patients with HCC have other treatment options that result in similar overall 5-year survival compared to LT, in combination with recent data suggesting that vast majority of transplant recipients with HCC derive little to no survival benefit, these data speak to the critical need to readdress current liver allocation policies for patients with HCC, while also identifying mechanisms to provide improved access to the waitlist for patients with decompensated cirrhosis who benefit the most.22
In addition to manifestations of ESLD influencing access to the waitlist, the underlying etiology of liver disease was a key factor in being waitlisted. The lower waitlisting in patients with alcohol-induced liver disease mirrors data from a single, large Veterans Health Administration medical center, and may reflect ongoing substance abuse or psychosocial contraindications that could not be ascertained in administrative data, or provider-level biases at the level of the referring physician and/or transplant center.26 In contrast, patients with primary sclerosing cholangitis had much higher rates of waitlisting in both cohorts which may reflect a similar phenomenon as seen in HCC (frequent receipt of MELD exception points), but also explained by preferential utilization of living donor LT in patients with PSC, or their limited number of medical co-morbidities.27
These conclusions are supported by several strengths. First, we had long-term follow-up of more than 80,000 patients with ESLD in two distinct, nationally representative administrative datasets with laboratory data on 20% of the HealthCore sample to complement the administrative data. Second, despite slight differences in the available data in the two datasets, the results were consistent, especially for the analyses on access to waitlisting for patients with versus without HCC, which provides greater confidence in the reliability of data than could be ascertained from a study using only one dataset. Third, the overall cohort was diverse with respect to socioeconomic status by including commercially insured patients and those receiving healthcare through state-sponsored Medicaid programs.
The study also has limitations. First, laboratory data were only available on a subset (≈20%) of HealthCore patients, and no Medicaid patients. Although this may have led to inclusion of patients with preserved liver synthetic function and low MELD scores, nearly 90% of the cohort had decompensated cirrhosis and portal hypertension, which is an indication for waitlisting per AASLD guidelines. Furthermore, the findings of increased waitlisting with HCC, adjusting for MELD score support the robustness of our findings. Second, we could not identify all medical co-morbidities that could impact a patient’s eligibility for waitlisting, such as extreme obesity or severe co-morbid conditions. Although this likely resulted in an overestimate of the number of transplant-eligible patients, this potential unmeasured confounding would not be expected to explain our between region, among-diagnosis, or among-indication (HCC vs non-HCC) comparisons, due to the robustness and strength of the associations between certain factors (notably HCC) and waitlisting. Additionally, we did adjust for baseline Charlson co-morbidity index. Third, we could not stage patients’ HCC (i.e., Milan or UCSF criteria). If anything, the rates of waitlisting for HCC were underestimated because our data would have included patients with tumors that were too small and not within Milan criteria (thus not eligible for standardized MELD exception points) or too large and a contraindication to LT. As a result, the true disparities in waitlisting rates between patients with HCC versus decompensated cirrhosis are likely much greater than the already large differences we report. Lastly, we could not determine whether a patient was referred for a transplant waitlist but was declined, or if a patient was evaluated and/or waitlisted as an inpatient vs outpatient.
In conclusion, the development of HCC greatly increases the probability that a patient with cirrhosis will be waitlisted for a LT. Further work is needed to determine whether these differences are attributable to variable referral practices at the level of the primary physician caring for these patients, or differences in decision-making at the level of the LT center. Nevertheless, the dramatic differences in waitlisting for patients with HCC, who are already at a significant advantage once waitlisted, raises the question of whether allocation policies need to consider not simply the impact to patients on the waitlist, but also patients who may be referred and/or evaluated for transplantation. Such considerations are critical in order to provide equitable access to a lifesaving LT, and to ensure that the design of the current transplant system does not systematically limit access for patients based on demographics, medical conditions, or geography.
Supplementary Material
Acknowledgments
Grant Support
Dr. Goldberg was funded by the National Institutes of Health (K08-DK098272).
This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C.
None of the authors have any potential conflicts of interest as it pertains to this manuscript. Dr. Goldberg was funded by the National Institutes of Health (K08-DK098272). This work was supported in part by Health Resources and Services Administration contract 234-2005-37011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Funding sources: Dr. Goldberg is funded by the National Institutes of Health (K08 DK098272 to Dr. Goldberg).
Abbreviations
- LT
Liver transplant
- MELD
Model for End-Stage Liver Disease
- HCC
Hepatocellular carcinoma
- ESLD
End-stage liver disease
- AASLD
American Association for the Study of Liver Diseases
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- HRSA
Health Resources and Services Administration
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
Footnotes
Conflicts of interest: None of the authors have any relevant financial, professional, and/or personal conflicts of interest with respect to this manuscript.
Writing Assistance: None
Author Contributions
David Goldberg: Study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, drafting and critical revision of the manuscript, statistical analysis
Benjamin French: Study concept and design, analysis and interpretation of the data, critical revision of the manuscript, statistical analysis
Craig Newcomb: Analysis and interpretation of the data, statistical analysis
Qing Liu: Analysis and interpretation of the data, statistical analysis
Gurvaneet Sahota: Study concept and design, acquisition of data, critical revision of the manuscript
Anna E. Wallace: Study concept and design, acquisition of data, critical revision of the manuscript
Kimberly A. Forde: Analysis and interpretation of the data, critical revision of the manuscript, statistical analysis
James D. Lewis: Study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, drafting and critical revision of the manuscript
Scott D. Halpern: Study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, drafting and critical revision of the manuscript
Disclosure
The authors have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gentry SE, Massie AB, Cheek SW, et al. Addressing geographic disparities in liver transplantation through redistricting. Am J Transplant. 2013;13:2052–8. doi: 10.1111/ajt.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldberg DS, French B, Sahota G, Wallace AE, Lewis JD, Halpern SD. Use of Population-Based Data to Demonstrate How Waitlist-Based Metrics Overestimate Geographic Disparities in Access to Liver Transplant Care. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2016 doi: 10.1111/ajt.13820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Medicare and Medicaid Services. [Accessed Accessed April 4, 2012];Medicaid Statistical Information System (MSIS) Tables. 2012 at http://www.cms.gov/MedicaidDataSourcesGenInfo/MSIS/list.asp.
- 4.Schelleman H, Bilker WB, Strom BL, et al. Cardiovascular events and death in children exposed and unexposed to ADHD agents. Pediatrics. 2011;127:1102–10. doi: 10.1542/peds.2010-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platt R, Wilson M, Chan KA, Benner JS, Marchibroda J, McClellan M. The new Sentinel Network--improving the evidence of medical-product safety. N Engl J Med. 2009;361:645–7. doi: 10.1056/NEJMp0905338. [DOI] [PubMed] [Google Scholar]
- 6.Heimbach JK, Hirose R, Stock PG, et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61:1643–50. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–65. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 8.Goldberg DS, Lewis JD, Halpern SD, Weiner MG, Lo Re V., 3rd Validation of a coding algorithm to identify patients with hepatocellular carcinoma in an administrative database. Pharmacoepidemiol Drug Saf. 2013;22:103–7. doi: 10.1002/pds.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg D, Lewis J, Halpern S, Weiner M, Lo Re V., 3rd Validation of three coding algorithms to identify patients with end-stage liver disease in an administrative database. Pharmacoepidemiology and drug safety. 2012;21:765–9. doi: 10.1002/pds.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–4. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo Re V, 3rd, Lim JK, Goetz MB, et al. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20:689–99. doi: 10.1002/pds.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–82. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 13.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18:561–9. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 14.Martin PDA, Feng S, Brown R, Fallon M. Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59:1144–65. doi: 10.1002/hep.26972. [DOI] [PubMed] [Google Scholar]
- 15.Kim WR, Therneau TM, Benson JT, et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology. 2006;43:345–51. doi: 10.1002/hep.21025. [DOI] [PubMed] [Google Scholar]
- 16.Jepsen P, Vilstrup H, Andersen PK. The clinical course of cirrhosis: The importance of multi-state models and competing risks analysis. Hepatology. 2014 doi: 10.1002/hep.27598. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–31. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515–20. doi: 10.1002/lt.21172. [DOI] [PubMed] [Google Scholar]
- 19.Health Resources and Services Administration Area Health Resources Files (AHRF) [Accessed 12/22/2015]; at http://ahrf.hrsa.gov/
- 20.Carr BG, Branas CC, Metlay JP, Sullivan AF, Camargo CA., Jr Access to emergency care in the United States. Ann Emerg Med. 2009;54:261–9. doi: 10.1016/j.annemergmed.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg D, French B, Abt P, Feng S, Cameron AM. Increasing disparity in waitlist mortality rates with increased model for end-stage liver disease scores for candidates with hepatocellular carcinoma versus candidates without hepatocellular carcinoma. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2012;18:434–43. doi: 10.1002/lt.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry K, Ioannou GN. Comparison of Liver Transplant-Related Survival Benefit in Patients With vs Without Hepatocellular Carcinoma in the United States. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 23.Northup PG, Intagliata NM, Shah NL, Pelletier SJ, Berg CL, Argo CK. Excess mortality on the liver transplant waiting list: unintended policy consequences and Model for End-Stage Liver Disease (MELD) inflation. Hepatology. 2015;61:285–91. doi: 10.1002/hep.27283. [DOI] [PubMed] [Google Scholar]
- 24.Schaubel DE, Guidinger MK, Biggins SW, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9:970–81. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merion RM, Schaubel DE, Dykstra DM, Freeman RB, Port FK, Wolfe RA. The survival benefit of liver transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5:307–13. doi: 10.1111/j.1600-6143.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- 26.Julapalli VR, Kramer JR, El-Serag HB. Evaluation for liver transplantation: adherence to AASLD referral guidelines in a large Veterans Affairs center. Liver Transpl. 2005;11:1370–8. doi: 10.1002/lt.20434. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg D, French B, Thomasson A, Reddy KR, Halpern SD. Waitlist survival of patients with primary sclerosing cholangitis in the model for end-stage liver disease era. Liver Transpl. 2011;17:1355–63. doi: 10.1002/lt.22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.