Abstract
Activation of Aβ-fibers by is an intrinsic feature of spinal cord stimulation (SCS) pain therapy. Cannabinoid receptor type 1 (CB1) is important to neuronal plasticity and pain modulation, but its role in SCS-induced pain inhibition remains unclear. Here, we showed that CB1 receptors are expressed in both excitatory and inhibitory interneurons in substantia gelatinosa (SG). Patch-clamp recording of the evoked excitatory postsynaptic currents (eEPSCs) in mice after spinal nerve ligation (SNL) showed that electrical stimulation of Aβ-fibers (Aβ-ES) using clinical SCS-like parameters (50 Hz, 0.2 millisecond, 10 μA) induced prolonged depression of eEPSCs to C-fiber inputs in SG neurons. Pretreatment with CB1 receptor antagonist AM251 (2μM) reduced the inhibition of C-eEPSCs by Aβ-ES in both excitatory and inhibitory SG neurons. We further determined the net effect of Aβ-ES on spinal nociceptive transmission in vivo by recording spinal local field potential (LFP) in SNL rats. Epidural SCS (50 Hz, Aβ-plateau, 5 minutes) attenuated C-fiber-evoked LFP. This effect of SCS was partially reduced by spinal topical application of AM251 (25 μg, 50 μl), but not CB2 receptor antagonist AM630 (100 μg). Finally, intrathecal pretreatment with AM251 (50 μg, 15 μl) in SNL rats blocked the inhibition of behavioral mechanical hypersensitivity by SCS (50 Hz, 0.2 millisecond; 80% of motor threshold, 60 minutes). Our findings suggest that activation of spinal CB1 receptors may contribute to synaptic depression to high-threshold afferent inputs in SG neurons after Aβ-ES and may be involved in SCS-induced inhibition of spinal nociceptive transmission after nerve injury.
Keywords: Aβ-fibers, neuropathic pain, dorsal horn, cannabinoid receptor, spinal cord stimulation
1. Introduction
Neuropathic pain constitutes a significant portion of chronic pain conditions. However, analgesic medications alone often provide insufficient neuropathic pain relief.21 Spinal cord stimulation (SCS) represents an important non-pharmacological treatment option for patients with refractory pain conditions by providing pain relief and improving quality of life.20,23,26,32 Despite some success, significant opportunities remain to improve the clinical efficacy of SCS. Notably, conventional SCS at 30–60 Hz has been associated with suboptimal efficacy, especially in so-called “mixed pain conditions,” and a fairly short-lived therapeutic effect.
Conventional SCS is thought to attenuate pain mainly by exciting low-thresholdAβ-fibers (with“paresthesia intensity”) that in turn activate spinal segmental and supraspinal pain-modulatory mechanisms.1,20,23,40,53 The fundamental mechanisms for transcutaneous electrical nerve stimulation and electrical acupuncture may also involve exciting Aβ-fibers. The superficial dorsal horn is an important site for pain transmission and modulation. Central processes of nociceptive afferent neurons (mostly C-fibers) principally terminate in the superficial dorsal horn.9,29,56 Yet, little is known about how repetitive stimulation of Aβ-fibers (mostly nonnociceptive) modulates nociceptive transmission in this region, especially under neuropathic pain conditions. Excitatory and inhibitory interneurons in substantia gelatinosa (SG) play crucial, yet different roles in integrating and modulating converging nociceptive inputs.19,38,59 Our recent research has shown that electrical stimulation of Aβ-fibers (Aβ-ES) at SCS-like parameters induces frequency-dependent depression of synaptic transmission between high-threshold afferents (C-fibers) and SG neurons.50 Further, this novel form of heterosynaptic depression occurs in both glutamatergic excitatory and GABAergic inhibitory neurons after nerve injury. However, the underlying neurochemical mechanisms and physiological implications ofAβ-ES-induced synaptic depression in SG neurons remain unclear.
Endocannabinoids, including anandamide and 2-arachidonoyl glycerol, are bioactive lipid signaling molecules that modulate nociceptive transmission and participate in prolonged synaptic plasticity that may affect duration of pain inhibition.31,45,62,65 Endocannabinoids can be rapidly produced on demand by the activity-dependent enzymatic cleavage of membrane phospholipids, and serve as retrograde messengers that mediate various types of neuronal plasticity and feedback modulation in the central nervous system.6,31,47,65 At the spinal level, endocannabinoids form an important endogenous mechanism that modulates nociceptive transmission primarily through cannabinoid type 1 (CB1) receptors,8,31,47 but they may also activate CB2 and transient receptor potential vanilloid (TRPV) receptors.25,31,36,55,60
Electrical stimulation may induce a release of endocannabinoids from both neuronal and glial sources.6,11,62,65 Intriguingly, stimulation of nonnociceptive afferents may induce endocannabinoid-dependent suppression of nociceptive transmission in invertebrates.61,63 CB1 receptors are expressed in neurons and astrocytes, whereas CB2 receptors are expressed mostly in microglia and macrophages.8,31,47 Although endocannabinoid activation of CB1 receptors generally leads to neuronal and pain inhibition,31,64,65 it may also facilitate pain transmission and potentially contribute to heterosynaptic pain sensitization induced by C-fiber inputs.45 Currently, the roles of cannabinoid receptors in SCS-induced pain inhibition remain unclear. Using a multidisciplinary approach in rodent models of neuropathic pain, we examined the roles of spinal CB1 receptors in synaptic depression and pain inhibition induced by Aβ-ES. We hypothesize that Aβ-ES at SCS-like parameters may activate CB1 receptors that suppress synaptic transmission in SG neurons, inhibit spinal nociceptive transmission, and attenuate neuropathic pain-related behavior.
2. Methods
All procedures were approved by the Johns Hopkins University Animal Care and Use Committee (Baltimore, MD, USA) as consistent with the National Institutes of Health Guide for the Use of Experimental Animals to ensure minimal animal use and discomfort. Animals received food and water ad libitum and were maintained on a 12-hour day–night cycle in isolator cages. All animals were euthanized at the end of the experiment by an intraperitoneal (i.p.) injection of sodium pentobarbital (100–300 mg).
2.1. Animals and surgery
2.1.1. Animals
Young adult mice (C57BL/6,5–6 weeks old) of both sexes were used in patch-clamp electrophysiological recording from spinal cord slices in vitro. Specifically, glutamic acid decarboxylase-green fluorescent protein (GAD1-GFP) mice were obtained directly from our vendor (Jackson Laboratory, Bar Harbor, ME) or interbred in our facility from breeding pairs obtained from the vendor. By crossing Rosa26-loxP-STOP-loxP (LSL)-TdTomato mice with vGlut2-Cre mice,4 we generated the offspring vGlut2-Cre:Rosa26-TdTomato mice (vGlut2-Td) in order to identify glutamatergic excitatory neurons with red fluorescence.50 Adult, male Sprague-Dawley rats (2–3 months old, Harlan Bioproducts for Science, Indianapolis, IN) were used for electrophysiological recording of spinal local field potential (LFP) in vivo and in animal behavioral tests.
2.1.2. Spinal nerve ligation (SNL)
Mice were anesthetized with 2.0% isoflurane (Abbott Laboratories, North Chicago, IL). The left L4 spinal nerve was exposed and ligated with a 9-0 silk suture and cut distally as described in our previous studies.28,44 The muscle layer was closed with 6-0 chromic gut suture and the skin closed with metal clips. In rats, the left L5 spinal nerve was tightly ligated with a 6-0 silk suture and cut distally as described previously.27 Care was taken not to pull the nerve or touch the L4 spinal nerve. The animals were monitored after surgery for signs of wound infection, inadequate food and water intake, or weight loss until the surgical site had healed.
2.2. Patch-clamp recording of evoked excitatory postsynaptic currents (eEPSCs) in vitro
2.2.1. Spinal cord slice preparation
Mouse spinal cord slices were prepared as described in our previous studies.33,50 Briefly, the lumbosacral segment of the spinal cord was rapidly removed with attached dorsal roots and placed in ice-cold, low-sodium Krebs solution that was saturated with 95%O2/5% CO2. After trimming and mounting the tissue on a tissue slicer (Vibratome VT1200, Leica Biosystems, Buffalo Grove, IL), we prepared 400-μm-thick transverse slices with attached dorsal roots.50 The slices were then incubated in preoxygenated low-sodium Krebs solution without kynurenic acid. The slices were allowed to recover at 34°C for 40 minutes and then at room temperature for an additional 1 hour before we began the experimental recordings.
2.2.2. Dorsal root stimulation in spinal cord slices
Aβ-ES
Because 50 Hz is the most commonly used frequency for SCS in clinic and has been validated in preclinical animal models of neuropathic pain,23,27,52-54 we focused on testing 50 Hz stimulation in the current study. To determine the stimulation strength that results predominantly in activation of Aβ-fibers, we measured the compound action potentials (APs) produced by increasing intensities of electrical stimulation at the dorsal root as in our previous study.50 Based on the stimulus-response curves of compound APs, we selected 10 μA with 0.2 millisecond pulse width as the intensity for Aβ-ES because stimulus at this strength recruited 40% of all Aβ-fibers and maximally avoided the activation of Aδ and C-fibers.50 This intensity was either comparable to or lower than those used for activation of Aβ-fibers in the dorsal root in previous studies.13,41 Aβ-ES was applied via a suction electrode to the attached distal root. Larger bore pipettes filled with Krebs solution were used for dorsal root stimulation.
Test stimulation
To evoke postsynaptic currents in SG neurons, we delivered paired-pulse test stimulation to the dorsal root that consisted of two synaptic volleys (500 μA, 0.1 millisecond) 400 milliseconds apart at a frequency of 0.05 Hz (3 tests/minute), as shown previously.50 This stimulus strength is sufficient to activate C-fibers. We used paired-pulse test stimulation so that we could calculate the paired-pulse ratio (PPR; 2nd amplitude/1st amplitude).66 A 0.1 millisecond-long 5 mV depolarizing pulse was used to measure R series and R input; cells were discarded if either of these values changed by more than 20%.
2.2.3. Whole-cell patch-clamp recording of eEPSCs in spinal cord slices
Spinal cord dorsal horn somatosensory maps undergo refinement over a critical postnatal period and are completed by the third postnatal week. To avoid developmental changes that may confound data interpretation during early postnatal stages, we conducted patch clamp recording in young adult mice (5–6 weeks). The gradual postnatal withdrawal of Aβ-fibers from superficial to deeper dorsal horn (laminae III and below) and the maturation of synaptic inputs through C-fibers have mostly completed by this time.2,22 Patch-clamp recording was conducted as described in our previous studies.33,50 Briefly, transverse slices with the dorsal root attached were submerged in a small-volume recording chamber (SD Instruments, San Diego, CA), perfused with room-temperature Krebs solution (in mM: 125 NaCl, 2.5 KCl, 26 NaHCO3, 1.25 NaH2PO4H2O, 1 MgCl2, 2 CaCl2, 25 glucose) bubbled with a continuous flow of 95% O2/5% CO2, and stabilized with a grid (Ala Scientific, Farmingdale, NY). Whole-cell patch-clamp recording of SG cells was carried out under oblique illumination with an Olympus fixed-stage microscope system (BX51, Melville, NY). Data were acquired with the pClamp 10 software and a Multiclamp amplifier (Molecular Devices, Sunnyvale, CA). Thin-walled glass pipettes (World Precision Instruments, Sarasota, FL) fabricated with a puller (P1000, Sutter, Novato, CA) had resistances of 3–6 MΩ and were filled with internal solution (in mM: 120 K-gluconate, 20 KCl, 2 MgCl2, 0.5 EGTA, 2 Na2-ATP, 0.5 Na2-GTP, and 20 HEPES). The cells were voltage clamped at -70 mV unless otherwise stated. Membrane current signals were sampled at 10 kHz and low-pass filtered at 2 kHz.
2.3. Extracellular recording of spinal local field potential in vivo
2.3.1. Compound AP recording from the sciatic nerve to calibrate epidural SCS
The rats were anesthetized initially with pentobarbital (45–50 mg/kg, i.p.). Then a tracheotomy was performed and mechanical ventilation (Kent Scientific Corporation, Litchfield, CT) was provided as described previously.27 In each experiment, we first determined the intensities of epidural SCS by recording the antidromic sciatic compound AP evoked by graded electrical stimulation (0.1–5.0 mA, 0.2 millisecond, biphasic) from epidural electrodes. A monopolar silver hook electrode was placed on the left sciatic nerve at mid-thigh level for recording compound APs. The reference electrode was placed in the nearby muscle. Both stimulating and compound AP recording areas were covered with mineral oil. We determined on-line the current thresholds that resulted in the first detectable Aα/β waveform (Aβ-threshold) and the peak Aα/β waveform (Aβ-plateau), without inducing an Aδor C-fiber waveform. Similar techniques have been used in previous studies.27,51,58
2.3.2. Epidural SCS
Because a significant portion of the pain inhibition from SCS is through activation of the dorsal columns,23,27 epidural Aβ-ES over the dorsal column may mimic some actions of SCS in vivo.7,32,43 To mimic the clinical actions of SCS and to correlate with findings in animal behavior studies, we used the same plate SCS electrode as that used in animal behavioral tests (Medtronic Inc., Minneapolis, MN)51,57 and provided bipolar stimulation using SCS-like parameters (50 Hz, 0.2 millisecond, Aβ-plateau intensity) over the dorsal aspect of T13-L1 spinal segments with the dura mater preserved.
2.3.3. Recording of spinal LFP
We recorded LFP in the lumbar dorsal horn with rats under isoflurane anesthesia (1.5%) using an experimental setup similar to that described in our previous studies.27,57 We partially removed the dura overlaying the recording segment (L4) so that the fine tip of the tungsten recording microelectrode would not get damaged as it was inserted into the superficial dorsal horn. The LFP evoked by C-fiber inputs (C-LFP) shows a long latency (90–130 milliseconds) and high threshold (7–13 V, 0.5 millisecond) and was recorded at a depth ranging from 200 to 500 μm below the surface. A bandwidth of 1–300 Hz was used to remove artifacts without altering the C-LFP. A real-time, computer-based data acquisition and processing system (CED Spike 2, Cambridge, UK) was used to collect analog data. Spinal LFP evoked by paired-pulse test stimulation (25 V, 0.5 millisecond, 400-millisecond interval, 1 test/minute) at the sciatic nerve was examined 10 minutes before SCS (baseline), during SCS, and 0–30 minutes after SCS. Because of the potential for sensitization after repetitive test stimulation and possible carryover effect with multiple stimulation sessions, SCS was not applied more than twice in each experiment. After the signal-to-noise ratio and stability of recording were checked at >60 minutes after the 1st SCS, effects of a 2nd SCS were tested. We always performed the pre-stimulation baseline test before the 2nd SCS to ensure recovery.
2.4. Behavioral tests
2.4.1. Mechanical hypersensitivity test
Hypersensitivity to punctuate mechanical stimulation was determined with the up-down method by using a series of von Frey filaments (0.38, 0.57, 1.23, 1.83, 3.66, 5.93, 9.13, 13.1 g).10 Briefly, the von Frey filaments were applied for 4 to 6 seconds to the test area between the footpads on the plantar surface of the hind paw. If a positive response occurred (e.g., abrupt paw withdrawal, licking, and shaking), the next smaller von Frey hair was used; if a negative response was observed, the next higher force was used. The test was continued until (1) the responses to five stimuli were assessed after the first crossing of the withdrawal threshold or (2) the upper/lower end of the von Frey hair set was reached before a positive/negative response had been obtained. The paw withdrawal threshold (PWT) was determined according to the formula provided by Dixon.17 Rats that showed impaired motor function after surgery or did not develop mechanical hypersensitivity (i.e., mechanical allodynia, >50% reduction from pre-SNL PWT) on the hind paw ipsilateral (left) to the nerve injury by day 5 post-SNL were excluded from subsequent study.
2.4.2. Spinal cord stimulation in animals
At 1 week after SNL, an SCS lead was placed epidurally through a small laminectomy at the T13 vertebra, as described in previous studies.51,57 The sterilized lead was placed at the T10-12 spinal levels, which corresponds to T13-L1 spinal cord region. A subcutaneous tunnel was used to position the proximal end of the electrode in the upper thoracic region, where it exited the skin and connected to an external stimulator (model 2100, A-M Systems, Sequim, WA). Animals were allowed to recover from surgery for >1 week. SCS and mechanical pain hypersensitivity were examined at 7–14 days after lead implantation.
We used a crossover design to minimize potential time and order effects of testing. For the 1st SCS, animals (n=11) were randomly selected to receive intrathecal infusion of CB1 receptor antagonist AM251 (50 μg, 15μl) or vehicle through lumbar puncture injection. One day later, to allow recovery from the previous treatment, the same group of animals was tested to the 2nd SCS after having the drug assignment switched. The data from the two tests were combined for analysis. A separate group of SNL rats (n=10) received AM251 (50 μg, 15μl) or vehicle treatment followed by sham SCS. We blinded the experimenter to the treatments (e.g., drug, SCS) to reduce selection and observation bias.
On each test day, rats were acclimated for 30 minutes before we measured the baseline PWT. Motor threshold (MoT) was determined first by slowly increasing the amplitude of 4 Hz electrical stimulation from zero until muscle contraction was observed in mid-lower trunk or hind limbs. We set the first and third contacts (rostral to caudal) of the four-contact lead as an anode and the second and fourth as a cathode (“twin-pairs” stimulation) as shown in our previous studies.51,57 Animals then received drug treatment, followed by pre-SCS PWT testing 30 minutes later. SCS (50 Hz, 0.2 millisecond, 80% MoT) or sham stimulation (0 mA) was then applied for 60 minutes. We examined PWTs at 30 and 60 minutes during the SCS (intra-SCS) and at 30 minutes after the completion of SCS to determine the carryover pain inhibitory effect. We used 80% of the MoT because it represents the maximum intensity of SCS that can be applied without causing discomfort in awake animals and it has been used in many previous studies.
2.5. Immunohistochemistry
The animals were deeply anesthetized with sodium pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 0.1 M phosphate-buffered saline (PBS; pH 7.4, 4°C) followed by fixative (4% formaldehyde and 14% [v/v] saturated picric acid in PBS, 4°C). Spinal cord tissues were cryoprotected in 20% sucrose for 24 hours before being serially cut into 15-μm sections and placed onto slides. The slides were incubated overnight at 4°C in primary antibodies: chicken antibody to GFP (GFP-1020, Aves Labs, 1:1,000) and rabbit polyclonal CB1 receptor antibody (10006590, Cayman, 1:500). Slides were incubated in secondary antibody at room temperature for 45 minutes. The secondary antibodies were diluted 1:100 in PBS and included donkey antibody to rabbit (711-295-152, Rhod Red-X-conjugated, Jackson ImmunoResearch, West Grove, PA), and goat antibody to chicken (103-225-155, Cy2-conjugated, Jackson ImmunoResearch). Only the neurons located at the superficial spinal cord (lamina I and lamina II) were counted. For the double-labeling studies, the percent of double-labeled neurons is expressed relative to the total number of labeled neurons. The spinal interneurons whose cell bodies were stained with CB1 receptor antibody or surrounded by CB1 receptor fluorescence ring were counted as CB1 receptor-positive. Tissues from different experimental groups were processed and analyzed together.
2.6. Drugs
For patch-clamp recording, all drugs were applied into the bath (i.e., extracellular) solution. (–)- Stock solutions were freshly prepared as instructed by the manufacturer. Drugs were purchased from Sigma-Aldrich (St. Louis, MO) or Tocris Bioscience (Bristol, UK). AM251 and AM630 (CB2 receptor antagonist) were purchased from Tocris Bioscience and dissolved into vehicle (8% DMSO, 5% TWEEN 80, and 87% saline).
2.7. Data analysis
For patch-clamp recording, we determined the peak amplitude of eEPSCs and PPR by using pClamp10 software. The evoked postsynaptic current corresponding to C-fiber inputs was distinguished on the basis of the latency and activation threshold. It is known that each spinal cord segment may receive C-fiber inputs from several segmental dorsal roots.46 Because of multi-segmental projections, branching, and dendrite arborization in the spinal cord, we cannot clearly distinguish the central terminals of the injured dorsal root ganglion neurons from those of the uninjured ones at each spinal level in spinal slices of SNL mice. Therefore, we performed the analysis on the combined eEPSC data from L4 and L5 spinal segments. Because vehicle and drug were tested in different experiments, we compared data between pre- and post-stimulation conditions, and between vehicle- and drug-treated groups at each time point using a two-way mixed model ANOVA.
In spinal LFP recording, LFP corresponding to A- and C-fiber activation can be distinguished on the basis of conduction velocity (CV) and activation threshold. The peak amplitude of A-LFP and area under the curve (AUC) of C-LFP were measured off-line. The PPR of C-LFP (AUC to 2nd pulse/AUC to 1st pulse) was calculated. We tested vehicle and drug in the same experiment, and compared these data between pre- and post-stimulation conditions and between vehicle and drug treatments using a two-way mixed model ANOVA.
To determine the PWT in animal behavior studies, we converted the pattern of positive and negative von Frey filament responses to a 50% threshold value using the formula provided by Dixon.17 The PWT was compared between the pre- and post-SCS conditions and between groups by using a two-way mixed model ANOVA.
In each study, we blinded the experimenter to the treatments (e.g., drug) to reduce selection and observation bias. STATISTICA 6.0 software (StatSoft, Inc., Tulsa, OK) was used to conduct all statistical analyses. The Tukey honestly significant difference (HSD) post-hoc test was used to compare specific data points. Bonferroni correction was applied for multiple comparisons. Two-tailed tests were performed, and numerical data are expressed as mean + SEM; P<0.05 was considered significant in all tests.
3. Results
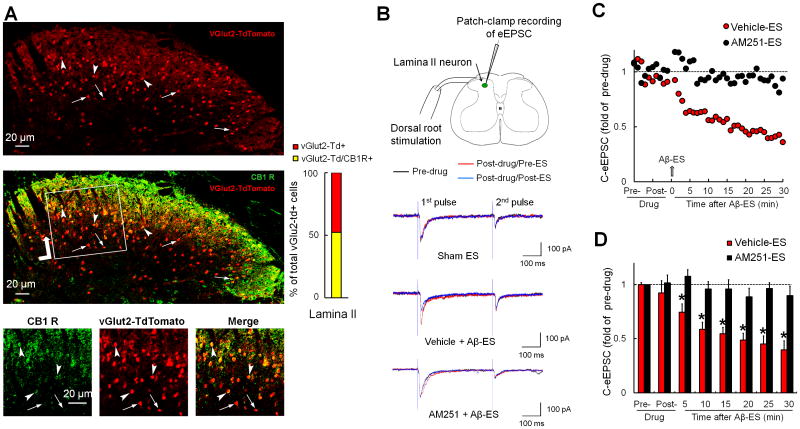
3.1. Inhibition of C-eEPSCs in excitatory SG neurons by Aβ-ES was blocked by AM251
Glutamatergic excitatory neurons were identified by red fluorescence (vGlut2-Td+) in spinal cord slices from vGlut2-Cre:Rosa26-TdTomato mice. Double-immunofluorescence labeling showed that 52.3% of vGlut2-Td+ SG neurons were positive for CB1 receptor immunoreactivity (Fig. 1A). We then conducted patch-clamp recording to examine whether CB1 receptor mediates the inhibition of eEPSCs by Aβ-ES in vGlut2-Cre:Rosa26-TdTomato mice at 1 week post-SNL. After measuring the baseline eEPSCs, we administered CB1 receptor antagonist AM251 (2μM, bath application) or vehicle for 5 minutes before applying Aβ-ES (50 Hz, 10 μA, 0.1 millisecond, 1 minute). The test pulse was applied at the dorsal root at an intensity (500 μA, 0.1 millisecond) sufficient to activate high-threshold afferent fibers (C-fibers, Fig. 1B). In line with our previous findings,50 Aβ-ES induced prolonged depression of eEPSCs in response to C-fiber inputs (C-eEPSCs) in vGlut2-Td+ neurons that were pretreated with vehicle (n=12, Fig. 1C). The peak amplitudes of C-eEPSCs during each 5-minute time period after Aβ-ES were averaged and then normalized to the respective pre-drug baseline for statistical analysis (Fig. 1D). The C-eEPSC amplitude was significantly decreased from pre-drug baseline in the vehicle-treated group at 5–30 minutes after Aβ-ES. However, pretreatment with AM251 (n=12) blocked the inhibition of C-eEPSC by Aβ-ES (Fig. 1C and D). Five minutes of AM251 alone did not affect C-eEPSCs as compared to pre-drug baseline. The access resistance remained largely unchanged during the experiment (data not shown), indicating a stable recording condition.
Figure 1. CB1 receptor antagonist AM251 blocks inhibition of evoked postsynaptic currents by 50 Hz Aβ-ES in excitatory neurons.
(A) Upper: Glutamatergic excitatory neurons were identified by red fluorescence vGlut2-TdTomato+ in spinal cord slices from vGlut2-Cre:Rosa26-TdTomato mice. Middle: A confocal image of a double-immunofluorescence–stained spinal cord slice shows that many vGlut2-Td+ cells in dorsal horn colocalize with CB1 receptor (CB1 R) immunoreactivity (green). Lower: A higher power view of the boxed region. Double-labeled neurons are indicated by arrowheads, and vGlut2-Td+ neurons negative for CB1 R immunoreactivity are indicated by arrows. Right: Percent of total vGlut2-Td+ cells in SG (lamina II) that are CB1 R+ (n=3 mice, 3-5 slices/animal). (B) Configuration for whole-cell patch-clamp recording in an SG neuron during dorsal root stimulation. Electrical conditioning stimulation of Aβ-fibers (Aβ-ES, 50 Hz, 10 μA, 0.1 millisecond, 1 minute) was administered to the ipsilateral dorsal root. Representative traces of evoked postsynaptic currents (eEPSC) in lamina I neurons in response to paired-pulse test stimulation (500 μA, 0.1 millisecond, 400 millisecond apart) before (black) and after (red) drug treatment, and after (blue) Aβ-ES or sham ES. (C) Time course of C-fiber eEPSC amplitudes before and after Aβ-ES in vehicle-pretreated (n=12) and AM251-preteated (2 μM, bath application, 5 minutes, n=12) groups. Error bars are omitted to improve clarity. (D) The amplitudes of C-fiber eEPSCs during each 5-minute period were averaged for analysis. Data are expressed as mean + SEM. Two-way mixed model ANOVA. *P<0.05 versus pre-drug.
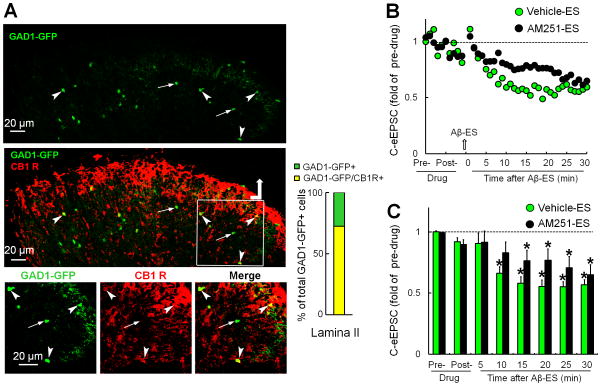
3.2. AM251 partially reduced the Aβ-ES–induced inhibition of C-eEPSCs in GABAergic interneurons in SG
We next examined if AM251 blocks the Aβ-ES–induced inhibition of C-eEPSCs specifically in excitatory, but not inhibitory, neurons. If so, combining low-dose CB1 receptor agonists with Aβ-ES may induce an excitatory neuron-preferred inhibition that leads to greater pain inhibition. GABAergic interneurons were identified by green fluorescence in spinal cord slices from GAD1-GFP mice. Double-immunofluorescence labeling showed that 71.4% of GAD1-GFP+ cells in SG express CB1 receptor immunoreactivity (Fig. 2A). The C-eEPSC amplitudes in GAD1-GFP+ neurons gradually decreased after Aβ-ES in the vehicle-pretreated group (n=12). The inhibitory effect of Aβ-ES was partially reduced by AM251 pretreatment (2μM, 5 minutes, n=13, Fig. 2B and C). AM251 alone did not affect C-eEPSCs at 5 minutes after treatment.
Figure 2. Blocking CB1 receptor with AM251 also attenuates the inhibition of GABAergic neurons by Aβ-ES.
(A) Upper: GABAergic neurons can be identified by green fluorescence in spinal cord slices of GAD1-GFP mice. Middle: A confocal image of a double-immunofluorescence–stained spinal cord slice shows that many GAD1-GFP+ neurons in superficial dorsal horn colocalize with CB1 receptor (CB1 R) immunoreactivity (red). Lower: A higher power view of the boxed region. Double-labeled neurons are indicated by arrowheads, and GAD1-GFP+ neurons negative for CB1 R immunoreactivity are indicated by arrows. Right: percent of total GAD1-GFP+ cells that are also CB1 R+ in SG (n=3 mice). (B) Time course of C-fiber eEPSC amplitudes before and after Aβ-ES in vehicle-pretreated (n=12) and AM251-preteated (2 μM, bath application, 5 minutes, n=13) groups. Error bars are omitted to improve clarity. (C) The amplitudes of C-fiber eEPSCs during each 5-minute period were averaged for analysis. Data are expressed as mean + SEM. Two-way mixed model ANOVA. *P<0.05 as compared with the pre-drug condition.
In a separate study, C-eEPSCs were not significantly changed during the 30 minutes after bath application of AM251 (2 μM, 5 minutes) followed by sham stimulation (0 mA) in vGlut2-Td+ (n=6) and GAD1-GFP+ neurons (n=6), as compared to the respective pre-drug baselines (data not shown).
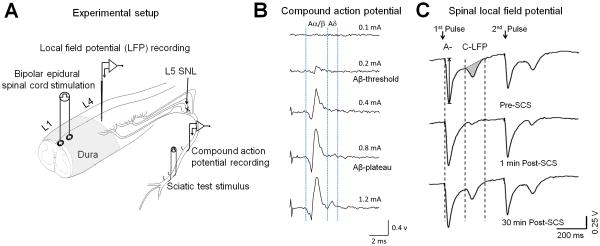
3.3. Epidural SCS decreased spinal LFP in rats after nerve injury
To examine the net effect of Aβ-ES on spinal nociceptive transmission in vivo, we recorded LFP from superficial dorsal horn in rats at 2-3 weeks post-SNL. To mimic SCS, we applied bipolar Aβ-ES through a pair of plate electrodes placed on the dura near T13-L1 spinal segment (Fig. 3A). Like in our previous studies,27,35,51 we calibrated the intensity of epidural SCS that activates only Aβ-fibers by recording compound APs at the sciatic nerve. Compound APs to graded epidural SCS can be separated into Aα/β- and Aδ-components based on latency and activation threshold. We determined current thresholds that resulted in the first detectable Aα/β waveform (Aβ-threshold) and the peak Aα/β waveform (Aβ-plateau), without inducing an Aδ-waveform (Fig. 3B). Spinal LFP evoked by paired-pulse test stimulation (25 V, 0.5 millisecond, 400-millisecond interval, 1 test/minute) at the sciatic nerve can be separated into early (A-LFP) and later (C-LFP) components that correspond to A-fiber and C-fiber inputs (Figs. 3C and 4A).
Figure 3. Epidural spinal cord stimulation (SCS) attenuates spinal local field potential (LFP) in vivo.
(A) Configuration for extracellular recording of spinal LFP and sciatic compound action potentials (APs) in rats after spinal nerve ligation (SNL). Bipolar SCS (50 Hz, 0.2 millisecond, 5 minutes) was delivered through a pair of plate electrodes placed on the dura near L1 spinal segment. (B) Examples of increasing epidural SCS stimulus intensities that activated compound APs. In each experiment, we determined on-line the current thresholds that resulted in the first detectable Aα/β waveform (Aβ-threshold) and the peak Aα/β waveform (Aβ-plateau), without inducing an Aδwaveform. (C) Example traces show spinal LFP evoked by paired-pulse test stimulation (25 V, 0.5 millisecond, 400-millisecond interval, 1 test/minute) before and after SCS. LFPs corresponding to A- and C-fiber activation were distinguished on the basis of conduction velocity. The peak amplitude of A-LFP and area under the curve (shaded area) of C-LFP were measured off-line.
Figure 4. CB1 receptor antagonist AM251 reduces the inhibition of spinal local field potential (LFP) induced by spinal cord stimulation (SCS).
(A) Example traces show that epidural SCS (50 Hz, 0.2 millisecond, A-plateau, 5 minutes) reduces C-fiber-evoked LFP (C-LFP) to paired-pulse test stimuli at the sciatic nerve (25 V, 0.5 millisecond, 400-millisecond interval, 1 test/minute). This reduction was diminished by spinal topical pretreatment with AM251 (25 μg, 100 μl, 30 minutes). (B) Time course of peak A-LFP amplitudes before and after SCS in rats with spinal nerve ligation (SNL). (C) The amplitudes of A-LFP during each 5-minute period were averaged for analysis. (D) Time course of C-LFP area under the curve (AUC) before and after SCS. SCS induced acute inhibition of C-LFP in SNL rats pretreated with vehicle (n=10). The effect of SCS was reduced by pretreatment with AM251 (25 μg, 100 μl, n=10). AUC of the C-LFP was normalized to baseline values. (E) The AUC of C-LFP during each 5-minute period was averaged for analysis. (F) The averaged paired-pulse ratios (2nd amplitude/1st amplitude) of C-LFP were increased at 0-10 minutes after SCS when spine was pretreated with vehicle, but not AM251. B, D: Error bars are omitted to improve clarity. C, E, F: Data are expressed as mean + SEM. Two-way mixed model ANOVA. *P<0.05 versus pre-SCS; #P<0.05 versus vehicle-SCS.
In the vehicle-pretreated group, A-LFP amplitude progressively decreased after epidural SCS (50 Hz, 0.2 millisecond, Aβ-plateau, 5 minutes, Fig. 4B and C). Importantly, the AUC of C-LFP showed an immediate and much greater reduction at 0-10 minutes after SCS. The inhibition of C-LFP by SCS was short-lasting and diminished after 15 minutes (Fig. 4D and E). The decrease in C-LFP after SCS was accompanied by an increased PPR (Fig. 4F), suggesting an inhibition of presynaptic neurotransmitter release by SCS.
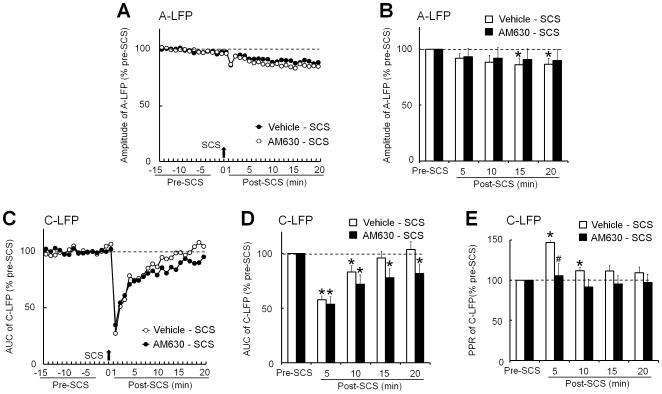
3.4. The inhibition of spinal LFP by epidural SCS was reduced by AM251 pretreatment
AM251, which was applied topically at the recording spinal segment (25μg, 30 minutes, n=10) partially blocked the epidural SCS-induced decrease in C-LFP (Fig. 4D and E). AM251 pretreatment also normalized the increased PPR of C-LFP after SCS (Fig. 4F). In contrast to AM251, spinal topical application of CB2 receptor antagonist AM630 (100 μg, n=10) did not block the inhibition of C-LFP by SCS, as compared to vehicle pretreatment (Fig. 5C and D), but it did normalize the increased PPR of C-LFP (Fig. 5E).
Figure 5. CB2 receptor antagonist AM630 does not block acute inhibition of spinal local field potential (LFP) induced by spinal cord stimulation (SCS).
(A) Time course of peak A-LFP amplitudes before and after SCS (50 Hz, 0.2 millisecond, A-plateau, 5 minutes) in rats with spinal nerve ligation (SNL). (B) The amplitudes of A-LFP during each 5-minute period were averaged for analysis. (C) Time course of C-LFP area under the curve (AUC) before and after SCS. SCS acutely inhibited C-LFP in SNL rats that received spinal topical pretreatment with vehicle (n=9, 30 minutes). Pretreatment with AM630 (100 μg, 100 μl, n=9) did not reduce the inhibition of C-LFP by SCS. AUC of the C-LFP was normalized to the baseline values. (D) The AUCs of C-LFP during each 5-minute period were averaged for analysis. (E) The averaged paired-pulse ratios (2nd amplitude/1st amplitude) of C-LFP were increased at 0-10 minutes after SCS when spine was pretreated with vehicle, but not AM630. A, C: Error bars are omitted to improve clarity. B, D, E: Data are expressed as mean + SEM. Two-way mixed model ANOVA. *P<0.05 versus pre-SCS; #P<0.05 versus vehicle-SCS.
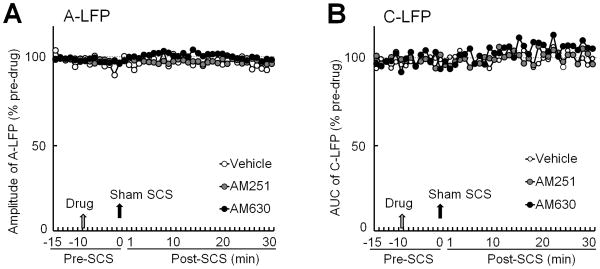
The decrease in A-LFP after SCS was reduced by AM251 pretreatment (Fig. 4B and C) but was mostly unaffected by AM630 (Fig. 5A and B). In a separate study, neither AM251 (25 μg, 10 minutes, n=6) nor AM630 (100 μg, n=6) changed the A-LFP or C-LFP at 0-30 minutes after sham SCS (0 mA, 5 minutes), as compared to vehicle (n=6, Fig. 6).
Figure 6. Effects of CB1 and CB2 receptor antagonists followed by sham spinal cord stimulation (SCS) on spinal local field potential (LFP).
(A) Time course of peak A-LFP amplitude before and after spinal topical application of vehicle, AM251 (25 μg, 100 μl, n=6), or AM630 (100 μg, 100 μl, n=6) followed by sham SCS (0 mA, 5 minutes) in nerve-injured rats. (B) Time course of C-LFP area under the curve (AUC) before and after the same treatment. Data are expressed as mean. Error bars are omitted to improve clarity.
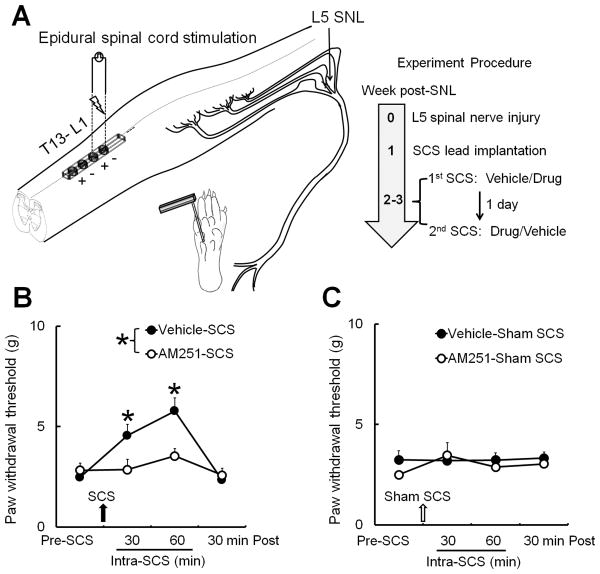
3.5. Intrathecal injection of AM251 blocked SCS-induced inhibition of mechanical hypersensitivity
Finally, we used a custom-made quadripolar electrode (Medtronic Inc.) to examine the net effect of AM251 on SCS-induced pain inhibition in SNL rats (Fig. 7A). Of the 30 rats that underwent SNL, three did not develop mechanical hypersensitivity by day 5 post-SNL. Of the 27 SNL rats that were implanted with an SCS lead, four showed impaired motor function, diminished mechanical hypersensitivity, or damage to the implanted lead before the drug trials. These rats were eliminated from the subsequent studies. Among the remaining 23 animals, two rats were eliminated because of later damage to the lead, undetectable MoT, or deteriorating health conditions. The PWT of the ipsilateral hind paw was significantly decreased from pre-injury level (21.5 g) in SNL rats (2.5–3.5 g) before SCS. In the vehicle-pretreated group (n=11), SCS significantly increased PWT from pre-SCS level at 30 and 60 minutes during SCS. However, the SCS-induced increase in PWT was diminished in SNL rats that received intrathecal pretreatment with AM251 (50 μg, 15μl, 30 minutes, n=11, Fig. 7B). SCS and drug treatment did not affect PWT of the contralateral hind paw (data not shown).The ipsilateral PWTs did not significantly change from pre-SCS level in SNL rats that received vehicle or AM251 pretreatment (50 μg, 15μl, 30 minutes, n=10) followed by sham SCS (Fig. 7C).
Figure 7. Intrathecal injection of CB1 receptor antagonist AM251 blocks spinal cord stimulation (SCS)-induced inhibition of mechanical hypersensitivity in nerve-injured rats.
(A) The miniature spinal cord lead with four contacts was implanted epidurally over the dorsal spinal cord at the T13–L1 spinal level. Mechanical test stimuli (von Frey filaments, 0.38–13.1 g) were applied to the mid-plantar area of the rat hind paw at 2-3 weeks after L5 spinal nerve ligation (SNL). (B) Paw withdrawal thresholds (PWTs) were measured before and at different time points after SCS (50 Hz, 80% motor threshold, 0.2 millisecond, constant current, 60 minutes). SCS increased PWTs at 30 and 60 minutes after stimulation in SNL rats that received vehicle pretreatment (n=11). This effect was blocked by intrathecal pretreatment with AM251 (n=11, 50 μg, 15 μl, 30 minutes). (C) In a separate group of SNL rats (n=10), neither AM251 nor vehicle treatment followed by sham SCS changed PWTs from pre-SCS level. B, C: Data are expressed as mean + SEM. Two-way mixed model ANOVA. *P<0.05 versus pre-SCS.
4. Discussion
This study demonstrates for the first time that AM251, a CB1 receptor antagonist, blocks the suppression of C-eEPSCs induced by Aβ-ES in SG neurons, and attenuates the inhibition of spinal C-LFP by 50 Hz SCS in vivo. Furthermore, intrathecal pretreatment with AM251 blocked the inhibition of mechanical hypersensitivity by SCS in nerve-injured rats. Accordingly, activation of spinal CB1 receptors may be an important mechanism that contributes to pain inhibition from conventional SCS.
We previously identified a novel form of synaptic depression between C-fiber and SG neurons that is induced by Aβ-ES,50 but the underlying neurochemical mechanism was unclear. CB1 receptors mediate various forms of synaptic plasticity in the central nervous system, and electrical stimulation might induce postsynaptic production of endocannabinoids, which act as retrograde messengers and activate presynaptic CB1 receptors to inhibit neurotransmitter release. Indeed, activation of CB1 receptors was shown to contribute to the depression of C-eEPSCs after high-intensity stimulation that activates nociceptive C-fibers.31 Unlike in the previous study, we used a much lower intensity of conditioning stimulation to activate only nonnociceptiveAβ-fibers, which induced prolonged inhibition of C-eEPSCs. Notably, AM251 reduced this novel form of Aβ-ES–induced heterosynaptic depression in both vGlut2-Td+ and GAD1-GFP+ neurons. To our knowledge, this is the first study to show that CB1 receptors participate in the prolonged inhibition of synaptic transmission in both excitatory and GABAergic inhibitory dorsal horn neurons after Aβ-fiber excitation. The respective neuronal network mechanisms underlying the synaptic changes induced by Aβ-fiber and C-fiber conditioning stimulation warrant further study.
Stimulation of Aβ-fibers may activate dorsal horn GABAergic interneurons, which in turn inhibit spinal nociceptive transmission.23,39 A small subset of GABAergic interneurons can be activated by Aβ-fiber inputs, and Aβ-ES briefly increases extracellular GABA level.12,13 Nevertheless, a recent contemporary view of gate control highlighted the complexity of spinal micro-circuitries that modulate nociceptive transmission.5 Our previous study also showed that blocking fast inhibitory neurotransmission mediated by GABA-A and glycine receptors, which may be important to pain gate-control, did not preclude the prolonged depression of C-eEPSCs after Aβ-ES.50 Therefore, the neurochemical mechanisms that underlie the synaptic plasticity induced by Aβ-ES may involve additional mechanisms. Differentiating the neurochemical mechanisms for inhibition of excitatory and inhibitory interneurons may help us to develop cell type-selective modulation, such as by combining SCS and adjuvant drugs that would primarily enhance the inhibition of excitatory dorsal horn neurons.
Endocannabinoids can participate in both short-term and long-term synaptic plasticity that would affect the duration of pain inhibition.31,45,62,65 Intriguingly, in invertebrates such as the medicinal leech, activities in nonnociceptive afferents depress transmission of nociceptive inputs converging onto the same postsynaptic neuron through endocannabinoid-dependent mechanisms.61,63 However, this mechanism has not been examined in mammals. In addition to different stimulation protocols, neuroanatomy, and neurochemistry, the depression of C-eEPSCs in mouse SG neurons after Aβ-ES is fundamentally different from that in leeches. First, unlike the motor neurons in leech that receive converging nonnociceptive and nociceptive inputs, most SG neurons in mice do not receive nonnociceptive inputs because Aβ-fibers primarily terminate in the deeper dorsal horn. Although it is possible that SG neurons may receive polysynaptic inputs from Aβ-fibers transmitted via interneurons, in most cases we did not observe such synaptic response (e.g., <15% of SG neurons show Aβ-eEPSCs). Importantly, AM251 attenuated the inhibitory effect of Aβ-ES in both excitatory and inhibitory interneurons that display only C-eEPSCs. Therefore, Aβ-ES may attenuate C-eEPSCs by activating the endocannabinoid system through a yet unidentified network mechanism. Second, C-fiber eEPSCs in leeches were depressed by stimulation of nonnociceptive neurons at a much lower frequency (1 Hz) than that used in current study (50 Hz). Our previous study showed that the lower frequency Aβ-ES (4 Hz) failed to suppress C-eEPSCs.50 Finally, endocannabinoid-mediated depression of nociceptive synapses and behavior was mediated by TRPV-like receptors in the leech.63 Yet, our findings suggest that the CB1 receptor contributes to profound synaptic depression of nociceptive inputs after Aβ-ES in SG neurons. Nevertheless, given that anandamide can activate both CB1 receptors and TRPV channels,31,36,55,60 it will be necessary to examine the roles of TRPV channels in Aβ-ES–induced synaptic depression and pain modulation.
Because 50 Hz Aβ-ES reduced synaptic transmission in both excitatory neurons and GABAergic interneurons, the net effect of Aβ-ES on spinal nociceptive transmission needs to be determined. The LFP comprises the responses of many cells, including excitatory postsynaptic potentials evoked in the dendrites and action potentials recorded from the cell body or axon terminals. Compared to single-unit recording from individual neurons, measuring C-LFP is a high-throughput approach for examining the broad and net spinal nociceptive transmission in vivo.18,48 We showed for the first time that epidural SCS inhibits C-LFP through activation of CB1 receptors in rats after nerve injury. CB1 receptors are densely expressed on the central terminals of primary afferent neurons and on postsynaptic neurons in the spinal cord.34 The SCS-induced decrease in C-LFP was associated with an increase in PPR, suggesting that a decrease in neurotransmitter release may contribute to the inhibition of C-LFP.50,66 Nevertheless, a large portion of vGlut2-Td+ excitatory neurons and GAD1-GFP+ inhibitory interneurons also express CB1 receptors; hence postsynaptic mechanisms cannot be ruled out. Future studies are needed to differentiate the roles of pre- and post-synaptic CB1 receptors in SCS-induced synaptic depression and pain inhibition, and to measure the dynamic changes of endocannabinoid level in the spinal cord after SCS using microdialysis.
We postulate that the decrease in synaptic efficiency induced by Aβ-ES may be physiologically relevant for pain inhibition by SCS. Conventional SCS is often applied to patients at the amplitude that elicits paresthesia over the painful area and titrated to the highest comfortable level. Paresthesia during SCS may stem largely from activation of low-threshold Aβ-fibers. SCS is thought to attenuate pain mainly by exciting dorsal column fibers that in turn drive activation of both spinal and supraspinal pain-inhibitory mechanisms. In line with this notion, epidural Aβ-ES that mimicked SCS acutely depressed C-LFP, suggesting a net inhibition of nociceptive transmission in vivo. However, the prolonged suppression of C-eEPSCs in SG neurons by Aβ-ES in vitro did not translate into a long carryover inhibition of C-LFP and pain behavior by SCS in vivo. The reasons for the generally short-lived carryover of pain inhibition after SCS remain unclear but may be due in part to a concurrent decrease in the excitability of GABAergic inhibitory neurons, which would limit the duration of pain inhibition. It is also possible that certain descending and trans-segmental facilitatory mechanisms are lost in recording of SG neurons from a spinal slice. Importantly, effects of SCS in vivo may reflect the combined actions on afferent terminals, superficial neurons, deep neurons, and interneurons in contact with them, which differ from those induced by Aβ-ES at the dorsal root in vitro. In previous studies, durations of carryover pain inhibition after SCS have been varied,24,37,57 possibly because of different SCS protocols (e.g., duration, single versus repetitive treatment), animal models, and experimental procedures.
Application of high-frequency alternating current waveforms to nerves may block conduction of APs.3 Previously, we showed that 50 Hz Aβ-ES at the dorsal column reduced Aα/β-compound APs recorded at peripheral nerves and inhibited wide-dynamic neuronal response to mechanical stimuli in SNL rats.27,51 These findings suggest that SCS may change conduction properties in Aα/β-fibers. However, these inhibitory effects were normally short-lived and mostly diminished at 15 minutes after conditioning stimulation. Yet, in our current study, epidural SCS induced a prolonged depression of A-LFP that remained at 30 minutes after SCS. This finding suggests that epidural SCS may also induce plasticity changes at the nonnociceptive synapses in dorsal horn. The physiological implication of prolonged A-LFP depression on pain remains to be examined, as the inhibition of mechanical hypersensitivity by SCS in animal behavior tests was rather brief.
Spinal application of AM251 reduced the depression of C-LFP and A-LFP, suggesting that spinal CB1 receptors may be involved in SCS-induced suppression of both low-threshold and high-threshold sensory transmission. The inhibition of neuropathic mechanical hypersensitivity by SCS was also reduced by intrathecal AM251, suggesting a role for CB1 receptors in SCS-induced pain inhibition. Although CB2 receptors are expressed mostly on non-neuronal cells and AM630 did not block the inhibition of C-LFP by SCS, it remains possible that CB2 receptors may play a role in SCS-induced pain inhibition, such as by regulating glial cells and cytokine release.
SCS has demonstrated only a moderate success rate for pain treatment (around 50% decrease in pain rating in 50-60% of patients) and shows high variability of efficacy between different pain conditions.14,15,30,32,42 Moreover, the inhibition of C-LFP and neuropathic pain behavior by SCS is brief and diminishes quickly after stimulation. Previous findings suggested that SCS inhibits pain through multiple neurochemical mechanisms.20,23,26,49,54 The spinal endocannabinoid system generally helps to maintain the balance of neuronal excitability and certainly has a therapeutic potential for pain treatment.16,31,65 Our study suggests that CB1 receptors may be another useful target for developing new treatment strategies, as CB1 receptor agonists or endocannabinoid degradation inhibitors might increase the efficacy of SCS and prolong pain inhibition.
Summary statement.
Electrical stimulation of Aβ-fibers may activate spinal CB1 receptors to suppress synaptic transmission to high-threshold afferent inputs in lamina II neurons, attenuate spinal nociceptive transmission, and inhibit mechanical hypersensitivity after nerve injury.
Acknowledgments
The authors thank Claire F. Levine, MS, ELS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University) for editing the manuscript. Electrodes for spinal cord stimulation used in animal behavior tests were generously provided by Medtronic, Inc. (Minneapolis, MN, USA). This work was facilitated by the Pain Research Core funded by the Blaustein Pain Fund and the Neurosurgery Pain Research Institute at the Johns Hopkins University.
Funding sources: This study was supported by a seed grant from the Johns Hopkins Blaustein Pain Research Fund (Y.G.) and was subsidized by grants from the National Natural Science Foundation of China: 81428008 (Y.W.) and the National Institutes of Health (Bethesda, Maryland, USA): NS70814 (Y.G.), NS26363 (S.N.R.).
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest. Louis P. Vera-Portocarrero is employed by Medtronic, Inc. However, none of the authors has a commercial interest in the material presented in this paper. There are no other relationships that might lead to a conflict of interest in the current study.
Author contributions. F.Y., Q.X., and B.S. performed most of the experiments and were involved in writing a draft manuscript. V.T., and S-Q H. also conducted some of the electrophysiology, molecular, and behavioral experiments. L.P.V., B.L., X.D., S.N.R., and Y.W. were involved in experimental design, data analysis, and discussion. Y.G. designed and directed the project and wrote the final manuscript.
References
- 1.Barchini J, Tchachaghian S, Shamaa F, Jabbur SJ, Meyerson BA, Song Z, Linderoth B, Saade NE. Spinal segmental and supraspinal mechanisms underlying the pain-relieving effects of spinal cord stimulation: an experimental study in a rat model of neuropathy. Neuroscience. 2012;215:196–208. doi: 10.1016/j.neuroscience.2012.04.057. [DOI] [PubMed] [Google Scholar]
- 2.Beggs S, Torsney C, Drew LJ, Fitzgerald M. The postnatal reorganization of primary afferent input and dorsal horn cell receptive fields in the rat spinal cord is an activity-dependent process. Eur J Neurosci. 2002;16:1249–58. doi: 10.1046/j.1460-9568.2002.02185.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhadra N, Lahowetz EA, Foldes ST, Kilgore KL. Simulation of high-frequency sinusoidal electrical block of mammalian myelinated axons. J Comput Neurosci. 2007;22:313–26. doi: 10.1007/s10827-006-0015-5. [DOI] [PubMed] [Google Scholar]
- 4.Borgius L, Restrepo CE, Leao RN, Saleh N, Kiehn O. A transgenic mouse line for molecular genetic analysis of excitatory glutamatergic neurons. Mol Cell Neurosci. 2010;45:245–57. doi: 10.1016/j.mcn.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Braz J, Solorzano C, Wang X, Basbaum AI. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–36. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burattini C, Battistini G, Tamagnini F, Aicardi G. Low-frequency stimulation evokes serotonin release in the nucleus accumbens and induces long-term depression via production of endocannabinoid. J Neurophysiol. 2014;111:1046–55. doi: 10.1152/jn.00498.2013. [DOI] [PubMed] [Google Scholar]
- 7.Buvanendran A, Lubenow TJ. Efficacy of transverse tripolar spinal cord stimulator for the relief of chronic low back pain from failed back surgery. Pain Physician. 2008;11:333–8. [PubMed] [Google Scholar]
- 8.Calignano A, La RG, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–81. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 9.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–80. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Zhang J, Li F, Qiu Y, Wang L, Li YH, Shi J, Pan HL, Li M. Endogenous anandamide and cannabinoid receptor-2 contribute to electroacupuncture analgesia in rats. J Pain. 2009;10:732–9. doi: 10.1016/j.jpain.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Cui JG, O'Connor WT, Ungerstedt U, Linderoth B, Meyerson BA. Spinal cord stimulation attenuates augmented dorsal horn release of excitatory amino acids in mononeuropathy via a GABAergic mechanism. Pain. 1997;73:87–95. doi: 10.1016/s0304-3959(97)00077-8. [DOI] [PubMed] [Google Scholar]
- 13.Daniele CA, MacDermott AB. Low-threshold primary afferent drive onto GABAergic interneurons in the superficial dorsal horn of the mouse. J Neurosci. 2009;29:686–95. doi: 10.1523/JNEUROSCI.5120-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deer TR, Krames E, Mekhail N, Pope J, Leong M, Stanton-Hicks M, Golovac S, Kapural L, Alo K, Anderson J, Foreman RD, Caraway D, Narouze S, Linderoth B, Buvanendran A, Feler C, Poree L, Lynch P, McJunkin T, Swing T, Staats P, Liem L, Williams K. The appropriate use of neurostimulation: new and evolving neurostimulation therapies and applicable treatment for chronic pain and selected disease states. Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:599–615. doi: 10.1111/ner.12204. [DOI] [PubMed] [Google Scholar]
- 15.Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, Buchser E, Burton A, Buvanendran A, Candido K, Caraway D, Cousins M, DeJongste M, Diwan S, Eldabe S, Gatzinsky K, Foreman RD, Hayek S, Kim P, Kinfe T, Kloth D, Kumar K, Rizvi S, Lad SP, Liem L, Linderoth B, Mackey S, McDowell G, McRoberts P, Poree L, Prager J, Raso L, Rauck R, Russo M, Simpson B, Slavin K, Staats P, Stanton-Hicks M, Verrills P, Wellington J, Williams K, North R. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17:515–50. doi: 10.1111/ner.12208. [DOI] [PubMed] [Google Scholar]
- 16.Devesa I, Ferrer-Montiel A. Neurotrophins, endocannabinoids and thermo-transient receptor potential: a threesome in pain signalling. Eur J Neurosci. 2014;39:353–62. doi: 10.1111/ejn.12455. [DOI] [PubMed] [Google Scholar]
- 17.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–62. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 18.Drdla-Schutting R, Benrath J, Wunderbaldinger G, Sandkuhler J. Erasure of a spinal memory trace of pain by a brief, high-dose opioid administration. Science. 2012;335:235–8. doi: 10.1126/science.1211726. [DOI] [PubMed] [Google Scholar]
- 19.Dubuisson D. Effect of dorsal-column stimulation on gelatinosa and marginal neurons of cat spinal cord. J Neurosurg. 1989;70:257–65. doi: 10.3171/jns.1989.70.2.0257. [DOI] [PubMed] [Google Scholar]
- 20.Falowski S, Sharan A. A review on spinal cord stimulation. J Neurosurg Sci. 2012;56:287–98. [PubMed] [Google Scholar]
- 21.Finnerup NB, Sindrup SH, Jensen TS. Recent advances in pharmacological treatment of neuropathic pain. F1000 Med Rep. 2010;2:52. doi: 10.3410/M2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fitzgerald M, Butcher T, Shortland P. Developmental changes in the laminar termination of A fibre cutaneous sensory afferents in the rat spinal cord dorsal horn. J Comp Neurol. 1994;348:225–33. doi: 10.1002/cne.903480205. [DOI] [PubMed] [Google Scholar]
- 23.Foreman RD, Linderoth B. Neural mechanisms of spinal cord stimulation. Int Rev Neurobiol. 2012;107:87–119. doi: 10.1016/B978-0-12-404706-8.00006-1. [DOI] [PubMed] [Google Scholar]
- 24.Gong WY, Johanek LM, Sluka KA. A Comparison of the Effects of Burst and Tonic Spinal Cord Stimulation on Hyperalgesia and Physical Activity in an Animal Model of Neuropathic Pain. Anesth Analg. 2016 doi: 10.1213/ANE.0000000000001161. [DOI] [PubMed] [Google Scholar]
- 25.Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–25. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan Y. Spinal cord stimulation: neurophysiological and neurochemical mechanisms of action. Curr Pain Headache Rep. 2012;16:217–25. doi: 10.1007/s11916-012-0260-4. [DOI] [PubMed] [Google Scholar]
- 27.Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- 28.He SQ, Li Z, Chu YX, Han L, Xu Q, Li M, Yang F, Liu Q, Tang Z, Wang Y, Hin N, Tsukamoto T, Slusher B, Tiwari V, Shechter R, Wei F, Raja SN, Dong X, Guan Y. MrgC agonism at central terminals of primary sensory neurons inhibits neuropathic pain. Pain. 2014;155:534–44. doi: 10.1016/j.pain.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 30.Kapural L. Spinal cord stimulation for intractable chronic pain. Curr Pain Headache Rep. 2014;18:406. doi: 10.1007/s11916-014-0406-7. [DOI] [PubMed] [Google Scholar]
- 31.Kato A, Punnakkal P, Pernia-Andrade AJ, von SC, Sharopov S, Nyilas R, Katona I, Zeilhofer HU. Endocannabinoid-dependent plasticity at spinal nociceptor synapses. J Physiol. 2012;590:4717–33. doi: 10.1113/jphysiol.2012.234229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, Thomson S, O'Callaghan J, Eisenberg E, Milbouw G, Buchser E, Fortini G, Richardson J, North RB. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132:179–88. doi: 10.1016/j.pain.2007.07.028. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, He SQ, Xu Q, Yang F, Tiwari V, Liu Q, Tang Z, Han L, Chu YX, Wang Y, Hin N, Tsukamoto T, Slusher B, Guan X, Wei F, Raja SN, Dong X, Guan Y. Activation of MrgC receptor inhibits N-type calcium channels in small-diameter primary sensory neurons in mice. Pain. 2014;155:1613–21. doi: 10.1016/j.pain.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang YC, Huang CC, Hsu KS. Therapeutic potential of cannabinoids in trigeminal neuralgia. Curr Drug Targets CNS Neurol Disord. 2004;3:507–14. doi: 10.2174/1568007043336833. [DOI] [PubMed] [Google Scholar]
- 35.Linderoth B, Gazelius B, Franck J, Brodin E. Dorsal column stimulation induces release of serotonin and substance P in the cat dorsal horn. Neurosurgery. 1992;31:289–96. doi: 10.1227/00006123-199208000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Lowin T, Straub RH. Cannabinoid-based drugs targeting CB1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res Ther. 2015;17:226. doi: 10.1186/s13075-015-0743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maeda Y, Wacnik PW, Sluka KA. Low frequencies, but not high frequencies of bi-polar spinal cord stimulation reduce cutaneous and muscle hyperalgesia induced by nerve injury. Pain. 2008;138:143–52. doi: 10.1016/j.pain.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 38.Meisner JG, Marsh AD, Marsh DR. Loss of GABAergic interneurons in laminae I-III of the spinal cord dorsal horn contributes to reduced GABAergic tone and neuropathic pain after spinal cord injury. J Neurotrauma. 2010;27:729–37. doi: 10.1089/neu.2009.1166. [DOI] [PubMed] [Google Scholar]
- 39.Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–9. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- 40.Meyerson BA, Linderoth B. Mode of action of spinal cord stimulation in neuropathic pain. J Pain Symptom Manage. 2006;31:S6–12. doi: 10.1016/j.jpainsymman.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Nakatsuka T, Ataka T, Kumamoto E, Tamaki T, Yoshimura M. Alteration in synaptic inputs through C-afferent fibers to substantia gelatinosa neurons of the rat spinal dorsal horn during postnatal development. Neuroscience. 2000;99:549–56. doi: 10.1016/s0306-4522(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 42.North R, Shipley J, Prager J, Barolat G, Barulich M, Bedder M, Calodney A, Daniels A, Deer T, DeLeon O, Drees S, Fautdch M, Fehrenbach W, Hernandez J, Kloth D, Krames ES, Lubenow T, North R, Osenbach R, Panchal SJ, Sitzman T, Staats P, Tremmel J, Wetzel T American Academy of Pain Medicine. Practice parameters for the use of spinal cord stimulation in the treatment of chronic neuropathic pain. Pain Med. 2007;8(Suppl 4):S200–S275. doi: 10.1111/j.1526-4637.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- 43.Olsson GL, Meyerson BA, Linderoth B. Spinal cord stimulation in adolescents with complex regional pain syndrome type I (CRPS-I) Eur J Pain. 2008;12:53–9. doi: 10.1016/j.ejpain.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Park U, Vastani N, Guan Y, Raja SN, Koltzenburg M, Caterina MJ. TRP vanilloid 2 knock-out mice are susceptible to perinatal lethality but display normal thermal and mechanical nociception. J Neurosci. 2011;31:11425–36. doi: 10.1523/JNEUROSCI.1384-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, Freund TF, Watanabe M, Filitz J, Koppert W, Schuttler J, Ji G, Neugebauer V, Marsicano G, Lutz B, Vanegas H, Zeilhofer HU. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–4. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto V, Derkach VA, Safronov BV. Role of TTX-sensitive and TTX-resistant sodium channels in Adelta- and C-fiber conduction and synaptic transmission. J Neurophysiol. 2008;99:617–28. doi: 10.1152/jn.00944.2007. [DOI] [PubMed] [Google Scholar]
- 47.Sagar DR, Kelly S, Millns PJ, O'Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 2005;22:371–9. doi: 10.1111/j.1460-9568.2005.04206.x. [DOI] [PubMed] [Google Scholar]
- 48.Sandkuhler J, Gruber-Schoffnegger D. Hyperalgesia by synaptic long-term potentiation (LTP): an update. Curr Opin Pharmacol. 2012;12:18–27. doi: 10.1016/j.coph.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schechtmann G, Song Z, Ultenius C, Meyerson BA, Linderoth B. Cholinergic mechanisms involved in the pain relieving effect of spinal cord stimulation in a model of neuropathy. Pain. 2008;139:136–45. doi: 10.1016/j.pain.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Sdrulla AD, Xu Q, He SQ, Tiwari V, Yang F, Zhang C, Shu B, Shechter R, Raja SN, Wang Y, Dong X, Guan Y. Electrical stimulation of low-threshold afferent fibers induces a prolonged synaptic depression in lamina II dorsal horn neurons to high-threshold afferent inputs in mice. Pain. 2015;156:1008–17. doi: 10.1097/01.j.pain.0000460353.15460.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shechter R, Yang F, Xu Q, Cheong YK, He SQ, Sdrulla A, Carteret AF, Wacnik PW, Dong X, Meyer RA, Raja SN, Guan Y. Conventional and Kilohertz-frequency Spinal Cord Stimulation Produces Intensity- and Frequency-dependent Inhibition of Mechanical Hypersensitivity in a Rat Model of Neuropathic Pain. Anesthesiology. 2013;119:422–32. doi: 10.1097/ALN.0b013e31829bd9e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smits H, van KM, Holsheimer J, Joosten EA. Experimental spinal cord stimulation and neuropathic pain: mechanism of action, technical aspects, and effectiveness. Pain Pract. 2013;13:154–68. doi: 10.1111/j.1533-2500.2012.00579.x. [DOI] [PubMed] [Google Scholar]
- 53.Song Z, Ansah OB, Meyerson BA, Pertovaara A, Linderoth B. The rostroventromedial medulla is engaged in the effects of spinal cord stimulation in a rodent model of neuropathic pain. Neuroscience. 2013;247:134–44. doi: 10.1016/j.neuroscience.2013.05.027. [DOI] [PubMed] [Google Scholar]
- 54.Song Z, Meyerson BA, Linderoth B. Spinal 5-HT receptors that contribute to the pain-relieving effects of spinal cord stimulation in a rat model of neuropathy. Pain. 2011;152:1666–73. doi: 10.1016/j.pain.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Starowicz K, Przewlocka B. Modulation of neuropathic-pain-related behaviour by the spinal endocannabinoid/endovanilloid system. Philos Trans R Soc Lond B Biol Sci. 2012;367:3286–99. doi: 10.1098/rstb.2011.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007;55:353–64. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 57.Yang F, Carteret AF, Wacnik PW, Chung CY, Xing L, Dong X, Meyer RA, Raja SN, Guan Y. Bipolar spinal cord stimulation attenuates mechanical hypersensitivity at an intensity that activates a small portion of A-fiber afferents in spinal nerve-injured rats. Neuroscience. 2011;199:470–80. doi: 10.1016/j.neuroscience.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 58.Yang F, Xu Q, Cheong YK, Shechter R, Sdrulla A, He SQ, Tiwari V, Dong X, Wacnik PW, Meyer R, Raja SN, Guan Y. Comparison of intensity-dependent inhibition of spinal wide-dynamic range neurons by dorsal column and peripheral nerve stimulation in a rat model of neuropathic pain. Eur J Pain. 2014;18:978–88. doi: 10.1002/j.1532-2149.2013.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–88. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan S, Burrell BD. Endocannabinoid-dependent LTD in a nociceptive synapse requires activation of a presynaptic TRPV-like receptor. J Neurophysiol. 2010;104:2766–77. doi: 10.1152/jn.00491.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan S, Burrell BD. Long-term depression of nociceptive synapses by non-nociceptive afferent activity: role of endocannabinoids, Ca(2)+, and calcineurin. Brain Res. 2012;1460:1–11. doi: 10.1016/j.brainres.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 62.Yuan S, Burrell BD. Endocannabinoid-dependent long-term depression in a nociceptive synapse requires coordinated presynaptic and postsynaptic transcription and translation. J Neurosci. 2013;33:4349–58. doi: 10.1523/JNEUROSCI.3922-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan S, Burrell BD. Nonnociceptive afferent activity depresses nocifensive behavior and nociceptive synapses via an endocannabinoid-dependent mechanism. J Neurophysiol. 2013;110:2607–16. doi: 10.1152/jn.00170.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu CZ, Mikusa JP, Fan Y, Hollingsworth PR, Pai M, Chandran P, Daza AV, Yao BB, Dart MJ, Meyer MD, Decker MW, Hsieh GC, Honore P. Peripheral and central sites of action for the non-selective cannabinoid agonist WIN 55,212-2 in a rat model of post-operative pain. Br J Pharmacol. 2009;157:645–55. doi: 10.1111/j.1476-5381.2009.00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol. 2013;27:64–80. doi: 10.1111/fcp.12008. [DOI] [PubMed] [Google Scholar]
- 66.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]