Abstract
Itch is a major indicator of psoriasis, but the underlying mechanisms behind this symptom are largely unknown. To investigate the neuronal mechanisms of psoriatic itch, we tested whether mice subjected to the imiquimod-induced psoriasis model exhibit itch-associated behaviors. Mice received daily topical applications of imiquimod to the rostral back skin for seven days. Imiquimod-treated mice exhibited a significant increase in spontaneous scratching behavior directed to the treated area as well as touch-evoked scratching (alloknesis). To characterize this model, we measured the mRNA expression levels of pruritogens and itch-relevant receptors/channels using real-time RT-PCR. The mRNA expression of MrgprA3, MrgprC11, and MrgprD decreased gradually over time in the dorsal root ganglion (DRG) cells. There was no significant change in the mRNA expression of TRPV1 or TRPA1 in DRG cells. TRPV4 mRNA expression was transiently increased in the DRG cells, while TRPM8 mRNA was significantly decreased. The mRNA expression levels of histidine decarboxylase and tryptophan hydroxylase 1, as well as the intensity of histamine and serotonin immunoreactivity, were transiently increased in the skin on day 2, returning to baseline by day 7. Histamine H1 receptor (H1R) antagonists, chlorpheniramine and olopatadine, significantly inhibited spontaneous scratching on day 2, but not day 7. Neither chlorpheniramine nor olopatadine affected alloknesis on day 2 or day 7. These results may reflect the limited antipruritic effects of H1R antagonists on human psoriasis. The imiquimod-induced psoriasis model appears to be useful for the investigation of itch and its sensitization in psoriasis.
Keywords: Psoriasis, chronic itch, histamine, alloknesis, scratching
Introduction
Psoriasis is a chronic inflammatory skin disease affecting up to 2–3% of the world’s population [19; 32]. Psoriasis is characterized by the presence of scaly skin plaques that display histological features including a thickened stratum corneum (hyperkeratosis), retention of nuclei within corneocytes (parakeratosis), and infiltration of inflammatory cells [10]. The term psoriasis is derived from the Greek word “psora,” meaning “itching condition.” Pruritus has been reported in 60–90% of psoriasis patients [30]. One of the aggravating factors of psoriatic itch is contact with clothes [33]. This phenomenon, known as alloknesis, results from the sensitization of itch signaling pathways and is an important aspect of chronic itch. As chronic itch is extremely difficult to treat, there is no universal treatment for psoriatic itch.
The pathogenesis of psoriasis has been investigated through the study of human as well as animal models. Repeated topical application of the drug imiquimod on the skin induces psoriasis-like inflammation in mice as well as humans and is considered a valid model for psoriasis [11; 13; 38]. The pathogenesis of itch in psoriasis is unclear. A recent study found that there is no correlation between the severity of the disease and the intensity of pruritus in patients with psoriasis [31].
Recent studies have revealed a variety of molecules which are relevant to itch signal transmission [1; 8; 21]. Inflammatory mediators including histamine and serotonin are known to act as pruritogens. However, histamine is less likely to be involved in psoriatic itch, as histamine H1 receptor (H1R) antagonists are reported to have limited or weak effects on psoriatic itch [34; 41]. Sensory neurons expressing Mas-related G-protein-coupled receptors (Mrgprs) mediate non-histaminergic itch [23; 24]. MrgprA3- and MrgprD-expressing neurons exhibited enhanced excitability in a mouse model of allergic contact dermatitis [29]. Additionally, thermosensitive TRP channels including TRPV1, TRPA1, TRPV4, and TRPM8 are activated downstreatm of GPCRs to mediate or modulate itch [5; 18; 35; 39].
In this study, we investigated whether an imiquimod-induced psoriasis mouse model exhibited spontaneous scratching as well as itch in response to light touch. We also characterized this model by measuring the gene expression levels of itch-relevant molecules. We further pharmacologically evaluated this model by testing the effects of H1R antagonists on spontaneous and touch-evoked itch.
Materials and Methods
Imiquimod application
Experiments were performed using adult male C57BL/6J mice (21–28 g) under a protocol approved by the Temple University Animal Care and Use Committee. Fur on the rostral back was trimmed with electric clippers and then removed with an electric shaver. The mice received a daily topical application of 62.5 mg Aldara cream (5% imiquimod) on the shaved back skin (2.5 cm × 2 cm) for seven consecutive days. Control mice were treated similarly with a control vehicle cream (Vaseline Lanette cream, Fargon).
Behavioral tests
Mice were habituated twice to a Plexiglas recording arena for 60 min before testing. Twenty to twenty-two hours after each topical application, mice were videotaped from above for 60 min. The number of videotaped scratch bouts was counted by a trained observer blinded to the treatment condition. A scratch bout was defined as one or more rapid back-and-forth hind paw motions directed toward and contacting the treated area, ending with licking or biting of the toes or placement of the hind paw on the floor. Hind paw movements directed away from the treated area (e.g., ear-scratching) and grooming movements were not counted [2; 6; 7]. Following the 60-min recording period, alloknesis was assessed as follows: the mouse received five separate innocuous mechanical stimuli delivered using a von Frey filament (bending force: 0.7 mN) to five randomly selected sites along the border of the cream application area. The 0.7 mN von Frey filament was selected because it does not elicit scratch bouts in naive mice and was the minimum strength to elicit scratch bouts when delivered to skin surrounding the site of either histamine injection or dry skin treatment [3]. The presence or absence of a positive response (a hindlimb scratch bout directed to the site of mechanical stimulation) was noted for each stimulus. The alloknesis score was the total number of positive responses elicited by the 5 stimuli (0 to 5). In H1R antagonist experiments, each animal received a subcutaneous injection of either vehicle (isotonic saline), chlorpheniramine (10 mg/kg; Alfa Aesar, Ward Hill, MA), or olopatadine (10 mg/kg; Sigma-Aldrich, St. Louis, MO) 30 min prior to the behavior recording on either day 2 or day 7.
Real-time qRT-PCR
Cervical dorsal root ganglia (DRG) and skin samples were isolated from mice, submerged in RNAlater (Qiagen, Valencia, CA), and stored at −80°C. Total RNA was extracted using Direct-zol RNA Mini Prep (Zymo Research, Irvine, CA). Reverse transcription of 0.5 μg total RNA was performed using ProtoScript® II First Strand cDNA Synthesis Kit (New England Biolabs, Ipswich, MA). Amplification of GAPDH cDNA was used for normalization. Real-time RT-PCR was performed using Fast Plus EvaGreen qPCR Master Mix (Biotium, Hayward, CA) on a 7500 Real Time PCR System (Applied Biosystems, Grand Island, NY). Forty cycles of amplification were performed involving sequential denaturation at 95°C for 5 s, annealing at 55–60°C for 5 s, and extension at 72°C for 33 s. Assays were validated using serial dilutions and confirmation of equal amplification efficiencies of the cDNA of interest and the GAPDH cDNA. Fold differences in expression were calculated using the comparative CT method by standardizing against GAPDH expression. All primer pairs are listed in Table 1. Gene expression level of enzymes that are responsible for synthesis of pruritogenic inflammatory mediators were measured in skin, while gene expression level of receptors/channels involved in itch transduction (e.g. Mrgprs and TRP channels) were measured in DRG.
Table 1.
List of primers; forward primer (FP) and reverse primer (RP)
| Gene Name | Primer Sequence (5′ → 3′) |
|---|---|
| GAPDH | FP: TCCACTGGCGTCTTCAC RP: GGCAGAGATGATGACCCTTTT |
| Histidine decarboxylase (HDC) | FP: CGTGAATACTACCGAGCTAGAGG RP: ACTCGTTCAATGTCCCCAAAG |
| Tryptophan hydroxylase 1 (TPH1) | FP: AACAAAGACCATTCCTCCGAAAG RP: TGTAACAGGCTCACATGATTCTC |
| MrgprA3 | FP: CTCAAGTTTACCCTACCCAAAGG RP: CCGCAGAAATAACCATCCAGAA |
| MrgprC11 | FP: ACTCTCTGCTACGGATCATTGA RP: TGATTGCTGCATTGCCTAAGATA |
| MrgprD | FP: TTTTCAGTGACATTCCTCGCC RP: GCACATAGACACAGAAGGGAGA |
| TRPV1 | FP: CCCGGAAGACAGATAGCCTGA RP: TTCAATGGCAATGTGTAATGCTG |
| TRPA1 | FP: GTCCAGGGCGTTGTCTATCG RP: CGTGATGCAGAGGACAGAGAT |
| TRPV4 | FP: CCTTGTTCGACTACGGCACTT RP: GGATGGGCCGATTGAAGACTT |
| TRPM8 | FP: ACAGACGTGTCCTACAGTGAC RP: GCTCTGGGCATAACCACACTT |
Immunohistochemistry
Animals were euthanized under sodium pentobarbital anesthesia, and the skin was acutely dissected. Skin was fixed in 4% paraformaldehyde followed by 30% sucrose, frozen in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek, Torrance, CA), and cut in 10-μm sections on a cryostat. The sections were incubated with 5% donkey serum and then immunostained with a rabbit histamine antibody (1:500; ImmunoStar Inc., Hudson, WI) and a rabbit serotonin antibody (1:1000; ImmunoStar Inc.) at 4°C overnight, followed by incubation with the corresponding secondary antibody conjugated with Alexa Fluor 488 (1:300; Life Technologies Inc., Grand Island, NY) for 2 hr. Specificity of the primary antibodies was confirmed by preabsorption of the antibodies with either histamine (Sigma) or serotonin (Alfa Aesar). For triple staining, sections were additionally stained with rhodamine-labeled avidin (1:200; Vector Laboratories), a marker for mast cells [9; 25], at room temperature for 45 minutes. All sections were counterstained with 4′,6-diamino-2-phenylindole (DAPI) in the mounting medium (Vector Laboratories, Burlingame, CA). Images were captured from 3–4 skin sections from each animal were imaged at 10X magnification. Images were evaluated by a trained observer blinded to the treatment condition. The intensity of immunofluorescence within the epidermis was measured using Image J (subtracting background fluorescence), and a mean immunofluorescence was determined for each mouse (4 mice per group).
Histology
Skin was fixed in 4% paraformaldehyde, embedded in paraffin and cut in 10-μm sections on a microtome. The sections were stained with hematoxylin and eosin (H&E) or toluidine blue using standard procedures. The number of mast cells per skin sample (total area of each image = 0.845 mm2) was quantified.
Data analysis
Between-group comparisons were made by one-way or two-way ANOVA followed by the Tukey post-test. In all cases p<0.05 was considered to be significant.
Results
Imiquimod or the control cream was applied on the rostral back skin for seven consecutive days (Fig. 1A). Imiquimod treatment elicited erythema, scaliness, thickening, and infiltration of inflammatory cells (Fig. 1B, C, D, E), consistent with previous studies [37; 38]. Toluidine blue staining showed that there was no change in the number of mast cells in the control (8.8±1.0 cells/mm2, n=3; Fig. 1F arrows) vs. the imiquimod-treated group (8.2±2.1 cells/mm2, n=3; Fig. 1G arrows).
Fig. 1.
Imiquimod treatment on the rostral back causes psoriasis-like skin lesions. A) Timeline for imiquimod-induced psoriasis-like skin inflammation model. Mice were treated with imiquimod every day for seven days (arrowheads). Behavior tests and/or skin dissection were conducted (arrows) 20–22 hours post-treatment. B) Photograph of representative shaved mouse before imiquimod treatment (day 0). C) Photograph of representative shaved mouse treated with imiquimod for seven days. D) Hematoxylin and eosin (H&E)-stained skin on day 0. E) H&E-stained skin on day 7. Scale bar indicates 500 μm. F) Toluidine blue-stained skin on day 0. G) Toluidine blue-stained skin on day 7. Scale bar indicates 500 μm. Arrows indicate mast cells.
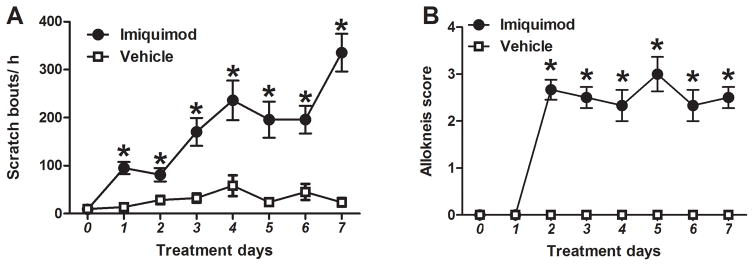
With imiquimod treatment, counts of spontaneous scratch bouts were consistently higher than in control mice and increased gradually over time (p<0.001, F(7, 94)=6.837; Fig. 2A). Vehicle-treated mice exhibited little spontaneous scratching, which increased only slightly over the tested time period. In imiquimod-treated mice, the alloknesis score increased significantly by day 2 and remained at a plateau through day 7 (p<0.001, F(7, 80)=23.111; Fig. 2B). Vehicle-treated mice exhibited an alloknesis score of 0 on all 7 test days.
Fig. 2.
Time-dependent changes in scratch bouts and alloknesis score in imiquimod-treated mice. A) Spontaneous scratching was measured on pretreatment day 0 and imiquimod treatment days 1–7. Black dots (●) and white squares (□) show, respectively, imiquimod- and vehicle- (Vaseline Lanette cream) treated groups. Error bars are SEM (n = 6–8). *p< 0.05, significant difference from day 0, two-way ANOVA followed by Bonferroni test. B) As in A for alloknesis score.
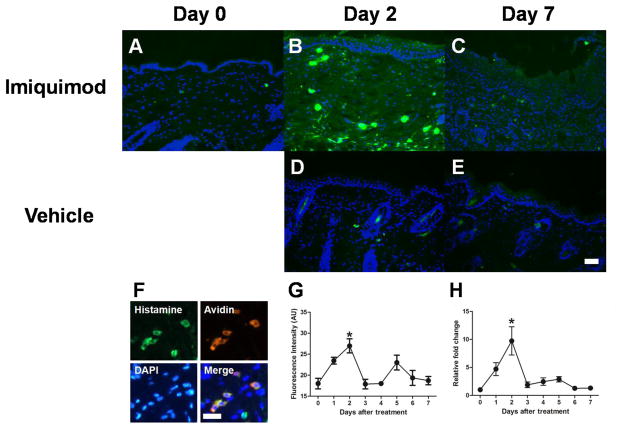
To investigate whether histamine is upregulated in the skin of imiquimod-treated mice, mRNA expression of histidine decarboxylase (HDC), the catabolic enzyme of histamine synthesis, was determined in the skin. A transient increase in HDC mRNA expression was observed, with maximal expression on day 2 (Fig. 3H). Histamine immunoreactivity was detected in mast cells as well as keratinocytes on day 0 (Fig. 3A). Fig. 3G shows a time course for fluorescence intensity of histamine in the epidermis of imiquimod-treated mice. The fluorescence intensity increased transiently on day 2 (Fig. 3B) and returned to the basal level by day 7 (Fig. 3C). Histamine fluorescence intensity did not change over time in the skin of vehicle-treated mice. Histamine immunoreactivity was completely abolished by preabsorption of primary antibody with histamine (data not shown). Seventy nine percent (n=4) of histamine-immunoreactive cells were colocalized with avidin, a marker for mast cells.
Fig. 3.
Time-dependent changes in histamine in imiquimod-treated mice. A–E) Typical examples of histamine (green) and DAPI (blue) expression in the skin treated with imiquimod on day 0 (A), day 2 (B), and day 7 (C), and vehicle on day 2 (D), and day 7 (E). Scale bar indicates 500 μm. F) Triple fluorescence immunohistochemistry for expressions of histamine (green), Avidin (red), and DAPI (blue). Scale bar indicates 250 μm. G) Immunofluorescence intensity of histamine in the epidermis was measured using ImageJ in imiquimod-treated mice. Error bars are SEM (n = 4). *p< 0.05, significant difference from day 0, one-way ANOVA followed by Tukey test. H) Levels of histidine decarboxylase mRNA in the skin were measured using RT-qPCR. Error bars are SEM (n = 6). *p< 0.05, significant difference from day0, one-way ANOVA followed by Tukey test.
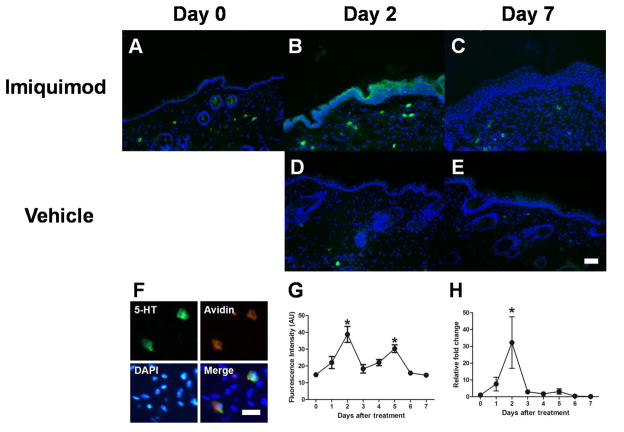
Another pruritogenic inflammatory mediator, serotonin, is synthesized by tryptophan hydroxylase 1 (TPH1). A transient increase in TPH1 mRNA expression was observed with maximal expression on day 2 (Fig. 4H). Serotonin immunoreactivity was detected in mast cells as well as keratinocytes on day 0 (Fig. 4A). Fig. 4G shows a time course for fluorescence intensity of serotonin in the epidermis of imiquimod-treated mice. The fluorescence intensity increased significantly on day 2 (Fig. 4B) and day 5, but returned to the basal level by day 7 (Fig. 4C). Serotonin fluorescence intensity did not change over time in the skin of vehicle-treated mice. Serotonin immunoreactivity was completely abolished by preabsorption of primary antibody with serotonin (data not shown). Ninety three percent (n=4) of serotonin-immunoreactive cells were colocalized with avidin, a marker for mast cells. There was no significant change in the number of histamine- or serotonin-immunoreactive mast cells over time in imiquimod-treated mice (histamine, 4.79–8.89 cells/mm2; serotonin, 3.34–6.19 cells/mm2).
Fig. 4.
Time-dependent changes in serotonin in imiquimod-treated mice. A–E) Typical examples of serotonin (green) and DAPI (blue) expression in the skin treated with imiquimod on day 0 (A), day 2 (B), and day 7 (C), and vehicle on day 2 (D), and day 7 (E). Scale bar indicates 500 μm. F) Triple fluorescence immunohistochemistry for expressions of serotonin (green), Avidin (red), and DAPI (blue). Scale bar indicates 250 μm. G) Immunofluorescence intensity of serotonin in the epidermis was measured using ImageJ in imiquimod-treated mice. Error bars are SEM (n = 4). *p< 0.05, significant difference from day 0, one-way ANOVA followed by Tukey test. H) Levels of tryptophan hydroxylase 1 mRNA in the skin were measured using RT-qPCR. Error bars are SEM (n = 6). *p< 0.05, significant difference from day 0, one-way ANOVA followed by Tukey test.
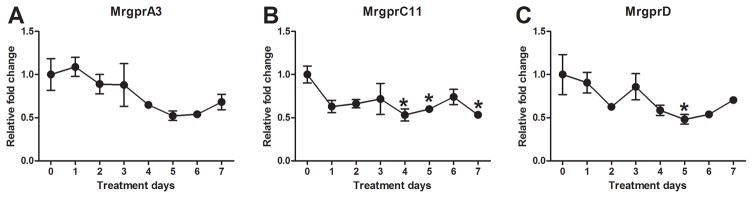
mRNA expression levels of MrgprA3 (Fig. 5A), MrgprC11 (Fig. 5B), and MrgprD (Fig. 5C) all decreased gradually over time in the DRG cells of imiquimod-treated mice. MrgprC11 mRNA showed a significant reduction on days 4, 5, and 7, while MrgprD mRNA was significantly reduced on day 5 alone.
Fig. 5.
Time-dependent changes in Mrgprs in imiquimod-treated mice. A–C) Levels of MrgprA3 (A), MrgprC11 (B), and MrgprD (C) in the DRG cells were measured using RT-qPCR. Error bars are SEM (n = 6). *p< 0.05, significant difference from day 0, one-way ANOVA followed by Tukey test.
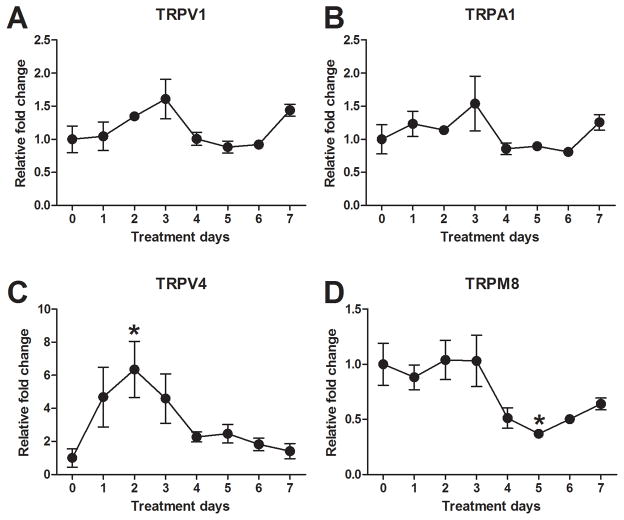
There was no significant change in the mRNA expression of TRPV1 (Fig. 6A) or TRPA1 (Fig. 6B) over time in imiquimod-treated mice. A transient increase in TRPV4 mRNA expression was observed in the DRG cells, with maximal expression on day 2 (Fig. 6C). Interestingly, TRPV4 mRNA decreased significantly over time in the skin of imiquimod-treated mice (data not shown). TRPM8 mRNA in the DRG cells began to decrease on day 4 with maximal reduction on day 5 (Fig. 6D).
Fig. 6.
Time-dependent changes in TRP channels in imiquimod-treated mice. AD) Levels of TRPV1 (A), TRPA1 (B), TRPV4 (C), and TRPM8 (D) in the DRG cells were measured using RT-qPCR. Error bars are SEM (n = 6). *p< 0.05, significant difference from day0, one-way ANOVA followed by Tukey test.
Finally, we investigated the effects of H1R antagonists on itch in this model. Mice were treated with either chlorpheniramine or olopatadine on day 2 of imiquimod treatment, when the histamine expression level had peaked, or day 7, when the histamine expression level had returned to baseline. The number of spontaneous scratch bouts was significantly lower in H1R antagonist-treated mice compared with vehicle-treated mice on day 2 (Fig. 7A). In contrast, there was no significant difference in the number of spontaneous scratch bouts on day 7 (Fig. 7A). Neither chlorpheniramine nor olopatadine inhibited alloknesis on either day 2 or day 7 (Fig. 7B).
Fig. 7.
Effects of histamine H1 receptor antagonists on ongoing (spontaneous) scratching and alloknesis score (touch-evoked scratching). A) A histamine H1 receptor antagonist (chlorpheniramine or olopatadine) or vehicle (isotonic saline) was subcutaneously injected on day 2 or day 7 in imiquimod-treated mice. Mice were videotaped from above for 60 min. White, gray, and black columns show, respectively, vehicle, chlorpheniramine, and olopatadine groups. Error bars are SEM (n = 6–8). *p< 0.05, significant difference from vehicle-treated group, one-way ANOVA followed by Tukey test. B) As in A, for alloknesis score.
Discussion
Chronic itch occurs in the patients with a variety of pathological conditions, including inflammatory skin, systemic, and neurological diseases [1]. Recent studies have revealed the neurotransmitters, receptors, and signal pathways involved in acute itch transduction. However, it is crucial for these findings to be tested in clinically relevant models that mimic specific pathological conditions. Although many animal models for itch in atopic dermatitis have been reported, the availability of animal models of itch related to other diseases is limited [8]. We previously reported that mouse models for itch associated with other skin diseases, such as atopic dermatitis and dry skin, exhibited spontaneous scratching and alloknesis, common manifestations of chronic itch [3; 4; 7]. However, the present study represents the first time that itch has been characterized in a model of psoriasis. We found that the imiquimod-induced psoriasis model exhibited spontaneous scratching and alloknesis, and we characterized the expression of several principal itch-related mediators across the timeline of the model, providing a framework for future work to examine the neuronal mechanisms underlying psoriatic itch and its sensitization.
The number of mast cells did not change over time in the imiquimod-induced psoriasis model. Previous studies reported that there was no significant change in the number of mast cells in lesional vs. non-lesional skin from psoriasis patients, though the number of mast cells was increased after elicitation of Koebner’s phenomenon, (the development of isomorphic pathological lesions in the traumatized, uninvolved skin of psoriasis patients) [28; 36]. Previous work has also found a pronounced infiltration of lymphocytes in lesional skin compared to non-lesional skin of psoriasis patients [28]. In the present study, we similarly observed an increase in lymphocyte infiltration in imiquimod-induced psoriatic skin.
The interstitial histamine concentration in psoriatic skin has previously been analyzed using microdialysis. Two studies showed that dialysate histamine levels in psoriatic skin were higher than those in the skin of healthy subjects [20; 28]. However, a later study reported that similar levels of histamine were observed in skin from healthy subjects, lesional psoriatic skin, and peri-lesional psoriatic skin using a novel rapid and sensitive chromatographic method [14]. In the present study, we found that the expression level of histamine peaked in the early phase and returned to the basal level in the late phase. Moreover, histamine H1 receptor antagonists inhibited spontaneous scratching in the early phase, but not the late phase, suggesting that histamine might play a role in spontaneous itch only in the initial stage of psoriasis.
Serotonin is involved in itch associated with dry skin through the 5-HT2 and possibly 5-HT7 receptors [2; 26; 40]. In the imiquimod-induced psoriasis model, we observed that serotonin was expressed by mast cells as well as keratinocytes. The expression levels of serotonin peaked in the early phase and returned to the basal level in the late phase. A similar chronological change of in serotonin expression was observed in human psoriasis patients [17]. Serotonin-positive expression was stronger in the stratum granulosum of the lesions in the progressive stage of psoriasis than in the static stage. Overall, serotonin does not appear to be a major itch mediator in the static phase of psoriasis.
It has recently been reported that Mrgprs present in subsets of small-diameter sensory neurons play an important role in itch [23; 24]. Unexpectedly, gene expression levels of MrgprA3, MrgprD, and MrgprC11 were decreased in the imiquimod-induced psoriatic itch model. It would be of interest to test whether Mrgpr-expressing neurons exhibit hyper-excitability associated with an increase in sodium current in the imiquimod-induced psoriasis model, as observed in a contact dermatitis model [29].
There are very few studies regarding the role of TRP channels in psoriatic itch. The RNA-seq database indicates a downregulation of TRPV4 mRNA in psoriatic skin [22]. The present study confirms that TRPV4 mRNA is downregulated in the skin of our imiquimod-induced psoriasis model. In contrast, TRPV4 gene expression was transiently increased in the DRG cells. This alteration paralleled the time course of serotonin expression in the skin. We previously showed that TRPV4 appears to function as a downstream transducer of the serotonin-induced itch signal [5]. Increased gene expression levels of TRPV4 in DRG cells may be related to increased expression of serotonin. In contrast, the cold-sensitive ion channel TRPM8 is believed to be involved in itch relief [12; 15; 16] and is negatively regulated following MrgprA3 agonist stimulation [35]. Interestingly, TRPM8 gene expression was decreased in the DRG cells of the imiquimod-induced psoriasis model. Altered TRPM8 expression may be involved in enhancement of itch in the primary sensory neurons through disinhibition of itch signaling. TRPV1 and TRPA1 are required for histaminergic and non-histaminergic itch, respectively. The proportion of TRPA1-expressing DRG neurons was increased in an IL-13-induced inflammatory skin model that exhibited spontaneous scratching [27]. Neither TRPV1 nor TRPA1 gene expression levels significantly changed in the DRG cells of our psoriasis model.
The present study reveals that imiquimod-treated mice represent a useful model to examine the neuronal mechanisms underlying itch and its sensitization in psoriasis for the following reasons: 1) Imiquimod-treated mice exhibit psoriasis-like skin conditions such as erythema, scales, epidermal thickness, and inflammatory cell infiltration, consistent with previous studies [37; 38]. 2) Imiquimod-treated mice exhibit an increase in spontaneous scratching, suggesting that chronic itch occurs in this model. Additionally, imiquimod-treated mice exhibit alloknesis, which is a sign of itch sensitization and is observed in human patients with psoriasis. 3) Histamine H1 receptor antagonists inhibited spontaneous scratching only on day 2 and not on day 7, and failed to inhibit alloknesis on either day 2 or day 7. This presumably reflects the limited anti-pruritic effects of H1R antagonists on psoriatic itch [34; 41]. The imiquimod-induced psoriatic itch model should prove useful in future studies to examine the mechanisms underlying histaminergic (as seen in the early phase) and non-histaminergic itch (as seen in the late phase) as well as the sensitization of itch signaling pathways in psoriasis.
Acknowledgments
The work was supported by grants from the National Institutes of Health (AR063228) and Pfizer. The authors thank Dr. Inami for preparation of cDNA samples. Y.G. has severed as a consultant for Pfizer.
References
- 1.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens MI, Carstens E. Enhanced scratching evoked by PAR-2 agonist and 5-HT but not histamine in a mouse model of chronic dry skin itch. Pain. 2010;151(2):378–383. doi: 10.1016/j.pain.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama T, Carstens MI, Ikoma A, Cevikbas F, Steinhoff M, Carstens E. Mouse model of touch-evoked itch (alloknesis) J Invest Dermatol. 2012;132(7):1886–1891. doi: 10.1038/jid.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens MI, Piecha D, Steppan S, Carstens E. Nalfurafine suppresses pruritogen- and touch-evoked scratching behavior in models of acute and chronic itch in mice. Acta Derm Venereol. 2015;95(2):147–150. doi: 10.2340/00015555-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akiyama T, Ivanov M, Nagamine M, Davoodi A, Iodi Carstens M, Ikoma A, Cevikbas F, Kempkes C, Buddenkotte J, Steinhoff M, Carstens E. Involvement of TRPV4 in serotonin-evoked scratching. J Invest Dermatol. 2016;136(1):154–60. doi: 10.1038/JID.2015.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama T, Merrill AW, Zanotto K, Carstens MI, Carstens E. Scratching behavior and Fos expression in superficial dorsal horn elicited by protease-activated receptor agonists and other itch mediators in mice. J Pharmacol Exp Ther. 2009;329(3):945–951. doi: 10.1124/jpet.109.152256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akiyama T, Nguyen T, Curtis E, Nishida K, Devireddy J, Delahanty J, Carstens MI, Carstens E. A central role for spinal dorsal horn neurons that express neurokinin-1 receptors in chronic itch. Pain. 2015;156(7):1240–1246. doi: 10.1097/j.pain.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bautista DM, Wilson SR, Hoon MA. Why we scratch an itch: the molecules, cells and circuits of itch. Nat Neurosci. 2014;17(2):175–182. doi: 10.1038/nn.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergstresser PR, Tigelaar RE, Tharp MD. Conjugated avidin identifies cutaneous rodent and human mast cells. J Invest Dermatol. 1984;83(3):214–218. doi: 10.1111/1523-1747.ep12263584. [DOI] [PubMed] [Google Scholar]
- 10.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5(9):699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 11.Fanti PA, Dika E, Vaccari S, Miscial C, Varotti C. Generalized psoriasis induced by topical treatment of actinic keratosis with imiquimod. Int J Dermatol. 2006;45(12):1464–1465. doi: 10.1111/j.1365-4632.2006.02980.x. [DOI] [PubMed] [Google Scholar]
- 12.Frolich M, Enk A, Diepgen TL, Weisshaar E. Successful treatment of therapy-resistant pruritus in lichen amyloidosis with menthol. Acta Derm Venereol. 2009;89(5):524–526. doi: 10.2340/00015555-0725. [DOI] [PubMed] [Google Scholar]
- 13.Gilliet M, Conrad C, Geiges M, Cozzio A, Thurlimann W, Burg G, Nestle FO, Dummer R. Psoriasis triggered by toll-like receptor 7 agonist imiquimod in the presence of dermal plasmacytoid dendritic cell precursors. Arch Dermatol. 2004;140(12):1490–1495. doi: 10.1001/archderm.140.12.1490. [DOI] [PubMed] [Google Scholar]
- 14.Guihen E, Ho WL, Hogan AM, O’Connell ML, Leahy MJ, Ramsay B, O’Connor WT. Rapid quantification of histamine in human psoriatic plaques using microdialysis and ultra high performance liquid chromatography with fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;880(1):119–124. doi: 10.1016/j.jchromb.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 15.Han JH, Choi HK, Kim SJ. Topical TRPM8 agonist (icilin) relieved vulva pruritus originating from lichen sclerosus et atrophicus. Acta Derm Venereol. 2012;92(5):561–562. doi: 10.2340/00015555-1244. [DOI] [PubMed] [Google Scholar]
- 16.Haught JM, Jukic DM, English JC., 3rd Hydroxyethyl starch-induced pruritus relieved by a combination of menthol and camphor. J Am Acad Dermatol. 2008;59(1):151–153. doi: 10.1016/j.jaad.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Huang J, Li G, Xiang J, Yin D, Chi R. Immunohistochemical study of serotonin in lesions of psoriasis. Int J Dermatol. 2004;43(6):408–411. doi: 10.1111/j.1365-4632.2004.02195.x. [DOI] [PubMed] [Google Scholar]
- 18.Imamachi N, Park GH, Lee H, Anderson DJ, Simon MI, Basbaum AI, Han SK. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106(27):11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Julia A, Tortosa R, Hernanz JM, Canete JD, Fonseca E, Ferrandiz C, Unamuno P, Puig L, Fernandez-Sueiro JL, Sanmarti R, Rodriguez J, Gratacos J, Dauden E, Sanchez-Carazo JL, Lopez-Estebaranz JL, Moreno-Ramirez D, Queiro R, Montilla C, Torre-Alonso JC, Perez-Venegas JJ, Vanaclocha F, Herrera E, Munoz-Fernandez S, Gonzalez C, Roig D, Erra A, Acosta I, Fernandez-Nebro A, Zarco P, Alonso A, Lopez-Lasanta M, Garcia-Montero A, Gelpi JL, Absher D, Marsal S. Risk variants for psoriasis vulgaris in a large case-control collection and association with clinical subphenotypes. Hum Mol Genet. 2012;21(20):4549–4557. doi: 10.1093/hmg/dds295. [DOI] [PubMed] [Google Scholar]
- 20.Krogstad AL, Lonnroth P, Larson G, Wallin BG. Increased interstitial histamine concentration in the psoriatic plaque. J Invest Dermatol. 1997;109(5):632–635. doi: 10.1111/1523-1747.ep12337620. [DOI] [PubMed] [Google Scholar]
- 21.LaMotte RH, Dong X, Ringkamp M. Sensory neurons and circuits mediating itch. Nat Rev Neurosci. 2014;15(1):19–31. doi: 10.1038/nrn3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B, Tsoi LC, Swindell WR, Gudjonsson JE, Tejasvi T, Johnston A, Ding J, Stuart PE, Xing X, Kochkodan JJ, Voorhees JJ, Kang HM, Nair RP, Abecasis GR, Elder JT. Transcriptome analysis of psoriasis in a large case-control sample: RNA-seq provides insights into disease mechanisms. J Invest Dermatol. 2014;134(7):1828–1838. doi: 10.1038/jid.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Sikand P, Ma C, Tang Z, Han L, Li Z, Sun S, LaMotte RH, Dong X. Mechanisms of itch evoked by beta-alanine. J Neurosci. 2012;32(42):14532–14537. doi: 10.1523/JNEUROSCI.3509-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Q, Tang Z, Surdenikova L, Kim S, Patel KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, Undem BJ, Kollarik M, Chen ZF, Anderson DJ, Dong X. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139(7):1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015;519(7542):237–241. doi: 10.1038/nature14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morita T, McClain SP, Batia LM, Pellegrino M, Wilson SR, Kienzler MA, Lyman K, Olsen AS, Wong JF, Stucky CL, Brem RB, Bautista DM. HTR7 Mediates Serotonergic Acute and Chronic Itch. Neuron. 2015;87(1):124–138. doi: 10.1016/j.neuron.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, Zheng T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol. 2013;191(11):5371–5382. doi: 10.4049/jimmunol.1300300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen LJ, Hansen U, Kristensen JK, Nielsen H, Skov PS, Nielsen HJ. Studies on mast cells and histamine release in psoriasis: the effect of ranitidine. Acta Derm Venereol. 1998;78(3):190–193. doi: 10.1080/000155598441503. [DOI] [PubMed] [Google Scholar]
- 29.Qu L, Fan N, Ma C, Wang T, Han L, Fu K, Wang Y, Shimada SG, Dong X, LaMotte RH. Enhanced excitability of MRGPRA3- and MRGPRD-positive nociceptors in a model of inflammatory itch and pain. Brain. 2014;137(Pt 4):1039–1050. doi: 10.1093/brain/awu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reich A, Szepietowski JC. Clinical Aspects of Itch: Psoriasis. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton (FL): 2014. [PubMed] [Google Scholar]
- 31.Roblin D, Wickramasinghe R, Yosipovitch G. Pruritus severity in patients with psoriasis is not correlated with psoriasis disease severity. J Am Acad Dermatol. 2014;70(2):390–391. doi: 10.1016/j.jaad.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Schon MP, Boehncke WH. Psoriasis. The New England journal of medicine. 2005;352(18):1899–1912. doi: 10.1056/NEJMra041320. [DOI] [PubMed] [Google Scholar]
- 33.Stinco G, Trevisan G, Piccirillo F, Pezzetta S, Errichetti E, di Meo N, Valent F, Patrone P. Pruritus in chronic plaque psoriasis: a questionnaire-based study of 230 Italian patients. Acta Dermatovenerol Croat. 2014;22(2):122–128. [PubMed] [Google Scholar]
- 34.Szepietowski JC, Reich A, Wisnicka B. Itching in patients suffering from psoriasis. Acta Dermatovenerol Croat. 2002;10(4):221–226. [PubMed] [Google Scholar]
- 35.Than JY, Li L, Hasan R, Zhang X. Excitation and modulation of TRPA1, TRPV1, and TRPM8 channel-expressing sensory neurons by the pruritogen chloroquine. J Biol Chem. 2013;288(18):12818–12827. doi: 10.1074/jbc.M113.450072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toruniowa B, Jablonska S. Mast cells in the initial stages of psoriasis. Arch Dermatol Res. 1988;280(4):189–193. doi: 10.1007/BF00513956. [DOI] [PubMed] [Google Scholar]
- 37.Uribe-Herranz M, Lian LH, Hooper KM, Milora KA, Jensen LE. IL-1R1 signaling facilitates Munro’s microabscess formation in psoriasiform imiquimod-induced skin inflammation. J Invest Dermatol. 2013;133(6):1541–1549. doi: 10.1038/jid.2012.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, Cornelissen F, Mus AM, Florencia E, Prens EP, Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182(9):5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 39.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14(5):595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamaguchi T, Nagasawa T, Satoh M, Kuraishi Y. Itch-associated response induced by intradermal serotonin through 5-HT2 receptors in mice. Neurosci Res. 1999;35(2):77–83. doi: 10.1016/s0168-0102(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 41.Yosipovitch G, Goon A, Wee J, Chan YH, Goh CL. The prevalence and clinical characteristics of pruritus among patients with extensive psoriasis. Br J Dermatol. 2000;143(5):969–973. doi: 10.1046/j.1365-2133.2000.03829.x. [DOI] [PubMed] [Google Scholar]