Abstract
Background
We investigate if switching from a ritonavir-boosted lopinavir (LPV/r)-based to an efavirenz-based antiretroviral therapy (ART) regimen is associated with beneficial bone development.
Methods
The CHANGES Bone Study follows HIV-infected children who participated in a non-inferiority randomized trial in Johannesburg, South Africa evaluating the safety and efficacy of pre-emptive switching to efavirenz (N=106) compared to remaining on LPV/r (N=113). HIV-uninfected children were also recruited. Whole body (WB) and lumbar spine bone mineral content (BMC) were assessed by dual-energy X-ray absorptiometry (DXA) at a cross-sectional visit. BMC Z-scores adjusted for sex, age, and height were generated. Physical activity (PA) and dietary intake were assessed. CD4 percentage and viral load were measured. We compared bone indices of HIV-infected to HIV-uninfected children and LPV/r to efavirenz by intent-to-treat.
Results
The 219 HIV-infected (52% male) and 219 HIV-uninfected (55% male) children were 6.4 and 7.0 years of age, respectively. Mean ART duration for HIV-infected children was 5.7 years. WB BMC Z-score was 0.17 lower for HIV-infected children compared to HIV-uninfected children after adjustment for PA, dietary vitamin D and calcium (p=0.03). WB BMC Z-score was 0.55 higher for HIV-infected children switched to efavirenz compared to those remaining on LPV/r after adjustment for PA, dietary vitamin D and calcium, CD4 percentage and viral load (p<0.0001).
Conclusion
South African HIV-infected children receiving ART have lower bone mass compared to HIV-uninfected controls. Accrued bone mass is positively associated with switching to efavirenz-based ART compared to remaining on LPV/r, providing additional rationale for limiting LPV/r exposure once viral suppression has been achieved.
Keywords: bone, antiretroviral therapy, efavirenz, children, pediatrics
Introduction
Advances in antiretroviral therapy (ART) have altered the disease course for the estimated 2.6 million children in the world living with HIV [1]. For those with perinatally-acquired HIV, initiation of ART early in life has resulted in declines in mortality and survival well into adulthood [2–4]. Developing management approaches that optimize long-term outcomes for those with perinatally-acquired HIV has emerged as an important area of clinical and public health research.
Among HIV-infected adults, the incidence of a number of chronic conditions, such as cardiovascular disease and osteoporosis, is increased [5]. The increased risk of osteoporosis and bone fractures in HIV-infected adults compared to the general population [6, 7] is likely due to multiple factors, including effects of HIV-1 viral proteins, inflammatory cytokines and ART on bone cells and bone turnover [8–13]. Lower bone mass accrual is also reported in HIV-infected children and adolescents [14–24]. While manifest later in life, osteoporosis has its origins in patterns of bone growth and turnover in childhood and adolescence when 85–90% of adult peak bone mass is attained [25]. Impaired skeletal growth during these critical periods may compromise bone microarchitecture and peak bone mass that are important determinants of bone strength and fracture risk in later life [26–28]. Few prior studies conducted in children, however, reflect current standards of care that emphasize initiation of ART early in life regardless of clinical or immunologic status. In addition, little is known about bone development among HIV-infected children in sub-Saharan Africa where >90% of HIV-infected youth live [1].
Ritonavir-boosted lopinavir (LPV/r) is recommended by the World Health Organization and widely used as part of the first line regimen for HIV-infected children. While ART regimens containing LPV/r are potent inhibitors of HIV replication and provide a high genetic barrier to emergence of drug resistance, there are a number of limitations for long-term use including poor palatability, dyslipidemias and lipodystrophy [29]. Our group has demonstrated the safety and efficacy of pre-emptive switching to non-nucleoside reverse transcriptase inhibitors in children on LPV/r who have well-suppressed virus [30–32]. Specifically, we recently reported the non-inferiority in virological control through 48 weeks of switching to EFV-based therapy compared to remaining on LPV/r [32]. The objective of the current study was to first to compare bone mass of South African HIV-infected children initiated on ART early in life to an HIV-uninfected control group and secondly, as follow-up of the children enrolled in the non-inferiority trial, to investigate whether a switch from LPV/r to efavirenz for HIV-infected children well-controlled on ART is associated with improved bone development.
Methods
Study population
We present a cross-sectional analysis of 219 HIV-infected and 219 HIV-uninfected children enrolled in the CHANGES Bone Study in Johannesburg, South Africa. The 219 HIV-infected children were enrolled into the study after completion of a non-inferiority randomized clinical trial evaluating the safety and efficacy of pre-emptive switching to efavirenz compared to remaining on LPV/r [32]. All children had been exposed to nevirapine used for prevention of mother-to-child transmission (PMTCT) and initiated on a protease inhibitor-based regimen, mostly LPV/r, at a mean age of 9 months. To be eligible for the clinical trial, children had to be virally suppressed on LPV/r at 3–5 years of age before being randomized to either remain on LPV/r or switch to efavirenz. A group of 219 healthy HIV-uninfected children were recruited for the present study from among eligible siblings or household members of HIV-infected study subjects or those attending the study site for routine outpatient health services. HIV-uninfected children with known chronic medical conditions, including known bone, renal, or liver disease, malabsorption syndrome, or inflammatory bowel disease, were excluded from enrollment.
Measurements and procedures
At the study visit, demographic data were collected and all participants underwent physical examinations to obtain anthropometric measures and to assess pubertal development. Weight was measured to the nearest 0.1 kg using a digital scale and standing height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Weight- (WAZ), height- (HAZ), and BMI-for-age (BAZ) z-scores were calculated using World Health Organization standards [33]. Underweight was defined as WAZ <-2 and stunted was defined as HAZ<-2. Pubertal status was assessed and graded by trained study physicians according the method of Tanner [34, 35]. Females were staged using the highest score of either breast or pubic hair development. At the visit, blood was drawn and CD4 percentages and HIV RNA levels for HIV-infected children were measured (Roche COBAS TaqMan HIV-1 test).
Bone area (BA) in cm2, bone mineral content (BMC) in grams, and areal bone mineral density (BMD) in grams/cm2 of the whole body (WB) (sub-total excluding the head) and lumbar spine (LS) (L1-4) were determined by dual-energy X-ray absorptiometry (DXA) using a Hologic Discovery Wi bone densitometer with Apex software version 3.4 (Hologic Inc, Bedford, MA, USA). Standard operational procedures for obtaining DXA images, data transfer, image reading, quality assurance, and data management were established involving the Department of Radiology of Rahima Moosa Mother and Child Hospital (Johannesburg, South Africa) and Image Reading Center (New York, NY). All scans were analyzed by a single technician blinded to the HIV status and treatments of the participants. LS scans performed in fast array mode were used for this analysis. DXA provides a measure of areal BMD, not volumetric BMD, that systematically underestimates bone mass-for-age in small-for-age children and the International Society for Clinical Densitometry recommends adjusting DXA results for short stature [36]; therefore, we calculated WB and LS BMC Z-scores adjusted for sex, age, race, and height-age Z-score based on reference norms from the United States Bone Mineral Density in Childhood Study [37]. This method accounts for considerable size deficits for children with perinatally-acquired HIV-infection, and correlates well with volumetric BMD determined by quantitative computed tomography [38, 39].
The frequency and duration of physical activities and sedentary behaviors were obtained with a validated interviewer-administered recall questionnaire that detailed all physical activity and inactivity over the past 7 days [40, 41]. The number of minutes of vigorous physical activity per week was calculated as previously described and the proportion of children meeting World Health Organization recommendations for physical activity was determined [42, 43]. Dietary intake was collected by an interviewer-administered 24 hour recall of the previous day’s intake. Total calcium (mg) and vitamin D (IU) intake per day was calculated by the FoodFinder3, software program which includes the South African Food Composition Database [44].
Signed informed consent was provided by each child’s parent or guardian. Children provided assent if they were at least 7 years old and able to understand. The study was approved by the Institutional Review Boards of Columbia University (New York, New York) and the University of the Witwatersrand (Johannesburg, South Africa).
Statistical analysis
We compared characteristics as well as WB and LS BMC, BMD, and BMC Z-scores between 1) HIV-infected and HIV-uninfected children and 2) HIV-infected children randomized to remain on LPV/r and HIV-infected children randomized to switch to efavirenz by intent-to-treat analysis. Linear regression was used to adjust comparisons of Z-scores between the HIV-infected and HIV-uninfected groups for physical activity and dietary vitamin D and calcium, as well to adjust comparisons of Z-scores between children on LPV/r and children on efavirenz for physical activity and dietary vitamin D and calcium, as well as viral load and CD4 percentage and current NRTI backbone (abacavir + lamivudine, stavudine + lamivudine, or zidovudine + lamivudine). All analyses were stratified by sex. Sensitivity analyses were conducted excluding the 12 children who were Tanner Stage 2 at the time of assessment. We also repeated all analyses “as-treated”, comparing bone outcomes for the HIV-infected children based on their current treatment groups (LPV/r vs. efavirenz). In addition, current duration on efavirenz as both a categorical (currently not on efavirenz, on efavirenz 0–24 months, on efavirenz 24 months or greater) and as a continuous variable was assessed. Where applicable, a chi-squared or Fisher’s exact test was used to compare proportions, a t-test was used to compare means, and a Wilcoxon rank sum test was used to compare medians. All p-values are 2-tailed and p-values <0.05 were considered statistically significant. All statistical calculations were performed using SAS version 9.4 (Cary, North Carolina, USA).
Results
Demographic and anthropometric characteristics of the 219 HIV-infected children (49% male) and 219 HIV-uninfected children (55% male) are presented in Table 1. HIV-infected children were younger than controls (mean 6.4 vs. 7.0 years). As expected, WAZ was lower in the HIV-infected group and 11% of the children were underweight compared to 3% in the HIV-uninfected group. Similarly, HAZ was lower in the HIV-infected group and 28% of the children were stunted compared to 9% in the HIV-uninfected group. HIV-infected children engaged in approximately 2 hours fewer of vigorous activity compared to HIV-uninfected children. Mean reported dietary intake of vitamin D (IU/day) and calcium (mg/day) was low in both groups.
Table 1.
Characteristics of 219 HIV-infected and 219 HIV-uninfected children in Johannesburg, South Africa
| Characteristic | HIV-infected (N=219) |
HIV-uninfected (N=219) |
P |
|---|---|---|---|
| Male, N (%) | 107 (48.9) | 120 (54.8) | 0.21 |
| Age in years, Mean (SD) | 6.4 (1.2) | 7.0 (1.5) | <0.0001 |
| Weight in kg, Mean (SD) | 19.2 (3.9) | 22.4 (5.4) | <0.0001 |
| WAZ, Mean (SD) | −0.83 (0.9) | −0.30 (1.1) | <0.0001 |
| Underweight, N (%) | 24 (11.0) | 6 (2.7) | 0.0007 |
| Height in cm, Mean (SD) | 110 (8.3) | 117 (9.6) | <0.0001 |
| HAZ, Mean (SD) | −1.40 (0.9) | −0.82 (0.9) | <0.0001 |
| Stunted, N (%) | 61 (27.9) | 19 (8.7) | <0.0001 |
| BMI, Mean (SD) | 15.7 (1.6) | 16.2 (2.1) | 0.002 |
| BAZ, Mean (SD) | 0.08 (1.0) | 0.27 (1.1) | 0.047 |
| Tanner Stage 1, N (%) | 215 (98.2) | 210 (95.9) | 0.16 |
| Vigorous physical activity in minutes per week, Median (IQR) |
480 (228, 900) | 630 (255, 1095) | 0.05 |
| Meets WHO physical activity guidelines, N (%) |
182 (83.5) | 183 (83.6) | 0.98 |
| Vitamin D in IU, Median (IQR) | 42 (14, 177) | 59 (25, 202) | 0.017 |
| Calcium in mg, Median (IQR) | 276 (155, 459) | 244 (154, 421) | 0.19 |
Abbreviations: WAZ – weight-for-age Z-score; HAZ – height-for-age Z-score; BMI – body mass index; BAZ – BMI-for-age Z-score
Characteristics of the HIV-infected children grouped according to original randomization are presented in Table 2. The children were enrolled into the present study 1–4 years (mean 2.1 years) after randomization in the clinical trial. All HIV-infected children were on a three drug ART regimen. In addition to either LPV/r or efavirenz, all children were receiving two nucleoside reverse transcriptase inhibitors (NRTIs). All were receiving lamivudine and either abacavir, zidovudine, or stavudine. None had any past or current use of tenofovir. None were receiving corticosteroids or antiepileptic medications. The mean (SD) duration on treatment for HIV-infected children was 5.7 (1.1) years (range 2.8–8.7). At the time of evaluation, 93.6% of children had an HIV-1 RNA quantity <400 copies/mL and a mean (SD) CD4 percentage 37.3 (7.1).
Table 2.
Characteristics of 219 HIV-infected children randomized to remain on a ritonavir-boosted lopinavir (LPV/r)-based regimen (N=113) or switch to an efavirenz-based regimen (N=106)
| Characteristic | LPV/r (N=113) |
Efavirenz (N=106) |
P |
|---|---|---|---|
| Male, N (%) | 53 (46.9) | 54 (50.9) | 0.55 |
| Age in years, Mean (SD) | 6.4 (1.3) | 6.3 (1.2) | 0.74 |
| Weight in kg, Mean (SD) | 19.0 (3.6) | 19.4 (4.1) | 0.49 |
| WAZ, Mean (SD) | −0.90 (0.9) | −0.76 (0.9) | 0.25 |
| Underweight, N (%) | 15 (13.3) | 9 (8.5) | 0.26 |
| Height in cm, Mean (SD) | 111 (8.6) | 110 (8.0) | 0.51 |
| HAZ, Mean (SD) | −1.36 (0.9) | −1.45 (0.9) | 0.48 |
| Stunted, N (%) | 30 (26.6) | 31 (29.3) | 0.66 |
| BMI, Mean (SD) | 15.4 (1.4) | 15.9 (1.7) | 0.018 |
| BAZ, Mean (SD) | −0.05 (0.9) | 0.21 (1.0) | 0.046 |
| Tanner Stage 1, N (%) | 113 (100.0) | 102 (96.2) | 0.053 |
| Vigorous physical activity in minutes per week, Median (IQR) |
455 (230, 900) | 505 (225, 870) | 0.99 |
| Meets WHO physical activity guidelines, N (%) | 92 (82.1) | 90 (84.9) | 0.58 |
| Vitamin D in IU, Median (IQR) | 39 (13, 168) | 43 (18, 183) | 0.78 |
| Calcium in mg, Median (IQR) | 290 (162, 504) | 252 (143, 455) | 0.13 |
| Age at ART start in months, Mean (SD) | 9.2 (6.7) | 8.5 (6.8) | 0.49 |
| Age at ART start in months, N (%) <6 6–12 12–24 >24 |
48 (42.5) 33 (29.2) 28 (24.8) 4 (3.5) |
58 (54.7) 23 (21.7) 23 (21.7) 2 (1.9) |
0.30 |
| Treatment duration in years, Mean (SD) | 5.7 (1.1) | 5.7 (1.1) | 0.98 |
| Time since randomization in years, Mean (SD) | 2.2 (0.6) | 2.1 (0.6) | 0.54 |
| Remained on randomized regimen at time of bone assessment, N (%) |
102 (90.3) | 99 (93.4) | 0.40 |
| Current NRTI backbone, N (%) Lamivudine, abacavir Lamivudine, zidovudine Lamivudine, stavudine |
82 (72.6) 4 (3.5) 27 (23.9) |
81 (76.4) 1 (0.9) 24 (22.6) |
0.50 |
| Plasma HIV-1 RNA <400 copies/mL, N (%) | 92.9 | 94.3 | 0.67 |
| CD4 percentage, Mean (SD) | 35.7 (6.6) | 39.0 (7.2) | 0.0006 |
Abbreviations: WAZ – weight-for-age Z-score; HAZ – height-for-age Z-score; BMI – body mass index; BAZ – BMI-for-age Z-score; ART – antiretroviral therapy
DXA results for HIV-infected and uninfected children are presented in Table 3. HIV-infected children had significantly lower WB mean BMC (415 vs. 490 g, p<0.0001) and BMD (0.51 vs. 0.55 g/cm2, p<0.001) compared to the HIV-uninfected group. The BMC Z-score was 0.15 lower in the HIV-infected group compared to the HIV-uninfected group (−0.95 vs. −0.80, p=0.046). The mean LS BMC and BMD were also significantly lower in the HIV-infected groups (14.4 vs 16.1 g, p<0.0001 and 0.46 vs. 0.49 g/cm2, p<0.0001, respectively) but no differences in LS BMC Z-score were observed. Differences in BMC and BMC Z-score at the WB and LS were similar when we excluded the children who were Tanner Stage 2 from the analysis.
Table 3.
Bone parameters by dual x-ray absorptiometry of 219 HIV-infected children (including 113 randomized to remain on LPV/r and 106 randomized to switch to efavirenz) and 219 HIV-uninfected children in Johannesburg, South Africa, Mean (SD)
| Measurement | HIV-infected (N=219) |
HIV-uninfected (N=219) |
P | LPV/r (N=113) |
Efavirenz (N=106) |
P |
|---|---|---|---|---|---|---|
| Total Body | ||||||
| BMC, g | 415 (97) | 490 (119) | <0.001 | 406 (98) | 425 (97) | 0.14 |
| BMC Z-score1 | −0.95 (0.83) | −0.80 (0.77) | 0.046 | −1.20 (0.82) | −0.68 (0.76) | <0.001 |
| BMD, g/cm2 | 0.51 (0.06) | 0.55 (0.07) | <0.001 | 0.50 (0.06) | 0.52 (0.06) | 0.016 |
| Lumbar Spine | ||||||
| BMC, g | 14.4 (3.1) | 16.1 (3.5) | <0.001 | 14.1 (3.0) | 14.8 (3.1) | 0.098 |
| BMC Z-score1 | −0.23 (0.89) | −0.32 (0.85) | 0.28 | −0.45 (0.89) | 0.01 (0.84) | 0.0001 |
| BMD, g/cm2 | 0.46 (0.06) | 0.49 (0.07) | <0.001 | 0.45 (0.06) | 0.47 (0.07) | 0.009 |
Note:
Z-scores from Bone Mineral Density in Childhood Study (adjusted for age, sex, race, and height-for-age Z-score)
Abbreviations: BMC – bone mineral content; BMD – bone mineral density
Children randomized to switch to efavirenz had a higher WB mean BMC (425 vs. 406 g, p=0.14) and BMD (0.52 vs. 0.50 g/cm2, p=0.016) compared to children randomized to remain on LPV/r. The mean LS BMC and BMD were also higher for children in the efavirenz vs. LPV/r group (14.8 vs. 14.1 g, p=0.098 and 0.47 vs. 0.45 g/cm2, p=0.009, respectively). The BMC Z-score for children in the LPV/r group was 0.52 lower than the children in the efavirenz group (−1.20 vs. −0.68, p<0.001).
After adjustment for physical activity and dietary vitamin D and calcium with linear regression, WB BMC Z-score remained 0.17 lower for HIV-infected children compared to HIV-uninfected children (p=0.03). WB BMC Z-score remained approximately 0.55 higher for HIV-infected children in the efavirenz compared to LPV/r group after adjustment for physical activity, dietary vitamin D and calcium, CD4 percentage and viral load (p<0.0001). LS BMC Z-score remained approximately 0.50 higher for HIV-infected children in the efavirenz compared to LPV/r group after adjustment for physical activity, dietary vitamin D and calcium, CD4 percentage and viral load (p=0.0001). WB and LS BMC Z-score differences between the LPV/r and efavirenz groups were similar after further adjustment for current NRTI backbone.
Additional analyses were performed stratified by sex. HIV-infected boys had lower WB BMC Z-score (−0.84 vs. −0.61, p=0.027) compared to HIV-uninfected boys but LS BMC Z-scores were not significantly different (−0.12 vs. −0.19, p=0.53). WB BMC Z-scores were significantly lower in boys on LPV/r compared to boys on efavirenz (−1.02 vs. −0.67, p=0.03). LS BMC Z-score was lower in boys on LPV/r compared to boys on efavirenz albeit not significant (−0.23 vs. −0.01, p=0.15). No statistically significant differences were observed between HIV-infected girls and HIV-uninfected girls in WB BMC Z-score (−1.05 vs. −1.02, p=0.79) or LS BMC Z-score (−0.33 vs −0.47, p=0.26). Girls on LPV/r had lower WB BMC Z-score (−1.37 vs. −0.69, p<0.001) and LS BMC Z-score compared to girls on efavirenz (−0.64 vs. 0.03, p=0.0002).
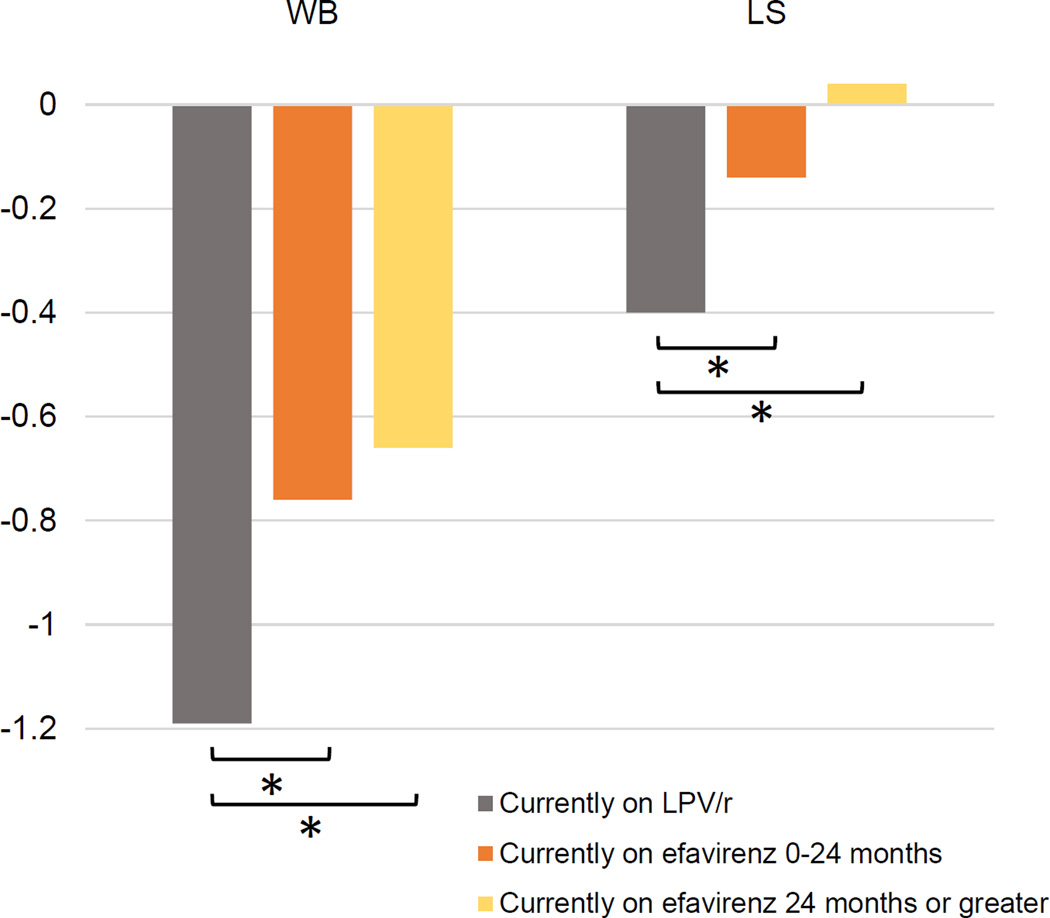
The treatment regimen differences were similar between the intent-to-treat analysis reported above and in as-treated analyses. Mean WB BMC Z-score of HIV-infected children currently on LPV/r, on efavirenz for 0–24 months, and on efavirenz for 24 months or greater are presented in Figure 1. There was a trend towards a greater mean WB BMC Z-score with increased duration on efavirenz (p<0.0001), although there was no significant difference in WB BMC Z-score between children on efavirenz for 0–24 months and 24 months or greater. In an additional regression analysis treating current time on efavirenz as a continuous variable, for every one month increase in current duration on efavirenz use, WB BMC Z-score increased by 0.02 (p=0.0002).
Figure 1.
Mean BMC-Z-score of the whole body (WB) and lumbar spine (LS) of HIV-infected infected children currently on LPV/r, on efavirenz for 0–24 months, and on efavirenz for 24 months or greater (*indicates p<0.05)
Discussion
Deficits in bone mass accrual are detectable in HIV-infected children who initiated ART early and have well-controlled disease. Furthermore, HIV-infected children maintained on a LPV/r-containing regimen ART regimen had lower accrued bone mass compared to those switched to an efavirenz-containing regimen. To our knowledge, this is the first assessment of bone mass in a population of HIV-infected children living in sub-Saharan Africa, the region where >90% of HIV-infected children reside [1].
The finding that bone mass is better among those switched from a LPV/r-based regimen to an efavirenz-based regimen suggests that some of the reduction in attained BMC observed in HIV-infected children compared to uninfected children may be related to LPV/r treatment. Lower bone mass associated with LPV/r has been previously reported [14, 23]. In addition, studies conducted in HIV-infected adults report bone loss in association with many but not all protease inhibitors. A meta-analysis of cross-sectional studies by Brown and Qaqish found an odds ratio for osteoporosis of 1.57 (95% CI 1.05–2.34) in HIV-infected persons treated with protease inhibitors compared with those on non-protease inhibitor-containing ART regimens [6]. The putative mechanisms for disruption of bone homeostasis by protease inhibitors are through direct toxicity to bone cells or bone cell precursors but may vary by specific drug agents [45]. In vitro and in vivo studies have demonstrated that a number of protease inhibitor agents (including ritonavir) inhibit osteoclastogenesis by impairing RANKL-induced signaling [11, 46]. Increased differentiation of peripheral blood mononuclear cells into osteoclasts by upregulation of growth factors and suppression of antagonist transcripts in vitro is also reported with ritonavir [47–49]. Other potential pathways involve drug-induced reductions in mesenchymal stem cell differentiation to osteoblasts as well as alterations in osteoblast gene expression and decreased bone formation [9, 50]. Protease inhibitors may also inhibit conversion of 25-hydroxyvitamin D to the bioactive metabolite 1,25-dihydroxyvitamin D by suppression of 25- and 1a-hydroxylase and thus impair bone formation via disruption of calcium homeostasis [51].
LPV/r, when used in combination with other agents, is a potent inhibitor of HIV replication and is widely used in young children. It was recommended initially as first-line ART for children because drugs used for PMTCT select NNRTI-resistant virus in the majority of infants with vertically-acquired HIV [52]. Furthermore, LPV/r was shown in randomized trials to have superior virological efficacy among both PMTCT-exposed and unexposed infants and young children [53]. For these reasons, LPV/r is recommended as part of first line ART for infants and young children (<3 years) by the World Health Organization [54]. However, LPV/r has a number of limitations of long-term use including poor palatability, dyslipidemia and lipodystrophy [29]. The greater BMC among children who switched to efavirenz from LPV/r we observed may provide additional rationale for limiting LPV/r exposure once viral suppression has been achieved [30, 31, 55].
In addition to ART, HIV-1 viral proteins may also have direct and indirect adverse effects on bone cells. In vitro studies demonstrate that exposure of osteoblast precursors to HIV proteins results in decreased osteogenesis [13], and exposure of osteoclast precursors to HIV proteins results increased osteoclast activation and differentiation [8]. HIV viral proteins have also been shown to induce T cell activation and elaboration of TNFα and RANKL, which induce osteoclastic bone resorption[8]. In our study, bone mass was reduced in HIV-infected children despite viral suppression. Residual low grade viral replication and immune activation are known to persist despite effective ART [56, 57]; therefore, the decreased BMD accrual in our HIV-infected children may be due to the effects of ART exposure as well as the direct effects of HIV viral replication and indirect effects of immune activation on bone cells.
Multiple studies report reduced bone mass accrual among children and adolescents with HIV compared to HIV-uninfected children [14, 21, 58]. These studies have assessed bone mass accrual largely by DXA. Whether these reductions in bone mass largely measured by DXA are solely due to smaller body size has not been established satisfactorily due mainly to inconsistencies in adjusting for smaller body size and selection of comparison group or reference norms. In this study, we observed differences between HIV infected and HIV-uninfected children in BMC Z-scores adjusted for age, sex, race, and HAZ, which account for body size difference, suggesting that decrements in bone mass are out of proportion to reductions in size. Neither do the decreases in bone accrual appear to be due to dietary intake of vitamin D and calcium or physical activity differences.
Although DXA does not distinguish between cortical (or compact) and trabecular bone, we found the differences between HIV-infected and uninfected subjects more consistently in WB BMC and BMD rather than LS similar to a report by Jacobson et al. [59]. Skeletal bone mass is comprised of 80% cortical bone which provides much of the mechanical strength and 20% trabecular bone, whereas the spine is approximately 20% cortical bone and 80% trabecular bone [60] and the distal ends of long bones (e.g. distal radius) is also comprised largely of trabecular bone. Although caution is warranted in interpretation, these findings suggest that during childhood, cortical bone accrual may be more vulnerable to effects of LPV/r. Studies that apply imaging methods such as quantitative computed tomography which provide measures of cortical and trabecular bone are needed to better understand and the importance of these findings to mechanical bone properties and strength and potential for increasing fracture risk.
In this sample the HIV-infected boys appear to have worse measures of bone mass compared to HIV-uninfected boys. This pattern of sex differences in bone outcomes with more unfavorable findings among boys has been reported by others especially after puberty. Jacobson et al. in a U.S. study of 48 perinatally HIV-infected males and females reported sex differences in post-pubertal bone measures, but not at earlier stages of biologic maturation [59]. Post-pubertal HIV-infected males had lower adjusted WB BMC, as well as adjusted WB and LS BMD than controls. Another study conducted in Brazil found no sex differences in patterns of bone measures between HIV-infected boys and girls [61]. Determining whether the sex differences we observed persist throughout the period of skeletal maturation is an important area of future research.
The inclusion of an HIV-uninfected comparison group is a strength. We chose to use population norms drawn from a large study in the United States as there are none available for children in this age group in South Africa or another African country which would have been preferable. Thus, our main outcomes cannot be used for identifying individual study subjects with low bone or estimating the prevalence of low bone mass (e.g. Z-score <-2) as there are important population differences in bone mass characteristics [37]. In addition, although we were able to measure other potential confounders including physical activity, and dietary vitamin D and calcium, these were based on self-report and are subject to non-differential misclassification. Although we were unable to assess independent effects of NRTI on bone, exposure to stavudine vs. abacavir vs. zidovudine did not attenuate the differences between LPV/r and efavirenz. Finally, we are limited by the cross-sectional nature of the study, as we did not have a measure of bone mass available at randomization in the trial.
Bone mass accrual during childhood and adolescence is a major determinant of adult peak bone mass, which in turn is an important determinant of osteoporosis later in life and lifetime fracture risk. Maximizing bone accumulation during childhood may have immediate benefit by reducing fractures in adolescence [62]; however potential deferred benefits of decreasing osteoporosis and fracture in older age are far more important [63]. Mathematical models indicate that relatively small increases (10%) in peak bone mass acquisition in healthy females could delay onset of osteoporosis by as many as 13 years [64], underscoring the importance of identifying strategies to optimize bone acquisition in HIV.
In conclusion, accrued bone mass is positively associated with switching to efavirenz-based ART compared to remaining on LPV/r and switching may help children achieve better peak bone mass. Use of “bone friendly” drugs may be beneficial for bone health in children with HIV.
Acknowledgments
The study was designed by SMA, RS, AC, LK, MTY. Data collection was carried out by RS, FP, NM. Data analysis was performed by SMA, SS, DJM, and MTY. Data was interpreted by SMA, SS, DJM, JJK, and MTY). Manuscript was prepared by SMA, SS, and MTY. All authors participated in the manuscript review and approved the final version of the text as submitted to AIDS (SMA, SS, RS, FP, NM, DJM, JJK, AC, LK, MTY).
Funding for this study was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD073977 and HD073952).
References
- 1.UNAIDS. UNAIDS report on the global AIDS epidemic. 2013 [Google Scholar]
- 2.Dollfus C, Le Chenadec J, Faye A, Blanche S, Briand N, Rouzioux C, et al. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10) Clin Infect Dis. 2010;51:214–224. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
- 3.Foster C, Judd A, Tookey P, Tudor-Williams G, Dunn D, Shingadia D, et al. Young people in the United Kingdom and Ireland with perinatally acquired HIV: the pediatric legacy for adult services. AIDS Patient Care STDS. 2009;23:159–166. doi: 10.1089/apc.2008.0153. [DOI] [PubMed] [Google Scholar]
- 4.Mirani G, Williams PL, Chernoff M, Abzug MJ, Levin MJ, Seage GR, 3rd, et al. Changing Trends in Complications and Mortality Rates Among US Youth and Young Adults With HIV Infection in the Era of Combination Antiretroviral Therapy. Clin Infect Dis. 2015;61:1850–1861. doi: 10.1093/cid/civ687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. A frailty-related phenotype before HAART initiation as an independent risk factor for AIDS or death after HAART among HIV-infected men. J Gerontol A Biol Sci Med Sci. 2011;66:1030–1038. doi: 10.1093/gerona/glr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown TT, Qaqish RB. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. Aids. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 7.Shiau S, Broun EC, Arpadi SM, Yin MT. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. Aids. 2013;27:1949–1957. doi: 10.1097/QAD.0b013e328361d241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fakruddin JM, Laurence J. HIV-1 Vpr enhances production of receptor of activated NF-kappaB ligand (RANKL) via potentiation of glucocorticoid receptor activity. Arch Virol. 2005;150:67–78. doi: 10.1007/s00705-004-0395-7. [DOI] [PubMed] [Google Scholar]
- 9.Malizia AP, Cotter E, Chew N, Powderly WG, Doran PP. HIV protease inhibitors selectively induce gene expression alterations associated with reduced calcium deposition in primary human osteoblasts. AIDS Res Hum Retroviruses. 2007;23:243–250. doi: 10.1089/aid.2006.0084. [DOI] [PubMed] [Google Scholar]
- 10.Mondy K, Yarasheski K, Powderly WG, Whyte M, Claxton S, DeMarco D, et al. Longitudinal evolution of bone mineral density and bone markers in human immunodeficiency virus-infected individuals. Clin Infect Dis. 2003;36:482–490. doi: 10.1086/367569. [DOI] [PubMed] [Google Scholar]
- 11.Wang MW, Wei S, Faccio R, Takeshita S, Tebas P, Powderly WG, et al. The HIV protease inhibitor ritonavir blocks osteoclastogenesis and function by impairing RANKL-induced signaling. J Clin Invest. 2004;114:206–213. doi: 10.1172/JCI15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wong BR, Besser D, Kim N, Arron JR, Vologodskaia M, Hanafusa H, et al. TRANCE, a TNF family member, activates Akt/PKB through a signaling complex involving TRAF6 and c-Src. Mol Cell. 1999;4:1041–1049. doi: 10.1016/s1097-2765(00)80232-4. [DOI] [PubMed] [Google Scholar]
- 13.Cotter EJ, Chew N, Powderly WG, Doran PP. HIV type 1 alters mesenchymal stem cell differentiation potential and cell phenotype ex vivo. AIDS Res Hum Retroviruses. 2011;27:187–199. doi: 10.1089/aid.2010.0114. [DOI] [PubMed] [Google Scholar]
- 14.DiMeglio LA, Wang J, Siberry GK, Miller TL, Geffner ME, Hazra R, et al. Bone mineral density in children and adolescents with perinatal HIV infection. Aids. 2013;27:211–220. doi: 10.1097/QAD.0b013e32835a9b80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hazra R, Gafni RI, Maldarelli F, Balis FM, Tullio AN, DeCarlo E, et al. Tenofovir disoproxil fumarate and an optimized background regimen of antiretroviral agents as salvage therapy for pediatric HIV infection. Pediatrics. 2005;116:e846–e854. doi: 10.1542/peds.2005-0975. [DOI] [PubMed] [Google Scholar]
- 16.Arpadi SM, Horlick M, Thornton J, Cuff PA, Wang J, Kotler DP. Bone mineral content is lower in prepubertal HIV-infected children. J Acquir Immune Defic Syndr. 2002;29:450–454. doi: 10.1097/00126334-200204150-00004. [DOI] [PubMed] [Google Scholar]
- 17.Giacomet V, Mora S, Martelli L, Merlo M, Sciannamblo M, Vigano A. A 12-month treatment with tenofovir does not impair bone mineral accrual in HIV-infected children. J Acquir Immune Defic Syndr. 2005;40:448–450. doi: 10.1097/01.qai.0000184860.62189.c8. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Zamproni I, Beccio S, Bianchi R, Giacomet V, Vigano A. Longitudinal changes of bone mineral density and metabolism in antiretroviral-treated human immunodeficiency virus-infected children. J Clin Endocrinol Metab. 2004;89:24–28. doi: 10.1210/jc.2003-030767. [DOI] [PubMed] [Google Scholar]
- 19.Mora S, Zamproni I, Giacomet V, Cafarelli L, Figini C, Vigano A. Analysis of bone mineral content in horizontally HIV-infected children naive to antiretroviral treatment. Calcif Tissue Int. 2005;76:336–340. doi: 10.1007/pl00020973. [DOI] [PubMed] [Google Scholar]
- 20.Zamboni G, Antoniazzi F, Bertoldo F, Lauriola S, Antozzi L, Tato L. Altered bone metabolism in children infected with human immunodeficiency virus. Acta Paediatr. 2003;92:12–16. doi: 10.1111/j.1651-2227.2003.tb00461.x. [DOI] [PubMed] [Google Scholar]
- 21.Puthanakit T, Saksawad R, Bunupuradah T, Wittawatmongkol O, Chuanjaroen T, Ubolyam S, et al. Prevalence and risk factors of low bone mineral density among perinatally HIV-infected Thai adolescents receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;61:477–483. doi: 10.1097/QAI.0b013e31826ea89b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stagi S, Bindi G, Galluzzi F, Galli L, Salti R, de Martino M. Changed bone status in human immunodeficiency virus type 1 (HIV-1) perinatally infected children is related to low serum free IGF-I. Clin Endocrinol (Oxf) 2004;61:692–699. doi: 10.1111/j.1365-2265.2004.02150.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson DL, Spiegelman D, Duggan C, Weinberg GA, Bechard L, Furuta L, et al. Predictors of bone mineral density in human immunodeficiency virus-1 infected children. J Pediatr Gastroenterol Nutr. 2005;41:339–346. doi: 10.1097/01.mpg.0000174468.75219.30. [DOI] [PubMed] [Google Scholar]
- 24.Rosso R, Vignolo M, Parodi A, Di Biagio A, Sormani MP, Bassetti M, et al. Bone quality in perinatally HIV-infected children: role of age, sex, growth, HIV infection, and antiretroviral therapy. AIDS Res Hum Retroviruses. 2005;21:927–932. doi: 10.1089/aid.2005.21.927. [DOI] [PubMed] [Google Scholar]
- 25.Sabatier JP, Guaydier-Souquieres G, Laroche D, Benmalek A, Fournier L, Guillon-Metz F, et al. Bone mineral acquisition during adolescence and early adulthood: a study in 574 healthy females 10–24 years of age. Osteoporos Int. 1996;6:141–148. doi: 10.1007/BF01623938. [DOI] [PubMed] [Google Scholar]
- 26.Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res. 2000;15:2245–2250. doi: 10.1359/jbmr.2000.15.11.2245. [DOI] [PubMed] [Google Scholar]
- 27.Theintz G, Buchs B, Rizzoli R, Slosman D, Clavien H, Sizonenko PC, et al. Longitudinal monitoring of bone mass accumulation in healthy adolescents: evidence for a marked reduction after 16 years of age at the levels of lumbar spine and femoral neck in female subjects. J Clin Endocrinol Metab. 1992;75:1060–1065. doi: 10.1210/jcem.75.4.1400871. [DOI] [PubMed] [Google Scholar]
- 28.Heaney RP, Abrams S, Dawson-Hughes B, Looker A, Marcus R, Matkovic V, et al. Peak bone mass. Osteoporos Int. 2000;11:985–1009. doi: 10.1007/s001980070020. [DOI] [PubMed] [Google Scholar]
- 29.Arpadi S, Shiau S, Strehlau R, Martens L, Patel F, Coovadia A, et al. Metabolic abnormalities and body composition of HIV-infected children on Lopinavir or Nevirapine-based antiretroviral therapy. Arch Dis Child. 2013;98:258–264. doi: 10.1136/archdischild-2012-302633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coovadia A, Abrams EJ, Stehlau R, Meyers T, Martens L, Sherman G, et al. Reuse of nevirapine in exposed HIV-infected children after protease inhibitor-based viral suppression: a randomized controlled trial. Jama. 2010;304:1082–1090. doi: 10.1001/jama.2010.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuhn L, Coovadia A, Strehlau R, Martens L, Hu CC, Meyers T, et al. Switching children previously exposed to nevirapine to nevirapine-based treatment after initial suppression with a protease-inhibitor-based regimen: long-term follow-up of a randomised, open-label trial. Lancet Infect Dis. 2012;12:521–530. doi: 10.1016/S1473-3099(12)70051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coovadia A, Abrams EJ, Strehlau R, Shiau S, Pinillos F, Martens L, et al. Efavirenz-Based Antiretroviral Therapy Among Nevirapine-Exposed HIV-Infected Children in South Africa: A Randomized Clinical Trial. Jama. 2015;314:1808–1817. doi: 10.1001/jama.2015.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Child Growth Standards. 2007 Available at: http://www.who.int/childgrowth/en/
- 34.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon CM, Leonard MB, Zemel BS. 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17:219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Zemel BS, Kalkwarf HJ, Gilsanz V, Lappe JM, Oberfield S, Shepherd JA, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–3169. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zemel BS, Leonard MB, Kelly A, Lappe JM, Gilsanz V, Oberfield S, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34:1044–1052. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 40.McVeigh J, Meiring R. Physical activity and sedentary behavior in an ethnically diverse group of South african school children. J Sports Sci Med. 2014;13:371–378. [PMC free article] [PubMed] [Google Scholar]
- 41.McVeigh JA, Norris SA, Pettifor JM. Bone mass accretion rates in pre- and early-pubertal South African black and white children in relation to habitual physical activity and dietary calcium intakes. Acta Paediatr. 2007;96:874–880. doi: 10.1111/j.1651-2227.2007.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong M, Shiau S, Yin MT, Strehlau R, Patel F, Coovadia A, et al. Decreased Vigorous Physical Activity in School-Aged Children with Human Immunodeficiency Virus in Johannesburg, South Africa. J Pediatr. 2016 doi: 10.1016/j.jpeds.2016.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. Geneva, Switzerland: World Health Organization; 2010. Global recommendations on physical activity for health. [PubMed] [Google Scholar]
- 44.FoodFinder3. Dietary Analysis Software. Parow Valley, Cape Town: Medical Research Council; 2002. [Google Scholar]
- 45.Moran CA, Weitzmann MN, Ofotokun I. The protease inhibitors and HIV-associated bone loss. Curr Opin HIV AIDS. 2016 doi: 10.1097/COH.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jain RG, Lenhard JM. Select HIV protease inhibitors alter bone and fat metabolism ex vivo. J Biol Chem. 2002;277:19247–19250. doi: 10.1074/jbc.C200069200. [DOI] [PubMed] [Google Scholar]
- 47.Modarresi R, Xiang Z, Yin M, Laurence J. WNT/beta-catenin signaling is involved in regulation of osteoclast differentiation by human immunodeficiency virus protease inhibitor ritonavir: relationship to human immunodeficiency virus-linked bone mineral loss. Am J Pathol. 2009;174:123–135. doi: 10.2353/ajpath.2009.080484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yin MT, Modarresi R, Shane E, Santiago F, Ferris DC, McMahon DJ, et al. Effects of HIV infection and antiretroviral therapy with ritonavir on induction of osteoclast-like cells in postmenopausal women. Osteoporos Int. 2011;22:1459–1468. doi: 10.1007/s00198-010-1363-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Santiago F, Oguma J, Brown AM, Laurence J. Noncanonical Wnt signaling promotes osteoclast differentiation and is facilitated by the human immunodeficiency virus protease inhibitor ritonavir. Biochem Biophys Res Commun. 2012;417:223–230. doi: 10.1016/j.bbrc.2011.11.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernandez-Vallejo SJ, Beaupere C, Larghero J, Capeau J, Lagathu C. HIV protease inhibitors induce senescence and alter osteoblastic potential of human bone marrow mesenchymal stem cells: beneficial effect of pravastatin. Aging Cell. 2013;12:955–965. doi: 10.1111/acel.12119. [DOI] [PubMed] [Google Scholar]
- 51.Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. Aids. 2003;17:513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- 52.Arrive E, Newell ML, Ekouevi DK, Chaix ML, Thiebaut R, Masquelier B, et al. Prevalence of resistance to nevirapine in mothers and children after single-dose exposure to prevent vertical transmission of HIV-1: a meta-analysis. Int J Epidemiol. 2007;36:1009–1021. doi: 10.1093/ije/dym104. [DOI] [PubMed] [Google Scholar]
- 53.Violari A, Lindsey JC, Hughes MD, Mujuru HA, Barlow-Mosha L, Kamthunzi P, et al. Nevirapine versus ritonavir-boosted lopinavir for HIV-infected children. N Engl J Med. 2012;366:2380–2389. doi: 10.1056/NEJMoa1113249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Geneva, Switzerland: World Health Organization; 2015. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Google Scholar]
- 55.Coovadia A, Abrams EJ, Strehlau R, Shiau S, Pinillos F, Martens L, et al. Efavirenz-based antiretroviral therapy among nevirapine-exposed HIV-infected children in South Africa: a randomized clinical trial. Under Review. 2015 doi: 10.1001/jama.2015.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 2010;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 57.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mora S, Sala N, Bricalli D, Zuin G, Chiumello G, Vigano A. Bone mineral loss through increased bone turnover in HIV-infected children treated with highly active antiretroviral therapy. Aids. 2001;15:1823–1829. doi: 10.1097/00002030-200109280-00011. [DOI] [PubMed] [Google Scholar]
- 59.Jacobson DL, Lindsey JC, Gordon CM, Moye J, Hardin DS, Mulligan K, et al. Total body and spinal bone mineral density across Tanner stage in perinatally HIV-infected and uninfected children and youth in PACTG 1045. Aids. 2010;24:687–696. doi: 10.1097/QAD.0b013e328336095d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lima LR, Silva RC, Giuliano Ide C, Sakuno T, Brincas SM, Carvalho AP. Bone mass in children and adolescents infected with human immunodeficiency virus. J Pediatr (Rio J) 2013;89:91–99. doi: 10.1016/j.jped.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 62.Clark EM, Ness AR, Bishop NJ, Tobias JH. Association between bone mass and fractures in children: a prospective cohort study. J Bone Miner Res. 2006;21:1489–1495. doi: 10.1359/jbmr.060601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ferrari SL, Chevalley T, Bonjour JP, Rizzoli R. Childhood fractures are associated with decreased bone mass gain during puberty: an early marker of persistent bone fragility? Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:501–507. doi: 10.1359/jbmr.051215. [DOI] [PubMed] [Google Scholar]
- 64.Hernandez CJ, Beaupre GS, Carter DR. A theoretical analysis of the relative influences of peak BMD age-related bone loss and menopause on the development of osteoporosis. Osteoporos Int. 2003;14:843–847. doi: 10.1007/s00198-003-1454-8. [DOI] [PubMed] [Google Scholar]