Abstract
Objective
Estimates for the contribution of transmission arising from acute HIV infections (AHI) to overall HIV incidence vary significantly. Furthermore, little is known about AHI-attributable transmission among people who inject drugs (PWID), including the extent to which interventions targeting chronic infections (e.g., highly active antiretroviral therapy [HAART] as prevention) are limited by AHI transmission. Thus, we estimated the proportion of transmission events attributable to AHI within the mature HIV epidemic among PWID in New York City (NYC).
Design
Modeling study
Methods
We constructed an interactive sexual and injecting transmission network using an agent-based model simulating the HIV epidemic in NYC between 1996–2012. Using stochastic microsimulations, we catalogued transmission from PWID based on the disease stage of index agents to determine the proportion of infections transmitted during AHI (in primary analyses, assumed to last three months).
Results
Our calibrated model approximated the epidemiological features of the mature HIV epidemic in NYC between 1996–2012. Annual HIV incidence among PWID dropped from approximately 1.8% in 1996 to 0.7% in 2012. Over the sixteen-year period, AHI accounted for 4.9% (10th/90th percentiles: 0.1%–12.3%) of incident HIV cases among PWID. The annualized contribution of AHI increased over this period from 3.6% in 1996 to 5.9% in 2012.
Conclusions
Our results suggest that, in mature epidemics such as NYC, between 3–6% of transmission events are attributable to acute HIV infection among people who inject drugs. Current HIV treatment as prevention strategies are unlikely to be substantially affected by AHI-attributable transmission among PWID populations in mature epidemic settings.
Keywords: People who inject drugs, PWID, Acute HIV Infection, HIV, treatment as prevention
Introduction
People who inject drugs (PWID) continue to be among the populations most heavily affected by HIV. Estimates suggest that 16 million people worldwide inject drugs [1], comprising approximately 0.2% of the global population [2]. Despite this relatively modest portion of the world population, PWID account for about 10% of incident HIV infections annually [3]. Many settings with mature PWID epidemics (e.g., New York City (NYC), France, and Vancouver) have provided examples of how to achieve stark reductions in HIV incidence [4]. One of the prominent features of these settings has been the expansion of highly active antiretroviral therapy (HAART) among PWID [4, 5], utilizing the principle of treatment as prevention (TasP) to decrease HIV transmission [6]. Prevention strategies based on TasP have played a key role in reducing HIV incidence among PWID in settings that encourage HIV testing, linkage to HIV care, and harm reduction services [5, 7].
There has been concern, however, regarding the ability of TasP interventions to address transmission events occurring early in HIV disease, due to the difficulty of early HIV diagnosis among many high risk groups (e.g., men who has sex with men [MSM]) [8–11]. Early detection may be difficult due to the large portion of individuals who are asymptomatic, or present with symptoms consistent with other infections, such as mononucleosis or influenza [12, 13]. Finally, PWID in particular face many barriers to HIV testing, including distrust of medical professionals, hostility at health centers, fear of prosecution, stigma, lack of HIV testing in substance abuse treatment programs, and competing concerns such as homelessness and poverty [14–17].
Although estimates for the precise infectiousness of individuals experiencing AHI have been notoriously difficult to produce—due in part to individual heterogeneity in viral load trajectories, HIV subtype, and immune response—evidence has consistently indicated an increased infectiousness when compared to chronic HIV infection [18–20]. One study examining heterosexual transmission in Malawi estimated that 38% of HIV transmission events were attributable to individuals within five months of infection, suggesting that strategies which focus on identifying and treating chronic infections (i.e., TasP) would be of limited effectiveness [9]. However, estimates of AHI-attributable transmission have ranged from just 2% to the majority, and have focused almost exclusively on non-drug-using populations, in which sex is the predominant mode of transmission [9, 10, 12, 18, 21–23]. Due to different risk behaviors, network structure, and distinct barriers to treatment and testing [24], these results may not be generalizable to PWID populations. For instance, studies have shown PWID networks to have high density [25], and rapid turnover of network members [26], each characteristics that may increase the likelihood of AHI transmission.
Using an agent-based microsimulation model, we sought to estimate the contribution of AHI-attributable transmission among PWID in an urban, mature HIV epidemic setting, based on data from NYC. For the purposes of our analysis, we define a mature epidemic setting as a setting with on-going, endemic HIV transmission within the PWID population, and with evidence of either stable or previously peaked HIV prevalence among PWID, many of which have been previously described [4].
Methods
Setting
The agent-based model (ABM) used for this analysis generated a virtual population of agents representing the demographics, characteristics, and risk-behavior of a large urban population experiencing a mature HIV epidemic over a sixteen-year period, as observed in NYC between 1996–2012. Previous studies have demonstrated the model’s capability to reproduce fundamental characteristics of the HIV epidemic in NYC, including HIV incidence, prevalence, and AIDS-related mortality [27, 28]. Model parameters were based on data collected from observational studies among PWID in NYC and elsewhere, and were subsequently calibrated to match HIV surveillance and observational estimates of the HIV epidemic in NYC from 1996–2012 (see Model Calibration in Supplemental Material). Although the model initializes the dynamic network in 1992, the first four years of the model simulation (i.e., the burn-in period) are not included in the results, as this period was necessary to permit time for certain parameters (e.g., the proportion of the HIV positive population experiencing acute infection) to reach a steady state, and to permit the calculation of annualized estimates that require at least 12 months of estimated output.
Model population/network
Agents within the model population represent individuals with given sets of demographic, behavioral, and clinical characteristics. These characteristics are stochastically assigned to each new agent during the simulation initiation or following the death of another agent (the population remains at a constant 100,000). The relative proportions of each key sub-population were chosen to represent the entire population of a mixed epidemic in a major urban setting (see Supplemental Material). We defined PWID as those who injected an illicit drug in the past month, estimating their initiation and cessation of drug injection using empirical data and previously published calibration procedures [27, 29]. We correspondingly categorized other agents based on their past-year usage or non-usage of other non-injection drugs (see Supplemental material). At model initialization 1.9% of agents are classified as PWID, approximately 43% of whom have HIV infection (approximating the highly endemic setting of NYC in 1992) [30, 31]. Also, agents are stratified by sex, and also by sexual behavior (e.g., heterosexuals, men who have sex with men [MSM], and women who have sex with women).
Relationships and risk behaviors within the agent network
Agents are represented by nodes, and relationships with other agents are represented by links (i.e., edges) between nodes, forming dyads or clusters through which HIV transmission can occur (see below). These links may represent an exclusively sexual relationship, a syringe and needle-sharing relationship (for PWID), or both, at any given time-step. Each discrete time-step represents the state of the network for one month. At the transition point between each time-step, the number of links per agent is determined stochastically by a series of network degree distributions, which are unique for each agent subpopulation, and parameterized using published sexual and injecting network data [32, 33]. Thus, relationships between agents are formed, retained, or broken during transitions between time-steps, representing an evolving population-based network.
Transmission of HIV within the agent population
All HIV negative agents who share a network connection (sexual or injection) with an HIV positive agent have the potential to acquire HIV infection. The risk of transmission is stochastic; the probability of a transmission event is based on key characteristics: (1) the type of risk behavior (sexual or injection); (2) the positive agent’s HIV disease stage (acute, chronic, or AIDS); (3) HAART treatment status of the positive agent; (4) the prevalence of use of a prophylaxis modality (e.g., condom use); and (5), if the risk behavior includes injection, the substance abuse treatment status of the infected agent and whether the agent has “access” to NSP. Furthermore, HAART treatment status is stratified by level of adherence (see HIV Disease Progression and Treatment in Supplemental Material) and risk from sexual transmission is stratified by agent characteristics (i.e., heterosexual, MSM or WSW).
In the model, HAART enrollment is based on empirical data (see Tables S2–S4 in Supplemental Material), subsequently PWID are less likely than the general HIV positive population to initiate HAART, however, those enrolled in substance abuse treatment have an increased probability of treatment initiation. The proportion of each agent class in the respective adherence categories can be found in Tables S2–S4 of the Supplemental Material. The effect of HAART adherence on HIV transmission risks was parameterized based on data from existing studies (see Table S5 in Supplemental Material).
The estimated duration of AHI has been studied previously [11, 12, 18, 34]. Although there is no consensus, and actual AHI duration among patients is believed to vary greatly, we assumed a three-month duration of AHI for the main analysis, as posited by Hollingsworth et al [18]. Estimates for the per-act probability of transmission across each stage of HIV disease and resulting from a given type of exposure (i.e., needle-sharing, sexual contact) have varied greatly throughout prior research [19, 20, 35–37]. Additionally, estimates for the relative transmission probability of those with acute versus chronic HIV infection have likewise been inconsistent [9, 18, 20, 23]. Although recent estimates for the relative infectiousness of AHI have decreased by about an order of magnitude from those published earlier [8, 20], no definitive estimate has emerged. For this model, per-act transmission probabilities were first approximated using empirical and meta-analytic data [35, 38, 39], and subsequently calibrated to fit model output (see HIV Transmission in Supplemental Material). Specifically, the relative infectiousness of acute versus chronic HIV infection was assumed to be 10-fold. This estimate lies approximately at the center of selected published estimates [8, 18, 20]. We conducted a series of sensitivity analyses to account for uncertainty in these estimates (see below).
AHI-attributable transmission among PWID
Given that PWID can interact with other agent classes, transmission between PWID and non-PWID agents creates the need for a specific interpretation of AHI-attributable events among the PWID population. To this end, our estimate is defined as the proportion of HIV transmissions arising from acutely infected PWID, out of all HIV transmissions arising from PWID. The results presented for the main analysis correspond to the mean output values over 500 Monte Carlo (MC) runs of the full model (and are presented alongside the 10th and 90th percentile values from these simulations).
Sensitivity Analyses
Sensitivity analyses were performed on factors suspected to strongly influence the main estimate for the proportion infections attributable to AHI. Namely, we conducted three series of sensitivity analyses, varying: the duration of AHI (1 month, 4 months, and 6 months, respectively); the rate of initiation of HAART (50% reduction and 200% increase compared to the main analysis); and the relative infectiousness of AHI as compared to chronic infection (3-fold, 5-fold, and 20-fold, respectively). Results from the sensitivity analyses represent the mean estimated values over 10 MC runs.
Results
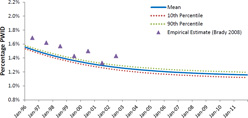
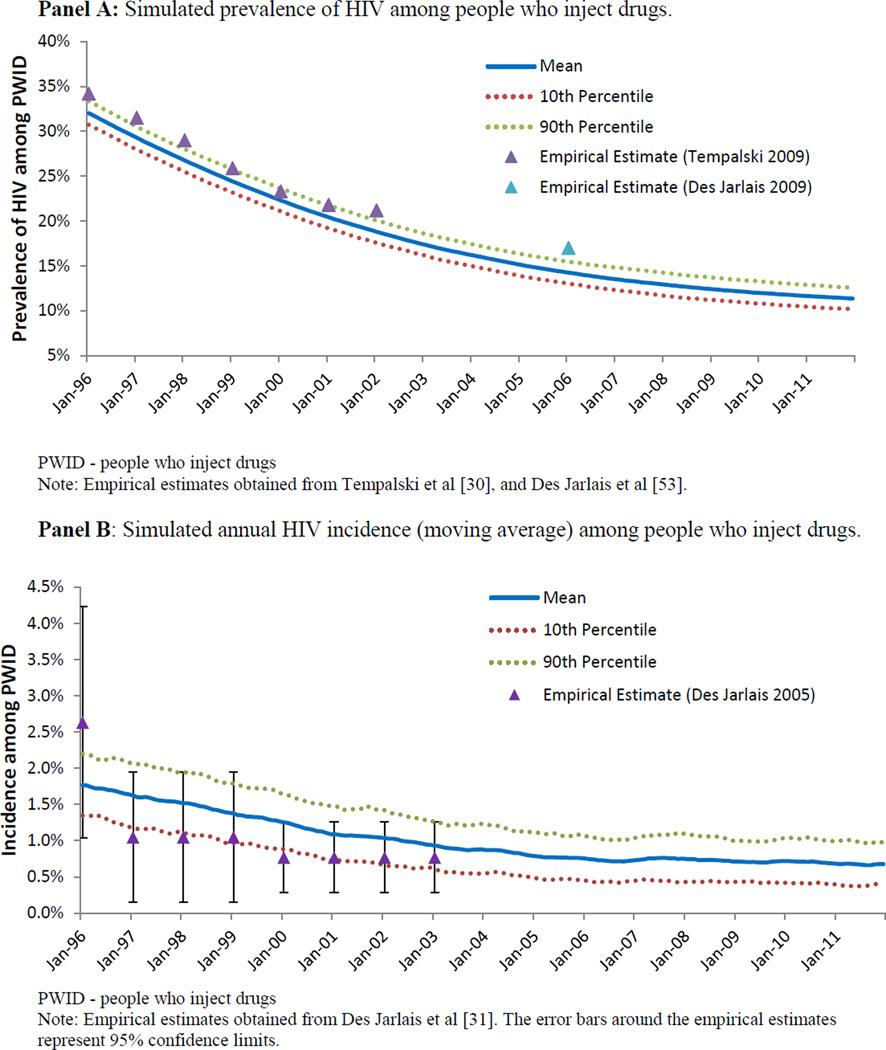
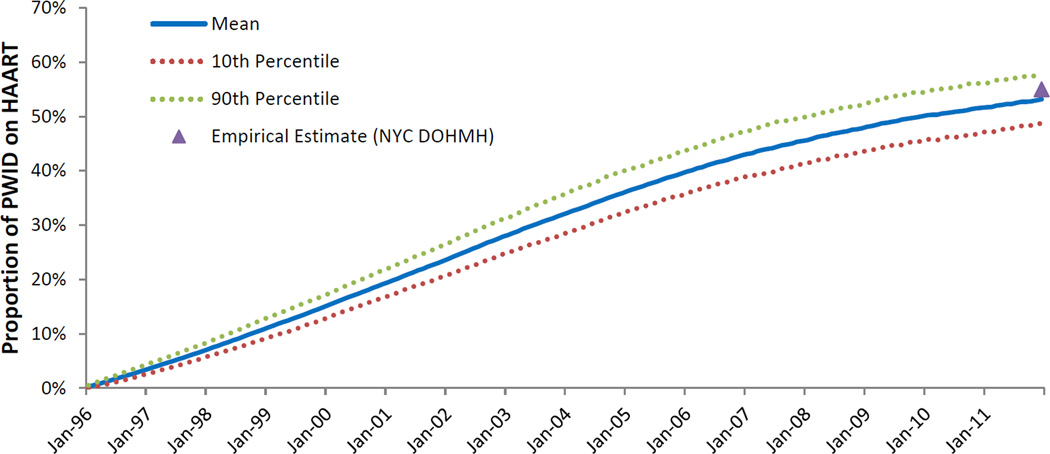
The ABM simulated a sixteen-year period of a mature HIV epidemic among PWID. The ABM was calibrated to match the characteristics of the mature HIV epidemic among PWID in NYC, and reproducing the epidemic trajectory in NYC from 1996 to 2012. During this time, we observed a decrease in the proportion of the population identified as PWID from 1.9% to 1.2% (Figure 1); we also observed a substantial decrease in HIV prevalence among PWID, from 43% to 10% (Figure 2, Panel A). HIV incidence among PWID decreased substantially over the course of the study period, from approximately 1.7 per 100 person-years in the beginning of 1996, to approximately 0.7 per 100 person-years at the end of 2011 (Figure 2, Panel B). As shown in Figure 3, HAART enrollment among PWID began in 1996 and steadily increased to approximately 52% by the end of the study period. Over the 16-year study period, HIV transmission via needle-sharing comprised about 69% of total HIV transmission arising from PWID, with the other 31% transmitted via sexual risk behavior. AIDS prevalence among PWID decreased from about 13% at the beginning of the simulation to 7% at the end of 2011. The estimated mean annual mortality rate for PWID over the sixteen-year study period was 3.3 per 100 person-years, a figure influenced heavily by AIDS-related mortality early in the study period. As HAART coverage increased, and the prevalence of AIDS and its associated mortality decreased, overall mortality among PWID decreased to approximately 2.4 per 100 person-years at the end of the study period.
Figure 1.
Percentage of the model population based on New York City that has injected drugs in the previous twelve months
PWID - people who inject drugs
Note: Empirical estimates obtained from Brady et al [52].
Figure 2.
Epidemiological output of an agent-based model representing HIV transmission among people who inject drugs in a mature HIV epidemic setting, based on New York City.
Figure 3.
Simulated proportion of HIV-positive people who inject drugs on highly-active antiretroviral therapy
HAART - highly active antiretroviral therapy; NYC DOHMH – New York City Department of Health and Mental Hygiene; PWID - people who inject drugs
Note: Empirical estimates obtained from the NYC DOHMH [54].
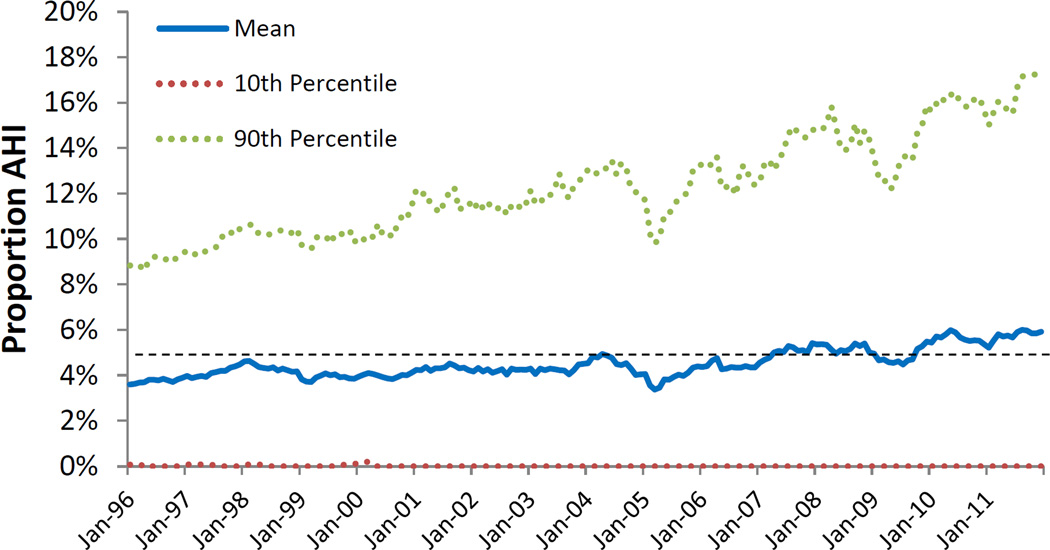
The contribution of AHI-attributable infections to total incident HIV infections arising from PWID over the lifetime of the model simulation is shown in Figure 4. Over the entire sixteen-year period, the cumulative transmission attributable to AHI accounted for 4.9% (10th/90th Percentiles: 0.1%–12.3%) of overall transmission from PWID. This result was not static, however, over the course of the sixteen-year period. Our estimates indicate the contribution of acute infection to overall transmission of HIV nearly doubled from 1996 to 2012, with estimates of 3.6% in 1996, and 5.9% at the end of 2011.
Figure 4.
The simulated proportion of all HIV transmission from people who inject drugs that is attributable to acute HIV infection
AHI – Acute HIV infection
Note: The dashed black line represents the cumulative estimate (4.9%) for the entire 16-year period. The values for the dotted red series (10th percentile) are zero for the majority of data points due to the rarity of acute transmission events in a small population over the course of a single month, i.e., the probability of observing an acute transmission event in the lowest decile of model runs for a given month is near zero.
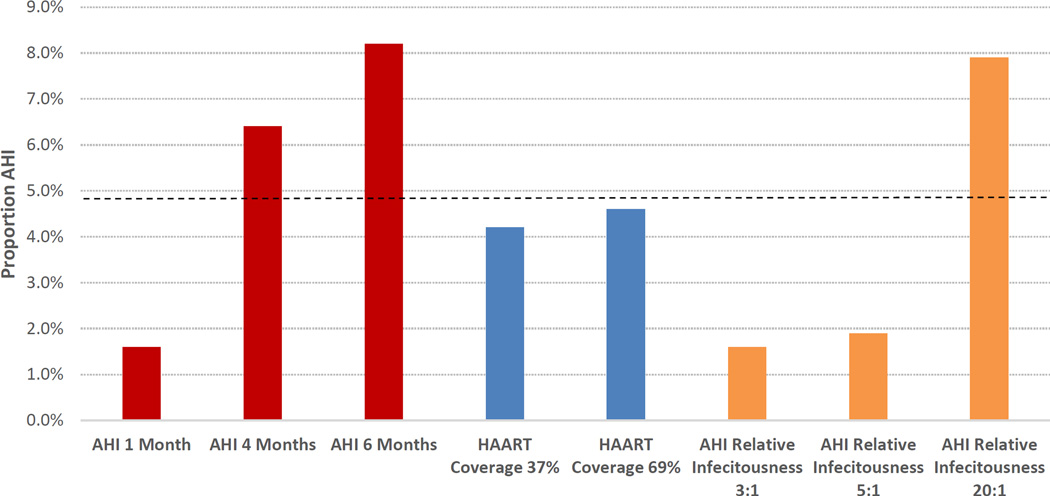
We conducted sensitivity analyses to examine the role of key model assumptions on the contribution of AHI-transmission among PWID. These results, all cumulative over the 16-year period, are presented alongside the estimate for the main analysis in Figure 5. When we reduced the assumed duration of AHI to one month, transmission attributable this stage accounted for 1.6% of total incidence from PWID, whereas when the duration was increased to 4 and 6 months, this estimate was elevated to 6.4% and 8.2%, respectively. The main estimate did not change significantly (from 4.9% to 4.2% and 4.6%, respectively) when the coverage of HAART among HIV-positive PWID was assumed to reach either 37% or 69% by 2011. When we adjusted the relative infectiousness of AHI to chronic infection (from 10:1 to ratios of 3:1, 5:1, and 20:1) AHI transmission accounted for 1.6%, 1.9%, and 7.9% of all HIV transmissions from PWID, respectively.
Figure 5.
Sensitivity analysis results for the simulated proportion of all HIV transmission from people who inject drugs that is attributable to acute HIV infection
AHI - acute HIV infection; HAART - highly active antiretroviral therapy
Note: The dashed black line represents the cumulative estimate (4.9%) for the entire 16-year period in the main analysis. The colors in the figure correspond to the type of sensitivity analysis (results for the AHI duration sensitivity analyses are in red, results for the HAART coverage sensitivity analyses are in blue, and results for the AHI relative infectious sensitivity analyses are in green). The baseline value for AHI duration (red bars) is three months, the baseline value for AHI relative infectiousness (orange bars) is 10:1, and the baseline HAART coverage (by the end of 2011) was 53%. All sensitivity analysis results are based on the mean estimates from 10 Monte Carlo runs.
Discussion
Using an agent-based modeling approach, we estimated the proportion of all HIV transmission events arising from AHI among PWID in a mature epidemic. During a twenty-year simulated time period, less than 10% of all HIV transmission occurred while the index agent was acutely-infected. Nonetheless, our results were sensitive to assumptions regarding the duration and relative infectiousness of AHI, demonstrating that additional empirical research is needed to investigate AHI dynamics among PWID.
This study demonstrates that, although a substantial amount of overall HIV transmission may occur during the highly-infectiousness period in the months following infection, our estimate is lower than many of those previously estimated for other high-risk populations, such as MSM [40, 41], and heterosexual couples in generalized epidemic settings [9, 22]. There are many reasons why the AHI attributable proportion may differ across high-risk populations. The most important of these reasons may by the relative maturity of the epidemics themselves [8, 42], which we could not examine here because we analyzed only a single mature epidemic. Other reasons include distinct network characteristics and dynamics (e.g., distribution and prevalence of concurrent relationships, total number of partners, rate of partner turnover), which have been shown to influence the transmission and spread of HIV infection among at-risk persons [43, 44], and thus may determine, in part, the contribution of AHI. For instance, the theory that chronically-infected individuals may act as network ‘firewalls,’ buffering acutely-infected individuals from the rest of the population, has been previously demonstrated [44, 45]. Further study is needed to determine whether this ‘firewall’ principle limited the contribution of AHI infection in the model. Differences in the biological susceptibility of specific transmission modes, behavioral adaptation (e.g., serosorting), and the coverage of HIV prevention modalities may also influence the magnitude of AHI transmission. Future modeling research should investigate the fundamental epidemiological, behavioral, and contextual differences between high-risk populations that likely elevate or diminish the role of AHI transmission.
In light of previous studies examining the contribution of AHI-transmission in other populations, the results presented here are further indication that its contribution to overall incidence will vary depending on specific population and epidemic characteristics. Moreover, it is likely that the AHI attributable fraction is highest in early-stage epidemics [46], and decreases as epidemics mature. Our analysis did not include the expansion stage of the HIV epidemic in NYC, however, we expect that had our study period included this epidemic phase, the AHI attributable fraction would have been greater. We observed an increasing contribution of AHI as the study period advanced, possibly due to a decreasing prevalence of chronic HIV infection among PWID over time, and increasing HAART coverage, which resulted in decreased transmission from agents in chronic infection. It should be noted that the relationship between HAART coverage and the contribution of acute infection is likely not monotonic, as decreased overall incidence through viral suppression also decreases the ratio of acute HIV cases to chronic. Although the influence of epidemic phase on the dynamics and force of infection has been demonstrated in the sexually transmitted infection literature [47], the possibility of recovery and reinfection for other infections makes the comparison to HIV difficult. Future research will seek to investigate and isolate the effect of epidemic phase and treatment coverage on AHI transmission dynamics among PWID.
The consequences of substantial acute-stage transmission of HIV on TasP has been debated considerably [8, 9, 11, 20, 23]. One recent analysis suggests that previous estimates of the relative infectiousness of AHI (compared to chronic infection), have been overstated [20]. Another study recently demonstrated that such early stage transmission does not have a long-term impact on the influence of HAART coverage on HIV incidence [8], although this has been contested [46]. It should be noted that differences, in particular, differences in epidemic phase (and study periods) suggests that direct comparison of our results with those published earlier may be difficult.
Virtually all research examining the contribution of AHI has been applied solely within the context of sexual transmission. Thus, although our study represents the first comprehensive investigation of the role of AHI transmission among PWID populations, the lack of empirical AHI transmission data specifically among PWID makes these results challenging to confirm [6]. Nonetheless, it should be noted that many of the published concerns regarding AHI-transmission apply directly to PWID populations, since many of the same barriers are present, such as difficulty identifying acutely infected individuals. Collectively, previous findings and ours suggest that transmission attributable to AHI is unlikely to substantially hamper the effectiveness of TasP-based interventions and other programs in mature epidemics. However, given that our results were sensitive to assumptions regarding the infectiousness and duration of AHI, efforts should be made to increase HIV testing frequency and linkage to care among PWID populations. For example, one study found that screening PWID enrolled in opioid agonist therapy every 3 to 6 months (to identify early HIV infection) is cost effective [48]. Additional strategies, including network-based interventions [46, 49, 50], are needed to identify PWID with AHI and link these individuals to treatment and care.
This study has a number of important limitations. The starkest limitation is the need to extrapolate several model parameters for PWID based largely on data from sexual modes of transmission; the per-act infectiousness of needle-sharing transmission (particularly when stratified by disease stage) is based on best available data. Also, the model does not account for heterogeneity in the viral load/infectiousness of those at the same disease stage, which has been shown elsewhere to vary significantly between individuals [51]. Additionally, there are some network parameter data that likely influence the main results, yet are not readily available, such as the rates of forming and dissolving sexual/needle-sharing partnerships. Although our model permits PWID to engage in different combinations of sexual and injection risk with other agents, the model does not account for difference in partner type (i.e., primary or secondary), which may affect the distribution of risk behavior within the population. Moreover, our assumption that PWID retain their sexual and injection partners for a minimum of one month may not reflect true behavior in the population. Finally, model assumptions regarding the initiation of non-injection drug use and injection drug use were based on the influence of sexual partners alone and did not reflect the potential for non-sexual partners to influence the initiation of drug use. This assumption reflected the structure of the network itself, with only sexual and drug-sharing relationships represented, and subsequently our model likely does not capture the influence of non-sexual relationships on the initiation of drug use in the population.
Critically, this study assessed the role of AHI transmission assuming the consistent application of prevention modalities and risk behavior, and therefore is not equipped to examine the role that any particular intervention, policy change, or structural modifications may have on this role, leaving the possibility that it may differ in settings with substantially different access to these interventions, or different risk environments. Given the effect that such interventions, i.e., increased HAART coverage, have had on estimates of HIV incidence among PWID in previous adaptions of the ABM [28], substantial differences in AHI-attributable transmission is possible. Similarly, as our setting represented a mature epidemic, with decreasing prevalence and stable incidence, these results may not necessarily be easily applied to settings experiencing expanding epidemics. Finally, the ABM does not account for some assortative mixing (e.g., by age, race), and since it does not factor in the age of PWID agents, we were not able to observe age-related morbidities among older PWID, such as liver disease or chronic hepatitis C infection.
Conclusions
We found that, although the contribution of acute infection may not substantially drive mature epidemics such as that investigated here, AHI may still be responsible for approximately one in twenty incident cases among PWID even in mature epidemics, indicating the need for development of new ways to detect those with early infection and elimination of structural barriers to early detection and treatment of PWID at high risk for HIV infection.
Supplementary Material
Acknowledgments
DJE proposed the study, with guidance from MNL, KHM, and BDLM. CW and MK developed the model software using the existing structure of an earlier model. DJE, MNL, KHM, SRF, SG, and BDLM developed the study methods. DJE drafted the manuscript, with substantial assistance and revisions from MNL, KHM, CW, MK, SG, SRF, and BDLM. This study was funded by research grants from the National Institute on Drug Abuse (R01-DA013336, F31-DA037808, DP2-DA040236).
References
- 1.Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- 2.Bureau USC. U.S. and World Population Clock. 2015 [Google Scholar]
- 3.Strathdee SA, Stockman JK. Epidemiology of HIV among injecting and non-injecting drug users: current trends and implications for interventions. Curr HIV/AIDS Rep. 2010;7:99–106. doi: 10.1007/s11904-010-0043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, Arasteh K. HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. AIDS. 2016;30:815–826. doi: 10.1097/QAD.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood E, Milloy MJ, Montaner JS. HIV treatment as prevention among injection drug users. Curr Opin HIV AIDS. 2012;7:151–156. doi: 10.1097/COH.0b013e32834f9927. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood E, Kerr T, Marshall BD, Li K, Zhang R, Hogg RS, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eaton JW, Hallett TB. Why the proportion of transmission during early-stage HIV infection does not predict the long-term impact of treatment on HIV incidence. Proc Natl Acad Sci U S A. 2014;111:16202–16207. doi: 10.1073/pnas.1323007111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers KA, Ghani AC, Miller WC, Hoffman IF, Pettifor AE, Kamanga G, et al. The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet. 2011;378:256–268. doi: 10.1016/S0140-6736(11)60842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS. 2007;21:1625–1629. doi: 10.1097/QAD.0b013e32826fb6a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen MS, Dye C, Fraser C, Miller WC, Powers KA, Williams BG. HIV treatment as prevention: debate and commentary--will early infection compromise treatment-as-prevention strategies? PLoS Med. 2012;9:e1001232. doi: 10.1371/journal.pmed.1001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. N Engl J Med. 2011;364:1943–1954. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zetola NM, Pilcher CD. Diagnosis and management of acute HIV infection. Infect Dis Clin North Am. 2007;21:19–48. vii. doi: 10.1016/j.idc.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Downing M, Knight K, Reiss TH, Vernon K, Mulia N, Ferreboeuf M, et al. Drug users talk about HIV testing: motivating and deterring factors. AIDS Care. 2001;13:561–577. doi: 10.1080/09540120120063205. [DOI] [PubMed] [Google Scholar]
- 15.Gyarmathy VA, Racz J, Neaigus A, Ujhelyi E. The urgent need for HIV and hepatitis prevention in drug treatment programs in Hungary. AIDS Educ Prev. 2004;16:276–287. doi: 10.1521/aeap.16.3.276.35435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- 17.Jurgens R, Csete J, Amon JJ, Baral S, Beyrer C. People who use drugs, HIV, and human rights. Lancet. 2010;376:475–485. doi: 10.1016/S0140-6736(10)60830-6. [DOI] [PubMed] [Google Scholar]
- 18.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 19.Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 20.Bellan SE, Dushoff J, Galvani AP, Meyers LA. Reassessment of HIV-1 acute phase infectivity: accounting for heterogeneity and study design with simulated cohorts. PLoS Med. 2015;12:e1001801. doi: 10.1371/journal.pmed.1001801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salomon JA, Hogan DR. Evaluating the impact of antiretroviral therapy on HIV transmission. AIDS. 2008;22(Suppl 1):S149–S159. doi: 10.1097/01.aids.0000327636.82542.87. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Raddad LJ, Longini IM., Jr No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22:1055–1061. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- 23.Williams BG, Granich R, Dye C. Role of acute infection in HIV transmission. Lancet. 2011;378:1913. doi: 10.1016/S0140-6736(11)61832-1. author reply 1914–1915. [DOI] [PubMed] [Google Scholar]
- 24.Vitek CR, Cakalo JI, Kruglov YV, Dumchev KV, Salyuk TO, Bozicevic I, et al. Slowing of the HIV epidemic in Ukraine: evidence from case reporting and key population surveys, 2005–2012. PLoS One. 2014;9:e103657. doi: 10.1371/journal.pone.0103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latkin C, Mandell W, Vlahov D, Oziemkowska M, Celentano D. People and places: behavioral settings and personal network characteristics as correlates of needle sharing. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:273–280. doi: 10.1097/00042560-199611010-00010. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann JP, Su SS, Pach A. Changes in network characteristics and HIV risk behavior among injection drug users. Drug Alcohol Depend. 1997;46:41–51. doi: 10.1016/s0376-8716(97)00038-0. [DOI] [PubMed] [Google Scholar]
- 27.Marshall BD, Paczkowski MM, Seemann L, Tempalski B, Pouget ER, Galea S, et al. A complex systems approach to evaluate HIV prevention in metropolitan areas: preliminary implications for combination intervention strategies. PLoS One. 2012;7:e44833. doi: 10.1371/journal.pone.0044833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall BD, Friedman SR, Monteiro JF, Paczkowski M, Tempalski B, Pouget ER, et al. Prevention and treatment produced large decreases in HIV incidence in a model of people who inject drugs. Health Aff (Millwood) 2014;33:401–409. doi: 10.1377/hlthaff.2013.0824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galai N, Safaeian M, Vlahov D, Bolotin A, Celentano DD, Study A. Longitudinal patterns of drug injection behavior in the ALIVE Study cohort, 1988–2000: description and determinants. Am J Epidemiol. 2003;158:695–704. doi: 10.1093/aje/kwg209. [DOI] [PubMed] [Google Scholar]
- 30.Tempalski B, Lieb S, Cleland CM, Cooper H, Brady JE, Friedman SR. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992–2002. J Urban Health. 2009;86:132–154. doi: 10.1007/s11524-008-9328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Des Jarlais DC, Perlis T, Arasteh K, Torian LV, Beatrice S, Milliken J, et al. HIV incidence among injection drug users in New York City, 1990 to 2002: use of serologic test algorithm to assess expansion of HIV prevention services. Am J Public Health. 2005;95:1439–1444. doi: 10.2105/AJPH.2003.036517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman SR, Neaigus A, Jose B, Curtis R, Goldstein M, Ildefonso G, et al. Sociometric risk networks and risk for HIV infection. Am J Public Health. 1997;87:1289–1296. doi: 10.2105/ajph.87.8.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman SR, Curtis R, Neaigus A, Jose B, Des Jarlais . Social Networks, Drug Injectors' Lives, and HIV/AIDS. New York, NY: Kluwer Academic; 1999. [Google Scholar]
- 34.Fraser C, Hollingsworth TD, Chapman R, de Wolf F, Hanage WP. Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci U S A. 2007;104:17441–17446. doi: 10.1073/pnas.0708559104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010;39:1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baggaley RF, Fraser C. Modelling sexual transmission of HIV: testing the assumptions, validating the predictions. Curr Opin HIV AIDS. 2010;5:269–276. doi: 10.1097/COH.0b013e32833a51b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hollingsworth TD, Laeyendecker O, Shirreff G, Donnelly CA, Serwadda D, Wawer MJ, et al. HIV-1 transmitting couples have similar viral load set-points in Rakai, Uganda. PLoS Pathog. 2010;6:e1000876. doi: 10.1371/journal.ppat.1000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baggaley RF, Boily MC, White RG, Alary M. Risk of HIV-1 transmission for parenteral exposure and blood transfusion: a systematic review and meta-analysis. AIDS. 2006;20:805–812. doi: 10.1097/01.aids.0000218543.46963.6d. [DOI] [PubMed] [Google Scholar]
- 39.Hudgens MG, Longini IM, Jr, Vanichseni S, Hu DJ, Kitayaporn D, Mock PA, et al. Subtype-specific transmission probabilities for human immunodeficiency virus type 1 among injecting drug users in Bangkok, Thailand. Am J Epidemiol. 2002;155:159–168. doi: 10.1093/aje/155.2.159. [DOI] [PubMed] [Google Scholar]
- 40.Pinkerton SD, Abramson PR. Implications of increased infectivity in early-stage HIV infection. Application of a Bernoulli-process model of HIV transmission. Eval Rev. 1996;20:516–540. doi: 10.1177/0193841X9602000502. [DOI] [PubMed] [Google Scholar]
- 41.Koopman JS, Jacquez JA, Welch GW, Simon CP, Foxman B, Pollock SM, et al. The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 42.Suthar AB, Granich RM, Kato M, Nsanzimana S, Montaner JS, Williams BG. Programmatic Implications of Acute and Early HIV Infection. J Infect Dis. 2015;212:1351–1360. doi: 10.1093/infdis/jiv430. [DOI] [PubMed] [Google Scholar]
- 43.Rothenberg R. HIV transmission networks. Curr Opin HIV AIDS. 2009;4:260–265. doi: 10.1097/COH.0b013e32832c7cfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Friedman SR, Kottiri BJ, Neaigus A, Curtis R, Vermund SH, Des Jarlais DC. Network-related mechanisms may help explain long-term HIV-1 seroprevalence levels that remain high but do not approach population-group saturation. Am J Epidemiol. 2000;152:913–922. doi: 10.1093/aje/152.10.913. [DOI] [PubMed] [Google Scholar]
- 45.Khan B, Dombrowski K, Saad M, McLean K, Friedman S. Network Firewall Dynamics and the Subsaturation Stabilization of HIV. Discrete Dyn Nat Soc. 2013;2013:720–818. doi: 10.1155/2013/720818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasylyeva TI, Friedman SR, Magiorkinis G. Prevention of early HIV transmissions might be more important in emerging or generalizing epidemics. Proc Natl Acad Sci U S A. 2015;112:E1515. doi: 10.1073/pnas.1424168112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ward H. Prevention strategies for sexually transmitted infections: importance of sexual network structure and epidemic phase. Sex Transm Infect. 2007;83(Suppl 1):i43–i49. doi: 10.1136/sti.2006.023598. [DOI] [PubMed] [Google Scholar]
- 48.Cipriano LE, Zaric GS, Holodniy M, Bendavid E, Owens DK, Brandeau ML. Cost effectiveness of screening strategies for early identification of HIV and HCV infection in injection drug users. PLoS One. 2012;7:e45176. doi: 10.1371/journal.pone.0045176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasylyeva TI, Friedman SR, Smyrnov P, Bondarenko K. A new approach to prevent HIV transmission: Project Protect intervention for recently infected individuals. AIDS Care. 2015;27:223–228. doi: 10.1080/09540121.2014.947913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman SR, Downing MJ, Jr, Smyrnov P, Nikolopoulos G, Schneider JA, Livak B, et al. Socially-integrated transdisciplinary HIV prevention. AIDS Behav. 2014;18:1821–1834. doi: 10.1007/s10461-013-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hecht FM, Hartogensis W, Bragg L, Bacchetti P, Atchison R, Grant R, et al. HIV RNA level in early infection is predicted by viral load in the transmission source. AIDS. 2010;24:941–945. doi: 10.1097/QAD.0b013e328337b12e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brady JE, Friedman SR, Cooper HL, Flom PL, Tempalski B, Gostnell K. Estimating the prevalence of injection drug users in the U.S. and in large U.S. metropolitan areas from 1992 to 2002. J Urban Health. 2008;85:323–351. doi: 10.1007/s11524-007-9248-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman SR. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City. Drug Alcohol Depend. 2009;101:88–91. doi: 10.1016/j.drugalcdep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 54.HIV Health and Human Services Planning Council of New York. HIV care cascades for New York City overall and Ryan White clinets: A first look. [Accessed June 23, 2016]; < http://www.nyhiv.org/pdfs/NACPresentation6-13-13.pdf.>. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.