Abstract
Background
The extent of interstitial fibrosis on kidney biopsy is regarded as a prognostic indicator and guide to treatment. Patients with extensive fibrosis are assigned to supportive treatments with the expectation that they have advanced beyond the point at which immunosuppressive or other disease modifying therapies would be of benefit. Our study highlights some of the limitations of using interstitial fibrosis to predict who will develop ESRD.
Methods
Analysis of 434 consecutive renal biopsies performed between 2001-2012 at a single center. We assessed the influence of various clinical factors along with fibrosis as predictors of ESRD and dialysis free survival in various patient groups.
Results
Interstitial fibrosis performed well overall as a predictor of progression to dialysis and on average patients with >50% fibrosis progressed more rapidly than those with either 25-49% or 0-24% fibrosis with a median time to dialysis of 1.2 years, 6.5 and >10 years respectively. In contrast, interstitial fibrosis was of less value as a predictor of disease progression in a subset of cases that included patients over the age of 70 and those with diabetic nephropathy on biopsy. Surprisingly, 13.9% of patients with normal renal function had 25-49% fibrosis and 5% had more than 50% fibrosis on biopsy and 5 years after undergoing biopsy 21% of patients with >50% fibrosis still remained dialysis free.
Conclusion
Renal fibrosis is an imperfect prognostic indicator for the development of ESRD and caution should be exercised in applying it too rigidly, especially in elderly or diabetic patients.
Keywords: chronic kidney disease, end stage renal disease, kidney biopsy, interstitial fibrosis, prognosis
Introduction
Early identification of individuals with renal disease and implementation of renoprotective treatment can slow or prevent progression to end stage renal disease (ESRD) reducing morbidity, mortality and improving quality of life. Renal biopsies are indicated to determine the underlying pathology, guide therapy, and to ascertain the degree of active (potentially reversible) and chronic (considered irreversible) changes[1-7]. Chronic renal damage is characterized by different morphological changes that show common findings in different renal diseases. In the glomerular compartment the major modifications are mesangial expansion and glomerulosclerosis; in the tubulointerstitial compartment the characteristic modifications are tubular atrophy, interstitial fibrosis, and a reduction in the number of capillaries [2, 4]. Although the majority of ESRD patients in the US have primary or secondary forms of glomerular disease, studies have suggested that it is actually the extent of accompanying histologic injury in the tubulointerstitium that correlates best with renal function decline [1,5-12]. Strictly applied, finding advanced fibrosis on renal biopsy might limit the use of beneficial but potentially toxic treatments based on the assumption of futility. In this study we assessed the influence of other factors along with fibrosis as predictors of progression to dialysis and examined the predictive value of interstitial fibrosis in various patient groups. The amount of interstitial fibrosis was taken directly from the biopsy report. We intentionally did not validate the reported fibrotic index using morphometric techniques because in real life clinical decisions are based on standard biopsy reports.
Methods
We performed a retrospective analysis of renal biopsies of adult patients at BUMC between January 2001 and July 2012. All patients who underwent a successful kidney biopsy in this period were included in our initial evaluation, but we excluded 6 patients from subsequent analyses who had acute, reversible renal failure. The following data were collected from the patients’ electronic medical record: demographic information, BMI, urine protein-to-creatinine ratio, and serum creatinine. GFR was estimated using the four variable MDRD equation for patients who were less than 70 years of age [13] and CKD-EPI Creatinine Equation for patients older than 70 years. The presence or absence of co-morbidities including hypertension, insulin dependent diabetes mellitus, non-insulin dependent diabetes mellitus, coronary artery disease, stroke, peripheral vascular disease and autoimmune disease were noted. The following diseases were grouped together under the autoimmune category: vasculitis, lupus nephritis, immune complex glomerulonephritis, and post-infectious glomerulonephritis. Histological findings were noted from the biopsy report and included the degree of glomerulosclerosis, and the percentage of tubular atrophy and interstitial fibrosis. If there was more than one pathological finding on the biopsy it was classified according to the most prevalent finding. Biopsy findings were categorized as having IgA nephropathy, autoimmune renal disease, diabetic nephropathy, or “other nephropathy” (focal segmental glomerulosclerosis, hypertensive glomerulosclerosis, collapsing glomerulopathy, primary membranous nephropathy, thin basement membrane disease, interstitial nephritis, minimal change disease, or amyloidosis).
Serum creatinine at the time of biopsy and last creatinine available during the study period and/or time of dialysis initiation or death were collected.
The two primary end points of the study were initiation of dialysis and the time from kidney biopsy to the initiation of dialysis. We also evaluated a composite outcome of death without reaching dialysis or dialysis as a secondary outcome measure. Only four patients declined dialysis.
We described study sample characteristics via means and standard deviations for continuous variables and counts and percentages for categorical variables. Demographic factors, comorbidities, and laboratory measures were assessed as possible predictors of dialysis. We used a t-test for continuous variables and Fisher's exact test for categorical variables to assess bivariate associations. Based on the current literature [14,15] we designed prediction models using clinical and biopsy variables to predict progression to dialysis. The following variables were included in the multivariable logistic model predicting the initiation of dialysis: demographic variables including age, sex, race, and BMI; comorbid conditions including IDDM, HTN, and composite of CAD, PVD, or stroke; laboratory variables at the time of the biopsy including GFR, creatinine, and proteinuria; biopsy findings including percentage of fibrosis, glomerulosclerosis, and nephropathy type. A parsimonious model was constructed using a backward elimination procedure with a 0.2 level to stay in the model. The model that includes fibrosis only was then evaluated. The discriminative ability of the models was described using C-statistic and ROC. The models’ fit was assessed using a Hosmer-Lemeshow test. The parsimonious model was compared to the fibrosis-only model using a likelihood ratio test. The effects were expressed via adjusted odds ratios (OR) with corresponding 95% confidence intervals. The predicted probability of patients reaching dialysis based on the model including fibrosis only was assessed in various subgroups. A t-test was used to compare predicted probability in patients who reached dialysis and patients who did not reach dialysis. We confirmed that there was a linear relationship between fibrosis and dialysis in our data (Suppl table 6). The amount of interstitial fibrosis was taken directly from the biopsy report prepared by an experienced, board certified nephropathologist using standard diagnostic criteria [1]. We intentionally did not validate the reported fibrotic index using morphometric techniques because clinical decisions are based on a standard biopsy report.
Time to the initiation of dialysis was assessed in patients grouped based on the amount of fibrosis. Kaplan-Meier curves and log-rank test were used in unadjusted analyses. The proportional hazard Cox-regression model was used to adjust for potential confounders; these included the same variables as the dialysis prediction model. The effects were expressed via adjusted hazard ratios (HR) with corresponding 95% CI. Analysis was performed using SAS 9.3 software (SAS Institute INC., Cary, NC, USA) and p value <0.05 was defined as statistically significant.
Results
Patient characteristics and risk factors for dialysis
We identified 434 native kidney biopsies done at Boston University Medical Center (BUMC) between 2001 and 2012. Baseline patient characteristics are summarized in Table 1. Over a median follow-up of 5.6 (0.46-11.82) years, 136 (31%) patients reached dialysis. Median time to dialysis was 0.86 (−0.03-10.6) years, including 6 patients who had dialysis immediately preceding the biopsy. During follow up 15 (3.5%) patients died and median time to death was 2.18 years (0-10.4). Mean age at the time of biopsy was 45±16 years. In our cohort 42% of the patients were black, 22% were white and 22% were Hispanic. The biopsies were performed in patients with various etiologies of chronic renal failure and a wide range of renal functions (creatinine 2.6±2.6 mg/dl and GFR 57±46 ml/min). Mean fibrosis at the time of biopsy was 31.2%±25.0 and mean glomerulosclerosis was 25.0%±24.5. Patients who reached dialysis during the follow up period were older (49±16 vs 43±16 years) and dialysis was more likely to occur in male patients (38% vs 24%). We noted a significant racial difference with the highest rate of dialysis in blacks (39%) and lower rates in whites (27%) and Hispanics (25%). Other clinical characteristics at baseline that were associated with a higher incidence of dialysis were hypertension (41% vs 10%), insulin-requiring diabetes mellitus (54% vs 28%), cardiovascular disease (48% vs 29%), higher levels of proteinuria (5.2±4.4 vs 3.3±4.2 g), especially nephrotic range proteinuria (47% vs 21%), higher creatinine (4.3±3.2 vs 1.8±1.9 mg/dl), and lower GFR (29.4±28.9 vs 70.5±46.6 ml/min). A history of autoimmune disease carried a lower risk of dialysis (23.6% vs 34.4%). As expected, more interstitial fibrosis (51.6%±23.7 vs 21.9%±19.4) and glomerular sclerosis (40.1%±28.4 vs 18.1%±18.8) were associated with dialysis. Patients with extensive interstitial fibrosis on biopsy but normal renal function had a median glomerulosclerosis score of 30 (range 5.7-66.7) that was similar to patients who had CKD. In unadjusted analysis FGG was significantly associated with progression to dialysis (p<0.001) but when adjusting for other factors FGG lost significance. Being on insulin treatment was a risk factor for dialysis but being on oral agents was not.
Table 1.
Patient characteristics of the overall cohort and a bivariate analysis comparing the dialysis group with the non-dialysis group.
| Characteristic | Overall (N=434) | Dialysis (N=136) | No Dialysis (N=298) | p-value |
|---|---|---|---|---|
| Age | ||||
| Mean ± SD | 45.1±16.3 | 48.7±16.2 | 43.4±16.1 | 0.002 |
| Median and Range | 44 (17-89) | 49 (20-83) | 42 (17-89) | |
| Gender | ||||
| Male | 226 (52.1%) | 86 (38.1%) | 140 (61.9%) | 0.002 |
| Female | 208 (47.9%) | 50 (24%) | 158 (76%) | |
| Race | ||||
| White | 96 (22.1%) | 26 (27.1%) | 70 (72.9%) | 0.045 |
| Black | 183 (42.2%) | 71 (38.8%) | 112 (61.2%) | |
| Hispanic | 95 (21.9%) | 24 (25.3%) | 71 (74.7%) | |
| Other | 60 (13.8%) | 15 (25%) | 45 (75%) | |
| BMI | ||||
| Mean ± SD | 28.9±7 | 28±6.7 | 29.3±7.1 | 0.070 |
| Median and Range | 27.2 (13.7-58.8) | 26.8 (17.2-53.8) | 27.7 (13.7-58.8) | |
| BMI | ||||
| Underweight (16-18.5) | 5 (1.2%) | 2 (40%) | 3 (60%) | 0.143 |
| Normal (18.5-25) | 129 (29.9%) | 50 (38.8%) | 79 (61.2%) | |
| Overweight (25-30) | 144 (33.3%) | 40 (27.8%) | 104 (72.2%) | |
| Obese (>30) | 154 (35.6%) | 43 (27.9%) | 111 (72.1%) | |
| Nephropathy | ||||
| Autoimmune | 124 (28.6%) | 29 (23.4%) | 95 (76.6%) | <0.001 |
| IgA | 58 (13.4%) | 17 (29.3%) | 41 (70.7%) | |
| Diabetic | 42 (9.7%) | 25 (59.5%) | 17 (40.5%) | |
| Other | 210 (48.4%) | 65 (31%) | 145 (69%) | |
| Interstitial Fibrosis (%) | ||||
| Mean ± SD | 31.2±25 | 51.6±23.7 | 21.9±19.4 | <0.001 |
| Median and Range | 25 (0-100) | 50 (5-100) | 20 (0-80) | |
| Glomerulosclerosis (%) | ||||
| Mean ± SD | 25±24.5 | 40.1±28.4 | 18.1±18.8 | <0.001 |
| Median and Range | 16.7 (0-100) | 40.6 (0-100) | 10.5 (0-77.3) | |
| Creatinine at the time of biopsy (mg/dl) | ||||
| Mean ± SD | 2.6±2.6 | 4.3±3.2 | 1.8±1.9 | <0.001 |
| Median and Range | 1.7 (0.4-20.3) | 3.2 (0.6-20.3) | 1.2 (0.4-15.2) | |
| GFR (MDRD) at the time of biopsy | ||||
| Mean ± SD | 57.6±46 | 29.4±28.9 | 70.5±46.6 | <0.001 |
| Median and Range | 42.5 (2.5-246.2) | 22.2 (2.5-153.1) | 61.1 (3.7-246.2) | |
| GFR at the time of biopsy | ||||
| 90+ mL/min | 107 (24.7%) | 8 (7.5%) | 99 (92.5%) | <0.001 |
| 60-89 mL/min | 65 (15%) | 9 (13.8%) | 56 (86.2%) | |
| 30-59 mL/min | 105 (24.2%) | 23 (21.9%) | 82 (78.1%) | |
| 15-29 mL/min | 85 (19.6%) | 48 (56.5%) | 37 (43.5%) | |
| <15 mL/min | 72 (16.6%) | 48 (66.7%) | 24 (33.3%) | |
| HTN | ||||
| Yes | 298 (68.8%) | 122 (40.9%) | 176 (59.1%) | <0.001 |
| No | 135 (31.2%) | 14 (10.4%) | 121 (89.6%) | |
| IDDM | ||||
| Yes | 59 (13.6%) | 32 (54.2%) | 27 (45.8%) | <0.001 |
| No | 374 (86.4%) | 104 (27.8%) | 270 (72.2%) | |
| NIDDM | ||||
| Yes | 42 (9.7%) | 13 (31%) | 29 (69%) | 0.999 |
| No | 391 (90.3%) | 123 (31.5%) | 268 (68.5%) | |
| CAD/PVD/CVA | ||||
| Yes | 63 (14.6%) | 30 (47.6%) | 33 (52.4%) | 0.003 |
| No | 369 (85.4%) | 105 (28.5%) | 264 (71.5%) | |
| AI disease | ||||
| Yes | 127 (29.4%) | 30 (23.6%) | 97 (76.4%) | 0.030 |
| No | 305 (70.6%) | 105 (34.4%) | 200 (65.6%) | |
| Proteinuria at time of biopsy (gr) | ||||
| Mean ± SD | 3.9±4.4 | 5.2±4.4 | 3.3±4.2 | <0.001 |
| Median and Range | 2.4 (0-34.4) | 4.2 (0-20.5) | 1.9 (0-34.4) | |
| Nephrotic Range Proteinuria | ||||
| Yes | 174 (40.1%) | 81 (46.6%) | 93 (53.4%) | <0.001 |
| No | 260 (59.9%) | 55 (21.2%) | 205 (78.8%) |
Data are presented as Mean ± SD, Median (Range). P<0.05 was considered significant. (BMI, Body Mass Index; IgA, Immunoglobulin A; GFR, Glomerular Filtration Rate; MDRD, Modification of Diet in Renal Disease; HTN, hypertension; IDDM, insulin dependent diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus; CAD, coronary artery disease; PVD, peripheral vascular disease; CVA, cerebrovascular accident; AI, autoimmune; SD, standard deviation).
The majority of the 172 patients who had a GFR >60 ml/min had less than 25% fibrosis on biopsy (139 or 81%), but surprisingly, 5% of these patients with near normal renal function had more than 50% fibrosis and 13.9% had 25-49% fibrosis (Table 2 and Figure 1). As expected[16], with decreasing GFR there were more patients in the >50% fibrosis group. Individual patient level data on association between percentage of fibrosis and GFR is shown in Figure 1B.
Table 2.
Number and percentage of patients with various degree of interstitial fibrosis, stratified by GFR.
| Interstitial Fibrosis <25% n (%) |
Interstitial Fibrosis 25-49% n (%) |
Interstitial Fibrosis >50% n (%) |
Total | |
|---|---|---|---|---|
| GFR 60+ | 139 (80.81) | 24 (13.95) | 9 (5.23) | 172 |
| GFR 30-59 | 29 (27.62) | 52 (49.52) | 24 (22.86) | 105 |
| GFR 15-29 | 12 (14.12) | 28 (32.94) | 45 (52.94) | 85 |
| GFR <15 | 19 (26.39) | 13 (18.06) | 40 (55.56) | 72 |
| Total | 199 | 117 | 118 | 434 |
(GFR, Glomerular Filtration Rate).
Figure 1. Correlation between GFR and degree of fibrosis.
A. Percentage of fibrosis according to GFR levels.
B. A scatter plot of the overall cohort mapping the association of GFR with percent fibrosis. Each dot represents a single patient. (GFR, Glomerular Filtration Rate).
Prediction of dialysis
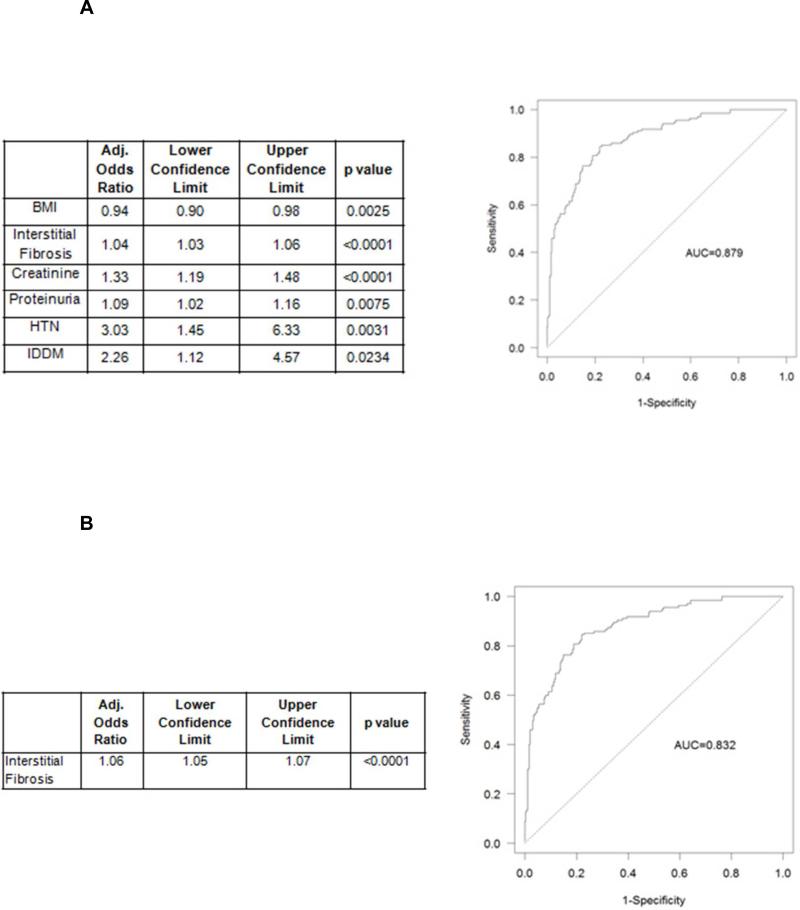
We designed a prediction model using demographic, clinical and biopsy variables collected at the time of biopsy to predict progression to dialysis (Figure 2A-B and Supplemental data, Table 1A-B). The model included the following six factors: percentage of fibrosis, BMI, GFR, proteinuria, and diagnoses of hypertension and IDDM (Figure 2A). The model has very good discriminative ability (C=0.879) and fit (Hosmer-Lemeshow p=0.565). Interestingly, only GFR below 30 ml/min was predictive of future dialysis (Supplemental data, Table 2A-C). The fibrosis alone model also had good characteristics with slightly lower discriminatory ability (C=0.832, Hosmer-Lemeshow p=0.174) (Figure 2B). However, the six-factor model was statistically superior (likelihood ratio test p<0.001) to the fibrosis alone model. ROCs for both models are shown on Figure 2A-B.
Figure 2. Dialysis prediction model.
Parsimonious prediction model for reaching dialysis. See Methods for a detailed description of the prediction model development.
2A: Dialysis prediction model using 6 variables: parsimonious 0.2 level (left panel) and receiver operator curve for 6 variable model predicting dialysis (right panel) (variables included: BMI, percentage of interstitial fibrosis, creatinine, proteinuria, HTN, IDDM). (AUC, area under the curve; BMI, Body Mass Index; GFR, Glomerular Filtration Rate; HTN, hypertension; IDDM, insulin dependent diabetes; CAD, coronary artery disease; DM, diabetes mellitus; IgA, Immunoglobulin A; DM, diabetes mellitus; IgA, Immunoglobulin A).
2B. Interstitial fibrosis alone dialysis prediction model (left panel) and receiver operator curve (ROC) for dialysis prediction model using interstitial fibrosis only.
Interstitial fibrosis and dialysis in different patient subgroups
We examined the association between the level of fibrosis and dialysis status in different clinical subgroups. It is critical to note that in all groups the range of fibrosis was wide. While fibrosis was a powerful predictor of dialysis risk overall (Table 3), it was not predictive for some individuals. Regardless of the patients’ diagnosis and demographics, there was a wide range of fibrosis observed in both the dialysis and non-dialysis groups. Some patients with 80% fibrosis on biopsy did not progress to ESRD (Table 3, “No dialysis” Range of fibrosis=0-80%; also see Table 1, Interstitial Fibrosis 0-80% in the “No dialysis” group). There was an age-associated increase in the level of fibrosis in the patients who did not progress to dialysis (Table 3; 14.8%<19.1%<29.5%<31.5) indicating that moderate levels of fibrosis did develop with aging and this was not necessarily indicative of dialysis risk. While in all three younger groups who progressed to ESRD the mean level of fibrosis was similar (51 to 53.1%), the mean percent of fibrosis of 43.3% was lower in older patients (>70) who progressed to dialysis. There were large statistically significant differences in fibrosis level between dialysis and non-dialysis patients for the three younger age groups (p<0.001). However, there was a much smaller difference in fibrosis level in the older patients and it did not reach statistical significance (p=0.148). The difference between the dialysis vs non-dialysis groups of patients with diabetic nephropathy on biopsy also did not reach statistical significance (54.2 vs. 43.5%, p=0.167).
Table 3.
Analysis of percentage fibrosis between dialysis group and no dialysis group for each patient characteristic.
| Characteristic | Frequency N (%) |
Dialysis (N=136) Mean±SD Median (Range) |
No Dialysis (N=298) Mean±SD Median (Range) |
p-value |
|---|---|---|---|---|
| Age | ||||
| 17-29 | 88 (20.3%) | 51.0±25.0 50.0 (5.0-95.0) |
14.8±17.2 10.0 (0.0-75.0) |
<0.001 |
| 30-49 | 177 (40.8%) | 52.1±25.3 55.0 (5.0-100.0) |
19.1±17.4 13.8 (0.0-80.0) |
<0.001 |
| 50-69 | 138 (31.8%) | 53.5±21.6 50.0 (10.0-90.0) |
29.5±20.6 27.5 (0.0-80.0) |
<0.001 |
| 70+ | 31 (7.1%) | 43.3±23.5 40.0 (5.0-80.0) |
31.5±20.3 30.0 (2.5-80.0) |
0.148 |
| Gender | ||||
| Male | 226 (52.1%) | 54.0±22.1 50.0 (5.0-100.0) |
24.9±20.4 20.0 (0.0-80.0) |
<0.001 |
| Female | 208 (47.9%) | 47.5±25.9 40.0 (5.0-90.0) |
19.3±18.2 10.0 (0.0-80.0) |
<0.001 |
| Race | ||||
| White | 96 (22.1%) | 43.4±21.6 40.0 (15.0-100.0) |
18.5±17.0 13.8 (0.0-75.0) |
<0.001 |
| Black | 183 (42.2%) | 52.3±24.2 50.0 (5.0-90.0) |
25.1±22.2 20.0 (0.0-80.0) |
<0.001 |
| Hispanic | 95 (21.9%) | 60.4±21.2 70.0 (20.0-95.0) |
20.6±18.0 15.0 (0.0-70.0) |
<0.001 |
| Other | 60 (13.8%) | 48.7±25.2 40.0 (5.0-90.0) |
21.4±17.0 20.0 (0.0-65.0) |
<0.001 |
| BMI | ||||
| Normal (18.5-25) | 134 (31.0%) | 49.7±24.6 50.0 (5.0-95.0) |
20.5±17.7 20.0 (0.0-75.0) |
<0.001 |
| Overweight (25-30) | 144 (33.3%) | 56.2±22.7 57.5 (17.5-90.0) |
21.1±17.6 20.0 (0.0-80.0) |
<0.001 |
| Obese (>30) | 154 (35.6%) | 50.3±23.3 50.0 (5.0-100.0) |
23.3±21.6 17.5 (0.0-80.0) |
<0.001 |
| Nephropathy | ||||
| Autoimmune | 124 (28.6%) | 43.8±24.6 40.0 (5.0-80.0) |
17.3±15.3 10.0 (0.0-65.0) |
<0.001 |
| IgA | 58 (13.4%) | 57.8±26.8 60.0 (17.5-100.0) |
18.1±15.3 15.0 (0.0-70.0) |
<0.001 |
| Diabetic | 42 (9.7%) | 54.2±22.2 50.0 (20.0-90.0) |
43.5±26.7 30.0 (10.0-80.0) |
0.167 |
| Other | 210 (48.4%) | 52.5±22.6 55.0 (5.0-90.0) |
23.5±20.1 20.0 (0.0-80.0) |
<0.001 |
| HTN | ||||
| Yes | 298 (68.8%) | 52.9±23.0 50.0 (5.0-100.0) |
27.0±20.3 20.0 (0.0-80.0) |
<0.001 |
| No | 135 (31.2%) | 40.7±27.3 32.5 (5.0-90.0) |
14.8±15.6 10.0 (0.0-80.0) |
<0.001 |
| IDDM | ||||
| Yes | 59 (13.6%) | 48.8±21.8 50.0 (5.0-90.0) |
35.8±25.2 30.0 (5.0-80.0) |
0.039 |
| No | 374 (86.4%) | 52.5±24.3 50.0 (5.0-100.0) |
20.6±18.3 16.3 (0.0-80.0) |
<0.001 |
| NIDDM | ||||
| Yes | 42 (9.7%) | 56.2±20.6 50.0 (20.0-100.0) |
26.9±21.4 25.0 (0.0-80.0) |
<0.001 |
| No | 391 (90.3%) | 51.1±24.0 50.0 (5.0-95.0) |
21.5±19.2 16.3 (0.0-80.0) |
<0.001 |
| CAD/PVD/CVA | ||||
| Yes | 63 (14.6%) | 50.2±23.8 45.0 (5.0-90.0) |
30.2±23.8 20.0 (2.5-80.0) |
0.002 |
| No | 369 (85.4%) | 51.8±23.7 50.0 (5.0-100.0) |
21.0±18.6 16.3 (0.0-80.0) |
<0.001 |
| AI disease | ||||
| Yes | 127 (29.4%) | 43.0±24.6 40.0 (5.0-80.0) |
17.2±15.2 10.0 (0.0-65.0) |
<0.001 |
| No | 305 (70.6%) | 54.3±22.9 60.0 (5.0-100.0) |
24.3±20.8 20.0 (0.0-80.0) |
<0.001 |
| GFR | ||||
| 60+ | 172 (39.6%) | 26.2±20.0 20.0 (5.0-80.0) |
12.6±13.3 10.0 (0.0-65.0) |
<0.001 |
| 30-59 | 105 (24.2%) | 43.0±17.2 40.0 (15.0-75.0) |
32.4±17.4 30.0 (5.0-80.0) |
0.011 |
| 15-29 | 85 (19.6%) | 54.4±21.8 60.0 (10.0-90.0) |
38.2±22.1 35.0 (2.5-80.0) |
0.001 |
| <15 | 72 (16.6%) | 62.0±21.7 65.0 (20.0-100.0) |
21.5±21.1 15.0 (0.0-80.0) |
<0.001 |
(BMI, Body Mass Index; IgA, Immunoglobulin A; HTN, hypertension; IDDM, insulin dependent diabetes mellitus; NIDDM, non-insulin dependent diabetes mellitus; CAD, coronary artery disease; PVD, peripheral vascular disease; CVA, cerebrovascular accident; AI, autoimmune disease; GFR, Glomerular Filtration Rate; SD, standard deviation); P<0.05 was considered significant.
Predictive value of interstitial fibrosis
We examined the value of interstitial fibrosis as a sole predictor of dialysis in different patient subgroups (Supplemental data, Table 3). In most groups fibrosis was a strong predictor of progression. However, we found three subgroups of patients in which fibrosis did not predict progression. Fibrosis was better as a predictor of dialysis in the younger age groups, but with increasing age the association became less strong. In older patients (>70 years), who represented 7.1% of our kidney biopsy cohort, fibrosis was not a statistically significant predictor of dialysis. Similarly, fibrosis was not a statistically significant predictor of dialysis in patients with diabetic nephropathy on biopsy or those receiving insulin therapy. In contrast, analysis of patients younger than 70 years who were non-diabetic showed that fibrosis is a good predictor of progression to ESRD both in our full prediction model (C=0.912) and in our fibrosis only model (C=0.866, see Supplemental Table 4).
Percentage of interstitial fibrosis and time to dialysis
We tested fibrosis as a predictor of time to dialysis (Table 4A and Figures 3 and 4). Patients were categorized by the level of fibrosis into three groups with 0-24 (n=199), 25-49 (n=117), or >50 (n=118) percent. As expected the group with >50% fibrosis progressed to ESRD more rapidly that those with either 25-49% or 0-24% fibrosis with a median time to dialysis of 1.2 years compared to 6.5 years and >10 years respectively (p<0.001) (Table 4 and Figure 4). Note that 2 and 5 years after undergoing biopsy 42% and 21% of patients with >50% fibrosis still remained dialysis free (Table 4A and Figure 4). Cox regression analysis showed that having >50% or 25-49% fibrosis significantly increased the risk of dialysis with hazard ratios of 7.2 (95%CI 3.5-14.8) and 2.7 (95%CI 1.3-5.3) respectively, compared to less than 25% fibrosis (Table 4B). Lower BMI, GFR<30 and proteinuria>3.5 g were the best predictors of progression to dialysis along with percent fibrosis. We found similar results using dialysis or death as end point instead of dialysis (Supplemental Table 5A-B and Supplemental Figure 1).
Table 4A.
Time to event (dialysis) analysis. Life table estimates of dialysis for 10-year follow-up from biopsy by severity of fibrosis at biopsy.
| Had Events Up to: | Interstitial Fibrosis: 0-24% (95% CI) | Interstitial Fibrosis: 25-49% (95% CI) | Interstitial Fibrosis:≥50% (95% CI) |
|---|---|---|---|
| 1 month | 1.0 (0.2-3.4) | 6.0 (2.7-11.4) | 18.7 (12.3-26.2) |
| 6 months | 1.0 (0.2-3.4) | 9.6 (5.1-15.9) | 33.8 (25.3-42.5) |
| 1 year | 3.4 (1.4-6.9) | 11.6 (6.5-18.3) | 45.4 (35.9-54.4) |
| 2 years | 6.1 (3.1-10.5) | 19.4 (12.4-27.6) | 58.3 (48.1-67.1) |
| 5 years | 8.0 (4.3-13.2) | 33.0 (23.0-43.3) | 79.1 (68.2-86.6) |
| 10 years | 21.8 (12.1-33.3) | 62.4 (45.0-75.7) | 89.6 (71.0-96.5) |
Fig 3.
Kaplan-Meier analysis of time-to-dialysis according to the level of interstitial fibrosis.
Fig 4. Correlation between fibrosis and time to dialysis.
A scatter plot of the overall cohort mapping the association of percentage fibrosis and time to dialysis. Each dot represents a single patient.
Table 4B.
Dialysis risk factor analysis. Cox proportional hazard model for time to dialysis.
| Adjusted HR | Lower Confidence Limit | Upper Confidence Limit | p-value | |
|---|---|---|---|---|
| TA: 25-49 vs. 0-24 | 2.7 | 1.3 | 5.3 | 0.003 |
| TA: 50+ vs. 0-24 | 7.2 | 3.5 | 14.8 | <0.001 |
| Age | 1.0 | 0.9 | 1.0 | 0.587 |
| Male vs. Female | 1.2 | 0.8 | 1.8 | 0.361 |
| White vs. Black | 1.5 | 0.8 | 2.6 | 0.147 |
| Hispanic vs. Black | 0.9 | 0.5 | 1.6 | 0.895 |
| BMI | 0.9 | 0.9 | 0.9 | 0.004 |
| FGG | 1.0 | 0.9 | 1.0 | 0.373 |
| GFR: 30-59 vs. 60+ | 0.9 | 0.4 | 1.9 | 0.950 |
| GFR: 15-29 vs. 60+ | 2.5 | 1.3 | 4.8 | 0.004 |
| GFR: <15 vs. 60+ | 8.3 | 4.3 | 15.8 | <0.001 |
| Protein: 3.5+ vs. <3.5 | 2.0 | 1.4 | 3.0 | <0.001 |
| HTN | 1.8 | 0.9 | 3.4 | 0.053 |
| CAD | 1.1 | 0.6 | 1.8 | 0.643 |
| Nephropathy: Diabetic vs. Autoimmune | 0.7 | 0.3 | 1.54 | 0.482 |
| Nephropathy: IgA vs. Autoimmune | 0.7 | 0.3 | 1.7 | 0.526 |
| Nephropathy: Other vs. Autoimmune | 0.6 | 0.3 | 1.1 | 0.118 |
(BMI, Body Mass Index; GFR, Glomerular Filtration Ratio; HTN, hypertension; CAD, coronary artery disease; IgA, Immunoglobulin A).
Discussion
Our study highlights some of the limitations of using the level of interstitial fibrosis on renal biopsy to predict who will develop ESRD. Several studies have shown that fibrosis on biopsy is an index of functional renal impairment and a major determinant in the progression of chronic kidney disease [1,5,6,10-12,17]. Our findings emphasize that although fibrosis performs well overall, it is of less value for a subset of individual cases. Regardless of patient characteristics, we observed a wide range of fibrosis both in patients who reached dialysis and who remained dialysis free. Surprisingly, some of the patients who had >80% fibrosis on the biopsy did not progress to ESRD during a 10-year follow up period. Over-reliance on fibrosis on biopsy may therefore erroneously identify patients as having advanced CKD at a time when interventions may still be effective.
Extent of fibrosis is a determining factor in designing clinical trials and in kidney transplantation. Patients with extensive histologic evidence of renal scarring with the percentage of fibrosis exceeding 50% are excluded from clinical trials because they are considered poor candidates for the evaluation of the effect of the therapy. In particular, they are routinely excluded from trials using medications that are designed to modulate immune response and functionally important inflammatory nephritis [18]. However, our data demonstrates that 42% and 21% of these patients are dialysis free at years 2 and 5, respectively, suggesting that some of those patients might also benefit from treatment. Patients who would benefit from a kidney transplant may have to remain on the waitlist because kidneys with mild interstitial fibrosis are being rejected for transplantation [19]. Our study conducted in native kidneys is consistent with several studies in the transplant literature showing that a mild to moderate degree of fibrosis on time-zero biopsy does not necessarily predict worse graft function in living or deceased donor kidney transplantation [20-23].
To the best of our knowledge this is the first study to identify specific patient groups where fibrosis does not perform well in predicting progression to ESRD: 1) in patients older than 70 years of age or 2) patients with diabetic nephropathy on biopsy. Our data show that aging leads to an increase in the level of fibrosis even in patients who don't progress to ESRD, making fibrosis a less reliable prognostic factor in the elderly. Age associated glomerulosclerosis may in part explain the weaker correlation in the elderly patients. A previous study from 1991 with a large cohort of 488 patients with diabetic nephropathy had suggested that fibrosis correlates with progression to ESRD [24]. In contrast, in our cohort of 42 patients with diabetic nephropathy on biopsy, the level of interstitial fibrosis was not a reliable predictor of progression. One possible explanation for the divergent finding is the changing nature of diagnosis and treatment of patients with diabetic nephropathy. Patients with poorly controlled diabetes and other obvious microvascular complications are frequently not biopsied. As a result we have a lower biopsy rate in these patients compared to other etiologies and this potentially could lead to sampling bias. Alternatively, the poor correlation between fibrosis and ESRD progression in our study could be because diabetic patients usually have some level of chronic vascular injury that can be patchy leading to variable levels of fibrosis in the tissue.
Another helpful clinical tool, in conjunction with predicting if a patient will progress to dialysis, is anticipating the amount of time a patient has until dialysis initiation (Tables 4A and Figures 3 and 4). This data could be used in clinical practice to give a rough estimate of dialysis free survival to our patients based on their level of fibrosis. In general, the group with more fibrosis progressed to ESRD more rapidly than those with less fibrosis, but again, individual variability was large regardless of biopsy diagnosis or patient characteristics.
We noted several expected findings that confirmed previous observations. Patients who reached dialysis during the follow-up period were older and more likely to be male. Hypertension, nephrotic range proteinuria, vascular disease and worse renal function at the time of biopsy were all risk factors for dialysis. Being on insulin treatment was a risk factor for dialysis, but being on oral agents was not, suggesting that easily controlled diabetics often don't progress to ESRD. History of autoimmune diseases carried a lower risk of dialysis likely because these groups of pathologies are potentially treatable.
All biopsies were read by one of our two pathologists, therefore the pathological findings are internally consistent. In general, the reliability of pathologist-based estimates of fibrosis improves the likelihood that our findings can be accurately reproduced in other centers. Furthermore, because interstitial fibrosis is one of the common final pathways in all kidney diseases, the results of this study are widely applicable.
Another important strength of our study is that its conclusions carry across racial lines. Our patient population was diverse: 42% of the patients were black, 22% were white and 22% were Hispanic. Renal disease disproportionately affects African-Americans therefore it is imperative to validate study findings in this population. Similar to previous studies we found that blacks were more likely to progress to ESRD regardless of their diagnosis.
Death rate was relatively low in our cohort. Only 15 (3.46%) patients died during the study period. Median time to death was 2.18 years. We speculate that very sick patients who died soon after presentation were not biopsied. The long duration of follow-up (median 5.6 years), large sample size, low rate of loss to follow-up, and availability of detailed medical history in electronic medical records are additional strengths.
In our cohort a large proportion of renal biopsies were done at CKD stage 3-4 with mean creatinine of 2.6±2.6 mg/dl. Most of these patients likely had renal impairment, proteinuria, or hematuria for a prolonged period of time before they were referred to a nephrologist and a biopsy was done.
The retrospective nature of this study is a limitation. Another potential limitation is that timing of the biopsy and dialysis initiation can be physician dependent and could contribute to lead time bias. In our opinion, in a university teaching hospital setting nephrologists follow the same guidelines so it is unlikely that provider variability had a large effect on renal outcome or timing of dialysis. Results for specific subgroups of patients were based on limited sample sizes and should be confirmed in a larger cohort. Last, our study results were derived from a single cohort and will require validation in an external cohort. While the inclusion of additional information in a predictive model resulted in statistically significant improvement in discriminatory ability, it isn't clear whether this improvement is clinically relevant. How multiple predictors could be used simultaneously in clinical practice also warrants further study.
Our study identifies some of the limitations in establishing disease prognosis and progression from a kidney biopsy obtained at one point in the course of a chronic disease affected by sampling error. Development of a non-invasive technique such as transient elastography for liver fibrosis [25-28] would be ideal for repeated, global assessment of renal fibrosis and to determine whether or not it correlates with functional deterioration. In the meantime, we should be cautious about the interpretation of renal fibrosis, especially in the elderly and those with diabetic nephropathy.
Supplementary Material
Acknowledgments
This research was partially supported by NIH grant 1KO8DK090143 (A.H.). We thank Prof. David J. Salant for his comments that greatly improved the manuscript.
Footnotes
Conflict of interest - Financial Disclosures: None
References
- 1.Kostadinova-Kunovska S, Petrusevska G, Jovanovic R, Grcevska L, Bogdanovska M, Polenakovic M. Morphological changes in the tubulointerstitial compartment in primary glomerulopathies. Prilozi. 2007;28:61–74. [PubMed] [Google Scholar]
- 2.Madaio MP. Renal biopsy. Kidney international. 1990;38:529–543. doi: 10.1038/ki.1990.236. [DOI] [PubMed] [Google Scholar]
- 3.Seron D, Moreso F, Fulladosa X, Hueso M, Carrera M, Grinyo JM. Reliability of chronic allograft nephropathy diagnosis in sequential protocol biopsies. Kidney international. 2002;61:727–733. doi: 10.1046/j.1523-1755.2002.00174.x. [DOI] [PubMed] [Google Scholar]
- 4.Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, Hemmelgarn B. Histopathologic features aid in predicting risk for progression of iga nephropathy. Clinical journal of the American Society of Nephrology : CJASN. 2010;5:425–430. doi: 10.2215/CJN.06530909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nath KA. Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis. 1992;20:1–17. doi: 10.1016/s0272-6386(12)80312-x. [DOI] [PubMed] [Google Scholar]
- 6.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005:S82–86. doi: 10.1111/j.1523-1755.2005.09915.x. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico G. Influence of clinical and histological features on actuarial renal survival in adult patients with idiopathic iga nephropathy, membranous nephropathy, and membranoproliferative glomerulonephritis: Survey of the recent literature. Am J Kidney Dis. 1992;20:315–323. doi: 10.1016/s0272-6386(12)70293-7. [DOI] [PubMed] [Google Scholar]
- 9.Meyer TW. Tubular injury in glomerular disease. Kidney international. 2003;63:774–787. doi: 10.1046/j.1523-1755.2003.00795.x. [DOI] [PubMed] [Google Scholar]
- 10.Nagy J, Trinn C, Deak G, Schmelzer M, Burger T. The role of the tubulointerstitial changes in the prognosis of iga glomerulonephritis. Klin Wochenschr. 1984;62:1094–1096. doi: 10.1007/BF01711380. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Tomino Y, Fukui M, Shirato I, Yaguchi Y, Ebihara I, Kubota M, Nakayama S, Koide H. Correlation between tubulointerstitial changes and prognosis in patients with primary membranous nephropathy. Nephron. 1994;67:362. doi: 10.1159/000187995. [DOI] [PubMed] [Google Scholar]
- 12.Ziyadeh FN. Significance of tubulointerstitial changes in diabetic renal disease. Kidney Int Suppl. 1996;54:S10–13. [PubMed] [Google Scholar]
- 13. http://mdrd.com/:
- 14.Cerqueira DC, Soares CM, Silva VR, Magalhaes JO, Barcelos IP, Duarte MG, Pinheiro SV, Colosimo EA, Simoes e Silva AC, Oliveira EA. A predictive model of progression of ckd to esrd in a predialysis pediatric interdisciplinary program. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:728–735. doi: 10.2215/CJN.06630613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, Levin A, Levey AS. A predictive model for progression of chronic kidney disease to kidney failure. Jama-J Am Med Assoc. 2011;305:1553–1559. doi: 10.1001/jama.2011.451. [DOI] [PubMed] [Google Scholar]
- 16.Mackensen-Haen S, Bader R, Grund KE, Bohle A. Correlations between renal cortical interstitial fibrosis, atrophy of the proximal tubules and impairment of the glomerular filtration rate. Clinical nephrology. 1981;15:167–171. [PubMed] [Google Scholar]
- 17.Park MH, D'Agati V, Appel GB, Pirani CL. Tubulointerstitial disease in lupus nephritis: Relationship to immune deposits, interstitial inflammation, glomerular changes, renal function, and prognosis. Nephron. 1986;44:309–319. doi: 10.1159/000184012. [DOI] [PubMed] [Google Scholar]
- 18.Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive iga nephropathy. Journal of the American Society of Nephrology : JASN. 2002;13:142–148. doi: 10.1681/ASN.V131142. [DOI] [PubMed] [Google Scholar]
- 19.Snoeijs MG, Buurman WA, Christiaans MH, van Hooff JP, Goldschmeding R, van Suylen RJ, Peutz-Kootstra CJ, van Heurn LW. Histological assessment of preimplantation biopsies may improve selection of kidneys from old donors after cardiac death. Am J Transplant. 2008;8:1844–1851. doi: 10.1111/j.1600-6143.2008.02318.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee AL, Kim YS, Lim BJ, Jeong HJ, Joo DJ, Kim MS, Huh KH. The impact of time-zero biopsy on early graft outcomes after living donor kidney transplantation. Transplantation proceedings. 2013;45:2937–2940. doi: 10.1016/j.transproceed.2013.08.081. [DOI] [PubMed] [Google Scholar]
- 21.Sund S, Reisaeter AV, Fauchald P, Bentdal O, Hall KS, Hovig T. Living donor kidney transplants: A biopsy study 1 year after transplantation, compared with baseline changes and correlation to kidney function at 1 and 3 years. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1999;14:2445–2454. doi: 10.1093/ndt/14.10.2445. [DOI] [PubMed] [Google Scholar]
- 22.Cockfield SM, Moore RB, Todd G, Solez K, Gourishankar S. The prognostic utility of deceased donor implantation biopsy in determining function and graft survival after kidney transplantation. Transplantation. 2010;89:559–566. doi: 10.1097/TP.0b013e3181ca7e9b. [DOI] [PubMed] [Google Scholar]
- 23.El-Husseini A, Sabry A, Zahran A, Shoker A. Can donor implantation renal biopsy predict long-term renal allograft outcome? American journal of nephrology. 2007;27:144–151. doi: 10.1159/000099944. [DOI] [PubMed] [Google Scholar]
- 24.Bohle A, Wehrmann M, Bogenschutz O, Batz C, Muller CA, Muller GA. The pathogenesis of chronic renal failure in diabetic nephropathy. Investigation of 488 cases of diabetic glomerulosclerosis. Pathology, research and practice. 1991;187:251–259. doi: 10.1016/s0344-0338(11)80780-6. [DOI] [PubMed] [Google Scholar]
- 25.Macias J, Camacho A, Von Wichmann MA, Lopez-Cortes LF, Ortega E, Tural C, Rios MJ, Merino D, Tellez F, Marquez M, Mancebo M, Pineda JA. Liver stiffness measurement versus liver biopsy to predict survival and decompensations of cirrhosis among hiv/hepatitis c virus-coinfected patients. Aids. 2013;27:2541–2549. doi: 10.1097/QAD.0b013e32836381f3. [DOI] [PubMed] [Google Scholar]
- 26.Singh S, Fujii LL, Murad MH, Wang Z, Asrani SK, Ehman RL, Kamath PS, Talwalkar JA. Liver stiffness is associated with risk of decompensation, liver cancer, and death in patients with chronic liver diseases: A systematic review and meta-analysis. Clin Gastroenterol H. 2013;11:1573–U1237. doi: 10.1016/j.cgh.2013.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awad Mel D, Shiha GE, Sallam FA, Mohamed A, El Tawab A. Evaluation of liver stiffness measurement by fibroscan as compared to liver biopsy for assessment of hepatic fibrosis in children with chronic hepatitis c. Journal of the Egyptian Society of Parasitology. 2013;43:805–819. [PubMed] [Google Scholar]
- 28.Kim BK, Fung J, Yuen MF, Kim SU. Clinical application of liver stiffness measurement using transient elastography in chronic liver disease from longitudinal perspectives. World J Gastroentero. 2013;19:1890–1900. doi: 10.3748/wjg.v19.i12.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.