Abstract
Hypertension is the most prevalent risk factor for cardiovascular disease caused by a persistent increase in arterial blood pressure that has lasting effects on the mechanical properties of affected tissues like myocardium and blood vessels. Our group recently discovered that gut dysbiosis is linked to hypertension in several animal models and humans; however, whether hypertension influences the gut’s mechanical properties remains unknown. In this study, we evaluated the hypothesis that hypertension increases fibrosis and thus mechanical properties of the gut. A custom indentation system was used to test colon samples from Wistar Kyoto (WKY) normotensive rats and Spontaneously Hypertensive Rats (SHR). Using force-displacement data, we derived an steady-state modulus metric to quantify mechanical properties of gastrointestinal tissue. We observed that SHR proximal colon has a mean steady-state modulus almost 3 times greater than WKY control rat colon (5.11 ± 1.58 kPa and 18.17 ± 11.45 kPa, respectively). These increases were associated with increase in vascular smooth muscle cells layer and collagen deposition in the intestinal wall in the SHR.
Keywords: Gastrointestinal tissue mechanics, Spontaneously Hypertensive Rat, Viscoelasticity, Colon, Bowel mechanics
1. Introduction
Hypertension (HTN) is a well-documented risk factor for cardiovascular disease and is one of the leading causes of death worldwide [1]. Causes of HTN include genetic predisposition, unhealthy lifestyle, and hormonal imbalances. Despite major advances in pharmacology and habitual lifestyle changes, HTN still retains a high prevalence rate. In efforts to improve our understanding of HTN, we recently discovered a link between HTN and disruption in microbes of the gut, known as gut dysbiosis [2]. Many of the causes of HTN, such as genetics, age, diet, and lifestyle differences, can cause disruptions in gastrointestinal microbiota [3]. Our group has found extensive evidence of gut dysbiosis in both the Spontaneously Hypertensive Rat (SHR) model and in patients with high blood pressure [2]. Since cardiovascular tissue stiffens in response to smooth muscle remodeling in HTN [1,4], and recognizing gastrointestinal (GI) tissue comprises smooth muscle cells to move waste and facilitate digestion [5], we hypothesized that the gut similarly will stiffen with HTN through increased matrix deposition and smooth muscle cell proliferation. Vascular smooth muscle remodeling has already been demonstrated in the SHR line [6], making this rat line a good model to investigate gastrointestinal smooth muscle remodeling as a result of gut dysbiosis and HTN.
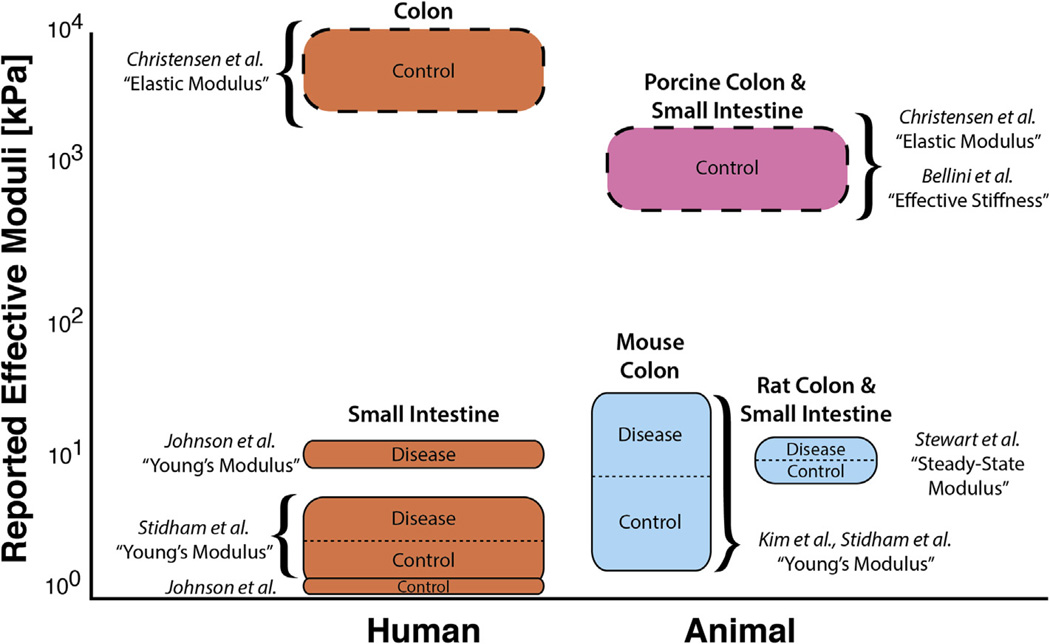
Mechanics of gastrointestinal tissue have been characterized using a variety of approaches, but the results vary widely among techniques and labs (Fig. 1). Some characterization methods yield variable results because the equipment was originally designed for traditional engineered materials, such as aluminum or titanium, that are orders of magnitude stiffer than biological tissue. Because of this, new equipment and methodologies have been developed, including redesigned test rigs and diverse techniques such as atomic force microscopy (AFM). AFM methods are useful to quantify properties at the nano- to micro-scale level and are appropriate for investigating cellular-level mechanics [13]. However, they are not suitable to characterize large areas of tissue that are micro- to milli-scale in size [14]. Additional variation in quantified properties can arise from erroneously fitting data to linear elastic constitutive models originally derived for materials such as ceramics or metals; the soft, viscous, and strain-rate sensitive nature of most organs and tissues further complicates attempts at characterization.
Fig. 1.
Reported values for moduli of gastrointestinal tissue span three orders of magnitude, ranging from <1 kPa to >6000 kPa depending on the method and analyses used. Uniaxial and biaxial test (dashed lines) results range from 850 to 6480 kPa (Christensen et al. [7] and Bellini et al. [8]), but tests using mesoscale compression/indentation methodology (solid line) showed a significantly lower modulus ranging from 0.3 to 58.5 kPa (Stidham et al. [9], Kim et al. [10], Johnson et al. [11], and Stewart et al., this work and small intestine data unpublished). Despite differences that can occur between species, age of the animal, and ex-vivo conditions [12], the high variability of this data over three orders of magnitude warrants more consistent testing and reporting methods for the characterization of soft tissue.
To properly characterize tissue-level mechanics, we have developed our own multi-scale indentation system to characterize soft, hydrated samples [15] that is sensitive enough to detect forces in the initial linear range of indentation. We and others have used similar indentation methods to determine mechanical properties of heart [15], muscle [16], brain [17], and other gastrointestinal tissue [14]. By modifying the well-developed Hertz contact model to identify a “steady-state modulus” with our multi-scale indenter, we have the capability to distinguish mechanical properties between biological samples to inform future mechanobiology experiments. Using the Spontaneously Hypertensive Rat as a well-accepted model of HTN, we find that steady-state modulus of HTN gut is increased over age-matched controls. Stained intestine from both control and SHR lines suggest increases in matrix deposition and smooth muscle proliferation may contribute to stiffening, but further experiments are warranted to understand the pathophysiological effects of dysbiosis on the gastrointestinal and cardiovascular systems.
2. Materials and methods
2.1. Specimen preparation
All animal procedures were approved by the University of Florida Institutional Animal Care and Use Committee. The SHR rat line was formed in the 1960s by breeding Wistar-Kyoto (WKY) rats with high blood pressure [18]. SHRs begin developing HTN around 5–6 weeks of age, develop cardiac failure at 18–24 months, and are frequently used for systemic hypertensive studies [19]. Proximal colon samples were tested from both normotensive control (WKY, n = 5) and SHR (n = 4) lines. At 20 weeks of age, WKY rats had a mean arterial pressure (MAP) of 99 ± 3 mmHg, and SHR had MAP of 158 ± 2 mmHg. Rats were euthanized and a 2–2.5 cm section of colon was excised. Freshly isolated tissue samples were placed in cold media solution consisting of Dulbecco’s Modified Eagle Medium (DMEM) and 5% Fetal Bovine Serum (FBS) and tested within 4 h of isolation.
Colon samples were cleaned initially by gently pushing feces out of the sample with a soft edge. Samples were then cut open longitudinally, rinsed in cold buffered saline, and placed flat on Petri dishes for testing. Tissues were handled and placed to ensure that the inner mucosa of the intestine remained on top (Fig. 2ii–iii). Sample thickness was approximately 1.5 mm. All samples were prepared and tested at room temperature (approximately 22 °C). Small aliquots (less than 1 mL) of the DMEM solution were added periodically during testing to prevent dehydration of the samples.
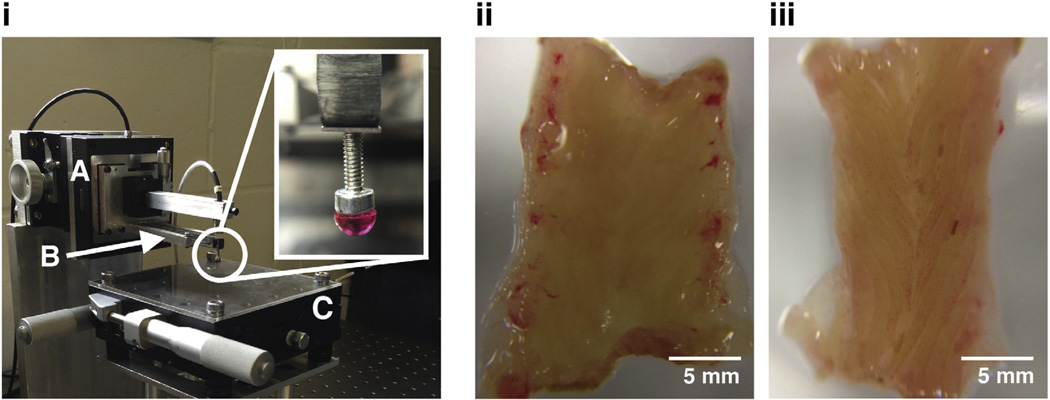
Fig. 2.
Custom-built indentation apparatus (i) used for indenting biological tissue samples. (A) Piezoelectric stage that moves cantilever base along an axis normal to the tissue surface. (B) Calibrated cantilever used for determining the normal force acting on the tip of the cantilever as the piezoelectric stages displaces the cantilever. Deflections of the cantilever are measured with a capacitive sensor. (C) X-Y stage as base for tissue sample. Micrometers enable indentations in different locations. (Inset) 3 mm-diameter rigid tip used in contact with tissue during indentation. Images of WKY (ii) and SHR (iii) colon samples isolated and cut open longitudinally for indentation. Samples were placed under the rigid tip for force-relaxation tests.
2.2. Indentation apparatus and force relaxation tests
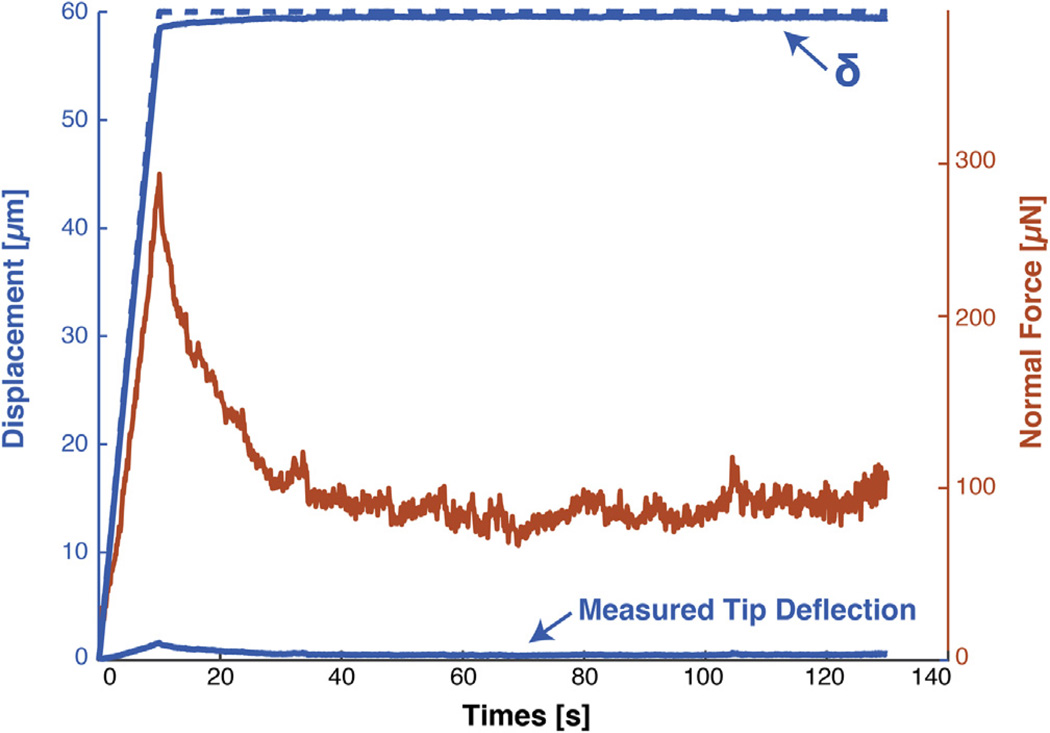
A custom cantilever-based indenter (Fig. 2i) [15,20] was used to indent tissue samples and record force relaxation over time. A piezoelectric stage (P-628.1CD, Physik Instrumente) displaced a soft titanium cantilever with a 3 mm-diameter rigid tip. Cantilever stiffness (175.4 N/m) was calibrated directly with small weights hung from the cantilever tip. A custom program in LabVIEW (National Instruments) was used to control indentation profile and to read deflection of cantilever tip with capacitive sensor (C8S-3.2-2.0 and compact driver CD1–CD6, Lion Precision) through a data acquisition card system (NI 9220 and cDAQ-9171, National Instruments). The cantilever base was driven to a depth of 60 µm at 6 µm/s and then held for 120 s (Fig. 3). Because isolated tissues from animals are hard and expensive to obtain, indentation profiles included a modest loading rate that would allow 10 s of data acquisition at 10 Hz with which to fit indentation models in addition to a relaxation phase that reached a quasi-steady state. This afforded flexibility in choosing models to describe tissue behavior with an efficient use of resources. Samples were indented at 4 different locations along the length of the tissue.
Fig. 3.
Our MSI is sensitive enough to detect ~10 nm-resolution deflections for stress-relaxation experiments on soft matter. Graph depicts the indentation and relaxation command profile for the piezoelectric stage (dashed blue line, left axis), deflection of the cantilever (solid blue bottom line, left axis), the cantilever tip displacement (δ, solid blue top line, left axis), and resulting calculated forces (orange line, right axis) determined from capacitive sensor values and cantilever stiffness. The ability to sensitively measure small forces and displacements allows for the quantification of mechanical properties using a Hertz model.
2.3. Immunohistochemical staining of tissues
Small intestines were rinsed, fixed in 4% paraformaldehyde, and embedded in Sakura Tissue-Tek Oct Compound (4583 OCT Compound; Sakura Finetek USA, Inc., Torrance, CA, USA).
Embedded tissues were cut on a transverse plane into 10 µm thick rings for hematoxylin and eosin (HE) and trichrome staining. Images were captured using an Olympus Model BX41 fluorescent microscope (Olympus, Tokyo, Japan).
3. Theory and calculations
3.1. Hertz contact model
The custom indentation system described in Section 2.2 utilizes a spherical tip to indent the surface of a tissue at a given rate. Using a capacitive probe to measure the deflection of the cantilever tip, force and displacement of the tip can be calculated over time. The Hertz contact mechanics model is frequently used to relate load and indentation depth and identify elastic modulus, EHertz, for indentation experiments as in Eq. (1) (See derivation and discussion by Vriend [21]):
| (1) |
where F = force as calculated by tip displacement multiplied by calibrated stiffness; R = radius of spherical indenter tip; δ = indentation depth calculated as stage movement minus deflection of tip; and ν = Poisson’s Ratio. This formulation has also been used for a variety of other soft matter indentation studies [22,23].
While the Hertz model is often applied to soft tissues and cells because indentation methods can be readily adapted to samples of arbitrary geometries [14,15,24,25], soft materials can violate assumptions of the original Hertz model [23,26,27]. Specifically, Hertzian contact theory assumes that the two materials in contact are isotropic, linearly elastic, and homogeneous. Biological tissues do not fit these assumptions as most are viscoelastic, have nonlinear material behavior, and are highly heterogeneous due to hierarchical tissue structure [28,29]. However, the millimeter-scale dimensions of our probe capture more homogeneous behavior than the typical nano/microscale of indentation, and the ~10 nm-resolution deflections measured with the capacitive probe on our MSI (Fig. 2) enable our indentations to be constrained to small deformations. Hertz theory also assumes no friction or adhesion exists between the surfaces, so we are careful to keep the tissues hydrated with media and we use an optically polished ruby tip to reduce friction effects. Complexities of adhesion are reduced by performing analysis of the indentation and relaxation phases but not the retraction phase where adhesive effects are high [26,30].
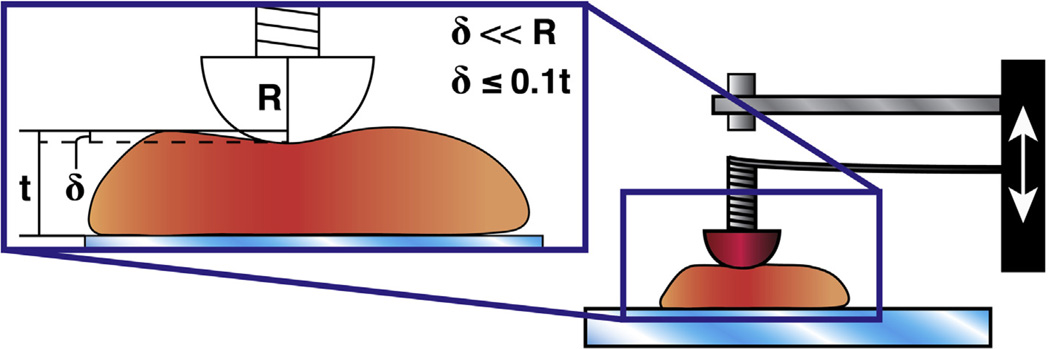
Even with the aforementioned limitations, the sensitivity and precision of our MSI allow us to control many of the depth and dimension parameters that are also constrained with the Hertz model. We find the Hertz model useful to approximate an effective modulus of biological soft matter for small indentations less than 10% of the total thickness of the sample being indented and much less than the diameter of the sphere used for indentation (Fig. 4) [27]. The radius constraint is often operationalized as less than 10% [22], though original Hertz calculations suggest that less than 1% is appropriate [27]. Similar to a viscoelastic “stress relaxation modulus” that looks at instantaneous changes in effective modulus during stress relaxation [31], we rearranged the Hertz contact model for parabolic contact area to determine the effective modulus at a given point in time:
| (2) |
where F = force as calculated by tip displacement multiplied by calibrated stiffness; R = radius of spherical indenter tip; δ = indentation depth calculated as stage movement minus deflection of tip; and ν = Poisson’s Ratio. In our experiments and analyses, R = 1.5 mm, δmax ≈ 60 µm, and ν = 0.4. A typical assumption in other work on gastrointestinal characterization is ν = 0.5 [32,33]; however, we used a Poisson’s ratio of 0.4 due to compressibility measured in similar tissues in our lab. Our uncertainty analysis indicates that error due to large changes in Poisson’s ratio is approximately 100 times less than the uncertainty on our force measurements, so we proceed with this estimate.
Fig. 4.
The Hertz contact model is valid only to certain indentation depths (δ) and is limited based on thickness of the sample (t) being tested and the radius (R) of the indentation tip. The figure shows a schematic of our MSI (right, described in Section 2.2) and a graphical representation of important restrictions on the Hertz model. Among other restrictions, the Hertz model will only be valid for small strains and indentation displacements such that δ ≪ R and δ ≤ 10% of the thickness of the sample [22,27].
3.2. Determination of steady-state modulus
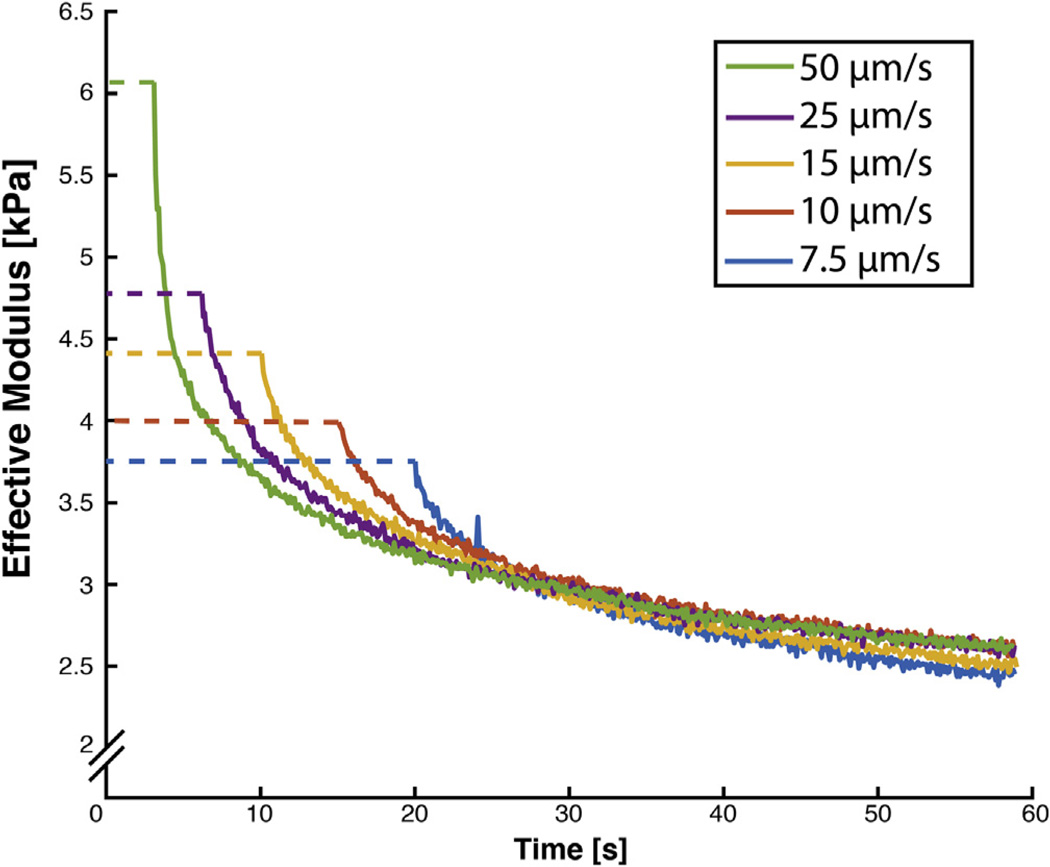
In viscoelastic stress relaxation experiments, the stress relaxation modulus Er is calculated at each time point for measured engineering stress, σ(t), and constant imposed strain, ε0: Er(t) = σ(t)/ε0. Similarly, we estimated the time-dependent Eeffective during our load relaxation phase using Eq. (2). After allowing ~2 min of load relaxation at a constant indentation depth, measured forces reach a steady-state independent of initial indentation rate (Fig. 5), similar to the stress relaxation modulus at t = ∞. Our thus-named steady-state modulus (SSM) is a common feature of many complex models though it goes by various titles, including the “aggregate modulus” of poroelastic model [34] and “indentation modulus” in an AFM study similar to ours [22,35].
Fig. 5.
Time-dependent effective modulus is similar to stress relaxation modulus and converges to a consistent steady-state value regardless of initial indentation rate. Indentations were performed at nearby locations (~0.5 mm away) after allowing ~5 min of recovery to minimize natural biological variation between measurements.
To calculate a reasonable SSM using a simple model, we chose the Standard Linear Solid (SLS) [36–38]. In the typical spring-dashpot model for the viscoelastic SLS, the single parallel spring, Ep, is a reasonable representation of SSM and thus the SLS provides a simple model to which to fit our data to obtain SSM.
| (3) |
where Eeffective is the effective, time-dependent modulus, Ep is the steady-state modulus after relaxation, Es is the initial or strain-rate dependent modulus, and η is the viscosity. To fit the experimental relaxation curve to the Standard Linear Solid model, three points were selected at the beginning, middle and end of the relaxation stage. The coefficients for the SLS model were found using an algebraic manipulation as explained in the Appendix, adapted from Moore et al. [38].
4. Experimental results and discussion
4.1. Evaluation of steady state modulus using indentation
While complex and sophisticated models are required to fully describe stress-strain behavior for all strain rates and loading configurations, we have experimentally determined that our steady-state modulus, derived from a Hertz-modified stress relaxation-type effective modulus, is consistent across strain rates (Fig. 5) and thus is a useful metric for simple comparisons of biological tissue.
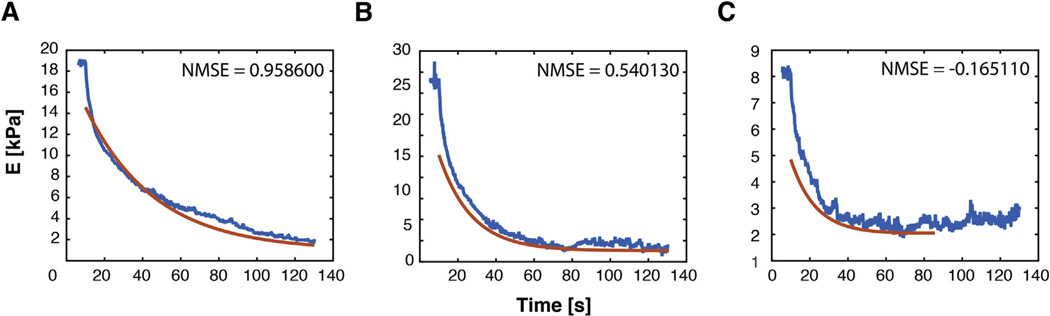
A simple SLS model can be used to fit effective modulus data during relaxation to estimate the steady-state modulus. SLS fit was evaluated using the Normalized Mean Square Error (NMSE) in the Goodness of Fit MATLAB function. Out of 36 indentations, 30 showed high values for NMSE, indicating good fit. Four data sets had NMSE numbers between 0.72 and 0.42 but still reflected reasonable SSM values upon inspection, and only two of them had negative NMSE values, which were discarded before further statistical analysis (Fig. 6). Since we have constrained ourselves to small indentations made possible by our precise capacitive sensor, linear behavior is a reasonable expectation and SLS equations thus provide a reasonable estimate of steady-state behavior. However, the SLS model does not recapitulate initial relaxation behavior well, indicating that non-linear, poroviscoelastic, and/or multiple element models would be required to recapitulate detailed constitutive behavior.
Fig. 6.
Normalized Mean Square Error (NMSE) is used to evaluate goodness of fit for algebraic determination of SSM. (A) Representative curve for high NMSE where 1 is a perfect fit. More than half of the fits yield a NMSE above 0.9. (B) Four indentations showed NMSE values between 0.72 and 0.42, indicating initial relaxation response was not perfectly captured by the SLS model. However, inspection reveals the fit through later relaxation times still approximates a reasonable asymptotic SSM. (C) Indentations that showed negative NMSE results (2 out of 36 indentations) were discarded, as negative values indicate a poor fit.
4.2. Steady-state moduli show a three-fold increase in SHR samples
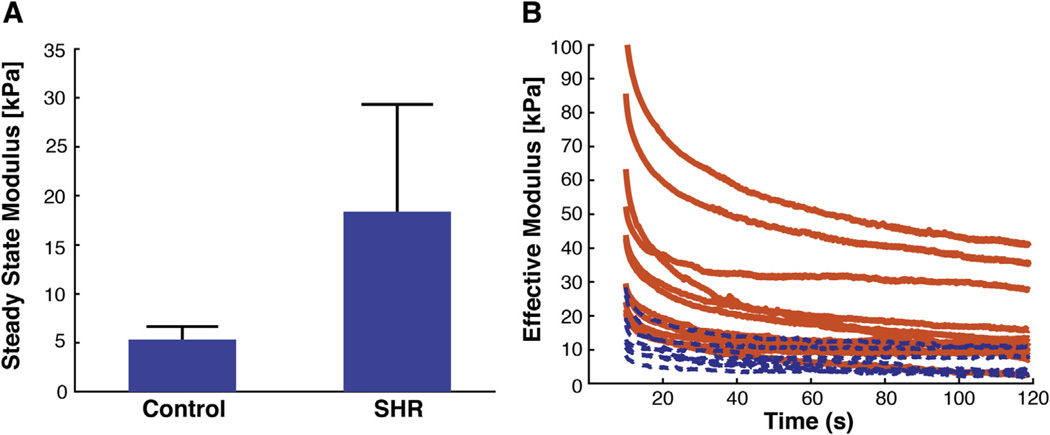
In normotensive WKY tissue samples, we observed a steady-state modulus of 5.11 ± 1.58 kPa while the SHR samples yielded a steady-state modulus of 18.17 ± 11.45 kPa, more than 3 times higher than in the WKY samples (Fig. 7). Previous methods to study the mechanical behavior of the colon have resulted in a wide range of values for modulus spanning 5–6000 kPa [7–12]. Our calculated values for steady-state moduli fall within this range, and our observation of stiffening with disease parallels findings from studies of colitis and Crohn’s Disease [9–11]. We are the first, however, to demonstrate this increase in mechanical properties for GI tissue in a hypertensive rat model.
Fig. 7.
SHR colon samples are 3 times stiffer than the normotensive WKY controls. A) Steady-state moduli of the WKY (control) and SHR colon samples (n = 5 animals for WKY and n = 4 animals for SHR with 3–4 indentations per animal) are 5.11 ± 1.58 kPa and 18.17 ± 11.45 kPa, respectively. Pooled means for all indentations were significantly different (p < 0.009), and average of means calculated for each animal were also significantly different (p = 0.05) based on the conservative Welch’s t-test. Error bars depict standard deviation. B) After a quasi-infinite time, tissues exhibit distinct steady-state properties during stress-relaxation. Control animals (dashed blue line) show a lower steady-state modulus than the SHR animals (solid orange line). Only a sub-set of the control curves are plotted for clarity but do depict the total range of values measured.
Importantly, the layered nature of the gut violates isotropy assumptions of the Hertz model, which confounds the causal conclusions we are able to draw from these experiments. Given the utility of indentation methods for characterizing small portions of soft tissue, future work should focus on appropriate contact models of layered materials. Indentations at different depths or with different shapes and sizes of tips may yield sufficient information to parse the properties of layered materials and thus provide useful information about the pathophysiology of different anatomical layers of gut or blood vessel. However, the range of methods required to derive coefficients of generalizable constitutive models may require a number and scope of tests unreasonable for limited availability, small size, and hydrated tissue samples. Prescriptive test protocols for biological tissue similar to ASTM test protocols for manufactured materials may be helpful for comparing across treatment conditions and designing engineered tissues.
4.3. Smooth muscle layer and collagen content increased in the SHR gut
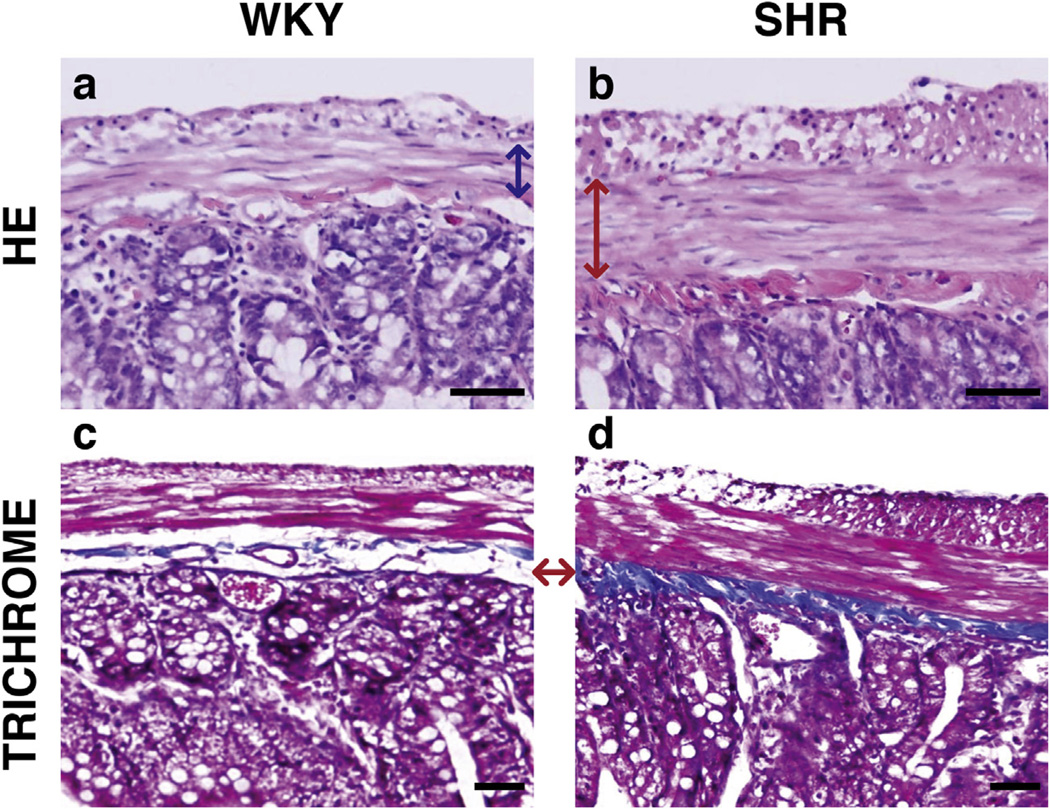
A large increase in the thickness of the muscularis layer and the density of the smooth muscle cells was observed in the SHR samples compared to the WKY normotensive control (Fig. 8a and b). Trichrome staining also showed an increase in the collagen layer deposition in the submucosa layer of the GI track (Fig. 8c and d). These increases in collagen and smooth muscle content along with changes in other extracellular matrix proteins of these two layers likely contributed to the increased stiffness that was observed through indentation. Small molecule inhibitors could be used in the future to relax smooth muscle tone prior to indentation to attempt to isolate independent contributions from matrix and cell stiffness. Future investigations are necessary to determine which mechanisms of stiffening and fibrosis are directly activated by HTN and thus responsible for the increased stiffness of SHR colon.
Fig. 8.
Representative transverse slices show an increase in smooth muscle proliferation and collagen deposition in SHR samples. Hematoxylin and Eosin staining (top row) shows thickened smooth muscle cell layer in the SHR (red arrow, 8b) from the WKY normotensive control (blue arrow, 8a). Trichrome stains (bottom row) show increased collagen deposition in the SHR sample (8d) in the submucosa layer than in the WKY sample (8c). The red arrow shows the corresponding layers in each sample, where the dense blue layer in the SHR sample is indicative of a higher density of collagen. Scale bars are 50 µm; serosa is featured at the top of each image.
Ultimately, this remodeling and stiffening response should be confirmed in other models of HTN as well. While SHR is used more frequently than other models, other HTN models with genetic, renal, and endocrine etiologies are also available such as the Dahl-rat, two-kidney one-clip, and DOCA model, respectively [19]. Gastrointestinal changes with HTN could likely depend on variations in diet, kidney function, and onset of heart failure seen across animal models. Our group has already identified similarities in HTN-related gut dysbiosis between human patients and SHRs, though, so we continue to investigate SHRs along with other small-molecule induced models of HTN [39].
Despite extensive investigations in the field of HTN over the last half a century, the role of gut physiology has been essentially ignored in blood pressure control and HTN. The gut is one of the highest vascularized organs and possess intricate neural and immune pathways, both critical in the development and establishment of HTN. Stiffness has been shown to increase permeability of blood vessels [40] and may have a similar effect on gut permeability. It is in this context that our observation is significant and persuades us to propose the following hypothesis: an inherent increase in gut permeability as a result of increased stiffness perpetuates a cascade of events in the gut involving inflammation and dysbiosis, which culminate in the development and establishment of high blood pressure.
The mechanism of HTN-linked increase in effective modulus of intestine remains speculative and needs to be investigated. We suggest that autonomic regulation of sympathetic nervous system innervating the gut could be critical in the initiation of this gut pathophysiology [41]. This view is supported by our data and evidence from the literature demonstrating an increase in sympathetic activity in pre-hypertensive humans and in animal models of HTN [42]. However, other possibilities such as a direct effect of angiotensin II (ANG II) on the gut cannot be ruled out. ANG II has been shown to induce fibrosis and hypertrophy of vessels and heart tissue in rodent models of HTN [43], and these pathologies are also present in the SHR model [44]. Finally, changes in the gut may be indirect and could be induced by increase in blood pressure.
Mechanisms of stiffening exist independent of traditional fibrotic events [14], so the causal relationship among gut dysbiosis, epithelial permeability, stiffness, and HTN inevitably will be complex. By developing methods and models to characterize the mechanical properties of hypertensive gut, we can now develop disease-mimicking in vitro platforms to control substrate mechanics independent of gut pathophysiology to determine causation. In vitro experiments in stiffness-matched systems will enable additional insight into the role of the gastrointestinal system in the development and maintenance of HTN.
5. Conclusion
Using a custom-based indentation apparatus, we derived models and methods to determine a steady-state modulus of biological tissue. Using this Hertz-derived approximation, we found that the effective moduli of colon samples from the SHR are nearly 3 times greater in magnitude compared to a normotensive WKY controls. The phenomena that causes the stiffening in relation to high blood pressure is unknown, but this finding reinforces previously reported links between HTN and the gastrointestinal system. In HTN, it has been proposed that the focus should be shifted from lowering arterial blood pressure to altering the remodeling of vascular smooth muscle cells [45]. Our data indicate that abnormal smooth muscle cell activity may be present in other organs as well, which reinforces the need for systemic approaches to understanding HTN that go beyond the cardiovascular system. Our mechanical characterization of large intestine also provides target values for engineered systems to investigate mechanisms of HTN-linked gastrointestinal pathophysiology.
Supplementary Material
Statement of Significance.
Mechanical characterization of biological materials can provide insight into health and disease of tissue. Recent investigations into a variety of cardiovascular pathologies show coincident changes in the microbiome and pathology of the gut. In this study, we sought to quantify changes in the gut in hypertension through mechanical characterization. Our methods and simple models for characterization, adapted from Hertz indentation models, prove useful to identify a meaningful steady-state modulus metric for small and irregular tissues from laboratory animals. Our data, for the first time, establish a stiffening of the gut wall in Spontaneously Hypertensive Rats. This observation suggests significant structural and functional changes in the gut correlate with hypertension, and future experiments are warranted to explore the specific causal relationship between dysbiosis, fibrosis, and stiffening in the gut during the development and maintenance of hypertension.
Acknowledgments
The authors gratefully acknowledge support from the NIH-NHLBI’s Cardiovascular Cell Therapy Research Network Skills Development Program (5UM1HL087366), UF’s Clinical and Translational Science Institute (UL1 TR000064), and NIH R01s (HL132448, HL033610 to MKR). A.R. acknowledges fellowship support from the Institute for Cell & Tissue Science and Engineering at the University of Florida.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.actbio.2016.08.045.
References
- 1.Shimoda LA, Laurie SS. Vascular remodeling in pulmonary hypertension. J. Mol. Med. 2013;91:297–309. doi: 10.1007/s00109-013-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howitt MR, Garrett WS. A complex microworld in the gut: gut microbiota and cardiovascular disease connectivity. Nat. Med. 2012;18:1188–1189. doi: 10.1038/nm.2895. [DOI] [PubMed] [Google Scholar]
- 4.Stenmark KR, Orton EC, Reeves JT, Voelkel NF, Crouch EC, Parks WC, et al. Vascular remodeling in neonatal pulmonary hypertension. Role of the smooth muscle cell. Chest. 1988;93:127S–133S. [PubMed] [Google Scholar]
- 5.Tokita Y, Akiho H, Nakamura K, Ihara E, Yamamoto M. Contraction of gut smooth muscle cells assessed by fluorescence imaging. J. Pharmacol. Sci. 2015;127:344–351. doi: 10.1016/j.jphs.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 6.Owens GK, Schwartz SM. Alterations in vascular smooth muscle mass in the spontaneously hypertensive rat. Role of cellular hypertrophy, hyperploidy, and hyperplasia. Circ. Res. 1982;51:280–289. doi: 10.1161/01.res.51.3.280. [DOI] [PubMed] [Google Scholar]
- 7.Christensen MB, Oberg K, Wolchok JC. Tensile properties of the rectal and sigmoid colon: a comparative analysis of human and porcine tissue. Springerplus. 2015;4:142. doi: 10.1186/s40064-015-0922-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellini C, Glass P, Sitti M, Di Martino ES. Biaxial mechanical modeling of the small intestine. J. Mech. Behav. Biomed. Mater. 2011;4:1727–1740. doi: 10.1016/j.jmbbm.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 9.Stidham RW, Xu J, Johnson LA, Kim K, Moons DS, McKenna BJ, et al. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology. 2011;141:819.e1–826.e1. doi: 10.1053/j.gastro.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K, Johnson LA, Jia C, Joyce JC, Rangwalla S, Higgins PDR, et al. Noninvasive ultrasound elasticity imaging (UEI) of Crohn’s disease: animal model. Ultrasound Med. Biol. 2008;34:902–912. doi: 10.1016/j.ultrasmedbio.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson LA, Rodansky ES, Sauder KL, Horowitz JC, Mih JD, Tschumperlin DJ, et al. Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm. Bowel Dis. 2013;19:891–903. doi: 10.1097/MIB.0b013e3182813297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watters DA, Smith AN, Eastwood MA, Anderson KC, Elton RA. Mechanical properties of the rat colon: the effect of age, sex and different conditions of storage. Q.J. Exp. Physiol. 1985;70:151–162. doi: 10.1113/expphysiol.1985.sp002887. [DOI] [PubMed] [Google Scholar]
- 13.Liao D, Sevcencu C, Yoshida K, Gregersen H. Viscoelastic properties of isolated rat colon smooth muscle cells. Cell Biol. Int. 2006;30:854–858. doi: 10.1016/j.cellbi.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Levental I, Levental KR, Klein EA, Assoian R, Miller RT, Wells RG, et al. A simple indentation device for measuring micrometer-scale tissue stiffness. J. Phys.: Condens. Matter. 2010;22:194120. doi: 10.1088/0953-8984/22/19/194120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubiano A, Qi Y, Guzzo D, Rowe K, Pepine C, Simmons C. Stem cell therapy restores viscoelastic properties of myocardium in rat model of hypertension. J. Mech. Behav. Biomed. Mater. 2016;59:71–77. doi: 10.1016/j.jmbbm.2015.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egorov V, Tsyuryupa S, Kanilo S, Kogit M, Sarvazyan A. Soft tissue elastometer. Med. Eng. Phys. 2008;30:206–212. doi: 10.1016/j.medengphy.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Dommelen JAW, van der Sande TPJ, Hrapko M, Peters GWM. Mechanical properties of brain tissue by indentation: interregional variation. J. Mech. Behav. Biomed. Mater. 2010;3:158–166. doi: 10.1016/j.jmbbm.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto K, Aoki K. Development of a strain of spontaneously hypertensive rats. Jpn. Circ. J. 1963;27:282–293. doi: 10.1253/jcj.27.282. [DOI] [PubMed] [Google Scholar]
- 19.Pinto YM, Paul M, Ganten D. Lessons from rat models of hypertension. Cardiovasc. Res. 1998;39:77–88. doi: 10.1016/s0008-6363(98)00077-7. [DOI] [PubMed] [Google Scholar]
- 20.Krick BA, Vail JR, Persson BNJ, Sawyer WG. Optical in situ micro tribometer for analysis of real contact area for contact mechanics, adhesion, and sliding experiments. Tribol. Lett. 2012;45:185–194. [Google Scholar]
- 21.Vriend NM. Derivation of Hertz Contact Law and Associated Pressures. [accessed June 20, 2016];Damtp.Cam.Ac.Uk. (n.d.). < http://www.damtp.cam.ac.uk/user/nv253/teaching/Hertz.pdf>. [Google Scholar]
- 22.Bush BG, Shapiro JM, DelRio FW, Cook RF, Oyen ML. Mechanical measurements of heterogeneity and length scale effects in PEG-based hydrogels. Soft Matter. 2015;11:7191–7200. doi: 10.1039/c5sm01210d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nalam PC, Gosvami NN, Caporizzo MA, Composto RJ, Carpick RW. Nanorheology of hydrogels using direct drive force modulation atomic force microscopy. Soft Matter. 2015;11:8165–8178. doi: 10.1039/c5sm01143d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chevalier NR, Gazguez E, Bidault L, Guilbert T, Vias C, Vian E, et al. How tissue mechanical properties affect enteric neural crest cell migration. Sci. Rep. 2016;6:20927. doi: 10.1038/srep20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta S. Analytical and numerical nanoindentation studies of compliant biomaterials and soft tissues (dissertation) Berkeley: University of California; 2008. [Google Scholar]
- 26.Shull KR. Contact mechanics and the adhesion of soft solids. Mater. Sci. Eng. R. 2002;36:1–45. [Google Scholar]
- 27.Hertz HR. Miscellaneous Papers. first. London: MacMillan and Co.; 1896. [Google Scholar]
- 28.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol. Motil. 2010;8:277–297. doi: 10.1111/j.1365-2982.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 29.Chevalier NR, Gazguez E, Dufour S, Fleury V. Measuring the micromechanical properties of embryonic tissues. Methods. 2016;94:120–128. doi: 10.1016/j.ymeth.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 30.Johnson KL, Kendall K, Roberts AD. Surface energy and the contact of elastic solids. Proc. R. Soc. London A. 1971;324:301–313. [Google Scholar]
- 31.Osswald TA. Understanding Polymer Processing: Processes and Governing Equations. first. Cincinnati: Hanser Publications; 2010. [Google Scholar]
- 32.Egorov VI, Schastlivtsev IV, Prut EV, Baranov AO, Turusov RA. Mechanical properties of the human gastrointestinal tract. J. Biomech. 2002;35:1417–1425. doi: 10.1016/s0021-9290(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 33.Hoeg HD, Slatkin AB, Burdick JW, Grundfest WS. Biomechanical modeling of the small intestine as required for the design and operation of a robotic endoscope; Proceedings of IEEE Conf. on Robotics and Automation; 2000. pp. 1599–1606. [Google Scholar]
- 34.Wang CC-B, Hung CT, Mow VC. An analysis of the effects of depth-dependent aggregate modulus on articular cartilage stress-relaxation behavior in compression. J. Biomech. 2001;34:75–84. doi: 10.1016/s0021-9290(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 35.Strange D, Fletcher TL, Tonsomboon K, Brawn H, Zhao X, Oyen ML. Separating poroviscoelastic deformation mechanisms in hydrogels. Appl. Phys. Lett. 2013;102 [Google Scholar]
- 36.Cowin SC, Doty SB. Tissue Mechanics. first. New York, NY: Springer; 2007. [Google Scholar]
- 37.Pruitt LA, Chakravartula AM. Mechanics of Biomaterials: Fundamental Principles for Implant Design. first. New York, NY: Cambridge University Press; 2011. [Google Scholar]
- 38.Moore SW, Keller RE, Koehl MA. The dorsal involuting marginal zone stiffens anisotropically during its convergent extension in the gastrula of Xenopus laevis. Development. 1995;121:3131–3140. doi: 10.1242/dev.121.10.3131. [DOI] [PubMed] [Google Scholar]
- 39.Santisteban MM, Zubcevic J, Qi Y, Yang T, Shenoy V, Cole-Jeffrey CT, et al. Hypertension-linked pathophysiological alterations in the gut. Circ. Res. doi: 10.1161/CIRCRESAHA.116.309006. submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci. Transl. Med. 2011;3:112–122. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santisteban MM, Kim S, Pepine CJ, Raizada MK. Brain-gut-bone marrow axis: implications for hypertension and related therapeutics. Circ. Res. 2016;118:1327–1336. doi: 10.1161/CIRCRESAHA.116.307709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zubcevic J, Jun JY, Kim S, Perez PD, Afzal A, Shan Z, et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension. 2014;63:542–550. doi: 10.1161/HYPERTENSIONAHA.113.02722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, et al. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65:1349–1355. doi: 10.1161/HYPERTENSIONAHA.115.05344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jang SW, Ihm SH, Choo EH, Kim OR, Chang K, Park CS, et al. Imatinib mesylate attenuates myocardial remodeling through inhibition of platelet-derived growth factor and transforming growth factor activation in a rat models of hypertension. Hypertension. 2014;63:1228–1234. doi: 10.1161/HYPERTENSIONAHA.113.01866. [DOI] [PubMed] [Google Scholar]
- 45.Fok H, Cruickshank JK. Future treatment of hypertension: shifting the focus from blood pressure lowering to arterial stiffness modulation? Curr Hypertens. Rep. 2015;17:569–568. doi: 10.1007/s11906-015-0569-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.