Abstract
Flavopiridol, roscovitine, and other inhibitors of Cyclin-Dependent Kinases (CDK) inhibit the replication of a variety of viruses in vitro while proving nontoxic in human clinical trials of their effects against cancer. Consequently, these and other Pharmacological CDK inhibitors (PCIs) have been proposed as potential antivirals. Flavopiridol potently inhibits all tested CDKs and inhibits the transcription of most cellular and viral genes. In contrast, roscovitine and other purine PCIs inhibit with high potency only CDK1, CDK2, CDK5, and CDK7, and they specifically inhibit the expression of viral but not cellular genes. The levels at which purine PCIs inhibit gene expression are unknown, as are the factors which determine their specificity for expression of viral but not cellular genes. We show herein that roscovitine prevents the initiation of transcription of herpes simplex virus type 1 (HSV-1) genes but has no effect on transcription elongation. We further show that roscovitine does not inhibit the initiation or elongation of cellular transcription and that its inhibitory effects are specific for promoters in HSV-1 genomes. Therefore, we have identified a novel biological activity for PCIs, i.e., their ability to prevent the initiation of transcription. We have also identified genome location as one of the factors that determine whether the transcription of a given gene is inhibited by roscovitine. The activities of roscovitine on viral transcription resemble one of the antiherpesvirus activities of alpha interferon and could be used as a model for the development of novel antivirals. The genome-specific effects of roscovitine may also be important for its development against virus-induced cancers.
Among human-pathogenic viruses, only members of the Corona-, Pox-, and Herpesviridae encode protein kinases (recently reviewed in reference 70). However, many viral functions are regulated by cellular protein kinases, and, consequently, many inhibitors specific for cellular protein kinases inhibit viral replication. For example, Pharmacological Cyclin-dependent kinase (CDK) inhibitors (PCIs) inhibit the replication of human-pathogenic viruses such as human immunodeficiency virus type 1 (70), human cytomegalovirus (9), varicella-zoster virus (54, 82a), Epstein-Barr virus (38), and herpes simplex virus types 1 (HSV-1) (73) and 2 (HSV-2) (70). Since PCIs are proving surprisingly safe for humans in clinical trials of their effects against cancer (25, 26), they have been repeatedly proposed as potential antiviral drugs (8, 13, 17, 19, 20, 24, 25, 38, 52, 53, 55, 56, 65, 69, 73, 84).
PCIs are a heterogeneous group of compounds that have in common their ability to preferentially inhibit CDKs involved in the cell cycle (CDK1, CDK2, CDK4, CDK6, and CDK7), transcription (CDK7 and CDK9), or neuronal functions (CDK5). PCIs also have the same mechanism of action; i.e., they compete with the ATP cosubstrate. However, different PCIs inhibit different sets of CDKs (recently reviewed in references 51 and 69). Flavopiridol (Flavo), for example, inhibits CDK1, CDK2, CDK4, CDK7, and CDK9 and probably also CDK6 and CDK5 (12, 13, 43). Flavo also inhibits other protein kinases (41, 58), activates the ATPase activity of multidrug resistance protein 1 (MRP1) (31), and binds double-stranded DNA (41). Purine-type PCIs, such as roscovitine (Rosco), inhibit with high potency only CDK1, CDK2, CDK5, and CDK7 (4, 29, 50, 72, 83). Although these PCIs also inhibit DYRK1a, ERK1, and ERK2, they do so with 50% inhibitory concentrations approximately 5- to 10-fold higher than those toward their target CDKs (4, 50, 83). Purine-type PCIs do not inhibit CDK4, CDK6, CDK8, or 57 other proteins, including an HSV-1-encoded protein kinase (4, 29, 50, 72, 83). Rosco may inhibit immunoprecipitated CDK9 (84), whose purity was not assessed, but a closely related PCI, purvalanol (Purv), does not bind to CDK9 with high affinity in cell extracts (72). Therefore, some purine-type PCIs may not be capable of inhibiting CDK9 efficiently in vivo.
Because of their effects on CDKs required for cell division, several PCIs are currently under development as antiproliferative drugs (for examples, see references 25 and 36 and C. Benson, F. Raynaud, A. O'Donnell, A. Gianella-Borradori, R. Westwood, S. McClue, P. Workman, and I. Judson, Abstr. 92nd Annu. Meet. Am. Assoc. Cancer Res., abstr. 1354, 2002). Because several PCIs inhibit viral replication in vitro, they have also been proposed as potential antiviral drugs (8, 13, 17, 19, 20, 24, 25, 38, 52, 53, 55, 56, 65, 69, 73, 84). However, not all biological activities of these drugs have been characterized, and purine- and nonpurine-type PCIs may even act via different mechanisms. For example, different PCIs inhibit viral replication at different levels. Flavo inhibits retroviral transcription (13), probably as a result of its effects on CDK9 (45), whereas Rosco inhibits herpesvirus DNA replication (9, 74), probably as a result of its effect on CDK2 (37). Surprisingly, Rosco and other purine PCIs also inhibit the accumulation of transcripts driven by promoters of HSV-1 (33), Epstein-Barr virus (38), adenovirus type 5 (23), human immunodeficiency virus type 1 (84), mouse mammary tumor virus (6), and murine leukemia virus (15).
The ability of any PCI to inhibit the accumulation of viral (or cellular) transcripts was discovered during an analysis of the effects of Rosco on HSV-1 replication (73, 75). HSV-1 gene expression is a highly regulated process that is initiated when a structural protein in HSV-1 virions (VP16) forms a complex with two constitutively expressed cellular proteins (HCF and Oct-1). These complexes bind to specific sequences (TAATGARAT) in the promoters of the five HSV-1 immediate-early (IE) genes and recruit cellular RNA polymerase II (RNA Pol II) to activate IE gene transcription. Two IE proteins, ICP0 and ICP4, then activate the transcription of a second subset of viral genes, the early (E) genes. However, there are no DNA sequences unique to the promoters of all E genes, and many non-HSV-1 promoters recombined into the viral genome are regulated as HSV-1 E promoters (i.e., they require IE proteins) (78, 79). Consequently, it is currently accepted that ICP0 and ICP4 activate transcription through indirect mechanisms that operate independently of promoter-specific sequences or transcription factors, which are the subject of intensive research (1, 7, 18, 19, 21, 22, 34, 54, 60, 80). ICP4 also inhibits the expression of IE genes, by binding with high affinity to sequences near their transcription start sites (35). The promoters of a third subset of HSV-1 genes, the so-called late (L) genes, are activated only after viral DNA replication, which itself requires E proteins. HSV-1 L genes encode the structural proteins of HSV-1 virions (such as VP16) and proteins required for viral encapsidation, envelopment, and egress.
The effects of PCIs on the accumulation of viral transcripts may be mediated by inhibition of any of the CDKs that regulate transcription. Transcription is activated by the binding of promoter-specific transcription factors to specific sequences in viral promoters. The promoter-specific factors recruit the general transcription factors and RNA Pol II to form preinitiation complexes. CDK8 inhibits transcription by phosphorylating promoter-bound transcription factors (30). CDK8 may also inhibit the recruitment of RNA Pol II by phosphorylating its carboxy-terminal domain (CTD) before it is recruited to promoters (82). Transcription begins when the RNA Pol II complexes are “released” from the preinitiation complexes, a function that is facilitated by CDK7 phosphorylation of the CTD (16, 76, 77). For RNA Pol II to transcribe sequences with complex structures, however, its processivity must be enhanced by further phosphorylation of the CTD by CDK9 (48, 49, 63). CDK1 and CDK2 are also capable of phosphorylating the CTD and may participate in transcription regulation (27, 42, 68, 85). The effects of PCIs on viral gene expression could also be mediated by transcript destabilization. Alternatively, PCIs may also inhibit the CDK-dependent chromatin remodeling that is often necessary to allow access of promoter-specific and general transcription factors to specific promoters (6, 86). No PCI is known to affect transcript stability, whereas Flavo inhibits early transcription elongation (13, 14, 39) and Rosco inhibits chromatin remodeling (6).
The mechanisms whereby purine-type PCIs inhibit the accumulation of viral transcripts have just started to be elucidated. Purine-type PCIs inhibit the expression of Pbx1 and Prep1, two transcription factors that are required for activation of the murine leukemia virus promoter (15). Rosco and another purine-type PCI, CVT-313, inhibit the remodeling of chromatin that is required for binding of the glucocorticoid receptor to its cognate sequences in the mouse mammary tumor virus promoter (6). Rosco also inhibits chromatin remodeling on the adenovirus type 12 E2 promoter by the viral transcription factor E1A12S (23). Rosco inhibits posttranslational modifications of two HSV-1 IE transcriptional regulators, ICP0 and ICP4 (1, 18) and the transcription-regulatory activity of ICP0 (18, 19). However, it also inhibits the accumulation of IE, E, and L HSV-1 transcripts in less than 2 h and even in the presence of cycloheximide (CHX) (73-75). Therefore, inhibition of the accumulation of HSV-1 transcripts by Rosco cannot be completely accounted for by inhibition of the expression of transcription factors, regulation of IE proteins, or, even less so, the action of promoter-specific transcription factors (except for inhibitors of general transcription, PCIs are the only drugs known to inhibit IE, E, and L transcription).
The mechanisms whereby Rosco inhibits viral gene expression remain only partially characterized. Considering that Rosco has been repeatedly proposed as a potential antiviral agent (8, 13, 17, 19, 24, 25, 38, 52, 55, 56, 65, 69, 73, 84) and is already in human clinical trials to assess its activity against proliferative diseases (10, 25, 40; Benson et al., Abstr. 92nd Annu. Meet. Am. Assoc. Cancer Res., 2002), the biological activities of Rosco must be fully characterized. Importantly, it is still unknown whether Rosco prevents or inhibits preinitiation, initiation, or elongation of HSV-1 transcription. It is also unknown whether these potential effects of Rosco on viral transcription are mediated by specific promoter sequences or transcription factors. Herein, we report the characterization of the mechanisms whereby Rosco inhibits the accumulation of HSV-1 transcripts. Rosco prevented the initiation of transcription of IE, E, and L HSV-1 genes but had no effect on ongoing viral transcription or on initiation or elongation of cellular transcription. Surprisingly, Rosco was specific for promoters in the HSV-1 genome, as opposed to specific for certain HSV-1 promoters. Moreover, its effects were independent of promoter-specific regulatory factors. We propose that kinases inhibited by Rosco are required to permit access of transcription proteins to the HSV-1 genome. Experiments to test this model are currently in progress.
MATERIALS AND METHODS
Cells and viruses.
Vero cells were maintained in Dulbecco's modified minium Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 50 mU of penicillin per ml, and 50 ng of streptomycin per ml. The low-passage (p10) HSV-1 strain KOS was used throughout this study. Viral stocks were propagated and titrated on monolayers of Vero cells.
HSV-1 infection.
Vero cells were infected with a multiplicity of infection (MOI) of 5 to 20 PFU of virus per cell (as described for each experimental procedure) in serum-free medium. After infection for 1 h at 37°C, viral inocula were removed and the cells were washed twice with cold phosphate-buffered saline (PBS; 1 mM KH2PO4, 154 mM NaCl, 3 mM Na2HPO4 [pH 7.4]) followed by addition of DMEM-5% FBS and different concentrations of Rosco, Purv, Flavo, CHX, actinomycin D (ActD), or α-amanitin. Infected cells were harvested at the times indicated in the description of each experiment.
To evaluate viral replication, harvested cells were freeze-thawed three times and then sonicated for three cycles of 30 s each, with intervals of 15 s. Subsequently, cell debris was centrifuged at 1,800 × g for 10 min. The supernatants containing the infectious virus were subjected to titer determination by standard plaque assay. When required, monolayers of cells were washed with PBS three times and then fixed with 1 ml of 10% formalin in PBS for 10 min. Subsequently, the cells were washed with PBS twice, fresh PBS was added, and the plates were stored at 4°C.
UV inactivation of HSV-1.
HSV-1 suspensions in PBS were subjected to UV at 3,000 mJ (UV Stratalinker 2400; Stratagene) for different lengths of time (30 s to 5 min). The original and UV-inactivated virus suspensions were titrated by standard plaque assay. Viral stocks inactivated by 4 orders of magnitude were used in all experiments.
Phages, cellular and viral DNA, and plasmids.
Recombinant phages harboring the 5′ end of selected HSV-1 genes were a generous gift from C. Spencer (Cross Cancer Institute, Alberta Cancer Board, Edmonton, Alberta, Canada). These phages contain DNA sense or antisense to HSV-1 transcripts ICP4 (IE), ICP27 (IE), ICP8 (E), UL36 (E), gC (L), and VP16 (L). Single-stranded phage DNA was isolated following standard polyethylene glycol precipitation methods. Isolated phage DNA was transferred to positively charged membranes (Gene Screen Plus; NEN Life Science, Boston, Mass.) by vacuum slot blotting.
HSV-1 and Vero cell DNA was isolated from purified HSV-1 virions or uninfected Vero cells, respectively, by proteinase K digestion, phenol-chloroform extraction, and ethanol precipitation. Viral and cellular DNAs were digested separately with HindIII. Fragments were separated by agarose gel electrophoresis and Southern blotted onto positively charged nylon membranes. Plasmid vector pA1-pICP0-LacZ (11,051 bp) containing the HSV-1 IE promoter ICP0 driving the expression of LacZ was a generous gift of A. Epstein (Université Claude Bernard Lyon 1, Lyon, France). Using this plasmid as the starting vector, an expression vector with the ICP0 promoter driving the expression of red fluorescent protein (RFP) was constructed. The RFP gene was derived from the pDsRed1-N1 vector (Clontech, Palo Alto, Calif.), and the ICP0 promoter was derived from pA1-pICP0-LacZ. The 720-bp SalI-Not1 RFP fragment was excised from the pDsRed1-N1 vector and subcloned following removal of the 3,489-bp SalI-NotI fragment containing the lacZ cassette. The resulting 8,282-bp construct was named pICP0-RFP5 and was used for transient transfection of Vero cells and for the creation of stably transfected cell lines.
Two probes were used for hybridization, RFP and ICP0. The RFP probe was prepared by digesting the pDsRed1-N1 plasmid with SalI and NotI and purifying the 720-bp fragment. The ICP0 probe was prepared by digesting the pRP0 plasmid (32) with XhoI and HindIII enzymes and purifying the 888-bp fragment. The probes were random primed as specified by the manufacturer (Amersham Biosciences, Piscataway, N.J.).
Transfection and selection.
Vero cells were transfected using Lipofectamine (Gibco BRL, Rockville, Md.) as specified by the manufacturer. Briefly, Vero cells were seeded at 60 to 70% confluence in six-well plates. The following day, the cells were transfected with 4 μg of pICP0-RFP5 and 0.6 μg of pcDNA. The latter plasmid contains the gene for G418 resistance. The cells were incubated with DNA-Lipofectamine at 37°C for 4 h, and then 1 volume of DMEM supplemented with 10% FBS was added. After 24 h, the medium was replaced with fresh medium supplemented with 5% FBS. For transient-transfection experiments, transfected cells were then trypsinized and seeded in replicate wells. For the construction of the stably transfected Vero cell lines, confluent cells were passaged 1:2 in medium supplemented with 800 μg of G418 per ml. After 10 to 15 days, when only the G418-resistant transfected cells had survived, the cells were expanded and the concentration of G418 was lowered to 400 μg/ml for maintenance of transfected cells. Transfected cells were cloned by limiting dilution in 96-well plates. Individual clones were screened for expression of RFP.
Drugs.
CHX stock (5 mg/ml) was prepared in serum-free DMEM. The stock was diluted to 50 μg/ml in DMEM-5% FBS and added to cells 1 h prior to infection. ActD stock (1 mg/ml) was prepared in ethanol and used at 10 μg/ml. Rosco was prepared in dimethyl sulfoxide as a 100 mM stock and was used in the concentration range of 25 to 100 μM, as indicated. Purv and Flavo were prepared in dimethyl sulfoxide as 10 mM stocks and used in the concentration ranges of 5 to 30 μM (Purv) or 31.25 to 500 nM (Flavo), as indicated. α-Amanitin was prepared as a 1-mg/ml stock in distilled water and used at 50 μg/ml in DMEM-5% FBS or 2 μg/ml in run-on transcription buffer.
Run-on analyses.
For each treatment, two 100-mm-diameter dishes containing approximately 6 × 106 Vero cells/dish were mock infected or infected with HSV-1 strain KOS at MOI of 20 PFU/cell. Following a 1-h adsorption at 37°C, inocula were removed and the infected monolayers were washed twice with PBS at 4°C. The cells were then treated for 5 h with DMEM-5% FBS supplemented with 100 μM Rosco or unsupplemented, starting at 1 h postinfection (p.i.), and harvested at 6 h p.i. Run-on assays were performed as described by Spencer, Rice, and coworkers (64, 81), with several modifications. The cells were rinsed with PBS at 4°C, trypsinized, and resuspended in 20 ml of DMEM-5% FBS. They were then pelleted by centrifugation, resuspended in hypotonic RSB buffer (10 mM Tris [pH 7.5], 10 mM NaCl, 5 mM MgCl2), and lysed with 0.5% (vol/vol) Nonidet P-40. Nuclei were isolated by differential centrifugation, resuspended in 150 μl of nuclear freezing buffer (50 mM Tris [pH 8.0], 5 mM MgCl2, 40% glycerol, 0.5 mM dithiothreitol [DTT]), and immediately snap-frozen and stored in liquid nitrogen. Afterward, 150 μl of thawed nuclear suspension was mixed with 150 μl of transcription run-on buffer (20 mM Tris [pH 8], 3 mM DTT, 20 mM MgCl2) plus 0.5mM each ATP, CTP, and UTP and 10 μCi of [α-32P]GTP. The final buffer concentrations were 30 mM Tris (pH 8.0), 1 mM DTT, 7.5 mM MgCl2, 20% glycerol, and 140 mM KCl. Transcription reactions proceeded at 30°C for 30 min and were then stopped by incubation with 50 μg (434 Worthington units) of DNase I (Invitrogen, Carlsbad, Calif.) for 15 min at 30°C. Proteins were degraded in SET buffer (10% sodium dodecyl sulfate [SDS], 100 mM Tris [pH 7.5], 50 mM EDTA) plus 100 μg of proteinase K at 37°C for 1 h. Total nuclear RNA was isolated by acid phenol-chloroform extraction and isopropanol precipitation. RNA was resuspended in 200 μl of TE buffer (10 mM Tris [pH 7.5], 1 mM EDTA) and denatured at 100°C for 5 min. Membranes containing phage or genomic DNA were prehybridized with 10 ml of rapid hybrid buffer (Amersham Biosciences, Piscataway, N.J.) at 60 or 37°C (for analyses of HSV-1 or cellular transcription, respectively). Denatured RNA was added to 5 ml of rapid hybrid buffer at the proper temperature and hybridized to membranes containing viral or cellular DNA at 60 or 37°C for 48 or 72 h (for analyses of HSV-1 or cellular transcription, respectively). The membranes were washed twice for 20 min in 300 mM NaCl-30 mM sodium citrate (2× SSC) supplemented with 0.1% SDS at room temperature. For analyses of HSV-1 transcription, the membranes were further washed for 15 min in 75 mM NaCl-7.5 mM sodium citrate (0.5× SSC) supplemented with 0.5% SDS at 50°C. They were then exposed to Kodak PhosphorImager screens. The membranes were imaged, and the signal hybridized to HSV-1 or Vero genomic fragments was quantitated using an FX molecular imager (Bio-Rad, Hercules, Calif.). Counts hybridized to the genomic fragments under each treatment were normalized to counts hybridized to the respective genomic fragments in the absence of any drug (control) and are expressed as a percentage of the control value. To evaluate whether transcription was performed by RNA Pol II, nucleic acids were precipitated after the proteinase K digestion in 10% ice-cold trichloroacetic acid (TCA). The precipitates were washed extensively in ice-cold 10% TCA, and the nucleic acid pellets were finally resuspended in 0.5% SDS. Radioactivity incorported into TCA-precipitable material was expressed as a percentage of the radioactivity used as the substrate in the run-on reactions (as [α-32P]GTP). Specific incorporation was then calculated by subtracting the percent radioactivity incorporated into TCA-precipitable material in the presence of 10 μg of ActD per ml (background) and is expressed as a percentage of the control value (no drug present in the run-on transcription reactions).
Northern blot analyses.
Monolayers containing 2 × 106 Vero cells in 100-mm-diameter tissue culture dishes were pretreated with CHX for 1 h and then infected with HSV-1 KOS at a MOI of 5 PFU/cell. The cells were washed with PBS containing CHX. Then DMEM-5% FBS containing CHX and different concentrations of Rosco, Purv, or Flavo was added. Total cellular RNA was extracted from mock- and HSV-1-infected cells at 3, 6, and 9 h p.i. using the guanidinium isothiocyanate method, and RNA concentrations were estimated spectrophotometrically at 260 nm. RNA samples were stored in isopropanol at −70°C. The RNA samples were centrifuged, resuspended, size fractionated on agarose-formaldehyde denaturing gels (30 μg of RNA/sample), and transferred to GeneScreen Plus nylon membranes for Northern blotting. Hybridizations were performed in rapid hybrid buffer for 4 h using RFP or ICP0 probes. Hybridization was performed at 65°C for RFP and at 80°C for ICP0. Hybridized membranes were washed twice for 15 min in 2× SSC-0.1% SDS at room temperature and once for 10 min in 0.5× SSC-0.5% SDS at 65°C. Hybridized and washed membranes were exposed to Kodak PhosphorImager screens and imaged using the FX molecular imager. The membranes were then stripped by treatment with 50% formamide-0.1% SDS-0.1× SSC preheated to 75°C, washed in the same buffer for 2 h at 68°C, and then washed with 0.5× SSC-0.5% SDS preheated to 65°C for 10 min. Stripped membranes were exposed and then used for rehybridization with a different probe.
RESULTS
Rosco prevents initiation of HSV-1 transcription but does not inhibit ongoing transcription.
Flavo inhibits ongoing viral and cellular transcription. Rosco inhibits the accumulation of five HSV-1 transcripts and consequently has been hypothesized to inhibit HSV-1 transcription as well (73-75). However, the effects of Rosco on viral transcription have not been evaluated directly. We tested the potential effects of Rosco on HSV-1 transcription by using run-on transcription assays. Vero cells were infected with 20 PFU of HSV-1 strain KOS per cell and treated with 100 μM Rosco at 1 h after infection or left untreated. The cells were harvested 5 h later, and their nuclei were isolated. Run-on transcription assays were performed as described by Spencer, Rice, and coworkers (64, 81) with several modifications. We tested the effects of Rosco on promoter-specific (i.e., sense) and non-promoter-specific (i.e., antisense) HSV-1 transcription by probing the run-on RNAs with single-stranded sense or antisense DNA to selected viral genes. The phages containing the probes were a generous gift of C. Spencer.
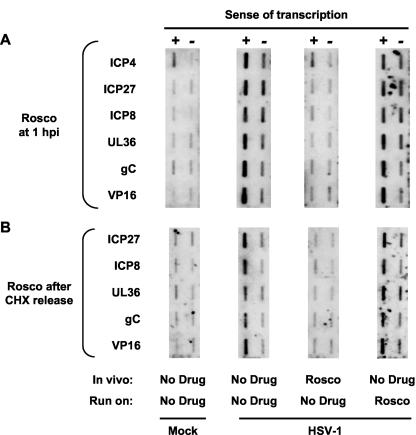
Run-on transcription assays performed with nuclei of mock-infected cells (negative controls) resulted in only low background levels of hybridization to viral genes, as expected. Run-on transcription assays performed with nuclei of HSV-1-infected cells (positive controls) resulted in abundant viral transcription, also as expected (Fig. 1A). Transcription occurred from both sense (promoter-specific) and antisense (non-promoter-specific) strands, which is characteristic of HSV-1 transcription (64, 81). Run-on assays performed with nuclei of cells infected in the presence of Rosco resulted in inhibition of transcription of all viral genes analyzed (Fig. 1A), even though no Rosco was present during the transcription reactions. Under these conditions, Rosco prevented transcription from both sense and antisense DNA strands. In contrast, run-on transcription assays performed in the presence of Rosco, but using nuclei of cells infected in the absence of the drug, resulted in no inhibition of either sense or antisense transcription (Fig. 1A). The concentration of Rosco used in these experiments inhibits CDK2 (and probably also CDK1, CDK5, and CDK7) in vitro in the presence of 500 μM ATP (72).
FIG. 1.
Rosco prevents the initiation of HSV-1 transcription but does not inhibit ongoing transcription. (A) Four membranes slot blotted with single-stranded DNA of the same sense as, or complementary to, six HSV-1 genes and probed with RNA isolated from run-on transcription reactions. Cells were mock infected (Mock), or infected with HSV-1 (HSV-1) in the presence of (In vivo) vehicle (No Drug) or Rosco (Rosco). Nuclei were isolated at 6 h p.i., and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug) or 100 μM Rosco (Rosco). RNA was purified and probed with membranes containing single-stranded DNA complementary to (+) or of the same sense as (−) two IE (ICP4 and ICP27), two E (ICP8 and UL36), and two L (gC and VP16) HSV-1 genes. The higher background in the ICP4 sense probe was consistently observed and is most probably due to cross-hybridization with cellular RNAs. (B) Four membranes slot blotted with single-stranded DNA of the same sense as, or complementary to, five HSV-1 genes and probed with RNA isolated from run-on transcription reactions. Cells were mock infected (Mock) or infected with HSV-1 (HSV-1) in the presence of CHX. At 6 h later, CHX-containing medium was removed and replaced with medium containing (In vivo) vehicle (No Drug), or Rosco (Rosco). Nuclei were isolated at 3 h after the change of medium, and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug) or 100 μM Rosco (Rosco). RNA was purified and probed with membranes containing single-stranded DNA complementary to (+) or of the same sense as (−) one IE (ICP27), two E (ICP8 and UL36), and two L (gC and VP16) HSV-1 genes. Transcription of ICP4 could not be evaluated in these experiments because the cross-hybridizing cellular RNAs are too strongly induced by CHX.
Transcription of HSV-1 E and L genes depends on previous expression of IE proteins, which is inhibited by Rosco (Fig. 1A) (75). Therefore, the experiments whose results are presented in Fig. 1A tested the direct effects of Rosco on transcription of IE genes but not on transcription of E or L genes. Moreover, IE proteins were present during transcription only when Rosco was added to the transcription reaction mixtures but not when Rosco was added to the infected cells (because Rosco prevents transcription of IE genes [Fig. 1A] [73]). To test the effects of Rosco on HSV-1 transcription in the presence of IE proteins, we used a CHX release experimental design. Vero cells were infected with HSV-1 in the presence of CHX for 5 h. At this time, CHX was removed and control or Rosco-containing medium was added. One of us (L.M.S.) has shown previously that high levels of all HSV-1 IE proteins are synthesized after removal of CHX following this procedure, from the IE transcripts overaccumulated during the 5 h in the presence of CHX (75).
Rosco prevented the initiation of transcription of IE, E, and L genes after a CHX release (Fig. 1B), which is consistent with its inhibitory effects on the accumulation of IE, E, and L transcripts in the presence of IE proteins (75). Rosco prevented the initiation of both sense and antisense transcription in the presence of IE proteins, as it did in their absence. Moreover, Rosco had no effect on ongoing transcription (Fig. 1B), as it had no effect on ongoing transcription in the absence of IE proteins (Fig. 1A). These results further revealed that Rosco inhibits the transactivation of HSV-1 promoters regardless of whether the promoters are regulated by cellular proteins and HSV-1 structural proteins such as VP16 (which enter the cell with the infecting virions) acting alone (Fig. 1A) or in combination with HSV-1 IE proteins (Fig. 1B). Therefore, the functions targeted by Rosco participate in activation of IE gene transcription by cellular proteins and HSV-1 structural proteins, as well as in regulation of IE gene transcription by HSV-1 IE proteins.
The effects of Rosco are specific for viral genes.
We concluded from the experiments in Fig. 1 that Rosco prevents the initiation of HSV-1 transcription but does not inhibit ongoing transcription. These effects could be specific for HSV-1 transcription or general to HSV-1 and cellular transcription. Therefore, we compared the effects of Rosco on viral and cellular transcription. HSV- or mock-infected cells were treated with Rosco or control medium for 5 h, their nuclei were isolated, and run-on transcriptions were performed. The RNAs purified from the transcription reaction mixtures were hybridized to membranes containing the entire genome of HSV-1 or Vero cells, and the hybridized signal was quantitated to assess the effects of Rosco on global HSV-1 or cellular transcription, respectively. Mock-infected cells were selected to analyze the effects of Rosco on cellular transcription because several HSV-1 proteins inhibit cellular transcription. Therefore, the inhibitory effects of Rosco on HSV-1 transcription in infected cells would complicate the analyses of its potential effects on cellular transcription.
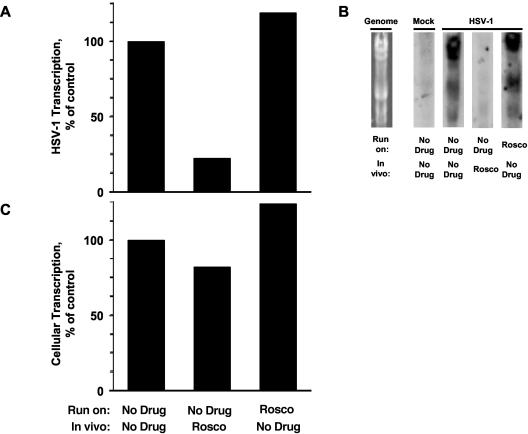
When cells were infected in the presence of Rosco, global HSV-1 transcription was inhibited, even though no drug was present in the transcription reaction mixtures (Fig. 2A and B). However, when mock infected cells were treated with Rosco, no major effects on global cellular transcription were detected (Fig. 2C). Furthermore, Rosco added to the run-on transcription reaction mixture had no effect on global HSV-1 or cellular transcription (Fig. 2), indicating again that Rosco does not inhibit ongoing transcription. These experiments further suggested that Rosco prevents the initiation of transcription of all HSV-1 genes, because we could not detect significant levels of transcription of any HSV-1 genome fragment in nuclei of cells infected in the presence of Rosco (Fig. 2B).
FIG. 2.
Rosco prevents the initiation of HSV-1 transcription but does not inhibit cellular transcription. (A) Bar graph showing the percentage of HSV-1 run-on transcription in the presence or absence of Rosco. Cells were infected with HSV-1 in the presence of (In vivo) vehicle (No Drug) or 100 μM Rosco (Rosco). Nuclei were isolated at 6 h p.i., and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug) or 100 μM Rosco (Rosco). RNA was purified and probed with membranes containing the entire HSV-1 genome as HindIII fragments. Radioactivity hybridized to the HSV-1 genome fragments was quantitated using a Bio-Rad molecular imager and the FX software package and is expressed as the percentage of transcription in the absence of Rosco in vivo or in vitro (first bar). (B) Four membranes Southern blotted with HindIII HSV-1 DNA fragments and probed with RNA isolated from run-on transcription reactions. A picture of a gel stained with ethidium bromide is presented on the left to show the positions of the HSV-1 genome fragments (Genome). Cells were mock infected (Mock) or infected with HSV-1 (HSV-1) in the presence of (In vivo) vehicle (No Drug) or Rosco (Rosco). Nuclei were isolated at 6 h p.i., and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug) or 100 μM Rosco (Rosco). RNA was purified and probed with membranes containing the entire HSV-1 genome as HindIII fragments. (C) Bar graph showing the percentage of global cellular run-on transcription in the presence or absence of Rosco. Cells were mock infected in the presence of (In vivo) vehicle (No Drug) or 100 μM Rosco (Rosco). Nuclei were isolated 6 h later, and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug) or 100 μM Rosco (Rosco). RNA was purified and probed with membranes containing the entire Vero cell genome as HindIII fragments. Radioactivity hybridized to the Vero cell genomic fragments was quantitated using a Bio-Rad molecular imager and FX software package and is expressed as percentage of transcription in the absence of Rosco in vivo or in vitro (first bar).
Although the reaction conditions used in our experiments favor transcription by RNA Pol II over transcription by RNA Pol I or III, either of these last two polymerases could have synthesized the cellular transcripts observed in the experiments the results of which are presented in Fig. 2C (only RNA Pol II synthesizes HSV-1 transcripts). Therefore, we tested next whether cellular transcription in the presence of Rosco was indeed performed by RNA Pol II. We purified nuclei from mock-infected cells treated with 100 μM Rosco or left untreated. Then we performed run-on reactions in the presence of no drug, α-amanitin, or Rosco and evaluated the global cellular transcription. Run-on transcription in nuclei of cells treated with Rosco in vivo or left untreated was equally inhibited (by 60 and 58% relative to control, respectively) by α-amanitin (Fig. 3). In contrast, Rosco added to the run-on transcription reaction mixtures failed to significantly inhibit transcription in nuclei of even cells treated with Rosco in vivo (Fig. 3). The highest level of inhibition by Rosco added in vitro (to nuclei of cells treated with Rosco in vivo) was that observed in the experiment presented in Fig. 3 (25% inhibition). Since α-amanitin inhibited cellular transcription by approximately 60% and most cellular transcription is performed by RNA Pol I or Pol III, these experiments also prove that the run-on conditions used in our assays strongly favor transcription by RNA Pol II over Pol I or Pol III.
FIG. 3.
Transcription of cellular genes in the presence of Rosco is performed by RNA Pol II. (A) Bar graph showing the percentage of global cellular run-on transcription in the presence or absence of Rosco. Cells were mock infected in the presence of (In vivo) vehicle (No Drug). Nuclei were isolated 6 h later, and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug), α-amanitin (αAma), or 100 μM Rosco (Rosco). RNA was then precipitated with TCA, and the percentage of radioactivity incorporated into RNA was calculated. Background incorporation was subtracted, and transcription is presented as the percentage of radioactivity incorporated into RNA in run-on reactions performed in the absence of any drug. (B) Bar graph showing the percentage of global cellular run-on transcription in the presence or absence of Rosco. Cells were mock infected in the presence of (In vivo) 100 μM Rosco (Rosco). Nuclei were isolated 6 h later, and run-on transcription reactions were performed in the presence of (Run on) vehicle (No Drug), α-amanitin (αAma), or 100 μM Rosco (Rosco). RNA was then precipitated with TCA, and the percentage of radioactivity incorporated into RNA was calculated. Background incorporation was subtracted, and transcription is presented as the percentage of radioactivity incorporated into RNA in run-on reactions performed in the absence of any drug. The actual levels of transcription for all run-on conditions (no drug, α-Ama, Rosco) were approximately 30% higher in nuclei of cells treated with Rosco in vivo than in nuclei of control cells, consistent with the data shown in Fig. 2C.
Inhibition of transcription by Rosco is specific for the HSV-1 genome.
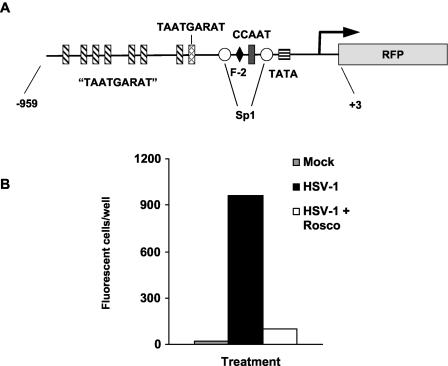
We concluded from the experiments presented in the previous sections that Rosco prevents the initiation of transcription of HSV-1 genes but not of cellular genes. Prevention of initiation of transcription of IE genes was especially surprising because Rosco does not inhibit the formation of the Oct-HCF-VP16 transactivating complex or the affinity of this complex for its cognate TAATGARAT sequences (33). Beyond specific promoters, however, the HSV-1 genome itself plays a major role in transcription regulation. For example, a variety of non-HSV-1 promoters recombined into the HSV-1 genome are regulated as HSV-1 E promoters (61, 78, 79). Also, transcription driven by the HSV-1 ICP0 promoter is inhibited by alpha interferon (α-IFN) when the promoter is in the HSV-1 genome but not when it is recombined in the cellular genome (57). We therefore tested whether inhibition of HSV-1 transcription by Rosco depends on the genome in which a promoter is located (i.e., viral or cellular). To this end, we constructed a recombinant plasmid in which the promoter of ICP0, including all known regulatory elements (Fig. 4A), drives the expression of a reporter RFP. In transient transfections, Rosco inhibited the transactivation of this ICP0 promoter by UV-inactivated HSV-1 (Fig. 4B), as expected since Rosco had been shown previously to inhibit the transactivation of another ICP0 reporter construct under similar circumstances (33).
FIG. 4.
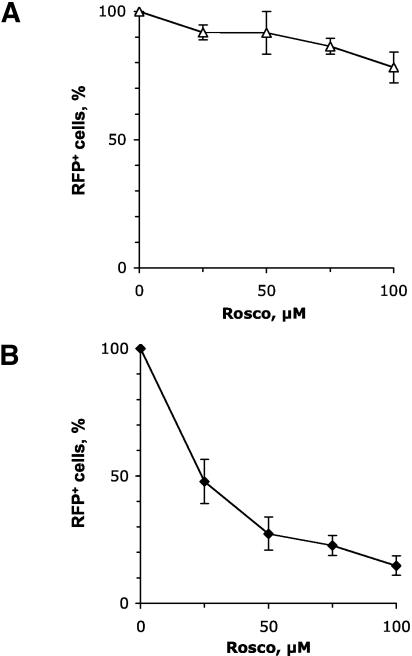
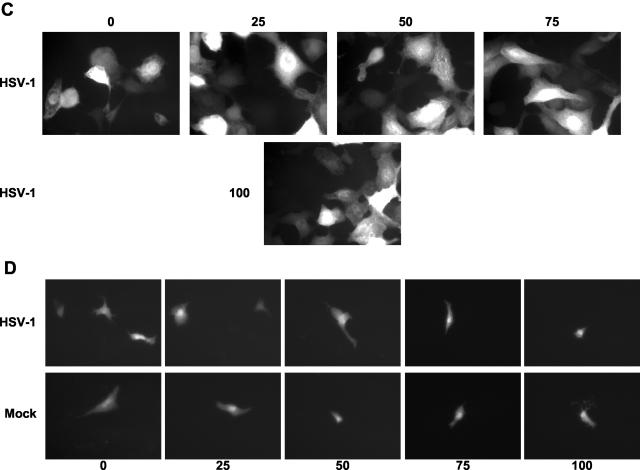
Rosco inhibits the expression of ICP0-driven RFP from a transiently transfected plasmid. (A) Diagram of the ICP0-RFP construct that was transiently or stably transfected into Vero cells. The major regulatory elements in the promoter are indicated. “TAATGARAT,” TAATGARAT-like sequences, in which one or two nucleotides deviate from the consensus TAATGARAT motif. (B) Vero cells were transiently transfected with pICP0-RFP5. Transfected cells were seeded in three individual wells and mock infected or infected with the equivalent of 2.5 PFU of UV-inactivated HSV-1 KOS in the presence of 0, 25, 50, 75, or 100 μM Rosco. At 24 h after infection, the cells were fixed and the number of cells expressing RFP was counted by UV microscopy.
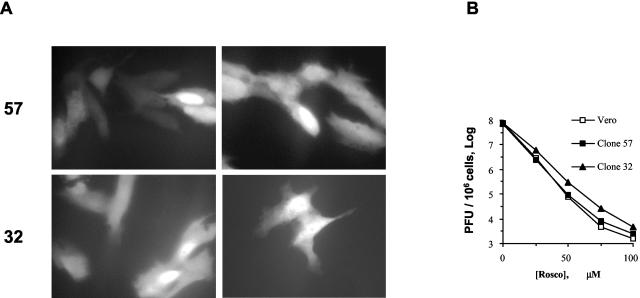
The ICP0 reporter construct was transfected into Vero cells, and stably transfected cells were selected and cloned. The stably transfected ICP0 promoter did not direct the expression of RFP in noninfected cells, but it was activated by HSV-1 in 13 of 110 clones analyzed (data not shown). Two of these clones were selected for further analyses. The ICP0 reporter construct was integrated into the cellular genome of the two clones as multiple copies per cell and was activated by virion proteins (Fig. 5A). Because the inhibitory concentrations of Rosco depend on the cell line, we also analyzed the potency of Rosco toward HSV-1 replication in the two clones and parental Vero cells. Both the clones and the parental Vero cells were more sensitive to Rosco than were other Vero cells used in previous studies. Rosco at 75 μM inhibited HSV-1 replication by 4 orders of magnitude in the two clones and the parental cells (Fig. 5B), in comparison to 100 μM Rosco, which was required in other Vero cells (73). Vero cells clone 57 and 32 displayed similar phenotypes throughout the experiments; results from clone 57 are presented herein.
FIG. 5.
Characterization of Vero clones 57 and 32 (stably transfected with pICP0-RFP5). (A) Clone 57 or 32 cells were infected with the equivalent of 0.5 PFU of UV-inactivated HSV-1 KOS per cell. At 24 h later, the cells were evaluated by optical and fluorescence microscopy. No plaques were visible, but a large fraction of Vero clone 57 or 32 cells expressed RFP. Representative UV micrographs are presented. Original magnification, 400 A. (B) Dose-response analysis of the sensitivity of HSV-1 replication to Rosco in parental Vero cells and clones 57 and 32. Cells were infected with 2.5 PFU of HSV-1 KOS per cell and treated with 0, 25, 50, 75, or 100 μM Rosco. At 24 h later, the cells were harvested and virus was isolated and subjected to titer determination by standard plaque assays. PFU per million cells is plotted against drug concentration.
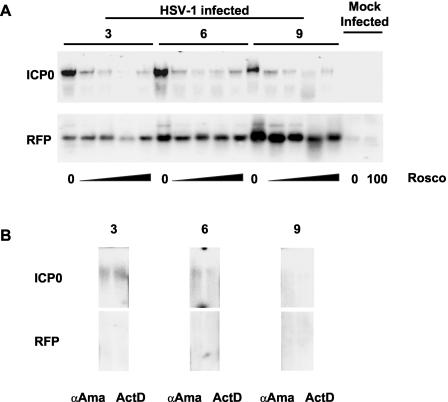
We next evaluated the effects of Rosco on the activation of ICP0 promoters recombined in the cellular genome. Clone 57 cells were infected with 10 PFU of HSV-1 per cell in the presence of CHX and 0, 25, 50, 75, or 100 μM Rosco. In the presence of CHX, the ICP0 promoter is activated only by cellular proteins and structural HSV-1 proteins such as VP16 (which enter the cell with the infecting virions). We included 100 μM Rosco in these experiments to compare these results with those presented in Fig. 1 to 3 and with previously published results (73-75). Infected cells were harvested at 3, 6 and 9 h p.i., and RNA was analyzed by Northern blotting.
Consistent with published results (73-75), all concentrations of Rosco efficiently inhibited transcription regulated by the ICP0 promoters in the viral genome (Fig. 6A). Surprisingly, however, Rosco did not show such efficient inhibition of transcription regulated by the ICP0 promoters recombined in the cellular genome (Fig. 6A). This differential sensitivity to Rosco suggested that RNA Pol I or Pol III, rather than RNA Pol II (which transcribes all HSV-1 genes), could have transcribed RFP from the cellular genome. However, α-amanitin inhibited the transcription of ICP0 and RFP as efficiently as ActD did (Fig. 6B). Therefore, both ICP0 and RFP were transcribed by RNA Pol II. From the experiments described in this section, we conclude that Rosco did not prevent the initiation of RNA Pol II transcription activated by ICP0 promoters recombined in the cellular genome.
FIG. 6.
Rosco does not inhibit RFP expression driven by ICP0 promoters recombined in the cellular genome. (A) Northern blot analyses of expression of ICP0 (ICP0 [top panels]) and RFP (RFP [bottom panels]) in Vero clone 57 cells infected with HSV-1 in the presence of CHX. Cells were infected with 5 PFU/cell (HSV-1 infected) and treated with CHX and 0, 25, 50, 75, or 100 μM Rosco. Mock-infected cells (Mock Infected), treated with CHX and 0 or 100 μM Rosco, were included as negative controls. Cells were harvested at 3, 6, and 9 h p.i. (lanes 3, 6, and 9), and RNA was extracted, resolved by gel electrophoresis, and blotted to nylon membranes. The membranes were then hybridized with RFP probe, stripped, and rehybridized with ICP0 probe. The decrease in signal in both RFP and ICP0 in the line for 75 μM Rosco at 3 h p.i. is an artifact due to experimental error and is not reproducible. (B) Northern blot analyses of expression of ICP0 (ICP0 [top panels]) and RFP (RFP [bottom panels]) in Vero clone 57 cells infected with HSV-1 in the presence of CHX. Cells were infected with 5 PFU/cell and treated with CHX and 50 μg of α-amanitin (αAma) per ml or 10 μg of actinomycin D (ActD) per ml. The cells were harvested at 3, 6, and 9 h p.i. (lanes 3,6, and 9), and RNA was extracted, resolved by gel electrophoresis, and blotted to nylon membranes. The membranes were then hybridized with RFP probe, stripped, and rehybridized with ICP0 probe.
Less specific PCIs inhibit transcription directed by the ICP0 promoter in either HSV-1 or cellular genomes.
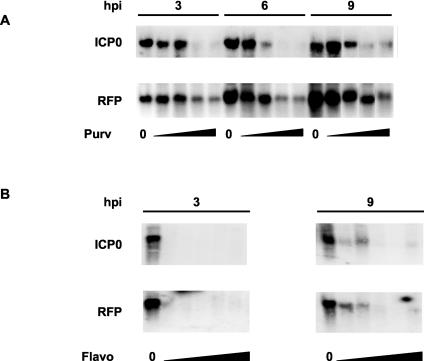
The specificity for HSV-1 genomes could be common to all PCIs or could be unique to Rosco, the PCI most specific for CDK1, CDK2, CDK5, and CDK7 (4, 29, 50, 72, 83). Therefore, we compared the effects of Rosco with those of Purv (a purine PCI that is less specific for CDK1, CDK2, CDK5, and CDK7 than is Rosco), and Flavo (a flavonoid PCI that preferentially inhibits CDK9 but further inhibits all other tested CDKs with similar potencies and many other protein kinases with variable potencies).
Cells were infected in the presence of CHX and 0, 5, 10, 20, or 30 μM Purv or 0, 31.25, 62.5, 125, 250, or 500 nM Flavo. Purv at 30 μM inhibits HSV-1 replication in Vero cells completely (72), and 125 to 500 nM Flavo completely inhibits the transcription of a variety of cellular and viral genes in vivo (14, 39). Purv, which is less selective than Rosco, prevented the transcription of ICP0 completely at 20 μM whereas it did not completely prevent the transcription of RFP at 30 μM. However, Purv prevented the transcription of RFP more efficiently than Rosco did (compare Fig. 7A with Fig 6A). Flavo, the least specific PCI tested, inhibited the transcription of ICP0-driven RFP at all concentrations tested, compared to the levels of RFP in the absence of any drug (Fig. 7B). Interestingly, Flavo appeared to prevent the transcription of ICP0 promoter-regulated RFP from the cellular at least as efficiently as it prevented the transcription of ICP0 promoter-regulated ICP0 from the viral genome. No ICP0 or RFP transcripts were detected in the presence of any concentration of Flavo at 3 h p.i., and only low levels were detected in the presence of 31.25 or 62.5 nM at 9 h p.i. in the experiments in Fig. 7B. However, very low levels of ICP0, but not of RFP, transcripts were occasionally detected at 3 h p.i. in repeats of this experiment. In contrast, RFP mRNAs were detected at 3 h p.i. in all concentrations of Rosco in all experiments, whereas almost no ICP0 transcripts were detected at 3, 6, or 9 h p.i. in the presence of any concentration of Rosco in any experiment (Fig. 6A, top panels).
FIG. 7.
Less specific PCIs, Purv and Flavo, inhibit RFP expression driven by ICP0 promoters recombined in the cellular genome. (A) Northern blot analyses of expression of ICP0 (ICP0 [top panels]) and RFP (RFP [bottom panels]) in Vero clone 57 cells infected with HSV-1 in the presence of CHX. Cells were infected with 5 PFU/cell and treated with CHX and 0, 5, 10, 20, or 30 μM Purv. The cells were harvested at 3, 6, and 9 h p.i. (lanes 3, 6, and 9), and RNA was extracted, resolved by gel electrophoresis, and blotted to nylon membranes. The membranes were then hybridized with RFP probe, stripped, and rehybridized with ICP0 probe. A composite picture is presented (the 10 and 20 μM samples at 9 h p.i. were switched in the original gel). (B) Northern blot analyses of expression of ICP0 (ICP0 [top panels]) and RFP (RFP [bottom panels]) in Vero clone 57 cells infected with HSV-1 in the presence of CHX. Cells were infected with 5 PFU/cell and treated with CHX and 0, 31.25, 62.5, 125, 250, or 500 nM Flavo. The cells were harvested at 3 or 9 h p.i. (lanes 3 and 9), and RNA was extracted, resolved by gel electrophoresis, and blotted to nylon membranes. The membranes were then hybridized with RFP probe, stripped, and rehybridized with ICP0 probe.
Inhibition of transcription by Rosco depends on genome-specific but not on promoter-specific factors.
In the experiments in Fig. 1B, we observed that Rosco inhibited the initiation of transcription of IE genes under conditions in which their promoters were regulated by cellular and virion proteins acting alone or in combination with IE proteins. In the experiments in Fig. 6A we evaluated the effects of Rosco on transcription from IE promoters recombined in the cellular genome only under conditions in which no IE proteins were present. At least two IE proteins regulate the activities of IE promoters in the viral genome, ICP0 and ICP4. Therefore, we evaluated next the effects of Rosco on the cellular copies of the ICP0 promoter in the presence of IE proteins. To this end, we used the CHX release experimental design used in the experiments presented in Fig. 1B. Parental Vero cells were transiently transfected with the ICP0-RFP construct. At 24 h later, transiently transfected Vero cells and Vero clone 57 cells were independently seeded into individual wells. Seeded cells were infected in the presence of CHX for 5 h, when CHX-containing medium was removed and replaced with medium containing 0, 25, 50, 75, or 100 μM Rosco. At 16 h later, we evaluated the expression of RFP by fluorescence microscopy, because the efficiency of transfection of Vero cells is too low to permit examination of RNA levels by Northern blotting.
As in all other experiments, Rosco inhibited HSV-induced expression of RFP (which is directed by the cellular copies of the ICP0 promoter) only inefficiently (Fig. 8A and C). The percentage of cells expressing RFP decreased only marginally when the concentration of Rosco was increased from 0 to 100 μM (from 95% of cells expressing RFP to 75% [Fig. 8A]), a decrease that is consistent with the effects of Rosco on the activity of the same reporter gene when it is regulated by structural and cellular proteins acting alone (Fig. 6A). In contrast, Rosco efficiently inhibited HSV-induced expression of RFP regulated by copies of ICP0 promoters in transiently transfected (i.e., extrachromosomal) plasmids (from 100% of transfected cells expressing RFP to less than 15% [Fig. 8B and D]), which is also consistent with the effects of Rosco on the same reporter gene when it is regulated only by structural proteins (Fig. 4B). Rosco did not inhibit the basal activity of the transiently transfected ICP0 promoter (Fig. 8D, bottom panels, and data not shown), which is consistent with the lack of inhibition of the basal activity of a transiently transfected ICP0 promoter observed in the experiments in Fig. 4B and others previously reported, in which a similar construct was used (33).
FIG. 8.
In the presence of IE proteins, Rosco does not inhibit RFP expression driven by ICP0 promoters recombined in the cellular genome. (A) Clone 57 Vero cells (stably transfected with pICP0-RFP5) were seeded in individual wells and mock infected or infected with 2.5 PFU of HSV-1 KOS per cell in the presence of CHX. At 6 h later, CHX-containing medium was replaced by fresh medium containing 0, 25, 50, 75, or 100 μM Rosco. At 24 h after infection, the cells were fixed and the number of RFP-positive cells was determined under UV microscopy. The percentage of RFP-positive (RFP+) cells is presented as the average of two independent experiments plotted against Rosco concentration. Error bars indicate range. (B) Vero cells were transiently transfected with pICP0-RFP5. Transfected cells were seeded in five individual wells and mock infected or infected with 2.5 PFU of HSV-1 KOS per cell in the presence of CHX. At 6 h later, CHX-containing medium was replaced by fresh medium containing 0, 25, 50, 75, or 100 μM Rosco. At 24 h after infection, the cells were fixed and the number of RFP-positive cells was determined under UV microscopy. The Percentage of RFP-positive (RFP+) cells in the presence of the different concentrations of Rosco (where the number of RFP-positive cells in the absence of Rosco is set at 100%), and corrected by efficiency of transfection, is presented as averages of two independent experiments and plotted against Rosco concentration. Error bars indicate range. (C) Clone 57 Vero cells (stably transfected with pICP0-RFP5) were seeded in individual wells and infected with 2.5 PFU of HSV-1 KOS per cell in the presence of CHX (HSV-1). At 6 h later, CHX-containing medium was replaced by fresh medium containing 0, 25, 50, 75, or 100 μM Rosco (panels 0, 25, 50, and 75). At 24 h after infection, the cells were fixed and RFP-positive cells were photographed under UV microscopy. Representative micrographs are presented. Original magnification, 200 A. (D) Vero cells were transiently transfected with pICP0-RFP5. Transfected cells were seeded in individual wells and mock infected (Mock) or infected with 2.5 PFU of HSV-1 KOS per cell in the presence of CHX (HSV-1). At 6 h later, CHX-containing medium was replaced by fresh medium containing 0, 25, 50, 75, or 100 μM Rosco (panels 0, 25, 50, 75, and 100). At 24 h after infection, the cells were fixed and RFP-positive cells were photographed under UV microscopy. Representative micrographs are presented. Original magnification, 100 A.
DISCUSSION
In the experiments described in this paper, we showed that Rosco prevents the initiation of transcription of HSV-1 genes but does not inhibit ongoing HSV-1 transcription. Furthermore, the inhibitory effects of Rosco on transcription regulated by the HSV-1 ICP0 promoter were shown to be independent of the specific regulatory factors (i.e., Oct1-HCF-VP16 acting alone or in combination with ICP0 and ICP4) but dependent on the location of the promoter to the HSV-1 genome. Thus, the mechanism of inhibition of viral transcription by Rosco is different from the mechanism previously described for a less specific PCI, Flavo.
It has been demonstrated previously that many promoters recombined into the HSV-1 genome are regulated as HSV-1 E promoters in that they require activation by HSV-1 IE proteins (78, 79). In contrast, HSV-1 promoters are generally regulated by similar mechanisms in HSV-1 or cellular genomes. For example, a variety of HSV-1 IE and E promoters recombined in a variety of cell lines still require activation by HSV-1 proteins (for examples, see references 59 and 62). In fact, the early studies of the regulation of HSV-I promoters were performed using HSV-1 promoters recombined in cellular genomes (for examples, see references 5 and 44). To the best of our knowledge, this is only the second demonstration that transactivation of a given HSV-1 IE promoter requires different factors depending on whether the promoter is in its natural location in the HSV-1 genome or recombined in the cellular genome. In this, study, we showed that functions inhibited by Rosco are required for transcription driven by the ICP0 promoter in the HSV-1 genome but not for transcription driven by this promoter recombined in the cellular genome. Previously, Nicholl and Preston (57) had shown that inhibition of ICP0 promoter-driven transcription by IFN-α has similar genome specificity. IFN-α efficiently inhibits transcription driven by ICP0 promoters in the HSV-1 genome but not by ICP0 promoters previously recombined in the genome of the infected cells. Intriguingly, one of the two most extensively characterized functions of IFN-α is induction of cell cycle arrest, which results from indirect inhibition of CDK activities. Two of the CDK activities inhibited by IFN-α are CDK1 and CDK2 (11, 46, 66, 67), which are also among the CDKs that are the most sensitive to inhibition by Rosco and not to Flavo (12, 13, 43). These coincidences in the effects of IFN-α and Rosco suggest that the differential inhibition of HSV-1 gene expression by IFN-α may be a result of the indirect effects of IFN-α on CDK activities, an intriguing possibility that remains to be tested. Regardless of the specifics of the mechanism of regulation, however, the unique transcriptional requirements of the HSV-1 genome could be exploited for the development of new antiviral drugs that specifically inhibit the transcription of extrachromosomal genomes in a promoter-independent manner.
Three major pieces of evidence led us to the conclusion that the effects of Rosco on HSV-1 transcription are independent of promoter-specific sequences or transcription factors. First, Rosco prevented equally well the transcription driven by IE and E promoters (Fig. 1 and 2) but had no major effects on the transcription driven by most cellular promoters (Fig. 2 and 3). No promoter sequences or transcription factors are known to be both common to all IE and E HSV-1 promoters and absent in most cellular promoters. Second, Rosco inhibited equally well the sense and antisense HSV-1 transcription, whereas antisense HSV-1 transcription is commonly thought to be largely independent of specific promoters. Third, Rosco prevented the initiation of transcription from IE promoters in the viral genome equally well when these promoters were regulated by cellular proteins and HSV-1 structural proteins acting alone or in combination with HSV-1 IE proteins. Therefore, inhibition was independent of the specific combination of proteins regulating these promoters.
From the comparison of the effects of Rosco, Purv, and Flavo on the transcription of genes in HSV-1 or cellular genomes (Fig. 6 and 7), we conclude that kinases which are efficiently inhibited by Rosco and Purv are required for preinitiation or initiation of transcription of the HSV-1 genome whereas kinases which are inhibited efficiently by Flavo, less efficiently by Purv, and even less efficiently by Rosco are required for transcription in general. Based on evidence published by others, it is likely that CDK9 is the kinase that is both inhibited by Flavo and required for cellular and viral transcription (13, 39). In contrast, the kinases that are inhibited by Rosco and required for transcription from the HSV-1 genome remain to be identified, although it is known that they are cellular, not viral, kinases. Rosco is highly selective in that among 68 proteins tested, it inhibited only CDK1, CDK2, CDK5, and CDK7 with high potency and DYRK1a, ERK1 and ERK2 with lower potency (recently reviewed in reference 70). Based on independent evidence, two of the Rosco-sensitive CDKs, CDK1 and CDK2, have been postulated to be required for HSV-1 transcription (2, 3, 71, 73), both these CDKs are also inhibited by IFN-α. Since the human genome encodes 518 potential protein kinases (47) and numerous other ATP-binding proteins, however, it is impossible to conclude from the experiments described in this paper which specific kinases are required for activation of HSV-1 transcription and inhibited by Rosco.
It is perhaps surprising that a drug which in vitro inhibits CDK7, and perhaps CDK9, has no major effects on initiation or elongation of global cellular transcription by RNA Pol II. However, it has been documented previously that Rosco (39) and a methylated derivative (15) have no major effect on the accumulation of most cellular RNA Pol II transcripts in vivo. Rosco induced the downregulation of the steady-state levels of only 35 (0.7%) of 5,032 transcripts analyzed, and Me-O-Rosco induced the downregulation of the steady-state levels of fewer than 261 (4.35%) of 6,000 transcripts analyzed. Recently, Gherardi et al. even analyzed steady-state levels of transcripts in the kidneys of mice treated with Rosco for 20 days (28). Consistent with the results obtained with cultured cells, Rosco had only very limited effects on cellular transcription in kidneys of mice. In fact, only 3 (0.055%) of 5,400 transcripts were downregulated more than twofold by Rosco whereas 18 others (0.33%) were actually upregulated by the drug. None of these previous studies evaluated whether the limited effects of Rosco on steady-state levels of cellular transcripts were consequence of Rosco having no major effects on transcription or of Rosco inhibiting transcription while inducing transcript stabilization at the same time. We have now demonstrated that Rosco has surprisingly limited effects on global cellular transcription by RNA Pol II (Fig. 2 and 3). However, the mechanisms whereby an inhibitor of CDK7 fails to inhibit most cellular transcription have yet to be identified. We can only speculate that Rosco may have only limited access to the pool of CDK7 engaged in cellular transcription. It should be highlighted that both we (this paper) and Lam et al. (39) have analyzed the effects of Rosco on cellular gene expression under conditions at which its effects on cell cycle progression are not yet evident (i.e., before cells can arrest in response to the drug). Therefore, these experiments did not evaluate whether transcription of genes that are normally transcribed in a cell cycle-regulated manner is inhibited by Rosco secondarily to the inhibition of cell cycle progression.
It had been shown previously that Rosco inhibits steady-state levels of five HSV-1 transcripts (73-75). In contrast, Rosco did not inhibit the steady-state levels of E transcripts when the drug was added after a release from a phosphonoacetic acid block (74). These results were hypothesized by one of us (L.M.S.) and coworkers to be an indirect effect, resulting from inhibition of the expression of vhs (74), an L-gene product which induces mRNA degradation. However, these results could also indicate that Rosco does not inhibit HSV-1 transcription if the drug is added after preinitiation complexes have already assembled. We show now that Rosco prevents the initiation of transcription of HSV-1 genes but does not inhibit ongoing HSV-1 transcription. Thus, the lack of effect of Rosco on E-gene transcription when the drug was added after a release from a phosphonoacetic acid block may well have resulted from the lack of effects of Rosco on ongoing HSV-1 transcription.
In conclusion, the results presented in this paper show that a highly specific PCI, Rosco, prevents the initiation of transcription regulated by HSV-1 promoters, independent of the mechanisms of activation of these promoters but dependent on the location of these promoters to the HSV-1 genome. In contrast, Rosco does not inhibit ongoing transcription of HSV-1 genes, or initiation or elongation of the transcription of cellular genes. Thus, we have identified a novel activity for PCIs, their ability to inhibit the initiation of transcription from promoters in extrachromosomal genomes. Our current efforts are directed at identifying the mechanisms whereby Rosco exerts this effect. Regardless of the specific mechanisms, however, the possibility of inhibiting transcription in a genome-dependent and promoter-independent manner could be exploited in the development of novel antiviral drugs. Moreover, the characterization of the mechanism of this new biological activity of PCIs may also have major implications for their development as drugs against cancer.
Acknowledgments
This work was supported by operating grant MOP 49551 from the Canadian Institute for Health Research (CIHR). Our laboratory was equipped with funds provided by the Alberta Heritage Foundation for Medical Research (AHFMR) and the Faculty of Medicine. L.M.S. is supported by a New Investigator Award from the CIHR and a Medical Scholar Award from the AHFMR.
We especially thank M. Schultz (University of Alberta) and all members of our laboratory for critically reading the manuscript and J. R. Smiley (University of Alberta) for extensive and productive discussions. We also acknowledge the anonymous reviewers for their valuable suggestions.
REFERENCES
- 1.Advani, S. J., R. Hagglund, R. R. Weichselbaum, and B. Roizman. 2001. Posttranslational processing of infected cell Proteins 0 and 4 of herpes simplex virus 1 is sequential and reflects the subcellular compartment in which the proteins localize. J. Virol. 75:7904-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2001. cdc2 cyclin-dependent kinase binds and phosphorylates herpes simplex virus 1 UL42 DNA synthesis processivity factor. J. Virol. 75:10326-10333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Advani, S. J., R. R. Weichselbaum, and B. Roizman. 2000. The role of cdc2 in the expression of herpes simplex virus genes. Proc. Natl. Acad. Sci. USA 97:10996-11001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain, J., H. McLauchlan, M. Elliott, and P. Cohen. 2003. The specificities of protein kinase inhibitors: an update. Biochem. J. 371:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batterson, W., and B. Roizman. 1983. Characterization of the herpes simplex virion-associated factor responsible for the induction of alpha genes. J. Virol. 46:371-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharjee, R. N., G. C. Banks, K. W. Trotter, H. L. Lee, and T. K. Archer. 2001. Histone H1 phosphorylation by Cdk2 selectively modulates mouse mammary tumor virus transcription through chromatin remodeling. Mol. Cell. Biol. 21:5417-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and is isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bresnahan, W. A., I. Boldogh, P. Chi, E. A. Thompson, and T. Albrecht. 1997. Inhibition of cellular Cdk2 activity blocks human cytomegalovirus replication. Virology 231:239-247. [DOI] [PubMed] [Google Scholar]
- 9.Bresnahan, W. A., I. Boldogh, E. A. Thompson, and T. Albrecht. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150-160. [DOI] [PubMed] [Google Scholar]
- 10.Buolamwini, J. K. 2000. Cell cycle molecular targets in novel anticancer drug discovery. Curr. Pharm. Des. 6:379-392. [DOI] [PubMed] [Google Scholar]
- 11.Bybee, A., and N. S. Thomas. 1992. The synthesis of p58cyclin A and the phosphorylation of p34cdc2 are inhibited in human lymphoid cells arrested in G1 by alpha-interferon. Biochim. Biophys. Acta 1137:73-76. [DOI] [PubMed] [Google Scholar]
- 12.Carlson, B. A., M. M. Dubay, E. A. Sausville, L. Brizuela, and P. J. Worland. 1996. Flavopiridol induces G1 arrest with inhibition of cyclin-dependent kinase (CDK) 2 and CDK4 in human breast carcinoma cells. Cancer Res. 56:2973-2978. [PubMed] [Google Scholar]
- 13.Chao, S. H., K. Fujinaga, J. E. Marion, R. Taube, E. A. Sausville, A. M. Senderowicz, B. M. Peterlin, and D. H. Price. 2000. Flavopiridol inhibits P-TEFb and blocks HIV-1 replication. J. Biol. Chem. 275:28345-28348. [DOI] [PubMed] [Google Scholar]
- 14.Chao, S. H., and D. H. Price. 2001. Flavopiridol inactivates p-TEFb and blocks most RNA polymerase II transcription in vivo. J. Biol. Chem. 276:31793-31799. [DOI] [PubMed] [Google Scholar]
- 15.Chao, S. H., J. R. Walker, S. K. Chanda, N. S. Gray, and J. S. Caldwell. 2003. Identification of homeodomain proteins, PBX1 and PREP1, involved in the transcription of murine leukemia virus. Mol. Cell. Biol. 23:831-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cismowski, M. J., G. M. Laff, M. J. Solomon, and S. I. Reed. 1995. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol. Cell. Biol: 15:2983-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coen, D. M., and P. A. Schaffer. 2003. Antiherpesvirus drugs: a promising spectrum of new drugs and drug targets. Nat. Rev. Drug Discovery 2:278-288. [DOI] [PubMed] [Google Scholar]
- 18.Davido, D. J., D. A. Leib, and P. A. Schaffer. 2002. The cyclin-dependent kinase inhibitor roscovitine inhibits the transactivating activity and alters the posttranslational modification of herpes simplex virus type 1 ICP0. J. Virol. 76:1077-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davido, D. J., W. F. Von Zagorski, G. G. Maul, and P. A. Schaffer. 2003. The differential requirement for cyclin-dependent kinase activities distinguishes two functions of herpes simplex virus type 1 ICP0. J. Virol. 77:12603-12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de la Fuente, C., A. Maddukuri, K. Kehn, S. Y. Baylor, L. Deng, A. Pumfery, and F. Kashanchi. 2003. Pharmacological cyclin-dependent kinase inhibitors as HIV-1 antiviral therapeutics. Curr. HIV Res. 1:131-152. [DOI] [PubMed] [Google Scholar]
- 21.Everett, R. D., and G. G. Maul. 1994. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 13:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D., M. Meredith, A. Orr, A. Cross, M. Kathoria, and J. Parkinson. 1997. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 16:1519-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fax, P., O. Lehmkuhler, C. Kuhn, H. Esche, and D. Brockmann. 2000. E1A12S-mediated activation of the adenovirus type 12 E2 promoter depends on the histone acetyltransferase activity of p300/CBP. J. Biol. Chem. 275:40554-40560. [DOI] [PubMed] [Google Scholar]
- 24.Fischer, P. M. 2003. CDK versus GSK-3 inhibition. A purple haze no longer? Chem. Biol. 10:1144-1146. [DOI] [PubMed] [Google Scholar]
- 25.Fischer, P. M., and A. Gianella-Borradori. 2003. CDK inhibitors in clinical development for the treatment of cancer. Expert Opin. Investig. Drugs 12:955-970. [DOI] [PubMed] [Google Scholar]
- 26.Fisher, P., J. Endicott, and L. Meijer. 2003. Cyclin-dependent kinase inhibitors, p. 235-248. In L. Meijer, A. Jézequel, and M. Roberge (ed.), Progress in cell cycle research, vol. 5. Life in progress, Roscoff, France. [PubMed] [Google Scholar]
- 27.Gebara, M. M., M. H. Sayre, and J. L. Corden. 1997. Phosphorylation of the carboxy-terminal repeat domain in RNA polymerase II by cyclin-dependent kinases is sufficient to inhibit transcription. J. Cell Biochem. 64:390-402. [PubMed] [Google Scholar]
- 28.Gherardi, D., V. D'Agati, T.-H. T. Chu, A. Barnett, A. Gianella-Borradori, I. H. Gelman, and P. J. Nelson. 2004. Reversal of collapsing glomerulopathy in mice with the cyclin-dependent kinase inhibitor CYC202. J. Am. Soc. Nephrol. 15:1212-1222. [DOI] [PubMed] [Google Scholar]
- 29.Gray, N. S., L. Wodicka, A. M. Thunnissen, T. C. Norman, S. Kwon, F. H. Espinoza, D. O. Morgan, G. Barnes, S. LeClerc, L. Meijer, S. H. Kim, D. J. Lockhart, and P. G. Schultz. 1998. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281:533-538. [DOI] [PubMed] [Google Scholar]
- 30.Hirst, M., M. S. Kobor, N. Kuriakose, J. Greenblatt, and I. Sadowski. 1999. GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol. Cell 3:673-678. [DOI] [PubMed] [Google Scholar]
- 31.Hooijberg, J. H., H. J. Broxterman, M. Heijn, D. L. Fles, J. Lankelma, and H. M. Pinedo. 1997. Modulation by (iso)flavonoids of the ATPase activity of the multidrug resistance protein. FEBS Lett. 413:344-348. [DOI] [PubMed] [Google Scholar]
- 32.Jordan, R., and P. A. Schaffer. 1997. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J. Virol. 71:6850-6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jordan, R., L. Schang, and P. A. Schaffer. 1999. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J. Virol. 73:8843-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi, Y., C. VanSant, and B. Roizman. 1997. Herpes simplex virus 1 alpha regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J. Virol. 71:7328-7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koop, K. E., J. Duncan, and J. R. Smiley. 1993. Binding sites for the herpes simplex virus immediate-early protein ICP4 impose an increased dependence on viral DNA replication on simple model promoters located in the viral genome. J. Virol. 67:7254-7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouroukis, C. T., A. Belch, M. Crump, E. Eisenhauer, R. D. Gascoyne, R. Meyer, R. Lohmann, P. Lopez, J. Powers, R. Turner, and J. M. Connors. 2003. Flavopiridol in untreated or relapsed mantle-cell lymphoma: results of a phase II study of the National Cancer Institute of Canada clinical trials group. J. Clin. Oncol. 21:1740-1745. [DOI] [PubMed] [Google Scholar]
- 37.Krude, T. 2000. Initiation of human DNA replication in vitro using nuclei from cells arrested at an initiation-competent state. J. Biol. Chem. 275:13699-13707. [DOI] [PubMed] [Google Scholar]
- 38.Kudoh, A., T. Daikoku, Y. Sugaya, H. Isomura, M. Fujita, T. Kiyono, Y. Nishiyama, and T. Tsurumi. 2004. Inhibition of S-phase cyclin-dependent kinase activity blocks expression of Epstein-Barr virus immediate-early and early genes, preventing Viral lytic replication. J. Virol. 78:104-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam, L., O. Pickeral, A. Peng, A. Rosenwald, E. Hurt, J. Giltnane, L. Averett, H. Zhao, R. Davis, M. Sathyamoorthy, L. Wahl, E. Harris, J. Mikovits, A. Monks, M. Hollingshead, E. Sausville, and L. Staudt. 2001. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biol. 2:0041.0041-0041.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laurence, V., S. Faivre, K. Vera, J. Pierga, C. Delbaldo, M. Bekradda, J. Armand, A. Gianella-Borradori, V. Dieras, and E. Raymond. 2002. Preliminary results of an ongoing phase I and pharmacokinetic study of CYC202, a novel oral cyclin-dependent kinases inhibitor, in patients with advanced malignancies. Eur. J. Cancer 38(Suppl. 7):49. [Google Scholar]
- 41.Leclerc, S., M. Garnier, R. Hoessel, D. Marko, J. A. Bibb, G. L. Snyder, P. Greengard, J. Biernat, Y.-Z. Wu, E.-M. Mandelkow, G. Eisenbrand, and L. Meijer. 2000. Indirubins inhibit glycogen synthase kinase-3beta and CDK5/p25, two protein kinases involved in abnormal Tau phosphorylation in Alzheimer's disease. A property common to most cyclin-dependent kinase inhibitors? J. Biol. Chem. 276:251-260. [DOI] [PubMed] [Google Scholar]
- 42.Leresche, A., V. J. Wolf, and J. M. Gottesfeld. 1996. Repression of RNA polymerase II and III transcription during M phase of the cell cycle. Exp. Cell Res. 229:282-288. [DOI] [PubMed] [Google Scholar]
- 43.Losiewicz, M. D., B. A. Carlson, G. Kaur, E. A. Sausville, and P. J. Worland. 1994. Potent inhibition of CDC2 kinase activity by the flavonoid L86-8275. Biochem. Biophys. Res. Commun. 201:589-595. [DOI] [PubMed] [Google Scholar]
- 44.Mackem, S., and B. Roizman. 1982. Structural features of the herpes simplex virus alpha gene 4, 0, and 27 promoter-regulatory sequences which confer alpha regulation on chimeric thymidine kinase genes. J. Virol. 44:939-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Majello, B., G. Napolitano, A. Giordano, and L. Lania. 1999. Transcriptional regulation by targeted recruitment of cyclin-dependent CDK9 kinase in vivo. Oncogene 18:4598-4605. [DOI] [PubMed] [Google Scholar]
- 46.Mandal, M., D. Bandyopadhyay, T. M. Goepfert, and R. Kumar. 1998. Interferon induces expression of cyclin-dependent kinase inhibitors p21WAF1 and p27Kip1 that prevent activation of cyclin-dependent kinase by CDK-activating kinase (CAK). Oncogene 16:217-225. [DOI] [PubMed] [Google Scholar]
- 47.Manning, G., D. B. Whyte, R. Martinez, T. Hunter, and S. Sudarsanam. 2002. The protein kinase complement of the human genome. Science 298:1912-1934. [DOI] [PubMed] [Google Scholar]
- 48.Marshall, N. F., J. Peng, Z. Xic, and D. H. Price. 1996. Control of RNA polymerase II elongation potential by a novel carboxyl-terminal domain kinase. J. Biol. Chem. 271:27176-27183. [DOI] [PubMed] [Google Scholar]
- 49.Marshall, N. F., and D. H. Price. 1995. Purification of P-TEFb, a transcription factor required for the transition into productive elongation. J. Biol. Chem. 270: 12335-12338. [DOI] [PubMed] [Google Scholar]
- 50.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Euro. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 51.Meijer, L., and E. Damiens. 2002. CDK inhibitors: small molecular weight compounds, p. 145-162. In H. Maruta (ed.), Tumor suppressing viruses, genes and drugs: innovative cancer therapy approaches. Academic Press, Inc., New York, N.Y.
- 52.Meijer, L., S. Leclerc, and M. Leost. 1999. Properties and potential-applications of chemical inhibitors of cyclin-dependent kinases. Pharmacol. Ther. 82:279-284. [DOI] [PubMed] [Google Scholar]
- 53.Moffat, J., M. McMichael, S. Leisenfelder, and S. Taylor. 2004. Viral and cellular kinases are potential antiviral targets and have a central role in varicella zoster virus pathogenesis. Biochim. Biophys. Acta 1697:225-231. [DOI] [PubMed] [Google Scholar]
- 54.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nelson, P. J., V. D. D'Agati, J.-M. Gries, J.-R. Suarez, and I. H. Gelman. 2003. Amelioration of nephropathy in mice expressing HIV-1 genes by the cyclin-dependent kinase inhibitor, flavopiridol. J. Antimicrob. Chemother. 51:921-929. [DOI] [PubMed] [Google Scholar]
- 56.Nelson, P. J., I. H. Gelman, and P. E. Klotman. 2001. Suppression of HIV-1 expression by inhibitors of cyclin-dependent kinases promotes differentiation of infected podocytes. J. Am. Soc. Nephrol. 12:2827-2831. [DOI] [PubMed] [Google Scholar]
- 57.Nicholl, M. J., and C. M. Preston. 1996. Inhibition of herpes simplex virus type 1 immediate-early gene expression by alpha interferon is not VP16 specific. J. Virol. 70:6336-6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oikonomakos, N. G., J. B. Schnier, S. E. Zographos, V. T. Skamnaki, K. E. Tsitsanou, and L. N. Johnson. 2000. Flavopiridol inhibits glycogen phosphorylase by binding at the inhibitor site. J. Biol. Chem. 275:34566-34573. [DOI] [PubMed] [Google Scholar]
- 59.Orberg, P. K., and P. A. Schaffer. 1987. Expression of herpes simplex virus type 1 major DNA-binding protein, ICP8, in transformed cell lines: complementation of deletion mutants and inhibition of wild-type virus. J. Virol. 61:1136-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panagiotidis, C. A., and S. J. Silverstein. 1999. The host-cell architectural protein HMG I(Y) modulates binding of herpes simplex virus type 1 ICP4 to its cognate promoter. Virology 256:64-74. [DOI] [PubMed] [Google Scholar]
- 61.Panning, B., and J. R. Smiley. 1989. Regulation of cellular genes transduced by herpes simplex virus. J. Virol. 63:1929-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pasick, J. M., and J. R. Smiley. 1988. Regulated expression of stably transfected herpes simplex virus thymidine kinase genes in continuous cell lines expressing a temperature-sensitive mutant form of the immediate-early protein ICP4. Virology 162:490-493. [DOI] [PubMed] [Google Scholar]
- 63.Peng, J., N. F. Marshall, and D. H. Price. 1998. Identification of a cyclin subunit required for the function of Drosophila P-TEFb. J. Biol. Chem. 273:13855-13860. [DOI] [PubMed] [Google Scholar]
- 64.Rice, S. A., M. C. Long, V. Lam, P. A. Schaffer, and C. A. Spencer. 1995. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J. Virol. 69:5550-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sadaie, M. R., R. Mayner, and J. Doniger. 2004. A novel approach to develop anti-HIV drugs: adapting non-nucleoside anticancer chemotherapeutics. Antiviral Res. 61:1-18. [DOI] [PubMed] [Google Scholar]
- 66.Sangfelt, O., S. Erickson, S. Einhorn, and D. Grander. 1997. Induction of Cip/Kip and Ink4 cyclin dependent kinase inhibitors by interferon-alpha in hematopoietic cell lines. Oncogene 14:415-423. [DOI] [PubMed] [Google Scholar]
- 67.Satomoto, K., M. Haisa, Y. Naomoto, N. Tanaka, and K. Orita. 1995. Cyclin A and Cdk2 kinase activity are suppressed by combined treatment with tumor necrosis factor-alpha and interferon-alpha. Biochem. Biophys. Res. Commun. 213:1115-1121. [DOI] [PubMed] [Google Scholar]
- 68.Schang, L., G.-J. Hwang, B. Dynlacht, D. Speicher, A. Bantly, P. Schaffer, A. Shelatifard, H. Ge, and R. Shiekhattar. 2000. Human PC4 is a substrate-specific inhibitor of RNA Polymerase II phosphorylation. J. Biol. Chem. 275:6071-6074. [DOI] [PubMed] [Google Scholar]
- 69.Schang, L. M. 2002. Cyclin dependent kinases as cellular targets for antiviral drugs. J. Antimicrob. Chemother. 50:779-792. [DOI] [PubMed] [Google Scholar]
- 70.Schang, L. M. 2004. Effects of pharmacological cyclin-dependent kinase inhibitors on viral transcription and replication. Biochim. Biophys. Acta 1697:197-209. [DOI] [PubMed] [Google Scholar]
- 71.Schang, L. M., A. Bantly, and P. A. Schaffer. 2002. Explant-induced reactivation of herpes simplex virus occurs in neurons expressing nuclear cdks2 and 4. J. Virol. 76:7724-7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schang, L. M., A. Bantly, M. Knockaert, F. Shaheen, L. Meijer, M. Malim, N. Gray, and P. A. Schaffer. 2002. Pharmacological cyclin-dependent kinase inhibitors inhibit replication of wild type and drug-resistant strains of HSV and HIV-1 by targeting cellular, not viral, proteins. J. Virol. 76:7874-7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schang, L. M., J. Phillips, and P. A. Schaffer. 1998. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J. Virol. 72:5626-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 2000. Roscovitine, a specific inhibitor of cellular cyclin-dependent kinases, inhibits herpes simplex virus DNA synthesis in the presence of viral early proteins. J. Virol. 74:2107-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schang, L. M., A. Rosenberg, and P. A. Schaffer. 1999. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J. Virol. 73:2161-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Serizawa, H., T. P. Makela, J. W. Conaway, R. C. Conaway, R. A. Weinberg, and R. A. Young. 1995. Association of Cdk-activating kinase subunits with transcription factor TFIIH. Nature 374:280-282. [DOI] [PubMed] [Google Scholar]
- 77.Shiekhattar, R., F. Mermelstein, R. P. Fisher, R. Drapkin, B. Dynlacht, H. C. Wessling, D. O. Morgan, and D. Reinberg. 1995. Cdk-activating kinase complex is a component of human transcription factor. Nature 374:283-287. [DOI] [PubMed] [Google Scholar]
- 78.Smibert, C. A., and J. R. Smiley. 1990. Differential regulation of endogenous and transduced beta-globin genes during infection of erythroid cells with a herpes simplex virus type 1 recombinant. J. Virol. 64:3882-3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smiley, J. R., C. Smibert, and R. D. Everett. 1987. Expression of a cellular gene cloned in herpes simplex virus: rabbit beta-globin is regulated as an early viral gene in infected fibroblasts. J. Virol. 61:2368-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith, C. A., P. Bates, R. Rivera-Gonzalez, B. Gu, and N. A. DeLuca. 1993. ICP4, the major transcriptional regulatory protein of herpes simplex virus type 1, forms a tripartite complex with TATA-binding protein and TFIIB. J. Virol. 67:4676-4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Spencer, C. A., M. E. Dahmus, and S. A. Rice. 1997. Repression of host RNA polymerase II transcription by herpes simplex virus type 1. J. Virol. 71:2031-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun, X., Y. Zhang, H. Cho, P. Rickert, E. Lees, W. Lane, and D. Reinberg. 1998. NAT, a human complex containing Srb polypeptides that functions as a negative regulator of activated transcription. Mol. Cell 2:213-222. [DOI] [PubMed] [Google Scholar]
- 82a.Taylor, S. L., P. R. Kinchington, A. Brooks, and J. F. Moffat. 2004. Roscovitine, a cyclin-dependent kinase inhibitor, prevents replication of varicella-zoster virus. J. Virol. 78:2853-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vesely, J., L. Havlicek, M. Strnad, J. J. Blow, A. Donella-Deana, L. Pinna, D. S. Letham, J. Kato, L. Detivaud, S. Leclerc, et al. 1994. Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem. 224:771-786. [DOI] [PubMed] [Google Scholar]
- 84.Wang, D., C. de la Fuente, L. Deng, L. Wang, I. Zilberman, C. Eadie, M. Healey, D. Stein, T. Denny, L. E. Harrison, L. Meijer, and F. Kashanchi. 2001. Inhibition of human immunodeficiency virus type 1 transcription by chemical cyclin-dependent kinase inhibitors. J. Virol. 75:7266-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang, L., L. Deng, K. Wu, C. de la Fuente, D. Wang, K. Kehn, A. Maddukuri, S. Baylor, F. Santiago, E. Agbottah, S. Trigon, M. Morange, R. Mahieux, and F. Kashanchi. 2002. Inhibition of HTLV-1 transcription by cyclin dependent kinase inhibitors. Mol. Cell. Biochem. 237:137-153. [DOI] [PubMed] [Google Scholar]
- 86.Workman, J. L., and R. E. Kingston. 1998. Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu. Rev. Biochem. 67:545-579. [DOI] [PubMed] [Google Scholar]