Abstract
Numerous health problems including acute critical illness, cancer, diseases associated with chronic inflammation, and neurological disorders often result in skeletal muscle weakness and fatigue. Disease-related muscle atrophy and fatigue is an important clinical problem because acquired skeletal muscle weakness can increase the duration of hospitalization, result in exercise limitation, and contribute to a poor quality of life. Importantly, skeletal muscle atrophy is also associated with increased morbidity and mortality of patients. Therefore, improving our understanding of the mechanism(s) responsible for skeletal muscle weakness and fatigue in patients is a required first step to develop clinical protocols to prevent these skeletal muscle problems. This review will highlight the consequences and potential mechanisms responsible for skeletal muscle atrophy and fatigue in patients suffering from acute critical illness, cancer, chronic inflammatory diseases, and neurological disorders.
Keywords: muscle atrophy, cancer, inflammation, critical illness, neurological disorders
INTRODUCTION
Many diseases (e.g., neuromuscular diseases, cancer, chronic inflammatory diseases, and acute critical illness) are associated with skeletal muscle atrophy, muscle weakness, and general muscle fatigue. This disease-induced muscle wasting and fatigue is an important clinical problem because skeletal muscle wasting (i.e., atrophy) is associated with increased morbidity and mortality and a decreased quality of life. Therefore, improving our understanding of the mechanism(s) responsible for skeletal muscle wasting and fatigue in patients is important in order to develop therapies to prevent these problems. This brief review will discuss the potential cause and consequences of skeletal muscle atrophy and fatigue resulting from four different disease conditions: 1) Intensive care-induced skeletal muscle weakness; 2) Cancer cachexia; 3) Chronic inflammatory disease-induced muscle weakness; and 4) neurological disorders. Because of space limitations, the details provided for each topic will be limited to a narrow scope defined within the introduction of each segment. We begin with a discussion of the muscle atrophy and weakness associated with critically ill patients during treatment in the intensive care unit.
INTENSIVE CARE-INDUCED SKELETAL MUSCLE WEAKNESS
Intensive care units (ICU) provide the medical care for critically ill patients that are hospitalized due to life threating injuries or illnesses. Unfortunately, severe skeletal muscle weakness and fatigue is common among critically ill patients. The generalized muscle weakness that develops in both limb and respiratory muscles during the course of ICU hospitalization has been termed “intensive care unit acquired weakness” (ICUAW) (44, 51). Note that the term ICUAW is only applied in cases of muscle weakness where the patient is noted to have clinically detected weakness with no plausible cause other than critical illness (51). Because ICUAW is an important complication of critical illness that increases both mortality and morbidity, research on this topic has rapidly expanded during the past decade. In the following segments, we briefly discuss the incidence and clinical outcomes of ICUAW, debate the mechanisms responsible for ICUAW, and discuss potential interventions to prevent ICUAW. We will discuss ICUAW in limb and respiratory muscles in separate segments; let's begin with a discussion of ICUAW in limb skeletal muscles.
ICUAW in limb skeletal muscles: incidence and clinical outcomes
The incidence of ICUAW in limb muscles depends upon the patient population and duration of the ICU stay. For example, muscle weakness is present in only 11% of patients treated in the ICU for 24 hours whereas the incidence of ICUAW increases to 24-55% of patients treated in the ICU for 7-10 days (44). Clearly, longer stays in the ICU are associated with an increased incidence of ICUAW (44, 51).
Patient populations that are at the greatest risk for developing ICUAW are individuals admitted to the ICU with sepsis, chronic systemic inflammation, hyperglycemia, and/or multiple organ failure (44). This is predicted because sepsis, diseases that produce chronic systemic inflammation, multiple organ failure, and diabetes are associated with skeletal muscle wasting (14, 36, 44). The mechanism(s) linking systemic inflammation to muscle wasting will discussed in detail in the third segment of this review. Moreover, older patients have a greater risk of developing ICUAW compared to young adults (44). Further, compared to men, women are more likely to develop ICUAW (25). The reasons for this gender difference in the risk of developing ICUAW remains unknown.
ICUAW has two major implications for clinical outcomes. In the short-term, ICUAW is associated with prolonged ICU and hospital stays and increased morbidity and mortality (44, 51). Several mechanisms may explain the increased mortality and morbidity associated with ICUAW including the associated respiratory muscle weakness which prolongs hospital stays and pharyngeal dysfunction that increases the risk of aspiration (44).
In reference to ICUAW and long-term clinical outcomes, recovery from ICUAW typically requires weeks to months in most patients (11). However, patients with severe cases of ICUAW may not achieve a complete recovery. For example, a recent study on the impact of ICUAW on physical function and health-related quality of life reveals that patients with severe cases of ICUAW display significant muscle weakness and muscle fatigue up to five years following release from the hospital (46). This persisting muscle weakness impairs physical function, quality of life, and the return to work in patients treated for critical illness (44).
Mechanisms responsible for ICUAW in limb skeletal muscles
The mechanisms responsible for ICUAW in limb muscles are complex and involve structural and functional changes in the nervous system and/or skeletal muscle fibers (44). Indeed, ICUAW evoked by critical illness can be due to axonal neuropathy, myopathy, or both (44). The mechanism(s) responsible for axonal degeneration in ICUAW patients is incompletely understood. Nonetheless, factors that are predicted to contribute to this problem include increased vascular permeability in the endoneurium (44). This increase in vascular permeability allows penetration of toxic factors in the nerve ends; the resulting endoneuron edema appears to impair energy metabolism in the axon resulting in axonal death (44). Hyperglycemia may also contribute to this process by inducing neural mitochondrial dysfunction. In this regard, there is evidence that aggressive control of blood glucose in ICU patients can reduce the risk of polyneuropathy (51). Nonetheless, a well-accepting clinical protocol for preventing ICU-associated polyneuropathy does not exist and additional research is required to better understand the pathophysiological links between critical illness and nervous system dysfunction.
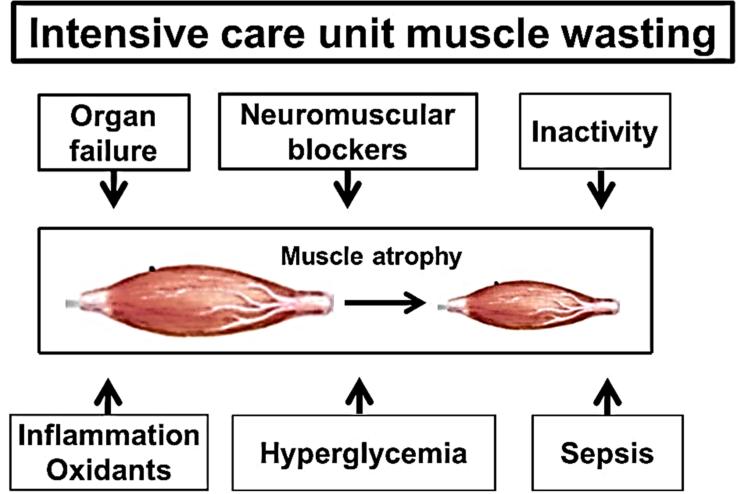
The factors that contribute to ICU-associated skeletal muscle wasting are complex and may vary due to differences in the duration of hospitalization and the type and severity of critical illness that resulted in ICU treatment (Figure 1). For example, in the absence of disease, prolonged bed rest results in skeletal muscle atrophy in healthy patients (72). Further, limb muscle wasting is common in many critical illnesses, particularly patients with sepsis, organ failure, hyperglycemia, and diseases associated with persistent systemic inflammation/oxidative stress (14, 36, 74, 75). Also, ICU patients treated with neuromuscular blockers are at increased risk for skeletal muscle wasting (51). In regard to cellular causes of ICU-induced muscle atrophy, the muscle wasting and weakness that occurs during ICU hospitalization is likely due to both an increase in proteolysis and a decrease in muscle protein synthesis (72, 74, 78).
Figure 1.
Key factors that contribute to intensive care unit-induced wasting of limb skeletal muscles.
Interventions to prevent ICUAW in limb skeletal muscles
Treatment of the cause of the acute illness is considered the first line of defense in preventing or minimizing ICUAW (44). For example, the aggressive treatment of sepsis is the cornerstone approach to protect against ICUAW in patients hospitalized for sepsis (44). Further, in theory, anti-inflammatory therapy could protect against ICUAW; however, the impact of specific anti-inflammatory therapy to protect against ICUAW has not been investigated in sufficient detail to determine whether this approach is effective against ICUAW.
Other protective strategies include the reduction of other known risk factors for ICUAW. For example, insulin treatment aimed at normalizing blood glucose levels has been reported to reduce both ICU-induced myopathy and neuropathy in select ICU patients (45, 91). Nonetheless, a subsequent multicenter trial revealed that intense therapy to maintain strict normoglycemia resulted in greater overall mortality compared to patients receiving smaller amounts of insulin in an effort to simply lower blood glucose levels without achieving normal blood insulin levels (37). Therefore, the wisdom of aggressive treatment of ICU patients to lower blood glucose levels remains a topic of debate (44).
Finally, ICU care for patients with severe illness involves bedrest where the patients are completely inactive; clearly, bed rest-induced immobilization contributes to ICUAW (51). While voluntary physical activity is not typically possible in critically ill patients, evidence exists that critically ill patients exposed to passive stretching of skeletal muscles (i.e., stretching performed by a therapist) can attenuate ICU-induced skeletal muscle atrophy (40). Moreover, several studies have performed electrical stimulation of limb muscles in the intensive care setting as a strategy to slow the rate of muscle atrophy during ICU care. In this regard, a recent review of this topic concludes that electrical muscle stimulation is a promising intervention but optimal training variables and safety of the intervention require additional study (70).
ICUAW in respiratory skeletal muscles: incidence and clinical outcomes
ICUAW also occurs in respiratory muscles when patients are treated with mechanical ventilation (MV). During the treatment for critical illness, MV is used to maintain sufficient pulmonary gas exchange in patients unable to sustain adequate alveolar ventilation without assistance. Common indications for MV include respiratory failure due to chronic obstructive pulmonary disease, heart failure, or patients recovering from surgery.
The number of patients receiving MV in the United States exceeds more than 800,000/year in the ICU (23). Although MV can be a life-saving measure, prolonged MV results in the rapid development of inspiratory muscle weakness due to both atrophy and contractile dysfunction(76). The principle muscle of inspiration in all mammals is the diaphragm and prolonged MV promotes diaphragmatic weakness in both animals and humans within the first 12-18 hours of ventilatory support (76). This detrimental impact of prolonged MV on the diaphragm has been termed ventilator-induced diaphragmatic dysfunction (VIDD) and VIDD is predicted to be a major contributor to problems in removing patients from the ventilator (i.e., weaning) (30, 92). The incidence of difficult weaning varies between different ICU's but can reach a level of ~30% for patients exposed to three or more days of MV (32, 59). The inability to wean patients from the ventilator is an important clinical problem because the failure to wean results in extended hospital stays and increased morbidity and mortality (31).
Mechanisms responsible for development of respiratory muscle weakness in ICU
Identical to other skeletal muscles, diaphragm fiber size is regulated by the balance between the rates of protein degradation and protein synthesis. It is established that proteolysis in the diaphragm is rapidly increased during full support MV (reviewed in (73, 76)) and prolonged MV also results in a decrease in protein synthesis in the diaphragm (82). Collectively, this MV-induced increase in proteolysis coupled with depressed protein synthesis results in the net loss of protein and diaphragm fiber atrophy. Because diaphragmatic atrophy occurs within the first 12-18 hours of prolonged MV, it is clear that the rapid onset of proteolysis plays a major role in the early stages of VIDD.
What factors trigger the early onset of proteolysis in the diaphragm during prolonged MV? Abundant evidence indicates that oxidative stress is a required up-stream activator of key proteolytic systems in the diaphragm (reviewed in (76)). Indeed, MV-induced oxidative stress appears to be required to activate calpain, caspase-3, and the ubiquitin-proteasome system of proteolysis during prolonged MV (76). While prolonged MV results in production of reactive oxygen species (ROS) from several sources, mitochondrial ROS production plays a key role (75).
Interventions to prevent ICU-induced respiratory muscle weakness
Given that VIDD is a key risk factor for difficult weaning from the ventilator, it is not surprising that numerous studies have explored therapeutic interventions aimed at protecting against MV-induced diaphragmatic weakness. In general, three different classes of pharmacological agents have shown promise toward protection against VIDD in animal models of prolonged MV. The pharmacological agents with promise include select antioxidants, protease inhibitors, and drugs that inhibit Janus kinase or nuclear factor-κB (NF-κB) signaling. A brief summary of evidence supporting the protective effects of these drugs against VIDD follows.
As discussed previously, prolonged MV results in oxidative stress in the diaphragm which is an upstream trigger to activate proteolytic activity in the diaphragm. It follows that pharmacological protection against MV-induced oxidative stress is a potential venue to protect against VIDD. In this regard, Betters et al. provided the first evidence that select antioxidants can protect against VIDD(10). This work demonstrated that animals treated with trolox, (water-soluble vitamin E analog) were protected against MV-induced protease activation and contractile dysfunction in the diaphragm. Subsequent reports have confirmed that trolox protects against MV-induced oxidative stress as well as MV-induced diaphragmatic atrophy and contractile dysfunction (58, 93). Further, the antioxidant N-acetylcysteine has also been reported to shield the diaphragm against MV-induced oxidative stress and contractile dysfunction (2). Finally, the mitochondrial-targeted antioxidant SS-31 can also protect the diaphragm against MV-induced oxidative stress and VIDD (71).
Because proteolysis plays a key role in development of MV-induced diaphragmatic atrophy and weakness within the first 12-18 hours of MV, numerous studies have explored the use of protease inhibitors to prevent VIDD. Specifically, the calpain inhibitors leupeptin and SJA 6017 both provide partial protection against VIDD (57, 68). Further, pharmacological inhibition of caspase-3 activity defends against the development of VIDD(68). In contrast, inhibition of the ubiquitin-proteasome system via expoxomicin provides limited protection against VIDD (84).
Recently it was revealed that pharmacological inhibition of the Janus kinase (JAK) and signal transducer and activator of transcription3 (STAT3) pathway protects against VIDD in rodents exposed to 12-18 hours of MV(83). It appears likely that inhibition of JAK/STAT3 signaling in the diaphragm during prolonged MV rescues the diaphragm from VIDD by preventing MV-induced increases in mitochondrial production of reactive oxygen species and the subsequent activation of proteases (83). The exact mechanism(s) to explain these findings remain unclear but may be linked to the fact that STAT3 translocated into the mitochondria can promote ROS production through modulation of components of the electron transport chain(83).
Activation of NF-κB in muscle fibers is associated with increased expression of the muscle specific E3 ligase and muscle atrophy. In this regard, inhibition of NF-κB signaling in the diaphragm by treating animals with SN50 (specific NF-κB inhibitor) or curcumin (natural phenol found in turmeric that inhibits NF-κB activation) has been shown to partially rescue the diaphragm from both MV-induced atrophy and contractile dysfunction (68).
Conclusions
In spite of the growing awareness of the clinical significance of ICUAW in both limb and respiratory muscles, no standard clinical treatments to prevent this problem are currently in place. Indeed, while significant research progress has been made in the development of pharmacological approaches to prevent VIDD in laboratory animals, none of the drugs that show experimental promise are currently approved by the Food and Drug Administration for use in humans. Clearly, developing clinically relevant treatment programs to prevent or delay ICUAW remains an important topic for future research.
CANCER CACHEXIA-INDUCED SKELETAL MUSCLE WEAKNESS AND FATIGUE
Many cancer patients suffer from skeletal muscle weakness and fatigue. In this section, we briefly introduce cancer cachexia, discuss the mechanisms responsible for muscle fatigue in cancer patients, and debate several potential interventions to prevent cancer-induced muscle weakness. We begin with an introduction to cancer cachexia.
What is cancer cachexia?
Cancer cachexia is a complex, multifactorial syndrome characterized by a progressive loss of skeletal muscle mass (with or without loss of fat mass) that cannot be fully reversed by conventional nutritional support and is associated with significant functional impairments (33). It can manifest early in disease with ~85% of patients with gastrointestinal and pancreatic cancers and 50-60% of patients with lung, colon and prostate cancer presenting with cachexia upon diagnosis. It is also evident in up to 80% of patients with advanced cancers of the colon, breast, sarcoma, lung, prostate, pancreas and gastrointestinal tract (26). Cachexia reduces quality of life (3), impairs the response to chemo- and radio-therapy (5) and increases mortality, with cachexia accounting for 20-30% of all cancer-related deaths (13). The devastating consequences of cancer cachexia highlight why muscle mass is critical for life itself. Skeletal muscle is not just an organ of locomotion but an essential organ of metabolism and survival.
Fatigue in cancer cachexia
In addition to muscle wasting and associated weakness, muscle fatigue is one of the most common and debilitating symptoms experienced by cancer patients. Severe fatigue, weakness and exercise intolerance has been reported in greater than 70% of cancer patients (21). Fatigue compromises quality of life, reduces independence and affects employment status, limits treatment options and increases morbidity. In fact, perceived fatigue is an independent predictor of quality of life and survival and has been rated to have a more negative impact on daily activities and quality of life than other cancer-related symptoms such as nausea, pain and depression (47). Fatigue occurs as a consequence of both the cancer itself and as a side effect of cancer treatments. It can be present at diagnosis, escalate during treatment and persist for six months and even up to two years after remission (47).
Mechanisms of fatigue in cancer cachexia
The etiology of fatigue in cancer cachexia is complex, encompassing both central and peripheral mechanisms (12). The central mechanisms relate to factors that influence motivation, including psychological distress and depression, pain, sleep disturbance and side effects of chemotherapies and radiotherapy including lethargy and general malaise. Specifically, dysregulation of the hypothalamic-pituitary-adrenal axis and five hydroxyl tryptophan (5-HT) neurotransmitter as well as circadian rhythm disruption have been implicated in cancer-related fatigue (8). Other (largely peripheral) mechanisms relate to factors that can influence muscular performance, either directly or indirectly affecting muscle structure-function. These include anemia, elevated circulating cytokines, dehydration, muscle fiber atrophy, and various cancer treatments. Interestingly, central effects such as neuronal inflammation and depressive-like behavior have been shown to contribute to reductions in voluntary activity and precede the loss of body and muscle mass in tumor-bearing mice (69).
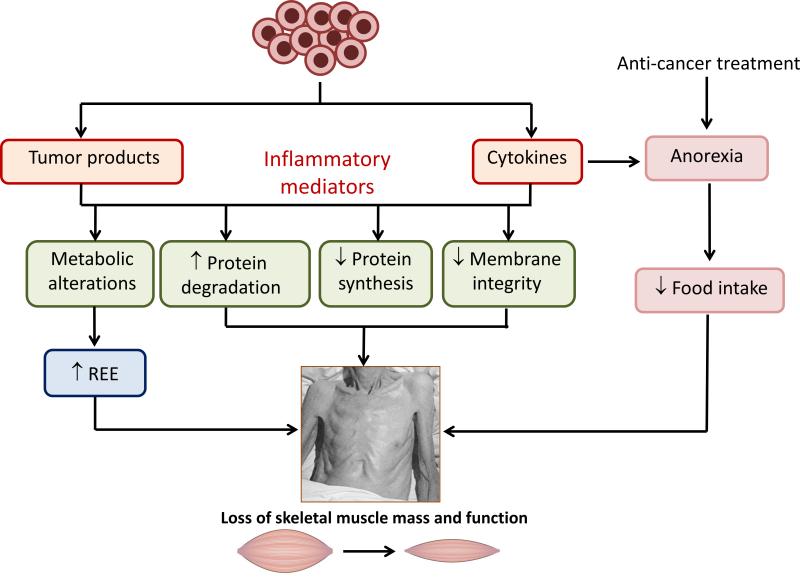
An overview of the potential peripheral mechanisms that lead to skeletal muscle wasting and weakness with cancer cachexia is depicted in Figure 2. Cancer cells release factors such as lipid mobilizing factor (LMF) and proteolysis-inducing factor (PIF) as well as cytokines including IL-6, TNF-α, IL-1β and IFN-γ that drive inflammatory signaling (see next section for more details). This systemic inflammation results in significant changes in metabolism including increasing resting energy expenditure, which increases by ~100-200 kcal/day even in the absence of physical activity (87). These aforementioned factors also compromise membrane integrity that disrupts signaling pathways responsible for cellular homeostasis causing a net loss of muscle because of increased protein degradation and decreased protein synthesis (7). Coupled with these events and especially with many cancer treatments, there is an associated anorexia leading to a reduction in nutrient intake of ~300-500 kcal/day (87). The resulting wasting and weakness is a very serious medical problem for which there is no effective treatment.
Figure 2.
Overview of the potential mechanisms leading to skeletal muscle wasting and weakness with cancer cachexia. Cancer cells release tumor-derived products and cytokines that drive an inflammatory response. These changes lead to metabolic alterations including a large increase in resting energy expenditure (REE). They also cause structural alterations which compromise membrane integrity and disrupt the signaling pathways involved in the maintenance of cellular homeostasis, leading to an increase in protein degradation and reduction in protein synthesis. The resulting overall loss of muscle mass and function (cancer cachexia) is exacerbated by a reduced nutrient intake due to anorexia (loss of desire to eat) induced by the pro-inflammatory environment and anti-cancer treatments such as chemo- and radio-therapy. Adapted from (6, 34).
Studying cancer cachexia using murine models
The impact of cancer cachexia and the efficacy of potential interventions can be studied using different rodent models, particularly tumor-bearing mouse models that exhibit varying magnitudes of muscle impairments. The severity of cachexia varies between models and also within models depending on the source of tumor cell lines. For example, mice inoculated with the same concentration of Colon-26 (C-26) cells from two different sources produce markedly different magnitudes of cachexia (64). Differences in storage conditions and passage number may account for these variances (69). Despite differing in the severity of muscle wasting (8-12% loss of muscle mass with LLC cells; 19-21% loss of muscle mass with C-26 cells), two of the most commonly used mouse models of cancer cachexia, the C-26 and Lewis Lung carcinoma (LLC) tumor-bearing mice both exhibit muscular fatigue (64, 65).
Given the complex nature of fatigue in cancer cachexia, it is appropriate in preclinical studies to utilize different approaches for measuring skeletal muscle impairments. Typically, these methods include rotarod performance, whole body grip strength and running performance on a motorized treadmill or voluntary (free) running wheel. In addition to physical capacity, these methods require voluntary engagement or motivation (19) and so both central and peripheral factors contribute to overall performance. Interestingly, one study reported that tumor-bearing mice did not demonstrate “fatigue” as assessed by levels of open-field locomotor activity, but did demonstrate “fatigue” as assessed by time spent immobile during a tail suspension test used to mimic depressive-like behavior (97). Since cancer-related fatigue can be associated with depressed mood, these findings highlight the importance of preclinical studies including tests of central and peripheral mechanisms. It is equally important to utilize tests specifically evaluating the force (strength) of isolated muscles or muscle groups in response to repeated stimulation (either in vitro or preferably in situ, with nerve and blood supply intact). A combination of these functional tests should be used to comprehensively evaluate the central and peripheral aspects of muscle fatigue in animal models of cancer cachexia. It should be noted when using grip strength as a measure of fatigue, whether the animal model exhibits kyphosis. For example, in severely cachectic C-26 tumor-bearing mice with a marked kyphosis remarkably similar to that in some of the more severe murine models of Duchenne muscular dystrophy (like the dystrophin-utrophin null dko mouse), grip strength may not be as severely affected as would be expected based on their overt frailty and weakness. In fact, the kyphosis can actually favor the animal's position on the grip meter to provide a biomechanical advantage that enables them to produce greater forces than expected.
Interventions for skeletal muscle wasting in cancer cachexia
Since the preservation of muscle mass is essential for survival and given the debilitating nature of fatigue in cancer cachexia, the goal of interventions should be to attenuate muscle atrophy and improve muscle strength, but not compromise muscle fatigue resistance. Although exercise is typically recommended for reducing muscle fatigue, its efficacy in cancer cachexia remains equivocal and exercise might not be a viable option for severely ill patients. Furthermore, the exercise response may be altered in cancer cachexia. For example, a single bout of low-frequency stimulation in mice revealed a disrupted metabolic signaling response in cachectic muscles (77).
Pharmacologic interventions that promote protein synthesis and/or reduce protein degradation are clearly important. Anabolic agents including the β-adrenoceptor agonists (β-agonists) clenbuterol and formoterol have potent anabolic actions for treating sarcopenia and muscular dystrophies in rodents. In aged rats with muscle wasting and weakness, treatment with β-agonists not only restored muscle mass and force production to the levels of adult rats, but in fact exceed them (81). However, despite the fast (extensor digitorum longus, EDL) and slow (soleus) muscles from treated rats being stronger, they fatigued more readily during repeated stimulation and recovered less well after cessation of stimulation (81). These impairments in fatigue resistance were directly attributable to the β-agonists inducing changes in muscle phenotype, specifically causing a shift from slow (oxidative) to fast (glycolytic) with a concomitant reduction in muscle succinate dehydrogenase (SDH) activity (81). Interventions that promote such a shift in phenotype might not be desirable for cancer cachexia if they exacerbate fatigue.
If the signaling pathways regulating protein synthesis and protein degradation are examined more closely, it is possible to identify multiple approaches with therapeutic potential for cancer cachexia. These include (but are not limited to) manipulation of insulin-like growth factor-I (IGF-I), proteolysis-inducing factor (PIF) signaling, myostatin/activin, TWEAK/Fn14, and signaling by the renin-angiotensin system (RAS). For this brief review, we will discuss the therapeutic potential of manipulating three of these signaling pathways for cancer cachexia; interfering with myostatin, RAS, and TWEAK/Fn14.
Myostatin inhibition
Considerable research over the last decade has evaluated the therapeutic potential of disrupting myostatin, a so-called negative regulator of skeletal muscle mass. Myostatin, originally termed growth and differentiation factor-8 (GDF-8), is a member of the transforming growth factor-β (TGF-β) superfamily. Myostatin negatively regulates skeletal muscle growth, an effect attributed to inhibition of both myoblast proliferation and differentiation (53). Livestock and humans with a loss-of-function mutation in the myostatin gene exhibit hyper-muscularity (60) and numerous studies have demonstrated that knocking out or knocking down myostatin, via genetic deletion or pharmacologic inactivation, leads to an increase in skeletal muscle mass and strength (53). Not surprisingly, there has been considerable interest in developing strategies to modulate myostatin activity in clinical conditions, including age-related muscle wasting, cancer cachexia, disuse atrophy, and muscular dystrophies and related diseases.
The myostatin inhibitory antibody PF-354 attenuated the loss of muscle mass and force in the LLC mouse model of cancer cachexia, and showed small but significant improvements in muscle mass and fatigue (force production during fatiguing stimulation of tibialis anterior, TA, muscles in situ) (65). These improvements were accompanied by increased SDH activity (in whole muscle cross-sections) and an increase in the proportion of oxidative muscle fibers (65). PF-354 also improved these endpoints in aged mice (67). Therefore, these studies have identified the therapeutic potential of antibody-directed myostatin inhibition for several muscle wasting disorders based on the ability to attenuate muscle atrophy and promote muscle growth but also to improve muscle fatigue resistance.
Activin A, another TGF-β ligand, binds the same transmembrane receptor as myostatin, and induces skeletal muscle atrophy and muscle fatigability (18). As a consequence, interventions inhibiting the signaling of both myostatin and activin A may be more effective than a single intervention. Indeed, the administration of soluble recombinant ActRIIB and other “ligand traps” have induced significant improvements in muscle mass and strength in animal models of cancer cachexia (98). Unfortunately, these interventions can inhibit TGF-β signaling in a ubiquitous manner and affect other tissues and processes, including reproduction and angiogensis, with some causing adverse and serious off-target effects (98). Studies are now focusing on targeting the intracellular ActRIIB signaling pathway as a potentially safer and effective way of inhibiting both myostatin and activin in cancer cachexia.
Renin-Angiotensin System inhibition
The renin-angiotensin system (RAS) is typically associated with the regulation of blood pressure and fluid homeostasis and has well-characterized effects, including vasoconstriction. Local RAS exist in multiple tissues including skeletal muscle and administration of the RAS peptide angiotensin II (Ang II) reduced muscle mass and induced muscle fiber atrophy in mice (80). Mice lacking the angiotensin type IA receptor (AT1A) have improved whole body and muscle function compared with wild type mice, despite having smaller muscles (64). However, inhibition of this pathway in C-26 tumor-bearing mice using the ACE inhibitor, perindopril, did not attenuate muscle wasting nor improve muscle maximum force production. Nonetheless, treatment with the ACE inhibitor did improve muscle fatigue (grip strength, rotarod performance, force during fatiguing stimulation of TA muscles in situ and diaphragm muscle strips in vitro); these effects appear to be due to a shift toward a more oxidative muscle phenotype, evident from increased SDH activity in muscle cross-sections (66).
Another strategy may be to target the alternative RAS axis, which involves conversion of Ang I to Angiotensin-(1-7) via two pathways: direct hydrolysis of Ang II to Ang-(1-7) via ACE2; and indirect hydrolysis of Ang I to Ang-(1-9) via ACE2 and the subsequent conversion of Ang-(1-9) to Ang-(1-7) via ACE. Ang-(1-7) signaling is mediated through the mitochondrial assembly receptor (MasR) and signaling via this pathway attenuates Ang II-induced atrophy and improves muscle strength and treadmill running performance in mice with muscular dystrophy (1, 61). However, the clinical benefits of targeting this pathway in cardiac cachexia have yet to be examined.
TWEAK/Fn14 inhibition
The inflammatory cytokine tumor necrosis factor (TNF)-like weak inducer of apoptosis (TWEAK) and its cognate receptor fibroblast growth factor-inducible 14 (Fn14) play multiple roles in proliferation, inflammation, and wound repair. TWEAK activates NF-κB signaling which leads to inflammation and fibrosis (48). Fn14 expression is increased after wounding and it has been said that tumors resemble ‘wounds that do not heal’ (28) with Fn14 present in high levels in solid tumours (20). TWEAK/Fn14 signaling also negatively regulates muscle growth and function (90) and consistent with these findings, C-26 tumor-bearing mice treated with an anti-Fn14 antibody lost less body and muscle mass and lived longer (48). Muscle fatigue (grip strength and force during fatiguing stimulation of TA muscles in situ) was also improved but not attributed to any change in muscle oxidative capacity. Although the mechanisms responsible for the improvement in fatigue with Fn14 inhibition have to be elucidated, these findings extend the role of Fn14 in wound repair and muscle development to involvement in the pathogenesis of cancer cachexia. They also demonstrate that anti-Fn14 antibodies may be a promising approach for improving muscle mass and strength, and reducing fatigability in cancer cachexia.
Conclusions
Despite a growing awareness of the debilitating consequences of cancer cachexia there is still no effective treatment for improving the muscle mass and muscle fatigue in this condition. The best candidates currently include myostatin inhibition and Fn14 inhibition which improve muscle mass, maximum force and muscle fatigue in preclinical models of cancer cachexia. Given that multiple signaling pathways contribute to the maintenance of muscle mass and function, it is logical that combination therapies are more likely to be needed to counter the multifactorial pathogenesis of cancer cachexia and produce successful outcomes for patients.
MUSCLE WEAKNESS IN CHRONIC INFLAMMATORY DISEASES
Skeletal muscle weakness, defined as a reduction in force or power in the absence of prior exercise -- is a major problem for many individuals afflicted with chronic inflammatory diseases including several types of cancer, rheumatoid arthritis, chronic heart failure, and chronic obstructive pulmonary disease (COPD). Weakness can interfere with basic activities of daily living, limit productivity in the workplace, impair one's mobility and range of motion, predispose individuals to fall-related injuries, and even restrict breathing. The literature shows that weakness is not simply an indirect product of illness caused by physical inactivity, poor nutrition, or physical discomfort. Rather, chronic inflammatory diseases appear to have direct, deleterious effects on skeletal muscles of the trunk and limbs. This section provides a brief discussion of the mechanism(s) responsible for skeletal muscle weakness in individuals afflicted with chronic inflammatory diseases.
Contractile dysfunction as a cause of weakness
Two parallel processes weaken skeletal muscle in chronic disease. Atrophy is the more familiar. In this process, myofiber size and muscle mass are reduced. These changes reflect an overall loss of myofibrillar protein that depresses force. Specifics of the atrophy process in the context of cancer are detailed elsewhere in this article. The second process that contributes to weakness is contractile dysfunction. Contractile dysfunction is characterized by loss of specific force, i.e., force per cross-sectional area, and may cause weakness in the absence of muscle atrophy. Contractile dysfunction is difficult to diagnose and is not well recognized in the clinical setting. However, human studies suggest contractile dysfunction is common in chronic diseases that range from chronic obstructive pulmonary disease or COPD (54) to heart failure (89) to rheumatoid arthritis or RA (43).
Over two decades ago, this concept was demonstrated by Helliwell and Jackson (43) who evaluated the relationship between weakness and muscle wasting in rheumatoid arthritis (RA). These investigators measured forearm muscle cross-sectional area and grip strength in 100 RA patients and 100 age- and sex-matched control subjects. Relative to controls, grip strength of RA patients was reduced (−58%) to a greater extent than forearm muscle cross-sectional area (−13%). Regression analysis confirmed that muscle cross-section accounted for less grip strength variation in RA patients than in control subjects. The authors concluded that muscle quality was diminished in RA patients.
Mechanism of weakness: A working hypothesis
Such findings raise obvious questions about the underlying mechanism. How do localized inflammatory events in the heart or lungs or distal joints cause contractile dysfunction in remote skeletal muscles? By defining this process, we may identify novel drug targets that can be used to develop new therapies that preserve muscle function in individuals with chronic inflammatory disease. Progress is being made. Human data suggest that pro-inflammatory cytokines are released from diseased tissues into the systemic circulation where they function as humoral mediators, exerting endocrine effects on skeletal muscle. Primary candidates for these endocrine stimuli include interleukin-6, C-reactive protein, sphingomyelinase, and tumor necrosis factor (TNF) with TNF being implicated most robustly. In parallel, evidence of oxidative and nitrosative stress suggests that reactive oxygen species (ROS) and nitric oxide (NO) derivatives also play an important role. Studies of cellular mechanism have led to the working hypothesis that TNF is a primary endocrine stimulus for contractile dysfunction in chronic inflammatory disease and that muscle-derived ROS and NO participate in post-receptor signaling events that depress specific force.
The Basic Concept: TNF, Oxidants, and Contractile Dysfunction
TNF acts directly on skeletal muscle to depress specific force, a unique property not shared by other pro-inflammatory cytokines. This decline in specific force has been observed following direct muscle exposure to exogenous TNF (4), systemic TNF administration (94), and stable TNF overexpression in transgenic mice (55). Decrements in specific force are evident within an hour after TNF exposure, arguing against a transcriptional mechanism, and can be detected at systemic concentrations that are too low to cause muscle atrophy (55).
In parallel, TNF stimulates muscle to produce cytosolic oxidants at an accelerated rate. This response is detectable in the cytoplasmic compartment within minutes (55). The rise in cytosolic oxidant activity can be inhibited by pretreatment with a nonspecific antioxidant, e.g., N-acetylcysteine (55) or Trolox (42); subsequent TNF exposure does not increase cytosolic oxidant activity and does not depress specific force. Such results underpin the concept that muscle-derived oxidants play a role in transducing the TNF signal and are essential mediators of contractile dysfunction stimulated by TNF.
Unraveling Mechanism: TNF/Oxidant Signaling
What receptor-mediated signaling events link TNF to the rise in oxidant activity? Which oxidant cascade is involved? Where are oxidants generated within muscle fibers? Such questions are fundamental to drug discovery efforts or therapeutic development. By identifying proteins that transduce the TNF signal and by defining the chemistry of key events, we can design interventions that interrupt the TNF/oxidant pathway and preserve muscle function. Prior research justifies this expectation. Proteins that regulate TNF signaling in muscle have been identified and blockade of the pathway can prevent contractile dysfunction.
First, TNF signaling is triggered by ligand/receptor interactions at the cell surface. Skeletal muscle fibers constitutively express two TNF receptors, the 55 kDa subtype 1 (TNFR1) and 75 kDa subtype 2 (TNFR2) (24). Each receptor subtype is a distinct gene product with unique structural and functional properties. Hardin and associates (2008) tested the involvement of both receptor subtypes in TNF/oxidant signaling by use of genetically-modified murine strains that were deficient in either TNFR1 (TNFR1−/−) or TNFR2 (TNFR2−/−). Relative to buffer-injected control animals, systemic administration of TNF to wild-type controls stimulated cytosolic oxidant activity in muscle fibers and depressed specific force. Muscles of TNFR1−/− animals were unaffected by TNF administration; neither oxidant activity nor specific force was different from muscles of buffer-injected controls. In contrast, muscles of TNFR2−/− mice responded to TNF in the same manner as wild-type controls; oxidant activity rose and specific force fell. These data demonstrate that muscle responses to TNF are triggered by activation of TNFR1 rather than TNFR2.
Second, early postreceptor events in the TNF/oxidant pathway include sphingolipid signaling. In a variety of cell types, ligand binding to TNFR1 stimulates signaling via neutral sphingomyelinase. This enzyme family metabolizes sphingomyelin, a component of cell membranes, to create ceramide, a bioactive sphingolipid with pro-inflammatory actions. Ferreira and co-workers (35) have shown that skeletal muscle responds similarly to exogenous sphingomyelinase or exogenous ceramide. Both stimuli increase cellular ceramide levels in muscle. This causes a rise in cytosolic oxidant activity and a drop in specific force, responses that are abolished by antioxidant pretreatment. Thus, direct augmentation of sphigomyelinase/ceramide signaling evokes a series of events in skeletal muscle that mimic the response to TNF. This set the stage for mechanistic studies by Moylan, et al. (63) who showed that TNF activates endogenous sphingomyelinase in skeletal muscle myotubes, increasing cellular ceramide content and cytosolic oxidant activity. A series of biochemical and functional analyses identified neutral sphingomyelinase 3 (nSMas3; gene Smpd4) as the dominant isoform of neutral sphingomyelinase expressed by skeletal muscle and the isoform that regulates TNF/ceramide signaling in muscle.
Which Oxidants Are Involved?
Like most eukaryotic cell types, skeletal muscle myofibers continually generate both ROS and NO derivatives. These two redox cascades contribute to general oxidant activity in the cell. They have similar chemistries and molecular targets. Plus they co-regulate many of the same physiologic processes. Yet the intracellular sources of ROS and NO can be quite distinct and their functional effects often oppose one another in biological systems. It is therefore useful to define the roles of these two cascades in any process regulated by redox signaling. However, most research studies that address TNF signaling in skeletal muscle have used nonspecific probes, e.g., redox assays, inhibitors, antioxidants, etc., that detect changes in general oxidant activity. The redox cascade that causes contractile dysfunction had not been defined until recently.
Stasko, et al. (85) systematically evaluated the relative importance of NO derivatives versus ROS. Data obtained using NO-specific probes make it clear that TNF stimulates production of NO derivatives by muscles of healthy mice. NO derivatives largely account for the rapid rise in general oxidant activity induced by TNF and are essential for the subsequent decline in specific force. Pharmacologic blockade of NO synthesis abolishes both responses to TNF. Both responses are also abolished by genetic deficiency of the neuronal-type NO synthase (nNOS), one of several NO synthase isoforms expressed by skeletal muscle fibers. These findings indicate that TNF stimulates nNOS to produce NO thereby depressing contractile function.
Surprisingly, Stasko and associates found that muscle-derived ROS are also required for TNF-induced dysfunction. Their data do not suggest that TNF stimulates ROS production. Rather, ambient ROS levels in the resting myofiber appear to play a permissive role. Selective depletion of cytosolic ROS does not alter the NO spike stimulated by TNF. It persists unabated. Yet ROS depletion preserves specific force, suggesting endogenous ROS are essential for the dysfunction stimulated by TNF. It is likely that these ROS derive from muscle mitochondria (56). Stasko and associates concluded that NO derivatives and ROS function as essential co-mediators of the contractile dysfunction stimulated by TNF. How might ROS and NO signaling be interdependent? The speculation is that ROS species, notably superoxide anions, react with NO to generate peroxynitrite (ONOO−). In turn, ONOO− functions as a common downstream mediator to depress specific force.
Force Depression by Oxidants
Functional studies suggest that TNF depresses specific force at the myofibrillar level. Using intact murine myofibers, Reid, et al. (79) showed that direct TNF exposure depresses tetanic force without altering tetanic calcium transients or resting calcium levels. The authors concluded that TNF acts downstream of voltage-dependent calcium release to lessen force production by muscle myofilaments. This interpretation was confirmed by Hardin and colleagues (42) who used to permeablized single fibers from TNF-treated mice to assess myofilament function directly. They showed that fibers from TNF-treated animals generate less specific force across a range of activating calcium concentrations. TNF depression of the calcium-force relationship is consistent with the actions of ceramide (36) and peroxynitrite (88), both of which depress the calcium-force curve in a manner similar to TNF.
Conclusions: an integrative model of muscle dysfunction
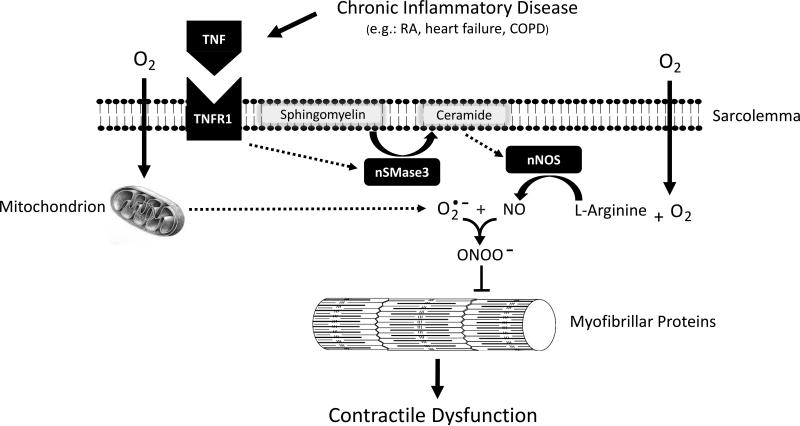
Integrating the available data, Figure 3 depicts a working model of the key events by which chronic inflammatory disease might cause contractile dysfunction in skeletal muscle. In this model, inflammation of a distal organ causes persistent elevation of circulating TNF which exerts endocrine actions on skeletal muscle myofibers. TNF binding to TNFR1 stimulates nSMase3-dependent ceramide signaling which increases NO production by nNOS. NO reacts with mitochondrial superoxide anions to form peroxynitrite. Finally, peroxynitrite acts on muscle myofilaments to depress specific force.
Figure 3.
Model of Contractile Dysfunction in Chronic Inflammatory Disease. Diagram depicts sequence of events by which chronic inflammatory disease is thought to depress specific force of skeletal muscle. Dashed arrows indicate steps in intracellular signaling that are undefined. RA, rheumatoid arthritis; COPD, chronic obstructive pulmonary disease; TNF, tumor necrosis factor; TNFR1, TNF receptor subtype 1; nSMase3, neutral sphingomyelinase 3; NO, nitric oxide; nNOS, neuronal-type NO synthase; , superoxide anion; ONOO−, peroxynitrite
Aspects of this model are intuitively appealing. Both nSMase3 and nNOS localize to the endofacial surface of the sarcolemma. This favors nSMase3 interaction with TNFR1, a transmembrane protein complex, and nNOS activation by ceramide, a highly lipophilic molecule. However, questions remain about the specifics of these interactions. For example, what intermediate proteins may be required for TNFR1/nSMase3 interaction? Does ceramide act directly on nNOS? Or is ceramide converted to a bioactive derivative that stimulates NO synthesis, e.g., sphingosine or sphingosine-1-phosphate? Such questions highlight the opportunities for continued research and greater mechanistic understanding in this field.
Finally, the current model identifies several proteins that could be targeted for development of future therapies. The model predicts that anti-TNF therapy or nNOS inhibitors might be useful in the clinic. Such drugs are available for human trials now and could be tested for their capacities to preserve muscle force. However, these compounds affect multiple organ systems and can have deleterious off-target actions. A drug that selectively inhibits TNF signaling in skeletal muscle would be preferable. nSMase3 provides a potential target for such drugs. nSMase3 does not have a well-defined role in non-muscle cell types and appears to be less critical for sphingolipid signaling. In theory, a selective nSMase3 inhibitor could blunt TNF signal transduction and weakness in skeletal muscle and have minimal side effects in other organs.
FATIGUE IN NEUROLOGICAL DISORDERS
In patients suffering from neurological disorders, fatigue can often have a major negative influence on the patient's quality of life and their ability to maintain employment. In the previous sections, fatigue is primarily described from a peripheral perspective (i.e., fatigue defined as a decline in performance). In neurological disorders, however, fatigue is often present before changes in muscle performance are observed. This observation suggests that the relationship between fatigue and performance is more complex. This section briefly discusses fatigue as a resultant of both disease-induced changes in performance and perception in patients with neurological disorders.
Fatigue-an evolving definition
Fatigue is generally conceptualized either in terms of ‘perception’ or a ‘decline in performance’. Persons suffering from neurological disorders generally describe fatigue in terms of perception; i.e. an unusual and unpredictable sensation, which differs from fatigue experienced by healthy persons, interferes with daily living and is often not related to effortful task performance. Enoka and colleagues proposed a framework to describe fatigue in which both constructs are represented (50). Fatigue that is perceived by an individual is affected by 1) factors which modulate perceptions of fatigability (e.g. affective factors like mood and arousal, but also homeostatic factors like energy status and hydration) and by 2) factors which affect performance fatigability (e.g. contractile state of muscles, voluntary activation, but also factors like afferent feedback, and cortical excitability)(50). These two constructs of fatigue are not necessarily independent.
Research efforts to evaluate the level of fatigue in patients have resulted in more than 30 questionnaires to quantify fatigue (29). These questionnaires can be uni- or multidimensional and can measure the impact, severity or the frequency of fatigue (29). Results obtained with fatigue questionnaires have provided important information regarding the incidence and impact of fatigue on the lives of patients suffering from neurological disorders. For example, in patients that experience a stroke, over a third of the patients reported fatigue within three months from the stroke (37, 50), seventy percent of patients with multiple sclerosis define fatigue as one of their worst symptoms (39, 52), and more than 70% of people with traumatic brain injury report increased levels of fatigue (15, 38). Hence, fatigue is an important, frequently observed, and disabling symptom in neurological disorders.
Fatigue in persons with multiple sclerosis
It is beyond the scope of this review to discuss the impact of fatigue in a large number of neurological disorders. Therefore, in this section, we will discuss multiple sclerosis (MS) as an example of a neurological disorder in which fatigue is a frequently occurring symptom. MS is a chronic disease of the central nervous system, characterized by inflammation, demyelination and neurodegeneration. As early as 1949, fatigue was recognized as an important symptom of MS (49). Fatigue is often one of the first symptoms to occur, and can be present years before the diagnosis of MS (9, 52). Although fatigue in MS has been studied for more than fifty years, mechanisms underlying fatigue are poorly understood.
Since perception of fatigue is a subjective parameter, several investigators have studied the association between MS and fatigue in an effort to develop objective performance measures. Although persons with MS differ from an age- and sex-matched healthy “control” population in both motor parameters (e.g., muscle force, walking ability, daily activity level, or gait parameters) as well as neuropsychological parameters (e.g., paced auditory serial addition test, symbol digit modalities test, or Stroop test) associations were often not observed, weak, or conflicting (95). Nevertheless, in recent experiments (86, 95, 96), we discovered three examples in which perceived fatigue was associated with ‘performance fatigability’ if measures of ‘perception of fatigability’ were simultaneously included in the explanatory model. These examples are briefly described in the next segment.
Associations between fatigue and fatigability in persons with multiple sclerosis
In persons with MS fatigue was quantified with questionnaires [the Fatigue Severity Scale (FSS) and the Modified Fatigue Impact Scale (MFIS)]. Additionally, subjects completed the Hospital anxiety and depression scale (HADS). Force measures were obtained during brief and sustained (2-minutes) maximal voluntary contractions (MVC) of the index finger abductor. In experiment one, relapsing-remitting MS patients (n=20) that were not prescribed fatigue-modulating medications were studied. As expected, persons with MS reported greater fatigue scores (86, 95, 96). Surprisingly, the force decline during the sustained contraction did not differ between persons with MS and controls. However, more than 70% of the variation in fatigue scores could be explained with a model including force decline during the sustained contraction, MVCs and the HADS depression score (86). In the second experiment, a large pool of subjects were studied that included patients using various fatigue-reducing medications and these results yielded lower correlations (95). Nonetheless, these findings explained 30-48% of the variation in fatigue scores; including MVC, force decline and depression scores in the model (95). In the third experiment, MS patients performed a sustained motor-cognitive dual-task until they were no longer able to maintain 30% MVC force. The results revealed that the combination of a fatigability measure (time until task failure), MVC, and a depression score could explain 53% of the variability in perceived fatigue (96).
In all three experiments the combination of measures of performance and fatigability along with measures of the perception of fatigability could explain more of the variation in fatigue scores than each of the individual scores by itself. Therefore, attempts to understand the mechanism(s) underlying fatigue in persons with MS should include measures that contain both constructs.
Fatigue in neurological disorders: hypothesis
Over a decade ago, Chaudhuri and Behan (17), proposed that fatigue was the consequence of an activation failure within the basal ganglia resulting in a disconnection between the thalamus and frontal areas of the brain. This theory was recently extended into the hypothesis that fatigue might develop as a consequence of a dopamine imbalance (27). Both too much and too little dopamine would affect connectivity/excitability within mesocorticolimbic pathway; linking areas known to be involved in reward-based decision (connecting the ventral tegmental area with the striatum, limbic areas and the prefrontal cortex) (27). The dopamine imbalance hypothesis also supports recent theories (22, 41, 62) in which fatigue is related to activation of the immune system. At least three pathways have been identified via which the peripheral immune signal can access the brain by way of a humoral, neural, and/or cellular pathway (16). These interactions between the immune system and the central nervous can eventually result in altered neurotransmission and neural processing, and disturbance of the neuronal environment (including availability of neurotransmitters)(16, 22).
Since determining a direct cause and effect link between circulating cytokines and fatigue is difficult, most evidence comes from indirect experiments. These results show that activation changes associated with inflammatory biomarkers, in brain areas involved in maintaining homeostasis (e.g., thalamus, insula, anterior cingulate cortex), effort-related cost-benefit analysis (e.g., basal ganglia, amygdala) and motivation (the medial prefrontal cortex)(22, 62). Most of these areas are also known to show abnormal activation patterns and/or structural impairments in patients suffering from fatigue (27). Interestingly, the brain areas in which the activity is modulated are known to be associated with both constructs of fatigue (i.e., perception of fatigability and performance fatigability).
Conclusions
Fatigue is an often reported symptom by persons suffering from neurological disorders. Despite the impact of fatigue on social and physical well-being, the mechanisms underlying fatigue are still poorly understood. Nonetheless, growing evidence data suggests that the causes of fatigue in persons with neurological disorders are multifactorial. Inclusion of measures which encompass both constructs of fatigue (i.e., factors modulating perception of fatigue and factors affecting performance fatigability) is essential for a multidisciplinary approach to explain fatigue. From a clinical perspective, focusing on one dimension of fatigue is inefficient and does not result in substantial progress in the understanding and treatment of fatigue in patient populations.
Acknowledgments
Scott K.Powers: The research on mechanical ventilation-induced diaphragm dysfunction in the Powers’ laboratory is supported by grants from the National Institutes of Health (NIAMS: R21AR064956, R01AR064189, and R21AR063805).
Gordon Lynch: KTM is supported by a NH&MRC Career Development Fellowship (#1023178).
The research on cancer cachexia was supported by project grant funding from the National Health and Medical Research Council of Australia (APP1041865).
Michael Reid: The author thanks Christine Coombes for graphic design and Delainie McNeil for administrative support. This research was supported by National Institutes of Health grant R01 AR055974.
Footnotes
Please note that the contents of this review do not constitute endorsement by the American College of Sports Medicine.
References
- 1.Acuña MJ, Pessina P, Olguin H, et al. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-b signalling. Hum Mol Genet. 2014;23(5):1237–49. doi: 10.1093/hmg/ddt514. [DOI] [PubMed] [Google Scholar]
- 2.Agten A, Maes K, Smuder A, Powers SK, Decramer M, Gayan-Ramirez G. N Acetylcysteine protects the rat diaphragm from the decreased contractility associated with controlled mechanical ventilation. Crit Care Med. 2011;39(4):777–82. doi: 10.1097/CCM.0b013e318206cca9. [DOI] [PubMed] [Google Scholar]
- 3.Ahlberg K, Ekman T, Gaston-Johansson F, Mock V. Assessment and management of cancer-related fatigue in adults. Lancet. 2003;362(9384):640–50. doi: 10.1016/S0140-6736(03)14186-4. [DOI] [PubMed] [Google Scholar]
- 4.Alloatti G, Penna C, Mariano F, Camussi G. Role of NO and PAF in the impairment of skeletal muscle contractility induced by TNF-alpha. Am J Physiol Regul Integr Comp Physiol. 2000;279(6):R2156–63. doi: 10.1152/ajpregu.2000.279.6.R2156. [DOI] [PubMed] [Google Scholar]
- 5.Andreyev HJ, Norman AR, Oates J, Cunningham D. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34(4):503–9. doi: 10.1016/s0959-8049(97)10090-9. [DOI] [PubMed] [Google Scholar]
- 6.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer. 2014;14(11):754–62. doi: 10.1038/nrc3829. [DOI] [PubMed] [Google Scholar]
- 7.Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100–6. doi: 10.1016/j.coph.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M. I'm so tired: biological and genetic mechanisms of cancer-related fatigue. Qual Life Res. 2010;19(10):1419–27. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berger JR, Pocoski J, Preblick R, Boklage S. Fatigue heralding multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2013;19(11):1526–32. doi: 10.1177/1352458513477924. [DOI] [PubMed] [Google Scholar]
- 10.Betters JL, Criswell DS, Shanely RA, et al. Trolox attenuates mechanical ventilation- induced diaphragmatic dysfunction and proteolysis. Am J Respir Crit Care Med. 2004;170(11):1179–84. doi: 10.1164/rccm.200407-939OC. [DOI] [PubMed] [Google Scholar]
- 11.Bolton CF, Breuer AC. Critical illness polyneuropathy. Muscle Nerve. 1999;22(3):419–24. doi: 10.1002/(sici)1097-4598(199903)22:3<419::aid-mus18>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Bower JE. Cancer-related fatigue-mechanisms, risk factors, and treatments. Nat Rev Clin Oncol. 2014;11(10):597–609. doi: 10.1038/nrclinonc.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruera E. ABC of palliative care. Anorexia, cachexia, and nutrition. British Medical Journal. 1997;315(7117):1219–22. doi: 10.1136/bmj.315.7117.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callahan LA, Supinski GS. Sepsis-induced myopathy. Crit Care Med. 2009;37(10 Suppl):S354–67. doi: 10.1097/CCM.0b013e3181b6e439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantor JB, Ashman T, Gordon W, et al. Fatigue after traumatic brain injury and its impact on participation and quality of life. The Journal of head trauma rehabilitation. 2008;23(1):41–51. doi: 10.1097/01.HTR.0000308720.70288.af. [DOI] [PubMed] [Google Scholar]
- 16.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2011;130(2):226–38. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363(1474-547; 9413):978–88. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 18.Chen JL, Walton KL, Winbanks CE, et al. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014;28(4):1711–23. doi: 10.1096/fj.13-245894. [DOI] [PubMed] [Google Scholar]
- 19.Clark YY, Wold LE, Szalacha LA, McCarthy DO. Ubiquinol reduces muscle wasting but not fatigue in tumor-bearing mice. Biol Res Nurs. 2015;17(3):321–9. doi: 10.1177/1099800414543822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Culp PA, Choi D, Zhang Y, et al. Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clin Cancer Res. 2010;16(2):497–508. doi: 10.1158/1078-0432.CCR-09-1929. [DOI] [PubMed] [Google Scholar]
- 21.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 22.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends in neurosciences. 2014;37(1):39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–71. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 24.De Bleecker JL, Meire VI, Declercq W, Van Aken EH. Immunolocalization of tumor necrosis factor-alpha and its receptors in inflammatory myopathies. Neuromuscul Disord. 1999;9(4):239–46. doi: 10.1016/s0960-8966(98)00126-6. [DOI] [PubMed] [Google Scholar]
- 25.De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA. 2002;288(22):2859–67. doi: 10.1001/jama.288.22.2859. [DOI] [PubMed] [Google Scholar]
- 26.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69(4):491–7. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 27.Dobryakova E, Genova HM, DeLuca J, Wylie GR. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Frontiers in neurology. 2015;6:52. doi: 10.3389/fneur.2015.00052. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 29.Elbers RG, Rietberg MB, Wegen EE, et al. Self-report fatigue questionnaires in multiple sclerosis, Parkinson's disease and stroke: a systematic review of measurement properties. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2012;21(6):925–44. doi: 10.1007/s11136-011-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eskandar N, Apostolakos MJ. Weaning from mechanical ventilation. Crit Care Clin. 2007;23(2):263–74, x. doi: 10.1016/j.ccc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Esteban A, Alia I, Ibanez J, Benito S, Tobin MJ. Modes of mechanical ventilation and weaning. A national survey of Spanish hospitals. The Spanish Lung Failure Collaborative Group. Chest. 1994;106(4):1188–93. doi: 10.1378/chest.106.4.1188. [DOI] [PubMed] [Google Scholar]
- 32.Esteban A, Frutos F, Tobin MJ, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med. 1995;332(6):345–50. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 33.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–95. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 34.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–66. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova-Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol. 2010;299(3):C552–60. doi: 10.1152/ajpcell.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira LF, Moylan JS, Stasko S, Smith JD, Campbell KS, Reid MB. Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers. J Appl Physiol (1985) 2012;112(9):1538–45. doi: 10.1152/japplphysiol.01269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 38.Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. The American Journal of Hospice & Palliative Care. 2014;31(5):562–75. doi: 10.1177/1049909113494748. [DOI] [PubMed] [Google Scholar]
- 39.Fisk JD, Pontefract A, Ritvo PG, Archibald CJ, Murray TJ. The impact of fatigue on patients with multiple sclerosis. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1994;21(0317-1671; 1):9–14. [PubMed] [Google Scholar]
- 40.Griffiths RD, Palmer TE, Helliwell T, MacLennan P, MacMillan RR. Effect of passive stretching on the wasting of muscle in the critically ill. Nutrition. 1995;11(5):428–32. [PubMed] [Google Scholar]
- 41.Hanken K, Eling P, Hildebrandt H. The representation of inflammatory signals in the brain - a model for subjective fatigue in multiple sclerosis. Frontiers in neurology. 2014;5:264. doi: 10.3389/fneur.2014.00264. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hardin BJ, Campbell KS, Smith JD, et al. TNF-alpha acts via TNFR1 and muscle-derived oxidants to depress myofibrillar force in murine skeletal muscle. J Appl Physiol (1985) 2008;104(3):694–9. doi: 10.1152/japplphysiol.00898.2007. [DOI] [PubMed] [Google Scholar]
- 43.Helliwell PS, Jackson S. Relationship between weakness and muscle wasting in rheumatoid arthritis. Ann Rheum Dis. 1994;53(11):726–8. doi: 10.1136/ard.53.11.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19:274. doi: 10.1186/s13054-015-0993-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermans G, Wilmer A, Meersseman W, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–9. doi: 10.1164/rccm.200605-665OC. [DOI] [PubMed] [Google Scholar]
- 46.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 47.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 48.Johnston AJ, Murphy KT, Jenkinson L, et al. Targeting of Fn14 prevents cancer-induced cachexia and prolongs survival. Cell. 2015;162(6):1365–78. doi: 10.1016/j.cell.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 49.Klenner FR. Fatigue, normal and pathological, with special consideration of myasthenia gravis and multiple sclerosis. Southern medicine and surgery. 1949;111(9):273–7. [PubMed] [Google Scholar]
- 50.Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: proposal for a unified taxonomy. Neurology. 2013;80(4):409–16. doi: 10.1212/WNL.0b013e31827f07be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kress JP, Hall JB. ICU-acquired weakness and recovery from critical illness. N Engl J Med. 2014;370(17):1626–35. doi: 10.1056/NEJMra1209390. [DOI] [PubMed] [Google Scholar]
- 52.Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Archives of Neurology. 1988;45(0003-9942; 4):435–7. doi: 10.1001/archneur.1988.00520280085020. [DOI] [PubMed] [Google Scholar]
- 53.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 54.Levine S, Nguyen T, Kaiser LR, et al. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med. 2003;168(6):706–13. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- 55.Li X, Moody MR, Engel D, et al. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation. 2000;102(14):1690–6. doi: 10.1161/01.cir.102.14.1690. [DOI] [PubMed] [Google Scholar]
- 56.Li YP, Atkins CM, Sweatt JD, Reid MB. Mitochondria mediate tumor necrosis factor- alpha/NF-kappaB signaling in skeletal muscle myotubes. Antioxid Redox Signal. 1999;1(1):97–104. doi: 10.1089/ars.1999.1.1-97. [DOI] [PubMed] [Google Scholar]
- 57.Maes K, Testelmans D, Powers S, Decramer M, Gayan-Ramirez G. Leupeptin inhibits ventilator-induced diaphragm dysfunction in rats. Am J Respir Crit Care Med. 2007;175(11):1134–8. doi: 10.1164/rccm.200609-1342OC. [DOI] [PubMed] [Google Scholar]
- 58.McClung JM, Kavazis AN, Whidden MA, et al. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol. 2007;585(Pt 1):203–15. doi: 10.1113/jphysiol.2007.141119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McConville JF, Kress JP. Weaning patients from the ventilator. N Engl J Med. 2012;367(23):2233–9. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- 60.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94(23):12457–61. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morales MG, Abrigo J, Meneses C, et al. The Ang-(1-7)/Mas-1 axis attenuates the expression and signalling of TGF-beta1 induced by AngII in mouse skeletal muscle. Clin Sci (Lond) 2014;127(4):251–64. doi: 10.1042/CS20130585. [DOI] [PubMed] [Google Scholar]
- 62.Morris G, Berk M, Galecki P, Walder K, Maes M. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Molecular neurobiology. 2016;53(2):1195–1219. doi: 10.1007/s12035-015-9090-9. [DOI] [PubMed] [Google Scholar]
- 63.Moylan JS, Smith JD, Horrell EM, et al. Neutral sphingomyelinase-3 mediates TNF-stimulated oxidant activity in skeletal muscle. Redox Biol. 2014;2:910–20. doi: 10.1016/j.redox.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murphy KT, Allen AM, Chee A, Naim T, Lynch GS. Disruption of muscle renin angiotensin system in AT −/− 1a mice enhances muscle function despite reducing muscle mass but compromises repair after injury. Am J Physiol. 2012;303(3):R321–31. doi: 10.1152/ajpregu.00007.2012. [DOI] [PubMed] [Google Scholar]
- 65.Murphy KT, Chee A, Gleeson BG, et al. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am J Physiol. 2011;301:R716–R26. doi: 10.1152/ajpregu.00121.2011. [DOI] [PubMed] [Google Scholar]
- 66.Murphy KT, Chee A, Trieu J, Naim T, Lynch GS. Inhibition of the renin-angiotensin system improves physiological outcomes in mice with mild or severe cancer cachexia. International Journal of Cancer. 2013;133(5):1234–46. doi: 10.1002/ijc.28128. [DOI] [PubMed] [Google Scholar]
- 67.Murphy KT, Koopman R, Naim T, et al. Antibody-directed myostatin inhibition in 21- mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J. 2010;24(11):4433–42. doi: 10.1096/fj.10-159608. [DOI] [PubMed] [Google Scholar]
- 68.Nelson WB, Smuder AJ, Hudson MB, Talbert EE, Powers SK. Cross-talk between the calpain and caspase-3 proteolytic systems in the diaphragm during prolonged mechanical ventilation. Crit Care Med. 2012;40(6):1857–63. doi: 10.1097/CCM.0b013e318246bb5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Norden DM, Bicer S, Clark Y, et al. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav Immun. 2015;43:76–85. doi: 10.1016/j.bbi.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parry SM, Berney S, Granger CL, Koopman R, El-Ansary D, Denehy L. Electrical muscle stimulation in the intensive care setting: a systematic review. Crit Care Med. 2013;41(10):2406–18. doi: 10.1097/CCM.0b013e3182923642. [DOI] [PubMed] [Google Scholar]
- 71.Powers SK, Hudson MB, Nelson WB, et al. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med. 2011;39(7):1749–59. doi: 10.1097/CCM.0b013e3182190b62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288(2):R337–44. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- 73.Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med. 2009;37(10 Suppl):S347–53. doi: 10.1097/CCM.0b013e3181b6e760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powers SK, Kavazis AN, McClung JM. Oxidative stress and disuse muscle atrophy. J Appl Physiol. 2007;102(6):2389–97. doi: 10.1152/japplphysiol.01202.2006. [DOI] [PubMed] [Google Scholar]
- 75.Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal. 2011;15(9):2519–28. doi: 10.1089/ars.2011.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol. 2013;305(5):R464–77. doi: 10.1152/ajpregu.00231.2013. [DOI] [PubMed] [Google Scholar]
- 77.Puppa MJ, Murphy EA, Fayad R, Hand GA, Carson JA. Cachectic skeletal muscle response to a novel bout of low-frequency stimulation. J Appl Physiol (1985) 2014;116(8):1078–87. doi: 10.1152/japplphysiol.01270.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310(15):1591–600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 79.Reid MB, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med. 2002;166(4):479–84. doi: 10.1164/rccm.2202005. [DOI] [PubMed] [Google Scholar]
- 80.Rezk BM, Yoshida T, Semprun-Prieto L, Higashi Y, Sukhanov S, Delafontaine P. Angiotensin II infusion induces marked diaphragmatic skeletal muscle atrophy. PLoS One. 2012;7(1):e30276. doi: 10.1371/journal.pone.0030276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ryall JG, Plant DR, Gregorevic P, Sillence MN, Lynch GS. b2-agonist administration reverses muscle wasting and improves muscle function in aged rats. J Physiol. 2004;555(Pt 1):175–88. doi: 10.1113/jphysiol.2003.056770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shanely RA, Van Gammeren D, Deruisseau KC, et al. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med. 2004;170(9):994–9. doi: 10.1164/rccm.200304-575OC. [DOI] [PubMed] [Google Scholar]
- 83.Smith IJ, Godinez GL, Singh BK, et al. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J. 2014;28(7):2790–803. doi: 10.1096/fj.13-244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Smuder AJ, Nelson WB, Hudson MB, Kavazis AN, Powers SK. Inhibition of the ubiquitin-proteasome pathway does not protect against ventilator-induced accelerated proteolysis or atrophy in the diaphragm. Anesthesiology. 2014;121(1):115–26. doi: 10.1097/ALN.0000000000000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stasko SA, Hardin BJ, Smith JD, Moylan JS, Reid MB. TNF signals via neuronal-type nitric oxide synthase and reactive oxygen species to depress specific force of skeletal muscle. J Appl Physiol (1985) 2013;114(11):1629–36. doi: 10.1152/japplphysiol.00871.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steens A, de Vries A, Hemmen J, et al. Fatigue perceived by multiple sclerosis patients is associated with muscle fatigue. Neurorehabilitation and neural repair. 2012;26(1):48–57. doi: 10.1177/1545968311416991. [DOI] [PubMed] [Google Scholar]
- 87.Stewart GD, Skipworth RJ, Fearon KC. Cancer cachexia and fatigue. Clin Med. 2006;6(2):140–3. doi: 10.7861/clinmedicine.6-2-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Supinski G, Stofan D, Callahan LA, Nethery D, Nosek TM, DiMarco A. Peroxynitrite induces contractile dysfunction and lipid peroxidation in the diaphragm. J Appl Physiol (1985) 1999;87(2):783–91. doi: 10.1152/jappl.1999.87.2.783. [DOI] [PubMed] [Google Scholar]
- 89.Szentesi P, Bekedam MA, van Beek-Harmsen BJ, et al. Depression of force production and ATPase activity in different types of human skeletal muscle fibers from patients with chronic heart failure. J Appl Physiol (1985) 2005;99(6):2189–95. doi: 10.1152/japplphysiol.00542.2005. [DOI] [PubMed] [Google Scholar]
- 90.Tajrishi MM, Zheng TS, Burkly LC, Kumar A. The TWEAK-Fn14 pathway: A potent regulator of skeletal muscle biology in health and disease. Cytokine Growth Factor Rev. 2014;25(2):215–25. doi: 10.1016/j.cytogfr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van den Berghe G, Schoonheydt K, Becx P, Bruyninckx F, Wouters PJ. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8):1348–53. doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 92.Vassilakopoulos T, Petrof BJ. Ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004;169(3):336–41. doi: 10.1164/rccm.200304-489CP. [DOI] [PubMed] [Google Scholar]
- 93.Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol. 2010;108(5):1376–82. doi: 10.1152/japplphysiol.00098.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wilcox PG, Wakai Y, Walley KR, Cooper DJ, Road J. Tumor necrosis factor alpha decreases in vivo diaphragm contractility in dogs. Am J Respir Crit Care Med. 1994;150(5 Pt 1):1368–73. doi: 10.1164/ajrccm.150.5.7952566. [DOI] [PubMed] [Google Scholar]
- 95.Wolkorte R, Heersema DJ, Zijdewind I. Muscle Fatigability During a Sustained Index Finger Abduction and Depression Scores Are Associated With Perceived Fatigue in Patients With Relapsing-Remitting Multiple Sclerosis. Neurorehabilitation and neural repair. 2015;29(8):796–802. doi: 10.1177/1545968314567151. [DOI] [PubMed] [Google Scholar]
- 96.Wolkorte R, Heersema DJ, Zijdewind I. Reduced Dual-Task Performance in MS Patients Is Further Decreased by Muscle Fatigue. Neurorehabilitation and neural repair. 2015;29(5):424–35. doi: 10.1177/1545968314552529. [DOI] [PubMed] [Google Scholar]
- 97.Yang M, Kim J, Kim JS, et al. Hippocampal dysfunctions in tumor-bearing mice. Brain Behav Immun. 2014;36:147–55. doi: 10.1016/j.bbi.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 98.Zhou X, Wang JL, Lu J, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–43. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]