Abstract
Background and Aims
Increased skeletal muscle ammonia uptake with loss of muscle mass adversely affects clinical outcomes in cirrhosis. Hyperammonemia causes reduced protein synthesis and sarcopenia but the cellular responses to impaired proteostasis and molecular mechanism of L-leucine induced adaptation to ammonia-induced stress were determined.
Methods
Response to activation of amino acid deficiency sensor, GCN2, in the skeletal muscle from cirrhotic patients and the portacaval anastomosis (PCA) rat were quantified. During hyperammonemia and L-leucine supplementation, protein synthesis, phosphorylation of eIF2α, mTORC1 signaling, L-leucine transport and response to L-leucine supplementation were quantified. Adaptation to cellular stress via ATF4 and its target GADD34 were also determined.
Results
Activation of the eIF2α kinase GCN2 and impaired mTORC1 signaling were observed in skeletal muscle from cirrhotic patients and PCA rats. Ammonia activated GCN2 mediated eIF2α phosphorylation (eIF2α–P) and impaired mTORC1 signaling that inhibit protein synthesis in myotubes and MEFs. Adaptation to ammonia-induced stress did not involve translational reprogramming by activation transcription factor 4 (ATF4) dependent induction of the eIF2α-P phosphatase subunit GADD34. Instead, ammonia increased expression of the leucine/glutamine exchanger SLC7A5, L-leucine uptake and intracellular L-leucine levels, the latter not being sufficient to rescue the inhibition of protein synthesis, due to potentially enhanced mitochondrial sequestration of L-leucine. L-leucine supplementation rescued protein synthesis inhibition caused by hyperammonemia.
Conclusions
Response to hyperammonemia is reminiscent of the cellular response to amino acid starvation, but lacks the adaptive ATF4 dependent integrated stress response (ISR). Instead, hyperammonemia-induced L-leucine uptake was an adaptive response to the GCN2-mediated decreased protein synthesis.
Keywords: amino acid transporter, solute linked carrier, General control non derepressible, eukaryotic initiation factor, mTOR1, sarcopenia
Graphical abstract
Introduction
The high clinical significance of sarcopenia in cirrhosis is well recognized and ammonia is a mediator of the liver muscle axis [1-4]. Impaired ureagenesis contributes to hyperammonemia in liver disease and the skeletal muscle is an alternate organ for ammonia disposal in cirrhosis [5]. Ammonia is a cellular stressor that results in decreased muscle protein synthesis but the underlying molecular mechanisms are not known [6]. Complex signaling pathways are activated in mammalian cells during hyperammonemia that contribute to impaired protein synthesis [3]. We previously reported increased phosphorylation and activation of general control nondepressible 2 (GCN2), an amino acid deficiency sensor, in the skeletal muscle in patients with alcoholic cirrhosis [7]. Activation of GCN2 results in reversible serine51 phosphorylation of the subunit of the eukaryotic initiation factor 2 (eIF2α), that results in global repression of mRNA translation and thus protein synthesis [8]. The potential mechanisms and consequences of GCN2 activation during hyperammonemia and cirrhosis are however, not known. The well recognized decreased plasma concentration of branched chain amino acids (BCAA) in cirrhosis is a potential mechanism of activation of GCN2 [9, 10]. However, a number of human studies on BCAA supplementation have not improved nutritional status or clinical outcomes of cirrhosis but the molecular responses to amino acid supplementation were not evaluated in these studies [11]. Of the BCAA, L-leucine is unique because in addition to activating mTORC1, its metabolic functions include being a substrate for oxidative degradation and for glutamate/glutamine synthesis for non-hepatic ammonia disposal [7, 12, 13]. Despite these beneficial effects of L-leucine, the lack of clinical efficacy of BCAA supplementation in improving nutritional status in cirrhosis may be related to decreased skeletal muscle uptake or increased metabolic demand so that higher doses of L-leucine are required for functional responses during hyperammonemia and cirrhosis.
Following phosphorylation of eIF2α, an adaptive integrated stress response (ISR) is mediated via upregulation of activating transcription factor 4 (ATF4) that results in translational recovery by protein phosphatase 1 (PP1) mediated dephosphorylation of phospho-eIF2α [8, 14, 15]. Activation of PP1 phosphatase requires the growth arrest and DNA damage- inducible protein 34 (GADD34/ Ppp1r15a) during cellular stress[8, 14]. Skeletal muscle cellular stress in cirrhosis and hyperammonemia are mediated by GCN2 and its target, eIF2α, but whether an ISR is activated and if L-leucine supplementation promotes skeletal muscle cellular translational recovery during hyperammonemia is not known.
In the present studies, we identified the skeletal muscle protein synthesis regulatory network and the mechanism(s) by which ammonia impairs skeletal muscle protein synthesis in patients with cirrhosis and in a comprehensive array of models of hyperammonemia. We also examined how L-leucine drives the cellular adaptation responses during hyperammonemia.
Methods
Details of the models and methods are described in the supplementary section. In brief, skeletal muscle from patients with cirrhosis and control subjects (Supplementary Table 1) and a separate cohort of cirrhotic patients and control subjects treated with an L-leucine enriched branched chain amino acid mixture (details published earlier [7]), gastrocnemius muscle from the hyperammonemic PCA rat and murine myotubes during hyperammonemia were studied using methods described by us earlier [3, 4, 16]. Additional studies were done in mouse embryonic fibroblasts from eIF2 kinase knockout mice and murine C2C12 myotubes with stable knockdown of GCN2 with shRNA. Ammonium acetate was used at 10mM concentration because this results in cellular concentrations similar to that in human cirrhosis and the hyperammonemic PCA rat [3, 17]. Primers for real time PCR are shown in Supplementary Table 2.
Results
Hyperammonemia inhibits protein synthesis
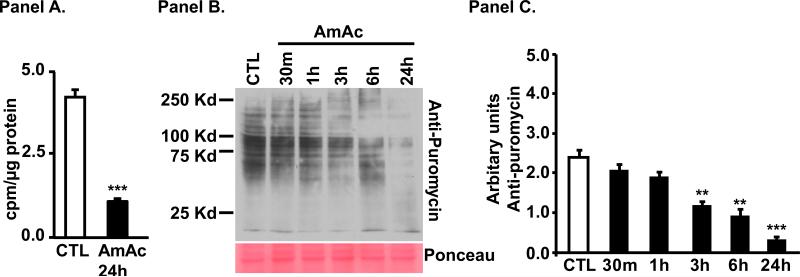
Following 24h of hyperammonemia, protein synthesis measured by incorporation of 3H-phenylalanine was significantly lower in murine myotubes (Fig. 1, Panel A). This was reproduced in the studies using puromycin incorporation that showed significantly and progressively lower protein synthesis after 3 h of hyperammonemia (Fig. 1, Panel B, C).
Fig. 1. Hyperammonemia impairs protein synthesis in myotubes.
Panel A. 3H phenylalanine incorporation was significantly lower in C2C12 murine myotubes treated with 10mM ammonium acetate for 24 h. Panel B. Representative immunoblots of protein synthesis quantified by puromycin incorporation showed reduced protein synthesis after 3 h exposure to ammonium acetate. Panel C. Densitometry of immunoblots of puromycin incorporation including all bands in the lane quantified. N=3 independent experiments. * p<0.05; ** p<0.01; *** p<0.001 vs. control (CTL).
Ammonia activates eIF2α kinase, GCN2 and impairs mTORC1 signaling in skeletal muscle
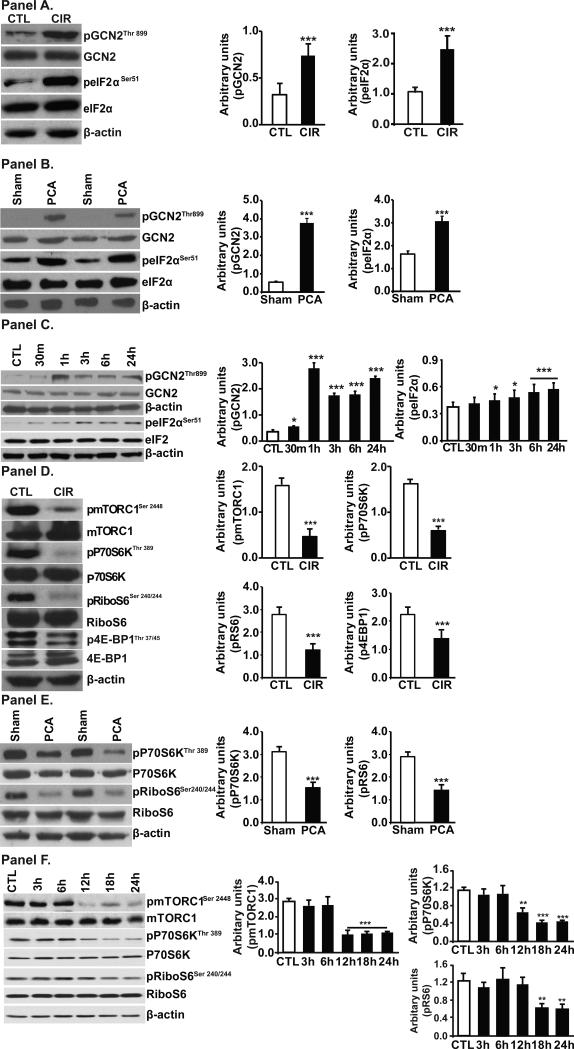
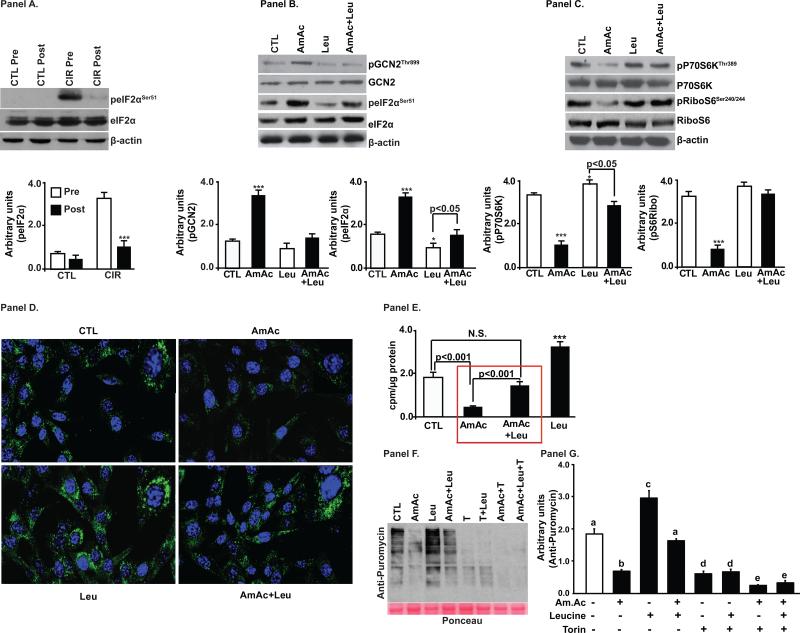
All patients with cirrhosis had increased plasma and skeletal muscle ammonia concentrations (Supplementary Table 1). GCN2 was activated and its target protein eIF2α was phosphorylated in the skeletal muscle of patients with cirrhosis compared to controls (Fig. 2, Panel A, Supplementary Fig. 1, Panel A), in the hyperammonemic PCA rat compared with the sham controls (Fig. 2, Panel B) and in murine myotubes treated with ammonium acetate (Fig. 2, Panels C). All patients with cirrhosis had elevated plasma ammonia and increased expression of phosphorylated GCN2. In addition to activation of the GCN2/eIF2α pathway, mTORC1 signaling was impaired in the skeletal muscle of human cirrhosis (Fig. 2, Panel D, Supplementary Fig. 1, Panels B, C, D), the hyperammonemic PCA rat (Fig. 2, Panel E) and C2C12 myotubes (Fig. 2 Panel F).
Fig. 2. Ammonia activates, amino acid sensor general control non-derepressible 2 (GCN2) and impairs mTORC1 signaling.
Panels A-C. Representative immunoblots and densitometry showed increased phosphorylated GCN2 and its target, eIF2α in skeletal muscle. Panels D-F. Representative immunoblots and densitometry showed ammonia impaired mTORC1 phosphorylation and signaling in skeletal muscle. Panel A and D. Patients with cirrhosis compared to controls (n=6 each). Panel B and E. PCA compared with sham operated, pair fed rats (n=5 each). Panel C and F. C2C12 myotubes during hyperammonemia (n=3 independent experiments). * p<0.05; ** p<0.01; *** p<0.001 vs. controls
Increased eIF2α phosphorylation did not trigger ISR
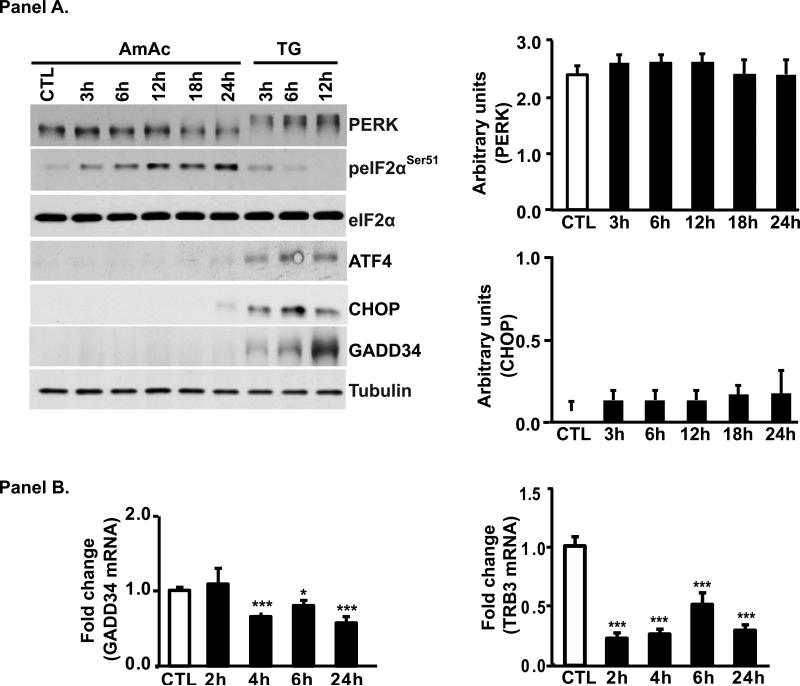
Inhibition of protein synthesis and activation of GCN2 with subsequent phosphorylation of eIF2α resemble the cellular response to amino acid starvation. However, the persistently high levels of phosphorylated eIF2α and decreased protein synthesis suggest impairment of the adaptive ISR response triggered by ATF4. Indeed ATF4 protein was not induced in myotubes treated with ammonia (Fig. 3, Panel A) but myotubes are able to translate ATF4 mRNA in response to ER stress inducer, Thapsigargin (TG), which activates another eIF2α kinase, PERK that is observed as a shift of protein band in SDS/PAGE (Fig. 3, Panel A). Electrophoresis shift of PERK protein by TG does represent activation was confirmed by reversal of the shift in response to the kinase inhibitor (GSK2656157) (Supplemental Fig. 2) that was similar to reports by others[18, 19]. These experiments show that failure to induce ATF4 during hyperammonemia is a specific ammonia-mediated effect. The unexpected lack of ATF4 induction during hyperammonemia was confirmed by the negligible induction of its target genes, CHOP. Consistent with the induction of ATF4 by TG, there was robust induction of CHOP in TG treated myotubes (Fig. 3, Panel A). Expression of TRB3 mRNA, another ATF4 target was also lower in ammonia-treated myotubes (Fig. 3, panel B). These data show that during hyperammonemia, there is defective ISR with lack of ATF4 induction. ISR downstream of ATF4 involves dephosphorylation of eIF2a and recovery of protein synthesis that depends on GADD34 phosphatase subunit [14]. Ammonia resulted in low expression of mRNA and protein expression of GADD34 while TG induced a robust expression of GADD34 protein (Fig. 3, Panels A and B). These data show that myotubes can mount an ISR to other stressors like TG and show that inability to mount an ISR is responsible for failure to reverse eIF2a phosphorylation and persistently low protein synthesis in myotubes during hyperammonemia. Impaired ATF4 program is a major difference in the cellular response to classical amino acid starvation and exposure to ammonia induced cellular response.
Fig. 3. Ammonia induced molecular response in myotubes.
Panel A. Representative immunoblots and densitometry of components of the stress response component GCN2 in C2C12 myotubes exposed to 10mM ammonium acetate or thapsigargin (TG) as positive control. PERK mobility shift with TG but not ammonium acetate. Persistent phosphorylation of eIF2α but target genes ATF4, CHOP, eIF2α phosphatase, GADD34 were not activated during hyperammonemia. Panel B. Real time PCR showed lower expression of GADD34 and TRB3 mRNA in C2C12 myotubes treated with 10 mM ammonium acetate for different times. * p<0.05; *** p<0.001 compared to untreated control cells.
Inhibition of protein synthesis by hyperammonemia is reversed by L-leucine
Amino acid starvation is the classical activator of GCN2 and phosphorylation of its downstream target eIF2α [20]. Consistent with our report that branched chain amino acid mixture supplemented with L-leucine reverses GCN2 activation in human cirrhosis [7], we observed in skeletal muscle that eIF2α phosphorylation was reversed by BCAA/LEU (Fig. 4, Panel A). Studies in murine myotubes demonstrated that L-leucine supplementation of the medium reversed hyperammonemia induced autophosphorylation/activation of GCN2 and phosphorylation of its target, eIF2α (Fig. 4, Panel B). L-leucine also reversed hyperammonemia-induced inhibition of mTORC1 signaling as observed by increased phosphorylation of P70S6K and ribosomal S6 protein (Fig. 4, Panel C). Immunofluorescence studies in C2C12 myotubes showed that hyperammonemia altered the condensed perinuclear localization of phospho-mTORC1 (Ser2448) to a diffuse cytoplasmic distribution. Supplementation of L-leucine during hyperammonemia prevented these changes in expression and distribution of mTORC1 (Fig. 4, panel D). We complemented these molecular signaling responses to L-leucine with functional studies on muscle protein synthesis during hyperammonemia. Incorporation of 3H-phenylalanine in myotubes showed that hyperammonemia-induced inhibition of protein synthesis was reversed by L-leucine (Fig. 4, Panel E) and the response was dependent on L-leucine concentration (Supplementary Fig. 3, Panels A,B). These experiments showed that L-leucine supplementation reversed ammonia induced inhibition of protein synthesis inhibition by regulating both mTORC1 and GCN2-eIF2α mediated mechanisms. To further dissect the molecular mechanism by which L-leucine rescues impaired protein synthesis during hyperammonemia, torin, an active site mTORC1 inhibitor was used. Torin dose titration showed that 100-400nM concentrations were effective in decreasing protein synthesis by impairing mTORC1 signaling without altering eIF2 α phosphorylation (Supplementary Fig. 4, Panels A,B). Consistent with our earlier observations (Fig. 4, Panel E), L-leucine supplementation in myotubes during hyperammonemia rescued ammonia induced impaired protein synthesis but this effect was significantly decreased by torin (Fig. 4, Panels F,G) and shows that mTORC1 signaling is the critical target for ammonia induced impaired protein synthesis.
Fig. 4. Decreased protein synthesis and impaired mTORC1 signaling during hyperammonemia are reversed by L-leucine.
Panel A. Increased phosphorylated eIF2α in the skeletal muscle of cirrhotics compared to controls (n=6 each) reversed by L-leucine enriched branched chain amino acid supplementation. Panel B. Increased phosphorylated GCN2 and its target, eIF2α in murine C2C12 myotubes during hyperammonemia reversed by L-Leucine. Panel C. Ammonia induced impaired mTOR signaling in C2C12 myotubes reversed by L-leucine. Panel D. Lower expression and diffuse cytoplasmic distribution of phospho-mTORC1 during hyperammonemia reversed by L-leucine. Panel E. L-leucine rescued hyperammonemia mediated impaired protein synthesis quantified by incorporation of 3H-phenylalanine in C2C12 myotubes. Panel F. Global protein synthesis in myotubes treated with ammonium acetate (AmAc), L-leucine (Leu) and torin. a-b,c p<0.01; b-c p<0.001; a,c-d p<0.001; b-d p<0.01.
Cross talk between GCN2 eIF2a kinase and mTORC1 during hyperammonemia
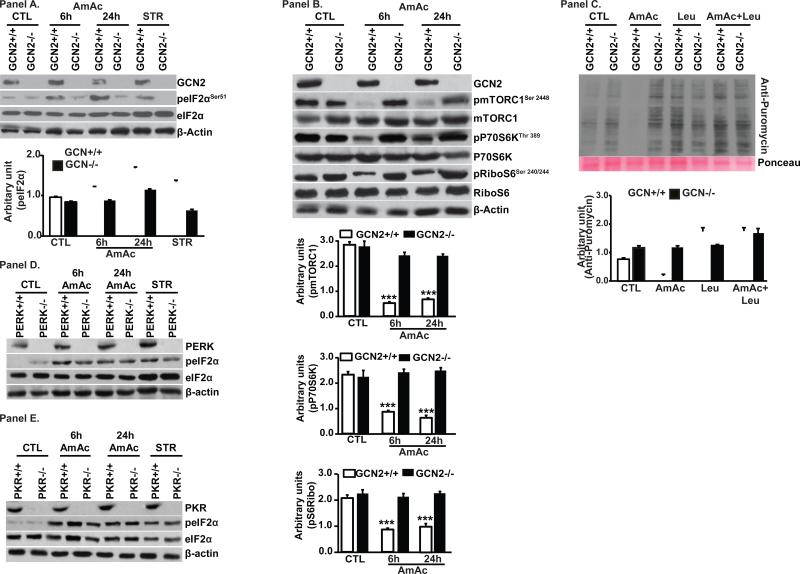
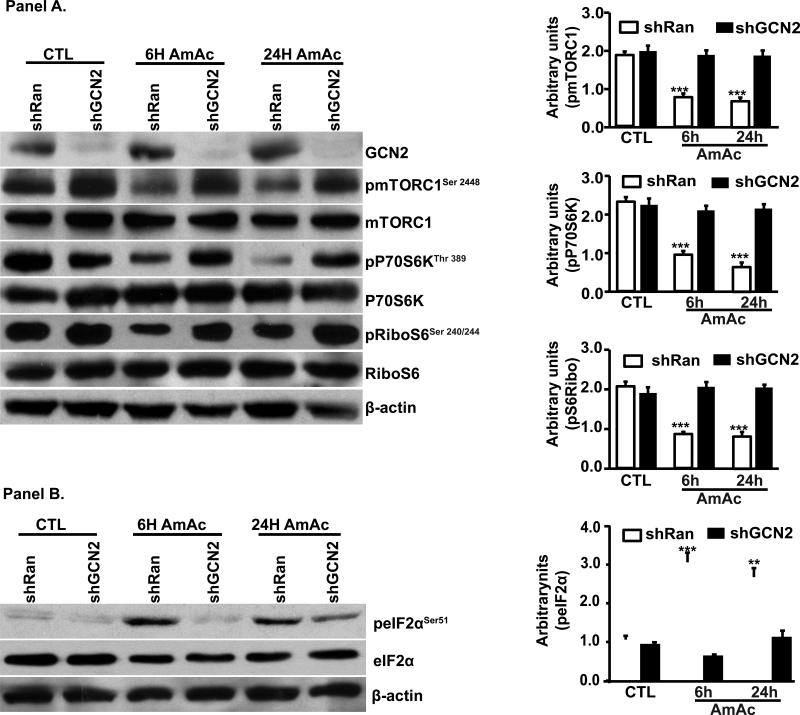
Since L-leucine reversed impaired protein synthesis during hyperammonemia by altering both GCN2/eIF2α and mTORC1 signaling response, we tested the crosstalk between these two pathways. We confirmed that GCN2 was the specific eIF2α kinase during hyperammonemia because ammonia treatment did not induce eIF2α phosphorylation in GCN2 deficient MEFs. Amino acid withdrawal was used as a positive control for GCN2 activation (Fig. 5, Panel A). Depletion of GCN2 also reversed ammonia-induced inhibition of mTORC1 signaling (Fig. 5, Panel B). Next we tested if the mechanism of ammonia induced GCN2 impairs protein synthesis rates, and indeed hyperammonemia decreased protein synthesis in wild type but not in GCN2 deficient MEFs (Fig. 5, Panel C). Additionally, L-leucine reversed impaired protein synthesis during hyperammonemia only wild type but not GCN2 deficient MEFs showing that the beneficial effects of L-leucine during hyperammonemia are mediated via GCN2 (Figure 5, Panel C). We then showed that ammonia mediated phosphorylation of eIF2α is dependent only on GCN2 kinase by treating MEFs deficient in PERK or PKR kinase. Absence of PERK or PKR did not reverse ammonia induced increased eIF2α phosphorylation (Fig. 5 D, E). These data led us to conclude that ammonia-induced inhibition of protein synthesis is not restricted to myotubes. Finally, we confirmed by silencing GCN2 in myotubes that ammonia induced impaired protein synthesis was GCN2 dependent. As expected, depleting GCN2 resulted in decreased phosphorylation of eIF2α and rescue of impaired mTORC1 signaling during hyperammonemia (Fig. 6, Panels A, B). These data allowed us to conclude that GCN2 kinase is the only known eIF2α kinase activated in response to hyperammonemia. Our observations also showed that in the 2 cellular models of MEFs and C2C12 myotubes, GCN2 depletion rescued mTORC1 signaling inhibited by hyperammonemia.
Fig. 5. Ammonia phosphorylates global protein repressor, eIF2α via GCN2.
Panel A. Immunoblots and densitometry from mouse embryonic fibroblasts (MEF) from GCN2−/− and GCN2+/+ exposed to 10mM ammonium acetate. Starvation (STR) with Krebs ringer bicarbonate buffer served as a positive control. * p<0.05, ** p<0.01 and *** p<0.001 compared to untreated GCN+/+ MEFs (CTL). Panel B. GCN2 deletion reverses ammonia mediated mTORC1 signaling. Panel C. Immunoblots and densitometry of puromycin incorporation as a measure of global protein synthesis in MEFs from GCN2−/− and GCN2+/+ mice exposed to 10mM ammonium acetate and L-leucine (n=3 independent experiments).*** p<0.001 vs. other groups.
Fig. 6. Impaired mTORC1 signaling during hyperammonemia rescued by GCN deletion in myotubes.
Panel A. Myotubes with stable deletion of GCN2 lower phosphorylation and activation of mTORC1 with decreased phosphorylated P70S6 kinase and ribosomal S6 protein. Panel B. Stable deletion of GCN2 blocked ammonia induced eIF2α phosphorylation.
Our studies also show that L-leucine decreased ammonia-induced phosphorylation of GCN2-eIF2α and rescued mTORC1 signaling. Silencing GCN2 blocked ammonia induced mTORC1 inhibition and decreased protein synthesis. L-leucine induced reversal of protein synthesis was blocked by inhibiting mTORC1 demonstrating the importance of mTORC1 in regulating protein synthesis during hyperammonemia.
Hyperammonemia induces the leucine exchanger SLC7A5
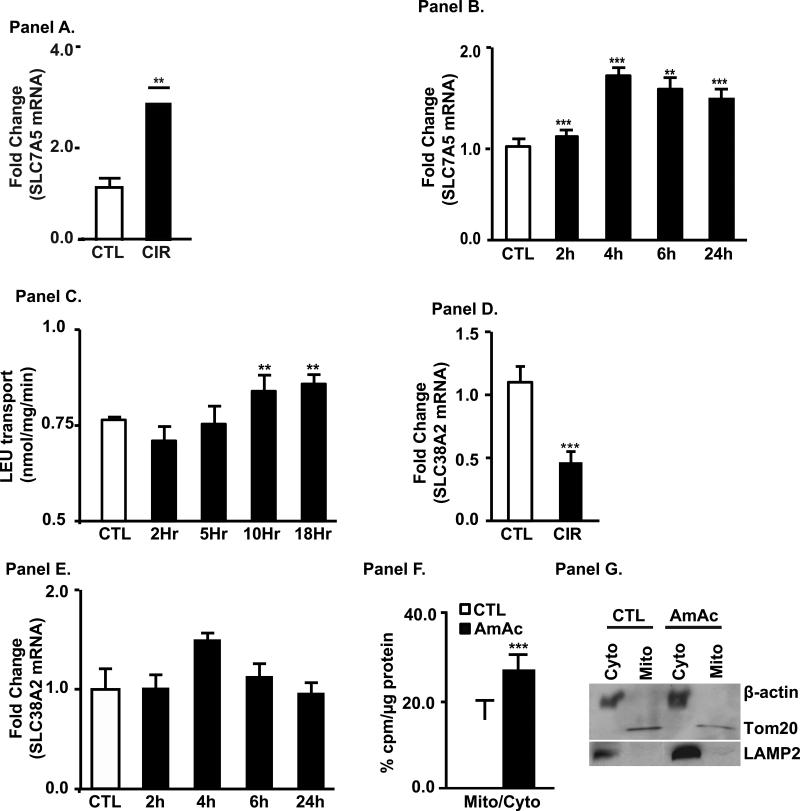
Data in this study and our previous report show that high doses of L-leucine are required to reverse ammonia induced inhibition of skeletal muscle protein synthesis and signaling perturbations[7]. Response to high dose L-leucine suggests that leucine uptake can be a limiting factor restricting the effectiveness of this treatment. ISR program induced in response to amino acid starvation includes numerous amino acid transporters and genes involved in amino acid biosynthesis [21, 22] including coordinated expression of functionally coupled transporters SLC7A5 and SLC 38A2 [23, 24]. However, hyperammonemic response lacks the ISR element that would therefore limit L-leucine supplementation as a therapeutic intervention. We therefore, tested expression of primary high-affinity L-leucine transporter SLC7A5/LAT1 in muscles of cirrhotic patients as well as in myotubes treated with ammonia and noted higher expression of mRNA than in corresponding control (Fig. 7, Panels A,B). Induction of mRNA was reflected in increased L-leucine uptake by SLC7A5/LAT1 (Fig. 7, Panel C). SLC7A5 acts as amino acid exchanger and BCAA uptake depends on efflux of other amino acids, and it is generally accepted that glutamine is a driver of BCAA uptake. We then tested if the major concentrative transporter of glutamine and other small neutral amino acids in myotubes was regulated by ammonia. We observed decreased or no induction in muscle biopsies from cirrhotic patients and myotubes treated with ammonia in vitro (Fig. 7, Panels D, E). These data showed that concentrative uptake of glutamine by SLC38A2/SNAT2, serving as an efflux substrate for SLC7A5, cannot account for the increased uptake of L-leucine. We evaluated changes in intracellular amino acid content in response to hyperammonemia, as expected glutamine levels were higher in stressed cells confirming that increased glutamine synthesis as a way to dispose ammonia (Supplementary Table 3). To our surprise intracellular L-leucine levels in stressed cells were higher than in untreated cells that raised question of how GCN2 is activated during hyperammonemia and how L-leucine reverses phosphorylation of GCN2-eIF2α. As GCN2 is activated in cytoplasm by uncharged tRNAs, we hypothesized that L-leucine is sequestered in a different compartment resulting in cytoplasmic deficiency. In cirrhosis and hyperammonemic states, there is greater oxidation of L-leucine to generate acetyl-CoA in the mitochondria. We therefore tested the mitochondrial uptake of L-leucine in ammonia treated myotubes. Fractionated mitochondria from ammonia treated cells had higher accumulation of labeled L-leucine as compared to untreated cells (Fig. 7, Panel F). Increased L-leucine uptake into the mitochondria, suggest an additional mechanism of the control of cytoplasmic levels of this amino acid.
Fig. 7. Increased expression and transport of leucine transporter, SLC7A5 (LAT1), in cirrhosis and hyperammonemia.
Panel A. Increased expression of SLC7A5 (LAT1) mRNA in skeletal muscle from cirrhosis vs. controls (n=6 in each group). Panel B. Increased expression of LAT1 mRNA in C2C12 cells during hyperammonemia. Panel C. Increased L-leucine transport into myotubes during hyperammonemia. Panel D. Decreased expression of SLC38A2 (SNAT2) mRNA in skeletal muscle from cirrhosis vs. controls (n=6 in each group). Panel E. Unaltered expression of SNAT2 during hyperammonemia in myotubes. Panel F. Mitochondrial uptake of 3H L-leucine was increased during hyperammonemia. Panel G. Mitochondrial (Tom20), lysosomal marker (LAMP2) and cytoplasmic markers (β-actin) showed pure mitochondrial preparations. ** p<0.01; *** p<0.001 compared to controls.
Discussion
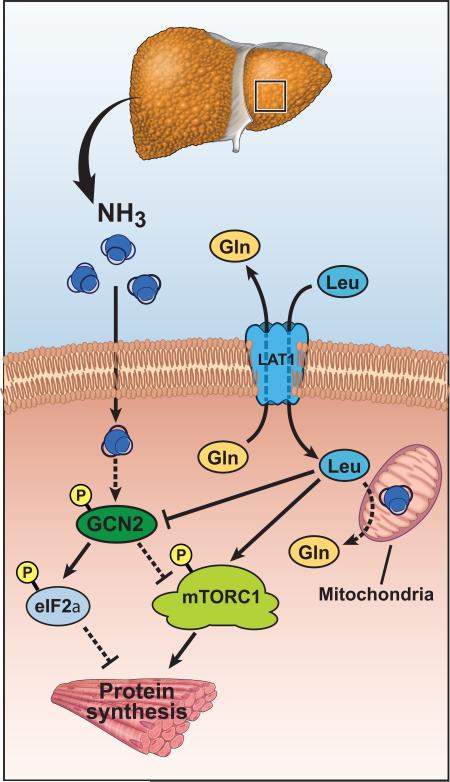
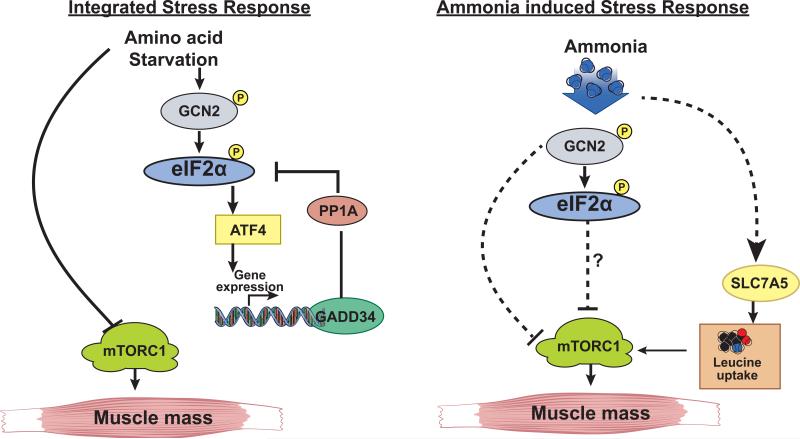
The present study demonstrates for the first time that skeletal muscle hyperammonemia induces a cellular response that resembles some features of amino acid starvation response without an integrated stress response. Of the eIF2α kinases, only GCN2 was activated by ammonia with phosphorylation of eIF2α and impaired muscle protein synthesis. Classical ISR response, with induction of ATF4 and GADD34 following eIF2α phosphorylation [8, 25], was not observed during hyperammonemia induced GCN2 activation. Both eIF2α phosphorylation and impaired mTORC1 signaling contributed to inhibition of protein synthesis and were reversed by L-leucine supplementation. Hyperammonemia resulted in increased expression of leucine transporter SLC7A5 and higher cellular concentrations of L-leucine in myotubes (Fig. 8).
Fig. 8. Schematic of hyperammonemic stress response.
Panel A. Amino acid deprivation or nutrient starvation activates GCN2 that in turn phosphorylates eIF2α. An adaptive response (integrated stress response) is initiated via ATF4 that upregulates a number of genes, including GADD34 to dephosphorylate and eIF2α to allow translational recovery. Panel B. Ammonia activates GCN2 and phosphorylates eIF2α resulting in impaired protein synthesis and increased transport of leucine. The adaptive response mediated by ATF4 and its targets including GADD34 that dephosphorylates peIF2α are however not activated with persistent phosphorylation of eIF2α and persistent impairment of protein synthesis.
Protein synthesis is critical for maintenance of skeletal muscle mass and the present studies in human subjects as well as rat and myotubes models show that hyperammonemia is a mediator of the liver-muscle axis. In skeletal muscle of patients with cirrhosis and the PCA rat, hyperammonemia is a consistent abnormality and results in a unique cellular stress response. Loss of the feedback negative loop of ISR during hyperammonemia was evidenced by failure to induce ATF4 protein levels and its target genes TRB3 and CHOP mRNA transcription. This is in contrast to the classical ISR response that was observed with thapsigargin that reversibly phosphorylated eIF2α with increased expression of ATF4 and its target genes [8, 14, 18, 22, 24, 26-28]. Activation of ATF4 is downstream of a number of signaling pathways and involves translational regulation, ATF4 protein stability as well as transcriptional component [8, 18, 25, 29] but the exact molecular regulation of ATF4 during hyperammonemia needs to be identified. The mechanism of skeletal muscle accumulation of ammonia has not been defined but may be related to the expression of ammonia transporters[30].
In addition to eIF2α phosphorylation, mTORC1 activation and signaling that result in translation of cap dependent mRNA and protein synthesis [31] was also inhibited by ammonia explaining the persistent suppression of protein suppression during hyperammonemia. Our highly novel observations in GCN−/− MEFs and myotubes with stable knockdown of GCN2 show that ammonia induced mTORC1 is reversed by silencing GCN2. These are consistent with recent data that GCN2 is a potential upstream inhibitor of mTORC1 signaling [32] and suggest that during hyperammonemia, activation of GCN2 impairs protein synthesis via eIF2α and mTORC1 dependent mechanisms. Others have reported that increased phosphorylation of eIF2a downregulates mTORC1 but this effect is mediated via ATF4 [20, 33]. Interestingly, lack of ATF4 induction during hyperammonemia suggests that GCN2/eIF2α dependent regulation of mTORC1 is mediated via ATF4-independent mechanism(s) without contribution of the adaptive ISR.
Our data that L-leucine supplementation reversed the molecular and functional perturbations due to hyperammonemia show that GCN2 activation and impaired protein synthesis are due to skeletal muscle L-leucine deficiency. These observations are, however, in contrast to previously published data that muscle concentrations of leucine are unaltered in cirrhosis [9, 10] and data from the current studies that L-leucine concentrations in myotubes were elevated during hyperammonemia. Thus, it would be necessary to reconcile the conflicting data of unaltered or elevated L-leucine concentrations and activation of GCN2 that is responsive to L-leucine supplementation. A potential explanation for such an observation is the compartmental localization of leucine. An increased metabolic demand for L-leucine has been reported in cirrhosis as an energy substrate to generate acetyl CoA as evidenced by increased leucine oxidation in cirrhosis [34]. This may be related to the impaired pyruvate dehydrogenase by ammonia that decreased acetyl CoA synthesis [35]. Another potential reason for the increased metabolic demand for leucine is the activation of the recently identified leucine-glutamate pathway [36] catalyzed by glutamate dehydrogenase that can detoxify ammonia [34]. Both leucine oxidation to generate acetyl-CoA and glutamate synthesis occur in the mitochondria[37, 38]. Our observation of increased mitochondrial transfer of L-leucine during hyperammonemia in the muscle provides support to the interpretation that L-leucine is compartmentalized to the mitochondria for metabolic adaptation. Supplementation with a high dose of L-leucine allows the metabolic demand in the mitochondria to be satisfied with resultant rescue of the molecular perturbations. Our data are consistent with previous reports that L-leucine stimulates skeletal muscle protein synthesis while providing novel mechanisms for the regulatory role of L-leucine during hyperammonemia[39].
Another novel component of these studies is identification of the cross talk between GCN2- eIF2α and mTORC1 during hyperammonemia. We observed that L-leucine reverses the GCN2- eIF2α phosphorylation as well as rescues the impaired mTORC1 signaling in the muscle during hyperammonemia. Our molecular dissection using silencing of GCN2 and specific mTORC1 inhibitor show that rescue of GCN2- eIF2α alone by L-leucine is not sufficient to reverse ammonia induced decreased protein synthesis and requires mTORC1 activation.
Finally, the molecular and functional effects of L-leucine are complemented by increased cellular uptake so that high dose L-leucine supplementation will be beneficial as noted in our studies. Our data show that 5mM leucine is beneficial during hyperammonemia and is in contrast to that reported earlier that doses above 2mM are not effective in increasing molecular responses in myotubes [40]. Response to a higher dose of L-leucine may be related to increased cellular and mitochondrial uptake as well as the high metabolic demand during hyperammonemia. Interestingly, unlike the physiological leucine transport that depends on glutamine transport via SLC38A2 [41], during hyperammonemia, cellular disposal of ammonia via glutamine precludes the need for transport of glutamine as evidenced by decreased or unaltered expression of SLC38A2. Our studies provide a mechanistic explanation for increased skeletal muscle L-leucine uptake during hyperammonemia via enhanced cellular glutamine. Our observations are also similar to that reported in cellular models overexpressing glutamine synthetase resulting in increased glutamine biosynthesis that resulted in increased uptake of L-leucine via SLC7A5/LAT1 [42].
Our comprehensive studies provide the molecular basis for sarcopenia in cirrhosis and demonstrate the mechanism of the novel cellular response to hyperammonemia. Activation of GCN2 with impaired ATF4 dependent stress response contributes to persistent eIF2α phosphorylation. Reversal of the hyperammonemic response and maladaptive molecular and functional responses by supplemental L-leucine provides the basis for rapid clinical translation of these studies. Our observations are also consistent with previous reports that sarcopenia worsens hepatic encephalopathy due, potentially, to decreased skeletal muscle ammonia disposal[43]. Even though hepatic encephalopathy is the most extensively studied consequence of hyperammonemia, our data provide evidence to the concept of ammonia being more than a neurotoxin[44]. The novel data in the present studies also lay the foundation for targeting ammonia disposal as a therapeutic strategy to reverse sarcopenia in cirrhosis.
Supplementary Material
Lay Summary.
Sarcopenia or skeletal muscle loss is the most frequent complication in cirrhosis but there are no treatments because the cause(s) of muscle loss in liver disease are not known. Results from laboratory experiments in animals, muscle cells were validated in human patients with cirrhosis to show that ammonia plays a key role in causing muscle loss in patients with cirrhosis. We identified a novel stress response to ammonia in the muscle that decreases muscle protein content that can be reversed by supplementation with the amino acid L-leucine.
Acknowledgements
We would like to thank Dr. Cynthia Tsien for assistance with the skeletal muscle samples in patients with cirrhosis who underwent liver transplantation and control subjects.
Supported in part by DK83414 and R21 AA 022742 (SD), DK53307 and DK60596 (MH) None of the authors have any conflict of interest with any entity except for being funded by the NIH.
List of Abbreviations
- αKG
alpha ketoglutarate
- BCAA/LEU
branched chain amino acids enriched with L-leucine
- CHOP
C/EBP homology protein
- eIF2α
eukaryotic initiation factor 2 alpha
- GADD34
growth arrest and DNA damage inducible protein 34
- GCN2
general control non depressible 2
- KRBB
Krebs Ringer bicarbonate buffer
- MEF
mouse embryonic fibroblasts
- mTORC1
mammalian target of rapamycin complex 1
- PCA
portacaval anastomosis
- PERK
PKR like eIF2α Kinase
- PKR
protein kinase RNA regulated
- SLC
solute linked carrier
- TG
thapsigargin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173. 173, e161. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, et al. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-kappaB-mediated mechanism. Proc Natl Acad Sci U S A. 2013;110:18162–18167. doi: 10.1073/pnas.1317049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, et al. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983–993. doi: 10.1152/ajpendo.00183.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatauret N, Desjardins P, Zwingmann C, Rose C, Rao KV, Butterworth RF. Direct molecular and spectroscopic evidence for increased ammonia removal capacity of skeletal muscle in acute liver failure. J Hepatol. 2006;44:1083–1088. doi: 10.1016/j.jhep.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Holecek M, Sprongl L, Tichy M. Effect of hyperammonemia on leucine and protein metabolism in rats. Metabolism. 2000;49:1330–1334. doi: 10.1053/meta.2000.9531. [DOI] [PubMed] [Google Scholar]
- 7.Tsien C, Davuluri G, Singh D, Allawy A, Ten Have GA, Thapaliya S, et al. Metabolic and molecular responses to leucine-enriched branched chain amino acid supplementation in the skeletal muscle of alcoholic cirrhosis. Hepatology. 2015;61:2018–2029. doi: 10.1002/hep.27717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 9.Montanari A, Simoni I, Vallisa D, Trifiro A, Colla R, Abbiati R, et al. Free amino acids in plasma and skeletal muscle of patients with liver cirrhosis. Hepatology. 1988;8:1034–1039. doi: 10.1002/hep.1840080509. [DOI] [PubMed] [Google Scholar]
- 10.Plauth M, Egberts EH, Abele R, Muller PH, Furst P. Characteristic pattern of free amino acids in plasma and skeletal muscle in stable hepatic cirrhosis. Hepatogastroenterology. 1990;37:135–139. [PubMed] [Google Scholar]
- 11.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan Y, Li F, Li Y, Tang Y, Kong X, Feng Z, et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids. 2015 doi: 10.1007/s00726-015-2067-1. [DOI] [PubMed] [Google Scholar]
- 13.Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, et al. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Novoa I, Zeng H, Harding HP, Ron D. Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol. 2001;153:1011–1022. doi: 10.1083/jcb.153.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 16.Dasarathy S, McCullough AJ, Muc S, Schneyer A, Bennett CD, Dodig M, et al. Sarcopenia associated with portosystemic shunting is reversed by follistatin. J Hepatol. 2011;54:915–921. doi: 10.1016/j.jhep.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDaniel J, Davuluri G, Hill EA, Moyer M, Runkana A, Prayson R, et al. Hyperammonemia results in reduced muscle function independent of muscle mass. Am J Physiol Gastrointest Liver Physiol. 2016;310:G163–170. doi: 10.1152/ajpgi.00322.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Starck SR, Tsai JC, Chen K, Shodiya M, Wang L, Yahiro K, et al. Translation from the 5' untranslated region shapes the integrated stress response. Science. 2016;351:aad3867. doi: 10.1126/science.aad3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang P, McGrath BC, Reinert J, Olsen DS, Lei L, Gill S, et al. The GCN2 eIF2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol Cell Biol. 2002;22:6681–6688. doi: 10.1128/MCB.22.19.6681-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J, Back SH, Hur J, Lin YH, Gildersleeve R, Shan J, et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 23.Evans K, Nasim Z, Brown J, Clapp E, Amin A, Yang B, et al. Inhibition of SNAT2 by metabolic acidosis enhances proteolysis in skeletal muscle. J Am Soc Nephrol. 2008;19:2119–2129. doi: 10.1681/ASN.2007101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krokowski D, Han J, Saikia M, Majumder M, Yuan CL, Guan BJ, et al. A self- defeating anabolic program leads to beta-cell apoptosis in endoplasmic reticulum stress-induced diabetes via regulation of amino acid flux. J Biol Chem. 2013;288:17202–17213. doi: 10.1074/jbc.M113.466920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dey S, Savant S, Teske BF, Hatzoglou M, Calkhoven CF, Wek RC. Transcriptional repression of ATF4 gene by CCAAT/enhancer-binding protein beta (C/EBPbeta) differentially regulates integrated stress response. J Biol Chem. 2012;287:21936–21949. doi: 10.1074/jbc.M112.351783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang HY, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guan BJ, Krokowski D, Majumder M, Schmotzer CL, Kimball SR, Merrick WC, et al. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eIF2alpha. J Biol Chem. 2014;289:12593–12611. doi: 10.1074/jbc.M113.543215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao XH, Krokowski D, Guan BJ, Bederman I, Majumder M, Parisien M, et al. Quantitative H2S-mediated protein sulfhydration reveals metabolic reprogramming during the integrated stress response. Elife. 2015:4. doi: 10.7554/eLife.10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress- inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda K, Takemasa T. Expression of ammonia transporters Rhbg and Rhcg in mouse skeletal muscle and the effect of 6-week training on these proteins. Physiol Rep. 2015:3. doi: 10.14814/phy2.12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar V, Pandey P, Sabatini D, Kumar M, Majumder PK, Bharti A, et al. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19:1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye J, Palm W, Peng M, King B, Lindsten T, Li MO, et al. GCN2 sustains mTORC1 suppression upon amino acid deprivation by inducing Sestrin2. Genes Dev. 2015;29:2331–2336. doi: 10.1101/gad.269324.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennis MD, McGhee NK, Jefferson LS, Kimball SR. Regulated in DNA damage and development 1 (REDD1) promotes cell survival during serum deprivation by sustaining repression of signaling through the mechanistic target of rapamycin in complex 1 (mTORC1). Cell Signal. 2013;25:2709–2716. doi: 10.1016/j.cellsig.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holecek M, Kandar R, Sispera L, Kovarik M. Acute hyperammonemia activates branched-chain amino acid catabolism and decreases their extracellular concentrations: different sensitivity of red and white muscle. Amino Acids. 2011;40:575–584. doi: 10.1007/s00726-010-0679-z. [DOI] [PubMed] [Google Scholar]
- 35.Cooper AJ, Lai JC. Cerebral ammonia metabolism in normal and hyperammonemic rats. Neurochem Pathol. 1987;6:67–95. doi: 10.1007/BF02833601. [DOI] [PubMed] [Google Scholar]
- 36.Schachter D, Sang JC. Regional differentiation in the rat aorta for a novel signaling pathway: leucine to glutamate. Am J Physiol. 1997;273:H1484–1492. doi: 10.1152/ajpheart.1997.273.3.H1484. [DOI] [PubMed] [Google Scholar]
- 37.Turner LV, Manchester KL. Effects of denervation on the activities of some tricarboxylic acid-cycle-associated dehydrogenases and adenine-metabolizing enzymes in rat diaphragm muscle. Biochem J. 1972;128:803–809. doi: 10.1042/bj1280803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul HS, Adibi SA. Effect of carnitine on branched-chain amino acid oxidation by liver and skeletal muscle. Am J Physiol. 1978;234:E494–499. doi: 10.1152/ajpendo.1978.234.5.E494. [DOI] [PubMed] [Google Scholar]
- 39.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 40.Atherton PJ, Smith K, Etheridge T, Rankin D, Rennie MJ. Distinct anabolic signalling responses to amino acids in C2C12 skeletal muscle cells. Amino Acids. 2010;38:1533–1539. doi: 10.1007/s00726-009-0377-x. [DOI] [PubMed] [Google Scholar]
- 41.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of System A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab. 2009;297:E822–829. doi: 10.1152/ajpendo.00330.2009. [DOI] [PubMed] [Google Scholar]
- 42.Bott AJ, Peng IC, Fan Y, Faubert B, Zhao L, Li J, et al. Oncogenic Myc Induces Expression of Glutamine Synthetase through Promoter Demethylation. Cell Metab. 2015;22:1068–1077. doi: 10.1016/j.cmet.2015.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merli M, Giusto M, Lucidi C, Giannelli V, Pentassuglio I, Di Gregorio V, et al. Muscle depletion increases the risk of overt and minimal hepatic encephalopathy: results of a prospective study. Metab Brain Dis. 2013;28:281–284. doi: 10.1007/s11011-012-9365-z. [DOI] [PubMed] [Google Scholar]
- 44.Rose CF. Ammonia: more than a neurotoxin? Liver Int. 2014;34:649–651. doi: 10.1111/liv.12518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.