Abstract
Pulmonary microvascular endothelial cells (PMECs) injury including apoptosis plays an important role in the pathogenesis of acute lung injury during sepsis. Our recent study has demonstrated that calpain activation contributes to apoptosis in PMECs under septic conditions. This study investigated how calpain activation mediated apoptosis and whether heat stress regulated calpain activation in lipopolysaccharides (LPS)-stimulated PMECs. In cultured mouse primary PMECs, incubation with LPS (1 µg/ml, 24 h) increased active caspase-3 fragments and DNA fragmentation, indicative of apoptosis. These effects of LPS were abrogated by pre-treatment with heat stress (43 °C for 2 h). LPS also induced calpain activation and increased phosphorylation of p38 MAPK. Inhibition of calpain and p38 MAPK prevented apoptosis induced by LPS. Furthermore, inhibition of calpain blocked p38 MAPK phosphorylation in LPS-stimulated PMECs. Notably, heat stress decreased the protein levels of calpain-1/2 and calpain activities, and blocked p38 MAPK phosphorylation in response to LPS. Additionally, forced up-regulation of calpain-1 or calpain-2 sufficiently induced p38 MAPK phosphorylation and apoptosis in PMECs, both of which were inhibited by heat stress. In conclusion, heat stress prevents LPS-induced apoptosis in PMECs. This effect of heat stress is associated with down-regulation of calpain expression and activation, and subsequent blockage of p38 MAPK activation in response to LPS. Thus, blocking calpain/p38 MAPK pathway may be a novel mechanism underlying heat stress-mediated inhibition of apoptosis in LPS-stimulated endothelial cells.
Keywords: Heat stress, Calpain, p38 MAPK, Apoptosis, Lipopolysaccharide
Introduction
Acute lung injury and its more severe form, acute respiratory distress syndrome, remain the important clinical problems affecting >190,000 patients per year in the USA [1, 2]. The most common cause of acute lung injury is bacterial infection resulting in the sepsis syndrome. Lipopolysaccharides (LPS) of Gram-negative bacteria have been suggested to be important pathogens responsible for acute lung injury [3, 4]. So far, there is no specific therapy and/or intervention available for acute lung injury and acute respiratory distress syndrome.
Pulmonary microvascular endothelial cells (PMECs) injury including apoptotic cell death has been recently suggested to contribute to acute lung injury in sepsis [5, 6]. Apoptosis in endothelial cells may induce microvascular dysfunction, which directly contributes to organ failure in sepsis [7, 8]. It has been reported that administration of a caspase inhibitor or vascular endothelial growth factor inhibits the LPS-induced endothelial cell apoptosis in vitro and improves the survival in mouse models of acute lung injury during sepsis [5, 9]. We have recently reported that calpain, a family of calcium-dependent thiol-proteases, plays an important role in promoting apoptosis in PMECs under septic conditions [10]. Furthermore, transgenic overexpression of calpastatin, an endogenous inhibitor of calpain, reduced acute lung injury in a mouse model of sepsis [11]. These studies suggest that calpain activation may represent an important mechanism for pulmonary endothelial cell apoptosis and acute lung injury in sepsis. However, the signalling mechanisms underlying calpain-mediated apoptosis and whether calpain can be modulated during sepsis remain to be determined.
Heat stress, as a physical stress, induces cellular heat shock responses, and protects cells from delayed injury stimulation, such as ischemia/reperfusion or oxidative injury [12–14]. Studies have shown that the heat shock response attenuates LPS-induced apoptosis in pulmonary artery endothelial cell apoptosis and reduces acute lung injury in sepsis [15–18]. However, the underlying mechanisms remain incompletely understood.
Mitogen-activated protein kinase (MAPK) has been implicated in the pathogenesis of sepsis [19–21]. There are three well-characterized subfamilies in MAPK family: the extracellular signal-regulated kinase (ERK1/2), p38 kinase, and the c-Jun NH2-terminal kinase (JNK1/2/3) [22]. Activation of all three subfamilies has been found to influence a multitude of cellular events such as cell growth and death, differentiation and inflammation in response to oxidative stress and LPS [23–25]. It has been shown that the blockage of calpain suppresses p38 phosphorylation in heat stress-induced male germ cell apoptosis [26]. However, it remains to be elusive whether heat stress and calpain influence MAPK signalling in preventing/promoting PMECs apoptosis under septic conditions.
In this study, we employed primary PMECs as a model to determine the signalling mechanisms by which heat stress prevented apoptosis in PMECs under septic conditions. Particularly, we examined our hypotheses that heat stress decreased calpain expression and activity, and subsequently blocked MAPK activation, thereby preventing apoptosis in LPS-induced PMECs.
Materials and methods
PMECs culture
Breeding pairs of C57BL/6 mice were purchased from the Jackson Laboratory and a breeding program was implemented at our animal care facilities. All animals were provided food and water ad libitum, and housed in a temperature and humidity controlled facility with 12-h light and dark cycles. All animals were used in accordance with the Canadian Council on Animal Care guidelines, and all experimental protocols were approved by the Animal Use Subcommittee at the University of Western Ontario. C57BL/6 mice (aged at 4–6 weeks, both male and female) were anaesthetized with ketamine (100 mg/kg)/xylazine (5 mg/kg, i.p.) and lung tissues were harvested. PMECs isolated from lung tissues were kindly provided by Dr. Sanjay Mehta’s lab and cultured as previously described [27], and all PMECs were used for the present study within five generations.
Reagents
LPS, calpain inhibitor-III (CI-III), caspase-3 inhibitor (Ac-DEVD-CHO), SB203580 and SP600125 were purchased from Sigma, Calbiochem or Life Technology.
Caspase-3 activity
Cells were homogenized in a cell lysis buffer and caspase-3 activity in cell lysates was measured using a caspase-3 fluorescent assay kit according to manufacturer’s protocol (BIOMOL Research Laboratories), as described previously [10].
Cellular DNA fragmentation
Cells were pre-labelled with BrdU for 24 h before treatments. DNA fragmentation was measured using a Cellular DNA Fragmentation ELISA kit (Roche Applied Science) according to the manufacturer’s instructions.
Calpain activity
Calpain activity was determined using a fluorescence substrate N-succinyl-LLVY-AMC (Cedarlane Laboratories) as described in our previous study [28].
Adenoviral infection of PMECs
Cultured PMECs were infected with recombinant adenoviruses containing mouse capn1 (Ad-capn1, SignaGen Laboratories), capn2 (Ad-capn2, Applied Biological Materials Inc.), or beta-gal (Ad-gal, Vector Bio-labs) as a control at a multiplicity of infection of 100 PFU/cell. Adenovirus-mediated gene transfer was implemented as previously described [29].
Western blot analysis
Protein samples were extracted from cultured PMECs. Equal amounts of protein were subjected to SDS-PAGE for separation. After transferring onto the PVDF membrane, immunoblotting was performed. Expressions of HSP27, HSP90, calpain-1, calpain-2, caspase-3, cleaved caspase-3, p38, phosphorylated p38, ERK1/2, phosphorylated ERK1/2, JNK1/2, phosphorylated JNK1/2 and GAPDH proteins were determined using respective specific antibodies (Cell Signalling, Cayman Chemical or Santa Cruz Biotechnology, 1/1000).
Statistical analysis
All data were given as mean + SD. For multi-group comparisons, a two-way ANOVA followed by Newman–Keuls test was performed. A value of p < 0.05 was considered statistically significant.
Results
Heat stress inhibits apoptosis in LPS-stimulated PMECs
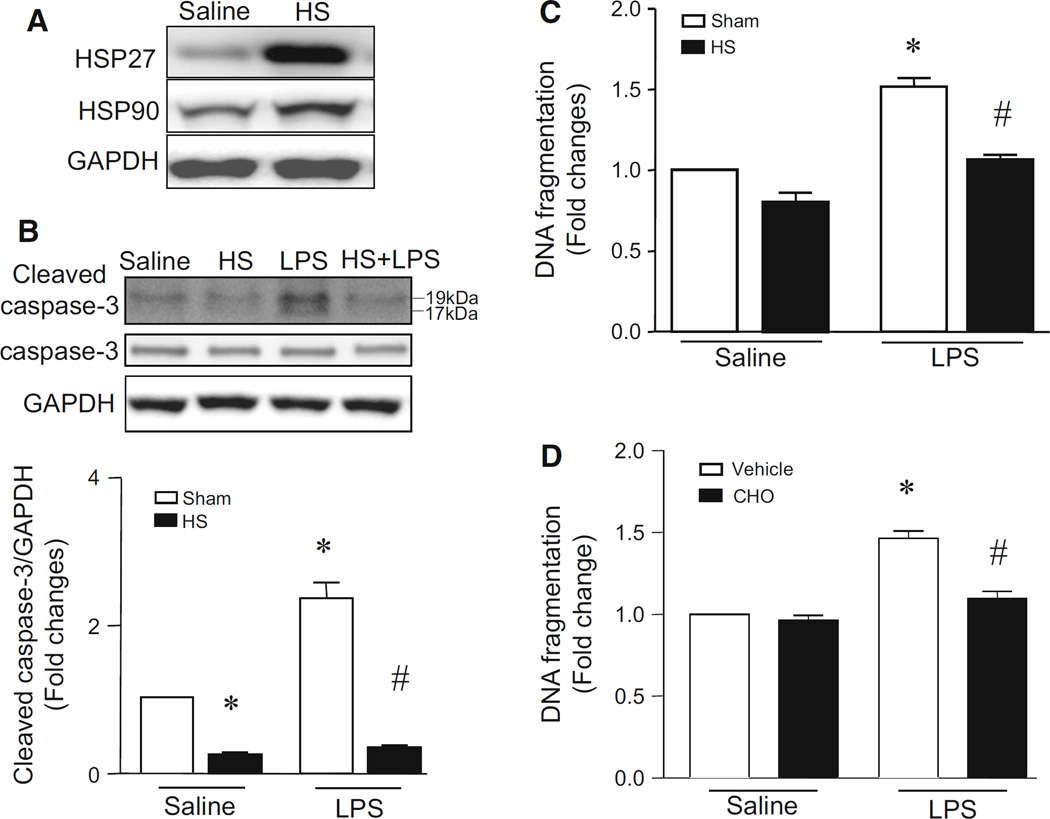
To determine the protective effect of heat stress on LPS-stimulated apoptosis, we pre-treated PMECs with heat stress (43 °C, 2 h) and then incubated them with LPS (1 µg/ml) at 37 °C for 24 h, treated them with heat stress (43 °C, 2 h) followed by incubation at 37 °C for 24 h, or incubated them with LPS (1 µg/ml) or saline for 24 h. Apoptosis was assessed by measuring cleaved caspase-3 fragments and DNA fragmentation. LPS increased the levels of cleaved caspase-3 fragments and DNA fragmentation, indicative of apoptosis (Fig. 1b, c). Heat stress induced a significant increase in heat shock proteins (e.g. HSP27 and HSP90) (Fig. 1a) and significantly inhibited LPS-induced apoptosis in PMECs (Fig. 1b, c). However, heat stress alone did not have any effect on apoptosis under normal condition (Fig. 1b, c). LPS-induced DNA fragmentation was prevented by Ac-DEVD-CHO caspase-3 inhibitor in PMECs (Fig. 1d). Together, these results demonstrate that heat stress inhibits LPS-induced apoptosis in PMECs.
Fig. 1.
Effects of heat stress on apoptosis in LPS-stimulated PMECs. Cultured PMECs were treated with either heat stress (HS, 43 °C for 2 h, then 37 °C for another 24 h), LPS (1 µg/ml) for 24 h, or with a combination of heat stress (HS, 43 °C for 2 h) and followed by LPS (1 µg/ml) in the presence of Ac-DEVD-CHO caspase-3 inhibitor (CHO) or vehicle for another 24 h. a A representative western blot from 3 different experiments shows increases in the protein levels of heat shock proteins (HSP27 and HSP90). b The levels of cleaved caspase-3 fragments were determined by western blot analysis. Up-panel is a representative western blot from 3 different experiments for total caspase-3 (−35KD) and cleaved caspase-3 fragments (17KD and 19KD) and lower panel is the quantification of cleaved caspase-3 fragments normalized to GAPDH. c and d DNA fragmentation was measured. Data are mean ± SD from 3 different experiments. A two-way ANOVA followed by Newman–Keuls test was performed. *p < 0.05 versus Sham + saline or vehicle + saline and #p < 0.05 versus Sham + LPS or LPS + vehicle
Heat stress decreases calpain expression and activation in PMECs
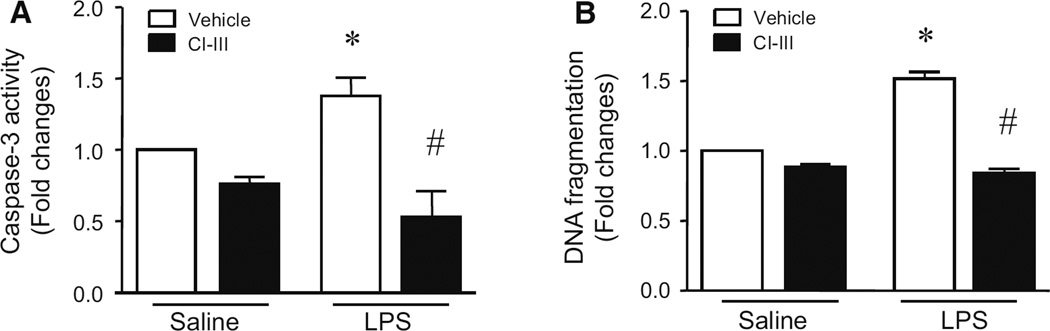
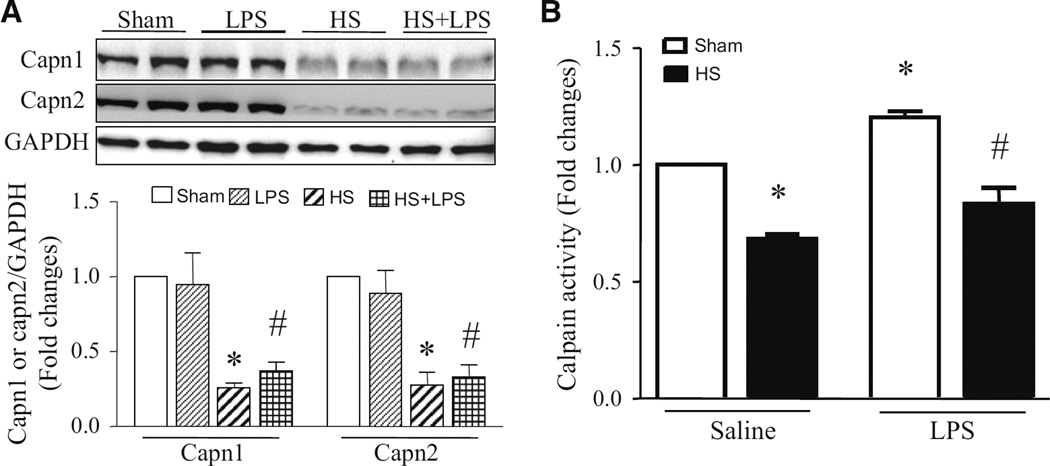
Our recent study has demonstrated that calpain activation contributes to apoptosis in PMECs under septic conditions (14). Consistently, incubation with calpain inhibitor-III (CI-III) decreased LPS-induced caspase-3 activity and DNA fragmentation in PMECs (Fig. 2). LPS increased calpain activity, but had no effect on the protein levels of calpain-1 and calpain-2 (Fig. 3a). Interestingly, heat stress significantly reduced the protein levels of calpain-1 and calpain-2 in PMECs (Fig. 3a), and prevented the increase in calpain activity induced by LPS (Fig. 3b). These results suggest that heat stress prevents LPS-induced apoptosis probably through down-regulation of calpain in PMECs.
Fig. 2.
Effects of calpain inhibitor-III on LPS-induced apoptosis in PMECs. Cultured PMECs were pre-treated with calpain inhibitor-III (CI-III) for 1 h and then stimulated with LPS (1 µg/ml) or saline for another 24 h. Cellular caspase-3 activity (a) and DNA fragmentation (b) were examined. Data are mean ± SD from 3 different experiments. A two-way ANOVA followed by Newman–Keuls test was performed. *p < 0.05 versus saline + vehicle and #p < 0.05 versus LPS + vehicle
Fig. 3.
Changes of calpain proteins and activity in PMECs stimulated with heat stress and LPS. Cultured PMECs were treated with either heat stress (HS, 43 °C for 2 h, then 37 °C for another 24 h), LPS (1 µg/ml) for 24 h, or with a combination of heat stress (HS, 43 °C for 2 h) and followed by LPS (1 µg/ml) for another 24 h. a The protein levels of capn1 and capn2 were determined by western blot analysis. Upper panel is a representative western blot for capn1 and capn2 protein from 3 different experiments and lower panel is the quantification of capn1 and capn2 relative to GAPDH. b Calpain activity was measured. Data are mean ± SD from 3 different experiments. A two-way ANOVA followed by Newman–Keuls test was performed. *p < 0.05 versus Sham + saline or Sham + Capn1 or Sham + Capn2 and #p < 0.05 versus Sham + LPS or LPS + Capn1 or LPS + Capn2
Heat stress attenuates calpain-induced apoptosis
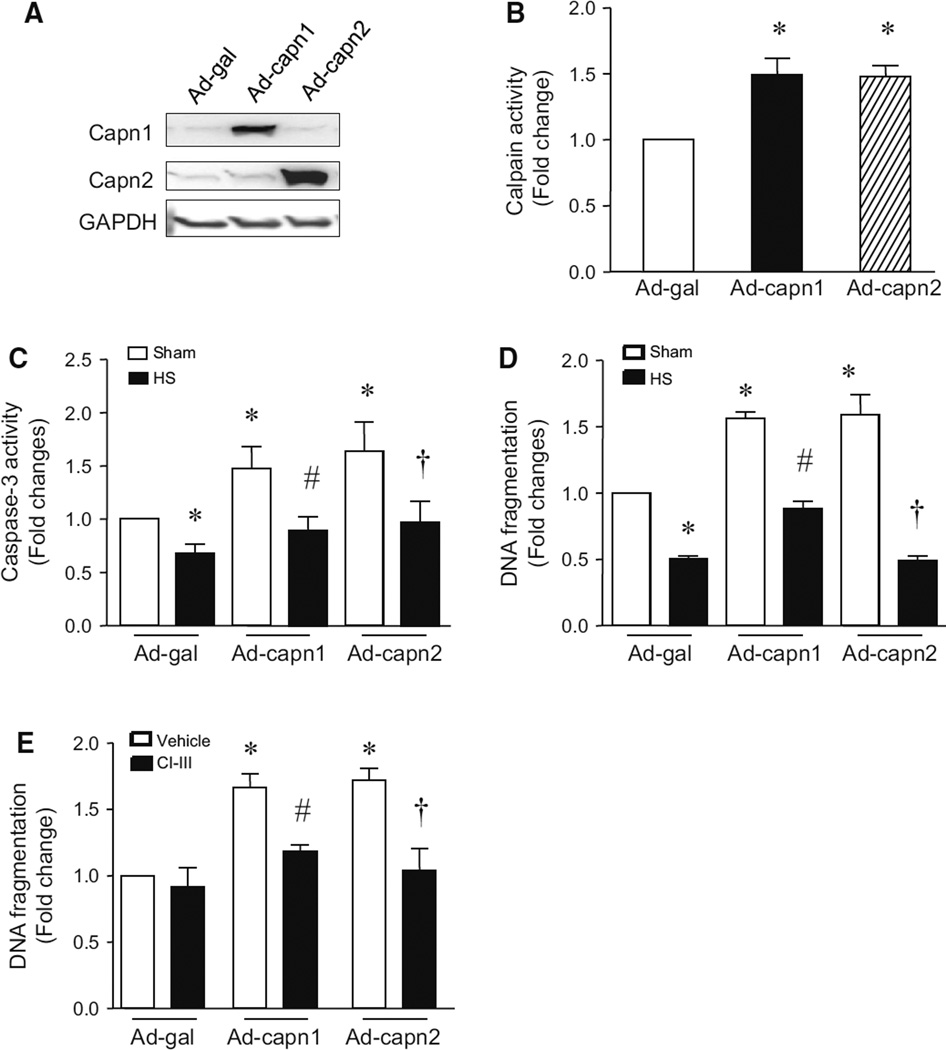
To provide further evidence to support the view that heat stress prevents LPS-induced apoptosis through calpain-dependent mechanisms, we investigated whether heat stress could inhibit calpain-induced apoptosis. To this end, we infected PMECs with Ad-capn1 or Ad-capn2 to up-regulate calpain-1 or calpain-2, respectively. Ad-gal was used as a control. 24 h after infection, the cells were exposed to heat stress at 43 °C for 2 h, and then at 37 °C for another 24 h. Infection with Ad-capn1 and Ad-capn2 increased the protein levels of calpain-1 and calpain-2, respectively (Fig. 4a). Enzymatic calpain activities were also increased in Ad-capn1 and Ad-capn2 infected PMECs as compared to Ad-gal (Fig. 4b). Up-regulation of calpain-1 and calpain-2 sufficiently induced apoptosis as evidenced by increased caspase-3 activity and DNA fragmentation. Heat stress significantly inhibited apoptosis in PMECs induced by up-regulation of calpain-1 and calpain-2 (Fig. 4c, d). DNA fragmentation induced by infection with Ad-capn1 and Ad-capn2 in PMECs was prevented by incubation with calpain inhibitor-III (Fig. 4e), suggesting that the induction of DNA fragmentation is related to calpain activities.
Fig. 4.
Effects of heat stress on calpain-induced apoptosis in PMECs. PMECs were infected with Ad-capn1, Ad-capn2 or Ad-gal for 24 h, and then stimulated with heat stress (43 °C for 2 h, then 37 °C for another 24 h) in the presence of calpain inhibitor-III (CI-III) or vehicle. a Representative western blots for capn1, capn2 and GAPDH from 3 different experiments. Cellular calpain activity (b), caspase-3 activity (c) and DNA fragmentation (d and e) were measured. Data are mean ± SD from 3 different experiments. A two-way ANOVA followed by Newman–Keuls test was performed. *p < 0.05 versus Ad-gal or Ad-gal + Sham or Ad-gal + vehicle, #p < 0.05 versus Ad-capn1 + Sham or Ad-capn1 + vehicle, and †p < 0.05 versus Ad-capn2 + vehicle
Heat stress inhibits LPS-induced MAPKs phosphorylation in PMECs
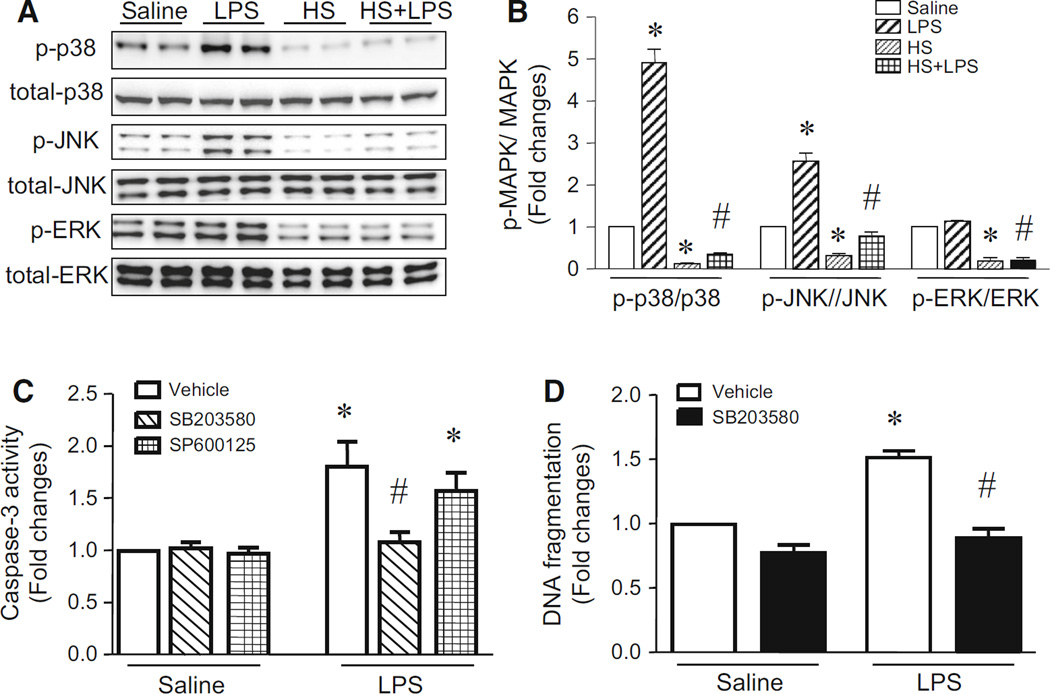
LPS has been reported to induce the phosphorylation of MAPK family members (p38, ERK1/2 and JNK1/2) in a wide variety of cell types [22, 30]. Activation of MAPKs has been implicated in apoptosis [22, 24, 31]. Similarly, we also showed that incubation with LPS for 24 h increased phosphorylation of p38 and JNK1/2 in PMECs while the levels of phosphorylated ERK1/2 remained unaltered (Fig. 5a, b). To determine whether p38 and JNK1/2 play a role in apoptosis, we exposed PMECs to LPS or saline in the presence of SB203580 (p38 inhibitor), SP600125 (JNK1/2 inhibitor) or vehicle for 24 h. Incubation with SB203580 prevented apoptosis in LPS-stimulated PMECs; however, SP600125 did not have any effect on LPS-induced apoptosis (Fig. 5c, d). This result demonstrates that p38 but not JNK1/2 contributes to apoptosis in LPS-stimulated PMECs.
Fig. 5.
Effect of heat stress on phosphorylation of MAPKs and role of MAPKs in apoptosis. a and b Cultured PMECs were treated with either heat stress (HS, 43 °C for 2 h, then 37 °C for another 24 h), LPS (1 µg/ml) for 24 h, or with a combination of heat stress (HS, 43 °C for 2 h) and followed by LPS (1 µg/ml) for another 24 h. Western blot analysis was performed. a Representative western blots for MAPKs and phosphorylated MAPKs from 3 different experiments. b Quantifications of phosphorylated MAPKs/total MAPKs. c and d PMECs were incubated with LPS or saline in combination with SB203580, SP600125 or vehicle for 24 h. Apoptosis was determined by measuring caspase-3 activity (c) and DNA fragmentation (d). Data are mean ± SD from 3 different experiments. A two-way ANOVA followed by Newman–Keuls test was performed. *p < 0.05 versus saline or saline + vehicle and #p < 0.05 versus LPS or LPS + vehicle
We also examined the effect of heat stress on phosphorylation of p38, JNK1/2 and ERK1/2 in LPS-induced PMECs. As shown in Fig. 5a, b, heat stress decreased basal and LPS-induced phosphorylation of p38, JNK1/2 and ERK1/2. Taken together, these results suggest that heat stress may inhibit apoptosis by blocking p38 MAPK signalling in LPS-stimulated PMECs.
Calpain activation promotes p38 phosphorylation
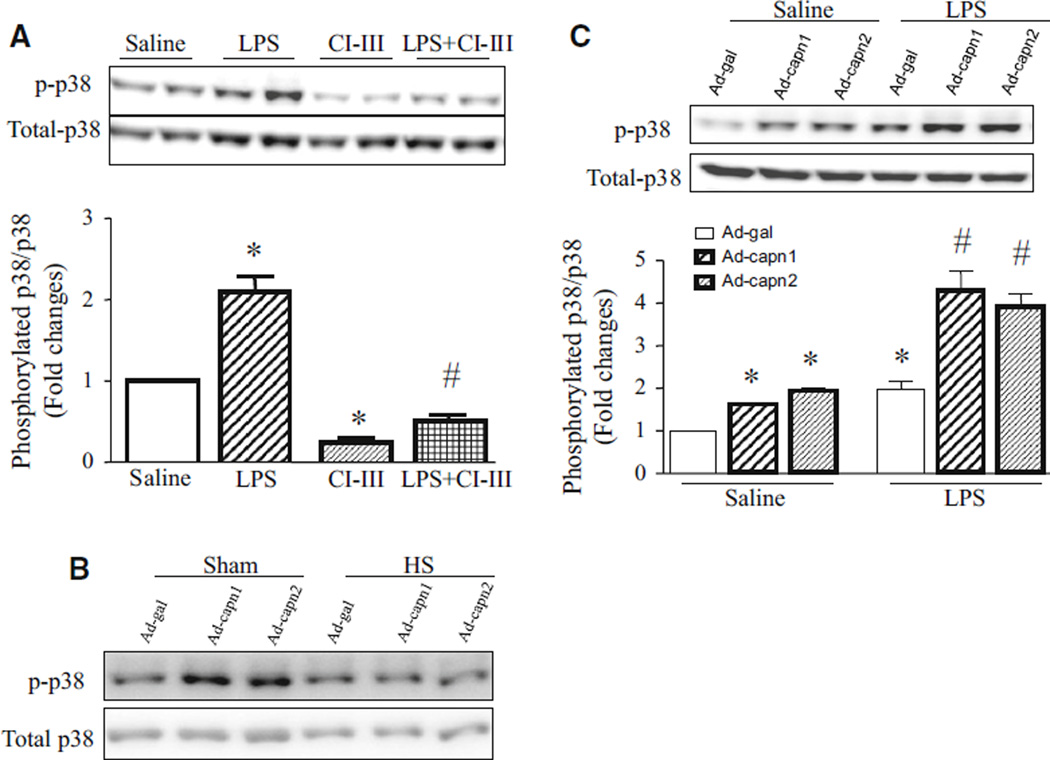
Having shown that heat stress decreased calpain expression and activity, and prevented p38 phosphorylation while both calpain and p38 contributed to apoptosis in response to LPS, we hypothesized that calpain activation induced p38 phosphorylation in promoting apoptosis. To examine this hypothesis, we incubated PMECs with LPS or saline in the presence of calpain inhibitor-III or vehicle. 24 h later, the phosphorylation of p38 was determined in PMECs. Consistently, LPS increased the levels of phosphorylated p38 whereas total p38 protein remained unchanged. However, incubation with calpain inhibitor-III prevented basal and LPS-induced phosphorylation of p38 in PMECs (Fig. 6a). This result suggests that calpain activation increases phosphorylation of p38 during LPS stimulation.
Fig. 6.
Effects of calpain inhibitor, p38 inhibitor and heat stress on p38 phosphorylation in LPS-treated PMECs. PMECs were pre-treated with calpain inhibitor-III (CI-III) or SB203580 (SB) for 1 h, or infected with Ad-capn1, Ad-capn2 or Ad-gal for 24 h, and then subjected to heat stress (HS) or incubated with LPS (1 µg/ml) or saline for another 24 h. a, b and c Upper panels are representative western blots for phosphorylated p38 and total p38 from 3 different experiments. Lower panels are quantifications for phosphorylated p38/total p38 (a and c). Data are mean ± SD from 3 different experiments. A two-way ANOVA followed by Newman–Keuls test was performed. *p < 0.05 versus saline or saline + Ad-gal, and #p < 0.05 versus LPS, LPS + Ad-gal or saline
To provide further evidence to support this finding, we investigated whether up-regulation of calpain-1 and calpain-2 was sufficient to induce phosphorylation of p38. To this end, we infected PMECs with Ad-capn1, Ad-capn2 or Ad-gal as a control, followed by heat stress. 24 h later, the levels of phosphorylated p38 relative to total p38 were significantly increased in both Ad-capn1 and Ad-capn2 infected PMECs as compared to Ad-gal (Fig. 6b, c). Heat stress abrogated the increase in phosphorylated p38 in prevented both Ad-capn1 and Ad-capn2 infected PMECs (Fig. 6b). In addition, infection with Ad-capn1 and Ad-capn2 further enhanced LPS-stimulated p38 phosphorylation in PMECs (Fig. 6c). Taken together, these studies demonstrate that calpain activation promotes p38 phosphorylation in PMECs under basal condition and LPS stimulation.
Discussion
In this study, we demonstrate for the first time that heat stress down-regulated calpain expression and activity, and subsequently prevented apoptosis in response to LPS. Calpain activation induces p38 MAPK phosphorylation and inhibition of p38 MAPK abrogated apoptosis induced by LPS in PMECs. Thus, heat stress inhibits LPS-induced apoptosis by blocking calpain/p38 MPAK pathway.
Severe heat stress has been reported to have a direct cytotoxic effect, such as in heatstroke [32, 33]. However, as a physical stress, heat stress may be protective under certain pathological conditions. For example, heat shock has been widely recognized to protect cardiomyocytes against ischemia/reperfusion-induced injury [34]. It is also observed that heat stress preconditioning prevents the coronary endothelial dysfunction induced by ischemia/reperfusion [35, 36]. The present study provides evidence demonstrating that heat stress inhibits apoptosis in LPS-stimulated mouse PMECs. This result is consistent with a previous report which showed that the heat-shock response attenuated LPS-mediated apoptosis in cultured sheep pulmonary artery endothelial cells [18]. Thus, heat stress may protect pulmonary micro- and macro-vascular endothelial cells in sepsis-associated acute lung injury. Indeed, heat pre-treatment has been demonstrated to attenuate inflammation and pulmonary microvascular leakage in mouse and rat models of endotoxemia or sepsis [37, 38].
An important finding of this study is that heat stress reduced basal and LPS-induced calpain protein and activity in PMECs. We further show that inhibition of calpain prevented LPS-induced apoptosis, which is agreement with our previous report [10]. Our observations are consistent with a model whereby heat stress inhibits LPS-induced apoptosis by down-regulating calpain in PMECs. Further evidence in support of this conclusion is that forced up-regulation of calpain-1 or calpain-2 sufficiently induced apoptosis in PMECs, which was inhibited by heat stress. It is currently unknown how heat stress down-regulates calpain expression in PMECs. However, it seems that heat stress negatively regulates post-transcription of calpain-1 and calpain-2 because both endogenous and exogenous expressions of calpain-1 and calpain-2 protein were decreased by heat stress. Nevertheless, future investigation is needed to clarify whether heat stress impacts on calpain mRNA stability, protein translation and/or degradation.
It is worthwhile to mention that in this study, heat stress also induced HSPs (HSP27 and HSP90) in PMECs. Since HSPs are known to be protective [39–41], it is possible, albeit speculated, that the induction of HSPs may be also involved in the inhibitory effect of heat stress on apoptosis in LPS-stimulated PMECs. Interestingly, calpain has been suggested to target and cleave HSPs [42, 43]. Whether this mechanism is also operative in LPS-induced PMECs apoptosis requires further investigation for clarification.
Another important finding of this study is that calpain promoted p38 activation in LPS-induced PMECs. Several lines of evidence support this conclusion. First, LPS induced p38 activation, which correlated with calpain activation. Second, inhibition of calpain prevented LPS-induced p38 activation. Third, forced up-regulation of calpain-1 or calpain-2 sufficiently induced p38 activation. Concurrently, heat stress inhibited LPS- or calpain-1/2 up-regulation-induced p38 activation in PMECs. Previous study has also demonstrated that calpain promotes p38 phosphorylation in heat stress-induced male germ cells [26]. We further show that activation of p38 mediated LPS-induced apoptosis as inhibition of p38 abrogated apoptosis induced by LPS. Taken together, our observation argues that calpain mediates apoptosis at least partly by activating p38 pathway in LPS-induced PMECs.
The other two subfamilies of MAPK family (ERK1/2 and JNK1/2) have been implicated in inflammatory response and apoptosis under septic conditions [31, 44]. We show that incubation with LPS for 24 h increased the levels of phosphorylated JNK1/2 but not ERK1/2 in LPS-stimulated PMECs. Both heat stress and calpain inhibition significantly reduced basal and LPS-induced JNK1/2 phosphorylation. This suggests that JNK1/2 signalling is negatively regulated by heat stress and calpain inhibition in PMECs. However, inhibition of JNK1/2 did not have any effect on apoptosis in LPS-stimulated PMECs. Similarly, the levels of phosphorylated ERK1/2 were reduced by heat stress in PMECs; however, inhibition of ERK1/2 did not decrease apoptosis (data not shown). Thus, these data exclude the involvement of JNK1/2 and ERK1/2 in LPS-induced apoptosis in PMECs.
In summary, this study demonstrates that heat stress prevented LPS-induced apoptosis in PMECs. This effect of heat stress was associated with down-regulation of calpain expression and activation, and subsequent blockage of p38 activation in response to LPS. Thus, blocking calpain/p38 pathway may be a novel mechanism for heat stress-mediated inhibition of apoptosis in LPS-stimulated endothelial cells.
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China [No. 81571940], the Project Team of Natural Science Foundation of Guangdong Province [No. s2013030013217], and the Project of Medical Research of PLA [No. BWS12J108], the Guangzhou Science and Technology Planning Project of China [No. 201504281714528], and the Heart & Stroke Foundation of Canada (Awarded to T.P.). T.P. is a recipient of a New Investigator Award from the Canadian Institutes of Health Research.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 2.Wheeler AP, Bernard GR. Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet. 2007;369:1553–1564. doi: 10.1016/S0140-6736(07)60604-7. [DOI] [PubMed] [Google Scholar]
- 3.Bannerman DD, Goldblum SE. Mechanisms of bacterial lipopolysaccharide-induced endothelial apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L899–L914. doi: 10.1152/ajplung.00338.2002. [DOI] [PubMed] [Google Scholar]
- 4.Rittirsch D, Flierl MA, Day DE, Nadeau BA, McGuire SR, et al. Acute lung injury induced by lipopolysaccharide is independent of complement activation. J Immunol. 2008;180:7664–7672. doi: 10.4049/jimmunol.180.11.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawasaki M, Kuwano K, Hagimoto N, Matsuba T, Kunitake R, et al. Protection from lethal apoptosis in lipopolysaccharide-induced acute lung injury in mice by a caspase inhibitor. Am J Pathol. 2000;157:597–603. doi: 10.1016/S0002-9440(10)64570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Shu R, Filippatos G, Uhal BD. Apoptosis in lung injury and remodeling. J Appl Physiol. 2004;97:1535–1542. doi: 10.1152/japplphysiol.00519.2004. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, et al. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- 8.Matute-Bello G, Winn RK, Martin TR, Liles WC. Sustained lipopolysaccharide-induced lung inflammation in mice is attenuated by functional deficiency of the Fas/Fas ligand system. Clin Diagn Lab Immunol. 2004;11:358–361. doi: 10.1128/CDLI.11.2.358-361.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namisaki T, Yoshiji H, Kojima H, Yoshii J, Ikenaka Y, et al. Salvage effect of the vascular endothelial growth factor on chemically induced acute severe liver injury in rats. J Hepatol. 2006;44:568–575. doi: 10.1016/j.jhep.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Hu H, Li X, Li Y, Wang L, Mehta S, et al. Calpain-1 induces apoptosis in pulmonary microvascular endothelial cells under septic conditions. Microvasc Res. 2009;78:33–39. doi: 10.1016/j.mvr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Zafrani L, Gerotziafas G, Byrnes C, Hu X, Perez J, et al. Calpastatin controls polymicrobial sepsis by limiting procoagulant microparticle release. Am J Respir Crit Care Med. 2012;185:744–755. doi: 10.1164/rccm.201109-1686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill RR, Gbur CJ, Jr, Fisher BJ, Hess ML, Fowler AA, et al. Heat shock provides delayed protection against oxidative injury in cultured human umbilical vein endothelial cells. J Mol Cell Cardiol. 1998;30:2739–2749. doi: 10.1006/jmcc.1998.0837. [DOI] [PubMed] [Google Scholar]
- 13.Gray CC, Amrani M, Yacoub MH. Heat stress proteins and myocardial protection: experimental model or potential clinical tool? Int J Biochem Cell Biol. 1999;31:559–573. doi: 10.1016/s1357-2725(99)00004-7. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto N, Shido O, Matsuzaki K, Katakura M, Hitomi Y, et al. Long-term heat exposure prevents hypoxia-induced apoptosis in mouse fibroblast cells. Cell Biochem Biophys. 2014;70:301–307. doi: 10.1007/s12013-014-9912-9. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda N, Takano Y, Kageyama S, Hatakeyama N, Shakunaga K, et al. Silencing of caspase-8 and caspase-3 by RNA interference prevents vascular endothelial cell injury in mice with endotoxic shock. Cardiovasc Res. 2007;76:132–140. doi: 10.1016/j.cardiores.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Stefanec T. Endothelial apoptosis: could it have a role in the pathogenesis and treatment of disease? Chest. 2000;117:841–854. doi: 10.1378/chest.117.3.841. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Murakami T, Kuwahara-Arai K, Tamura H, Hiramatsu K, et al. Human anti-microbial cathelicidin peptide LL-37 suppresses the LPS-induced apoptosis of endothelial cells. Int Immunol. 2011;23:185–193. doi: 10.1093/intimm/dxq471. [DOI] [PubMed] [Google Scholar]
- 18.Wong HR, Mannix RJ, Rusnak JM, Boota A, Zar H, et al. The heat-shock response attenuates lipopolysaccharide-mediated apoptosis in cultured sheep pulmonary artery endothelial cells. Am J Respir Cell Mol Biol. 1996;15:745–751. doi: 10.1165/ajrcmb.15.6.8969269. [DOI] [PubMed] [Google Scholar]
- 19.Abraham E. Alterations in cell signaling in sepsis. Clin Infect Dis. 2005;41:S459–S464. doi: 10.1086/431997. [DOI] [PubMed] [Google Scholar]
- 20.Jarrar D, Chaudry IH, Wang P. Organ dysfunction following hemorrhage and sepsis: mechanisms and therapeutic approaches (Review) Int J Mol Med. 1999;4:575–583. doi: 10.3892/ijmm.4.6.575. [DOI] [PubMed] [Google Scholar]
- 21.Strassheim D, Park JS, Abraham E. Sepsis: current concepts in intracellular signaling. Int J Biochem Cell Biol. 2002;34:1527–1533. doi: 10.1016/s1357-2725(02)00049-3. [DOI] [PubMed] [Google Scholar]
- 22.Cobb MH. MAP kinase pathways. Prog Biophys Mol Biol. 1999;71:479–500. doi: 10.1016/s0079-6107(98)00056-x. [DOI] [PubMed] [Google Scholar]
- 23.Braz JC, Bueno OF, Liang Q, Wilkins BJ, Dai YS, et al. Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J Clin Investig. 2003;111:1475–1486. doi: 10.1172/JCI17295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- 25.Peng T, Lu X, Feng Q. NADH oxidase signaling induces cyclooxygenase-2 expression during lipopolysaccharide stimulation in cardiomyocytes. FASEB J. 2005;19:293–295. doi: 10.1096/fj.04-2289fje. [DOI] [PubMed] [Google Scholar]
- 26.Lizama C, Lagos CF, Lagos-Cabre R, Cantuarias L, Rivera F, et al. Calpain inhibitors prevent p38 MAPK activation and germ cell apoptosis after heat stress in pubertal rat testes. J Cell Physiol. 2009;221:296–305. doi: 10.1002/jcp.21868. [DOI] [PubMed] [Google Scholar]
- 27.Farley KS, Wang LF, Law C, Mehta S. Alveolar macrophage inducible nitric oxide synthase-dependent pulmonary microvascular endothelial cell septic barrier dysfunction. Microvasc Res. 2008;76:208–216. doi: 10.1016/j.mvr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Ma J, Zhu H, Singh M, Hill D, et al. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes. 2011;60:2985–2994. doi: 10.2337/db10-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen E, Fan J, Chen R, Yee SP, Peng T. Phospholipase Cgamma1 signalling regulates lipopolysaccharide-induced cyclooxygenase-2 expression in cardiomyocytes. J Mol Cell Cardiol. 2007;43:308–318. doi: 10.1016/j.yjmcc.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- 31.Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 32.Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346:1978–1988. doi: 10.1056/NEJMra011089. [DOI] [PubMed] [Google Scholar]
- 33.Moulin M, Arrigo AP. Long lasting heat shock stimulation of TRAIL-induced apoptosis in transformed T lymphocytes. Exp Cell Res. 2006;312:1765–1784. doi: 10.1016/j.yexcr.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Joyeux-Faure M, Arnaud C, Godin-Ribuot D, Ribuot C. Heat stress preconditioning and delayed myocardial protection: what is new? Cardiovasc Res. 2003;60:469–477. doi: 10.1016/j.cardiores.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Amrani M, Corbett J, Allen NJ, O’Shea J, Boateng SY, et al. Induction of heat-shock proteins enhances myocardial and endothelial functional recovery after prolonged cardioplegic arrest. Ann Thorac Surg. 1994;57:157–160. doi: 10.1016/0003-4975(94)90385-9. [DOI] [PubMed] [Google Scholar]
- 36.Joyeux M, Bouchard JF, Lamontagne D, Godin-Ribuot D, Ribuot C. Heat stress-induced protection of endothelial function against ischaemic injury is abolished by ATP-sensitive potassium channel blockade in the isolated rat heart. Br J Pharmacol. 2000;130:345–350. doi: 10.1038/sj.bjp.0703312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Kelly C, Chen H, Leahy A, Bouchier-Hayes D. Thermotolerance protects against endotoxin-mediated microvascular injury. J Surg Res. 2001;95:79–84. doi: 10.1006/jsre.2000.5896. [DOI] [PubMed] [Google Scholar]
- 38.Heidemann SM, Glibetic M. Heat stress protects against lung injury in the neutropenic, endotoxemic rat. Inflammation. 2005;29:47–53. doi: 10.1007/s10753-006-8969-4. [DOI] [PubMed] [Google Scholar]
- 39.Brinton MR, Tagge CA, Stewart RJ, Cheung AK, Shiu YT, et al. Thermal sensitivity of endothelial cells on synthetic vascular graft material. Int J Hyperthermia. 2012;28:163–174. doi: 10.3109/02656736.2011.638963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mestril R, Dillmann WH. Heat shock proteins and protection against myocardial ischemia. J Mol Cell Cardiol. 1995;27:45–52. doi: 10.1016/s0022-2828(08)80006-5. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Luo D, Miao R, Bai L, Ge Q, et al. Hsp90-Akt phosphorylates ASK1 and inhibits ASK1-mediated apoptosis. Oncogene. 2005;24:3954–3963. doi: 10.1038/sj.onc.1208548. [DOI] [PubMed] [Google Scholar]
- 42.Averna M, Stifanese R, De Tullio R, Salamino F, Pontremoli S, et al. In vivo degradation of nitric oxide synthase (NOS) and heat shock protein 90 (HSP90) by calpain is modulated by the formation of a NOS-HSP90 heterocomplex. FEBS J. 2008;275:2501–2511. doi: 10.1111/j.1742-4658.2008.06394.x. [DOI] [PubMed] [Google Scholar]
- 43.Sahara S, Yamashima T. Calpain-mediated Hsp70.1 cleavage in hippocampal CA1 neuronal death. Biochem Biophys Res Commun. 2010;393:806–811. doi: 10.1016/j.bbrc.2010.02.087. [DOI] [PubMed] [Google Scholar]
- 44.Mizumura K, Gon Y, Kumasawa F, Onose A, Maruoka S, et al. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates lipopolysaccharide-induced tissue factor expression in pulmonary microvasculature. Int Immunopharmacol. 2010;10:1062–1067. doi: 10.1016/j.intimp.2010.06.006. [DOI] [PubMed] [Google Scholar]