Abstract
Human immunodeficiency virus type 1 (HIV-1) Gag multimerization and membrane binding are required for particle formation. However, it is unclear what constitutes a minimal plasma membrane-specific targeting signal and what role the matrix (MA) globular head and other Gag domains play in membrane targeting. Here, we use membrane flotation and microscopic analysis of Gag deletion mutants to demonstrate that the HIV-1 MA globular head inhibits a plasma membrane-specific targeting signal contained within the six amino-terminal MA residues. MA-mediated inhibition is relieved by concentration-dependent Gag multimerization and imparts a high degree of cooperativity on Gag-membrane association. This cooperativity may confer temporal and spatial regulation on HIV-1 assembly.
Various protein, nucleic acid, and lipid components are assembled during retroviral particle morphogenesis, but the Gag precursor is the only viral protein that is required for particle formation. While most retroviruses assemble on cellular membranes, the temporal relationship between the association of Gag with itself and the association of Gag with membranes can vary. Human immunodeficiency virus type 1 (HIV-1) Gag multimerization occurs primarily on cellular membranes (3, 23), but some degree of multimerization may occur prior to membrane association (1, 11), and it is controversial whether Gag multimerization is necessary for membrane binding (8, 12, 19-21). Here, we show that Gag multimerization is indeed required for efficient membrane binding in cells, because it overcomes an activity in the globular head of HIV-1 matrix (MA) that inhibits membrane association. These effects confer concentration dependence on the HIV-1 Gag-membrane interaction and are consistent with the notion that multimerization triggers the “myristoyl switch” (18, 22, 25), thereby imparting a high degree of cooperativity on HIV-1 Gag trafficking to membranes.
HIV-1 Gag binding to cellular membranes is cooperative.
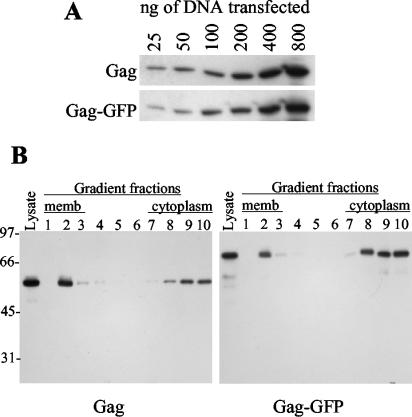
Previous studies have employed membrane flotation and immunofluorescence assays to determine the degree to which HIV-1 Gag is membrane associated in cells at steady state, with varied results (3-5, 11, 16, 20). To reexamine this issue, we expressed an HIV-1 Gag-green fluorescent protein (GFP) fusion protein by using a pCR3.1-based expression vector (Invitrogen) containing codon-optimized HIV-1 Gag (6) and enhanced GFP (Clontech) sequences. A similar vector expressed unfused Gag. In 293T cells, unfused Gag and Gag-GFP were expressed at similar levels (Fig. 1A) and formed extracellular particles with similar efficiencies (data not shown). Moreover, Gag and Gag-GFP associated with cell membranes to similar degrees. This conclusion was based on membrane flotation analyses, carried out by loading lysates of transfected cells at the bottom of a 90%-65%-10% sucrose step gradient, centrifuging them at 35,000 rpm in an SW41 rotor for 18 h, and performing a Western blot analysis of gradient fractions (20) (Fig. 1B). Thus, the GFP tag should provide a good surrogate for Gag localization within cells.
FIG. 1.
HIV-1 Gag and Gag-GFP expression and membrane association. (A) 293T cells were transfected with the indicated amounts of HIV-1 Gag or Gag-GFP expression plasmid, and cell lysates were subjected to Western blot analysis using an HIV-1 Gag-specific monoclonal antibody. (B) Dounce-homogenized cells transfected with 100 ng of Gag or Gag-GFP expression plasmids per well were subjected to membrane flotation analysis. The leftmost lane in each panel was loaded with an aliquot of the total cell lysate, while the remaining lanes contain samples of proteins precipitated from each of 10 fractions of the sucrose gradient, beginning with the least-dense (membrane [memb]-containing) fractions on the left and ending with the most-dense cytoplasm-containing fractions on the right. The panels show Western blots probed with an HIV-1 Gag-specific monoclonal antibody, and the scale to the left of the blots indicates the positions of molecular weight markers (in thousands).
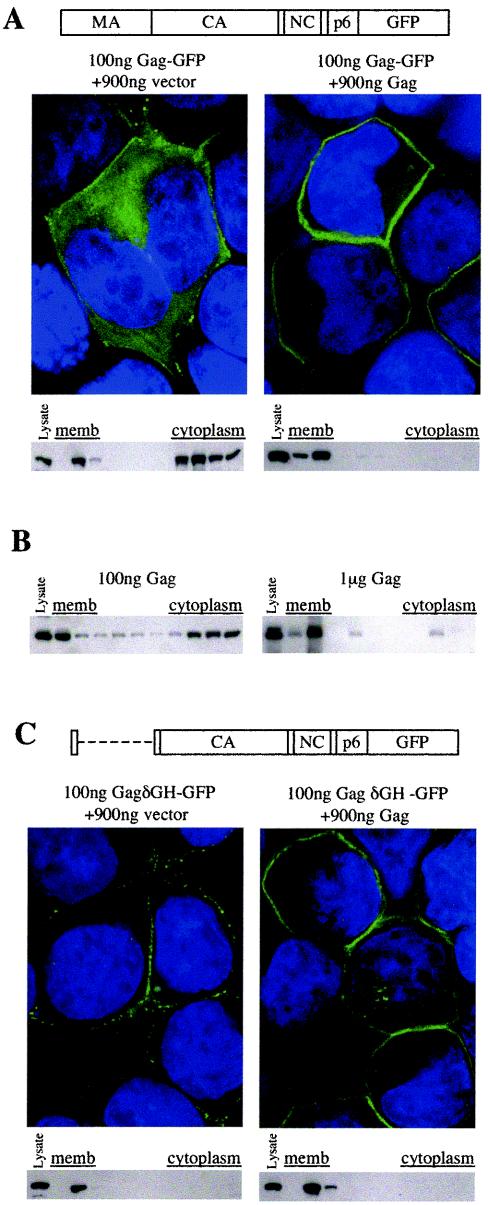
At low levels of expression (100 ng of expression plasmid transfected per 5 × 105 cells per well of a 24-well plate), the fraction of Gag or Gag-GFP that associated with membranes was modest (Fig. 1B and 2A). However, if the number of GFP tags was held constant (by transfecting a fixed quantity [100 ng] of the Gag-GFP expression plasmid) and the total level of Gag was varied (by cotransfecting 900 ng of a control vector or a Gag expression plasmid), the fraction of Gag that was membrane associated varied dramatically (Fig. 2A). Membrane flotation assays and deconvolution microscopy, performed as previously described (10, 20), showed that Gag was largely diffuse and cytoplasmic at low expression levels but almost entirely associated with the plasma membrane at high expression levels (Fig. 2A). Similarly, flotation analysis revealed that unfused Gag was almost entirely associated with cell membranes at high, but not at low, expression levels (Fig. 2B). Thus, HIV-1 Gag trafficking to the membranes is cooperative.
FIG. 2.
Cooperative association of Gag with the plasma membrane (memb). (A) A schematic representation of the intact Gag-GFP fusion protein is shown. CA, capsid; NC, nucleocapsid. 293T cells were transfected with 100 ng of a plasmid expressing Gag-GFP, along with 900 ng of either an empty expression vector (left panels) or a Gag expressionplasmid (right panels). For each combination, deconvolved microscopic images of representative cells are shown, with Gag-GFP localization shown in green and DAPI (4′,6′-diamidino-2-phenylindole) staining of cell nuclei shown in blue. Western blots of gradient fractions following membrane flotation analysis, probed with an anti-GFP monoclonal antibody, are shown for each combination of transfected plasmids. The leftmost lane of each blot contains an aliquot of unfractionated cell lysate. (B) Membrane flotation-Western blot analysis of unfused Gag expressed at low levels (100 ng of expression plasmid per transfection; left panel) or high levels (1 μg of expression plasmid per transfection; right panel). Blots were probed with an anti-HIV-1 Gag monoclonal antibody, and the leftmost lane of each blot contains an aliquot of unfractionated cell lysate. (C) A schematic representation of the GagδGH-GFP fusion protein, which lacks MA residues 7 to 110, is shown. 293T cells were transfected and analyzed by deconvolution microscopy and membrane flotation-Western blotting with an anti-GFP monoclonal antibody as described for panel A.
The globular head of MA inhibits and induces cooperativity in Gag-membrane interactions.
To determine which features of Gag were responsible for cooperative membrane binding, we first focused on the MA globular head because point mutations in this domain can enhance membrane binding (5, 13, 16). Moreover, large deletions in, or the complete removal of, MA are compatible with HIV-1 assembly and replication (7, 17). A fusion protein, termed GagδGH-GFP, containing a deletion of Gag residues 7 through 110, which encode the MA globular head, was almost completely membrane bound, even at low Gag expression levels (Fig. 2C). Thus, removal of the HIV-1 MA globular head relieves a barrier to membrane association at low Gag expression levels (compare Fig. 2A and C). Furthermore, GagδGH-GFP localized primarily to the plasma membrane, indicating that the MA globular head is dispensable for specific Gag targeting. While the levels of association of GagδGH-GFP with membrane were not quantitatively different under conditions of high Gag coexpression, the more punctate distribution of GagδGH-GFP was converted to a uniform Gag-GFP-like membrane distribution by coexpression with excess unfused Gag protein, suggesting that the two proteins formed heteromultimers. Despite the differential distribution, the deletion in the GagδGH Gag protein was compatible with infectious virion production in the absence of the HIV-1 gp41 cytoplasmic tail. Indeed, a proviral plasmid carrying a GFP reporter gene in place of Nef and the GagδGH Gag protein generated 9.8 × 105 ± 2.1 × 105 infectious units/ml upon transfection of 293T cells, while an otherwise identical proviral plasmid encoding an intact Gag protein generated 3.1 × 106 ± 0.1 × 106 infectious units/ml. A similar lack of requirement of the MA globular head in spreading HIV-1 replication has been previously reported by using similar proviral plasmid-based constructs (17).
A 6-residue myristoylated peptide sequence at the amino terminus of HIV-1 Gag is a specific plasma membrane targeting signal.
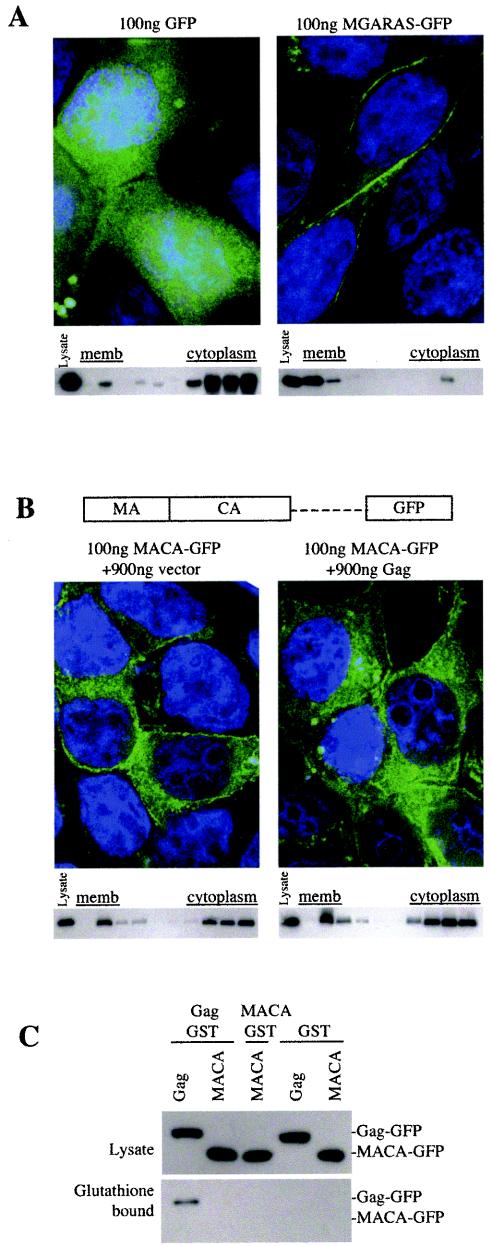
Previously, sequences within the globular head of HIV-1 MA, including basic residues within MA residues 15 to 31, have been reported to be important for targeting Gag specifically to the plasma membrane (2, 14, 15, 24). However, because Gag proteins entirely lacking the MA globular head appeared to target the plasma membrane (Fig. 2C), we examined which HIV Gag sequences were responsible for this specificity. We first fused a 6-residue sequence from the amino terminus of MA (MGARAS) that can support myristoylation and HIV-1 particle formation (7) directly to GFP. Biochemical and microscopic assays showed that these residues were fully sufficient to direct GFP specifically to the plasma membrane and that they did so more efficiently than did the intact HIV-1 Gag protein (Fig. 3A).
FIG. 3.
A small peptide sequence at the amino terminus of HIV-1 Gag is a fully functional plasma membrane (memb) targeting signal whose activity is masked in the context of a poorly multimerizing Gagprotein. (A) 293T cells were transfected with 100 ng of a GFP expression plasmid (left panels) or 100 ng of a plasmid expressing GFP fused to the amino-terminal HIV-1 Gag residues MGARAS (right panels). For each protein, deconvolved microscopic images of representative cells are shown. In addition, Western blots of gradient fractions following membrane flotation analysis that were probed with an anti-GFP monoclonal antibody are shown. The leftmost lane of each blot contains an aliquot of unfractionated cell lysate. (B) A schematic representation of the MACA-GFP fusion protein, which lacks the p2-NC-p1-p6 Gag domain, is shown. 293T cells were transfected with 100 ng of a plasmid expressing this protein, along with 900 ng of either an empty expression vector (left panels) or a Gag expression plasmid (right panels). Shown are deconvolution microscopic images and the results of membrane flotation-Western blot analysis with an anti-GFP monoclonal antibody carried out as described for panel A. The leftmost lane of each blot contains an aliquot of unfractionated cell lysate. (C) Western blot analysis of 293T cell lysates and glutathione-bound proteins following transfection of 293T cells with plasmids expressing Gag and/or MACA fused to GFP or GST, as indicated, and precipitation with glutathione-agarose beads. Blots were probed with anti-GFP monoclonal antibody. MACA-GST and unfused GST were expressed at least as abundantly as Gag-GST (data not shown).
HIV-1 Gag multimerization is required to overcome the inhibitory effect of the MA globular head on plasma membrane binding.
Previously, studies that investigated the requirement for HIV-1 Gag multimerization in membrane association have reached opposing conclusions (12, 19). Based on the fact that HIV-1 Gag contains a fully functional plasma membrane targeting signal (Fig. 2C and 3A), whose activity was dependent on the concentration of Gag only when the MA globular head was present (Fig. 2A), we reasoned that multimerization might be required for efficient membrane binding in this context. Consistent with this idea, a truncated Gag-GFP, termed MACA-GFP, which lacked the C-terminal Gag sequences bound to membrane with about the same efficiency as intact Gag at low concentrations (Fig. 3B). However, unlike that of wild-type Gag-GFP, MACA-GFP membrane binding could not be enhanced by an increased Gag concentration (Fig. 3B), because MACA-GFP lacks sequences necessary for Gag multimerization, as assessed by a previously described glutathione S-transferase (GST)-fusion protein coprecipitation assay (9) (Fig. 3C).
Conclusions.
There are at least two ways in which Gag multimerization may induce membrane binding. Multimerization of low-affinity membrane-binding moieties may increase the overall membrane-binding affinity of the complex. Alternatively, multimerization may induce an increase in the intrinsic membrane-binding affinity of each Gag molecule. Clearly, each Gag monomer contains a signal that is fully sufficient to direct a heterologous protein to the plasma membrane (Fig. 3A). However, the MA globular head partly masks the activity of this signal, particularly at low concentrations of Gag (Fig. 2). This inhibitory property is relieved at high Gag concentrations, but only if Gag is able to multimerize (Fig. 2A, 3B, and 3C). While this paper was in preparation, the structures of myristoylated MA in monomeric and trimeric forms were solved (22), and they confirm the previously proposed notion that MA can exist in two conformations, distinguished by the degree of myristate exposure (25). Moreover, MA trimerization was correlated with the “myristate-exposed” conformation (22). Thus, it seems likely that at low concentrations in the cell cytoplasm, Gag is primarily monomeric, with myristate being concealed by the MA globular head, and is therefore largely cytoplasmic. As the Gag concentration is increased, a greater fraction is driven into multimeric forms, resulting in myristate exposure and efficient membrane binding. Such a mechanism might be exploited by HIV-1 to confine Gag-membrane binding and virus assembly in infected cells to a short “burst” and/or to specific cellular locations where high levels of Gag expression are reached.
Acknowledgments
We thank Kyriacos Mitrophanous for the codon-optimized HIV-1 Gag gene.
This work was supported by a grant from the NIH (RO1AI50111). T.H. is the recipient of an AmFAR Scholar Award. P.D.B. is an Elizabeth Glaser Scientist of the Elizabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Burniston, M. T., A. Cimarelli, J. Colgan, S. P. Curtis, and J. Luban. 1999. Human immunodeficiency virus type 1 Gag polyprotein multimerization requires the nucleocapsid domain and RNA and is promoted by the capsid-dimer interface and the basic region of matrix protein. J. Virol. 73:8527-8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freed, E. O., J. M. Orenstein, A. J. Buckler-White, and M. A. Martin. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermida-Matsumoto, L., and M. D. Resh. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorchid, A., R. Halwani, M. A. Wainberg, and L. Kleiman. 2002. Role of RNA in facilitating Gag/Gag-Pol interaction. J. Virol. 76:4131-4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kiernan, R. E., A. Ono, and E. O. Freed. 1999. Reversion of a human immunodeficiency virus type 1 matrix mutation affecting Gag membrane binding, endogenous reverse transcriptase activity, and virus infectivity. J. Virol. 73:4728-4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotsopoulou, E., V. N. Kim, A. J. Kingsman, S. M. Kingsman, and K. A. Mitrophanous. 2000. A Rev-independent human immunodeficiency virus type 1 (HIV-1)-based vector that exploits a codon-optimized HIV-1 gag-pol gene. J. Virol. 74:4839-4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, P. P., and M. L. Linial. 1994. Efficient particle formation can occur if the matrix domain of human immunodeficiency virus type 1 Gag is substituted by a myristylation signal. J. Virol. 68:6644-6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Serrano, J., A. Yarovoy, D. Perez-Caballero, and P. D. Bieniasz. 2003. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl. Acad. Sci. USA 100:12414-12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin-Serrano, J., T. Zang, and P. D. Bieniasz. 2003. Role of ESCRT-I in retroviral budding. J. Virol. 77:4794-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morikawa, Y., D. J. Hockley, M. V. Nermut, and I. M. Jones. 2000. Roles of matrix, p2, and N-terminal myristoylation in human immunodeficiency virus type 1 Gag assembly. J. Virol. 74:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ono, A., D. Demirov, and E. O. Freed. 2000. Relationship between human immunodeficiency virus type 1 Gag multimerization and membrane binding. J. Virol. 74:5142-5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono, A., and E. O. Freed. 2004. Cell-type-dependent targeting of human immunodeficiency virus type 1 assembly to the plasma membrane and the multivesicular body. J. Virol. 78:1552-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ono, A., J. M. Orenstein, and E. O. Freed. 2000. Role of the Gag matrix domain in targeting human immunodeficiency virus type 1 assembly. J. Virol. 74:2855-2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paillart, J.-C., and H. G. Göttlinger. 1999. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of Gag membrane targeting. J. Virol. 73:2604-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Gottlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Resh, M. D. 2004. A myristoyl switch regulates membrane binding of HIV-1 Gag. Proc. Natl. Acad. Sci. USA 101:417-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandefur, S., V. Varthakavi, and P. Spearman. 1998. The I domain is required for efficient plasma membrane binding of human immunodeficiency virus type 1 Pr55Gag. J. Virol. 72:2723-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spearman, P., R. Horton, L. Ratner, and I. Kuli-Zade. 1997. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 71:6582-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spearman, P., J.-J. Wang, N. Vander Heyden, and L. Ratner. 1994. Identification of human immunodeficiency virus type 1 Gag protein domains essential to membrane binding and particle assembly. J. Virol. 68:3232-3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang, C., E. Loeliger, P. Luncsford, I. Kinde, D. Beckett, and M. F. Summers. 2004. Entropic switch regulates myristate exposure in the HIV-1 matrix protein. Proc. Natl. Acad. Sci. USA 101:517-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tritel, M., and M. D. Resh. 2000. Kinetic analysis of human immunodeficiency virus type 1 assembly reveals the presence of sequential intermediates. J. Virol. 74:5845-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou, W., and M. D. Resh. 1996. Differential membrane binding of the human immunodeficiency virus type 1 matrix protein. J. Virol. 70:8540-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]