Abstract
Phenolic compounds are phytochemicals with high health-promoting properties. Carrot is a vegetable highly worldwide consumed although its phenolic content is low compared to other plant products. The aim of this work was to evaluate changes in phenolic compounds in carrots caused by abiotic stresses. The phenylalanine ammonia-lyase (PAL) activity, phenolic compounds and total antioxidant capacity (TAC) changes during storage up to 72 h at 15 °C after wounding (shredding), 9 kJ UV-C m−2 pretreatment and hyperoxia (80 kPa) conditions of carrots were studied. Shredding and hyperoxia storage induced the highest phenolic compounds and TAC enhancements. Accumulation of phenolic compounds in shredded carrots could be structured in the following phases: 1st phase (<24 h): unchanged phenolic compounds levels with minimum PAL activity; 2nd phase (24–48 h): moderate phenolic increases (≈600–700 mg CAE kg−1 accumulated in 24 h) concurring with the greatest increase of PAL activity; 3nd phase (48–72 h): high phenolic increases (≈1600–2700 mg CAE kg−1, accumulated in 24 h) while a moderate increment of PAL activity was registered. Although UV-C pretreatment of shreds reduced phenolic accumulation, 600 % increments were still registered in those samples stored under hyperoxia conditions for 72 h. However, the contents of chlorogenic acid at 72 h were 1.4-fold higher in irradiated shreds under hyperoxia compared to the same samples under air conditions.
Keywords: UV-C radiation, Wounding, Hyperoxia, Phenolic compounds, Antioxidants
Introduction
The actual interest of better eating habits might be explained by the increasing of life expectancy or the high costs of health care, and need to satisfy population groups with special needs such as elderly and children (Siró et al. 2008). High intakes of fruit and vegetables have been proven to prevent a grand array of diseases such as degenerative disorders, cancer, cardiovascular among others (Slavin and Lloyd 2012). Nowadays, foods are not only intended to feed, but also to prevent chronic and nutritional-related diseases as well as to improve overall human well-being, mainly linked to the crescent consumer’s knowledge on functional foods. Enhancement of the health-promoting properties of fruit and vegetables will add value and create new opportunities, even with recent economical drawbacks. Therefore, there is a need to provide technologies to handle fresh products with enhanced health-promoting properties (Jongen 2002).
Carrot (Daucus carota L.) is a popular vegetable among broad strata of the population. The popularity of this vegetable is mainly due to its sensory characteristics and nutritional compounds. Furthermore, carrots do not contribute with high calories intake, however they play a significant source of nutrients, such as carotenoids, vitamins (A, E) and antioxidants on human diet (Sharma et al. 2011). Phenolic compounds are great antioxidants related to several health-promoting properties such as anti-inflammatory, antitumoral, as well as preventing neurodegenerative and chronic disorders. Moreover, those compounds contribute to sensory features to food products. Nowadays, health recommendations rely on a diet rich in multiple antioxidant compounds than one used based on a single antioxidant (Shahidi and Ambigaipalan 2015). Plant products such as carrots have been proposed as biofactories of phenolic compounds through different mechanisms induced by abiotic stresses (Cisneros-Zevallos 2003). Concisely, phenylalanine ammonia-lyase (PAL) is the key enzyme of primary (shikimate) and secondary (phenylpropanoid) pathways and is, therefore, involved in the biosynthesis of polyphenolic compounds (Dixon and Paiva 1995). It is well reported that this enzyme is induced by an array of biotic and abiotic stress-induced mechanisms, such as wounding, radiation exposure, hyperoxia storage, water stress, chilling injury, low minerals, hormones and pathogen attack, among others (Alegria et al. 2012; Avena-Bustillos et al. 2012; Becerra-Moreno et al. 2012; Jacobo-Velázquez et al. 2011). Consequently, such postharvest abiotic stresses enhance the levels of phenolic compounds like caffeoylquinic (CQA) acid, ferulic acid and their derivates as a defense mechanism of the plant (Jacobo-Velázquez et al. 2011). Previous studies have shown that single application of wounding, low UV-C doses and hyperoxia storage enhanced phenolic content on carrots and other plant products (Artés-Hernández et al. 2009a, b; Cisneros-Zevallos 2003; Martínez-Hernández et al. 2013b; Martínez-Hernández et al. 2011; Sánchez-Rangel et al. 2013). Nonetheless, to the best of our knowledge, the combined effect of wounding, moderate UV-C radiation and hyperoxia atmospheres on the phenolic compounds levels and related total antioxidant capacity (TAC) has not been studied yet. Accordingly, this work studied the singular and combined effects of UV-C pretreatment and hyperoxia storage on PAL activity, phenolic compounds and related TAC during storage of whole and shredded carrots at 15 °C.
Materials and methods
Plant material preparation
Fresh carrots (Daucus carota L., cvs. group Nantes, cv. Soprano) were bought in a local market (Cartagena, Spain) on April 6th. According to producer specifications, carrots were harvested on the first week of April in Villena area (northwest area of Alicante region, Spain) without any postharvest treatment, but washing, previous expedition to the market. Carrots were transported to the Pilot Plant of the Technical University of Cartagena where they were stored in a cold room at 5 °C until the next day when the experiment was conducted. Plant material was carefully inspected, selecting those with similar visual appearance and size (14–15 cm long and 2–3 cm diameter). Then, carrots (unpeeled) were sanitized in a cold room (8 °C) with chlorine (100 ppm NaClO; 5 °C; pH 6.5 ± 0.1) for 2 min, rinsed with tap water at 5 °C for 1 min and drained in a perforated basket for 1 min. A ratio of 300 g plant material: 5 L chlorine was used. Carrots were wounded to shreds (2 mm × 3 mm × 40–60 mm) with a food processor (FreshExpress+, Moulinex, Lyon, France). Unwounded carrots were used as control (hereinafter ‘whole’). Approximately 9 kg of shreds and 9 kg of whole carrots were prepared for the experiment. Immediately after wounding all samples were submitted to wounding, UV-C radiation and hyperoxia storage treatments as described below.
Abiotic stress treatments
UV-C pretreatment
The UV-C treatment chamber consisted of a reflective stainless steel chamber with two banks (one bank suspended horizontally over the radiation vessel and the other placed below it) being fitted to each bank 15 unfiltered germicidal emitting lamps (>80 % emitted spectrum at λ = 254.7 nm; TUV 36W/G36 T8, Philips, Eindhoven, The Netherlands) which has been previously described (Artés-Hernández et al. 2009a, b). Whole or shredded carrots were placed between the two lines of UV-C lamps at 17.5 cm above and below over a 35 mm thick bi-oriented polypropylene (PP) film mounted on a polystyrene (PS) net (130 × 68 cm) that minimized blockage of the UV-C radiation. The applied UV-C intensity of 67.6 W m−2 was calculated as the mean of 18 UV-C readings on each side of the net using a VLX 254 radiometer at λ = 254 nm (Vilber Lourmat, Marne la Vallee, France). Thus, both sides received the same UV-C intensity. The UV-C light intensity was kept constant and the applied dose was varied by altering the exposure time at the fixed distance. A UV-C radiation treatment of 9 kJ m−2 (exposure time of 139 s) was applied. Non-irradiated samples were used as ‘control’.
Hyperoxia storage
Samples to be stored under hyperoxia conditions were placed in plastic containers (30 cm diameter, 60 cm height) connected to a humidified air-flow-through system of either air or a gas mixture containing 80 kPa O2 (balanced with N2). The gas entering the containers was previously passed through a water trap giving a humidity close to saturation in order to greatly minimize water losses (Jacobo-Velázquez et al. 2011; McLaughlin and O’Beirne 1999) and avoid additional phenolic biosynthesis owed to water stress (Becerra-Moreno et al. 2015). In order to ensure a good air flow through carrot shreds, these samples were distributed in opened plastic petri dishes (8.5 cm diameter, 1 cm height). The CO2 partial pressures were kept <0.15 kPa to avoid any physiological effect exerted by CO2 such as anaerobic metabolism (Surjadinata and Cisneros-Zevallos 2003). Gas treatments were applied at 15 °C for up to 72 h in darkness. Sampling was conducted every 12 h with 3 replicates per treatment. Every replicate per treatment and sampling time consisted of approximately 100 g of carrots (in the case of whole carrots each 100 g-replicate was composed from three different carrot units). Samples were stored in reclosable PP bags zipper-locking at −80 °C until further analysis.
Analyses
UV-C transmittance through carrot tissue
Carrot sections (1.5 cm × 1.5 cm) with different thickness (0.10–3.00 mm) from internal and external tissue were prepared with a scalpel. Thickness of carrot sections was measured with a digital caliper (500-302 Series, Mitutoyo, Aurora IL, USA). Subsequently, carrot sections were carefully attached over the radiometer, placed on the net of the UV-C treatment chamber, and UV-C intensity was measured with (I) and without (I 0) the carrot section. For every thickness, five identical (±0.02 mm) sections were prepared representing five replicates. UV-C transmittance (T) for every carrot thickness was calculated using Eq. (1).
| 1 |
Relative electrolyte leakage
Relative electrolyte leakage (REL) was measured according to the method described by Martínez-Hernández et al. (2016) but with modifications. A 10-g carrot shreds portion was placed in a glass bottle (100 mL capacity) and 70 mL of 0.2 M mannitol (Sigma Aldrich, Steinheim, Germany) were added. For whole samples, a carrot was placed in a glass bottle (1 L capacity) and 800 mL of 0.2 M mannitol were added. The electrical conductivity of the bathing mannitol solution was measured with an electrical conductivity meter (GLP32, Crison, Alella, Spain) after 60 min (C0) of incubation with orbital shaking (Stuart SSL1, Osa, UK) at a speed of 60 cycles min−1. Then, the samples were heated at 121 °C for 20 min in an autoclave and the conductivity (C) of the bathing mannitol solution was measured after cooling at room temperature. The REL was calculated using Eq. (2). Three replicates per treatment were analyzed.
| 2 |
Color
Color was determined using a colorimeter (Minolta CR-300 Series, Japan) calibrated with a white reference plate (light source C), 2° observer and 8-mm viewing aperture. Measurements were recorded using the standard tristimulus parameters (L, a, b) of the CIE Lab system on three equidistant points of each replicate. Three color readings were taken on three parts of the same sample and all three measurements were automatically averaged by the device and recorded.
Whitening and browning are the main color degradation processes occurred in wonded (fresh-cut) carrots. Accordingly, whitening index (WI) and browning index (BI) were calculated from CIE Lab parameters according to Eqs. (3) and (4) as previously described (Castillejo et al. 2015; Martínez-Hernández et al. 2016; Palou et al. 1999).
| 3 |
| 4 |
Complimentary, total color differences (∆E) is a colorimetric parameter extensively used to characterize the variation of colors during processing and storage of food products (Martínez-Hernández et al. 2013a). ∆E was calculated according to Eq. (5).
| 5 |
Phenylalanine ammonia-lyase (PAL)
PAL activity was analyzed according to Ke and Saltveit (1986) with modifications. Concisely, 2 g carrot tissue samples were mixed with polyvinylpolypyrrolidone (Sigma, St Louis, MO, USA) (0.2 g) and homogenized (Ultra Turrax® model 18T, IKA-Werke GmbH & Co. KG, Germany) in cold 50 mM borate buffer (pH 8.5) containing 400 μL L−1 β-mercaptoethanol (Sigma, St Louis, MO, USA). Homogenates were filtered through four layers of cheesecloth and then centrifuged at 10,000×g for 20 min at 4 °C. Supernatants were used as enzyme extract. Two sets of UV-Star well plates (Greiner Bio-One, Frickenhausen, Germany) containing 69 μL of PAL extract plus 200 µL ultrapure water were prepared for every sample and pre-incubated at 40 °C for 5 min. Afterwards, 30 μL of either water (blank) or 100 mM l-phenylalanine substrate solution (freshly prepared before assay) were added to each of the well for every sample set. The absorbances of sample sets were measured at 290 nm, using a Multiscan plate reader (Tecan Infininte M200, Männedorf, Switzerland), at time 0 and after 1 h of incubation at 40 °C. The PAL activity was calculated as μmol of t-cinnamic acid synthesized kg−1 fresh weight (fw) h−1 using a t-cinnamic acid (Sigma, St Louis, MO, USA) standard curve (0–6.75 mM).
Phenolic compounds
Extraction to determine phenolic compounds and TAC extract was conducted by homogenization (Ultra Turrax®) of 2 g of sample in 8 mL methanol (Sigma, St Louis, MO, USA) for 20 s under ice-water bath. Subsequently, extracts were centrifuged at 13,500×g for 20 min at 4 °C and supernatants were collected and analyzed. Extracts for individual phenolic compounds were further filtered through a 0.22 µm polyethersulphone filter and stored at −80 °C in amber vials until Ultra High-Performance liquid chromatography (UHPLC) analysis.
Total phenolic content (TPC) was analyzed by Folin–Ciocalteu reagent method as previously described (Martínez-Hernández et al. 2011). Briefly, a 19 µL aliquot of TPC extract was placed on a 96 PS flat bottom well plate (Greiner Bio-One, Frickenhausen, Germany) and 29 µL of Folin–Ciocalteu reagent 1N (Sigma, St Louis, MO, USA) were added. Samples were incubated for 3 min in darkness at room temperature. After incubation, 192 µL of a solution containing Na2CO3 (4 g L−1) and NaOH (20 g L−1) were added and the reaction was carried out for 1 h at room temperature in darkness, measuring the absorbance at 750 nm using the Multiscan plate reader. TPC was expressed as chlorogenic acid equivalents (ChAE) in mg kg−1 fw. Each of the three replicates was analyze by triplicate.
Analyses of individual phenolic compounds were conducted as previously described (Alegria 2015) with some modifications. Briefly, samples of 20 µL were analyzed using an UHPLC instrument (Shimadzu, Kyoto, Japan) equipped with a DGU-20A degasser, LC-30AD quaternary pump, SIL-30AC autosampler, CTO-10AS column heater and SPDM-20A photodiode array detector. The UHPLC system was controlled by the software LabSolutions (Shimadzu, v. 5.42 SP5). Chromatographic analyses were carried out onto a Kinetex C18 column (100 mm × 4.6 mm, 2.6 µm particle size; Phenomenex, Macclesfield, UK) with a KrudKatcher Ultra HPLC guard column (Phenomenex, Macclesfield, UK). The column temperature was maintained at 25 °C. The mobile phase was acidified water (A; formic acid to final pH 2.3) and acidified methanol (B; formic acid to final pH 2.3). The flow rate was 1.5 mL min−1. Gradient program used was 0/88, 1.2/88, 2.4/85, 8.3/70, 9.4/50, 11.8/50, 20.8/55, 22.0/60 (min/ % phase A). Then, column equilibration was conducted at 0 % A for 2.2 min. Chromatograms were recorded at 320 nm. Phenolic acids were quantified as standards of chlorogenic acid (3-CQA), ferulic acid (Sigma, St Louis, MO, USA), isochlorogenic acid A (3,5-CQA) and C (4,5-CQA) (ChromaDex, Irvine, CA, USA). The calibration curves were made with at least six data points. The results were expressed as mg kg−1 fw. Each of the three replicates was analyzed by duplicate.
Total antioxidant capacity
The extracts were analyzed for TAC according to Brand-Williams et al. (1995) with slight modifications (Martínez-Hernández et al. 2013b). Briefly, a solution of 0.7 mM 2,2-diphenyl-1-picrylhydrazil (DPPH) (Sigma, St Louis, MO, USA) in methanol was prepared 2 h before the assay and adjusted to 1.1 (nm) immediately before use. A 21 µL aliquot of the previously described extract was placed on a 96 PS flat-bottom well plate and 194 µL of DPPH was added. The reaction was carried out for 30 min at room temperature in darkness and the absorbance at 515 nm was measured using the Multiscan plate reader. Results were expressed as Trolox (Sigma, St Louis, MO, USA) equivalent antioxidant capacity kg−1 fw. Each of the three replicates was analyze by triplicate.
Statistical analyses
A complete randomized design in triplicate, with two-way ANOVA (treatment × storage), by Post Hoc Tuckey HSD tests, were used with SPSS software (v. 21, IBM, USA). Possible synergistic effects of the stresses combinations were studied with Limpel’s formula (Eq. 6) according to (Richer 1987), where the effectiveness of a combination of treatments exceeds the prediction of the effectiveness of their additive action.
| 6 |
Results
UV-C transmittance through carrot tissue
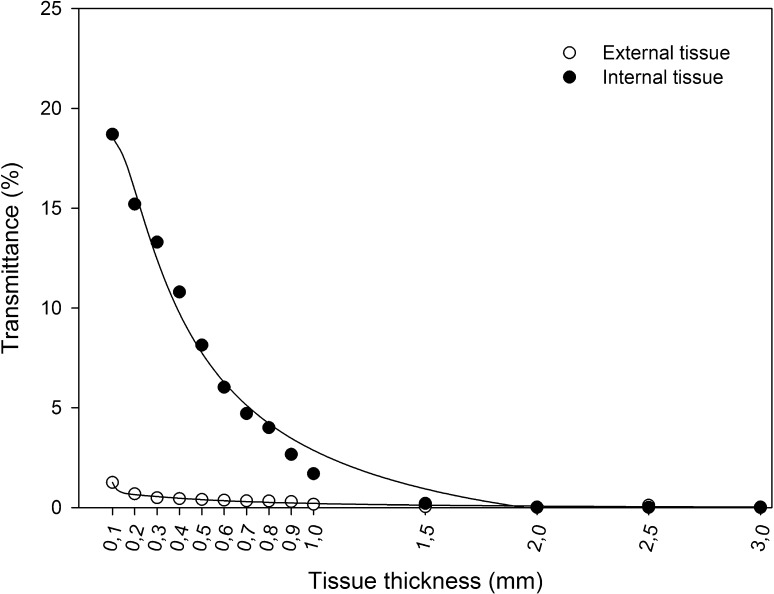
UV-C radiation showed a low transmittance through carrot tissue. Accordingly, carrot sections prepared with the lowest thickness (0.1 mm) showed a transmittance <20 %. Carrot external tissue showed a low UV-C transmittance compared to internal tissue (Fig. 1). Accordingly, external tissue sections of 0.1 mm thickness showed a transmittance of 1.25 % while the same thickness for internal tissue showed a transmittance of 18.7 %.
Fig. 1.
UV-C transmittance of internal and external carrot tissue sections with different thickness. Symbols represent experimental data and lines represent fitted data with polynomial inverse third order (R2 > 0.98)
Relative electrolyte leakage
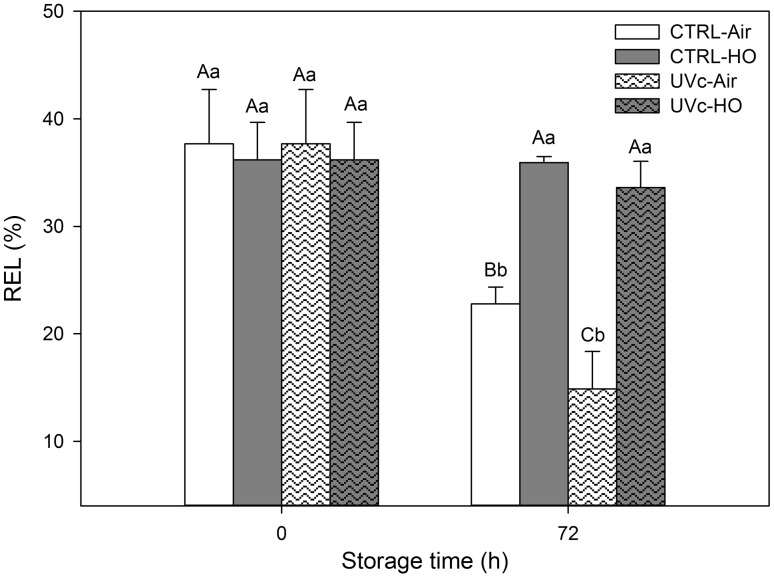
Initial REL of whole and shredded carrots was 0.9 ± 0.2 and 36.9 ± 0.9 %, respectively, without significant (p < 0.05) differences among irradiated and non-irradiated samples on processing day (Fig. 2). No significant (p < 0.05) REL differences among whole carrots were found at 72 h (data not shown). Attending to shredded samples, REL values of air-stored ones showed 13–19 lower units compared to hyperoxia conditions. Among air-stored shreds, irradiated ones showed the lowest REL with 14.9 ± 3.5 %. Non-irradiated shreds stored under air conditions registered intermediate REL of 32.8 ± 1.6 % at 72 h.
Fig. 2.
Relative electrolyte leakage (REL) of shredded carrots treated with different postharvest abiotic stresses (UV-C and hyperoxia storage) at time 0 and after 72 h of storage at 15 °C (n = 3 ± SD). Different capital letter denotes significant differences (p < 0.05) among different treatments for the same sampling day. Different lowercase letters denote significant differences (p < 0.05) among different sampling days for the same treatment
Color
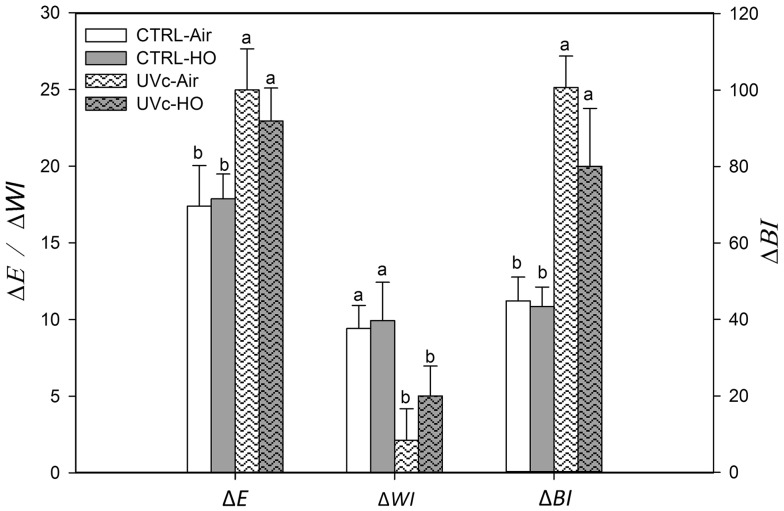
Whole carrots did not show significant (p < 0.05) color changes throughout storage (data not shown). Attending to shredded samples, the applied UV-C dose only induced mild color changes of ∆E = 6.2 with a slight browning of ∆BI = 80.4 on processing day (data not shown). Initial WI of 22.3 was not significantly (p < 0.05) changed after 72 h with final ΔWI ranging from 2 to 10 (Fig. 3). Irradiated shreds showed higher ∆E (23–25) and browning (∆BI = 80–101) after 72 h compared to control carrots regardless of gas treatments (Fig. 3).
Fig. 3.
Total color (ΔE), whitening (ΔW) and browning differences (ΔBI) of shredded carrots treated with different postharvest abiotic stresses (UV-C and hyperoxia storage) after 72 h of storage at 15 °C (n = 3 ± SD). Different letters denote significant differences (p < 0.05) among different treatments
Phenylalanine ammonia-lyase activity
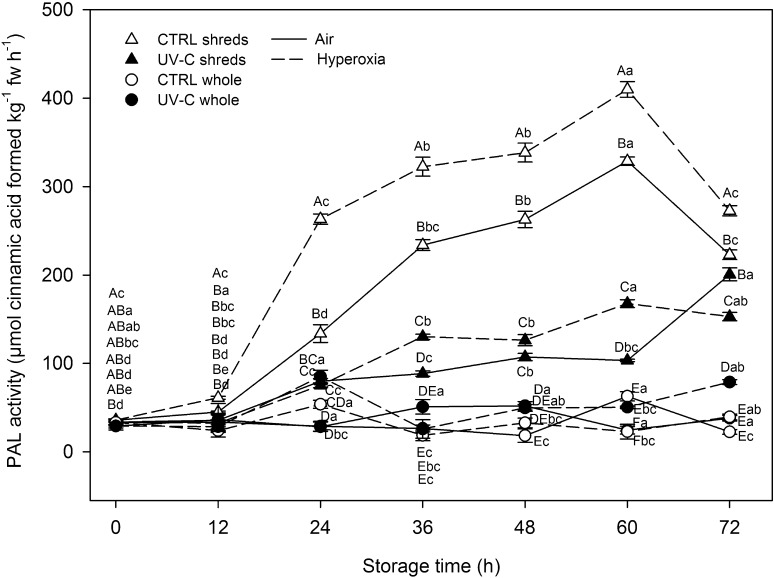
Carrots showed an initial PAL activity of 39.5 ± 9.6 µmol cinnamic acid formed kg−1 fw h−1 without significant (p < 0.05) differences among treatments (Fig. 4). PAL activity of hyperoxia-stored shreds early increased after 12 h registering an activity 71 % higher compared to their respective initial levels. However, the remaining shredded samples did not achieve significant changes of PAL activity after 12 h. In general, PAL activity of shreds registered a continuous increase throughout storage reaching maximum levels at 60 h. Accordingly, air-stored shreds registered PAL activities 820 % higher after 60 h while hyperoxia-stored shreds showed 1050 % enhanced PAL activities after 60 h regarding their initial levels.
Fig. 4.
Phenylalanine ammonia lyase activity of carrots treated with different postharvest abiotic stresses (wounding, UV-C and hyperoxia storage) during storage up to 72 h at 15 °C (n = 3 ± SD). Different capital letters denote significant differences (p < 0.05) among different treatments for the same sampling day. Different lowercase letters denote significant differences (p < 0.05) among different sampling days for the same treatment
UV-C pretreatment resulted in 4 and threefold reduced enhancements of PAL activities after 60 h under air and hyperoxia conditions, respectively, compared to non-irradiated carrots. PAL activity of non-irradiated shreds was approximately 30 % reduced from 60 to 72 h regardless of the storage atmosphere. PAL activities of UV-C-pretreated shreds followed a continuous increment throughout storage, although in a lower rate compared to non-irradiated shreds. In the same way, UV-C-pretreated shreds stored under air conditions registered a 94 % increment of PAL activity from 60 to 72 h, while the PAL activity of the same samples remained unchanged under hyperoxia conditions during that period.
Phenolic compounds
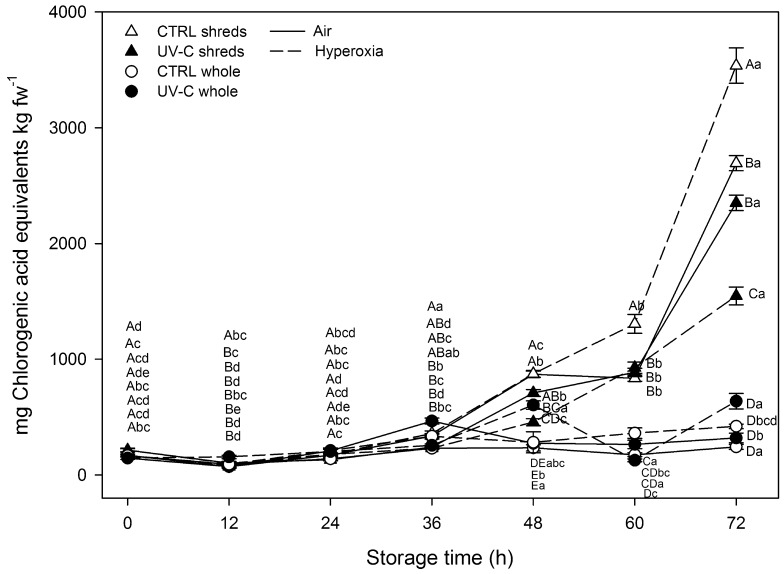
The initial TPC of whole carrots was 166.4 ± 11.7 mg ChAE kg fw−1 (Fig. 5). The major individual phenolic compounds identified were 3-CQA, 3,5-CQA, 4,5-CQA and ferulic acid (Table 1). These phenolic compounds accounted 67.5, 15.5, 14.8 and 2.2 % of the sum of individual phenolics, respectively. The phenolic contents of carrots were unchanged (p < 0.05) immediately after wounding and UV-C radiation on processing day, although these levels increased throughout storage of stressed samples.
Fig. 5.
Total phenolic content of carrots treated with different postharvest abiotic stresses (wounding, UV-C and hyperoxia storage) during storage up to 72 h at 15 °C (n = 3 ± SD). Different capital letters denote significant differences (p < 0.05) among different treatments for the same sampling day. Different lowercase letters denote significant differences (p < 0.05) among different sampling days for the same treatment
Table 1.
Individual phenolic compounds of carrots treated with different postharvest abiotic stresses (wounding, UV-C and hyperoxia storage) during storage up to 72 h at 15 °C (n = 3 ± SD)
| Storage (h) | 0 | 12 | 24 | 36 | 48 | 60 | 72 |
|---|---|---|---|---|---|---|---|
| 3-CQA (mg kg −1 fw) | |||||||
| Whole | |||||||
| AIR | 36.6 ± 13.3 Bc | 65.6 ± 15.0 Abc | 74.6 ± 0.7 Cb | 60.4 ± 6.8 Fbc | 59.7 ± 2.4 Fbc | 48.1 ± 8.7 Fbc | 150.7 ± 49.8 Da |
| AIR-UVc | 48.2 ± 2.3 Ad | 42.7 ± 3.5 Bd | 115.1 ± 9.1 Bd | 250.0 ± 33.4 Bd | 149.8 ± 5.2 Ca | 196.4 ± 54.6 BCb | 330.3 ± 10.5 BCc |
| HO* | 36.6 ± 13.3 Be | 36.2 ± 1.5 Be | 85.2 ± 19.3 Ccd | 364.9 ± 25.1 Aa | 79.7 ± 5.0 Ed | 98.1 ± 16.1 EFc | 124.6 ± 0.5 Db |
| HO-UVc | 48.2 ± 2.3 Ae | 80.3 ± 19.7 Ad | 158.4 ± 27.8 Ac | 161.8 ± 6.9 Cc | 304.3 ± 1.6 Ab | 319.3 ± 14.0 Ab | 354.9 ± 13.8 Ba |
| Shredded | |||||||
| AIR | 27.5 ± 0.3 Cd | 34.9 ± 4.0 Bd | 69.5 ± 9.5 CDc | 71.5 ± 6.1 Fc | 160.4 ± 11.8 Ca | 133.6 ± 19.6 DEb | 146.6 ± 6.6 Dab |
| AIR-UVc | 43.5 ± 2.2 ABd | 38.3 ± 0.5 Bd | 51.8 ± 2.0 DEd | 58.7 ± 8.2 Fd | 200.4 ± 19.5 Ba | 164.7 ± 7.1 CDb | 117.1 ± 29.4 Dc |
| HO | 27.5 ± 0.3 Cf | 42.9 ± 8.6 Bef | 69.2 ± 1.0 CDe | 137.7 ± 1.1 Dd | 190.4 ± 4.4 Bc | 249.5 ± 61.3 Bb | 299.3 ± 7.3 Ca |
| HO-UVc | 43.5 ± 2.2 ABd | 46.0 ± 1.8 Bd | 42.9 ± 0.9 Ed | 115.6 ± 16.0 Ec | 125.8 ± 18.6 Dc | 197.0 ± 14.0 BCb | 421.2 ± 12.2 Aa |
| Ferulic acid (mg kg −1 fw) | |||||||
| Whole | |||||||
| AIR | 1.2 ± 0.9 ABb | 1.5 ± 0.9 Ab | 1.6 ± 0.2 DEb | 0.2 ± 0.2 Cb | 2.1 ± 0.7 Cb | 2.2 ± 0.1 Eb | 12.6 ± 3.0 Aa |
| AIR-UVc | 2.2 ± 0.4 Ac | 1.3 ± 0.6 BCc | 3.9 ± 0.5 Bb | 7.2 ± 3.0 Aa | 3.9 ± 0.6 Bb | 5.1 ± 0.6 Bb | 7.8 ± 0.4 BCa |
| HO | 1.2 ± 0.9 ABb | 1.3 ± 0.0 BCb | 0.0 ± 0.0 Db | 2.2 ± 1.6 Bb | 4.4 ± 1.1 Ba | 0.0 ± 0.3 Ca | 3.8 ± 0.0 CDa |
| HO-UVc | 2.2 ± 0.4 Ad | 2.3 ± 0.0 Ad | 7.6 ± 0.9 Ab | 2.3 ± 0.2 Bd | 8.6 ± 0.9 Aa | 5.1 ± 0.4 Bc | 8.7 ± 0.9 ABa |
| Shredded | |||||||
| AIR | 0.4 ± 0.0 Bd | 0.3 ± 0.0 Ed | 0.9 ± 0.6 DEcd | 1.1 ± 0.2 BCc | 2.9 ± 0.7 BCb | 3.1 ± 0.8 Db | 4.4 ± 0.5 BCDa |
| AIR-UVc | 1.0 ± 0.2 Bd | 0.7 ± 0.1 DEd | 0.7 ± 0.5 Ed | 1.2 ± 0.0 BCcd | 3.6 ± 1.1 BCa | 2.9 ± 0.8 DEab | 2.1 ± 0.2 Dbc |
| HO | 0.4 ± 0.0 Be | 0.7 ± 0.5 DEe | 2.9 ± 0.4 Cc | 1.6 ± 0.2 BCd | 2.2 ± 0.7 Ccd | 6.2 ± 0.5 Ab | 9.0 ± 0.0 ABa |
| HO-UVc | 1.0 ± 0.2 Bd | 1.0 ± 0.1 CDd | 1.0 ± 0.2 DEcd | 2.1 ± 0.3 Bbcd | 2.2 ± 1.7 Cbc | 3.1 ± 0.3 Db | 6.8 ± 0.2 BCa |
| 3,5-CQA (mg kg −1 fw) | |||||||
| Whole | |||||||
| AIR | 8.4 ± 0.0 Be | 8.7 ± 0.3 Acd | 9.0 ± 0.2 BCb | 9.2 ± 0.3 BCa | 8.7 ± 0.1 Acd | 8.5 ± 0.1 Cde | 8.8 ± 0.1 Dbc |
| AIR-UVc | 8.7 ± 0.0 Bc | 8.6 ± 0.1 ABc | 8.7 ± 0.2 BCbc | 15.1 ± 0.7 BCa | 8.6 ± 0.0 Ac | 8.7 ± 0.0 Cc | 9.4 ± 0.8 CDb |
| HO | 8.4 ± 0.0 Bc | 8.7 ± 0.0 ABbc | 8.7 ± 0.1 Cbc | 11.4 ± 1.2 Ca | 9.9 ± 2.0 Ab | 8.5 ± 0.1 Cbc | 8.5 ± 0.0 Dbc |
| HO-UVc | 8.7 ± 0.0 Bbc | 8.7 ± 0.1 Abc | 9.5 ± 0.5 Aab | 10.3 ± 1.0 Aa | 9.3 ± 0.1 Ab | 8.3 ± 0.8 Cc | 8.1 ± 0.6 Dc |
| Shredded | |||||||
| AIR | 9.0 ± 0.3 Abc | 8.6 ± 0.0 ABc | 8.7 ± 0.0 BCc | 8.7 ± 0.1 BCc | 9.1 ± 0.5 Abc | 9.5 ± 0.1 Bb | 10.3 ± 0.4 Ca |
| AIR-UVc | 8.6 ± 0.2 Ba | 8.6 ± 0.0 Ba | 8.7 ± 0.1 Ca | 8.6 ± 0.2 Ca | 9.4 ± 0.3 Aa | 9.6 ± 0.5 Ba | 9.4 ± 1.8 CDa |
| HO | 9.1 ± 0.3 Acd | 8.6 ± 0.0 ABd | 8.9 ± 0.1 BCcd | 8.9 ± 0.1 BCcd | 9.4 ± 0.4 Ac | 10.6 ± 0.6 Ab | 16.4 ± 1.2 Aa |
| HO-UVc | 8.6 ± 0.2 Bd | 8.7 ± 0.0 ABd | 9.0 ± 0.0 Bd | 10.2 ± 0.8 Bb | 9.1 ± 0.4 Acd | 9.7 ± 0.1 Bbc | 11.9 ± 0.6 Ba |
| 4,5-CQA (mg kg −1 fw) | |||||||
| Whole | |||||||
| AIR | 8.0 ± 0.3 Ab | 4.9 ± 0.7 Bc | 3.7 ± 0.2 CDd | 2.7 ± 0.1 Cc | 4.9 ± 0.0 Dc | 4.0 ± 0.2 EFd | 18.0 ± 0.1 Aa |
| AIR-UVc | 8.7 ± 0.1 Bc | 4.0 ± 0.6 Bd | 4.7 ± 1.0 Bd | 15.5 ± 0.6 Aa | 6.0 ± 1.0 Cc | 6.6 ± 1.4 Bc | 7.7 ± 1.3 Fb |
| HO | 8.0 ± 0.3 Ac | 4.9 ± 0.9 Bd | 4.3 ± 0.1 BCd | 8.6 ± 1.6 Ba | 6.8 ± 0.0 Cbc | 7.1 ± 0.1 Abc | 7.5 ± 0.1 Db |
| HO-UVc | 8.7 ± 0.1 Bcd | 8.0 ± 3.5 Abc | 9.0 ± 0.4 Ab | 3.0 ± 0.1 Ee | 16.7 ± 1.2 Aa | 4.4 ± 0.3 Ede | 5.9 ± 0.1 Ecd |
| Shredded | |||||||
| AIR | 3.4 ± 0.2 Cb | 3.3 ± 0.1 Bb | 3.6 ± 0.2 DEb | 3.2 ± 0.1 DEb | 4.9 ± 0.9 DEa | 3.8 ± 0.0 Fb | 5.5 ± 0.5 EFa |
| AIR-UVc | 3.8 ± 0.4 Cc | 3.5 ± 0.1 Bc | 3.1 ± 0.1 Ec | 3.1 ± 0.1 Ec | 8.0 ± 0.5 Ba | 5.4 ± 0.2 Cb | 4.9 ± 1.0 CDb |
| HO | 3.4 ± 0.2 Ccd | 3.7 ± 0.5 Bcd | 3.3 ± 0.1 DEd | 3.9 ± 0.2 CDc | 4.7 ± 0.2 DEb | 4.9 ± 0.3 Db | 9.2 ± 0.7 Ba |
| HO-UVc | 3.8 ± 0.4 Ccd | 3.4 ± 0.1 Bd | 3.6 ± 0.1 DEcd | 3.4 ± 0.3 DEd | 4.0 ± 0.5 Ec | 4.8 ± 0.3 Db | 8.4 ± 0.1 BCa |
Different capital letter denotes significant differences (p < 0.05) among different treatments for the same sampling day. Different lowercase letter denotes significant differences (p < 0.05) among different sampling days for the same treatment
* HO hyperoxia storage, 3-CQA 3-caffeoylquinic acid, 3,5-CQA 3,5-dicaffeoylquinic acid, 4,5-CQA 4,5-dicaffeoylquinic acid
Regarding whole carrots, a UV-C pretreatment induced maximum TPC enhancements of 220 and 315 % after 36 and 48 h in air and hyperoxia storage conditions, respectively, and then followed by a general decrease. This behavior was also observed in non-irradiated shreds, which registered ≈400–410 % higher TPC contents after 48 h compared to initial levels regardless of the atmosphere conditions. Whole irradiated samples showed 120 and 340 % higher TPC contents after 72 h under air and hyperoxia storage conditions, respectively, compared to their respective initial levels. TPC of non-irradiated whole carrots remained unchanged (p < 0.05) throughout storage. Correspondingly, the highest 3-CQA enhancements were registered by irradiated whole samples with 585 (air) and 636 % (hyperoxia) after 72 h.
Wounding of non-irradiated carrots enhanced TPC by ≈1490 % after 72 h under air conditions. Hyperoxia benefit on TPC was only significantly observed after 60 h reaching non-irradiated samples the maximum TPC enhancements, ≈2000 %, after 72 h. Similarly, non-irradiated shreds stored under hyperoxia conditions registered approximately fivefold higher 3,5-CQA enhancements after 72 h compared to air-stored non-irradiated shreds. Furthermore, the rest of phenolic compounds of non-irradiated shreds registered 2–3-fold higher accumulation under hyperoxia conditions compared to air conditions after 72 h. The combination of wounding and hyperoxia stresses showed a synergistic effect after 72 h since the observed TPC enhancement (2000 %) was higher than that calculated according to Limpel’s formula (1620 %).
The UV-C pretreatment, in shreds, induced lower TPC accumulation throughout storage, contrary to non-irradiated shreds. Accordingly, UV-C-pretreated shreds registered 1.5 and 3.2-fold lower TPC enhancements after 72 h under air and hyperoxia conditions, respectively, compared to non-irradiated shreds. Similarly, 3,5-CQA/4,5-CQA registered 2 and 6-fold lower accumulation in irradiated shreds under air and hyperoxia conditions, respectively, compared to non-irradiated shreds. However, irradiated shreds under hyperoxia conditions showed 1.4-fold higher 3-CQA content compared to non-irradiated samples stored under air conditions for 72 h. Meanwhile, no great differences between air and hyperoxia conditions were observed in the UV-C-induced lower levels of the rest of individual phenolic compounds.
Total antioxidant capacity
The initial TAC of whole carrots was 135.7 ± 45.4 mg Trolox kg−1 fw (Table 2). Wounding of carrots increased initial TAC by ≈610 % just after processing on day 0. Similarly, UV-C treatment of whole and shredded samples induced 269 and 16 % higher TAC on processing day. The combination of wounding and UV-C did not show a synergistic effect since the observed TAC enhancement of carrots treated with both stresses combined (720 %), compared to untreated whole ones, was lower than TAC enhancement calculated according to Limpel’s formula (870 %) being then considered as an additive effect.
Table 2.
Total antioxidant capacity of carrots treated with different postharvest abiotic stresses (wounding, UV-C and hyperoxia storage) during storage up to 72 h at 15 °C (n = 3 ± SD)
| Storage (h) | 0 | 12 | 24 | 36 | 48 | 60 | 72 |
|---|---|---|---|---|---|---|---|
| Whole | |||||||
| AIR | 135.7 ± 45.4 Dd | 234.5 ± 35.2 Dcd | 351.8 ± 74.1 Ebcd | 481.6 ± 24.5 Dabc | 619.3 ± 59.1 Cab | 368.8 ± 44.1 Gbcd | 740.7 ± 92.6 Ea |
| AIR-UVc | 500.0 ± 44.0 Cc | 475.6 ± 41.6 Cc | 470.6 ± 42.1 DEc | 314.2 ± 31.5 DEcd | 241.3 ± 42.1 Dd | 844.4 ± 39.8 Eb | 1511.9 ± 43.1 CDa |
| HO* | 135.7 ± 45.4 Dc | 365.2 ± 32.6 Dbc | 476.1 ± 54.0 DEab | 349.5 ± 43.1 Ebc | 197.9 ± 39.6 Dbc | 619.5 ± 76.8 Fa | 732.6 ± 70.4 Ea |
| HO-UVc | 500.0 ± 44.0 Cc | 515.6 ± 49.5 Cc | 548.7 ± 88.2 CDEc | 378.2 ± 21.0 DEd | 169.7 ± 29.7 Dd | 1071.7 ± 55.4 Db | 1432.8 ± 33.9 Da |
| Shredded | |||||||
| AIR | 959.6 ± 103.1 Bb | 847.2 ± 54.5 Bc | 742.1 ± 47.2 CDd | 841.3 ± 45.6 Cc | 983.3 ± 32.4 Bb | 1490.4 ± 42.8 Ca | 1580.2 ± 41.4 CDa |
| AIR-UVc | 1112.7 ± 152.3 Ab | 1004.2 ± 87.6 Ab | 1046.6 ± 52.9 ABb | 1198.5 ± 87.2 Ab | 1229.0 ± 26.4 Ab | 1878.7 ± 24.7 Ba | 2049.6 ± 115.8 Ba |
| HO | 959.6 ± 103.1 Bd | 898.2 ± 87.5 Bd | 816.3 ± 49.6 BCd | 1042.2 ± 92.1 BCcd | 1158.4 ± 43.1 Ac | 2063.2 ± 64.4 Ab | 2668.1 ± 128.1 Aa |
| HO-UVc | 1112.7 ± 152.3 Abc | 1085.3 ± 95.6 Abc | 1232.1 ± 34.0 Ab | 1106.2 ± 36.8 ABbc | 967.3 ± 32.2 Bc | 1336.8 ± 42.7 Cb | 1863.2 ± 55.3 BCa |
Different capital letter denotes significant differences (p < 0.05) among different treatments for the same sampling day. Different lowercase letter denotes significant differences (p < 0.05) among different sampling days for the same treatment
* HO hyperoxia storage
TAC of all carrots showed a constant increase throughout storage, registering maximum increases in the last 24 h of storage. Whole samples registered maximum TAC increases of 7.0 and 2.4–2.8-fold regarding their respective initial levels in non-irradiated air-stored and hyperoxia and/or UV-C stressed ones, respectively. However, final TAC of shreds was higher than that of whole shreds. Accordingly, shreds stored under air and hyperoxia registered TAC of 2050 and 2668 mg Trolox kg−1 fw at 72 h, respectively. However, UV-C pretreatment of shreds induced 30 and 40 % lower TAC levels at 72 h under air and hyperoxia conditions, respectively, compared to the respective non-irradiated samples at 72 h. TAC changes throughout storage were highly correlated (R2 = 0.70) to TPC.
Discussion
Carrot has been widely used as a model system to understand the effect of different postharvest abiotic stresses on the phenylpropanoid metabolism due to the great enhancement of phenolic compounds observed, with high antioxidant capacity, compared to other vegetables (Cisneros-Zevallos 2003). UV-C is a sanitizing method used in fresh-cut products as a sustainable alternative to conventional NaOCl (Martínez-Hernández et al. 2015b). Food safety legislation of fresh-cut products is regulated for Escherichia coli, Salmonella spp. and Listeria monocytogenes (EC_1441/2007 2007). Inactivation kinetics of these three pathogens by UV-C has been recently modeled in fresh-cut products (Martínez-Hernández et al. 2015a). Therefore, intermediate doses of 9–10 kJ m−2 are needed to ensure the legislated food safety criteria of a fresh-cut product (Martínez-Hernández et al. 2011; Martínez-Hernández et al. 2015a). In that sense, a UV-C dose of 9 kJ m−2 was selected in this experiment achieving a sanitizing effect of approximately 1.5 log units for mesophiles and yeasts and molds (data not shown). The effect of this moderate UV-C dose, single or combined with other abiotic stresses like hyperoxia storage and wounding, on the phenolic content and related antioxidant capacity and PAL activity are firstly reported in this study, to the best of our knowledge.
The initial TPC of non-wounded carrot (166.4 mg GAE kg fw−1) was similar to previous data being hydroxycinnamic acids and their derivatives the major phenolic compounds found (Alegria et al. 2012). According to phenolic profile, chlorogenic acid was the major compound found accounting approximately 70 % of the sum of individual phenolic compounds. As expected, application of the studied abiotic stresses did not immediately affect PAL activity of samples on processing day, and consequently the phenolic contents. However, TAC was apparently increased immediately after UV-C radiation and, in a greater extend, after wounding (showing the combination of both stresses an additive effect according to Limpel’s formula). TAC was highly correlated (R2 = 0.70) to TPC throughout storage of samples as previously found (Cisneros-Zevallos 2003). Accordingly, the observed higher TAC immediately after wounding and UV-C pretreatment may be an experimental artifact resulted from higher extraction of other antioxidant compounds of carrots such as carotenoids due to increased cell wall depolymerization (Alegria et al. 2012; Bhat et al. 2007).
Wounding and hyperoxia storage of carrots at 15 °C induced phenolic compounds enhancements which were well correlated to TAC and explained by the observed changes of PAL activity. The phenolic compounds accumulation after wounding and hyperoxia stresses has been related to PAL activation being proposed ATP and reactive oxygen species as signaling molecules (Jacobo-Velázquez et al. 2011). Furthermore, phenolic compounds in wounded plants are produced in part as a mechanism to support the biosynthesis of lignin (Becerra-Moreno et al. 2015). Accordingly, a REL decrease was observed in air-stored shreds after 72 h possibly due to such lignification process being this lignification probably inhibited in hyperoxia-stored samples. This study shows a detailed register of phenolic contents and related antioxidant capacity in stressed carrots in 12-h intervals. Accordingly, phenolic accumulation in shredded carrots during storage at 15 °C could be divided into three different phases: 1st phase, <24 h: unchanged phenolic compounds levels with minimum PAL activity; 2nd phase, 24–48 h: moderate phenolic increments (≈600–700 mg CAE kg−1 accumulated in 24 h) concurring with the greatest increase of PAL activity; 3nd phase, 48–72 h: high phenolic increments (≈1600–2700 mg CAE kg−1 accumulated in 24 h) while a moderate increment of PAL activity was registered. The observed lower increase of PAL activity from 48 to 60 h and subsequent intense decrease in non-irradiated shreds may be a result of a feedback modulation or due to the diversion of the synthetic capacity of the cell to the production of other proteins (Alegria 2015; Boerjan et al. 2003; Saltveit 2000). Maximum phenolic content of non-irradiated shreds at 72 h may be the delayed consequence of maximum PAL activity which is the first key enzyme in the phenylpropanoid pathway. The combination of wounding and hyperoxia stresses showed a synergistic effect with phenolic accumulation of 2000 % after 72 h previously described in carrot shreds (Jacobo-Velázquez et al. 2011).
Combination of moderate UV-C dose and subsequent hyperoxia storage reduced TPC increments throughout storage compared to non-irradiated samples. However, the content of chlorogenic acid in irradiated samples under hyperoxia was slightly higher (1.4-fold) compared to non-irradiated samples after 72 h under high hyperoxia. Similarly, UV-C-irradiated carrot bagasse showed higher 3-CQA content compared to non-irradiated bagasse after 48 h at 25 °C being not correlated to TPC data which were statistically similar between non-irradiated and irradiated samples (Sánchez-Rangel et al. 2013). Low UV-C doses (≤2.5 kJ m−2) applied as single treatment in carrot shreds induced phenolic accumulations of approximately 20–35 % after 5–8 days at 5 °C (Alegria et al. 2012). On the other side, UV-C radiation treatments (4.5–6 kJ m−2) and hyperoxia storage (90–100 kPa O2 balanced with nitrogen) of fresh-cut broccoli Bimi® and tatsoi baby leaves did not induce significant enhancements of total phenolic content and total antioxidant capacity which were even reduced during storage at 5 °C up to 19 days (Martínez-Hernández et al. 2013b; Tomás-Callejas et al. 2012). Latter finding may be explain by the high ascorbic acid content of broccoli Bimi® and tatsoi (Samuolienė et al. 2012) contrary to carrot, showing vegetables with low ascorbic acid content higher phenolic accumulation after abiotic stresses (Reyes et al. 2007). A possible explanation for the hereby found lower phenolic accumulation in irradiated samples with moderate UV-C dose, compared to non-irradiated samples, may be a partial PAL denaturation by such UV-C dose delaying the stress-enhanced activity of this enzyme. A subsequent PAL reactivation may occur as observed in air-stored irradiated shreds from 60 to 72 h which agrees with enhanced phenolic levels of these samples in that period. However, this great PAL reactivation from 60 to 72 h was not observed in irradiated shreds stored under hyperoxia conditions. Such absence or delayed PAL reactivation beyond 72 h may be a consequence of oxidative detrimental effects of hyperoxia storage on this enzyme. In contrast to shredded samples, whole UV-C-pretreated carrots experimented a mild phenolic accumulation peak early during storage, contrary to unchanged phenolic contents of non-irradiated samples. Carrot peel has a very high UV-C protective effect since peel with 0.1 mm-thickness only allowed penetration of 1.25 % of total UV-C applied. However, a layer of internal tissue with 0.1 mm-thickness allowed UV-C penetration of 18.7 %. Accordingly, carrot peel in whole samples reduced hypothetical damage to PAL but somehow allowed transmission of stress-induced signal with consequent observed phenolic accumulation. The hereby found phenolic peak of irradiated whole samples was later observed in hyperoxia-stored samples compared to air conditions due to previously supposed partial damage of PAL after UV-C radiation.
Color is the main sensory parameter decisive on the visual consumer election of fresh-cut carrots on retail surfaces. Whitening and browning and are the main color degradation processes occurred in wounded (fresh-cut) carrots. Carrot browning was early related to enzymatic oxidation of polyphenolic compounds (Chubey and Nylund 1969) being its occurrence in UV-C-treated products owed to the increased peroxidase (POD) activity (Tomás-Barberán and Espín 2001). On the other side, whitening mechanism was deeply studied in carrots being attributed in a first reversible physical stage to dehydration and lately to an irreversible physiological response involving activation of phenolic metabolism and production of lignin (Cisneros-Zevallos et al. 1995). The hereby registered WI ranges were not visually detected as previously reported by a sensory panel test (Alegria et al. 2012). The low whitening changes (ΔWI) after 72 h registered in carrots were greatly reduced by the supply of humidified gas to the containers containing carrot samples during storage avoiding samples dehydration. The slightly higher color changes due to browning in irradiated samples after 72 h of storage may be owed to the pre-activated POD during UV-C pretreatment (Tomás-Barberán and Espín 2001). Orange color of carrots is owed to its high carotenoid content representing β-carotene approximately 80 % of the total content of these natural pigments (Rodriguez-Concepcion and Stange 2013). Total carotenoids contents have been reported to be increased by 2–3-fold after wounding, heat shock or UV-C radiation in shredded carrots (Alegria et al. 2012). However, last authors did not report noticeable visual color differences in samples during storage comparing to freshly-cut carrots. Conclusively, no color changes were detected in whole carrots while mild color changes were registered in shredded samples after 72 h which would not lead to an organoleptic rejection of these stressed samples.
Microbiological analyses of stressed carrots after 72 h revealed total mesophilic and yeasts and molds loads of whole and shredded samples lower than 6.0 and 6.5 log cfu g−1, respectively, without significant differences among treatments (data not shown). Accordingly, these microbiological levels were below the threshold limit (7 log cfu g−1) to define fresh-cut products shelf life (Gilbert et al. 2000).
Chlorogenic acid, the main phenolic compound in carrots, is an ester of caffeic acid with quinic acid with great antioxidant capacity compared to other phenolic compounds (Castelluccio et al. 1995). To prevent or slow the oxidative damage in humans induced by free radicals sufficient amounts of phenols as antioxidants need to be consumed with foods. Carrots occupy the sixth place among the list of most consumed vegetables in the American diet, although the total phenolic content of this vegetable is almost the lowest one (Chun et al. 2005). Accordingly, proposed postharvest abiotic stresses can highly increase the phenolic levels of carrots leading to greater ingestion of these antioxidant compounds from this popular and highly consumed vegetable.
Conclusion
Wounding and moderate UV-C pretreatment of carrots greatly increased the activity of the phenylalanine ammonia-lyase, the key enzyme in the biosynthesis pathway of phenolic compounds, with subsequent increments of 1000–1500 % of total phenolic content after 72 h at 15 °C. A hyperoxia storage even augmented those total phenolic increments up to 2000 %, being also partially benefited by a mild water stress, although the pretreatment with UV-C reduced PAL activity favored by a higher electrolyte leakage. Accordingly, this study provides a detailed photograph (12 h intervals) of phenolic accumulation after synergistic effects of those postharvest abiotic stresses. The application of such stresses may be used as a postharvest tool to greatly increase the health-promoting properties of carrots meeting food safety aspects related to the used moderate UV-C dose.
Acknowledgments
Authors are grateful to the Spanish Ministry Economy and Competitiveness (Project AGL2013-48830-C2-1-R) and FEDER for financial support. We are grateful to V. Díaz-López for his skillful technical assistance.
References
- Alegria C (2015) Heat shock and UV-C abiotic stress treatments as alternative tools to promote fresh-cut carrot quality and shelf-life. PhD Thesis University of Lisbon
- Alegria C, Pinheiro J, Duthoit M, Gonçalves EM, Moldão-Martins M, Abreu M. Fresh-cut carrot (cv. Nantes) quality as affected by abiotic stress (heat shock and UV-C irradiation) pre-treatments. Lebensm Wiss Technol. 2012;48:197–203. doi: 10.1016/j.lwt.2012.03.013. [DOI] [Google Scholar]
- Artés-Hernández F, Escalona VH, Robles PA, Martínez-Hernández GB, Artés F. Effect of UV-C radiation on quality of minimally processed spinach leaves. J Sci Food Agric. 2009;89:414–421. doi: 10.1002/jsfa.3460. [DOI] [Google Scholar]
- Artés-Hernández F, Escalona VH, Robles PA, Martínez-Hernández GB, Artés F. Effect of UV-C radiation on quality of minimally processed spinach leaves. J Sci Food Agric. 2009;89:414–421. doi: 10.1002/jsfa.3460. [DOI] [Google Scholar]
- Avena-Bustillos RJ, Du WX, Woods R, Olson D, Breksa AP, 3rd, McHugh TH. Ultraviolet-B light treatment increases antioxidant capacity of carrot products. J Sci Food Agric. 2012;92:2341–2348. doi: 10.1002/jsfa.5635. [DOI] [PubMed] [Google Scholar]
- Becerra-Moreno A, Benavides J, Cisneros-Zevallos L, Jacobo-Velázquez DA. Plants as biofactories: glyphosate-induced production of shikimic acid and phenolic antioxidants in wounded carrot tissue. J Agric Food Chem. 2012;60:11378–11386. doi: 10.1021/jf303252v. [DOI] [PubMed] [Google Scholar]
- Becerra-Moreno A, Redondo-Gil M, Benavides J, Nair V, Cisneros-Zevallos L, Jacobo-Velázquez DA. Combined effect of water loss and wounding stress on gene activation of metabolic pathways associated with phenolic biosynthesis in carrot. Front Plant Sci. 2015;6:837. doi: 10.3389/fpls.2015.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat R, Sridhar KR, Tomita-Yokotani K. Effect of ionizing radiation on antinutritional features of velvet bean seeds (Mucuna pruriens) Food Chem. 2007;103:860–866. doi: 10.1016/j.foodchem.2006.09.037. [DOI] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W, Cuvelier M, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Castelluccio C, Paganga G, Melikian N, Bolwell GP, Pridham J, Sampson J, Rice-Evans C. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher plants. FEBS Lett. 1995;368:188–192. doi: 10.1016/0014-5793(95)00639-Q. [DOI] [PubMed] [Google Scholar]
- Castillejo N, Martínez-Hernández GB, Gómez PA, Artés F, Artés-Hernández F (2015) Red fresh vegetables smoothies with extended shelf life as an innovative source of health-promoting compounds. J Food Sci Technol. doi:10.1007/s13197-015-2143-2 [DOI] [PMC free article] [PubMed]
- Chubey BB, Nylund RE. Surface browning of carrots. Can J Plant Sci. 1969;49:421–426. doi: 10.4141/cjps69-070. [DOI] [Google Scholar]
- Chun OK, Kim D-O, Smith N, Schroeder D, Han JT, Lee CY. Daily consumption of phenolics and total antioxidant capacity from fruit and vegetables in the american diet. J Sci Food Agric. 2005;85:1715–1724. doi: 10.1002/jsfa.2176. [DOI] [Google Scholar]
- Cisneros-Zevallos L. The use of controlled postharvest abiotic stresses as a tool for enhancing the nutraceutical content and adding-value of fresh fruits and vegetables. J Food Sci. 2003;68:1560–1565. doi: 10.1111/j.1365-2621.2003.tb12291.x. [DOI] [Google Scholar]
- Cisneros-Zevallos L, Saltveit ME, Krochta JM. Mechanism of surface white discoloration of peeled (minimally processed) carrots during storage. J Food Sci. 1995;60:320–323. doi: 10.1111/j.1365-2621.1995.tb05664.x. [DOI] [Google Scholar]
- Dixon RA, Paiva NL. Stress-induced phenylpropanoid metabolism. Plant Cell. 1995;7:1085–1097. doi: 10.1105/tpc.7.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EC_1441/2007 (2007) Commission regulation (EC) No 1441/2007 of 5 December 2007 amending Regulation (EC) No 2073/2005 on microbiological criteria for foodstuffs. Official J Eur Union L322:12–29
- Gilbert RJ, et al. Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale. Phls advisory committee for food and dairy products. Commun Dis Public Health. 2000;3:163–167. [PubMed] [Google Scholar]
- Jacobo-Velázquez DA, Martínez-Hernández GB, del C. Rodríguez S, Cao C-M, Cisneros-Zevallos L. Plants as biofactories: physiological role of reactive oxygen species on the accumulation of phenolic antioxidants in carrot tissue under wounding and hyperoxia stress. J Agric Food Chem. 2011;59:6583–6593. doi: 10.1021/jf2006529. [DOI] [PubMed] [Google Scholar]
- Jongen W. Fruit and vegetable processing: Improving quality. Cambridge: Woodhead Publishing Limited; 2002. [Google Scholar]
- Ke D, Saltveit ME. Effects of calcium and auxin on russet spotting and phenylalanine ammonia-lyase activity in iceberg lettuce. HortScience. 1986;21:1169–1171. [Google Scholar]
- Martínez-Hernández GB, Gómez PA, Pradas I, Artés F, Artés-Hernández F. Moderate UV-C pretreatment as a quality enhancement tool in fresh-cut bimi® broccoli. Postharvest Biol Technol. 2011;62:327–337. doi: 10.1016/j.postharvbio.2011.06.015. [DOI] [Google Scholar]
- Martínez-Hernández GB, Artés-Hernández F, Colares-Souza F, Gómez PA, García-Gómez P, Artés F. Innovative cooking techniques for improving the overall quality of a kailan-hybrid broccoli. Food Bioprocess Technol. 2013;6:2135–2149. doi: 10.1007/s11947-012-0871-0. [DOI] [Google Scholar]
- Martínez-Hernández GB, Artés-Hernández F, Gómez PA, Formica AC, Artés F. Combination of electrolysed water, UV-C and superatmospheric o2 packaging for improving fresh-cut broccoli quality. Postharvest Biol Technol. 2013;76:125–134. doi: 10.1016/j.postharvbio.2012.09.013. [DOI] [Google Scholar]
- Martínez-Hernández GB, Huertas J-P, Navarro-Rico J, Gómez PA, Artés F, Palop A, Artés-Hernández F. Inactivation kinetics of foodborne pathogens by UV-C radiation and its subsequent growth in fresh-cut kailan-hybrid broccoli. Food Microbiol. 2015;46:263–271. doi: 10.1016/j.fm.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Martínez-Hernández GB, Navarro-Rico J, Gómez PA, Otón M, Artés F, Artés-Hernández F. Combined sustainable sanitising treatments to reduce escherichia coli and salmonella enteritidis growth on fresh-cut kailan-hybrid broccoli. Food Control. 2015;47:312–317. doi: 10.1016/j.foodcont.2014.07.029. [DOI] [Google Scholar]
- Martínez-Hernández GB, Amodio ML, Colelli G. Potential use of microwave treatment on fresh-cut carrots: physical, chemical and microbiological aspects. J Sci Food Agric. 2016;96:2063–2072. doi: 10.1002/jsfa.7319. [DOI] [PubMed] [Google Scholar]
- McLaughlin CP, O’Beirne D. Respiration rate of a dry coleslaw mix affected by storage temperature and respiratory gas concentrations. J Food Sci. 1999;64:116–119. doi: 10.1111/j.1365-2621.1999.tb09872.x. [DOI] [Google Scholar]
- Palou E, López-Malo A, Barbosa-Cánovas GV, Welti-Chanes J, Swanson BG. Polyphenoloxidase activity and color of blanched and high hydrostatic pressure treated banana puree. J Food Sci. 1999;64:42–45. doi: 10.1111/j.1365-2621.1999.tb09857.x. [DOI] [Google Scholar]
- Reyes LF, Villarreal JE, Cisneros-Zevallos L. The increase in antioxidant capacity after wounding depends on the type of fruit or vegetable tissue. Food Chem. 2007;101:1254–1262. doi: 10.1016/j.foodchem.2006.03.032. [DOI] [Google Scholar]
- Richer DL. Synergism—a patent view. Pestic Sci. 1987;19:309–315. doi: 10.1002/ps.2780190408. [DOI] [Google Scholar]
- Rodriguez-Concepcion M, Stange C. Biosynthesis of carotenoids in carrot: an underground story comes to light. Arch Biochem Biophys. 2013;539:110–116. doi: 10.1016/j.abb.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Saltveit ME. Wound induced changes in phenolic metabolism and tissue browning are altered by heat shock. Postharvest Biol Technol. 2000;21:61–69. doi: 10.1016/S0925-5214(00)00165-4. [DOI] [Google Scholar]
- Samuolienė G, Brazaitytė A, Sirtautas R, Sakalauskienė S, Jankauskienė J, Duchovskis P, Novičkovas A (2012) The impact of supplementary short-term red led lighting on the antioxidant properties of microgreens. In 2012 International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 649–656. doi:10.17660/ActaHortic.2012.956.78
- Sánchez-Rangel JC, Benavides J, Jacobo-Velázquez DA (2013) Production of nutraceuticals in carrot bagasse using abiotic stresses. In 2013 International Society for Horticultural Science (ISHS), Leuven, Belgium, pp 1475–1479. doi:10.17660/ActaHortic.2013.1012.199
- Shahidi F, Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—a review. J Functio Foods. 2015;18, Part B:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- Sharma KD, Karki S, Thakur NS, Attri S. Chemical composition, functional properties and processing of carrot—a review. J Food Sci Technol. 2011;49:22–32. doi: 10.1007/s13197-011-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siró I, Kápolna E, Kápolna B, Lugasi A. Functional food. Product development, marketing and consumer acceptance—a review. Appetite. 2008;51:456–467. doi: 10.1016/j.appet.2008.05.060. [DOI] [PubMed] [Google Scholar]
- Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr: Int Rev J. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surjadinata B, Cisneros-Zevallos L. Modeling wound-induced respiration of fresh-cut carrots (Daucus carota L.) J Food Sci. 2003;68:2735–2740. doi: 10.1111/j.1365-2621.2003.tb05797.x. [DOI] [Google Scholar]
- Tomás-Barberán FA, Espín JC. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric. 2001;81:853–876. doi: 10.1002/jsfa.885. [DOI] [Google Scholar]
- Tomás-Callejas A, Otón M, Artés F, Artés-Hernández F. Combined effect of uv-c pretreatment and high oxygen packaging for keeping the quality of fresh-cut tatsoi baby leaves. Innov Food Sci Emerg Technol. 2012;14:115–121. doi: 10.1016/j.ifset.2011.11.007. [DOI] [Google Scholar]