Abstract
A rapid and selective analytical method was developed to simultaneously quantify seven polyphenolic compounds (gallic acid, catechin, epicatechin, quercetin, kaempferol, syringic acid and p-coumaric acid). 15 phenolics of diverse groups in 80 % ethanolic extracts of jacquemont’s hazelnut (Corylus jacquemontii) kernels and its byproducts from western Himalaya using ultra-performance liquid chromatography coupled with tandem mass spectrometry (UPLC–MS/MS) were identified. The developed analytical method showed excellent linearity, repeatability and accuracy. Total phenols concentrations were found to be 4446, 1199 and 105 mg gallic acid equivalent (GAE)/Kg of dried extract for jacquemont’s hazelnut skin, hard shell and kernels respectively. Antioxidant potential of defatted, raw jacquemont’s hazelnut skin, hard shell and kernel extracts assessed by 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2′-diphenyl-1-picrylhydrazyl (DPPH) methods were increased in a dose-dependent manner. The IC50 values were observed as 23.12, 51.32, 136.46 and 45.73, 63.65, 169.30 μg/ml for jacquemont’s hazelnut skin, hard shell, kernels by DPPH and ABTS assays, respectively. The high phenolic contents in jacquemont’s hazelnut skin contributed towards their free radical scavenging capacities.
Electronic supplementary material
The online version of this article (doi:10.1007/s13197-016-2329-2) contains supplementary material, which is available to authorized users.
Keywords: Corylus jacquemontii, UPLC–MS/MS, Hazelnut extracts, Phenolics, Antioxidant activity
Introduction
Jacquemont’s hazelnut, Corylus jacquemontii (syn. Corylus colurna var. lacera, Betulaceae), commonly known as Thangi or Thankoli, a deciduous tree around 21 m high, flowers from April to May and seeds ripen from September to October, is one of the most esteemed tree nut of the western Himalayan region (Badhwar and Fernandez 2011). Recently, the US Food and Drug Administration (FDA) recognized edible nuts as “heart healthy” foods. Frequent nut intake is associated with low risk of cardiovascular disease and cancer (Surh 2003; Hertog et al. 1993; Ness and Powles 1997). Accumulation of high content of good quality fat, high level of dietary fiber and bioactive molecules, makes edible tree nuts a qualifying candidate of healthy diets (Sabate et al. 2006; Ros and Mataix 2006; Salas-Salvado et al. 2006; Esatbeyoglu et al. 2013: Arya et al. 2016). The antioxidant efficacies of diverse nuts and their byproducts were globally explored and several published communications demonstrated nut byproducts as a very rich source of natural phenolics with potential bioactivities (Shahidi et al. 2007; Blomhoff et al. 2006; Wijeratne et al. 2006; Sang et al. 2002; Yurttas et al. 2000).
Hazelnuts (all varieties) are typically consumed whole (raw, with skin or roasted, without skin) and its paste is extensively used as a major ingredient in a variety of processed food products of dairy and bakery. Hazelnuts also have extensive applications in development of outputs other than foods, including cosmetics and pharmaceuticals (Santamaria et al. 2003; Sullivan et al. 2014). The complete fruit consist of kernel covered outside by a hard shell (Suppl. Figure 1). Hard shell has commercial value and currently used as a heating source upon burning. Skin, hard shell, green leafy cover and tree leaves are byproducts derived from processes like roasting, cracking, shelling and harvesting respectively (Shahidi et al. 2007; Delgado et al. 2010).
Edible food products obtained from plant sources contains a broad range of phytochemicals and phenolics which have diverse biological activities like antioxidant, anti-carcinogenic, anti-mutagenic and anti-proliferative etc. (Singh et al. 2016a, b; Shahidi and Naczk 2004; Yang et al. 2005; Rana and Bhushan 2016; Abuajah et al. 2015). Hazelnuts also have phytochemicals of different classes including tannins, carotenoids, and polyphenols. These metabolites have excellent antioxidative properties and ability to reduce risk of certain type of cancers, coronary heart disease (CHD), cardiovascular disease (CVD), stroke, atherosclerosis, osteoporosis, inflammation and other oxidative stress associated ailments (Watson 2003; Hertog et al. 1993; Ness and Powles 1997; Shahidi and Naczk 2004; Agnihotri et al. 2008). The prevalent phenolics accumulates in Corylus avellana kernels and its byproducts are catechin, gallic acid, sinapic acid, caffeic acid, p-coumaric acid, ferulic acid, their esters and flavonoids (Shahidi et al. 2007; Del Rio et al. 2011). Various other bioactive phenols have also been characterized in hazelnut leaves and foliar buds (Oliveira et al. 2007). However, literature survey revealed that jacquemont’s hazelnut got very less attention than C. avellana. So it is an urgent need to examine the unexplored jacquemont’s hazelnut for its health-related benefits (antioxidant potential) and polyphenolic composition.
In this study we examined the antioxidant activity of jacquemont’s hazelnut kernels and it’s byproducts using 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) and 2,2′-diphenyl-1-picrylhydrazyl (DPPH) assays. Also a rapid and selective ultra performance liquid chromatography coupled to diode array detector-electrospray ionisation quadrupole time-of-flight mass spectrometry (UPLC–DAD–ESI–Q-TOF–MS) method was developed and satisfactorily validated for chemical characterization and quantification of phenolic compounds in jacquemont’s hazelnut.
Materials and methods
Chemicals
Gallic acid, catechin, epicatechin, syringic acid, p-coumaric acid, kaempferol, quercetin, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), 2,2′-diphenyl-1-picrylhydrazyl (DPPH), and ascorbic acid were purchased from Sigma-Aldrich India. All the solvents i.e. acetonitrile, methanol, water and formic acid used for UPLC–MS analysis were of LC–MS grade and solvents used in the extraction process, etc. of analytical grade, purchased from J. T. Baker USA.
Collection, extraction and freeze-drying of hazelnut fruit
Jacquemont’s hazelnut (C. jacquemontii) fruits were purchased in early September 2013, from tehsil Pangi, district Chamba in Himachal Pradesh (India). The fruits were dried in dark for 6 days using the oven at 30 °C. Afterwards; the fruits were put in plastic bags and frozen to −4 °C until further analyzed. The jacquemont’s hazelnuts was thawed, manually cracked, the hard shells, hazelnut kernels and skins were grinded separately. Jacquemont’s hazelnut kernels, hard shell, and skin were separately then blended with hexane to separate the fat present in the samples at room temperature. Defatted samples were subsequently air-dried for 16 h and stored at −4 °C until used for further analyses.
Preparation of 80 % ethanolic extracts
Defatted samples (kernels, hard shell and skin) were extracted in triplicate using 80:20 (v/v) ethanol/water mixtures (sample: solvent (0.15:1, w/v) under reflux conditions in a thermostated water bath at 70 °C for 30 min. After each extraction, the individual supernatants were collected and dried in a rota-evaporator at 45 °C. Afterwards, the remaining water in concentrated extracts were removed by lyophilization for 48 h at −50 °C and 0.054 mbar. Finally, the crude 80 % ethanolic extracts after flowing nitrogen were stored at −4 °C in culture tubes.
UPLC/ESI/QTOF/MS condition
The equipment used was of Waters Acquity UPLC hyphenated to Waters Q-TOF micro mass system (Waters, Milford, MA, USA), controlled by mass Lynx v4.1 software. The chromatographic separation was carried out on Waters BEH C18 (2.1 mm × 100 mm, 1.7 μm) column at 30 °C. The mobile phase consists of water (0.1 % formic acid) as solvent A and acetonitrile (0.1 % formic acid) as solvent B, with gradient and isocratic programme (Suppl. Table 1). Flow rate was 300 μl/min; injection volume was 1 μl. The method run time was 15 min. The detector wavelength range is kept between 200 and 490 nm. All chromatograms were analyzed at 265 nm. The ionisation source used was ESI with +ve mode polarity, capillary voltage 3.1 kV, cone voltage 22 V, source block temperature 80 °C, desolvation temperature 200 °C, cone gas flow 50 L h−1, desolvation gas flow 400 L h−1.
Preparation of sample and standard solutions
A stock solution of all standard compounds was prepared by dissolving accurately weighed portion in methanol. The concentration of each standard stock solution was 1 mg/ml. The aliquot amount of each stock solution was mixed and diluted with methanol to prepare a standard mixture. This standard mixture was further diluted with methanol to provide a series of ten different concentrations in the range of 0.579–142.85 ng/ml for all standard compounds. For quantification, the concentration of jacquemont’s hazelnut extracts (skin, hard shell and kernel) prepared were of 10 mg/ml in methanol. All the solutions were filtered through 0.2 μm filter and stored at −4 °C before analysis.
UPLC method validation
Validation of newly developed UPLC method included assessment of selectivity, linearity, LOD, LOQ, precision and recovery. Selectivity of peaks of compounds in the sample was assessed by comparing the retention time with those of the standards run individually. Calibration curve was constructed by plotting peak areas of individual components versus their respective concentrations. The LOD and LOQ for each phenolic compounds were evaluated by injecting the diluted standard solutions till the signal to noise ratio (S/N), was about 3 for LOD and 10 for LOQ. To study the repeatability and reproducibility of the developed analytical method, intra- and inter-day precisions test were also performed. For intra-day variability test, a sample was analyzed six times per day, whereas for inter-day, the sample was analyzed for three consecutive days in triplicate. Recovery was evaluated to check the accuracy of the method. Mixed standard solutions with three different concentrations were prepared and spiked to 10 mg of 80 % ethanolic extract. The resultant samples were further sonicated, filtered with a 0.2 μm filter, made up to 2 ml, and analysed using the proposed developed analytical method.
Total phenolic contents assay
Total phenolic contents were estimated by Folin–Ciacalteu colorimetric method (Gao et al. 2000). Jacquemont’s hazelnut kernels and its byproducts 80 % ethanolic extracts (100 μl) were mixed with 0.2 ml of Folin–Ciacalteu reagent and 2 ml of H2O and incubated at room temperature for 3 min. Following the addition of 1 ml of 20 % sodium carbonate to the each sample, total phenolic contents were evaluated after 1 h of incubation at room temperature. The absorbance of the resulting blue color was measured at 765 nm. Quantification was done with respect to the standard curve of gallic acid (0.02–0.1 mg/ml). Phenolic contents were expressed as: gallic acid equivalent (GAE) using the equation based on the calibration curve: y = 0.0234x + 0.109, r 2 = 0.998, where x was the absorbance and y was the GAE at a final concentration of 0.1 mg/ml. The results were express as GAE (mg/Kg) of the dried extract (Suppl. Table 2).
DPPH radical scavenging assay
Radical scavenging activity of jacquemont’s hazelnut 80 % ethanolic extracts, against stable DPPH radical, were evaluated by DPPH assay with slight modification (Kalia et al. 2008: Kant et al. 2013). The effect of antioxidants on DPPH-radical-scavenging test reflects the hydrogen donating capacity of a compound. When radical form of DPPH scavenged by an antioxidant through donation of hydrogen, DPPH becomes a stable molecule, which leads to a color change from purple to yellow and a decrease in absorbance was measured at 517 nm. Stock solution of 1 mg/ml for ascorbic acid, jacquemont’s hazelnut skin, hard shell and kernel were prepared in methanol, respectively. Different amount of concentration (20, 40, 60, 80, 100 μg/ml) for ascorbic acid, jacquemont’s hazelnut skin, hard shell and kernels were prepared in methanol having final volume 100 μl, to which 2 ml of the 0.100 mM DPPH solution prepared in methanol added. The mixtures were shaken vigorously, allowed to stand at 25 °C in the dark for 30 min, and a decrease in absorbance of the resulting solutions monitored at 517 nm (Shimadzu 2450) against a blank consisted of 100 µl of methanol and 2 ml of DPPH solution. All measurements were done in triplicate. Inhibition of free radical DPPH in percent I (%) was calculated as follows:
where, A b was the absorbance of control reaction and A s was absorbance of test compound. The sample concentration providing 50 % inhibitions (IC50) were calculated by plotting inhibition percentages against concentrations of samples (Table 1).
Table 1.
DPPH and ABTS radical scavenging capacities of 80 % ethanolic extract of jacquemont’s hazelnut kernel and its byproducts
| Inhibition (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Conc. (μg/ml) | DPPH assay | ABTS assay | ||||||
| Hazelnut skin | Hazelnut hard shell | Hazelnut kernel | AAa | Hazelnut skin | Hazelnut hard shell | Hazelnut kernel | AAa | |
| 20.0 | 40.06 ± 3.9 | 22.16 ± 1.9 | 7.50 ± 0.7 | 55.82 ± 1.4 | 22.22 ± 1.8 | 11.8 ± 0.7 | 7.18 ± 0.8 | 42.01 ± 1.5 |
| 40.0 | 77.87 ± 2.1 | 36.66 ± 3.7 | 17.76 ± 2.6 | 83.75 ± 1.4 | 40.06 ± 3.9 | 29.61 ± 1.4 | 15.71 ± 1.7 | 58.19 ± 0.8 |
| 60.0 | 92.95 ± 0.7 | 56.65 ± 3.4 | 24.79 ± 0.4 | 95.76 ± 1.5 | 73.32 ± 0.8 | 47.37 ± 2.1 | 19.23 ± 0.8 | 78.37 ± 1.1 |
| 80.0 | 97.12 ± 0.9 | 79.10 ± 1.6 | 30.73 ± 1.6 | 97.17 ± 0.9 | 86.37 ± 1.1 | 64.09 ± 1.3 | 23.14 ± 2.1 | 95.74 ± 0.9 |
| 100 | 97.78 ± 0.5 | 92.57 ± 0.6 | 35.79 ± 0.5 | 99.45 ± 0.3 | 96.52 ± 1.1 | 82.94 ± 1.2 | 31.52 ± 1.3 | 98.51 ± 1.5 |
| IC50 | 23.12 ± 1.4 | 51.32 ± 1.2 | 136.46. ± 0.9 | 11.18 ± 0.5. | 45.73 ± 1.2 | 63.65 ± 0.5 | 169.30 ± 0.5 | 27.08 ± 0.3 |
aAscorbic acid
ABTS radical scavenging assay
ABTS•+ scavenging activity was carried out with a slight modification from defined method (Kalia et al. 2008). The radical cations were prepared by reacting 7 mM aqueous ABTS with 2.45 mM potassium persulphate. The mixture was allowed to stand in the dark at room temperature for 16 h before use, by which the ABTS turned blue-green. The ABTS•+ solution was diluted with methanol to an absorbance of 0.700 ± 0.020 at 734 nm. Different amount of concentration (20, 40, 60, 80, 100 μg/ml) for ascorbic acid, jacquemont’s hazelnut skin, hard shell and kernels were prepared in methanol having final volume 100 μl, to which 2 ml of the ABTS∙+ solution prepared in methanol added. The absorbance was recorded after 4 min. The IC50 and percentage inhibition of absorbance at 734 nm calculated (Table 1). All measurements were done in triplicate. Inhibition of ABTS∙+ in percent I (%) was calculated as follows.
where, A b was absorbance of the control reaction and A s was absorbance of test compound. The sample concentration providing 50 % inhibitions (IC50) were calculated by plotting inhibition percentages against concentrations of sample.
Statistical analysis
All experimental results were expressed as mean ± standard deviation (SD) using statistical analysis with Sysstat (Chicago, III., USA) version.
Results and discussion
Yield
The extract yield after lyophilization of jacquemont’s hazelnut kernel, skin and hard shell in 80 % ethanolic extracts were 24.8 ± 0.7, 100.0 ± 1.4 and 27.1 ± 0.5 g/Kg of defatted samples, respectively (Suppl. Table 2). A similar extract yields were also previously reported in hazelnut kernels (C. avellana) and its byproducts (Shahidi et al. 2007).
Assessment of total phenolic contents
The total phenolic contents of hazelnut extracts are summarized in Suppl. Table 2. Among all the hazelnut extracts, 80 % ethanolic skin extract had the highest phenolic contents (4446.0 ± 1.2 GAE/Kg dried extract wt.), followed by hard shells (1199.0 ± 3.2 GAE/Kg dried extract wt.) and kernels (105.0 ± 0.6 GAE/Kg dried extract wt.). Total phenolic contents were also reported in hazelnut kernels (C. avellana) (Jakopic et al. 2011). The total phenol contents were ranged from 70 to 478 mg GAE/Kg in hazelnut different cultivars when extraction was done in methanol. Delgado et al. (2010) also reported total phenolic contents with boiling water for 30 min, of 44.3 ± 7.7 mg GAE/g extract, and with 80 % (v/v) aqueous acetone solution for 24 h, 36.2 ± 8.8 mg GAE/g extract was reported. Contini et al. (2008) examined total phenolic contents of woody hardshell, a byproduct from C. avellana. The phenolic contents were ranged from 56.6 to 72.2 mg GAE/g extract in different solvents. Comparison of our findings and previous results revealed that total phenolic contents values vary due to differences in extraction solvent, extraction time and the basis used for measuring.
Antioxidant capacity of hazelnut extracts
DPPH assay revealed the inhibitory concentration (IC50) of jacquemont’s hazelnut extracts varied from 23.12 ± 1.4 to 136.46 ± 0.9 μg/ml. The jacquemont’s hazelnut skin extract displayed the highest antioxidant capacity (23.12 ± 1.4 μg/ml), followed by hardshell (51.32 ± 1.2 μg/ml) and kernel (136.46 ± 0.9 μg/ml) (Table 1). These results indicate that jacquemont’s hazelnut kernels and its byproducts, 80 % ethanolic extracts exhibited potential free-radical scavenging capacity, which was comparable to positive control ascorbic acid. Our report concerning DPPH-radical scavenging activity (IC50) of the extracts were not comparable with the earlier results published by (Shahidi et al. 2007). These authors evaluated DPPH scavenging activity of the extracts and a reference antioxidant (catechin) by testing 50 and 100 ppm solutions, obtaining nearly 100 % of DPPH scavenging for both the hazelnut by-product extracts. The antioxidant potential of 80 % ethanolic extract of hazelnut kernels (C. avellana) had been reported previously (Alasalvar et al. 2006). The IC50 value obtained was higher (0.504 mg/ml) than our study (136.46 ± 0.9 μg/ml). In addition, radical scavenging activities of hazelnut kernels (C. avellana) also vary due to differences in extraction solvents (Delgado et al. 2010; Alasalvar et al. 2006).
ABTS assay revealed inhibitory concentration (IC50) of jacquemont’s hazelnut extracts ranged from 45.73 ± 1.2 to 169.30 ± 0.5 μg/ml. The jacquemont’s hazelnut skin extract demonstrated excellent antioxidant capacity (45.73 ± 1.2 μg/ml), followed by hardshell (63.65 ± 0.5 μg/ml) and kernel (169.30 ± 0.5 μg/ml). IC50 values of jacquemont’s hazelnut kernel and its byproducts were comparable to standard ascorbic acid (Table 1).
UPLC/MS/MS analysis
Optimization of chromatographic conditions
A fast and sensitive UPLC–ESI–MS/MS method was developed and validated for simultaneous quantification of seven phenolic compounds in jacquemont’s hazelnut kernels and its byproducts. To obtain optimum elution conditions, various UPLC conditions, including mobile phase composition, such as acetonitrile: methanol, acetonitrile: water, methanol: water and water (0.1 % formic acid): acetonitrile with column temperatures 25, 30, 35, 40 °C were assessed. Because of sharper peak shape and better baseline separation, acetonitrile was selected as the most suitable organic solvent. The introduction of formic acid into the mobile phase improved the sensitivity and separation efficiency. Finally, the adoption of aqueous 0.1 % formic acid (A) and acetonitrile 0.1 % formic acid (B), and column temperature 30 °C with gradient and isocratic elution was necessary to ensure better baseline separation of all phenolic constituents. Moreover, several chromatographic columns were also tested to achieve better separation, when BEH C18 analytical column (2.1 mm × 100 mm), with particle size 1.7 μm used, separation efficiency was excellent (Suppl. Table 1). Various wavelength (265, 280 nm) for chromatographic analysis were evaluated but relative absorption of phenolics was best at 265 nm. Also, very low injection volume (1 μl) was used for analysis, because of good absorption response of phenolics in less sample loading.
Linearity and calibration curves
Linearity of the method was evaluated using ten different concentrations of standard mixture prepared in methanol keeping the injection volume constant. The calibration curve of all standard compounds was constructed by plotting the peak area ratio (y), versus their concentration (x) (Table 2).
Table 2.
Linear regression equations, test ranges, LODs, LOQs, intra and inter-day for quantified compounds
| Component | Regression equationa | r2b | Test range (ng/ml) | LODc (ng/ml) | LOQd (ng/ml) | Intra-day (ng/ml) RSDe (%) | Inter-day (ng/ml) RSD (%) |
|---|---|---|---|---|---|---|---|
| Catechin | y = 7.5769x − 12.345 | 0.99 | 8.93–142.85 | 1.77 | 5.31 | 0.88 | 0.60 |
| Epicatechin | y = 13.675x + 26.547 | 0.99 | 4.46–142.85 | 0.73 | 2.19 | 0.61 | 0.59 |
| Quercetin | y = 48.136x − 1.7448 | 0.99 | 1.1–17.85 | 0.29 | 0.87 | 0.64 | 0.65 |
| Kaempferol | y = 165.28x − 12.111 | 0.99 | 0.55–17.85 | 0.32 | 0.96 | 0.95 | 0.52 |
| Gallic acid | y = 149.52x − 158.06 | 0.97 | 1.11–17.85 | 1.27 | 3.81 | 2.30 | 0.75 |
| P-Coumaric acid | y = 73.729x + 0.8202 | 1.0 | 0.57–17.85 | 0.26 | 0.78 | 0.74 | 0.45 |
| Syringic acid | y = 75.158x + 2.2644 | 0.99 | 2.23–17.85 | 0.27 | 0.81 | 0.56 | 0.74 |
aThe regression equation were represented as y = mx + c. y, peak area; x, concentration of compounds (ng/ml)
b r2 is the correlation coefficient of the equation
c LOD, limit of detection, S/N = 3
d LOQ, limit of quantification, S/N = 10
e RSD (%) = (SD/mean) × 100
Limit of detection (LOD) and limit of quantification (LOQ)
LOD and LOQ for all compounds ranged between 0.26–1.77 and 0.78–5.31 ng/ml respectively (Table 2).
Repeatability and Recovery
The results, summarized in Table 2, showed that the values of precision and accuracy were within the acceptable limit and indicated that the developed analytical method was highly accurate and precise.
Recovery of each phenolics examined by performing three replicate analysis of samples spiked with 3 different concentrations at low, medium and high levels (20, 40 and 60 ng/ml). The recovery for each analyte determined at selected concentrations by spiking 80 % ethanolic extract with standard working solutions. Mean recovery results were in range of 88.1–111.0 % for all seven compounds and RSD < 4.76 % revealed that developed analytical method was reproducible with excellent recovery having all the values within the acceptable limit Table 3. Over all, these results indicated that extraction of phenolic compounds from jacquemont’s hazelnut kernels and its byproducts was nearly complete during sample processing.
Table 3.
Extract recovery of quantified phenolic components
| Recovery (n = 3) | |||||
|---|---|---|---|---|---|
| Component | Original (ng/ml) | Spiked (ng/ml) | Found (ng/ml, mean ± SD) | Recoverya (%) | RSDb (%) |
| Catechin | 13.12 | 20.0 | 32.32 ± 0.05 | 96.0 | 0.15 |
| 40.0 | 48.39 ± 0.21 | 88.1 | 0.43 | ||
| 60.0 | 73.89 ± 0.54 | 101.2 | 0.73 | ||
| Epicatechin | 1.75 | 20.0 | 21.18 ± 0.29 | 97.1 | 1.36 |
| 40.0 | 41.04 ± 0.33 | 98.2 | 0.80 | ||
| 60.0 | 59.84 ± 0.37 | 96.8 | 0.61 | ||
| Quercetin | 2.05 | 20.0 | 23.37 ± 0.12 | 106.6 | 0.51 |
| 40.0 | 40.33 ± 0.10 | 95.7 | 0.24 | ||
| 60.0 | 60.74 ± 0.13 | 97.8 | 0.21 | ||
| Kaempferol | 3.20 | 20.0 | 22.56 ± 0.04 | 96.8 | 0.17 |
| 40.0 | 43.80 ± 0.04 | 101.5 | 0.09 | ||
| 60.0 | 65.44 ± 0.03 | 103.7 | 0.04 | ||
| Gallic acid | 1.41 | 20.0 | 23.61 ± 0.05 | 111.0 | 0.21 |
| 40.0 | 42.67 ± 0.07 | 103.1 | 0.16 | ||
| 60.0 | 61.40 ± 0.08 | 99.9 | 0.13 | ||
| p-Coumaric acid | 0.24 | 20.0 | 19.99 ± 0.67 | 98.7 | 3.35 |
| 40.0 | 39.97 ± 1.46 | 99.3 | 3.65 | ||
| 60.0 | 59.54 ± 1.08 | 98.8 | 1.81 | ||
| Syringic acid | 0.32 | 20.0 | 21.0 ± 1.00 | 103.4 | 4.76 |
| 40.0 | 40.67 ± 0.50 | 100.8 | 1.22 | ||
| 60.0 | 61.37 ± 0.51 | 101.7 | 0.83 | ||
a Recovery (%) = (detected amount − original amount)/spiked amount × 100
b RSD (%) = (SD/mean) × 100
Quantitative analysis by UPLC–DAD
The quantity of all the seven phenolic compounds investigated for jacquemont’s hazelnut different extracts are summarized in Table 4. Jacquemont’s hazelnut skin, showed the highest antioxidant capacity and total phenolic contents, contained high concentration of catechin (414.0 ± 2.9 mg/Kg dried extract wt.), followed by kaempferol (32.0 ± 20.0 mg/Kg dried extract wt.), quercetin (20.3 ± 4.9 mg/Kg dried extract wt.), and epicatechin (17.9 ± 2.6 mg/Kg dried extract wt) Del Rio et al. (2011), also analyzed the quantity of phenolic compounds in hazelnut skin (C. avellana) aqueous extracts in different cultivars reported a high catechin content (99.9 ± 5.7–200.9 ± 5.1 mg/100 g). The quantity of quercetin and gallic acid reported in our study was similar to previous results (Del Rio et al. 2011). Kaempferol in jacquemont’s hazelnut skin, which was found to be higher than other hazelnut species (Table 4). The quantity of 9.3 ± 2.4, 3.24 ± 1.5, 3.22 ± 1.8 mg/Kg of gallic acid, syringic acid and kaempferol, respectively, could be responsible for potent antioxidant activity of jacquemont’s hardshell. It was very interesting to observe that kaempferol and quercetin identified in jacquemont’s hardshell was previously not detected in hardshell of other hazelnut species. The antioxidant activity of jacquemont’s hazelnut kernel obtained in this study was found to be higher than early study (Alasalvar et al. 2006); it could be due to the presence of catechin (15.9 ± 1.4 mg/Kg dried extract wt.) and gallic acid (2.99 ± 1.7 mg/Kg dried extract wt.). Jakopic et al. (2011) reported higher gallic acid content in hazelnut kernel of C. avellana different cultivars (0.07 to 0.52 mg/Kg).
Table 4.
Quantification of phenolic compounds in jacquemont’s hazelnut and its byproducts
| Extract | Gallic acid | Catechin | Syringic acid | Epicatechin | p-Coumaric acid | Quercetin | Kaempferol |
|---|---|---|---|---|---|---|---|
| Hazelnut kernel | 2.99 ± 1.7 | 15.9 ± 1.4 | ND | 1.16 ± 1.6 | ND | 0.54 ± 0.5 | 0.31 ± 0.5 |
| Hazelnut skin | 14.1 ± 4.6 | 414.0 ± 2.9 | ND | 17.9 ± 2.6 | 2.46 ± 0.5 | 20.3 ± 4.9 | 32.0 ± 20.0 |
| Hazelnut hard shell | 9.3 ± 2.4 | Traces | 3.24 ± 1.5 | Traces | 0.30 ± 1.5 | 1.50 ± 3.8 | 3.22 ± 1.8 |
Data expressed as mg/Kg dried extract wt. as mean ± SD (n = 3); ND not detected
UPLC–DAD–ESI–MS/MS analysis
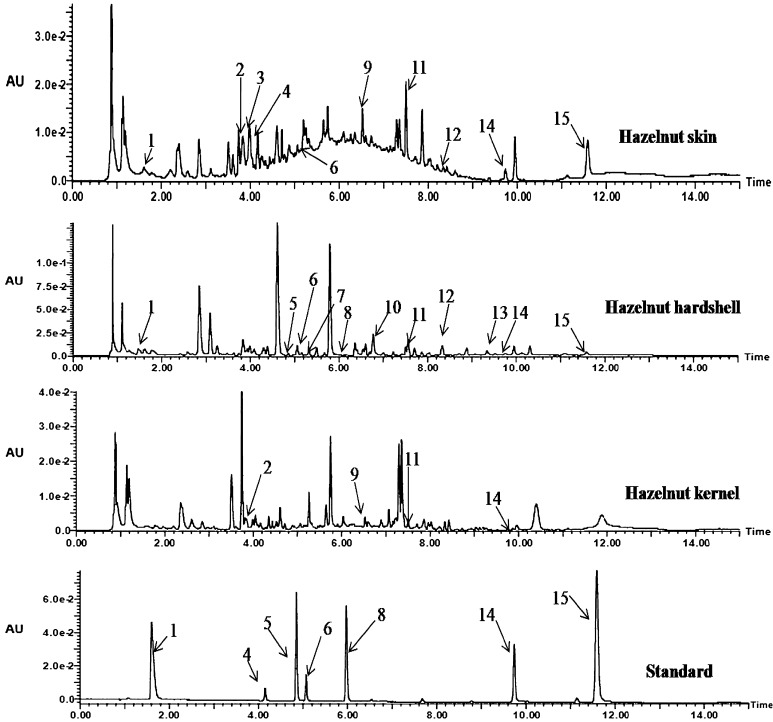
For characterization of individual phenolics in 80 % ethanolic extracts of jacquemont’s hazelnut kernels, hard shell and skin, UPLC tandem MS was used (Fig. 1 shows a typical chromatogram). Phytochemicals detected in UPLC and MS data were summarized in Table 5. The major group of phenols in jacquemont’s hazelnut extracts were phenolic acids and flavan-3-ols. Peak 1 (Rt = 1.61 min), identified as gallic acid having mass spectral data of [M + H]+ at m/z 171, the MS2 fragment ion at m/z 145 and co-chromatography with the standard. Jakopic et al. (2011) had previously reported the presence of gallic acid in C. avellana kernel. Peak 2 (Rt = 3.86 min), had a [M + H]+ at m/z 579, and MS2 fragment ions at m/z 289, 213 and 163 (Jakopic et al. 2011). This compound identified as procyanidin dimer. Peak 3 (Rt = 4.00), produced molecular ion [M + H]+ at m/z 867, and MS2 fragment at [M + H]+ m/z 579. This compound was identified as procyanidin trimer. In present experiment, the procyanidin dimer and trimer were not detected in jacquemont’s hazelnut hard shell (Jakopic et al. 2011). Peaks 4 (Rt = 4.15 min) and 6 (Rt = 5.06) had positively charged molecular ion [M + H]+ at m/z 291 and MS2 fragment at m/z 139 (Jakopic et al. 2011). Comparison of retention time and UV with the standards, assigned peak 4 as (+)-catechin and peak 6 as (−)-epicatechin. (+)-Catechin was also identified in jacquemont’s hazelnut hard shell in trace amount (Suppl. Figure 17). Peak 5 (Rt = 4.86 min), with [M + H]+ at m/z 199, had MS2 fragment at m/z 155, this compound identified as syringic acid, which was further confirmed by comparison of retention time and UV with the standard compound. In present experiment, syringic acid was not detected in jacquemont’s hazelnut kernel and skin extracts. Peak 7 (Rt = 5.38 min), with [M + H]+ at m/z 271 and [M + Na]+ at m/z 293, also yielded MS2 fragment at m/z 179, was characterized as apigenin by comparing fragmentation pattern with literature (Lin and Harnly 2012). Apigenin was not detected in jacquemont’s hazelnut skin and kernel extracts (Suppl. Figure 17). Peak 8 (Rt = 5.98 min), with [M + H]+ at m/z 165, had MS2 fragments at m/z 145 and 137, this compound identified as p-coumaric acid, which was further confirmed by comparison with retention time and UV of standard compound. p-Coumaric acid was not detected in jacquemont’s hazelnut kernel extract. Quercetin hexoside (Rt = 6.64, peak 9) was detected in jacquemont’s hazelnut skin and kernel extracts, with [M + H]+ at m/z 465, and MS2 fragment at m/z 303, a characteristic fragment of quercetin (Abu-Reidah et al. 2015). Peak 10 (Rt = 6.77 min), was identified as myricetin, having [M + H]+ at m/z 319 and MS2 fragment at m/z 301, after a loss of 18 amu as water (Abu-Reidah et al. 2015). Peak 11 (Rt = 7.56 min), was characterized as quercetin rhamnoside in all jacquemont’s hazelnut extracts having [M + H]+ at m/z 449, which after the loss of rhamnoside moiety yielded a characteristic fragment of quercetin at m/z 303 (Abu-Reidah et al. 2015) (Suppl. Figure 17). Peak 12 (Rt = 8.34 min) having [M + H]+ at m/z 433, and yield MS2 fragment at m/z 287, this compound identified as kaempferol rhamnoside (Abu-Reidah et al. 2015). Kaempferol rhamnoside was detected in jacquemont’s hazelnut hard shell and skin extracts. Peak 13 (Rt = 9.39) had [M + H]+ at m/z 331, and MS2 fragments at m/z 313 and 303. Ellagic acid having [M + H]+ at m/z 303 as a major fragment. This compound was tentatively characterized as dimethylellagic acid. Dimethylellagic acid was identified in jacquemont’s hazelnut hard shell extract (Suppl. Figure 17). Peak 14 (Rt = 9.74 min), with [M + H]+ at m/z 303 was identified as quercetin, which further identified by comparison with retention time and UV of standard compound (Abu-Reidah et al. 2015). Quercetin was present in all jacquemont’s hazelnut extracts. Peak 15 (Rt = 11.6 min), with [M + H]+ at m/z 287 characterized as kaempferol, which was further identified by comparison with retention time and UV of standard compound (Abu-Reidah et al. 2015) (Fig. 1 and Suppl. Figures 5.1–5.3 and 17).
Fig. 1.
UPLC chromatogram of jacquemont’s hazelnut skin, hard shell, kernel 80 % ethanolic extracts and used standards at 265 nm. Gallic acid (1), Proanthocyanidin B dimer (2), Proanthocyanidin B trimer (3), Catechin (4), Syringic acid (5), Epicatechin (6), Apigenin (7), p-Coumaric acid (8), Quercetin hexoside (9), Myricetin (10), Quercetin rhamnoside (11), Kaempferol rhamnoside (12), Dimethylellagic acid (13), Quercetin (14), Kaempferol (15)
Table 5.
Phytochemical compounds detected and characterized in jacquemont’s hazelnut using UPLC–DAD/QTOF–MS in positive mode at 265 nm
| Peak | Rt (min) | λmax | [M + H]+(m/z) | Mass fragments | Tentative identification | Part | References |
|---|---|---|---|---|---|---|---|
| 1 | 1.61 | 269 | 171.0623 | 145 | Gallic acida | All | Jakopic et al. (2011) |
| 2 | 3.86 | 278 | 579.1534 | 289 | Proanthocyanidin B dimer | Skin, kernel | Jakopic et al. (2011)) |
| 3 | 4.00 | 203, 281 | 867.2124 | 579, 450 | Proanthocyanidin B trimer | Skin | Jakopic et al. (2011) |
| 4 | 4.15 | 203, 278 | 291.0864 | 139 | Catechina | All | Jakopic et al. (2011) |
| 5 | 4.86 | 273 | 199.0993 | 137 | Syringic acida | Hard shell | |
| 6 | 5.06 | 279 | 291.0813 | 267, 139 | Epicatechina | Skin, kernel | |
| 7 | 5.38 | 279 | 271.0592 | 293, 179 | Apigenin | Hard shell | Lin and Harnly (2012) |
| 8 | 5.98 | 252, 297 | 165.0967 | 145, 137 | p-Coumaric acida | Hard shell, Skin | |
| 9 | 6.64 | 278 | 465.1006 | 303 | Quercetin hexoside | Skin,kernel | Abu-Reidah et al. (2015) |
| 10 | 6.77 | 273 | 319.0449 | 301 | Myricetin | Hard shell | Abu-Reidah et al. (2015) |
| 11 | 7.56 | 339 | 449.1079 | 303 | Quercetin rhamnoside | All | Abu-Reidah et al. (2015) |
| 12 | 8.34 | 264 | 433.1129 | 287 | Kaempferol rhamnoside | Hard shell, Skin | Abu-Reidah et al. (2015) |
| 13 | 9.39 | 218 | 331.0425 | 313, 303 | Dimethylellagic acid | Hard shell | Robbins et al. (2014) |
| 14 | 9.74 | 256, 376 | 303.0509 | 267 | Quercetina | All | Abu-Reidah et al. (2015) |
| 15 | 11.62 | 362 | 287.0532 | 309, 269 | Kaempferola | All | Abu-Reidah et al. (2015) |
a Identified by comparison with standard compound
All these findings including antioxidant potential and a new natural source of catechins makes jacquemont’s hazelnut kernels and its byproducts an excellent candidate for human health benefits. The overall antioxidative effect of jacquemont’s hazelnut extracts was different and may depend on the type of individual phenols present in different extracts. It was previously documented that hazelnut skin of C. avellana had a high concentration of catechin compared to the hard shell and kernels (Shahidi et al. 2007; Del Rio et al. 2011). This finding is also in agreement with present study observation on jacquemont’s hazelnut. Catechin found to be directly linked to protection against several chronic diseases, with mechanisms of action, possibly involving antioxidant, anti-inflammatory, and vasoactive (Scalbert et al. 2005; Vita 2005). Beside catechin as a major component, C. jacquemontii also contains apigenin (7), dimethyl ellagic acid (13), quercetin rhamnoside (11), quercetin hexoside (9) and kaempferol rhamnoside (12) as other bioactive metabolites. These phenolic compounds had been reputed in literature as potentially related to diverse health benefits. In this study, proanthocyanidins were also detected in jacquemont’s hazelnut. Prior to their excellent antioxidant potential, proanthocyanidins may reduce the risk of cardiovascular diseases by improving lipid homeostasis. Investigational and clinical research demonstrated frequent intake of procyanidin-rich foods could improve endothelial dysfunction and decrease vascular oxidative stress associated with major cardiovascular risk factors such as hypertension (Del Rio et al. 2011). Comparison of the present study with previous published reports strongly suggests jacquemont’s hazelnuts could potentially be considered as an excellent and readily available source of natural antioxidants. Due to very high concentration of catechin and other phenolic compounds, this can be incorporated into new health related products that promote human health and well-being.
Conclusion
A rapid and sensitive UPLC method coupled with mass spectrometry was developed and satisfactorily validated for chemical characterization and quantification of phenolic compounds in jacquemont’s hazelnut. Jacquemont’s hazelnut different parts were found to be rich with polyphenols and food derived flavonoids and their derivatives which play diverse roles such as antimutagenic and anticarcinogenic effects in vitro and in vivo, antioxidant, antimicrobial, anti-allergic and anti-inflammatory agents and help in the prevention of cardiovascular diseases, osteoporosis, neurodegenerative disease and diabetes mellitus. The lack of natural phenolics in quantity in the western diet is increasing the demand for natural phenolic and flavonoid supplements. Different part’s extracts of jacquemont’s hazelnut can be used as a source of phenolic supplements. Promising results received in present study also suggest that different parts extract of jacquemont’s hazelnut can be considered for detailed pharmacological studies to develop new natural antioxidant products for the treatment of diverse oxidative stress-related diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors gratefully acknowledge Dr. Sanjay Kumar, Director, CSIR-IHBT Palampur (HP), India, for continuous encouragement and for providing the necessary facilities for the investigation. Thanks are also due to UGC for awarding senior research fellowship to Mr Ashish Kumar. Authors are also grateful to CSIR, New Delhi for funding BSC-0106 and BSC-0209 projects under which the work was carried out.
References
- Abuajah CI, Ogbonna AC, Osuji CM. Functional components and medicinal properties of food: a review. J Food Sci Technol. 2015;52:2522–2529. doi: 10.1007/s13197-014-1396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Reidah IM, Ali-Shtayeh MS, Jamous RM, Arráez-Román D, Segura-Carretero A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015;166:179–191. doi: 10.1016/j.foodchem.2014.06.011. [DOI] [PubMed] [Google Scholar]
- Agnihotri VK, ElSohly HN, Khan SI, Smillie T, Khan IA, Walker LA. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry. 2008;69:2061–2066. doi: 10.1016/j.phytochem.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Alasalvar C, Karamac M, Amarowicz R, Shahidi F. Antioxidant and antiradical activities in extracts of hazelnut kernel (Corylus avellana L.) and hazelnut green leafy cover. J Agric Food Chem. 2006;54:4826–4832. doi: 10.1021/jf0601259. [DOI] [PubMed] [Google Scholar]
- Arya SS, Salve AR, Chauhan S. Peanuts as functional food: a review. J Food Sci Technol. 2016;53:31–41. doi: 10.1007/s13197-015-2007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhwar SRL, Fernandez RR. Edible wild plants of Himalayas. Delhi: Daya publishing house; 2011. pp. 444–445. [Google Scholar]
- Blomhoff R, Carlsen MH, Andersen LF, Jacobs DR. Health benefits of nuts: potential role of antioxidants. Br J Nutr. 2006;9:S52–S60. doi: 10.1017/BJN20061864. [DOI] [PubMed] [Google Scholar]
- Contini M, Baccelloni S, Massantini R, Anelli G. Extraction of natural antioxidants from hazelnut (Corylus avellana L.) shell and skin wastes by long maceration at room temperature. Food Chem. 2008;110:659–669. doi: 10.1016/j.foodchem.2008.02.060. [DOI] [Google Scholar]
- Del Rio D, Calani L, Dall Asta M, Brighenti F. Polyphenolic composition of hazelnut skin. J Agric Food Chem. 2011;59:9935–9941. doi: 10.1021/jf202449z. [DOI] [PubMed] [Google Scholar]
- Delgado T, Malheiro R, Pereira JA, Ramalhosa E. Hazelnut (Corylus avellana L.) kernels as a source of antioxidants and their potential in relation to other nuts. Ind Crop Prod. 2010;32:621–626. doi: 10.1016/j.indcrop.2010.07.019. [DOI] [Google Scholar]
- Esatbeyoglu T, Wray V, Winterhalter P. Identification of two novel prodelphinidin a-type dimers from roasted hazelnut skins (Corylus avellana L.) J Agric Food Chem. 2013;61:12640–12645. doi: 10.1021/jf404549w. [DOI] [PubMed] [Google Scholar]
- Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides) during maturation. J Agric Food Chem. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- Hertog MGL, Feskens EJM, Hollman PCH, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-U. [DOI] [PubMed] [Google Scholar]
- Jakopic J, Petkovsek MM, Likozar A, Solar A, Stampar F, Veberic R. HPLC–MS identification of phenols in hazelnut (Corylus avellana L.) kernels. Food Chem. 2011;124:1100–1106. doi: 10.1016/j.foodchem.2010.06.011. [DOI] [Google Scholar]
- Kalia K, Sharma K, Singh HP, Singh B. Effects of extraction methods on phenolic contents and antioxidant activity in aerial parts of Potentilla atrosanguinea Lodd. and quantification of its phenolic constituents by RP-HPLC. J Agric Food Chem. 2008;56:10129–10134. doi: 10.1021/jf802188b. [DOI] [PubMed] [Google Scholar]
- Kant K, Walia M, Agnihotri VK, Pathania V, Singh B. Evaluation of antioxidant activity of Picrorhiza kurroa (leaves) extracts. Indian J Pharm Sci. 2013;75:324–329. doi: 10.4103/0250-474X.117438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LZ, Harnly JM. LC-PDA-ESI/MS Identification of the phenolic components of three compositae spices: chamomile, Tarragon, and Mexican Arnica. Nat Prod Commun. 2012;7:749–752. [PMC free article] [PubMed] [Google Scholar]
- Ness AR, Powles JW. Fruit and vegetables, and cardiovascular disease: a review. Int J Epidemiol. 1997;26:1–13. doi: 10.1093/ije/26.1.1. [DOI] [PubMed] [Google Scholar]
- Oliveira I, Sousa A, Valentao P, Andrade PB, Ferreira ICFR, Ferreres F, Bento A, Seabra R, Estevinho L, Pereira JA. Hazel (Corylus avellana L.) leaves as source of antimicrobial and antioxidative compounds. Food Chem. 2007;105:1018–1025. doi: 10.1016/j.foodchem.2007.04.059. [DOI] [Google Scholar]
- Rana S, Bhushan S. Apple phenolics as nutraceuticals: assessment, analysis and application. J Food Sci Technol. 2016;53:1727–1738. doi: 10.1007/s13197-015-2093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins KS, Ma Y, Lenny Wells M, Greenspan P, Pegg RB. Separation and characterization of phenolic compounds from US pecans by liquid chromatography—tandem mass spectrometry. J Agric Food Chem. 2014;62:4332–4341. doi: 10.1021/jf500909h. [DOI] [PubMed] [Google Scholar]
- Ros E, Mataix J. Fatty acid composition of nuts—implications for cardiovascular health. Braz J Nutr. 2006;96:S29–S35. doi: 10.1017/BJN20061861. [DOI] [PubMed] [Google Scholar]
- Sabate J, Ros E, Salas-Salvado J. Nuts: nutrition and health outcomes. Braz J Nutr. 2006;96:S1–S2. doi: 10.1017/BJN20061857. [DOI] [PubMed] [Google Scholar]
- Salas-Salvado J, Bullo M, Perez-Heras A, Ros E. Dietary fibre, nuts and cardiovascular diseases. Braz J Nutr. 2006;96:S46–S51. doi: 10.1079/BJN20061700. [DOI] [PubMed] [Google Scholar]
- Sang S, Lapsley K, Jeong WS, Lachance PA, Ho CT, Rosen RT. Antioxidantive phenolic compounds isolated from almond skins (Prunus amygdalus Batsch) J Agric Food Chem. 2002;50:2459–2463. doi: 10.1021/jf011533+. [DOI] [PubMed] [Google Scholar]
- Santamaria RI, Soto C, Zuniga ME, Chamy R, Lopez-Munguia A. Enzymatic Extraction of Oil from Gevuina avellana, the Chilean hazelnut. J Am Oil Chem Soc. 2003;80:33–36. doi: 10.1007/s11746-003-0646-8. [DOI] [Google Scholar]
- Scalbert A, Johnson IT, Saltmarsh M. Polyphenols: antioxidants and beyond. Am J Clin Nutr. 2005;81:S215–S217. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- Shahidi F, Naczk M. Phenolics in food and nutraceuticals. Boca Raton: CRC Press; 2004. [Google Scholar]
- Shahidi F, Alasalvar C, Liyana-Pathirana CM. Antioxidant phytochemicals in hazelnut kernel (Corylus avellana L.) and hazelnut byproducts. J Agric Food Chem. 2007;55:1212–1220. doi: 10.1021/jf062472o. [DOI] [PubMed] [Google Scholar]
- Singh B, Singh JP, Kaur A, Singh N. Bioactive compounds in banana and their associated health benefits—a review. Food Chem. 2016;206:1–11. doi: 10.1016/j.foodchem.2016.03.033. [DOI] [PubMed] [Google Scholar]
- Singh JP, Kaur A, Singh N, Nim L, Shevkani K, Kaur H, Arora DS. In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT Food Sci Technol. 2016;65:1025–1030. doi: 10.1016/j.lwt.2015.09.038. [DOI] [Google Scholar]
- Sullivan GT, Ozman-Sullivan SK, Akbasli O, Sahin M. A tribute to the hazelnut plant (Corylus spp.)—the multiple uses of nature’s magnificent gifts. Acta Hortic. 2014;1052:371–376. doi: 10.17660/ActaHortic.2014.1052.51. [DOI] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005;81:S292–S297. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- Watson RR. Functional foods and nutraceuticals in cancer prevention. Oxford: Blackwell Publishing; 2003. [Google Scholar]
- Wijeratne SSK, Abou-Zaid MM, Shahidi F. Antioxidant polyphenols in almond and its coproducts. J Agric Food Chem. 2006;54:312–318. doi: 10.1021/jf051692j. [DOI] [PubMed] [Google Scholar]
- Yang J, Halim L, Liu RH (2005) Antioxidant and antiproliferative activities of common nuts. In: Presented at the Institute Of Food Technologists annual meeting and food expo, New Orleans, LA, 16–20 July 2005, paper 035-05
- Yurttas HC, Schafer HW, Warthesen JJ. Antioxidant activity of nontocopherol hazelnut (Corylus spp.) phenolics. J Food Sci. 2000;65:276–280. doi: 10.1111/j.1365-2621.2000.tb15993.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.