Abstract
A lethal disease of koi and common carp (species Cyprinus carpio) has afflicted many fish farms worldwide since 1998, causing severe financial losses. Morbidity and mortality are restricted to common carp and koi and appear in spring and autumn, when water temperatures are 18 to 28°C. We have isolated the virus causing the disease from sick fish, propagated it in koi fin cell culture, and shown that virus from a single clone causes lethal disease in carp and koi upon infection. Intraperitoneal virus injection or bathing the fish in virus-containing water kills 85 to 100% of the fish within 7 to 21 days. This virus is similar to the previously reported koi herpesvirus; however, it has characteristics inconsistent with the herpesvirus family, and thus we have called it carp interstitial nephritis and gill necrosis virus. We examined the pathobiology of this disease in carp by using immunohistochemistry and PCR. We found large amounts of the virus in the kidneys of sick fish and smaller amounts in liver and brain. A rapid increase in the viral load in the kidneys was detected by using both immunofluorescence and semiquantitative PCR. Histological analyses of fish at various times after infection revealed signs of interstitial nephritis as early as 2 days postinfection, which increased in severity up to 10 days postinfection. There was severe gill disease evidenced by loss of villi with accompanying inflammation in the gill rakers. Minimal focal inflammation was noted in livers and brains. This report describes the etiology and pathology of a recently described viral agent in fish.
Since 1998 many carp and koi farms have been afflicted by a disease with a high mortality rate, resulting in a drastic reduction in production. This disease is caused by a viral agent (1, 4, 9, 15, 16) and has been observed in many farms in the northern and western regions of the United States (9), Europe (1, 18), Israel, Indonesia, Japan, and Korea (14). The rapid spread of this disease is probably due to the intensive worldwide trade of these splendid fish, mostly without veterinary supervision. The disease appears in open-air ponds during spring and fall, with clinical signs including fatigue, gasping movements in shallow water, gill necrosis, sunken eyes, pale patches on skin, and increased mucus secretion (4, 9). Studies performed under laboratory conditions confirmed that the virus induces the disease only at the permissive temperature of 18 to 28°C (5, 9, 15). Although the disease is highly contagious, its morbidity and mortality are restricted to koi and common carp populations. Other fish of the tilapia group (Oreochromis niloticus), silver perch (Bidyanus bidyanus), silver carp (Hypophthalmichthys molitrix), gold fish (Carassius auratus), and grass carp (Ctenopharyngodon idella) were found to be fully resistant to the disease, even after long cohabitation with sick fish at the permissive temperature (15).
This disease has been described previously by Hedrick and coworkers, who isolated the causative pathogen, the koi herpesvirus (KHV). In agreement with previous studies of KHV (1, 4, 5, 9), the virus that we isolated and designated carp interstitial nephritis and gill necrosis virus (CNGV) (15, 16) has an icosahedron-shaped core of 100 to 110 nm, is an enveloped virus, and bears thread-like structures (tegument) on the core surface resembling those of the herpesvirus (M. Hutoran et al., submitted for publication). Although we believe KHV and CNGV to be the same, we have found that CNGV contains an asymmetrical electron-dense region within the viral core, which is probably the genomic nucleoprotein complex, and that its genome is a double-stranded DNA molecule of 270 to 290 kbp (Hutoran et al., submitted), which is larger than those of all known Herpesviridae members (3). Since the viral genome sequences determined so far, including part of the thymidine kinase gene (6) (GenBank accession numbers AY208988 to -91, AJ535112, and AF411803), contain only small fragments (16 to 45 bp), similar to the case for other DNA viral genomes, we believe that it is too early to classify the virus phylogenetically. Thus, although CNGV may be similar or identical to KHV, we believe that it is preferable to designate it according to its pathological manifestation as CNGV.
The morphology of KHV/CNGV and its genomic DNA sequences have been intensively studied (4-6, 15, 16). However, there is little information about its effect in tissue culture, the pathogenesis of the disease, target organs of the virus, and the way it spreads in fish organs. This study was undertaken to elucidate these important points. Here we show that (i) CNGV propagates and induces severe cytopathic effects at 3 to 5 days postinfection (p.i.) in fresh koi fin cell (KFC) cultures; (ii) cloned CNGV harvested from KFC cultures induces the same disease upon inoculation of healthy koi and common carp, with a mortality rate of 75 to 95%; (iii) the amounts of CNGV DNA and specific viral antigens in the kidney and blood increase during the first 7 days p.i.; and (iv) the virus induces progressive pathogenic effects in the kidney, liver, and gills, as shown by histological and immunohistochemical examinations.
MATERIALS AND METHODS
Fish.
Common carp with an average weight of 50 g (approximately 4 months old) were grown in 100- or 500-liter tanks. The water temperature was maintained at 22 to 24°C, and fresh water was supplied at 0.5 or 0.9 liter/min.
Fish infection.
Infection was carried out by cohabitation, bathing, or injection.
(i) Cohabitation.
Two symptomatic fish from the stock of sick fish continuously maintained at Dor Research Station (14) were added to a 500-liter tank containing 30 healthy fish and left for 24 h.
(ii) Bathing.
Healthy fish were kept in a 20-liter tank, to which virus was added to achieve a final concentration of ∼30 PFU/ml. After 50 min under these conditions, the fish were transferred into large tanks. The control group was immersed in water containing medium harvested from uninfected KFC cultures under the same conditions.
(iii) Injection.
A 0.2-ml volume of culture medium containing 100 PFU of isolated virus was injected intraperitoneally into healthy fish.
Cell cultures.
Cultures were prepared as previously described by Hasegawa et al. (8) and Neukirch et al. (13). Briefly, caudal fins were removed from 50 g of koi fish under anesthesia, bathed in 1% sodium hypochloride solution for 1 min, and rinsed in 70% ethyl alcohol for a few seconds. The fins were then washed three times for 0.5 min each in phosphate-buffered saline (PBS) containing penicillin and streptomycin. They were transferred to petri dishes and extensively minced with scissors, and small semidry tissue pieces of approximately 1 mm3 were placed in dry 50-ml culture flasks (Nunc). After 60 min of incubation at room temperature, the clumps adhering to the flasks were covered with culture medium containing 60% Dulbecco's modified Eagle's medium, 20% Leibovitz (L-15) medium, 10% fetal calf serum (Biological Industries, Kibbutz Beit Haemek, Israel), and 10% tryptose phosphate (Difco) and supplemented with 1% HEPES and antibiotics. Cells grew to form a monolayer over a period of 10 to 14 days in a 22°C incubator supplemented with 5% CO2. The monolayer cultures were trypsinized and transferred into new flasks with fresh medium.
Purification of virus from culture medium.
Medium harvested from infected KFC cultures was cleared of cells and cell debris by centrifugation for 10 min at 10,000 × g. The virus was then pelleted by centrifugation in a Beckman Ti-60 rotor for 50 min at 100,000 × g. Pellets were suspended in PBS and loaded on a 15 to 65% (wt/vol) sucrose gradient prepared in PBS and centrifuged for 60 min at 110,000 × g in a Beckman SW28 rotor. Bands were aspirated from tubes, diluted 10-fold in PBS, and repelleted. The pellets were suspended in PBS and frozen at −70°C for further investigation.
Antibodies.
Anti-CNGV serum was generated by immunizing a rabbit with 0.1 mg of purified CNGV emulsified 1:1 in Freund's complete adjuvant. The rabbit was boosted three additional times at 10- to 14-day intervals with 0.05 mg of purified CNGV mixed 1:1 with Freund's incomplete adjuvant and bled three times between 7 and 10 weeks after the first immunization to collect antiserum. In order to reduce nonspecific background, the antisera were absorbed into a mixture of KFCs and koi fish powder prepared from muscles and kidneys of healthy fish, as described by Harlow and Lane (7).
Detection of viral DNA by semiquantitative PCR.
With heparinized syringes, blood samples (0.2 ml each) were taken from four anesthetized fish daily for 7 days, at which point the fish were euthanatized and their kidneys were removed. The kidney and blood samples were incubated with proteinase K (100 μg/ml) in lysis buffer (20 μg of RNase per ml, 0.5% sodium dodecyl sulfate, and 0.1 M EDTA in 10 mM Tris [pH 8]) for 4 h at 55°C, followed by inactivation at 70°C for 20 min. The DNA was then phenol extracted, precipitated with ethanol according to standard procedures, and resuspended in double-distilled water (17). DNA concentrations were determined by spectrophotometry. Primers CNGV-543 (5′CGACCGACTTCGTCATCAAAG3′) and CNGV-652 (5′GGACATCAATGGAGGAACGGA3′) were used to amplify the CNGV (109 bp), while 18S-702 (5′GGACGAAAGCGAAAGCATTTG3′) and 18S-902 (5′TTTGACAACCATACTCCCCCC3′) were used to amplify the 18S ribosomal DNA (200 bp) as an internal control. PCR amplification was performed with a DNA thermal cycler (MJ Research). PCR conditions were as follows: initial denaturation at 94°C for 5 min, followed by denaturation at 95°C for 30 s, annealing at 60°C for 30 s, extension at 72°C for 30 s, and a final extension reaction at 72°C for 5 min. For determination of the linearity of PCRs, replicate PCRs were performed with increasing numbers of cycles (20 to 40 cycles) and different amounts of DNA (0.01 to 5 μg). Twenty-six cycles were carried out to amplify 18S ribosomal DNA, and 35 cycles were carried out in order to detect the viral DNA. DNA was amplified in a total volume of 20 μl including 1 μg of total DNA extracted from kidney or blood cells, 2 μl of 10× buffer (Promega), 1.5 mM MgCl2, 0.01% (wt/vol) bovine serum albumin (BSA), a 100 μM concentration of each deoxynucleoside triphosphate, a 0.25 μM concentration of each primer, and 2 U of Taq DNA polymerase (Promega).
Indirect immunofluorescence microscopy.
At 16 days after cohabitation, sick fish were anesthetized and their kidneys, livers, and brains were removed and used for the preparation of touch imprint slides. Organs taken from healthy fish were used as controls. These were fixed for 10 min in 100% methanol, washed with PBS, and blocked by incubation for 60 min with low-fat milk containing 50% fetal calf serum. The slides were then incubated for 60 min with rabbit preimmune serum or anti-CNGV serum (diluted 1:10,000 in PBS), washed with PBS, incubated for 60 min with fluorescein isothiocyanate-conjugated swine anti-rabbit antibodies (Dako), washed with PBS, and analyzed with a fluorescence microscope equipped with a 40× objective.
Histological analysis.
For histological analysis, brain, gill, liver, and kidney tissue samples were fixed overnight in 4% buffered formaldehyde and embedded in paraffin. Five-micrometer sections were stained with hematoxylin and eosin. Selected sections were also stained with Gram, periodic acid-Schiff, and Ziehl-Neelsen stains.
Immunohistochemistry.
Fish infected by intraperitoneal injection of 200 PFU of CNGV per ml were used for immunohistochemistry. Formalin-fixed, paraffin-embedded tissue samples were cut into 5-μm sections, deparaffinized in xylene, and rehydrated through a series of graded ethanol solutions. Sections were incubated for 1 h with polyclonal rabbit anti-CNGV serum diluted at 1:30,000 in CAS block (Zymed Laboratories) at room temperature. Antibodies were detected by using the Envision+ kit (DAKOCytomation, Glostrup, Denmark) according to the manufacturer's instructions. 3-Amino-9-ethyl-carbazole was used as the chromogen. As a control, sections were incubated with nonimmune rabbit serum at the same dilution. For competition experiments the antiserum was preincubated with purified virus or BSA for 30 min before application to the tissue sections.
Electron microscopy.
For ultrastructural studies, kidneys from healthy or infected fish were removed, dissected, rapidly immersed in 2.5% glutaraldehyde in cacodylate buffer, postfixed in osmium tetraoxide, and embedded in Epon. Ultrathin sections were cut with an Ultracut E microtome (Reichert-Jung, Vienna, Austria). Sections were stained with lead citrate and uranyl acetate and viewed by transmission electron microscopy (Philips 420 electron microscope at 80 kV).
RESULTS
Cloned CNGV is sufficient to induce mortal disease in carp.
Virus was isolated from sick fish by cocultivation of KFCs with cells from kidney of sick fish (15, 16), and aliquots were used to infect KFC cultures. The virus isolated from sick fish produces typical plaques on KFCs at 4 to 6 days p.i. (Fig. 1). Cytopathic effect appears in KFC cultures at 3 to 5 days p.i. At 2 to 3 days p.i. the volume of the infected cells increases and abundant endoplasmic vacuoles appear in the cells. At a later stage, cells become round, die, and detach from the substrate. Significant amounts of virus are released into the culture medium at 3 to 5 days p.i. In order to determine whether a virus isolated from a single plaque (cloned virus) is sufficient to induce the carp disease, we infected KFC cultures with decreasing titers of the virus. Following absorption, the cultures were overlaid with agar and incubated at 22 to 23°C for 5 days. The number of plaques decreased proportionally to the virus dilutions, displaying a one-hit curve, demonstrating that a single virus is sufficient to generate each plaque (results not shown). Virus from a single plaque was propagated, purified, and injected into healthy fish as described in Materials and Methods, and 91% of fish injected with ∼102 PFU of the cloned virus died within 21 days following infection (Fig. 2). All the diseased fish showed the classic clinical symptoms of CNGV infection, including gasping movements, sunken eyes, and swimming in shallow water. In addition, we randomly sampled four fish at 2, 4, 6, 8, and 10 days p.i. for histological analysis. All infected fish showed typical changes of CNGV infection (see below). Control fish injected with medium harvested from uninfected cultures remained healthy throughout the 30-day experiment.
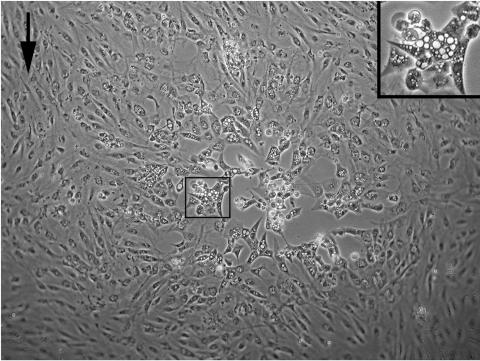
FIG. 1.
Cytopathic effect induced by CNGV. KFCs were infected with CNGV and incubated at a permissive temperature of 22°C. At 4 to 5 days p.i., cytopathic effect can be observed. Cells become enlarged and develop abundant endoplasmic vacuoles (center box; the inset at upper right shows the boxed area at a higher magnification). The arrow indicates uninfected cells.
FIG. 2.
Mortality of fish infected with cloned virus. Fish (n = 50; average weight, 100 g) were injected with 0.2 ml of a solution containing approximately 102 PFU of the cloned virus. The control group was composed of fish (n = 50; average weight, 100 g) injected with 0.2 ml of a solution containing medium harvested from uninfected cultures.
The cloned virus was also used to infect fish by bathing as described in Materials and Methods. Ninety-one percent of the fish exposed to virus in the water died between 10 and 24 days p.i., while all of the control fish remained healthy during the entire experimental period. These results demonstrate that the virus can be transmitted by injection or via water and that virus cloned from a single plaque is sufficient to induce the carp disease.
Identification of CNGV DNA in blood and kidneys of infected fish.
To follow the kinetics of the virus's appearance, healthy fish (50 ± 10 g) were cohabitated with sick fish or bathed for 1 h in water containing CNGV at a final concentration of 30 PFU/ml. Noninfected fish kept under identical conditions served as a control group. Five fish from each group were randomly sampled at each time point.
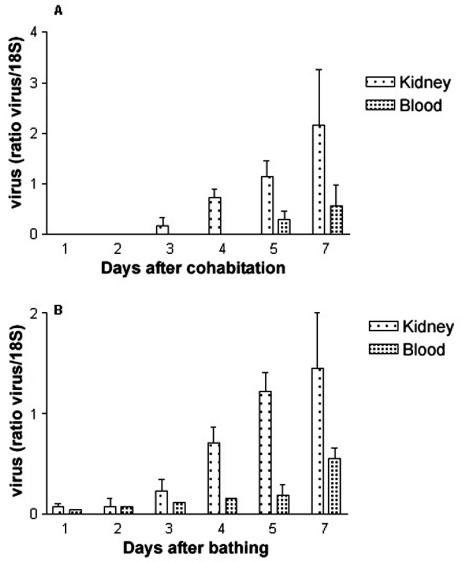
Using semiquantitative PCR, we found that the amount of viral DNA in extracts of both kidney and blood cells from fish infected by cohabitation or bathing increased throughout the testing period (Fig. 3). CNGV DNA was detected at low levels in blood and kidney as early as 1 day after exposure of fish to the virus by bathing (Fig. 3B), whereas it was not detected until day 3 or 5 in kidney or blood cells of fish infected by cohabitation and was absent in kidney and blood of uninfected fish (Fig. 3A).
FIG. 3.
Kinetics of the appearance of viral DNA in infected fish. Semiquantitative PCR analysis of CNGV DNA was performed on DNA extracted from fish blood samples or kidneys following infection by cohabitation (A) or bathing (B). The CNGV DNA levels are normalized against those of 18S ribosomal DNA in the same sample. Each bar represents the mean for five fish ± standard error of the mean.
Identification of CNGV in fish organs.
To search for viral protein in fish organs, we generated a rabbit anti-CNGV antiserum. The rabbit anti-CNGV serum bound to touch imprints of kidney taken from symptomatic fish at 16 days p.i. by cohabitation but not to those from uninfected fish, indicating that the virus is present in kidneys of infected fish (Fig. 4A and D). We also used immunofluorescence with rabbit anti-CNGV serum to assess the presence of virus in the brains and livers of sick fish. Staining in touch imprints from these organs was significant but was lower than that found in kidney imprints (Fig. 4B and C). Preimmune rabbit serum did not bind to touch imprints from any of these organs, demonstrating the specificity of the interaction (data not shown). These experiments suggest that viral proteins are extensively expressed in kidney of diseased fish and less so in the brain and liver. These results are in agreement with our experiments isolating virus from diseased fish organs (results not shown) and with our semiquantitative PCR experiments (Fig. 3), both of which show that the kidney harbors large quantities of infectious virus.
FIG. 4.
Immunofluorescence assay for the presence of CNGV in fish organs. Touch imprints of kidney (A), liver (B), and brain (C) from infected fish and of kidney from healthy fish (D) were incubated with rabbit anti-CNGV serum (1:1,000). Antibodies were detected by using fluorescein isothiocyanate-conjugated swine anti-rabbit antibodies.
Histopathology.
To further characterize the disease process, we infected fish by bathing and analyzed the histopathological changes induced by viral infection in several organs at various time points following infection. Pathological changes were noted in the gills as early as 2 days p.i., as evidenced by a loss of lamellae accompanied by a mixed inflammatory cell infiltrate (Fig. 5). However, these changes were not present in all of the filaments, and considering the frequent involvement of the gills in numerous inflammatory processes (T. T. Poppe, Histopathology workshops: notes and images, EAFP Histology Workshop, 2001), these findings are not diagnostic at this early stage of infection. From 6 days p.i. onward, changes in the gills became more pronounced, with complete effacement of the gill architecture accompanied by severe inflammation in nearly all of the filaments. Congestion of the gills' central venous sinus was also evident at this stage. Similar changes were evident at 8 and 10 days p.i. Many infectious diseases induce a pronounced effect in the gill filaments (2, 10-12). Interestingly, in fish infected with CNGV, the gill rakers showed effects that were more easily recognizable than the changes observed in the filaments. These include increased subepithelial inflammation and congestion of the blood vessels in the gill arch (Fig. 5D to F). The inflammatory process was accompanied by attenuation of the rakers' height. As early as 6 days p.i., focal sloughing of the surface epithelium was noted (Fig. 5). This was even more marked at later time points assayed (not shown).
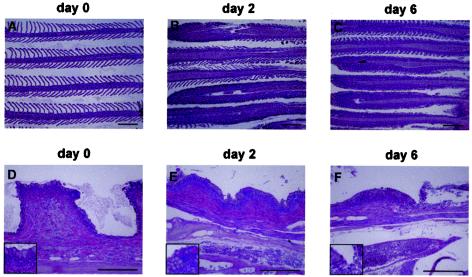
FIG. 5.
CNGV induces gill inflammation as early as 2 days p.i. Carps were infected with CNGV and harvested at the indicated days p.i. Gills were collected and subjected to histological analyses. (A to C) Gill filaments. Normally gill filaments are slender structures containing numerous lamellae (A). As early as 2 days p.i. (B), many lamellae are infiltrated by inflammatory cells. At 6 days p.i. (C) and onwards, all lamellae are heavily infiltrated. (D to F) Gill rakers. As early as 2 days p.i. (E), an increased inflammatory infiltrate is present in the subepithelial zone. In addition, at the bottom of the photomicrograph a congested vessel in the gill arch is seen. At 6 days p.i. (F), the inflammatory process is more pronounced, with sloughing of the overlying epithelium (upper right). This is accompanied by increased congestion and edema. All of the sections were stained with hematoxylin and eosin. The insets in the lower left corners are of areas in the centers of the respective photomicrograph. Bars, 200 μm.
While gill injury precedes all other histological changes, it is plausible, as argued before (9), that this effect is caused by secondary infections. To assess whether bacterial, fungal, or parasitic infectious agents are involved in this process, we stained histological sections of gills at different time points with Gram, periodic acid-Schiff, and Ziehl-Neelsen stains, which facilitate the detection of microorganisms. No increase in the amount of microorganisms was detected on days 2 to 6 following infection. This is in contrast with the marked inflammatory response in the gills that is evident as early as 2 days p.i. Only at 8 and 10 days p.i., when focal denudation of the gill epithelium occurs, were increased numbers of microorganisms noted (data not shown), suggesting that the primary gill disease induced by CNGV predisposes the fish to secondary infections.
In addition to the gills, the most prominent pathological changes were noted in the kidneys (Fig. 6). A mild peritubular inflammatory infiltrate was evident as early as 2 days p.i. On day 6, a heavy interstitial inflammatory infiltrate was observed, along with congestion of blood vessels. At 8 days p.i. the infiltrates were more severe and were accompanied by a feathery degeneration of the tubular epithelium in many nephrons, together with the presence of intraepithelial lymphocytes (Fig. 6E to H). As early as 6 days p.i., large cells with a foamy distended cytoplasm and a few intranuclear inclusion bodies were scattered among the inflammatory interstitial cells (Fig. 6G to I). These “foamy” cells are reminiscent of the cytopathic effect observed in infected cultured cells (Fig. 1). Liver analysis showed mild inflammatory infiltrates, located mainly in the parenchyma, while brain sections showed focal meningeal and parameningeal inflammation.
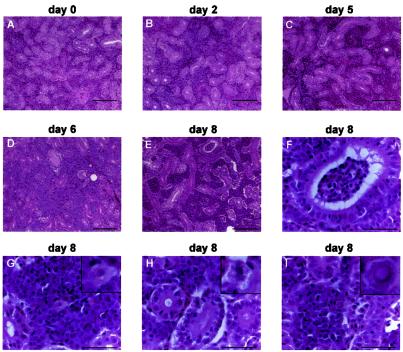
FIG. 6.
Progressive interstitial nephritis induced by CNGV. Kidneys from infected carp were collected at the indicated days p.i., and tissue sections were stained with hematoxylin and eosin. Note increased interstitial infiltration by inflammatory cells as the disease progresses. At 8 days p.i. epithelial vacuolization is also noted. (A to E) Low-power photomicrographs (bars, 100 μm). (F to I) High-power photomicrographs (bars, 40 μm). Note renal tubular inflammation (F), cytoplasmic vacuolization in a white blood cell (G), epithelial cytopathic effect (H), and a rare intranuclear inclusion body in an inflammatory cell (I). In panels G to I, the insets in the upper right corners are of cells in the centers of the respective photomicrograph.
Detection of CNGV in fish tissues by immunohistochemistry.
As noted above, both PCR and immunofluorescence detect viral particles in kidneys, with the latter method also showing that such particles are present in brains and livers of diseased fish. To verify these findings and to identify the specific cells in which viral proteins are expressed, we performed immunohistochemical analyses of kidneys, brains, livers, spleens, and gills from infected and healthy fish. Kidneys from healthy fish showed no staining with anti-CNGV antiserum (Fig. 7A). As early as 2 days p.i., viral proteins were detected in the kidney by the rabbit anti-CNGV serum, in conjunction with the appearance of the lymphoid inflammatory infiltrate in the renal interstitium (data not shown). At this stage of infection, stained cells were detected only in the interstitium, most of them large and corresponding to the foamy cytomegalic cells described above. The number of immunostained interstitial cells was maximal at 6 days p.i. (Fig. 7B) and remained constant through 10 days p.i. Interestingly, at 10 days p.i., when hematoxylin and eosin staining shows maximal feathery degeneration of the tubular epithelium, viral proteins were also detected in some of the tubular epithelial cells (Fig. 7C). This finding suggests that the virus is not restricted to cells of the hematolymphoid lineage.
FIG. 7.
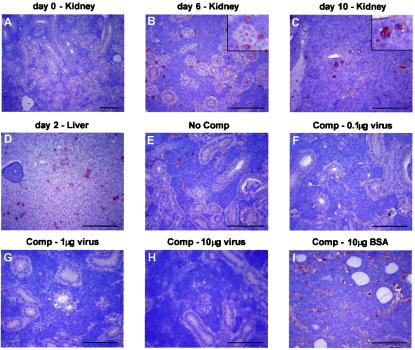
Immunohistochemical staining of carp tissues with antiserum against CNGV (A to D). Tissue sections from kidney or liver of healthy carp (A) or carp sacrificed at the indicated days p.i. (B to D) were incubated with antiserum against CNGV. Note that while at day 6 only interstitial cells are stained, at day 10 viral proteins are also detected in the epithelial cells. To demonstrate the specificity of the staining reaction, the serum was incubated with increasing amounts of CNGV protein (F to H) or BSA (I) or was not preincubated (E) prior to its application to the tissue sections. Bar, 100 μm. The insets in panels B and C show enlargements. Comp, competition.
No virus-positive cells were detected in a serial section from each kidney that was immunostained with preimmune rabbit serum and served as a negative control. To further verify the specificity of the immunohistochemical method, we preincubated the antiserum with purified virus. Preincubation with BSA served as a control. Figure 7E to I show serial sections of a kidney from an infected fish at 8 days p.i. that were incubated with untreated anti-CNGV antiserum (Fig. 7E) or with anti-CNGV antiserum preincubated with 0.1, 1, or 10 μg of viral proteins (Fig. 7F to H). Preincubation with increasing amounts of viral protein decreased the staining intensity and, at high concentrations, abolished staining. In contrast, preincubation of the antiserum with BSA at the same concentrations (Fig. 7I) did not decrease the staining intensity.
Immunohistochemistry with anti-CNGV antibodies showed that the number of infected cells in the gills of diseased fish increased between 2 and 10 days p.i. (data not shown). However, a few positive cells were detected in all healthy fish assayed. This may represent subclinical disease in these fish, cross-reaction of the antiserum with a similar virus that is commonly present in carp gills, or, alternatively, nonviral proteins present in gills that nonspecifically reacted with our antiserum.
Detection of CNGV in fish tissues by electron microscopy.
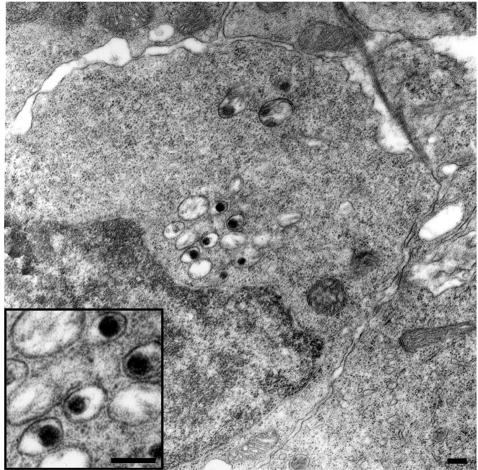
To better characterize the cells which are infected by CNGV, sections from infected kidneys were analyzed by electron microscopy (Fig. 8). Viral particles were detected in the cytoplasm of occasional cells in the interstitium of diseased kidneys. The particles had a dark, electron-dense core surrounded by a halo, similar to the case of the particles detected in infected cultured cells and in sections of purified virus preparation (data not shown). The frequency of virus-containing cells was similar to the frequency of positively stained cells in the immunohistochemical analysis. The typical virus-bearing cell has an oval, somewhat irregular nucleus contour and abundant cytoplasm. The main cytoplasmic organelles include numerous free ribosomes, a Golgi apparatus, a few mitochondria, and some lysosomes. Neither filaments nor junctional complexes were apparent. Thus, these cells most probably represent monocytes/macrophages. We were unable to detect infected cells in the peripheral blood.
FIG. 8.
Ultrastructural appearance of CNGV particles in infected kidney. Shown is a detail of an infected cell, at 8 days p.i., harboring several cytoplasmic viral particles with round-electron dense cores (magnified in the inset). Bars, 200 nm.
DISCUSSION
A lethal disease of common carp and koi is caused by a virus here designated CNGV, according to its pathogenic effect in fish. A very similar pathogen was previously isolated from fish suffering from the same symptoms and was designated KHV by Hedrick et al. (9). The virus that we have isolated is most probably the same virus that was described by Hedrick et al. (9). However, we believe that CNGV is a more appropriate name, since the virus has several characteristics not found in Herpesviridae. Previous studies have focused on the evolutionary relationship of this virus to other large viruses, by using morphological studies, characterization of the genomic DNA molecule, and sequencing the viral genome (4-6). Although a detailed understanding of the cytopathic effect, the pathogenesis, and the pathogenic effect induced by the virus is extremely important for virus characterization, little is known in this regard. Our report addresses this issue and describes molecular, histological, and immunological findings essential for the characterization of the disease induced by CNGV.
In order to demonstrate that a single plaque-derived clone is sufficient to induce the disease, we first proved that the number of plaques induced by CNGV is directly related to its dilution, i.e., it yields a single-hit curve. Thus, a single infectious unit is sufficient to produce each plaque. We then showed that virus isolated from a single plaque induces the disease in fish and that no helper virus or additional microorganism is required. The virus isolated from sick fish induced a cytopathic effect and typical plaques in KFCs: it increased the cell volume, induced the formation of abundant cytoplasmic vacuoles in the infected cells, and rendered the cells round shaped before they detached from the substrate. It is noteworthy that infected cells with foamy cytoplasm were also observed in kidneys of diseased fish, suggesting that the production of abundant vacuoles is determined by the virus and not by the target cells.
PCR of DNA from various tissues revealed that viral DNA is present in blood and kidneys of infected fish at very early time points after infection but appears in brain, liver, and spleen only in the late stages of the disease (6) (data not shown). Using semiquantitative PCR, we showed that the viral DNA is present in the kidneys of infected fish. These results are supported by both the immunofluorescence and immunohistochemical studies. Viral DNA was detected in the kidney and blood as early as 3 and 5 days postcohabitation, respectively. Infection of fish by bathing appears to be more efficient than that by cohabitation, since viral DNA was detected in the kidney and blood as early as 1 day postexposure. However, as in the case of infection by cohabitation, the amount of viral DNA in the kidney began to increase at 3 days p.i. and that in the blood began to increase at 5 or 7 days p.i.
The kidney was found to be the organ in which the virus propagates most efficiently, by both immunohistochemistry and touch imprint techniques. Interestingly, while immunohistochemical staining revealed only occasional positive cells, our touch imprint analysis detected many more positive cells. This may be explained in several ways. First, the touch technique samples cells that detach easily from the tissue surface. It is possible, therefore, that infected white blood cells are more abundant and more prone to detachment than other cell types, yet all cell types are equally represented in the immunohistochemical sections. Second, touch imprints were performed from terminally sick fish at 16 days p.i., while fish used for immunohistochemistry were euthanatized at 6 to 10 days p.i. Finally, formalin fixation, which interferes with antigen-antibody recognition, may result in labeling only of cells bearing very high levels of viral antigens. At present, we cannot conclusively determine the intracellular localization of the viral propagation. Since this is a DNA virus, one might expect evidence for intranuclear replication. However, electron microscopy, as well immunological detection methods, localized viral particles and/or proteins to the cytoplasm of the infected cells. This interesting aspect of CNGV propagation remains to be solved.
The virus remains infective in water for at least 4 h (15), explaining the highly contagious nature of the virus in ponds. It is not yet known how the virus enters the fish body, i.e., whether through the gills or through the intestine (9, 15). Our histological analysis supports the possibility that the virus enters the fish body through the gills, replicates there, and induces mucosal sloughing and necrosis. It is conceivable that gill injury induced by the virus significantly contributes to fish morbidity. However, host-virus interaction in the gills still needs to be elucidated in order to understand the pathogenic mechanisms responsible for gill injury. The possibility that the virus replicates in the diseased gills and is then shed into the water is in agreement with the rapid and efficient spread of this contagious disease. From the gills, the virus is rapidly transferred to the kidneys, where it resides in white blood cells and induces severe interstitial nephritis. Other organs assayed, including the brain and liver, are relatively spared.
Localization of the virus within white blood cells raises the intriguing possibility that the virus is rapidly transferred to the viscera via infected white blood cells. The virus can then multiply in the kidney and infect the epithelial cells there. Another important question is whether the virus resides in the gills. Despite the technical difficulties mentioned above, the large amounts of virus in this organ suggest that the virus can multiply in the gills. It can then be released into the water either through shedding or together with the sloughed epithelial and inflammatory cells resulting from severe local inflammation. The ability to invade the fish through the gills, multiply there, and then be released through the water is favorable for the virus. This is analogous to respiratory viruses in mammals that infect the respiratory epithelium, replicate there, and are spread through air droplets and aerosol. This may turn out to be the most common means of spreading of aquatic viruses.
Acknowledgments
This work was supported by grant 02-0082 from the Chief Scientist's Office, Ministry of Agriculture and Rural Development, Israel.
We thank S. Amir for editing the manuscript and Tamara Golub and Norma Kidess for excellent technical assistance.
REFERENCES
- 1.Body, A., F. Lieffrig, G. Charlier, and A. Collard. 2000. Isolation of virus-like particles from Koi (Cyprinus carpio) suffering gill necrosis. Bull. Eur. Assoc. Fish Pathol. 20:87-88. [Google Scholar]
- 2.Brudeseth, B. E., J. Castric, and O. Evensen. 2002. Studies on pathogenesis following single and double infection with viral hemorrhagic septicemia virus and infectious hematopoietic necrosis virus in rainbow trout (Oncorhynchus mykiss). Vet. Pathol. 39:180-189. [DOI] [PubMed] [Google Scholar]
- 3.Davison, A. J. 2002. Evolution of the herpes viruses. Vet. Microbiol. 86:69-88. [DOI] [PubMed] [Google Scholar]
- 4.Gilad, O., S. Yun, K. B. Andree, M. A. Adkison, A. Zlotkin, H. Bercovier, A. Eldar, and R. P. Hedrick. 2002. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Dis. Aquat. Organ. 48:101-108. [DOI] [PubMed] [Google Scholar]
- 5.Gilad, O., S. Yun, M. A. Adkison, K. Way, N. H. Willits, H. Bercovier, and R. P. Hedrick. 2003. Molecular comparison of isolates of an emerging fish pathogen, koi herpesvirus, and the effect of water temperature on mortality of experimentally infected koi. J. Gen. Virol. 84:2661-2668. [DOI] [PubMed] [Google Scholar]
- 6.Gray, W. L., L. Mullis, S. E. LaPatra, J. M. Groff, and A. Goodwin. 2002. Detection of koi herpesvirus DNA in tissues of infected fish. J. Fish Dis. 25:171-178. [Google Scholar]
- 7.Harlow, E., and D. Lane. 1988. Antibodies: a laboratory manual, p. 632-633. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 8.Hasegawa, S., T. Somamoto, C. Nakayasu, T. Nakanishi, and N. Okamoto. 1997. A cell line (CFK) from fin of isogeneic ginbuna crusian carp. Fish Pathol. 32:127-128. [Google Scholar]
- 9.Hedrick, R. P., O. Gilad, S. Yun, J. Spangenberg, G. Marty, R. Nordhausen, M. Kebus, H. Bercovier, and A. Eldar. 2000. A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of common carp. J. Aquat. Anim. Health 12:44-55. [DOI] [PubMed] [Google Scholar]
- 10.Lee, N. S., Y. Nomura, and T. Miyazaki. 1999. Gill lamellar pillar cell necrosis, a new birnavirus disease in Japanese eels. Dis. Aquat. Organ. 37:13-21. [DOI] [PubMed] [Google Scholar]
- 11.Neukirch, M. 1984. An experimental study of the entry and multiplication of viral haemorrhagic septicemia virus in rainbow trout (Oncorhynchus mykiss L.), after water-borne infection. J. Fish Dis. 7:231-234. [Google Scholar]
- 12.Neukirch, M. 1986. Demonstration of persistent viral haemorrhagic septicemia (VHS) virus in rainbow trout after experimental waterborne infection. J. Vet. Med. 33:471-476. [DOI] [PubMed] [Google Scholar]
- 13.Neukirch, M., K. Bottcher, and S. Bunnajirakul. 1999. Isolation of a virus from Koi with altered gills. Bull. Eur. Assoc. Fish Pathol. 19:221-224. [Google Scholar]
- 14.Oh, M. J., S. J. Jung, T. J. Choi, H. R. Kim, K. Rajendran, Y. J. Kim, M. A. Park, and S. K. Chun. 2001. A viral disease occurring in cultured carp Cyprinus carpio in Korea. Fish Pathol. 36:147-151. [Google Scholar]
- 15.Perelberg, A., M. Smirnov, M. Hutoran, A. Diamant, Y. Bejerano, and M. Kotler. 2003. Epidemiological description of a new viral disease afflicting cultured Cyprinus carpio in Israel. Israeli J. Aquaculture 55:5-12. [Google Scholar]
- 16.Ronen, A., A. Perelberg, J. Abramowitz, M. Hutoran, S. Tinman, I. Bejerano, M. Steinitz, and M. Kotler. 2003. Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio. Vaccine 21:4677-4684. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook, J., and D. W. Russell. 2001. Molecular cloning, p 6.7-6.10. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Walster, C. 1999. Clinical observations of severe mortalities in Koi carp, Cyprinus carpio, with gill disease. Fish Vet. J. 3:54-58. [Google Scholar]