Abstract
Docetaxel chemotherapy for metastatic castration resistant prostate cancer patients has been thought palliative because the radiological response rate is low and durable response is rare. The patient was a 64-year-old man who was diagnosed with cT3aN0M0 prostate cancer and underwent external beam radiation therapy as the initial treatment. He underwent androgen deprivation therapy and 8 cycles of docetaxel chemotherapy. His PSA level decreased and became undetectable and the disease was confirmed to be stable by radiological examination. We report a rare case that a metastatic castration resistant prostate cancer patient with multiple bone metastases has durable radiological and biochemical response.
Keywords: Docetaxel, metastatic castration resistant prostate cancer, prostatic neoplasms, multiple bone metastasis
Introduction
Though new chemotherapeutic agents, such as cabazitaxel, abiraterone acetate and enzalutamide, have been placed on the market, docetaxel-based chemotherapy still remains as one of the standard treatments for patients with metastatic castration resistant prostate cancer (mCRPC). In the TAX327 study, docetaxel chemotherapy significantly prolonged survival among men with CRPC [1]. However, docetaxel chemotherapy for mCRPC patients is still thought to be palliative; the radiological response rate is low and durable response is rare. It was reported in the study that the median survival of patients treated with docetaxel every 3 weeks was 18.9 months and that 50% decrease in the serum PSA level was attained in 35% of the cases. And it was also reported that radiological response rates is about 12% and duration of the therapy is about 8 months [1]. Some prostate cancer patients treated with androgen deprivation therapy (ADT) get complete remission, but only a few patients with mCRPC achieve remission [2]. We describe a rare case of a mCRPC patient treated with 8 cycles of docetaxel chemotherapy has durable radiological and complete biochemical response.
Case presentation
This patient was a 64-year-old man who presented with a high serum level of PSA of 8.13 ng/ml. A biopsy was performed and pathologic analysis revealed adenocarcinoma of the prostate with a Gleason Score 3+2. Magnetic resonance imaging (MRI) revealed that the tumor had grown outside the prostate. Computed tomography (CT) and bone scan showed no evidence of metastasis. Imaging examination revealed stage cT3aN0M0 prostate cancer. As a result, the patient underwent external beam radiation therapy (70Gy) as the initial treatment. His PSA decreased to a nadir value of 0.03 ng/ml. Two years after radiation therapy, his PSA level sharply increased to 16.36 ng/ml. He was prescribed ADT. Five years later, CT and bone scan were performed because his PSA rose to 3.30 ng/ml; these revealed multiple bone metastases in the ribs and left pubic bone. He was then prescribed zoledronic acid. Six months after that, his PSA rose to 69.01 ng/ml, and bone scan showed more multiple bone metastases at the shoulder blade, left humerus and vertebrae as well as ribs and left pubic bone. Then, the patient was started on docetaxel, 75 mg/m2, with prednisone according to the standard protocol plus zoledronic acid. PSA temporarily decreased to 37.10 ng/ml after the first cycle of chemotherapy. However, after the second cycle of docetaxel, PSA markedly increased to 182.21 ng/ml. After the third cycle of docetaxel, PSA suddenly decreased to 3.5 ng/ml within a month. He received 8 cycles of docetaxel chemotherapy in total. Bone scan showed that bone metastases at ribs and left pubic bone were still present after chemotherapy, but MRI revealed that viability of the lesions had become weaker (Figure 1). His PSA level gradually went down becoming undetectable and the disease was radiologically confirmed to be stable for 18 months after the last cycle of chemotherapy.
Figure 1.
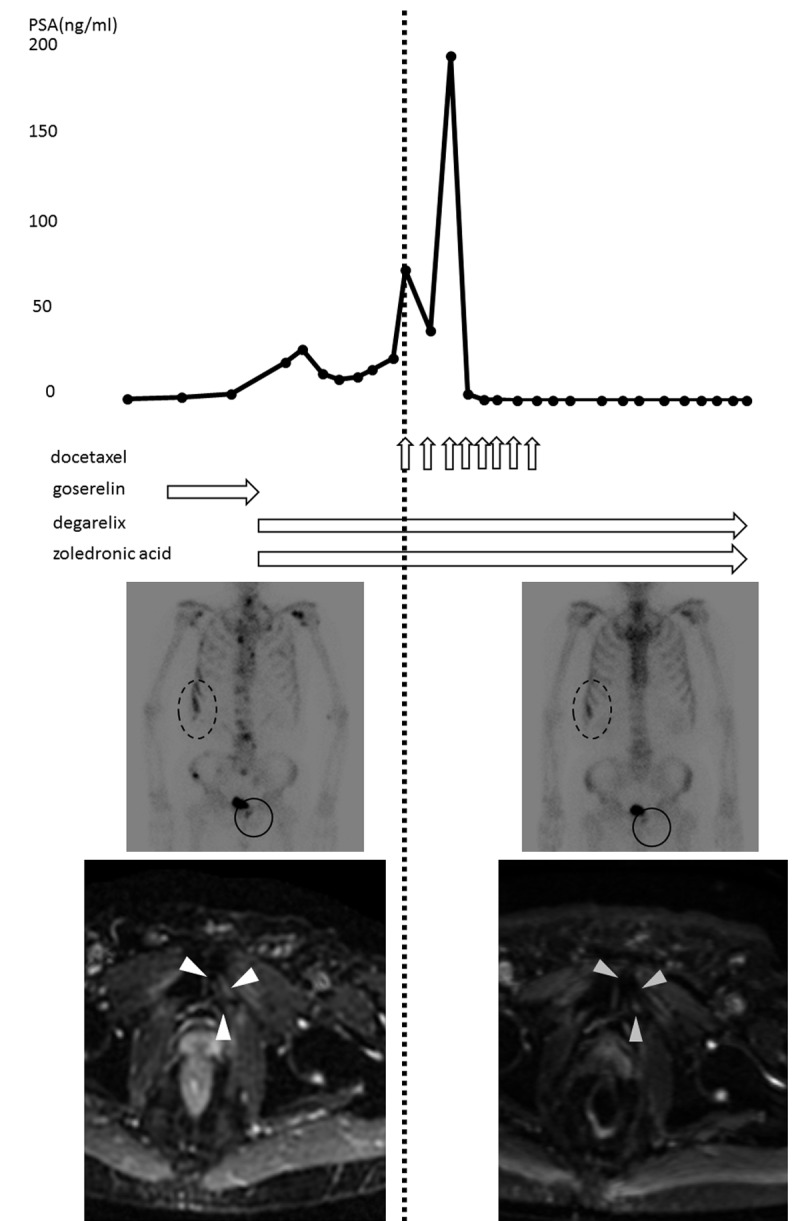
The graph illustrates time-course changes of serum PSA levels and therapeutic agents. The images on the left side of the dotted line are bone scan and MRI (left pubic bone) before docetaxel-based chemotherapy. And the images on the right side are those obtained after chemotherapy. Bone metastases are indicated by circles and triangles surrounding them.
Discussion
We describe a rare case that a mCRPC patient treated with 8 cycles of docetaxel chemotherapy has durable radiological and biochemical response, and we believe that it is not reported that mCRPC patients with multiple bone metastases achieved long response after docetaxel chemotherapy not only biochemically but radiologically.
In our patient, his PSA level markedly elevated after the 2nd cycle of docetaxel chemotherapy; it rose 37.10 ng/ml to 182.21 ng/ml (about 490%) from the previous cycle. And PSA suddenly decreased to 3.5 ng/ml after the next cycle. The initial elevation of a tumor marker serum level above its baseline value followed by steady decline is known as therapy-related flare-up. Flare-up of tumor markers after chemotherapy has been reported in some kinds of cancers: alpha-fetoprotein and human chorionic gonadotropin in patients with a testicular tumor, carcinoembryonic antigen in those with a gastrointestinal tumor [3]. PSA flare-up is well-known to occur in patients with prostate cancer when they are started on ADT because the serum level of testosterone is also up. On the other hand, the mechanism of PSA flare-up after chemotherapy is thought to be due to tumor lysis. The incidence of PSA flare-up after chemotherapy has been reported to be between 7.6 and 13.6%. The median percentage of PSA flare-up is about 15.4-61.5% from baseline (2.3-404%). In most patients PSA flare-up occurred after the first cycle, but in 2 of 8 cases it occurred after the second or third cycle [4]. Patients who experience PSA flare-up show no statistically significant differences in biochemical and clinical responses compared with the patients who show an immediate PSA response [4]. A minimum of 3 cycles before removing patients from a docetaxel-based regimen is recommended by the Prostate Cancer Clinical Trials Working Group [5], if there is no clinical signs indicating progression.
Docetaxel chemotherapy for metastatic castration-resistant prostate cancer patients has been also thought palliative; the radiological response rate is low and durable response is rare. Some prostate cancer patients treated with ADT get complete remission, but only a few patients with mCRPC achieve remission. Until now, Daverede L et al. reported that a case of a mCRPC patient with retroperitoneal lymphnode metastases who had durable response to docetaxel chemotherapy [2]. To the best of my knowledge, it has not previously reported in the literature that durable response to docetaxel chemotherapy of mCRPC with multiple bone metastases.
Conclusion
We reported a case of a mCRPC patient treated with 8 cycles of docetaxel chemotherapy has durable radiological and complete biochemical response. It is rare mCRPC patients with multiple bone metastases achieved long response after docetaxel chemotherapy not only biochemically but radiologically.
The patient experienced PSA flare-up after the second cycle of chemotherapy in this case. It is recommended a minimum of 3 cycles before removing patients from a docetaxel-based regimen, when the patient presents no signs of progression.
Disclosure of conflict of interest
None.
Abbreviations
- ADT
androgen deprivation therapy
- CT
Computed tomography
- mCRPC
metastatic castration resistant prostate cancer
- MRI
Magnetic resonance imaging
- PSA
prostate specific antigen
References
- 1.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA TAX 327 Investigators. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 2.Daverede L, Ralph C, Jagdev SP, Trigonis I, Trainor S, Harnden P, Weston M, Paul A, Vasudev NS. Metastatic castrate-resistant prostate cancer with a late, complete and durable response to docetaxel chemotherapy: a case report. J Med Case Rep. 2014;8:122. doi: 10.1186/1752-1947-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundle SD, Marathe AS, Chelladurai M. Transient therapy-related surge in serum tumor biomarkers: Characterizing behavior and postulating its biologic role. Crit Rev Oncol Hematol. 2013;86:15–22. doi: 10.1016/j.critrevonc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Nelius T, Filleur S. PSA surge/flare-up in patients with castration-refractory prostate cancer during the initial phase of chemotherapy. Prostate. 2009;69:1802–7. doi: 10.1002/pros.21024. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ, Dreicer R, Petrylak D, Kantoff P, Basch E, Kelly WK, Figg WD, Small EJ, Beer TM, Wilding G, Martin A, Hussain M Prostate Cancer Clinical Trials Working Group. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 2008;26:1148–59. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]


