Abstract
The dosage of 18F-FDG must be sufficient to ensure adequate PET image quality. For younger patients and research controls, the lowest possible radiation dose should be used. The purpose of this study was to find a protocol for FDG-PET of the brain with reduced radiation dose and preserved quantitative characteristics. Eight patients with neurodegenerative disorders and nine controls (n=17) underwent FDG-PET/CT twice on separate occasions, first with normal-dose (3 MBq/kg), and second with low-dose (0.75 MBq/kg, 25% of the original). Five additional controls (total n=22) underwent FDG-PET twice, using normal-dose and ultra-low-dose (0.3 MBq/kg, 10% of original). All subjects underwent MRI. Ten-minute summation images were spatially normalized and intensity normalized. Regional standard uptake value ratios (SUV-r) were calculated using an automated atlas. SUV-r values from the normal- and low-dose images were compared pairwise. No clinically significant bias was found in any of the three groups. The mean absolute difference in regional SUV-r values was 0.015 (1.32%) in controls and 0.019 (1.67%) in patients. The ultra-low-dose protocol produced a slightly higher mean difference of 0.023 (2.10%). The main conclusion is that 0.75 MBq/kg (56 MBq for a 75-kg subject) is a sufficient FDG dose for evaluating regional SUV-ratios in brain PET scans in adults with or without neurodegenerative disease, resulting in a reduction of total PET/CT effective dose from 4.54 to 1.15 mSv. The ultra-low-dose (0.5 mSv) could be useful in research studies requiring serial PET in healthy controls or children.
Keywords: PET, FDG, neuroimaging, neurodegeneration, methodology
Introduction
Fluorodeoxyglucose labeled with 18F (FDG) is the most common tracer for positron emission tomography (PET). It has an established clinical role in neurology, oncology and internal medicine, and is a powerful research tool. The dosage of FDG must be sufficient to ensure adequate image quality for clinical diagnostics or acquisition of quantitative information. The number of counts, and the signal-to-noise ratio, are functions of administered dose and scan time [1]. An FDG-PET examination induces a moderate but not insignificant dose of ionizing radiation to the patient and to the nuclear medicine staff. There is a stochastically increased risk of malignancy that is related to the radiation dose [2]. For many patients, this is of minor significance due to high age and concurrent disease, but for younger patients and healthy research subjects, a reduction of administered activity is highly desirable [https://ec.europa.eu/energy/sites/ener/files/documents/099_en.pdf]. The prospect of repeated scans for evaluating progress rate or effect of an intervention would also be a strong incentive for decreasing the dose.
The reproducibility of FDG-PET brain scans has been studied extensively during the late twentieth century, mostly on scanners with inferior resolution and sensitivity compared to those currently used [3]. In one study from 1995, absolute values of glucose metabolic rates varied from 6.4 to 12.5% between scans in the frontal cortex and 6.8 to 14.7% in the basal ganglia [4]. A later report deals with measuring reproducibility from scans performed on separate occasions on a GE Advance scanner [3]. The findings include test-retest differences in the metabolic rate of 2.47-9.85% in subcortical regions of interest, which are considered to have a larger variability than large cortical regions. Test-retest differences in functional imaging such as FDG-PET arise partly from imperfections in the imaging technology, but also from physiological variations in the subject such as current homeostasis, cognitive state, and minimal motor activity [5]. Even under perfect conditions, test-retest differences in such examinations will never equal zero.
Current guidelines for conducting FDG brain examinations contain a recommended dose interval [6,7]. The European Association of Nuclear Medicine (EANM) recommends an administered dose of 125-250 MBq [6], and the Society of Nuclear Medicine (SNM) recommends a dose of 185-740 MBq [7]. The exact dose given depends on the available PET scanner and local clinical practice. Thus, there are considerable differences in dosage routines across institutions.
Recent research efforts regarding FDG dose reduction include a BMI-based dosage for whole body FDG-PET [8], as well as an MRI-driven statistical prediction of a normal dose FDG-PET brain [9]. The latter is based on a virtual low dose protocol created by a shortened scan after administering a normal FDG dose. To the best of our knowledge, no modern publication has shown true low-dose FDG-PET of the brain with preserved image quality.
A low-dose FDG-PET can be simulated by using list-mode acquisition after administering a normal dose, and reducing the amount of collected data. However, merely demonstrating the virtual feasibility of dose reduction through simulations is not likely to change clinical routine, and evidence of clinically acceptable diagnostic images with true low dose is needed for a widespread adoption of dose reduction. This is especially important in order to increase availability of FDG-PET in younger patients with higher vulnerability to radiation. Similarly, a cautious approach to radiation ethics limits the use of FDG-PET in research protocols, and a demonstration of statistical equivalence with true low-dose on the regional level is required for introduction of serial FDG-PET in healthy volunteers.
The aim of this study was to find a protocol for FDG-PET of the adult brain with a considerably decreased radiation dose and preserved quantitative results in healthy controls and patients with regional metabolic deficits. The hypothesis was that low and ultra-low doses of FDG are sufficient for assessing SUV-ratios of cortical regions.
Material and methods
Subjects
Twenty-four subjects were originally enrolled in the study, and 22 (8 patients and 14 controls) successfully completed the study. One control withdrew due to a panic attack, and one control had an inconclusive scan due to dosage error.
Eight patients (mean age 71.4) who had received a diagnosis of neurodegenerative dementia disease were prospectively enrolled from the memory clinic at the geriatric department. Four patients were diagnosed with frontotemporal dementia (behavioural variant n=3, semantic dementia n=1), two patients with Lewy body dementia, and one each with Alzheimer’s disease and corticobasal degeneration, respectively. The diagnoses imply that all patients had neurodegenerative diseases with assumed (or previously proven) regional hypometabolism, identifiable on FDG-PET.
Fourteen cognitively normal controls (mean age 71.9) were recruited through advertising. A structured interview was performed to exclude subjects with cognitive dysfunction or history of cerebral disease, memory tests were normal, and a physical examination showed no signs of neurological deficits.
All eight patients and nine of the controls (n=17) were assigned to a low-dose (LD) protocol and underwent PET/CT, as described below. The remaining five controls were assigned to an ultra-low dose-protocol (ULD) and were scanned on an ECAT PET scanner. Information about the included subjects is summarized in Table 1. The study was approved by the Regional Board of Medical Ethics, and all subjects provided written informed consent. For most patients, a present family member co-signed the consent.
Table 1.
Included subjects
| Subject | Diagnosis | Gender | Age | 1st dose | 2nd dose | Scanner |
|---|---|---|---|---|---|---|
| 1 | Control | M | 70 | 314 | 87 | PET/CT |
| 2 | Control | M | 69 | 280 | 72 | PET/CT |
| 3 | Control | M | 78 | 220 | 56 | PET/CT |
| 4 | Control | M | 84 | 298 | 84 | PET/CT |
| 5 | Control | M | 64 | 261 | 63 | PET/CT |
| 6 | Control | F | 70 | 215 | 58 | PET/CT |
| 7 | Control | F | 68 | 240 | 54 | PET/CT |
| 8 | Control | F | 77 | 190 | 45 | PET/CT |
| 10 | Control | M | 68 | 273 | 26 | PET |
| 11 | Control | F | 67 | 277 | 29 | PET |
| 12 | Control | F | 73 | 177 | 16 | PET |
| 13 | Control | M | 73 | 262 | 21 | PET |
| 14 | Control | M | 68 | 259 | 27 | PET |
| 15 | Control | F | 78 | 155 | 35 | PET/CT |
| 16 | SD | M | 82 | 264 | 62 | PET/CT |
| 17 | bvFTD | M | 76 | 244 | 49 | PET/CT |
| 18 | bvFTD | M | 56 | 248 | 66 | PET/CT |
| 19 | AD | M | 69 | 227 | 54 | PET/CT |
| 20 | bvFTD | F | 59 | 161 | 35 | PET/CT |
| 21 | LBD | M | 74 | 207 | 57 | PET/CT |
| 22 | CBD | F | 84 | 164 | 49 | PET/CT |
| 23 | LBD | M | 71 | 239 | 47 | PET/CT |
Diagnosis (SD=semantic dementia, bvFTD=behavioural variant frontotemporal dementia, AD=Alzheimer’s disease, LBD=Lewy body dementia, CBD=corticobasal degeneration). 1st dose represents the normal dose scan, in MBq. 2nd dose represents the low-dose/ultra-low-dose scan, also in MBq. The ULD controls were scanned on a separate scanner, as described in the methods section. Note the absence of control no. 9, (n=22).
MRI protocol
The subjects were scanned using a 3T MRI scanner (Achieva; Philips Medical Systems, the Netherlands) with a protocol that included a 3D T1-weighted gradient echo sequence (3D turbo field echo), and a T2-weighted fluid attenuated inversion recovery (FLAIR) sequence. The images were used to rule out unknown pathology and for spatial registration of the PET images, as described below.
FDG-PET protocol
All subjects underwent two FDG-PET scans on separate occasions. All scans were acquired 35-45 min after tracer injection. A routine scan with an injected dose of 3 MBq/kg FDG was performed first. The average administered activity was 235 MBq (effective dose from FDG: 4.47 mSv). The second scan was either a low-dose (LD) scan using 25% of the normal dose (mean administered activity 57 MBq, effective dose from FDG: 1.08 mSv), or an ultra-low-dose (ULD) scan using 10% of the normal dose (mean 24 MBq, effective dose from FDG: 0.46 mSv). In effect, the LD dosage was 0.75 MBq/kg and the ULD dosage was 0.3 MBq/kg.
All eight patients and nine controls (n=17) were assigned to the LD protocol and scanned on a Discovery ST (GE Healthcare, Waukesha, WI, USA) PET/CT scanner in 3D acquisition mode. This scanner consists of 24 rings of 420 bismuth germanate (BGO) detectors with detector dimensions 6.3×6.3×30 mm, resulting in 47 image planes with a plane separation of 3.27 mm and a total axial field of view of 15.4 cm. Images were reconstructed using ordered-subsets expectation maximization (OSEM; 2 iterations, 21 subsets) with a 2.14 mm FWHM post-filter, applying appropriate corrections including attenuation correction based on a low-dose CT scan. These studies included a low-dose CT with an average effective dose of 0.07 mSv (Dose length product 12-15 mGycm), so the total effective doses were 4.54 mSv for ND and 1.15 mSv for LD.
The remaining five controls, assigned to the ULD protocol, were instead scanned on an ECAT Exact HR+ PET scanner (Siemens, Knoxville, TN, USA). For those subjects, images were reconstructed using normalization and attenuation-weighted OSEM (6 iterations, 8 subsets) applying a 4-mm Hann filter, with attenuation correction based on a 10-min transmission scan with rotating 68Ge rod sources.
The scanning and examination routine were the same for both scans for each subject. Voxel size of the resulting images was 2×2×3.7 and 2×2×2.4 mm for PET/CT and PET images, respectively, and spatial resolution was approximately 6 mm for both scanners. The average time interval between the two PET examinations was 26 days.
Image post processing
To allow for automatic quantification, the images were spatially normalized to a common reference space (MNI standard space [10] combined with an MR T1 template from the International Consortium for Brain Mapping [ICBM-152, http://packages.bic.mni.mcgill.ca/tgz/mni-models_icbm152-lin-1.0.tar.gz.]. To minimize the potential bias of the FDG dose, all PET images were initially co-registered to each subject’s T1-weighted MR images with rigid registration, using the superior anatomical detail of the MR images for spatial normalization. The MR images were first normalized using a global affine registration, followed by a non-rigid registration as implemented by the software (FSL) [11], allowing for local deformations. The calculated transform for MR images was then applied to the co-registered PET images. After PET images were transformed into reference space, they were intensity-normalized using a global reference value derived from the thresholded mean values of all voxels within a brain mask.
Regional standardized uptake value ratios (SUV-r) were calculated by dividing each region of interest with the global value of the thresholded brain mask, using an atlas derived from the automated anatomic labeling (AAL) atlas [11]. Volumes of interest were the anterior cingulate, frontal, lateral temporal, parietal, and a combined (precuneus + posterior cingulate) cortex from each side.
Statistical analysis
SUV-r values extracted from the normal- and low-dose scans were compared pairwise. The differences were tested for normal distribution using Shapiro-Wilks test and visualized with normal probability plots for detection of outliers. Bland-Altman plots and boxplots were calculated for visual evaluation of variance, bias, and trends. Constant and proportional biases in Figure 2A-C were tested with single sample t-tests and simple regressions, respectively. Dell Statistica, version 13 (Dell Inc. 2015, software.dell.com) was used for calculations and graph production.
Figure 2.
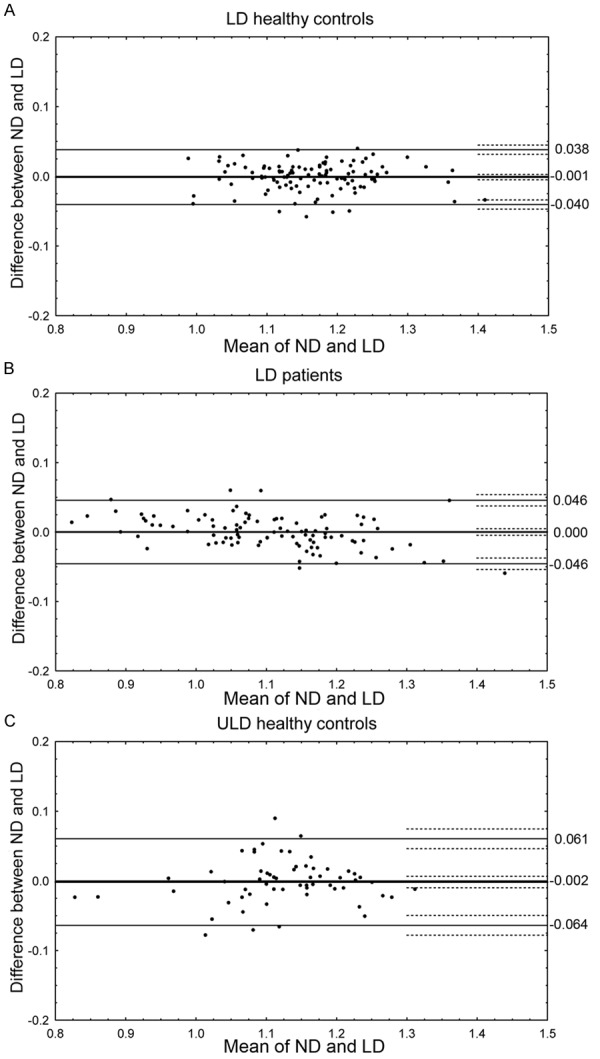
(A-C) SUV-r differences by group. (A-C) show Bland-Altman plots of regional SUV-r values from low-dose controls (A), low-dose dementia patients (B), and ultra-low-dose controls (C). Thick lines represent the bias and the adjacent dashed lines the confidence limits of the bias. Thin lines represent the upper and lower 1.96 standard deviation range and the adjacent dashed lines the confidence limits of the range. (Note: the two low SUV-r values in C originate from the occipital cortex).
Results
The normal- and low-dose images were compared pairwise for assessment of similarity. Sample images of the 10-minute summation images (before post-processing) are shown in Figure 1.
Figure 1.
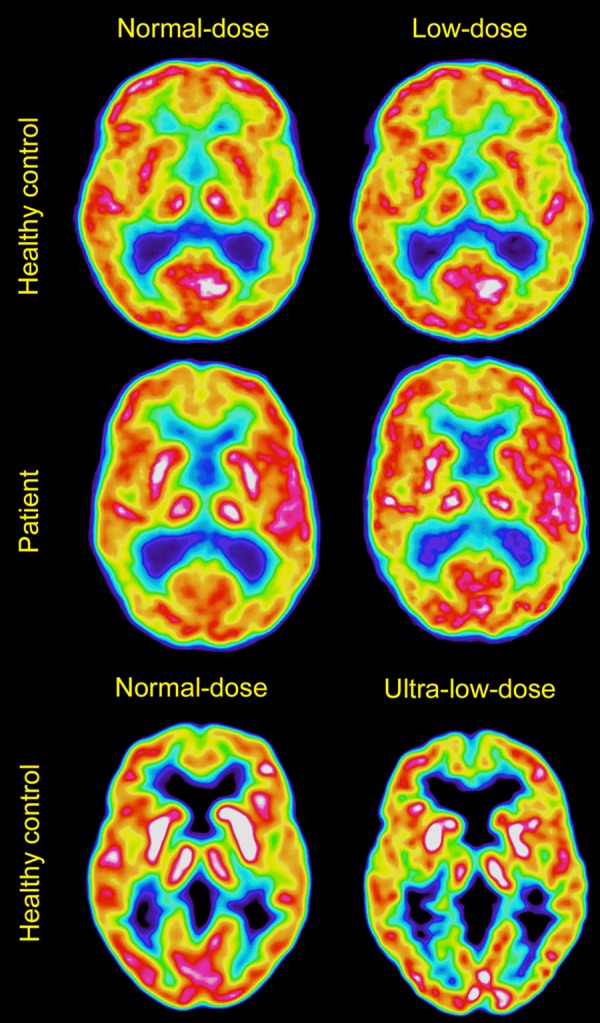
Sample images. Shows a healthy control (subject 4, top row), and a patient with Alzheimer’s disease (subject 19, middle row), with normal-dose images to the left and low-dose images to the right. The bottom row shows normal- and ultra-low-dose images from a healthy control (subject 10). Images are produced with VOIager, using Sokoloff color scheme.
Regional SUV-r values are plotted in Bland-Altman graphs, sorted by group in Figure 2A-C. For LD controls (Figure 2A), the bias was -0.001 (confidence interval (CI) -0.005 to 0.003). For LD patients (Figure 2B), the bias was 0.000 (CI -0.005 to 0.005). For ULD controls (Figure 2C), the bias was -0.002 (CI -0.010 to 0.007). In all groups, the confidence interval of the bias included zero, and t-tests confirmed that no significant constant bias was present. Regression showed no significant proportional bias in the low dose control group or in the ultra low dose group (Figure 2A, 2C). In the patient group (Figure 2B), a slight but significant proportional bias was shown (r2=0.18, p < 0.001).
The difference between the SUV-r values from the two PET/CT examinations in the LD groups are shown in a boxplot sorted by cortical regions in Figure 3. The ranges of the differences are within 0.06, and the median values close to zero for all regions. Shapiro-Wilks test confirmed a normal distribution of the differences in all three groups, and normal probability plots revealed no deviant outliers.
Figure 3.
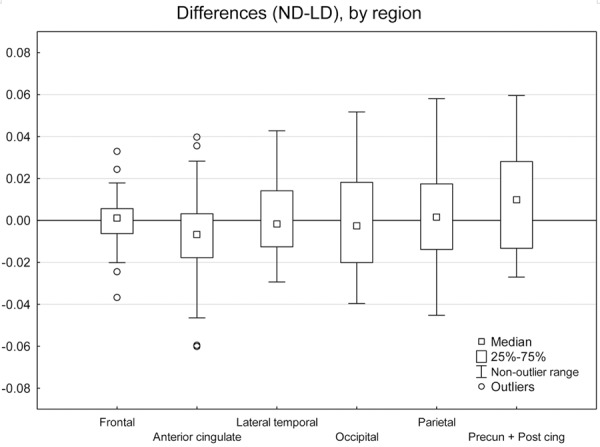
SUV-r differences by region. Shows boxplots of differences in SUV-r (shown on the y-axis), comparing normal dose and low dose (patients and controls combined), sorted by cortical region. Precun + Post cing = combined region of precuneus and posterior cingulate gyrus.
Absolute values of the difference in each region (ND-LD) were calculated and expressed as a percentage of the normal dose. Regional averages sorted by group are shown in Table 2. The low-dose protocol produced a mean absolute difference in regional SUV-r values of 0.015 (1.32%) in controls and 0.019 (1.67%) in patients with neurodegenerative disorders. The ultra-low-dose protocol produced a slightly higher mean difference of 0.023 (2.10%), with the largest differences noted in the anterior cingulate cortices.
Table 2.
Mean absolute differences per region
| Region | LD controls | LD patients | ULD controls |
|---|---|---|---|
| Left anterior cingulate | 1.02% (0.05-2.65) | 2.14% (0.44-5.65) | 4.71% (2.96-7.32) |
| Right anterior cingulate | 1.39% (0.48-3.64) | 2.08% (0.02-5.66) | 4.20% (0.21-8.39) |
| Left frontal | 0.79% (0.18-1.51) | 1.10% (0.02-3.33) | 1.05% (0.32-1.57) |
| Right frontal | 0.77% (0.23-2-72) | 0.83% (0.18-2.11) | 1.54% (0.53-3.16) |
| Left lateral temporal | 1.31% (0.74-2.60) | 1.58% (0.70-2.24) | 2.05% (0.42-3.82) |
| Right lateral temporal | 0.55% (0.01-2.04) | 1.64% (0.13-3.93) | 1.42% (0.44-2.93) |
| Left occipital | 1.85% (0.21-4.37) | 1.76% (0.54-3.26) | 0.94% (0.33-1.55) |
| Right occipital | 1.95% (0.37-4.42) | 1.35% (0.05-2.87) | 1.58% (0.84-2.28) |
| Left parietal | 1.63% (0.17-3.48) | 1.68% (0.38-4.38) | 3.61% (0.47-6.21) |
| Right parietal | 1.70% (0.12-5.04) | 1.76% (0.23-2.87) | 1.01% (0.10-2.08) |
| Left precuneus + post cing | 1.25% (0.52-2.90) | 2.04% (0.44-3.76) | 1.68% (0.07-5.43) |
| Right precuneus + post cing | 1.62% (0.50-3.89) | 2.12% (0.49-5.12) | 1.42% (0.17-3.50) |
| Mean | 1.32% (0.01-5.04) | 1.67% (0.02-5.66) | 2.10% (0.07-8.39) |
The columns represent the low-dose healthy controls, the low-dose patients, and the ultra-low-dose controls, respectively. Each row represents the average absolute difference in SUV-r value from the normal dose in a cortical region, expressed as a percentage, with the range in parentheses. All mean values higher than 3% are marked in bold for easy reference. Post cing=posterior cingulate gyrus.
Discussion
The main finding of this study is that 0.75 MBq/kg (56 MBq for a 75-kg subject) is a sufficient FDG dose for assessing SUV ratios in cortical regions, in PET scans of adults with or without neurodegenerative disease, without increasing scan time. The discrepancies in SUV-r values between normal and low dose were minor, both for patients and healthy controls, and no constant bias was found. There was a slight proportional bias in the patient group, resulting in <3% underestimation in regions with lowest activity and <3% overestimation in regions with highest activity. As no proportional bias was identified in the other groups, this finding is not necessarily an effect of dose reduction. The detected bias was minimal and could not be considered relevant from a diagnostic point of view, both because the other groups did not show any such bias and because much larger changes than 3% are generally required to define regional uptake as pathological.
In the search for methods to track disease progression rate in clinical trials, many studies have explored the rate of atrophy and the rate of ventricular dilatation, as summarized by Frisoni et al. [12]. Yearly reduction of hippocampal volume has been found to larger in patients than age-matched controls [12]. In future studies, repeated low-dose FDG-PET examinations might provide a similar quantitative “rate-of-progression” measurement for cortical metabolism, which would be useful in identifying pathology at early stages of disease and monitoring disease progression rate.
Normal-dose FDG-PET has regional test-retest differences in the order of several percent in previous publications [3,4]. In this perspective, the current study shows promising similarities between the normal- and low-dose studies. This suggests that a low-dose scan performed for follow-up in a clinical situation could be compared to a previous, normal-dose scan. Considering the ND-LD comparison as a test-retest situation, the current results represent a substantial improvement of accuracy when compared to historical data using higher injected doses. Most likely, this is an effect of improvements in scanner technology and image reconstruction methodology. The current data were reconstructed using OSEM, while previously published data were based on reconstructions using filtered back projection. Another important difference is that the current data is extracted using software-defined volumes of interest from an MRI-driven spatial normalization, instead of manually drawn regions. The current study included an MRI-driven spatial normalization to achieve highest possible level of matching between normal- and low-dose derived data. However, the authors’ experience is that PET-driven registrations are adequate for clinical purposes and would like to emphasize that MRI is not necessary for conducting low-dose FDG scans.
This study was conducted using early generations of BGO-based scanners. The HR+ and the Discovery PET/CT were installed 12 and 8 years prior to this study. Considering the technological advantages of more modern equipment, the results from this study should be applicable to any PET facility.
The low-dose CT included for attenuation correction in this study had an effective dose of 0.07 mSv, thus far less than the effective dose from the FDG. This highlights that the potential dose reduction in the current set-up can be achieved mainly by decreasing the amount of administered activity. Using a PET/MRI instead of PET/CT would decrease the dose slightly further, but not as much as the suggested FDG reduction.
The ULD protocol of 0.3 MBq/kg (22.5 MBq for a 75-kg subject), revealed slightly larger differences between examinations, but still without significant bias. This protocol should be considered for research applications, especially in young individuals and paradigms requiring serial scanning over time. The upper limit of radiation dose in adults aged 18-50 for research purposes has been set to 10 mSv in several European countries. Taking into account the dose from the low-dose CT (0.07 mSv), the number of potentially permissible PET/CT scans effectively increases from one or two per subject to eight for LD and 18 for ULD. As established in the European Commission report, the assumed risk regarding ionizing radiation in children below the age of 18 is generally considered 3 times higher than for adults aged 18-50 [https://ec.europa.eu/energy/sites/ener/files/documents/099_en.pdf]. Consequently, FDG-PET with a normal dose is currently not allowed for pediatric research. This study implies that up to six ULD scans are ethically acceptable, and this could facilitate studies in currently largely unknown areas such as the metabolism of the developing brain.
The main limitation of this study was the relatively small cohort. A larger cohort and absolute quantification of the results (such as metabolic rate of glucose consumption) might give a more exact correlation coefficient. The usefulness of such a coefficient might be limited to situations in which quantitative values are necessary. Establishing the accuracy of low-dose protocols for the purpose of absolute quantification requires further studies. The current study compares relatively large regions of interest, and the ability to detect small lesions with low FDG-dose has not been evaluated. This question would require a separate study with a specific study design.
Another important limitation was the lack of normal dose reproducibility data from the scanners used in the study. A full cohort of test-retest examinations using a normal dose twice in the same setting as that in which the current subjects were examined would provide an interesting reliability measure that would put the low-dose comparisons in a meaningful perspective.
In conclusion, the dose of FDG required for evaluating cortical SUV-ratios in PET scans can be reduced, without loss of diagnostic accuracy, by a factor of at least 4 in clinical practice and by a factor of 10 when multiple scans are considered in the same individual for research purposes. This will reduce radiation burden to patients and staff.
Acknowledgements
Many thanks to the staff of the radiology department, and the staff of the PET center, especially Mimmi Lidholm and Lars Lindsjö.
References
- 1.de Groot EH, Post N, Boellaard R, Wagenaar NR, Willemsen AT, van Dalen JA. Optimized dose regimen for whole-body FDG-PET imaging. EJNMMI Res. 2013;3:63. doi: 10.1186/2191-219X-3-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang B, Law MW, Khong PL. Whole-body PET/CT scanning: estimation of radiation dose and cancer risk. Radiology. 2009;251:166–174. doi: 10.1148/radiol.2511081300. [DOI] [PubMed] [Google Scholar]
- 3.Schaefer SM, Abercrombie HC, Lindgren KA, Larson CL, Ward RT, Oakes TR, Holden JE, Perlman SB, Turski PA, Davidson RJ. Six-month test-retest reliability of MRI-defined PET measures of regional cerebral glucose metabolic rate in selected subcortical structures. Hum Brain Mapp. 2000;10:1–9. doi: 10.1002/(SICI)1097-0193(200005)10:1<1::AID-HBM10>3.0.CO;2-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman S, Dethy S, Lotstra F, Biver F, Stanus E, Wikler D, Hildebrand J, Mendlewicz J, Luxen A. Basal ganglia and frontal lobe glucose metabolism. A reproducibility positron emission tomography study. J Neuroimaging. 1995;5:219–226. doi: 10.1111/jon199554219. [DOI] [PubMed] [Google Scholar]
- 5.Berti V, Mosconi L, Pupi A. Brain: normal variations and benign findings in fluorodeoxyglucose-PET/computed tomography imaging. PET Clin. 2014;9:129–140. doi: 10.1016/j.cpet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Nagren K, Darcourt J, Kapucu OL, Tatsch K, Bartenstein P, Van Laere K European Association of Nuclear Medicine Neuroimaging Committee. EANM procedure guidelines for PET brain imaging using [18F] FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–2110. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- 7.Waxman AD, Herholz K, Lewis DH, Herscovitch P, Minoshima S, Mountz JM Consensus GID. Society of Nuclear Medicine procedure guideline for FDG PET brain imaging. Soc Nucl Med (Version 1.0) . 2009 [Google Scholar]
- 8.Sanchez-Jurado R, Devis M, Sanz R, Aguilar JE, del Puig Cozar M, Ferrer-Rebolleda J. Whole-body PET/CT studies with lowered 18F-FDG doses: the influence of body mass index in dose reduction. J Nucl Med Technol. 2014;42:62–67. doi: 10.2967/jnmt.113.130393. [DOI] [PubMed] [Google Scholar]
- 9.Kang J, Gao Y, Shi F, Lalush DS, Lin W, Shen D. Prediction of standard-dose brain PET image by using MRI and low-dose brain [18F] FDG PET images. Med Phys. 2015;42:5301. doi: 10.1118/1.4928400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans AC, et al. 3D statistical neuroanatomical models from 305 MRI volumes. N Sci Sym Med Ima Con. 1993:1813–1817. vol.1813. [Google Scholar]
- 11.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 12.Frisoni GB, Fox NC, Jack CR Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]


