Abstract
Measles virus hemagglutinin (MVH) residues potentially responsible for attachment to the wild-type (wt) MV receptor SLAM (CD150) have been identified and localized on the MVH globular head by reference to a revised hypothetical structural model for MVH (www.pepscan.nl/downloads/measlesH.pdb). We show that the mutation of five charged MVH residues which are conserved among morbillivirus H proteins has major effects on both SLAM downregulation and SLAM-dependent fusion. In the three-dimensional surface representation of the structural model, three of these residues (D505, D507, and R533) align the rim on one side of the cavity on the top surface of the MVH globular head and form the basis of a single continuous site that overlaps with the 546-548-549 CD46 binding site. We show that the overlapping sites fall within the footprint of an anti-MVH monoclonal antibody that neutralizes both wt and laboratory-vaccine MV strains and whose epitope contains R533. Our study does not exclude the possibility that Y481 binds CD46 directly but suggests that the N481Y mutation of wt MVH could influence, at a distance, the conformation of the overlapping sites so that affinity to CD46 increases. The relevance of these results to present concepts of MV receptor usage is discussed, and an explanation is proposed as to why morbillivirus attachment proteins are H, whereas those from the other paramyxoviruses are HN (hemagglutinin-neuraminidase).
Measles virus (MV), a member of the genus Morbillivirus in the family Paramyxoviridae of the order Mononegavirales, possesses two glycoproteins in its envelope: the hemagglutinin (H) protein, responsible for attachment to the cellular receptors, and the fusion (F) protein, which mediates the fusion of the viral and host membranes (12, 51). Expression of the MV glycoproteins at the surface of the infected host cell also leads to cell-to-cell fusion, resulting in the formation of multinuclear giant cells (syncytium formation), which is the hallmark of paramyxoviral infections. MV fusion has been shown to depend upon the coexpression of the two glycoproteins (53), and it is believed that the fusion helper function of the MVH protein depends upon a specific and physical interaction with the MVF protein mediated via the latter's cysteine-rich region (52).
The host range of MV is restricted to humans and certain large primates. MV has been shown to use two cellular proteins as receptors: CD46 and SLAM/CD150 (8, 9, 18, 28, 47). CD46 is a member of the RCA family of proteins, which control the complement cascade, whereas SLAM is a CD2 member of the immunoglobulin superfamily and plays a regulatory role in lymphocyte activation. Although CD46 is ubiquitously expressed on all human nucleated cells, the expression of SLAM appears to be restricted to certain cells of hematopoietic origin, including activated B and T lymphocytes and activated dendritic cells and monocytes (47). It is now generally accepted that whereas laboratory and vaccine strains use both SLAM and CD46 as their cellular receptors, wild-type (wt) MV strains appear to use only SLAM. This presents an enigma, as many human tissues in which MV propagates, including respiratory epithelium, where the infection commences, are SLAM negative. Although the existence of a third receptor for MV (14, 26, 30, 45, 50) cannot be ruled out owing to the failure of anti-CD46 antibodies to systematically block the entry of wt MV in SLAM-negative cells, an alternative explanation has recently been proposed: despite data suggesting that CD46 is not used as a cellular receptor by wt MV strains (4), evidence that these strains have the capacity to bind CD46 at low affinity is accumulating (25, 27). Whether the induction of a more potent immune response (and immunosuppression) by wt relative to vaccine MV strains is related in part to differential receptor use is at present unknown.
It has been known since 1996 that the MVH residue Y481 is important for attachment to CD46 (1, 17, 21, 39), but recently, evidence that other residues can also play a role has accumulated: an MVH mutant in which five alanines replaced the 473-to-477 region was not functional in a hemadsorption assay (31), the S546G substitution has been shown to increase hemadsorption and CD46 binding (5, 23), and residues S548 and F549, which are present in both wt and laboratory-vaccine MV strains, appear to represent a low-affinity CD46 binding site (27).
Since the identification in 2000 of SLAM as the wt MV receptor, the search for residues on MVH responsible for binding SLAM has become a priority. We have used two approaches to identify such amino acids. First, profiting from a study (48) showing that SLAM also acts as a cellular receptor for other members of the genus Morbillivirus, we initially targeted charged amino acids—which can be expected to be exposed at the surface and hence available for interaction with ligands—that are conserved on the globular heads of morbillivirus H proteins. We then extended our mutagenesis studies to include amino acids in the primary structure vicinal to those charged residues identified as potentially playing crucial roles in SLAM attachment. Our second approach involved mapping the epitope of monoclonal antibody (MAb) 55, a neutralizing anti-MVH MAb (11) that is effective against both laboratory-vaccine and wt strains of MV. Presumably MAb 55 exerts its effect by interfering with receptor attachment. Unless this antibody causes a conformational change upon binding, it can be expected that its epitope will be close to, or even coincide with, part of the receptor binding site. The neutralization of wt MV strains suggests that this antibody targets SLAM attachment. However, MAb 55 is equally effective against laboratory strains which use both SLAM and CD46. If the SLAM and CD46 binding sites are topologically distinct in the MVH tertiary structure, then laboratory-vaccine strains should still be able to attach to CD46 in the presence of MAb 55. The fact that they cannot raises the possibility that the CD46 and SLAM binding sites may be very close together on the surface of MVH. The existence of overlapping sites but distinct interacting surfaces for the two receptors on MVH was recently proposed by Santiago et al. (38). These authors found that a panel of anti-MVH MAbs inhibited the binding of both receptors equally and that soluble versions of SLAM and CD46 competed each other out in binding MVH.
We have used the downregulation of SLAM and CD46 as an indirect marker of their interaction with MVH: expression of the MVH protein alone is sufficient to induce internalization of both SLAM and CD46 (29, 46). As MV fusion is dependent upon receptor attachment (53), mutant MVH proteins with modified receptor downregulation were further tested to investigate whether their fusion promotion capacities had also been affected. Our results, interpreted using the three-dimensional (3D) surface representation of the revised MVH structural model (www.pepscan.nl/downloads/measlesH.pdb), show that three charged residues (D505, D507, and R533), whose mutation affects both SLAM downregulation and SLAM-dependent fusion, align the rim on one side of the cavity present on the top surface of the MVH globular head. The notion that these residues form the basis of the MVH SLAM binding site is given credence by our finding that one of them (R533) is part of the epitope of MAb55. Other MVH residues that also appear to play roles in SLAM attachment extend the potential site in a continuous manner so that it overlaps on the surface of the MVH with residues 546, 548, and 549, recently identified as a second CD46 binding site on MVH (27). The possible implications of these findings for MV receptor usage are discussed below.
MATERIALS AND METHODS
Cells and viruses.
HeLa, B95a, Vero, and Vero.SLAM cells were propagated in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 0.1 mg of streptomycin/ml, 10 mM HEPES, and 10% fetal calf serum. HeLa (human epitheloid cervical carcinoma) and Vero (African green monkey kidney fibroblast) cells express a CD46 molecule usable by MV as a cellular receptor. Vero.SLAM cells express human SLAM in addition to simian CD46. B95a cells (an Epstein-Barr virus-transformed marmoset B-cell line) express simian SLAM, but the CD46 molecule expressed on these cells cannot be used as a cellular receptor by MV, as it lacks the first short consensus repeat (SCR), which has been shown to be essential for binding MVH (3, 16, 22, 24). The Hallé strain of MV, MV escape mutants, the Copenhagen strain of vaccinia virus (VV), and recombinant VV expressing the MVF protein (VV-MVF) were propagated and titrated in Vero cells.
Antibodies.
The murine MAbs used in this study were anti-MVH MAbs 55 (11) and BH59 (a gift from C. P. Muller), anti-CD46 MAbs 13/42 and 11/88 (both gifts from J. Schneider-Schaulies), and anti-SLAM MAb IPO3 (Interchim). The secondary antibody used was a fluorescein isothiocyanate-conjugated goat anti-mouse antibody (Dako). MAb 13/42 recognizes SCR1 of CD46, which contains the MVH binding domain, whereas MAb 11/88 recognizes SCR4 of CD46.
Production and analysis of MAb 55 escape mutants.
Vero cells in a six-well plate were infected with MV (Hallé strain) at a multiplicity of infection of 0.01. Anti-MVH MAb 55 (2 μl of ascites fluid) was added 1 h later. After 6 days, the cultures were freeze-thawed, and the viruses were reselected by infecting further Vero cells and cultivating them in the presence of MAb 55. The virus was then plaque purified on Vero cells in the presence of MAb 55. Total RNA was extracted from Vero cells infected with MV Hallé or an MV escape mutant by using RNA NOW (Ozyme, St. Quentin, France). MVH genes were amplified by one-step reverse transcription (RT)-PCR (Titan One-Step; Roche Diagnostics) using the primers 5′-CGGTAGTTAATTAAAACTTAGGGTGCAAGATCATCCAC-3′ and 5′-ATGCCTGACGTCTGGGTGACATCATG-3′. The RT-PCR products were entirely sequenced using the Big Dye Terminator kit (Applied Biosystems) on an Applied Biosystems 3000 sequencer.
Production and expression of MVH mutants.
Site-directed mutagenesis (QuickChange; Stratagene) was performed on the MV Hallé H gene (10) cloned in the pCopak plasmid, which contains a modified form of the VV H6 promoter (33). The mutated plasmids were then amplified and purified on a CsCl gradient. HeLa cells were first infected with VV or VV-MVF for 2 h in 1 ml of Dulbecco's modified Eagle's medium-0% fetal calf serum and then transfected by a mix of Lipofectamine (Invitrogen) and 3 to 5 μg of mutated pCopakHH.
CD46 and SLAM expression analysis.
For the analysis of the MVH mutants, confluent HeLa cells in six-well plates were infected with VV and transfected by pCopakHH as described above. Eighteen hours later, 106 B95a cells were added, and 4 h later, the cells were harvested. For the analysis of the MAb 55 escape mutants, Vero or Vero.SLAM cells were infected with MV Hallé or each of the escape mutants. The cells were stained for 10 min with the anti-MVH MAbs 55 and BH59, the anti-CD46 MAbs 13/42 and 11/88, or the anti-SLAM MAb IPO3; washed; and then stained for 10 min with fluorescein isothiocyanate-conjugated goat anti-mouse antibody. The stained cells were analyzed by flow cytometry using a FACScan machine (Becton Dickinson). HeLa and B95a cells were differentially analyzed depending on their morphologies. Cells positively stained with propidium iodide were excluded from the analysis.
Analysis of fusion helper function of MVH mutants.
Either confluent HeLa cells or a coculture of HeLa and B95a cells was infected with VV-MVF and transfected by pCopakHH as described above. The cells were observed with a light microscope 18 to 24 h later for the presence of syncytia.
Sequence alignments.
Multiple sequence alignments were performed using the Genetic Computer Group's PILEUP algorithm (7). Alignments were edited using MegAlign (DNASTAR Inc.). Secondary-structure predictions were performed using the profile-based neural network prediction program PHD and the threading program TOPITS (36, 37). Multiple sequence alignments were performed using several representative sequences of neuraminidase family members and morbillivirus H proteins. The following hemagglutinin-neuraminidase (HN) and H sequences were used for analysis and comparison: bovine parainfluenza 3 virus (accession number P06167), Sendai virus strain HVJ (P06863), human parainfluenza virus strain Toshiba (P25466), Simian virus 5 isolate LN (P28885), mumps virus strain Miyahara (P11235), Newcastle disease virus strain Beaudette C/45 (P32884), rinderpest virus strain L (p09460), MV strain Edmonston (P08362), phocine distemper virus isolate DK88-4A (P28882), canine distemper virus strain Onderstepoort (P24306), dolphin morbillivirus (Q66411), and peste de petits ruminants virus strain 75/1 (Q98603). Model building and visualization were done using DeepView (Swiss PDBViewer) version 3.7 and SwissModel (13, 32). For two loops that could not be created automatically, the program LOOP SEARCH in the SYBYL package (Tripos Associates, St. Louis, Mo.) was used. Model verification was done using WhatCheck (15).
RESULTS
Generation of a refined higher-resolution hypothetical model structure for the MVH globular head.
In a previous study, it was predicted that the morbillivirus H protein folds as a β-propeller (20). This fold adopts a symmetrical structure based on six twisted antiparallel β sheets (coded B1 to B6), with four strands each (coded S1 to S4). The sheets are radially arranged like the blades of a propeller around an axis through the center of the molecule. The loops are numbered according to the strands they connect, e.g., L23 is the loop that connects strands 2 and 3. A shallow cavity, located on the top surface of the globular head near the center, is equivalent to the structure that forms the active site of the paramyxovirus HN where sialic acid binds. The β-propeller model of morbillivirus H was based on a very low sequence homology (<6%) with the influenza neuraminidase protein. Typically, molecules with a β-propeller fold are characterized by extreme sequence diversity, and this makes homology modeling a great challenge. However, this task has been greatly facilitated by the recently published structure (6) of the HN protein of Newcastle disease virus (NDV), which is a more reliable template for homology modeling. As several very important active-site residues were missing from the previous model, and in the sequence database several other supposedly conserved residues that were thought to be involved in neuraminidase activity appeared not to be conserved, there was no further need for biasing of the alignment based on presumed conserved catalytic residues.
Thus, the revised alignment is based only on sequence homology, secondary-structure prediction, and protein-folding compatibility and not on the assumption that the morbillivirus H has catalytic residues in common with other neuraminidases. PHD predictions were determined for morbillivirus H sequences, and the H sequences were aligned with NDV HN using the H3P2 substitution matrix (a five-dimension substitution matrix with three dimensions for the homologous structure and two dimensions for the probe sequence), which includes secondary-structure parameters (34, 37). The locations of the strands according to the modified alignment now agree much better with the PHD secondary-structure predictions, and because the modified alignment is no longer discontinuous, it fits better with alignment programs that are based on fold recognition (36). In order to obtain a refined globular structure, homology modeling was performed with the new alignment (Fig. 1) and the more homologous template using the automated protein-modeling software on the SWISS-MODEL protein-modeling server (41). For the large 16-residue loop β1L23, the homologous loop of influenza neuraminidase was used. On the top of the β-propeller, in the two adjacent loops β4L01 and β4L23, a deletion and an insertion, respectively, had to be created. The automated software failed to build a model with this insertion and deletion. Thus, these two loops were introduced separately as described in Materials and Methods. The seven rectangles in the alignment indicate the seven regions which are similar to the alignment described by Vongpunsawad et al. (49). The regions indicated by the rectangles are spaced by seven dissimilar regions that deviate from the alignment of Vongpunsawad et al. (49). In general, the differences are caused by the emphasis we have put on the conservation of cysteine bridges, which are all conserved between the morbillivirus H and paramyxovirus HN proteins according to our alignment, and sequence conservation in β-strands. The majority of the differences between the two alignments are found in dissimilar regions 1 and 5. Dissimilar region 3 is also very different, but it does not have a great impact on the overall structure because it corresponds to the large β3L23 loop. The shift in dissimilar region 4 results in a completely different position for β-strands β4S1 and S2. In order to retain the conservation of these two strands, we had to make a large deletion in β4L01 and an insertion in β4L23. The large deletion in β4L01 of the template structure allowed us to accommodate the large insertion in β4L23. We have interpreted the results generated by the mutagenesis-expression studies of MVH in terms of this revised structural model of the morbillivirus H protein.
FIG. 1.
Alignment of sequences of several morbillivirus H proteins, tupaia paramyxovirus H protein, and several paramyxovirus HN proteins (see Materials and Methods). The crystal structure of NDV HN was used as a template for the 3D model of MVH. Secondary-structure elements of the crystal structure of NDV HN (6) are indicated by bars and correspond to numbered α-helices (α) and strands (S) in each β-sheet (β). Residues within 1 distance unit from CSFV are boxed. Gaps are indicated by dashes. The large rectangles indicate the parts of the alignment that are identical to the alignment of Vongpunsawad et al. (49).
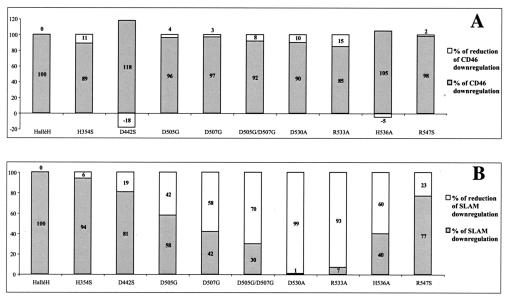
Mutation of residues D505, D507, and H536 strongly reduces SLAM downregulation, but mutation of D530 and R533 abolishes SLAM downregulation completely.
Tatsuo et al. reported that canine distemper virus and rinderpest virus also use SLAM as their cellular receptor and predicted that this is also the case for the other members of the genus Morbillivirus (48). We thus formulated the hypothesis that if SLAM is the cellular receptor for all the morbilliviruses, the residues responsible for attachment to the receptor should be conserved among MV, canine distemper virus, rinderpest virus, peste de petits ruminants virus, and phocine distemper virus. Acting on the premise that the interaction with SLAM potentially involves residues present on the protein surface, we targeted charged amino acids that are conserved on the globular heads of the H proteins from the five different morbilliviruses: R253, E256, E293, H354, R355, D442, D505, D507, D530, R533, H536, and R547. These 12 residues were mutated to either alanine or serine, and in two cases (D505 and D507) to glycine, with the change D505G being present in a natural MV mutant. The capacity of each MVH mutant to induce the downregulation of SLAM was then monitored as described in Materials and Methods. We used the Hallé strain of MVH for this study (10). As laboratory strains of MV, such as Hallé, use both SLAM and CD46 as cellular receptors, the effects of these mutations on CD46 downregulation could also be monitored as an internal control.
We found that the R253, E256, E293, and R355 mutants were not expressed at the cell surface: the residues between 253 and 256 (RVFE) are conserved among all the morbillivirus H proteins and presumably play an important role in the maintenance of the H protein tertiary structure; a similar supposition can be made for E293 and R355, which are part of the conserved HRG sequence on a bulge of the central strand of sheet 3 that points directly to the center of the molecule. The expression levels of the mutant H proteins were calculated by measuring the mean fluorescence intensity for each. This varied between 86 and 112% relative to the expression of the nonmutated H protein. The effect of each mutation on SLAM and CD46 downregulation as measured by flow cytometry was calculated in relation to the values obtained for the nonmutated H protein (Fig. 2). Figure 2 shows that mutating H354, D442, and R547 did not have a significant effect on the downregulation of SLAM (i.e., <25%). SLAM downregulation was strongly reduced by the mutations D505G, D507G, D505G/D507G (by 42, 58, and 70%, respectively), and H536A (60%) and was essentially abolished in the cases of D530A and R533A (99 and 93%, respectively). These mutations had minimal effects on CD46 downregulation.
FIG. 2.
Effects of mutating conserved charged H residues on the induction of CD46 (A) and SLAM (B) cell surface downregulation as measured by flow cytometry. The percentages of downregulation and its counterpart, reduction of downregulation, were calculated with respect to the values obtained for the nonmutated MV Hallé H protein (100 and 0%, respectively).
Mutating D505, D507, D530, R533, and H536 has no effect on the CD46-dependent fusion promotion capacity of the Hallé H protein.
We then investigated the effects of these mutations on the fusion promotion capacity of the MVH protein. The Hallé strain of MV can use both CD46 and SLAM as cellular receptors. If these residues are responsible for attachment to SLAM but not CD46, their mutation in the Hallé MVH protein should not compromise the protein's capacity to promote viral fusion in CD46-expressing cells. The lack of an effect on the downregulation of CD46 by the mutation of residues 505, 507, 530, 533, and 536 suggests that these residues do not play a role in the attachment to CD46, and as expected, syncytium formation was observed when each of the mutant proteins was coexpressed with the Hallé F protein in HeLa cells (CD46+) (not shown).
Mutating D530 or R533 abolishes the SLAM-dependent fusion capacity of the Hallé H protein.
A single mutation (Y481N) in the Hallé H protein has been shown to abolish interaction with CD46, and thus syncytium formation, in HeLa cells (1, 17, 21). A series of double mutants—Y481N/D505G, Y481N/D507G, etc.—were constructed and tested for the capacity to promote viral fusion when coexpressed with the Hallé F protein. We found that (i) these Y481N-containing mutants lost their CD46-dependent fusion promotion capacities in HeLa cells (not shown) and (ii) although the SLAM-dependent fusion promotion capacities of the mutants Y481N/D505G, Y481N/D507G, Y481N/D505G/D507G, and Y481N/H536A were maintained in the HeLa/B95a coculture system, those of the double mutants Y481N/D530A and Y481N/R533A were abolished (Fig. 3).
FIG. 3.
Effects of mutating residues 505, 507, 530, 533, and 536 on the SLAM-dependent fusion capacity of MV Hallé H protein already carrying the Y481N mutation (HH Y481N). Fusion was induced by the coexpression of the mutants (expressed from the plasmid pCopak) with MVF in the HeLa/B95a cell coculture. The cells were observed 18 to 24 h after transfection.
Alanine mutation of the 521-to-537 region reveals that it contains residues implicated in both SLAM and CD46 binding.
Residues D530 and R533 are situated on the loop which connects the second and third strands of β-sheet 5. Considering their apparent importance for SLAM binding, we decided to investigate the consequences of mutating (to alanine) each residue on β5S2 and the preceding loop: residues 521 to 537. Figure 4 shows that although the mutation of residues 532 to 537 had little effect on CD46 downregulation, the mutation of residues L522, Y524, L526, Y529, and T531, which are far in terms of primary sequence from the two CD46 binding sites identified in MVH, led to reductions in CD46 downregulation of 29, 48, 38, 40, and 34%, respectively. Mutating the residues that are neighbors of D530 and R533 in the primary sequence—Y529, T531, and S532—resulted in important reductions in SLAM downregulation (85, 40, and 58%, respectively), but mutating residues 522 to 527 also resulted in significant reductions in SLAM downregulation: 58, 81, 49, and 77% for L522, Y524, V525, and A527, respectively. It is noteworthy that the mutation of Y524, Y529, and T531 led to considerable reductions in the downregulation of both CD46 and SLAM. If, in general, SLAM downregulation appears to be more sensitive to mutation than CD46 downregulation, this could be explained in part by a study showing that MVH has a higher affinity for CD46 than for SLAM (38).
FIG. 4.
Effects of mutating residues in the region 521 to 537 on the induction of CD46 (A) and SLAM (B) cell surface downregulation as measured by flow cytometry. The percentages of downregulation and its counterpart, reduction of downregulation, were calculated as described in the legend to Fig. 1. Data relating to the conserved residues 530, 533, and 536 are presented in Fig. 1.
R533 is part of the epitope of a neutralizing anti-H MAb.
The Hallé MV strain, like all vaccine and wt strains that we have tested, is neutralized by the anti-MVH MAb 55 (11). As MVH is the attachment protein for MV, it can be assumed that MAb 55 achieves neutralization by blocking cellular receptor attachment. Giraudon and Wild found that MAb 55 not only passively protected against the Hallé strain in a mouse model but also inhibited hemagglutination (11). This suggests that the antibody targets CD46 attachment, as hemagglutination (of CD46-expressing simian erythrocytes) depends upon the interaction MVH and CD46. However, the neutralizing effect of MAb 55 on both wt and laboratory MV strains suggests that the antibody also blocks attachment to SLAM. This raises the possibility that the CD46 and SLAM binding sites are topologically close, if not overlapping, on the MVH. Mapping the MAb 55 epitope thus became a priority and was achieved through the production of MAb 55 escape mutants (see Materials and Methods). Hallé strain mutants whose replication was not impeded by MAb 55 were isolated, and the genes encoding their H proteins were cloned by RT-PCR and sequenced. Comparison of these sequences with the original Hallé H sequence showed that each escape mutant contained two mutations: T469A and R533G. Subsequent introduction of these mutations singly into MVH showed that the latter mutation alone was responsible for the loss of staining by MAb 55 observed with the MAb 55 escape mutants (Fig. 5A). Although the MAb 55 escape mutants and the H protein carrying the R533G mutation were not stained by MAb 55 (Fig. 5A), they were stained by another MVH-specific antibody, MAb BH59 (Fig. 5A). R533 does not appear to be essential for the MAb 55 epitope, as the mutations R533A and R533S have only a minor effect on MAb 55 staining (Fig. 5A). However, we found that the double mutant R533S/E535A results in a lack of staining for MAb 55, although the individual mutations do not have this effect. Subsequent experiments in which Vero and Vero.SLAM cells were infected with the escape mutants showed that, although the capacity of the virus to induce CD46-dependent syncytium formation or to downregulate CD46 was not affected (Fig. 5B), the internalization of SLAM in Vero.SLAM cells was only 12% of that obtained with the Hallé virus (Fig. 5C). When residue R533 was mutated in vitro to glycine, alanine, or serine, CD46-dependent syncytium formation and CD46 downregulation were unaffected but downregulation of SLAM was abolished (Fig. 5B and C). Moreover, the double mutant Y481N/R533G could not induce syncytium formation in the coculture system (Fig. 3). Hence, residue R533, whose mutation results in the abolishment of SLAM downregulation, is also part of the epitope of a neutralizing anti-MVH MAb.
FIG. 5.
MV Hallé strain MAb 55 escape mutants and MV Hallé H protein T469 and R533 mutants. (A) Comparative analysis of MAb 55 and MAb BH59 staining. Shown are effects on the induction of CD46 (B) or SLAM (C) cell surface downregulation as measured by flow cytometry. The percentages of CD46 or SLAM downregulation and its counterpart, reduction of downregulation, were calculated with respect to the values obtained for the MV Hallé strain or the nonmutated MV Hallé H protein.
MVH globular-head residues whose mutation affects SLAM downregulation align in 3D as a continuous site which overlaps with the 546-548-549 CD46 binding site.
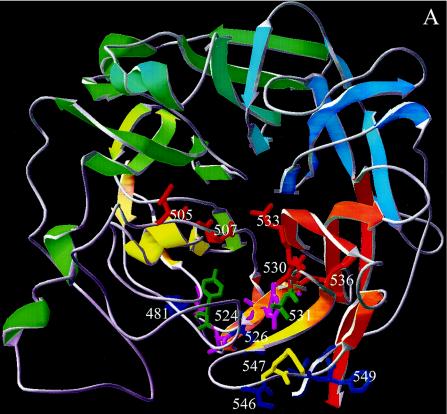
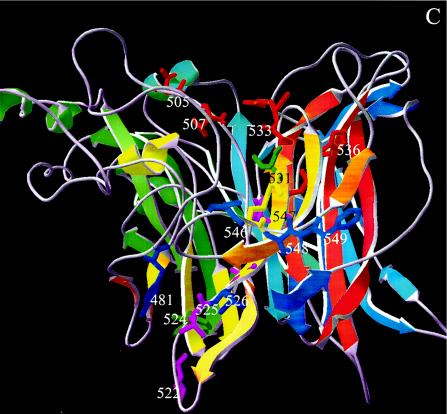
Examination of the wire frame representation of the revised structural model for MVH (www.pepscan.nl/downloads/measlesH.pdb) reveals that residues 505 and 507 are very close to residues 530, 533, and 536 on two loops (β5L01 and β5L23, respectively) (Fig. 6A and C). The revised structural model's 3D surface representation reveals, however, that residues 505, 507, and R533 are exposed prominently on the top surface of the β-propeller, so that in 3D a continuous site is formed which aligns the ridge of the cavity (Fig. 6B and D). The surface representation suggests that whereas residue R533 is available on the protein surface for ligand binding, D530 is buried. However, the predicted 3D structure of the short stretch preceding R533 is not very precise. NDV HN, which was used as a template, has a large unique loop that had to be deleted for the MVH model. This meant that the automated protein-modeling server was able to build a loop only after the alignment was modified in such a way that D530 was no longer on the exposed β5L23 loop but on β5S2, just preceding the loop; as a consequence, D530 is not exposed on the surface. However, if a similar template structure were available, or some manual modifications of the automatically constructed structure could be made, then D530 would probably be positioned in the exposed β5L23 loop. Alternatively, D530 does not participate directly in binding to SLAM, and mutation of this residue leads to a local conformational change that perturbs the position of R533, the residue that appears to have paramount importance in SLAM binding.
FIG. 6.
(A) 3D model of β-propeller domain of measles H viewed along the quasi-sixfold axis (top view). Secondary-structure elements in the ribbon diagram are colored blue to red from the N to the C terminus. D505, D507, D530, R533, and H536, charged residues that are conserved among the different morbilliviruses and whose mutation significantly reduces SLAM downregulation, are colored red. Residues Y524 and T531, whose mutations have a significant effect on both CD46 and SLAM downregulation, are colored light green. Residue R547, whose mutation resulted in reductions of 2 and 23%, respectively, for CD46 and SLAM downregulation, is colored yellow. Residues L522, V525, and A527, whichappear to have an indirect effect on SLAM binding, are colored purple. Residues Y481, L526, and 546, 548, and 549, which are potentially involved in CD46 binding, are colored dark blue. (B) Same as panel A, top view, with a surface representation. The orange line indicates the reach of a hypothetical antibody footprint of MAb 55 (radius, 13 Å) with R533 at the center of the epitope. (C) Side view that is rotated 270° around the x axis compared with panel A. (D) Same as panel C, side view, with a surface representation.
Strikingly, the revised structural model's 3D surface representation reveals (Fig. 6B and D) that the continuous site formed by D505, D507, and R533 is extended by residues T531 and R547, and this overlaps with residues 546, 548, and 549, recently identified as a second CD46 binding site in MVH (23). Although R547 resides in the center of the 546-548-549 CD46 binding site (in terms of both primary structure and tertiary structure), mutation of this residue has only a minimal effect on CD46 downregulation. Mutation of R547 does, however, cause a 23% reduction in SLAM downregulation (Fig. 2). Furthermore, mutation of T531, the residue that in the structural model's 3D surface representation makes liaison between R533 and R547, causes significant reduction in the downregulation of both CD46 and SLAM (34 and 40%, respectively). Support for the hypothesis that these residues do indeed represent overlapping SLAM and CD46 binding sites is provided by the MAb 55 footprint: assuming the standard diameter of a MAb footprint to be 26 Å, a footprint for MAb 55 centered on R533 encompasses the overlap made with residues 546, 548, and 549 (Fig. 6B and D).
Residues 524 to 526 are very close in 3D to Y481 in the MVH globular head.
Although in the revised structural model's 3D surface representation (Fig. 6B and D) most residues in the 521-to-537 region are predicted to be buried, those predicted to be on the surface (524 to 526) are very close in 3D to Y481, the residue shown to be essential for the MVH CD46 binding phenotype (1, 17, 21). In fact, according to the model, Y481 actually contacts Y524 (Fig. 7), a residue whose mutation has an effect on both CD46 and SLAM downregulation.
FIG. 7.
Distances (in angstroms) of residues L522, Y524, and L526 relative to Y481.
DISCUSSION
Although the initial aim of this study was to identify MVH residues potentially responsible for attachment to the MV cellular receptor SLAM, it has also afforded new insights regarding the molecular basis of MV receptor usage in general. This has been achieved by elucidating the results generated by the mutagenesis-expression studies of MVH in terms of a revised hypothetical structural model for the protein (www.pepscan.nl/downloads/measlesH.pdb).
Identification of MVH residues that potentially participate in interaction with SLAM.
When Giraudon and Wild (11) generated a panel of MAbs specific for MVH, they found that the binding of each MAb was competed to some degree by all the others. This suggests that all the MAbs target a particular region of MVH rather than disparate domains. The most neutralizing of these MAbs, MAb 55, is effective against all strains of MV. If MAb 55 functions by blocking receptor attachment, this points to SLAM being the target, as it is the cell receptor used by all strains of MV. However, laboratory and vaccine strains of MV, which use both SLAM and CD46, are also neutralized by MAb 55. A possible explanation for this would be proximity of the binding sites for SLAM and CD46 on the surface of the MVH protein. The existence of overlapping sites but distinct interacting surfaces for the two receptors on MVH was recently proposed by Santiago et al. (38). These authors found that a panel of anti-MVH MAbs inhibited the binding of both receptors equally and that soluble versions of SLAM and CD46 competed each other out in binding MVH. We thus considered the possibility that the neutralizing anti-MVH MAb 55 could target SLAM binding primarily but might also have an effect on CD46 binding if the binding sites of SLAM and CD46 overlapped. The epitope mapping of MAb 55 strongly suggests that R533 participates directly in the interaction with SLAM. The R533G mutation has no effect on CD46 binding, and indeed, the fact that selection of the escape mutant was made on CD46+ Vero cells confirms this. In order to discover which MVH residues associate with R533 to form a potential SLAM binding site, we made reference to the revised structural model for MVH (www.pepscan.nl/downloads/measlesH.pdb). We needed to differentiate those residues which are buried and whose mutation alters the conformation of the site from those present on the surface, which have the potential to interact directly with SLAM. The structural model's 3D surface representation predicts that of the five conserved charged amino acids whose mutations significantly affect SLAM downregulation, only three are present on the surface: D505, D507, and R533. Interestingly, these three residues align the rim of the cavity on the top surface of the MVH globular head, and the continuous site they form is extended by two residues, T531 and then R547, so that it contacts residues 546, 548, and 549, recently identified as a second CD46 binding site in MVH (27). Furthermore, mutation of R547, one of the 12 charged residues conserved among the morbilliviruses, which resides, in terms of both primary and tertiary structure, in the center of the 546-548-549 CD46 binding site, has only a minimal effect on CD46 downregulation. R547 mutation does, however, result in a moderate reduction in SLAM downregulation, and mutation of T531, which makes liaison between R547 and R533, leads to significant reductions in the downregulation of both receptors.
A recent study dedicated to the production of recombinant MV strains specific for either SLAM or CD46 (49) sought to identify MVH residues that play roles in SLAM-dependent fusion. For this study, reference was made to a new theoretical structural model for MVH, which like ours (www.pepscan.nl/downloads/measlesH.pdb) uses the NDV HN protein structure as a template (see Materials and Methods for a comparison of the two models). Evidently, it should be borne in mind that the two structural models are both theoretical, and confirmation of the roles of particular residues awaits a definitive structural determination for MVH. Although by being restricted to a SLAM-dependent fusion assay as a means to identify residues playing roles in SLAM attachment, residues important for the interaction with MVF (as yet unknown) will also be targeted, Vongpunsawad et al. (49) identified seven “SLAM-relevant ” amino acids: Y529, D530, T531, R533, F552, Y553, and P554. Vongpunsawad et al. showed that these residues form two clusters on the surface of the MVH globular head. As far as the first cluster is concerned, our results suggest that Y529 and T531 play roles in the attachment of both CD46 and SLAM but that Y529 and D530 are not solvent exposed. Although Vongpunsawad et al. did not identify residues D505 and D507 as potentially playing roles in binding SLAM, our results suggest that these residues initiate a single continuous site that is extended by residues R533, T531, and R547. There is evidence that the two residues are part of the epitope of the anti-MVH MAb 80-II-B2 (42). Furthermore, in a study which sought to determine a direct estimate of the spontaneous mutation rate of MV (40), five MAb 80-II-B2 escape mutants were isolated with single-residue mutations located at amino acids 505 (two mutants), 507 (two mutants), and 530 (one mutant). The natural mutation D505G has been proposed to be responsible for the lack of reactivity by the vaccine strain CAM-70 to this antibody (19); natural mutations in these residues can probably be tolerated in vaccine strains, as they can also use CD46 for entry. It might be expected that the residues making up the second cluster of SLAM-relevant residues described by Vongpunsawad et al. (F552, Y553, and P554) would be conserved among the different morbilliviruses, but this is not the case, except for P554, whose mutation could be problematic for the conformation of MVH.
Molecular basis of MV receptor usage.
The fact that both the SLAM site and the 546-548-549 CD46 binding site are within a theoretical footprint for MAb 55 centered on R533 provides a plausible explanation as to how this antibody can block both SLAM and CD46 attachment and provides support for the prediction made by Santiago et al. (38) that the binding sites for SLAM and CD46 overlap on the MVH globular head. What is somewhat surprising, however, is that the CD46 binding residues concerned are 546, 548, and549 rather than Y481. The N481Y mutation occurs upon adaptation of wt MV strains to CD46+ Vero cells (4, 35, 43). Adaptation involves the acquisition of phenotypic markers associated with laboratory-vaccine strains: CD46-dependent fusion, CD46 downregulation, and hemadsorption (21). Ten years ago, Shibahara et al. (43) showed that three wt MV strains that acquired the hemadsorption phenotype following passaging in Vero cells had point mutations in the H protein: two had N481Y, and one had S546G. Li and Qi (23) subsequently showed that the S546G substitution is also associated with the acquisition of hemadsorption and CD46 binding. Evidently both mutations increase affinity for CD46. But how? Santiago et al. (38) correctly predicted that SLAM binding was likely to be localized on the top of the H protein propeller but that Y481 would localize to the side. In order to explain their finding that the SLAM and CD46 sites overlap, Santiago et al. suggested that the first repeat of CD46 could make contact with Y481, with the second repeat making contact adjacent to the SLAM binding site. However, we propose that another explanation is possible: the S546G and N481Y mutations induce conformational changes in the MVH globular head. It was recently demonstrated that residues 548 and 549 appear to represent a low-affinity CD46 binding site that is present on the H proteins of both wt and laboratory-vaccine MV strains (27). However, our present results suggest that in laboratory-vaccine strains these residues represent a high-affinity CD46 binding site. If this switch from low- to high-affinity CD46 binding depends upon a conformational change, it could be achieved locally by the S546G mutation and at distance by the N481Y mutation. Our results concerning the mutation of other MVH residues in the vicinity of Y481 give support to this concept. According to the structural model, Y481 is very close in 3D to the 521-to-529 region and even appears to be in contact with another tyrosine (Y524) whose mutation causes significant reductions in the downregulation of not only CD46 but also SLAM. L526 is also close to Y481, and Shingai et al. (44) recently reported that the mutation S526L can induce CD46 attachment in the H protein from a particular subacute sclerosing panencephalitis MV strain called Osaka-2. Our results showing that the reverse mutation, L526S, reduces CD46 downregulation confirm that this residue plays a role in CD46 attachment. As Y524 and L526 are predicted to be on the surface of the globular head, they could theoretically be part of an extensive CD46 (and SLAM) binding site, but our finding that other residues in the 521-to-529 region, whose mutations also have an effect on the downregulation of the two receptors, are predicted to be buried suggests that this is probably not the case. Although evidence of a conformational change in MVH induced by the N481Y mutation is lacking, this could explain the puzzling results of Bieback et al. (2), who found that this point mutation abolishes the SLAM- and CD46-independent capacity of wt MV to induce Toll-like receptor 2 activation.
Are morbillivirus attachment proteins H rather than HN because they bind SLAM rather than sialic acid?
A dramatic consequence of the revised morbillivirus H model (www.pepscan.nl/downloads/measlesH.pdb) is that although the NA (neuraminidase) fold is conserved, all NA active site residues except R533 are lost. As this conserved residue is the equivalent of a major residue involved in binding the carboxylate of sialic acid in NA active sites, this raises the possibility that members of the genus Morbillivirus have adapted the sialic acid binding site on their attachment protein to binding SLAM via evolution. One possible way in which morbilliviruses could prevent readsorption of progeny virus would be by removal of the cellular receptor from the infected cell's surface: MVH-induced receptor downregulation could fill this role.
The present study lends more credence to the concept that the capacity of MV to interact with CD46 is an intrinsic in vivo property rather than an in vitro adaptation. Whether low-affinity attachment to CD46 plays a role in wt MV infection of SLAM-negative tissues remains to be elucidated, but the overlapping of CD46 and SLAM binding sites on MVH could facilitate switching of receptor usage during wt MV infection.
Acknowledgments
We thank Gert Bolt for stimulating discussions, Claude Muller for the anti-MVH MAb BH59, Jürgen Schneider-Schaulies for anti-CD46 MAbs 13/42 and 11/88, and Glaucia Paranhos-Baccala for hospitality.
This work was supported by a grant (HHCO2F) from the Rhône-Alpes Région. N.M. was supported by a scholarship from the Rhône-Alpes Région and a grant from the Fondation Recherche Médicale (FRM). R.B. is a CNRS scientist.
REFERENCES
- 1.Bartz, R., U. Brinckmann, L. M. Dunster, B. K. Rima, V. ter Meulen, and J. Schneider-Schaulies. 1996. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology 224:334-337. [DOI] [PubMed] [Google Scholar]
- 2.Bieback, K., E. Lien, I. M. Klagge, E. Avota, J. Schneider-Schaulies, W. P. Duprex, H. Wagner, C. J. Kirschning, V. ter Meulen, and S. Schneider-Schaulies. 2002. Hemagglutinin protein of wild-type measles virus activates Toll-like receptor 2. J. Virol. 76:8729-8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz, C. J., D. Koller, P. Devaux, C. Mumenthaler, J. Schneider-Schaulies, W. Braun, D. Gerlier, and R. Cattaneo. 1997. Mapping of the primary site of measles virus to its receptor CD46. J. Biol. Chem. 272:22072-22079. [DOI] [PubMed] [Google Scholar]
- 4.Buckland, R., and T. F. Wild. 1997. Is CD46 the cellular receptor for measles virus? Virus Res. 48:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Buckland, R. 1999. Molecular basis of the interaction of measles virus with its cellular receptors: implications for vaccine development. Recent Res. Dev. Virol. 1:467-484. [Google Scholar]
- 6.Crennel, S., T. Takimoto, A. Portner, and G. Taylor. 2000. Crystal structure of the multifunctional paramyxovirus hemagglutinin-neuraminidase. Nat. Struct. Biol. 7:1068-1074. [DOI] [PubMed] [Google Scholar]
- 7.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dörig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 9.Erlenhöfer, C., W. J. Wurzer, S. Loffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2002. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerald, C., R. Buckland, R. Barker, G. Freeman, and T. F. Wild. 1986. Measles virus hemagglutinin gene: cloning, complete nucleotide sequence analysis and expression in COS cells. J. Gen. Virol. 67:2695-2703. [DOI] [PubMed] [Google Scholar]
- 11.Giraudon, P., and T. F. Wild. 1985. Correlation between epitopes on hemagglutinin of measles virus and biological activities: passive protection by monoclonal antibodies is related to their hemagglutination activity. Virology 144:46-58. [DOI] [PubMed] [Google Scholar]
- 12.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, A. M. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 13.Guex, N., and M. C. Peitsch. 1997. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714-2723. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto, K., N. Ono, H. Tatsuo, H. Minagawa, M. Takeda, K. Takeuchi, and Y. Yanagi. 2002. SLAM (CD150)-independent measles virus entry as revealed by recombinant virus expressing green fluorescent protein. J. Virol. 76:6743-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooft, R. W., G. Vriend, C. Sander, and E. E. Abola. 1996. Errors in protein structures. Nature 381:272. [DOI] [PubMed] [Google Scholar]
- 16.Hsu, E. C., R. E. Dörig, F. Sarangi, A. Marcil, C. Iorio, and C. D. Richardson. 1997. Artificial mutations and natural variations in the CD46 molecules from human and monkey cells define regions important for measles virus binding. J. Virol. 71:6144-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu, E. C., F. Sarangi, C. Iorio, M. S. Sidhu, S. A. Udem, D. L. Dillehay, W. Xu, P. A. Rota, W. J. Bellini, and C. D. Richardson. 1998. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 72:2905-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu, E. C., C. Iorio, F. Sarangi, A. A. Khine, and C. D. Richardson. 2001. CDw150 (SLAM) is a receptor for lymphotropic strains of measles virus and may account for the immunosuppressive properties of this virus. Virology 279:9-21. [DOI] [PubMed] [Google Scholar]
- 19.Hummel, K. B., and W. J. Bellini. 1995. Localization of monoclonal antibody epitopes and functional domains in the hemagglutinin protein of measles virus. Virology 69:1913-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Langedijk, J. P. M., F. J. Daus, and J. T. van Oirschot. 1997. Sequence and structure alignment of Paramyxoviridae attachment proteins and discovery of enzymatic activity for a morbillivirus hemagglutinin. J. Virol. 71:6155-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lecouturier, V., J. Fayolle, M. Caballero, J. Carabana, M. L. Celma, R. Fernandez-Munoz, and R. Buckland. 1996. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J. Virol. 70:4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lecouturier, V., A. Rizzitelli, J. Fayolle, L. Daviet, T. F. Wild, and R. Buckland. 1999. Interaction of measles virus (Hallé strain) with CD46: evidence that a common binding site on CD46 facilitates both CD46 downregulation and MV infection. Biochem. Biophys. Res. Commun. 264:268-275. [DOI] [PubMed] [Google Scholar]
- 23.Li, L., and Y. Qi. 2002. A novel amino acid position in hemagglutinin glycoprotein of measles virus is responsible for hemadsorption and CD46 binding. Arch. Virol. 147:775-786. [DOI] [PubMed] [Google Scholar]
- 24.Manchester, M., J. E. Gairin, J. B. Patterson, J. Alvarez, M. K. Liszewski, D. S. Eto, J. P. Atkinson, and M. B. A. Oldstone. 1997. Measles virus recognizes its receptor, CD46, via two distinct binding domains within SCR1-2. Virology 233:174-184. [DOI] [PubMed] [Google Scholar]
- 25.Manchester, M., D. S. Eto, A. Valsamakis, P. B. Liton, R. Fernandez-Munoz, P. A. Rota, W. J. Bellini, D. N. Forthal, and M. B. A. Oldstone. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manchester, M., K. A. Smith, D. S. Eto, H. B. Perkin, and B. E. Torbett. 2002. Targeting and hematopoietic suppression of human CD34+ cells by measles virus. J. Virol. 76:6636-6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massé, N., T. Barrett, C. P. Muller, T. F. Wild, and R. Buckland. 2002. Identification of a second major site for CD46 binding in the hemagglutinin protein from a laboratory strain of measles virus (MV): potential consequences for wild-type MV infection. J. Virol. 76:13034-13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naniche, D. G., T. F. Wild, C. Rabourdin-Combe, and D. Gerlier. 1993. Measles virus hemagglutinin induces downregulation of gp57/67, a molecule involved in virus binding. J. Gen. Virol. 74:1073-1079. [DOI] [PubMed] [Google Scholar]
- 30.Oldstone, M. B. A., D. Homann, H. Lewicki, and D. Stevenson. 2002. One, two, or three step: measles virus receptor dance. Virology 299:162-163. [DOI] [PubMed] [Google Scholar]
- 31.Patterson, J. B., F. Scheiflinger, M. Manchester, T. Yilma, and M. B. A. Oldstone. 1999. Structural and functional studies of the measles virus hemagglutinin: identification of a novel site required for CD46 interaction. Virology 256:142-151. [DOI] [PubMed] [Google Scholar]
- 32.Peitsch, M. C. 1996. ProMod and Swiss-Model: Internet-based tools for automated comparative protein modelling. Biochem. Soc. Trans. 24:274-279. [DOI] [PubMed] [Google Scholar]
- 33.Perkus, M. E., K. Limbach, and E. Paoletti. 1989. Cloning and expression of foreign genes in vaccinia virus, with a host range selection system. J. Virol. 63:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rice, D. W., and D. Eisenberg. 1997. A 3D-1D substitution matrix for protein fold recognition that includes predicted secondary structure of the sequence. J. Mol. Biol. 276:1026-1038. [DOI] [PubMed] [Google Scholar]
- 35.Rima, B. K., J. A. P. Earle, K. Baczko, V. ter Meulen, U. G. Liebert, C. Carstens, J. Carabana, M. Caballero, M. L. Celma, and R. Fernandez-Munoz. 1997. Sequence divergence of measles virus hemagglutinin during natural evolution and adaptation to cell culture. J. Gen. Virol. 78:97-106. [DOI] [PubMed] [Google Scholar]
- 36.Rost, B. 1995. TOPITS: threading one-dimensional predictions into three-dimensional structures, p. 314-321. In C. Rawlings, D. Clark, R. Altman, L. Hunter, T. Lengauer, and S. Wodak (ed.), The Third International Conference on Intelligent Systems for Molecular Biology (ISMB), Cambridge, United Kingdom, July 16-19, 1995. AAAI Press, Menlo Park, Calif. [PubMed]
- 37.Rost, B. 1996. PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol. 266:525-539. [DOI] [PubMed] [Google Scholar]
- 38.Santiago, C., E. Björling, T. Stehle, and J. M. Casasnovas. 2002. Distinct kinetics for binding of the CD46 and SLAM receptors to overlapping sites in the measles virus hemagglutinin protein. J. Biol. Chem. 277:32294-32301. [DOI] [PubMed] [Google Scholar]
- 39.Schneider, U., V. von Messling, P. Devaux, and R. Cattaneo. 2002. Efficiency of measles virus entry and dissemination through different receptors. J. Virol. 76:7460-7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrag, S. J., P. A. Rote, and W. J. Bellini. 1999. Spontaneous mutation rate of measles virus: direct estimation based on mutations conferring monoclonal resistance. J. Virol. 73:51-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheshberadaran, H., E. Norrby, and K. W. Rammohan. 1985. Monoclonal antibodies against five structural components of measles virus. II. Characterization of five cell lines persistently infected with measles virus. Arch. Virol. 83:251-268. [DOI] [PubMed] [Google Scholar]
- 43.Shibahara, K., H. Hotta, Y. Katayama, and M. Homma. 1994. Increased binding activity of measles virus to monkey red blood cells after long-term passage in Vero cultures. J. Gen. Virol. 75:3511-3516. [DOI] [PubMed] [Google Scholar]
- 44.Shingai, M., M. Ayata, H. Ishida, I. Matsunaga, Y. Katayama, T. Seya, H. Tatsuo, Y. Yanagi, and H. Ogura. 2003. Receptor use by vesicular stomatitis virus pseudotypes with glycoproteins of defective variants of measles virus isolated from brains of patients with subacute sclerosing panencephalitis. J. Gen. Virol. 84:2133-2143. [DOI] [PubMed] [Google Scholar]
- 45.Takeuchi, K., N. Miyajima, N. Nagata, M. Takeda, and M. Tashiro. 2003. Wild-type measles virus induces large syncytium formation in primary human small airway epithelial cells by a SLAM(CD150)-independant mechanism. Virus Res. 94:11-16. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka, K., H. Minagawa, M.-F. Xie, and Y. Yanagi. 2002. The measles virus hemagglutinin downregulates the cellular receptor SLAM (CD150). Arch. Virol. 147:195-203. [DOI] [PubMed] [Google Scholar]
- 47.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 48.Tatsuo, H., N. Ono, and Y. Yanagi. 2001. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 75:5842-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vongpunsawad, S., N. Oezgun, W. Braun, and R. Cattaneo. 2004. Selectively receptor-blind measles viruses: identification of residues necessary for SLAM- or CD46-induced fusion and their localization on a new hemagglutinin structural model. J. Virol. 78:302-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Waku Kouomou, D., and T. F. Wild. 2002. Adaptation of wild-type measles to tissue culture. J. Virol. 76:1505-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wild, T. F., and R. Buckland. 1995. Functional aspects of envelope-associated measles virus proteins, p. 51-62. In V. ter Meulen and M. A. Billeter (ed.), Measles virus. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 52.Wild, T. F., J. Fayolle, P. Beauverger, and R. Buckland. 1994. Measles virus fusion: role of the cysteine-rich region of the fusion protein. J. Virol. 68:7546-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wild, T. F., E. Malvoisin, and R. Buckland. 1991. Measles virus: both the hemagglutinin and fusion glycoproteins are required for fusion. J. Gen. Virol. 72:439-442. [DOI] [PubMed] [Google Scholar]