Abstract
Background
The occurrence of brain death in patients with hypoxic-ischaemic brain injury after resuscitation from cardiac arrest creates opportunities for organ donation. However, its prevalence is currently unknown.
Methods
Systematic review. MEDLINE via PubMed, ISI Web of Science and the Cochrane Database of Systematic Reviews were searched for eligible studies (2002–2016). The prevalence of brain death in adult patients resuscitated from cardiac arrest and the rate of organ donation among brain dead patients were summarised using a random effect model with double-arcsine transformation. The quality of evidence (QOE) was evaluated according to the GRADE guidelines.
Results
26 studies [16 on conventional cardiopulmonary resuscitation (c-CPR), 10 on extracorporeal CPR (e-CPR)] included a total of 23,388 patients, 1830 of whom developed brain death at a mean time of 3.2 ± 0.4 days after recovery of circulation. The overall prevalence of brain death among patients who died before hospital discharge was 12.6 [10.2–15.2] %. Prevalence was significantly higher in e-CPR vs. c-CPR patients (27.9 [19.7–36.6] vs. 8.3 [6.5–10.4] %; p < 0.0001). The overall rate of organ donation among brain dead patients was 41.8 [20.2–51.0] % (9/26 studies, 1264 patients; range 0–100 %). The QOE was very low for both outcomes.
Conclusions
In patients with hypoxic-ischaemic brain injury following CPR, more than 10 % of deaths were due to brain death. More than 40 % of brain-dead patients could donate organs. Patients who are unconscious after resuscitation from cardiac arrest, especially when resuscitated using e-CPR, should be carefully screened for signs of brain death.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-016-4549-3) contains supplementary material, which is available to authorized users.
Keywords: Cardiac arrest; Brain death; Anoxia-ischemia, brain; Organ donation
Introduction
Despite recent improvements [1] mortality after cardiopulmonary resuscitation (CPR) remains high. About two-thirds of patients admitted to hospital [1] or an intensive care unit (ICU) [2] after cardiac arrest die before hospital discharge. Most of these deaths are due to hypoxic-ischaemic brain injury [3, 4] and result from active withdrawal of life-sustaining treatment (WLST) based on prognostication of survival with a poor neurological outcome [5, 6]. However, in some resuscitated patients hypoxic-ischaemic brain injury can result in a total loss of clinical brain function, i.e. in brain death [7].
Despite being an unfavourable outcome for the individual patient, brain death creates opportunities for organ donation [4, 8], which may represent an additional benefit from CPR in terms of lives saved or improved. In fact, transplanted organs retrieved from brain-dead patients after cardiac arrest have a similar success rate as organs retrieved from patients who have died from other causes [8–10]. The 2015 American Heart Association Guidelines for Post-Cardiac Arrest Care [11] recommend that all patients who are resuscitated from cardiac arrest but who subsequently progress to brain death should be evaluated for organ donation (Class I, Level of Evidence B). Despite this, the prevalence of brain death following cardiac arrest is only rarely reported and it has never been systematically reviewed. Knowing the epidemiology of brain death after cardiac arrest could help intensivists to organize a timely screening and identification of potential organ donors after resuscitation.
The primary aim of the present systematic review is to measure the reported prevalence of brain death in adult patients resuscitated from cardiac arrest as compared to other causes of death. The secondary aim of this review is to measure the rate of organ donation in these patients.
Materials and methods
Data reporting in this review is consistent with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [12]. The MOOSE checklist [13] was adopted for study design and manuscript preparation.
Review questions
The review questions were formulated following the PICO scheme (population, intervention, comparator, outcome) as follows:
Among adults who are admitted to hospital after successful resuscitation from cardiac arrest (P), what is the prevalence of brain death at hospital discharge (O)?
Among adults who develop brain death after successful resuscitation from cardiac arrest (P), what is the rate of organ donation (O)?
In addition, a preliminary screening of the literature suggested potential differences in the epidemiology of brain death between patients resuscitated with extracorporeal CPR (e-CPR) as compared to patients resuscitated with conventional CPR (c-CPR). Therefore, we planned a comparison between these two subgroups of patients.
Inclusion and exclusion criteria
All studies published as full-text articles in indexed journals which reported the prevalence of brain death in adult (≥18 years old) patients resuscitated from cardiac arrest occurred either in-hospital (in-hospital cardiac arrest, IHCA) or out-of-hospital (out-of-hospital cardiac arrest, OHCA) were considered for inclusion. No restrictions of language or publication status were imposed. Publication date was restricted to studies published after 2002 in order to better reflect current practice which includes targeted temperature management (TTM) [14, 15] and post-resuscitation care bundles [16]. Reviews, case reports and studies published in abstract form were excluded. Studies including patients with non-hypoxic causes of brain death or patients with hypoxic coma from causes other than cardiac arrest (respiratory arrest, asphyxia, drowning, hanging) were excluded. Studies including donors after circulatory death were excluded.
Search strategy and study selection
MEDLINE via PubMed was searched using the keywords “heart arrest” (MESH) OR “cardiac arrest” (MESH) AND “brain death”. ISI Web of Science and the Cochrane Database of Systematic Reviews were searched using the search strings “cardiac arrest” AND “brain death”. The search was iterated until April 30, 2016. The websites of relevant journals were searched to identify relevant studies in press. The reference lists of relevant studies were screened to identify other studies of interest.
Data extraction and analysis
For each study included in the final analysis, the following data were extracted: patients’ age and sex; location of cardiac arrest (IHCA or OHCA); witnessed status; cause of cardiac arrest; initial cardiac rhythm; CPR technique (c-CPR vs. e-CPR); cardiac arrest duration, defined as the interval between collapse to either return of spontaneous circulation (ROSC) or to the start of extracorporeal circulation; hospital mortality; mode of death; rate and timing of brain death; rate of organ donation. Whenever possible, the authors of the original studies where contacted to retrieve missing data.
Data of the study populations were summarized using proportions and weighted means. The mean and standard deviations in individual studies were estimated from median and interquartile range, when needed, according to the method described by Wan et al. [17]. Pooled estimates of continuous variables were made using the inverse variance method and reported as mean ± standard error (SE). The DerSimonian–Laird random effects model [18] was used to account for heterogeneity. For categorical variables, proportions with 95 % confidence intervals (CIs) were calculated using the Freeman–Tukey double-arcsine transformation [19]. The heterogeneity of pooled data was estimated by calculating the Q and I 2 statistics and it was regarded as significant when p < 0.05 or I 2 > 50 %. Statistical analyses were performed using MedCalc software [20] version 16.4.1 (MedCalc Software bvba, Ostend, Belgium), Comprehensive Meta-Analysis version 2.2.064 (Biostat Inc. Englewood NJ USA) and MetaXL version 5.1 (EpiGear International, http://www.epigear.com).
Grading
The quality of evidence was independently and blindly assessed by two authors (C.S., S.D’A.) on the basis of the presence of limitations (risk of bias), indirectness, inconsistency and imprecision according to the grading of recommendations assessment, development and evaluation (GRADE) criteria [21–23]. Given the observational nature of included studies, the quality of evidence was initially graded as low [24]. Inconsistency across studies was graded as serious when heterogeneity was significant (p < 0.1 or I 2 > 50 %). Imprecision was graded as serious when either the lower or the upper bound of the CIs was respectively less than or greater than 20 % of the point estimate of the prevalence. Limitations of individual studies were assessed using a modified version of the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies delivered by the US National Institutes of Health, March 2014 version [25] (ESM Table 1). Disagreements between assessors were resolved by consensus.
Results
Study selection
The initial search on PubMed, ISI Web of Science and the Cochrane Database of Systematic Reviews yielded 862 records, and 177 additional records were identified through forward search (Fig. 1). After duplicate removal and abstract screening, 274 articles were considered for full-text analysis. Among them, 248 were excluded because they did not fulfil inclusion criteria. These studies with reasons for their exclusion are listed in the Appendix. The remaining 26 studies (total 23,388 patients) were included in our review. The overall quality of evidence was very low (ESM Table 2).
Fig. 1.
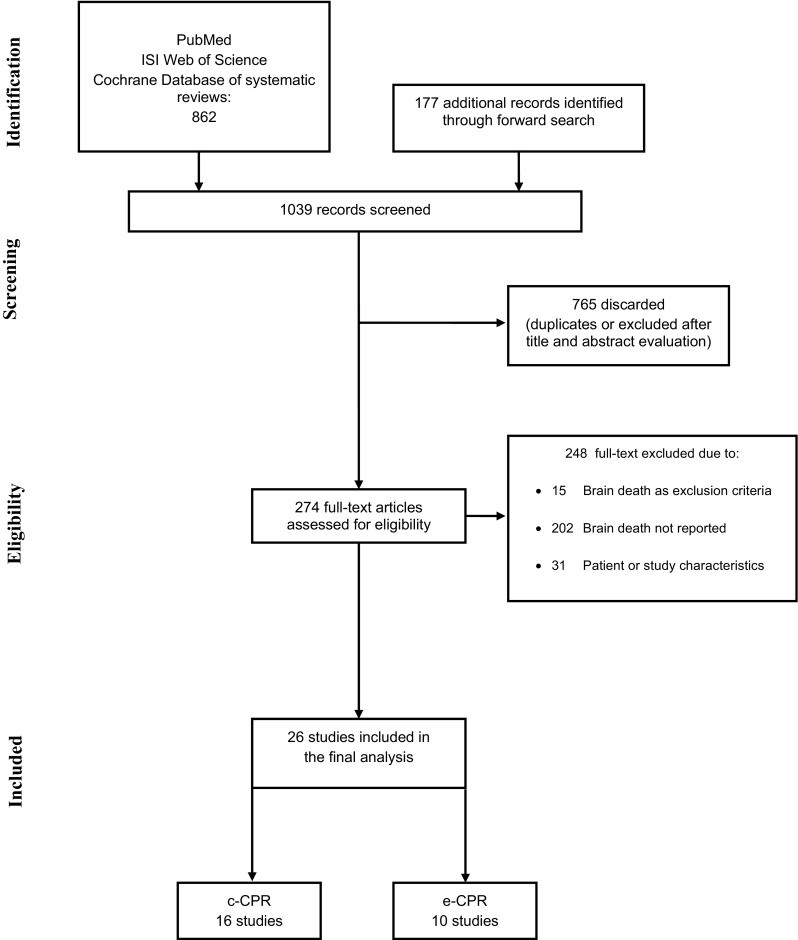
Flow chart of study selection
Patient characteristics
Sixteen out of 26 studies included patients resuscitated using c-CPR [4–6, 8, 14, 26–36] while ten studies included patients resuscitated using e-CPR [37–46]. The characteristics of these two patient subgroups are described in Table 1.
Table 1.
Patients’ characteristics in the included studies
| Author, year [reference] | IHCA or OHCA | No. of patients | Males, n (%) | Age, yeara | VF/pVT, n (%) | Witnessed, n (%) | TTM, n (%) |
|---|---|---|---|---|---|---|---|
| c-CPR | |||||||
| Adrie, 2008 [8] | OHCA | 246 | 174 (70.7) | 55.2 ± 17.6 | 62 (25.2) | 198 (80.5) | 71 (28.9) |
| Bernard, 2002 [14] | OHCA | 77 | 52 (67.5) | 66.5 ± 9.7 | 77 (100) | 73 (94.8) | 48 (55.8) |
| Calderon, 2014 [26] | Mixed | 72 | 42 (58.3) | 59.0 ± 16 | 31 (43.1) | N/A | 72 (100) |
| Dragancea, 2013 [6] | Mixed | 159 | 109 (68.6) | 67 ± 13.7 | 97 (60.5) | N/A | 159 (100) |
| Elmer, 2016 [27] | OHCA | 4265 | 2740 (64.2) | 65 (53–77) | 1835 (43) | 2809 (65.9) | 1858 (43.8) |
| Geocadin, 2006 [5] | Mixed | 58 | 41 (70.7) | 57.2 ± 15 | N/A | N/A | N/A |
| Greer, 2013 [28] | Mixed | 200 | 124 (62) | 59.9 ± 16.5 | 68 (34) | N/A | 39 (19.5) |
| Grossestreuer, 2013 [29] | OHCA | 194 | 114 (58.8) | 57.0 ± 16 | 76 (40) | N/A | 194 (100) |
| Lemiale, 2013 [4] | OHCA | 1152 | 842 (73.1) | 58.4 ± 15.4 | 654 (56.8) | 1054 (87.7) | 764 (66.3) |
| Mentzelopoulos, 2013 [30] | IHCA | 268 | 183 (68.3) | 63 ± 18.2 | 45 (16.8) | 247 (92.2) | 68 (25.4) |
| Mulder, 2014 [31] | OHCA | 154 | 104 (67.5) | 59.0 ± 16 | 83 (53.9) | 154 (100) | 154 (100) |
| Nielsen, 2013 [32] | OHCA | 939 | 761 (81) | 64 ± 12.6 | 752 (80.1) | 839 (89.4) | 939 (100) |
| Peberdy, 2003 [33] | IHCA | 14,720 | 8390 (57) | 67.0 ± 15 | 3680 (25) | 12,659 (86) | N/A |
| Rundgren, 2010 [34] | Mixed | 95 | 68 (71.6) | 65 (50–74) | 57 (60) | N/A | 95 (100) |
| Sivaraju, 2015 [35] | N/A | 100 | 59 (59) | 62.3 ± 16.2 | 33 (33) | N/A | 100 (100) |
| Stammet, 2009 [36] | Mixed | 45 | 30 (66.7) | 56 ± 17 | 22 (48.9) | N/A | 45 (100) |
| Total c-CPR | 22,744 | 13,833 (60.8) | 60.9 ± 0.86 b | 7572 (33.3) | 18,033 (82.6) | 4601 (57.8) | |
| e-CPR | |||||||
| Avalli, 2012 [37] | Mixed | 42 | 33 (78.6) | 64.8 ± 11.7 | 28 (66.7) | 42 (100) | 42 (100) |
| Fagnoul, 2013 [38] | Mixed | 24 | 14 (58.3) | 48 (38–55) | 10 (41.7) | 22 (91.7) | 17 (70.8) |
| Lamhaut, 2013 [39] | OHCA | 7 | 6 (85.7) | 42 ± 16 | 5 (71.4) | 7 (100) | 7 (100) |
| Le Guen, 2011 [40] | OHCA | 51 | 46 (90.2) | 42 ± 15 | 32 (62.7) | 51 (100) | 51 (100) |
| Massetti, 2005 [41] | Mixed | 40 | 23 (57.5) | 42 ± 15 | N/A | N/A | N/A |
| Megarbane, 2007 [42] | Mixed | 17 | 5 (29.4) | 47 (27–57) | 0 | 17 (100) | 17 (100) |
| Megarbane, 2011 [43] | Mixed | 66 | 51 (77.3) | 46 (39–55) | 30 (45.5) | 66 (100) | 66 (100) |
| Pozzi, 2016 [44] | OHCA | 68 | 50 (73.5) | 43.7 ± 11.4 | 32 (47.1) | 68 (100) | 68 (100) |
| Rousse, 2015 [45] | OHCA | 32 | 23 (71.9) | 43.2 ± 14.3 | 19 (59.4) | 32 (100) | 32 (100) |
| Thiagarajan, 2009 [46] | N/A | 297 | 195 (65.7) | 52 (35–64) | N/A | N/A | N/A |
| Total e-CPR | 644 | 466 (72.4) | 47.6 ± 1.44 b* | 156 (50.8)* | 305 (99.3)* | 300 (97.7)* | |
| Overall | 23,388 | 14,299 (61.1) | 57.5 ± 0.74 b | 7728 (33) | 18,338 (82.9) | 4901 (59.2) | |
Total percentages are referred to studies with available data
c-CPR conventional cardiopulmonary resuscitation, e-CPR extracorporeal cardiopulmonary resuscitation, IHCA in-hospital cardiac arrest, N/A not available, OHCA out-of-hospital cardiac arrest, TTM targeted temperature management, VF/pVT ventricular fibrillation/pulseless ventricular tachycardia
* p < 0.0001 vs. c-CPR
aMean ± standard deviation or median (interquartile range)
bPooled estimate of the mean ± SE
Eleven out of 26 studies (42.3 %) included only patients resuscitated from OHCA, two (7.7 %) included only patients resuscitated from IHCA, 11 studies (42.3 %) included both IHCA and OHCA patients while in two studies (7.7 %) the location of cardiac arrest was not specified. The cause of arrest was cardiac in three studies (11.5 %), cardiac or respiratory in 17 studies (65.4 %), while in six studies (23.1 %) the cause of arrest was not specified. Patients had a mean age of 57.5 (±0.74) years and 14,299 (61.1 %) were male. Patients resuscitated with e-CPR were significantly younger than those resuscitated with c-CPR (47.6 vs. 60.9 years; p < 0.001). The first recorded rhythm was shockable in 7728 (33.0 %) patients (7572/22,686 [33.3 %] c-CPR patients vs. 156/307 [50.8 %] e-CPR patients; p < 0.001). The overall duration of cardiac arrest was 42.4 ± 1.5 min (19/26 studies; Table 2). Patients resuscitated with e-CPR had a significantly longer duration of arrest than those resuscitated with c-CPR (94.7 ± 2.9 vs. 24.0 ± 1.5 min; p < 0.0001).
Table 2.
Duration of cardiac arrest in included studies
| Author, year [reference] | Duration of cardiac arrest, mina |
|---|---|
| Adrie, 2008 [8] | 32.0 ± 6.6 |
| Bernard, 2002 [14] | 25.7 ± 8.1 |
| Dragancea, 2013 [6] | 20 ± 26.4 |
| Elmer, 2016 [27] | 23.3 ± 0.15 |
| Lemiale, 2013 [4] | 24.1 ± 0.6 |
| Mentzelopoulos, 2013 [30] | 16.1 ± 8.0 |
| Mulder, 2014 [31] | 23 ± 1.4 |
| Nielsen, 2013 [32] | 27.4 ± 8.0 |
| Rundgren, 2010 [34] | 21.3 ± 1.2 |
| Sivaraju, 2015 [35] | 14.7 ± 8.1 |
| Stammet, 2009 [36] | 25 [3–90] |
| c-CPR | 24.0 ± 1.7 |
| Avalli, 2012 [37] | 69.1 ± 3.9 |
| Fagnoul, 2013 [38] | 57.7 ± 0.8 |
| Lamhaut, 2013 [39] | 79.0 ± 5.7 |
| Massetti, 2005 [41] | 105.0 ± 7.0 |
| Megarbane, 2007 [42] | 129.7 ± 11.7 |
| Megarbane, 2011 [43] | 151.7 ± 5.6 |
| Pozzi, 2016 [44] | 85.4 ± 2.6 |
| Rousse, 2015 [45] | 115.5 ± 3.5 |
| e-CPR | 94.7 ± 2.9* |
| Overall | 42.4 ± 1.5 |
Pooled data are reported as mean ± SE
* p < 0.0001 vs. c-CPR
aMean ± standard deviation, median (interquartile range), or median [range]
In 15/26 studies all patients were treated using TTM. In seven studies TTM was used in part of the patient population (range 19.5–70.8 %). In the remaining four studies temperature management was not reported. Use of TTM was significantly more common in e-CPR vs. c-CPR studies (300/307 [97.7 %] vs. 4601/7966 [57.8 %] patients; p < 0.001).
Outcomes
Among 23,388 patients, 17,779 (76.0 %) died in hospital. Brain death was diagnosed at mean time of 3.2 ± 0.4 days after recovery of circulation (11/26 studies; Table 3). The rates of brain death ranged from 0 to 16.3 % of total population in studies conducted on patients resuscitated with c-CPR (Table 4) and from 5.9 to 42.9 % in studies conducted in patients resuscitated with e-CPR (Table 4). Among patients who died, the estimated pooled prevalence of brain death was 12.6 [10.2–15.2] %. This corresponded to 8.9 [7.0–11.0] % of patients resuscitated from cardiac arrest. The prevalence of brain death was significantly higher in patients resuscitated with e-CPR than in patients resuscitated with c-CPR, both as a percentage of total deaths (27.9 [19.7–36.6] vs. 8.3 [6.5–10.4] %) and as a percentage of total patients (21.9 [16.6–27.5] vs. 5.4 [3.9–7.1] %; p < 0.0001 for both).
Table 3.
Timing of brain death
| Author, year [reference] | Days after arresta |
|---|---|
| Adrie, 2008 [8] | 2.5 (2.0–4.2) |
| Avalli, 2012 [37] | 3 (3–4) |
| Bernard, 2002 [14] | 3 [2–4] |
| Calderon, 2014 [26] | 3.8 ± 1.7 |
| Dragancea, 2013 [6] | 5.0 ± 1.3 |
| Fagnoul, 2013 [38] | 0.1 [0.1–2] |
| Lemiale, 2013 [4] | 5 [3–6] |
| Nielsen, 2013 [32] | 3.1 ± 1.2 |
| Pozzi, 2016 [44] | 1.3 ± 2.1 |
| Rundgren, 2010 [34] | 5.3 ± 1.5 |
| Stammet, 2009 [36] | 2 (1–3) |
| Overall (mean ± SE) | 3.2 ± 0.4 |
SE standard error
aMean ± standard deviation, median (interquartile range), or median [range]
Table 4.
Rates of mortality, brain death and organ donation in the included studies
| Author, year [reference] | No. of patients | Mortality, n (%) | Brain death rate | Organ donation rate | ||||
|---|---|---|---|---|---|---|---|---|
| n | % Of total patients | % Of deaths | n | % Of brain deaths | % Of deaths | |||
| c-CPR | ||||||||
| Adrie, 2008 [8] | 246 | 210 (85) | 40 | 16.3 | 19.0 | 19 | 47.5 | 9.1 |
| Bernard, 2002 [14] | 77 | 45 (58) | 2 | 2.6 | 4.4 | |||
| Calderon, 2014 [26] | 72 | 46 (64) | 8 | 11.1 | 17.4 | |||
| Dragancea, 2013 [6] | 159 | 84 (53) | 4 | 2.5 | 4.8 | 4 | 100 | 4.8 |
| Elmer, 2016 [27] | 4265 | 2775 (65) | 305 | 7.2 | 11.0 | |||
| Geocadin, 2006 [5] | 58 | 48 (83) | 1 | 1.7 | 2.1 | |||
| Greer, 2013 [28] | 200 | 180 (90) | 20 | 10.0 | 11.1 | |||
| Grossestreuer, 2013 [29] | 194 | 109 (56) | 4 | 2.1 | 3.7 | |||
| Lemiale, 2013 [4] | 1152 | 768 (67) | 94 | 8.2 | 12.2 | |||
| Mentzelopoulos, 2013 [30] | 268 | 149 (56) | 0 | 0 | 0 | |||
| Mulder, 2014 [31] | 154 | 78 (51) | 8 | 5.2 | 10.3 | |||
| Nielsen, 2013 [32] | 939 | 411 (44) | 18 | 1.9 | 4.4 | |||
| Peberdy, 2003 [33] | 14,720 | 12,217 (83) | 1177 | 8.0 | 9.6 | 159 | 13.5 | 1.3 |
| Rundgren, 2010 [34] | 95 | 43 (45) | 3 | 3.2 | 7.0 | 3 | 100 | 3.2 |
| Sivaraju, 2015 [35] | 100 | 71 (71) | 7 | 7.0 | 9.9 | |||
| Stammet, 2009 [36] | 45 | 22 (49) | 3 | 6.7 | 13.6 | |||
| Total c-CPR | 22,744 | 17,256 (75.9) | 1694 | 5.4 [3.9–7.1] | 8.3 [6.5–10.4] | 185 | 59.2 [18.0–95.7] | 4.8 [0.4–11.5] |
| e-CPR | ||||||||
| Avalli, 2012 [37] | 42 | 31 (73.8) | 12 | 28.6 | 38.7 | 5 | 41.7 | 16.1 |
| Fagnoul, 2013 [38] | 24 | 18 (75.0) | 5 | 20.8 | 27.8 | 1 | 20 | 5.6 |
| Lamhaut, 2013 [39] | 7 | 6 (85.7) | 3 | 42.9 | 50 | 2 | 66.7 | 33.3 |
| Le Guen, 2011 [40] | 51 | 49 (96.1) | 10 | 19.6 | 20.4 | |||
| Massetti, 2005 [41] | 40 | 32 (80) | 15 | 37.5 | 46.9 | |||
| Megarbane, 2007 [42] | 17 | 14 (82.4) | 1 | 5.9 | 7.1 | |||
| Megarbane, 2011 [43] | 66 | 65 (98.5) | 6 | 9.1 | 9.2 | 3 | 50 | 4.6 |
| Pozzi, 2016 [44] | 68 | 62 (91.2) | 14 | 20.6 | 22.6 | 0 | 0 | 0 |
| Rousse, 2015 [45] | 32 | 30 (93.8) | 9 | 28.1 | 30 | |||
| Thiagarajan, 2009 [46] | 297 | 216 (72.7) | 61 | 20.5 | 28.2 | |||
| Total e-CPR | 644 | 523 (81.2) | 136 | 21.9 [16.6–27.5]* | 27.9 [19.7–36.6]* | 11 | 29.4 [4.3–60.8] | 7.6 [0.5–17.8] |
| Overall | 23,388 | 17,779 (76.0) | 1830 | 8.9 [7.0–11.0] | 12.6 [10.2–15.2] | 196 | 41.8 [20.2–51.0] | 5.8 [2.1–10.9] |
Pooled rates are reported in italics as point estimate (95 %CIs)
c-CPR conventional cardiopulmonary resuscitation, e-CPR extracorporeal cardiopulmonary resuscitation
p < 0.0001 vs. c-CPR
Rates of organ donation were reported in 9/26 studies. Donation rates ranged from 0 to 100 % of brain deaths and from 0 to 33.3 % of total deaths. The estimated pooled rates were 41.8 [20.2–51.0] and 5.8 [2.1–10.9] %, respectively (9/26 studies, 1264 patients; Table 4). There was no significant difference in the rate of organ donation between studies on patients resuscitated with e-CPR and those on patients resuscitated with c-CPR (p = 0.544 and 0.471, respectively). The heterogeneity within and across subgroups was significant for both brain death rate and organ donation rate (ESM Figs. 1, 2).
One study [8] reported the outcome of organs (kidneys and livers) retrieved from patients with brain death after cardiac arrest and compared it with that of organs retrieved from patients with brain death due to a primary brain injury (head injury or stroke). This study found no significant difference in 5-year survival rates of transplanted organs between these two groups.
Only one study [8] specifically investigated early predictors of brain death. In that study, none of the clinical or laboratory findings distinguished the patients with brain death from those who died without a diagnosis of brain death.
Discussion
In studies included in our review, 8.9 % of patients initially resuscitated from cardiac arrest developed brain death. They represented more than 12 % of total patients who died before hospital discharge. The prevalence of brain death in patients resuscitated with e-CPR was more than three times higher than in those resuscitated with c-CPR, despite similar mortality rates. e-CPR patients were significantly younger and had significantly higher rates of witnessed arrest or shockable rhythms compared to c-CPR patients, which reflects that e-CPR programs use restrictive indications to select patients with favourable characteristics who are most likely to benefit [47]. However, in our review e-CPR patients had significantly longer arrest times than c-CPR patients, because e-CPR is used when c-CPR fails to restore spontaneous circulation and it requires longer to initiate [48]. If the severity of hypoxic-ischaemic neuronal death is proportional to the duration of arrest [49], this may explain the higher prevalence of brain death in e-CPR patients. However, since the mechanism of brain death has not been described in studies included in our review, we could not exclude other possible explanations. For example, in some patients resuscitated using e-CPR, brain death could have been due to cerebral haemorrhage induced by anticoagulation needed to maintain extracorporeal circulation.
Our study shows that brain death is relatively common after cardiac arrest. Since none of the indices currently used to report outcome after cardiac arrest, such as cerebral performance categories (CPC), modified Rankin Scale (mRS) or Glasgow Outcome Scale (GOS), distinguishes between death due to neurological causes (as brain death or death after WLST because of severe brain damage) and death from other causes, such as irreversible cardiovascular collapse or multiorgan failure, we suggest that those indices should be modified to include this information (e.g. CPC 5b to indicate brain death). We also suggest that the authors should be encouraged to report the detailed mechanism of death in post-resuscitation studies.
In studies included in our review the pooled rate of organ donation from brain dead patients after cardiac arrest was 41 %. According to recent studies [50, 51] each year approximately 167,000 OHCA patients are treated by the emergency medical services in the USA. Of these, 41,750 (25 %) are successfully resuscitated and admitted to an ICU. On the basis of the results of our review, we estimate that 3716 [95 %CI 2923–4593] patients yearly will evolve to brain death, of whom 1553 could potentially donate organs. With this perspective, intensive life support of patients with severe post-resuscitation brain injury should be maintained for a sufficiently long time to detect not only the occurrence of cerebral recovery but also to determine progression to brain death.
The diagnosis of brain death was made at a mean of 3 days and up to 6 days after ROSC in studies included in our review, which is consistent with the fact that neuronal death occurring after global brain ischaemia is typically delayed [52, 53]. Delayed massive cerebral oedema leading to brain death and occurring at 48–72 h after ROSC has also been described [54]. Since both TTM and sedation/paralysis used to maintain TTM may interfere with clinical neurological examination [55, 56], it is reasonable to evaluate resuscitated patients for occurrence of brain death after rewarming and cessation of interference from sedation (see suggested algorithm in Fig. 2). This will occur at 48–72 h from ROSC in most patients [57]. Nevertheless, brain death can be suspected even during sedation or paralysis on the basis of clinical signs such as fixed, dilated pupils, diabetes insipidus, and acute cardiocirculatory changes that suggest cerebral herniation.
Fig. 2.
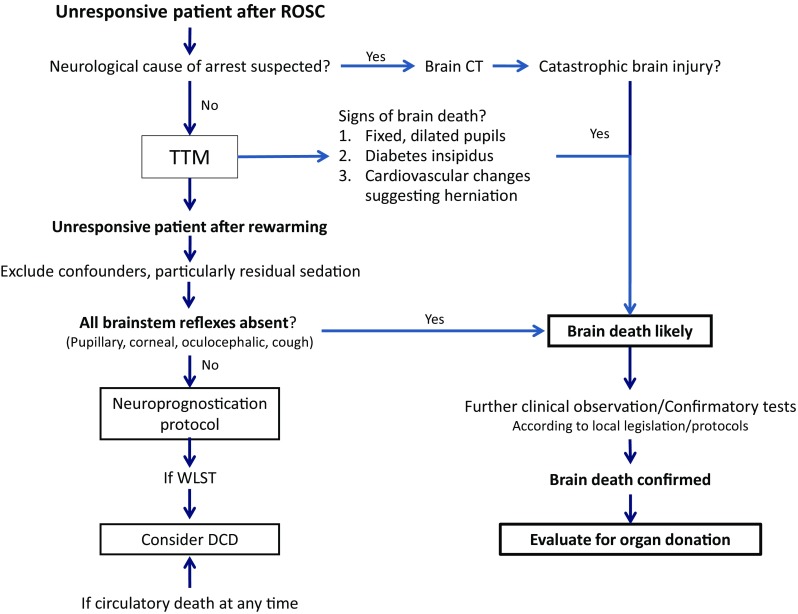
Suggested algorithm for brain death screening after cardiac arrest. In a resuscitated patient who is unresponsive after rewarming from targeted temperature management (TTM), and after having excluded confounders, brain death is suspected if brainstem reflexes are all absent. Brain death can be suspected earlier if a catastrophic brain injury is demonstrated on CT or if the patient shows signs like fixed, dilated pupils, diabetes insipidus, or cardiovascular changes suggesting herniation. Brain death is confirmed by clinical observation and/or by confirmatory tests like apnoea, a flat EEG or absent cerebral blood flow, according to local legislation or protocols. Organ donation is considered after ascertainment of brain death. In cases where circulatory death occurs, either spontaneously or as a consequence of withdrawal of life-sustaining treatment (WLST), donation after circulatory death (DCD) can be considered. For the European Resuscitation Council and the European Society of Intensive Care Medicine (ERC–ESICM) recommended neuroprognostication protocol, see Ref. [55]
In some cases, occurrence of brain death has been reported within 1 day after cardiac arrest. This is common when cardiac arrest is due to neurological causes, most commonly a subarachnoid haemorrhage [58, 59]. When a neurological rather than a cardiac cause of arrest is suspected, a brain computerized tomography (CT) immediately after ROSC is recommended [56]. If brain CT demonstrates a catastrophic brain injury, an early evaluation for brain death may be considered.
Clinical examination for ascertainment of brain death usually requires the absence of all brainstem reflexes [60]. Confirmatory tests such as electroencephalogram or evaluation of cerebral blood flow may be required when confounders cannot be excluded or when requested by local legislation [61]. Whenever circulatory death occurs, either spontaneously or as a consequence of WLST, donation after circulatory death (DCD) may be considered, according to local legislation and practices.
The potential for organ donation in patients resuscitated from cardiac arrest has important ethical implications. From one side, our review showed that the general principle of providing “CPR to save lives” is not limited to the life of the patient who is being resuscitated but it extends also to the potential recipients of organs retrieved from resuscitated patients who proceed to brain death. From the opposite side, however, if CPR was started with the only aim of organ procurement, the community-level beneficence represented by organ donation would potentially conflict with the general principle of individual non-maleficence (“first, do not harm”) [62]. We think a broader ethical and public debate is necessary on that issue.
Our study has important limitations. Firstly, its primary outcome measure, the prevalence of brain death, was reported in only 10 % of studies screened for inclusion, so we cannot exclude a selection bias. In addition, some variables like the timing of brain death and the rate of organ donation were reported in less than 50 % of included studies. However, this shows that brain death after cardiac arrest is underreported in current literature, and confirms the utility of our review.
Secondly, our analysis used aggregated data, so our ability to investigate the factors associated with the occurrence of brain death after cardiac arrest was limited. Our pooled analysis showed a significant heterogeneity, which likely reflects differences in terms of case mix, treatment and possibly criteria for the diagnosis of brain death. An individual patient data meta-analysis will be needed to adjust adequately for these confounders. In particular, we could not investigate the association between location of cardiac arrest (IHCA vs. OHCA) and the prevalence of brain death, because in 13/26 (50 %) studies the location of cardiac arrest was either mixed or not specified. Similarly, we could not investigate the association between use of TTM and occurrence of brain death. This is also because—in order to reflect current post-resuscitation practice—we restricted our analysis to studies published after 2002, in whom most of the patients received TTM. Future studies investigating different temperature management strategies after CPR might include the occurrence of brain death among their endpoints to specifically address this question.
Thirdly, our study did not include DCD, which in some countries [63] represents a major source of organ donation after cardiac arrest. This is because our study was focused only on the epidemiology of brain death after cardiac arrest, whose prevalence is underreported, and whose importance as a source of organ donation may consequently have been underrecognised [8, 10].
Conclusions
Despite being only rarely reported in current literature, brain death represented more than 10 % of deaths in the 23,388 adult cardiac arrest patients included in our review. This rate was significantly higher after extracorporeal than after conventional CPR, probably because of the higher severity of anoxic-ischaemic brain injury in that group of patients. More than 40 % of patients brain dead after cardiac arrest donated organs. Given this potential for organ donation, patients who are unconscious after resuscitation from cardiac arrest, especially when resuscitated using e-CPR, should be carefully screened for brain death before deciding on withdrawal of life support.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors gratefully thank the colleagues who provided data from their studies: Dr. Leonello Avalli, Department of Anaesthesiology and Intensive Care, Cardiac Surgery Unit, S. Gerardo Hospital, Milano-Bicocca University, Monza, Italy; Dr Mehran Monchi, Centre Hospitalier Général de Melun, Paris area, France; Dr. Niklas Nielsen, Department of Anaesthesia and Intensive Care, Intensive Care Unit, Helsingborg Hospital, Helsingborg, Sweden; Dr. Matteo Pozzi, Department of Cardiac Surgery, “Louis Pradel” Cardiologic Hospital, Claude Bernard University, Lyon, France; Dr. Malin Rundgren, Department of Intensive and Perioperative Care, Skåne University Hospital, Lund, Sweden.
Compliance with ethical standards
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- 1.Fugate JE, Brinjikji W, Mandrekar JN, Cloft HJ, White RD, Wijdicks EF, Rabinstein AA. Post-cardiac arrest mortality is declining: a study of the US national inpatient sample 2001 to 2009. Circulation. 2012;126:546–550. doi: 10.1161/CIRCULATIONAHA.111.088807. [DOI] [PubMed] [Google Scholar]
- 2.Nolan JP, Laver SR, Welch CA, Harrison DA, Gupta V, Rowan K. Outcome following admission to UK intensive care units after cardiac arrest: a secondary analysis of the ICNARC case mix programme database. Anaesthesia. 2007;62:1207–1216. doi: 10.1111/j.1365-2044.2007.05232.x. [DOI] [PubMed] [Google Scholar]
- 3.Laver S, Farrow C, Turner D, Nolan J. Mode of death after admission to an intensive care unit following cardiac arrest. Intensive Care Med. 2004;30:2126–2128. doi: 10.1007/s00134-004-2425-z. [DOI] [PubMed] [Google Scholar]
- 4.Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, Carli P, Mira JP, Nolan J, Cariou A. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39:1972–1980. doi: 10.1007/s00134-013-3043-4. [DOI] [PubMed] [Google Scholar]
- 5.Geocadin RG, Buitrago MM, Torbey MT, Chandra-Strobos N, Williams MA, Kaplan PW. Neurologic prognosis and withdrawal of life support after resuscitation from cardiac arrest. Neurology. 2006;67:105–108. doi: 10.1212/01.wnl.0000223335.86166.b4. [DOI] [PubMed] [Google Scholar]
- 6.Dragancea I, Rundgren M, Englund E, Friberg H, Cronberg T. The influence of induced hypothermia and delayed prognostication on the mode of death after cardiac arrest. Resuscitation. 2013;84:337–342. doi: 10.1016/j.resuscitation.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Geocadin RG, Eleff SM. Cardiac arrest resuscitation: neurologic prognostication and brain death. Curr Opin Crit Care. 2008;14:261–268. doi: 10.1097/MCC.0b013e3282fd68ea. [DOI] [PubMed] [Google Scholar]
- 8.Adrie C, Haouache H, Saleh M, Memain N, Laurent I, Thuong M, Darques L, Guerrini P, Monchi M. An underrecognized source of organ donors: patients with brain death after successfully resuscitated cardiac arrest. Intensive Care Med. 2008;34:132–137. doi: 10.1007/s00134-007-0885-7. [DOI] [PubMed] [Google Scholar]
- 9.Sandroni C, Adrie C, Cavallaro F, Marano C, Monchi M, Sanna T, Antonelli M. Are patients brain-dead after successful resuscitation from cardiac arrest suitable as organ donors? A systematic review. Resuscitation. 2010;81:1609–1614. doi: 10.1016/j.resuscitation.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Orioles A, Morrison WE, Rossano JW, Shore PM, Hasz RD, Martiner AC, Berg RA, Nadkarni VM. An under-recognized benefit of cardiopulmonary resuscitation: organ transplantation. Crit Care Med. 2013;41:2794–2799. doi: 10.1097/CCM.0b013e31829a7202. [DOI] [PubMed] [Google Scholar]
- 11.Callaway CW, Donnino MW, Fink EL, Geocadin RG, Golan E, Kern KB, Leary M, Meurer WJ, Peberdy MA, Thompson TM, Zimmerman JL. Part 8: post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2015;132:S465–S482. doi: 10.1161/CIR.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 15.HACA Working group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 16.Sunde K, Pytte M, Jacobsen D, Mangschau A, Jensen LP, Smedsrud C, Draegni T, Steen PA. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73:29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67:974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 20.Schoonjans F, Zalata A, Depuydt CE, Comhaire FH. MedCalc: a new computer program for medical statistics. Comput Methods Programs Biomed. 1995;48:257–262. doi: 10.1016/0169-2607(95)01703-8. [DOI] [PubMed] [Google Scholar]
- 21.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schunemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schunemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. doi: 10.1016/j.jclinepi.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, Norris SL, Williams JW, Jr, Atkins D, Meerpohl J, Schunemann HJ. GRADE guidelines: 4. Rating the quality of evidence-study limitations (risk of bias) J Clin Epidemiol. 2011;64:407–415. doi: 10.1016/j.jclinepi.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 25.National Heart Lung and Blood Institute Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. http://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/cohort. Accessed 11 July 2015
- 26.Calderon LM, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC. Combining NSE and S100B with clinical examination findings to predict survival after resuscitation from cardiac arrest. Resuscitation. 2014;85:1025–1029. doi: 10.1016/j.resuscitation.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, Herren H, Jasti J, Kudenchuk PJ, Scales DC, Stub D, Richardson DK, Zive DM. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016;102:127–135. doi: 10.1016/j.resuscitation.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greer DM, Yang J, Scripko PD, Sims JR, Cash S, Wu O, Hafler JP, Schoenfeld DA, Furie KL. Clinical examination for prognostication in comatose cardiac arrest patients. Resuscitation. 2013;84:1546–1551. doi: 10.1016/j.resuscitation.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grossestreuer AV, Abella BS, Leary M, Perman SM, Fuchs BD, Kolansky DM, Beylin ME, Gaieski DF. Time to awakening and neurologic outcome in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84:1741–1746. doi: 10.1016/j.resuscitation.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Mentzelopoulos SD, Malachias S, Chamos C, Konstantopoulos D, Ntaidou T, Papastylianou A, Kolliantzaki I, Theodoridi M, Ischaki H, Makris D, Zakynthinos E, Zintzaras E, Sourlas S, Aloizos S, Zakynthinos SG. Vasopressin, steroids, and epinephrine and neurologically favorable survival after in-hospital cardiac arrest: a randomized clinical trial. JAMA. 2013;310:270–279. doi: 10.1001/jama.2013.7832. [DOI] [PubMed] [Google Scholar]
- 31.Mulder M, Gibbs HG, Smith SW, Dhaliwal R, Scott NL, Sprenkle MD, Geocadin RG. Awakening and withdrawal of life-sustaining treatment in cardiac arrest survivors treated with therapeutic hypothermia. Crit Care Med. 2014;42:2493–2499. doi: 10.1097/CCM.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, Horn J, Hovdenes J, Kjaergaard J, Kuiper M, Pellis T, Stammet P, Wanscher M, Wise MP, Aneman A, Al-Subaie N, Boesgaard S, Bro-Jeppesen J, Brunetti I, Bugge JF, Hingston CD, Juffermans NP, Koopmans M, Kober L, Langorgen J, Lilja G, Moller JE, Rundgren M, Rylander C, Smid O, Werer C, Winkel P, Friberg H. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369:2197–2206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 33.Peberdy MA, Kaye W, Ornato JP, Larkin GL, Nadkarni V, Mancini ME, Berg RA, Nichol G, Lane-Trultt T. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58:297–308. doi: 10.1016/S0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 34.Rundgren M, Westhall E, Cronberg T, Rosen I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38:1838–1844. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- 35.Sivaraju A, Gilmore EJ, Wira CR, Stevens A, Rampal N, Moeller JJ, Greer DM, Hirsch LJ, Gaspard N. Prognostication of post-cardiac arrest coma: early clinical and electroencephalographic predictors of outcome. Intensive Care Med. 2015;41:1264–1272. doi: 10.1007/s00134-015-3834-x. [DOI] [PubMed] [Google Scholar]
- 36.Stammet P, Werer C, Mertens L, Lorang C, Hemmer M. Bispectral index (BIS) helps predicting bad neurological outcome in comatose survivors after cardiac arrest and induced therapeutic hypothermia. Resuscitation. 2009;80:437–442. doi: 10.1016/j.resuscitation.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 37.Avalli L, Maggioni E, Formica F, Redaelli G, Migliari M, Scanziani M, Celotti S, Coppo A, Caruso R, Ristagno G, Fumagalli R. Favourable survival of in-hospital compared to out-of-hospital refractory cardiac arrest patients treated with extracorporeal membrane oxygenation: an Italian tertiary care centre experience. Resuscitation. 2012;83:579–583. doi: 10.1016/j.resuscitation.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Fagnoul D, Taccone FS, Belhaj A, Rondelet B, Argacha JF, Vincent JL, Backer DD. Extracorporeal life support associated with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation. 2013;84:1519–1524. doi: 10.1016/j.resuscitation.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Lamhaut L, Jouffroy R, Soldan M, Phillipe P, Deluze T, Jaffry M, Dagron C, Vivien B, Spaulding C, An K, Carli P. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation. 2013;84:1525–1529. doi: 10.1016/j.resuscitation.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 40.Le Guen M, Nicolas-Robin A, Carreira S, Raux M, Leprince P, Riou B, Langeron O. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit Care. 2011;15:R29. doi: 10.1186/cc9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massetti M, Tasle M, Le Page O, Deredec R, Babatasi G, Buklas D, Thuaudet S, Charbonneau P, Hamon M, Grollier G, Gerard JL, Khayat A. Back from irreversibility: extracorporeal life support for prolonged cardiac arrest. Ann Thorac Surg. 2005;79:178–183. doi: 10.1016/j.athoracsur.2004.06.095. [DOI] [PubMed] [Google Scholar]
- 42.Megarbane B, Leprince P, Deye N, Resiere D, Guerrier G, Rettab S, Theodore J, Karyo S, Gandjbakhch I, Baud FJ. Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest (BD 1pt/14) Intensive Care Med. 2007;33:758–764. doi: 10.1007/s00134-007-0568-4. [DOI] [PubMed] [Google Scholar]
- 43.Megarbane B, Deye N, Aout M, Malissin I, Resiere D, Haouache H, Brun P, Haik W, Leprince P, Vicaut E, Baud FJ. Usefulness of routine laboratory parameters in the decision to treat refractory cardiac arrest with extracorporeal life support. Resuscitation. 2011;82:1154–1161. doi: 10.1016/j.resuscitation.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Pozzi M, Koffel C, Armoiry X, Pavlakovic I, Neidecker J, Prieur C, Bonnefoy E, Robin J, Obadia JF. Extracorporeal life support for refractory out-of-hospital cardiac arrest: should we still fight for? A single-centre, 5-year experience. Int J Cardiol. 2016;204:70–76. doi: 10.1016/j.ijcard.2015.11.165. [DOI] [PubMed] [Google Scholar]
- 45.Rousse N, Robin E, Juthier F, Hysi I, Banfi C, Al Ibrahim M, Coadou H, Goldstein P, Wiel E, Vincentelli A. Extracorporeal life support in out-of-hospital refractory cardiac arrest. Artif Organs. 2015 doi: 10.1111/aor.12655. [DOI] [PubMed] [Google Scholar]
- 46.Thiagarajan RR, Brogan TV, Scheurer MA, Laussen PC, Rycus PT, Bratton SL. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87:778–785. doi: 10.1016/j.athoracsur.2008.12.079. [DOI] [PubMed] [Google Scholar]
- 47.Conseil français de réanimation cardiopulmonaire (CFRC) et al. Guidelines for indications for the use of extracorporeal life support in refractory cardiac arrest. Annales Françaises d’Anesthésie et de Réanimation. 2009;28:187–190. doi: 10.1016/j.annfar.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 48.Soar J, Nolan JP, Bottiger BW, Perkins GD, Lott C, Carli P, Pellis T, Sandroni C, Skrifvars MB, Smith GB, Sunde K, Deakin CD, Adult advanced life support section Collaborators European resuscitation council guidelines for resuscitation 2015: section 3. Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 49.Bjorklund E, Lindberg E, Rundgren M, Cronberg T, Friberg H, Englund E. Ischaemic brain damage after cardiac arrest and induced hypothermia–a systematic description of selective eosinophilic neuronal death. A neuropathologic study of 23 patients. Resuscitation. 2014;85:527–532. doi: 10.1016/j.resuscitation.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2015 doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 51.Elmer J, Torres C, Aufderheide TP, Austin MA, Callaway CW, Golan E, Herren H, Jasti J, Kudenchuk PJ, Scales DC, Stub D, Richardson DK, Zive DM. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation. 2016 doi: 10.1016/j.resuscitation.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horn M, Schlote W. Delayed neuronal death and delayed neuronal recovery in the human brain following global ischemia. Acta Neuropathol. 1992;85:79–87. doi: 10.1007/BF00304636. [DOI] [PubMed] [Google Scholar]
- 53.Petito CK, Feldmann E, Pulsinelli WA, Plum F. Delayed hippocampal damage in humans following cardiorespiratory arrest. Neurology. 1987;37:1281–1286. doi: 10.1212/WNL.37.8.1281. [DOI] [PubMed] [Google Scholar]
- 54.Bergman R, Tjan DH, Adriaanse MW, van Vugt R, van Zanten AR. Unexpected fatal neurological deterioration after successful cardio-pulmonary resuscitation and therapeutic hypothermia. Resuscitation. 2008;76:142–145. doi: 10.1016/j.resuscitation.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, Horn J, Nolan JP, Rossetti AO, Soar J. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1816–1831. doi: 10.1007/s00134-014-3470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine guidelines for post-resuscitation care 2015: section 5 of the European Resuscitation Council guidelines for resuscitation 2015. Resuscitation. 2015;95:202–222. doi: 10.1016/j.resuscitation.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 57.Paul M, Bougouin W, Geri G, Dumas F, Champigneulle B, Legriel S, Charpentier J, Mira JP, Sandroni C, Cariou A. Delayed awakening after cardiac arrest: prevalence and risk factors in the Parisian registry. Intensive Care Med. 2016;42:1128–1136. doi: 10.1007/s00134-016-4349-9. [DOI] [PubMed] [Google Scholar]
- 58.Arnaout M, Mongardon N, Deye N, Legriel S, Dumas F, Sauneuf B, Malissin I, Charpentier J, Pene F, Baud F, Chiche JD, Mira JP, Cariou A. Out-of-hospital cardiac arrest from brain cause: epidemiology, clinical features, and outcome in a multicenter cohort. Crit Care Med. 2015;43:453–460. doi: 10.1097/CCM.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 59.Sandroni C, Dell’Anna AM. Out-of-hospital cardiac arrest from neurologic cause: recognition and outcome. Crit Care Med. 2015;43:508–509. doi: 10.1097/CCM.0000000000000777. [DOI] [PubMed] [Google Scholar]
- 60.Shemie SD, Baker A. Uniformity in brain death criteria. Semin Neurol. 2015;35:162–168. doi: 10.1055/s-0035-1547538. [DOI] [PubMed] [Google Scholar]
- 61.Citerio G, Crippa IA, Bronco A, Vargiolu A, Smith M. Variability in brain death determination in europe: looking for a solution. Neurocrit Care. 2014;21:376–382. doi: 10.1007/s12028-014-9983-x. [DOI] [PubMed] [Google Scholar]
- 62.Swain GR, Burns KA, Etkind P. Preparedness: medical ethics versus public health ethics. J Public Health Manag Pract. 2008;14:354–357. doi: 10.1097/01.PHH.0000324563.87780.67. [DOI] [PubMed] [Google Scholar]
- 63.Cheetham OV, Thomas MJ, Hadfield J, O’Higgins F, Mitchell C, Rooney KD. Rates of organ donation in a UK tertiary cardiac arrest centre following out-of-hospital cardiac arrest. Resuscitation. 2016;101:41–43. doi: 10.1016/j.resuscitation.2016.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


