Abstract
Macrophages represent an essential part of innate immunity, and the viral infection of macrophages results in the release of multiple proinflammatory mediators, such as nitric oxide (NO), cytokines, and chemokines. This study was undertaken to define the molecular mechanism of macrophage activation in response to rabies virus (RV) infection. In RAW264 murine macrophage cells, a well-characterized macrophage model, RV replication was strictly restricted, whereas cell proliferation was significantly enhanced upon RV inoculation. Transcriptional analyses for the expression of inducible forms of NO synthase (iNOS), cytokines, and chemokines revealed that RV virions potentiate the gene expression of iNOS and CXC chemokine ligand 10 (CXCL10), a major chemoattractant of T helper cell type 1. However, RV stimulation had little or no effect on the expression profiles of proinflammatory cytokines and other types of chemokines. In macrophages stimulated with UV-inactivated RV virions, as well as infectious viruses, the phosphorylation of extracellular signal-regulated kinase (ERK) 1 and 2, members of the mitogen-activated protein kinase family, was significantly induced. Specific inhibitors of MAPK/ERK kinase reduced the RV-induced production of NO and CXCL10. Furthermore, the RV-induced activation of the ERK1/2 pathway was severely impaired by the neutralization of the endosomal and lysosomal pH environment with lysosomotropic agents, indicating that endocytosis is a key step leading to the activation of ERK1/2 signaling. Taken together, these results suggest that the ERK1/2-mediated signaling pathway plays a cardinal role in the selective activation of macrophages in response to RV virions, thereby regulating cellular functions during virus infection.
Rabies virus (RV) is a negative-strand RNA virus belonging to the Rhabdoviridae family, genus Lyssavirus. Most RV strains are highly neurotropic, which usually causes a fatal infection in warm-blooded animals, and viral replication primarily occurs in neurons as a cellular target (43). It has been reported that RV replicates in muscle cells prior to the invasion of the peripheral nervous system and central nervous system (CNS) in vivo (62). In vitro, it is known that a highly neurovirulent RV strain, challenge virus standard (CVS), and an attenuated strain, high egg passage (HEP)-Flury (hereafter called HEP), infect a variety of cell types, including nonneuronal cells (26, 82, 84, 85).
It is well known that infection of experimental animals with nonpathogenic RV strains triggers a strong antiviral immune response (90). Recent studies have demonstrated that the strong antiviral immune response elicited by attenuated RV infection is closely related to the induction of apoptosis in infected cells due to the expression of viral glycoprotein (61, 76). Several groups have attempted to develop vaccine vehicles using RV-based vectors. It has been reported that the RV vectors expressing foreign antigens induce strong humoral and cellular responses against other kinds of viral pathogens, such as human immunodeficiency virus type 1 and hepatitis C virus, in the mouse model (54, 55, 79, 81). The high efficacy of RV in inducing an antiviral immune response may also be due to the stimulation of immunocytes by RV components. Lafon and colleagues demonstrated that the nucleocapsid of RV acts as a viral superantigen, which activates T lymphocytes bearing particular V beta subsets of T cell receptor via its binding to major histocompatibility complex class II (2, 47, 48).
A major component of innate immunity against infection, particularly virus infection, is the macrophage. Macrophages play a principal role in the uptake of pathogens into intracellular compartments via endocytosis and phagocytosis. The enzymolysis of exogenous particles in the acidic environment of endosomal-lysosomal vesicles finally leads to the induction of various cellular responses (8, 23, 58). At the local sites of infection, macrophages induce proinflammatory responses aiming at the elimination of invading pathogens and infected cells. During virus infection, the activated macrophages secrete various kinds of proinflammatory mediators, such as proinflammatory cytokines, including interleukin-1β (IL-1β) and IL-6; chemokines; and nitric oxide (NO) (24, 29, 52). In the process leading to macrophage activation, signaling pathways mediated by the mitogen-activated protein kinase (MAPK) superfamily have been shown to play pivotal roles in regulating cellular functions (22). For mammalian cells, several different subgroups of the MAPK subfamily have been identified, such as the extracellular signal-regulated kinases 1 and 2 (ERK1/2), c-Jun N-terminal kinase, and p38 MAPK (22, 45). ERK1/2, members of the MAPK family, are primarily activated by stimuli such as growth factors, cytokines, and phagocytosis, while p38 MAPK is involved in the MAPK activation induced by environmental stresses, such as bacterial endotoxins, proinflammatory cytokines, osmotic shock, UV irradiation, and virus infections (5, 22, 30, 37, 56, 73, 74, 93).
Previous studies have demonstrated that some RV strains infect bone marrow macrophages and several macrophage-like cell lines (41, 77). However, RV replication depends on virus strains and cell types and tends to be severely impaired compared to that in neuronal cells (77). It has also been reported by others that RV virions are taken up by murine peritoneal macrophages into intracellular compartments, in which they are actively destroyed (86). Furthermore, immunohistochemistry and electron microscopy studies have proven that large numbers of macrophages are recruited to the peripheral sites of RV inoculation in vivo (14). Recently, Claassen and colleagues have demonstrated that peripherally administrated RV antigens are taken up mainly by macrophages and are localized in the lymph nodes and spleen (15). From these lines of evidence, it is likely that RV interacts with macrophage-like cells at the peripheral sites of infection. However, macrophage responses to RV remain largely unknown.
In the present study, we have examined the transcription patterns of inducible NO synthase (iNOS), cytokines, and chemokines in macrophages stimulated with neurovirulent CVS and attenuated HEP viruses. We demonstrate that RV virions stimulate iNOS gene expression and NO production, although viral replication is strictly arrested. We also show that the gene expression and protein release of CXCL10 (chemokine CXC ligand 10)/IP-10 (interferon-inducible protein-10 kDa) are selectively enhanced in RV-stimulated macrophages but that RV stimulation has little or no effect on the expression profiles of proinflammatory cytokines and other chemokines. Furthermore, it has been shown that the induction of the iNOS and CXCL10 genes in RV-stimulated macrophages is mediated through the activation of the MAPK signaling cascade that involves ERK1/2. Our findings suggest an essential role for the ERK1/2-mediated signaling cascade in regulating the selective activation of antiviral functions of macrophages in response to RV virions.
MATERIALS AND METHODS
Reagents and antibodies.
Highly purified bovine serum albumin (BSA; fatty acid free), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide (MTT), DAPI (4′,6-diamidino-2-phenylindole), 1,4-diazabicyclo-2,2,2-octane, lipopolysaccharide (LPS) from Escherichia coli (serotype O111:B4), bafilomycin A1 (BA1), and ammonium chloride (NH4Cl) were purchased from Sigma (St. Louis, Mo.). PD98059, a selective inhibitor of MAPK/ERK kinase 1 (MEK1); U0126, a potent and specific inhibitor of MEK1/2; U0124, an inactive analogue of U0126 used as a negative control; and SB202190, a specific inhibitor of p38 MAPK, were purchased from EMD Biosciences, Inc. (San Diego, Calif.). Rabbit antibodies against phosphorylated forms of ERK1/2 and p38 MAPK were obtained from Santa Cruz Biotechnology (Hercules, Calif.). Anti-ERK1/2 and anti-α-tubulin antibodies, as well as horseradish peroxidase-linked antibodies, were purchased from Sigma. Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (MAbs) specific for RV nucleoprotein (N) were purchased from Centocor, Inc. (Malvern, Pa.).
Cells.
A murine macrophage cell line, RAW264, was obtained from RIKEN Cell Bank (Tsukuba, Japan). Murine cell lines derived from T lymphoma (EL4) and B lymphoma (A20) were kindly provided by the Cell Resource Center for Biomedical Research, Tohoku University (Sendai, Japan). Murine neuroblastoma (NA; C1300 clone) and RAW264 cells were cultivated in Dulbecco's modified Eagle's medium (DMEM) (Sigma) supplemented with 10% heat-inactivated fetal calf serum (Invitrogen, Carlsbad, Calif.), penicillin (100 U/ml), and streptomycin (100 μg/ml). EL4 and A20 cells were grown in RPMI 1640 medium (Sigma) containing the above-mentioned additives. All cell cultures were maintained at 37°C in a humidified incubator containing 5% CO2 in air.
Viruses.
The pathogenic CVS-11 (hereafter called CVS) and nonpathogenic HEP strains of RV were propagated in NA cells as previously described (80). Preparation of RV virions was performed essentially as described before (39). Briefly, virions in the culture supernatant of the RV-infected NA cells were purified by polyethylene glycol (no. 6,000) precipitation, followed by sucrose density ultracentrifugation. Finally, the RV virions were purified and resuspended in DMEM, which contained 0.2% BSA instead of serum (test medium), by using ultrafiltration with an Amicon Ultra-15 centrifugal filter device (Millipore, Billerica, Mass.) according to the manufacturer's instructions. Virus titers were determined by a focal infectivity assay using the FITC-coupled anti-N protein MAbs (see below). Alternatively, purified viruses were inactivated by UV light irradiation for 15 min just prior to the experiments.
Measurement of virus replication.
NA and RAW264 cells, which had been plated in 96-well culture plates (4 × 105 cells/well), were incubated with 10 focus-forming units (FFU) of viruses per cell suspended in test medium for 2 h at 37°C, washed, and overlaid with culture medium. At different times after inoculation, culture supernatants were separated by centrifugation at 5,000 × g for 5 min and subjected to virus titration on NA cell monolayers.
Immunofluorescence.
Fluorescent staining of cultured cells was performed essentially as described in previous papers (64, 66). Briefly, cells were incubated with or without viruses, washed, and overlaid with culture medium in the same procedure as for the virus replication assay. After a 48-h incubation period, the cells were washed with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde in PBS for 10 min, and then permeabilized with 0.2% Triton X-100 in PBS for 5 min. The cells were stained with FITC-coupled MAbs specific for the viral N protein and with DNA dye (DAPI; 0.1 μg/ml). Samples were overlaid with a solution containing 90% glycerol, 2.3% 1,4-diazabicyclo-2,2,2-octane, and 20 mM Tris-HCl (pH 8.0) and were examined under a fluorescent microscope (Eclipse TE200; Nikon, Tokyo, Japan).
Cell proliferation assay.
The proliferation of RV-infected cells was measured by using a colorimetric MTT assay as described previously (65). The cells, which had been plated at a density of 2 × 104 per well in the flat-bottom 96-well culture dishes, were incubated in test media with or without RV (10 FFU/cell) for 2 h at 37°C, washed, and further incubated. After incubation of the cells for the appropriate times, MTT solution was added to the culture supernatants at a final concentration of 0.5 mg per ml, and the cells were incubated at 37°C. After a 4-h incubation period, the culture supernatants were decanted and the formazan precipitates were solubilized in 150 μl each of dimethyl sulfoxide. The optical density of each sample was determined on a spectrophotometer at a wavelength of 550 nm.
RT-PCR analysis.
Semiquantitative reverse transcription (RT)-PCR analysis was carried out as described previously (7, 68). The cells were cultured in test media with or without a virus inoculum (10 FFU/cell) at 37°C for 2 h, washed, and further incubated at 37°C. After incubation for the appropriate times, total RNA was isolated from the 1.5 × 106 cells by using the RNA extraction reagent ISOGEN (Nippon Gene, Tokyo, Japan) according to the manufacturer's recommendations. The first-strand cDNA was generated from 5 μg of total RNA prepared as described above in a final volume of 50 μl and was subjected to PCR amplification of the specific cDNA sequences by using an RNA PCR kit (AMV) and Ex Taq DNA polymerase (Takara Bio Inc., Shiga, Japan) according to the manufacturer's protocol. Oligonucleotides were synthesized on the basis of the published primers for cDNA amplification of GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (68), iNOS (71), cytokines (27, 36, 71, 72), and chemokines (68, 46); their specificities and optimum PCR conditions have been described previously. To control possible contamination of genomic DNAs in total RNA extracts, primer sequences for GAPDH were separated by introns so that the amplified products from genomic DNAs would be longer than the amplified cDNAs (68). The absence of contaminating DNA was also verified by PCR analysis using RNA preparations not treated with reverse transcriptase and cDNA reactions. To adjust the relative amounts of total cDNAs in each sample, GAPDH-specific PCRs were performed with serially fivefold-diluted cDNA preparations, and the amplified products were separated by using 1% agarose gel electrophoresis and visualized by ethidium bromide staining. The digital images of DNA bands were prepared by using a FAS-III system (Toyobo, Osaka, Japan), and the band intensities were measured at the steps of template dilution that resulted in a linear relationship between the initial cDNA input and the PCR product by using Scion Image (Scion Corp., Frederick, Md.) according to the manufacturer's recommendations. The relative mRNA levels of iNOS, cytokines, and chemokines were then measured by PCR by using the specific primers and serial fivefold-dilution series of cDNA templates, which had been normalized with reference to the amount of GAPDH cDNA, followed by the image analyses described above.
Measurement of NO production and CXCL10 release.
Synthesis of NO was measured as the stable end product of nitrite (NO2−) in culture supernatants with the Griess reagent (25). RAW264 cells were plated at a density of 4 × 105 per well in 24-well culture dishes and incubated in virus suspensions containing 4 × 106 FFU of RV at 37°C for 2 h. The cells were washed twice and further incubated in culture medium for 24 h, and then 100 μl (each) of the culture fluids was allowed to react with an equal volume of Griess reagent (1% sulfanilamide, 0.1% naphthylethylenediamine dihydrochloride, and 2.5% phosphoric acid) at room temperature for 10 min. The absorbances of assay samples were measured spectrophotometrically at a wavelength of 550 nm. Nitrite concentrations were calculated by comparison with a standard curve derived from the reaction of NaNO2 under assay conditions. For the determination of CXCL10 levels in culture supernatants, cells were incubated in the presence or absence of RV inoculum (10 FFU/cell), washed, and overlaid with culture medium as described above. After incubation for the appropriate times, the culture fluids were separated, and the levels of CXCL10 protein content were determined by using an enzyme-linked immunosorbent assay kit (Quantikine mouse IP-10 immunoassay; R&D Systems Inc., Minneapolis, Minn.) according to the manufacturer's instructions. As to the inhibition of vacuolar pH acidification, cells were preincubated in the presence of ammonium chloride or BA1 for 2 h at 37°C at concentrations that did not cause any cytotoxicity, and then the cultures were continued in culture media including these drugs. At 24 h after incubation, the concentrations of nitrite and CXCL10 in the culture fluids were determined as described above.
Western blot analysis.
The activation of MAPK pathways in macrophages was measured by immunoblotting using antibodies against phosphorylated forms of ERK1/2 and p38 MAPK as described elsewhere (44). Cells were incubated in test media with or without RV (10 FFU/cell), which had been left untreated or inactivated with UV irradiation, for 2 h at 37°C, washed, and overlaid with test media. Alternatively, cells were incubated in the presence of LPS (50 ng/ml) for 2 h. As to the inhibition of vacuolar pH acidification, cells were preincubated for 2 h at 37°C with DMEM containing ammonium chloride or BA1 at concentrations that did not cause any cytotoxicity just before stimulation, and then the cultures were continued in test media including these drugs. At the appropriate time points, the cells were washed with PBS and lysed directly with lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, protease inhibitors (Complete Mini; Roche, Penzberg, Germany), and phosphatase inhibitor cocktails (Sigma). Extracts were clarified by centrifugation at 12,000 × g for 10 min at 4°C. Each sample, containing 15 μg of proteins, was separated under reducing conditions in 0.4% sodium dodecyl sulfate (SDS)-12% polyacrylamide gels, and was transferred to polyvinylidene difluoride membranes (Millipore) as described previously (65, 66). The blots were blocked for 1 h with 2% BSA in Tris-buffered saline (20 mM Tris-HCl, pH 7.4, 0.15 M NaCl) containing 0.05% Triton X-100 (TBST) and incubated with the primary antibodies in TBST. The proteins were reacted with the horseradish peroxidase-linked secondary antibodies and visualized with enhanced-chemiluminescence Western blotting detection reagent (Amersham Biosciences, Piscataway, N.J.) and photographed using an enhanced-chemiluminescence minicamera (Amersham Biosciences).
Inhibition of MAPK pathways.
Inhibition of MAPK activation in macrophages was carried out as described elsewhere (83). Briefly, cells were incubated for 1 h at 37°C in test media containing PD98059, U0126, U0124 (10 μM each), or SB202190 (20 μM) prior to the experiment and were subjected to the above-mentioned analyses. Under these conditions, the MAPK inhibitors did not induce any cytotoxic effects as judged by a dye exclusion assay using trypan blue (63).
Statistics.
The significance of differences between groups was statistically determined by Student's t test.
RESULTS
Viral replication and macrophage proliferation following RV inoculation.
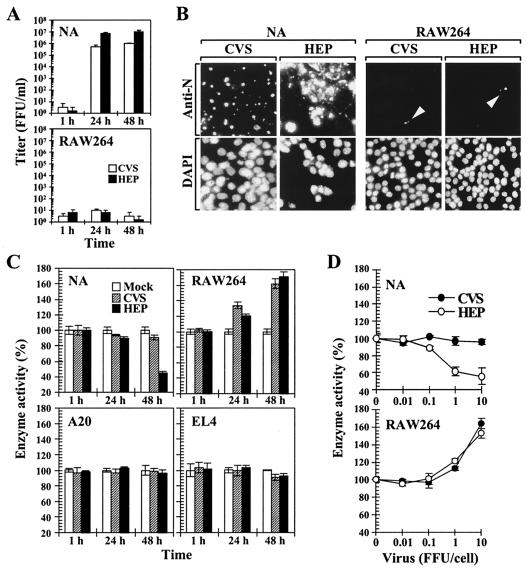
In the present study, we sought to systemically analyze the macrophage responses against RV by using RAW264 cells, in which the cellular signaling mechanisms in response to various stimuli have been extensively characterized (13, 59, 92). Previous studies demonstrated that several RV strains are able to infect macrophage-like cells and that viral multiplication in macrophages depends on the strains of viruses and the types of macrophages (41, 77). We initially examined the growth characteristics of pathogenic CVS and nonpathogenic HEP strains in RAW264 cells. NA and RAW264 cells were inoculated with CVS and HEP at a multiplicity of infection (MOI) of 10 (10 FFU/cell), and the progeny viruses in culture supernatants were titrated at the appropriate time points (Fig. 1A). In neuronal NA cells, both viruses exhibited a marked increase in virus titers, reaching a near-plateau level at 24 h postinfection. In contrast, the onset of RV replication in RAW264 cells was not observed even 48 h after inoculation. To further examine the defective growth of these RV strains in macrophages, the expression patterns of viral N protein, which accumulates in the cytoplasm of RV-infected cells, were assessed by indirect immunofluorescence assay. Cells were inoculated with CVS and HEP viruses (10 FFU/cell), and the viral N protein and cell nuclei were stained with anti-N MAbs and the DNA dye DAPI, respectively, 48 h after inoculation (Fig. 1B). In NA cells, strong signals of N proteins were observed in most of the cells. On the other hand, the majority of the RV-inoculated RAW264 cells did not exhibit any signs of N protein expression, and the fluorescent signals of the N proteins, which were much weaker than those observed in NA cells, were seen in <1% of the total cell population (Fig. 1B). These results indicate that the viral replication and protein expression of both CVS and HEP strains are strictly impaired in RAW264 macrophages.
FIG. 1.
Viral replication and cell proliferation following RV inoculation. (A) Multiplication of RV in neuroblastoma (NA) and macrophage (RAW264) cell lines. Cells were inoculated with the pathogenic CVS or attenuated HEP strain of RV (10 FFU/cell). At the indicated time points, progeny viruses in culture supernatant fluids were titrated on NA cell monolayers. The values are averages of six independent experiments. The error bars indicate standard deviations. (B) Indirect immunofluorescence assay of viral N protein in RV-inoculated cells. Cells were incubated with CVS and HEP viruses at an MOI of 10 for 2 h and then further incubated. After 48 h of incubation, the cells were fixed and stained for the viral N proteins (top) and cell nuclei (bottom) by using FITC-coupled anti-N MAbs and DAPI, respectively. Samples were examined by using a fluorescent microscope. The experiments were repeated three times, and representative areas of each culture are shown. The arrowheads point to the accumulation of viral N proteins in cells. Magnification, ×100. (C) Effect of RV on cell proliferation. NA, T-cell derived EL4, B-cell-derived A20, and RAW264 cells were inoculated with RV at an MOI of 10. At different times after incubation, the activities of mitochondrial succinate dehydrogenase in each culture were measured by MTT assay as described in the text. (D) Dose-dependent stimulation of cell proliferation by RV. Cells were inoculated with various doses of RV as indicated. At 48 h after incubation, the enzyme activities in each sample were determined by MTT assay. The percentages of enzyme activity were calculated with reference to the values for the mock-inoculated cells. The data are averages of six independent experiments, and the error bars indicate standard deviations.
In order to elucidate the possibility that RV affects the characteristics of macrophages, we next examined the proliferation of macrophages after RV inoculation by measuring the activity of the mitochondrial enzyme succinate dehydrogenase. To compare the proliferation of macrophages with that of other cell types, NA, A20, EL4, and RAW264 cells were incubated with RV (10 FFU/cell), and the enzyme activities of each sample were determined by using an MTT assay at the appropriate time points. When NA cells were inoculated with CVS viruses, the enzyme activity was slightly decreased, while the HEP-infected NA cells showed marked reduction in enzyme activity (Fig. 1C). In the B-cell line A20, the enzyme activity was not affected by RV inoculation compared to that in the mock-inoculated cells (Fig. 1C). In the T-cell line EL4, the mitochondrial activity was slightly decreased (by ∼10%) compared to mock-inoculated cells (P < 0.005) 48 h after inoculation. Unlike other cell types tested, RAW264 macrophages exhibited a significant increase in enzyme activity compared to the mock-treated control 24 and 48 h after inoculation (P < 0.001) (Fig. 1C). As shown in Fig. 1D, when macrophages were treated with increasing amounts of RV, their enzyme activities were promoted in a dose-dependent manner. The increase in enzyme activity of the RV-stimulated macrophages was proportional to the number of cells and was blocked by the neutralization of viruses with anti-RV antiserum (data not shown). These results indicate that RV induces the proliferation of RAW264 macrophages, although viral replication is arrested in the cells.
RV facilitates NO production in macrophages.
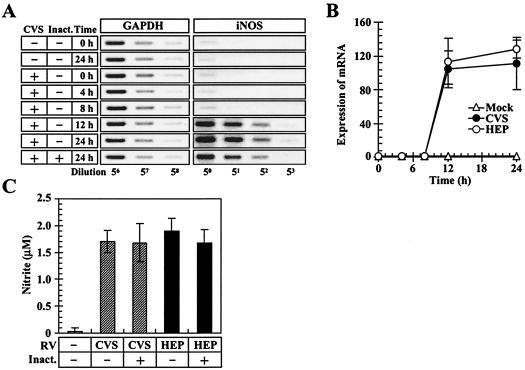
Based on the above-mentioned results, we hypothesized that some cellular functions of macrophages might be activated in response to RV. To assess this possibility, we examined NO production in the RV-stimulated cells as a major characteristic feature of macrophage activation (Fig. 2). NO is a short-lived diffusible molecule, which is primarily released from the activated macrophages, and its generation is catalyzed by iNOS (60). In the experiments shown in Fig. 2A, RAW264 cells were preincubated with or without CVS viruses (10 FFU/cell) for 2 h, and the expression of iNOS mRNA was monitored by RT-PCR methods. Prior to the measurement of iNOS mRNA, the relative amount of total cDNA in each sample was normalized with reference to the expression levels of the housekeeping gene for GAPDH. In the mock-stimulated macrophages, low levels of iNOS mRNA were expressed and did not vary during the course of incubation tested. In contrast, when macrophages were stimulated with CVS, the strong signals of the amplified products were detected at highly diluted concentrations of PCR templates compared to those of mock-stimulated cells 12 and 24 h after incubation, and an increase in iNOS transcription was also observed for the cells which had been stimulated with the UV-inactivated virions (Fig. 2A). In the experiments shown in Fig. 2B, the relative amounts of iNOS mRNA in macrophages were monitored during the course of incubation. In the RV-stimulated macrophages, the expression levels of iNOS mRNA steeply increased between 8 and 12 h after incubation and were >100-fold higher than those in the mock-stimulated cells at 12 h postinoculation (P < 0.001). At 12 and 24 h after incubation, the levels of iNOS transcription in the RV-stimulated macrophages were slightly lower than those in the HEP-stimulated cells, but the difference was not statistically significant. Figure 2C shows the biosynthesis of NO in macrophages. Cells were unstimulated or stimulated with infectious viruses or UV-inactivated virions, and the concentrations of nitrite (NO2−), a stable end product of NO, in culture fluids were measured 24 h after incubation. The nitrite concentrations in culture supernatants of macrophages stimulated with the infectious CVS and HEP viruses were 1.68 and 1.89 μM, respectively, and were significantly higher than those in unstimulated macrophages (0.034 μM; P < 0.001) (Fig. 3C). In the macrophages stimulated with UV-inactivated virions, NO production was slightly decreased compared to that in the infectious-RV-stimulated cells, but the difference was not statistically significant (Fig. 3C). Taken together, these results suggest that both virulent and avirulent RV viruses are able to stimulate the expression of the iNOS gene, resulting in an efficient generation of NO.
FIG. 2.
RV stimulates iNOS expression and NO biosynthesis in macrophages. (A) Expression of iNOS gene in macrophages. RAW264 cells were preincubated with (+) the infectious and UV-inactivated (Inact.) virions of CVS for 2 h, washed, and further incubated. At the indicated time points, total RNAs were extracted from each culture, and the first-strand cDNAs were generated. The relative amount of total cDNA from each sample was normalized with reference to GAPDH cDNAs, and PCR amplifications for GAPDH and iNOS sequences were performed by using serially fivefold-diluted cDNA preparations as described in the text. The PCR products were electrophoresed and stained. The data are from one of three independent experiments with similar results. (B) Measurement of relative amounts of iNOS mRNA in macrophages. Cells were stimulated with RV (10 FFU/cell) for 2 h, and immediately thereafter (0 h) and at the indicated time points, RT-PCR analyses specific for iNOS mRNA were carried out as described for panel A. The band intensity of each PCR product was measured by using densitometry, and the results are shown as the n-fold increase in the amount of iNOS mRNA with reference to the mRNA levels at 0 h after incubation. Mean values and standard deviations from the results of three independent experiments are shown. (C) NO production in RV-stimulated macrophages. Cells were mock stimulated or stimulated with (+) infectious and UV-inactivated virions, and the concentrations of nitrite were measured as described in the text. The data are means of three independent experiments, and the error bars indicate standard deviations.
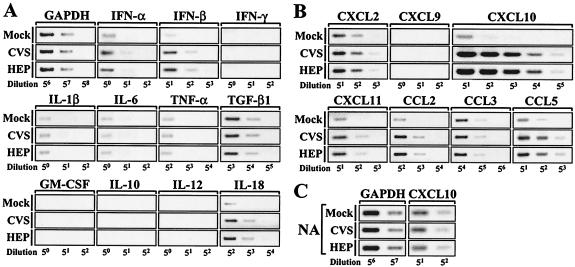
FIG. 3.
Transcription profiles of cytokines and chemokines in RV-stimulated cells. (A and B) RAW264 macrophages were incubated in the absence or presence of CVS or HEP virus (10 FFU/cell) for 2 h, washed, and further incubated for 24 h. Total RNAs were extracted from each culture, and the mRNAs of cytokines (A) and chemokines (B) were examined by using RT-PCR analyses as described in the legend to Fig. 2. (C) NA cells were inoculated with CVS at an MOI of 10, and RT-PCR analyses of CXCL10 mRNA were performed 24 h after infection as described in the text. The data are from one of three independent experiments with similar results.
Expression of cytokine and chemokine genes induced by RV.
The enhanced iNOS gene expression and NO production in the RV-stimulated cells imply that RV activates the antiviral cellular response. To examine whether RV affects the expression patterns of cytokines and chemokines, RAW264 cells were left unstimulated or stimulated with RV (10 FFU/cell), and the amounts of cytokine and chemokine mRNAs were examined by RT-PCR 24 h after incubation (Fig. 3). As shown in Fig. 3A, upon RV stimulation, the band intensities for antiviral cytokines (alpha and beta interferons [IFN-α and -β]) and IL-18, a cytokine involved in T helper cell type 1 (Th1) activation (40), were slightly increased, while the transcription patterns for IFN-γ, IL-1β, IL-6, tumor necrosis factor alpha (TNF-α), transforming growth factor β1, granulocyte-macrophage colony-stimulating factor, IL-10, and IL-12 remained unchanged.
Chemokines are low-molecular-weight and structurally related molecules that are involved in chemotaxis and activation of leukocytes at the site of inflammation, and these proteins can be divided into four subfamilies, designated C, CC, CXC, and CX3C chemokine ligands, based on the positions of their cysteine residues (94). Figure 3B shows the transcription patterns of two major chemokine subfamilies, CXC (CXCL2, -9, -10, and -11) and CC (CCL2, -3, and -5) chemokine ligands, in macrophages. Of the chemokines tested, the gene expression of CXCL10/IP-10 was notably induced in the RV-stimulated cells compared to that in the mock-stimulated cells. We also observed slight increases in band intensities for CXCL11/I-TAC (interferon-inducible T-cell chemoattractant), CCL2/MCP-1 (monocyte chemoattractant protein 1), and CCL5/RANTES (regulated upon activation, normal T-cell expressed and secreted). As shown in Fig. 3C, the induction of CXCL10 transcription was not seen in neuronal NA cells, which are permissive for RV infection. To assess the gene expression profiles more quantitatively, RT-PCR analyses were independently repeated three times, and the relative amounts of mRNAs were measured by using densitometry as described above (Table 1). In NA cells, the changes in mRNA expression of cytokines and chemokines were from 0.3- to 1.9-fold compared to the mock-infected control. At this time point, the gene expression patterns in the HEP-infected cells were similar to those in the CVS-infected cells (data not shown). When RAW264 macrophages were stimulated with RV, the relative amounts of CXCL10 mRNA were >200-fold higher than that in the mock-stimulated cells (P < 0.001). In contrast to the strong induction of CXCL10 expression in the RV-stimulated macrophages, the increases in mRNA levels for other cytokines and chemokines were from 1.1- to 7.0-fold. Thus, these results indicate that the expression of the CXCL10 gene is selectively potentiated in the RV-stimulated macrophages, whereas RV does not induce dramatic increases in the expression of other types of cytokines and chemokines.
TABLE 1.
Cytokine and chemokine mRNA expression in RV-stimulated cellsa
| Target | Expression of mRNA (n-fold)b
|
||
|---|---|---|---|
| NA | RAW264
|
||
| CVS | CVS | HEP | |
| GAPDH | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.0 |
| IFN-α | 1.6 ± 0.1 | 3.4 ± 0.7 | 3.2 ± 0.5 |
| IFN-β | 1.1 ± 0.1 | 4.6 ± 1.2 | 5.3 ± 1.3 |
| IFN-γ | − | − | − |
| IL-1β | − | 1.0 ± 0.1 | 1.1 ± 0.2 |
| IL-6 | 1.9 ± 0.2 | − | − |
| TNF-α | − | 1.2 ± 0.2 | 1.0 ± 0.2 |
| GM-CSF | − | − | − |
| TGF-β1 | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.0 ± 0.1 |
| IL-10 | − | − | − |
| IL-12 | 1.0 ± 0.3 | − | − |
| IL-18 | − | 4.1 ± 0.2 | 5.0 ± 1.9 |
| CXCL2 | 1.6 ± 0.2 | 2.0 ± 0.1 | 3.3 ± 0.4 |
| CXCL9 | 1.1 ± 0.4 | − | − |
| CXCL10 | 1.0 ± 0.2 | 222.1 ± 37.4 | 240.2 ± 46.3 |
| CXCL11 | − | 4.1 ± 0.7 | 3.5 ± 0.4 |
| CCL2 | 0.9 ± 0.1 | 6.2 ± 0.7 | 3.8 ± 0.8 |
| CCL3 | − | 3.5 ± 0.5 | 1.9 ± 0.0 |
| CCL5 | 0.3 ± 0.1 | 6.7 ± 0.6 | 7.0 ± 0.8 |
Relative amounts of mRNA transcripts in RV-inoculated cells were measured by using RT-PCR and densitometry and are shown as n-fold increase with respect to mock-inoculated controls, as described in the text. Mean values and standard deviations from three independent experiments are shown.
−, PCR product was not detected.
CXCL10 release from RV-stimulated macrophages.
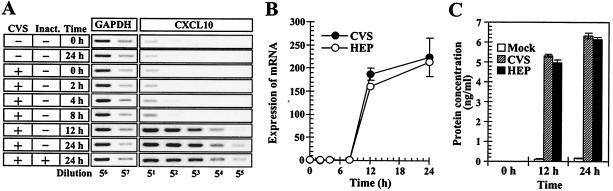
Analyses for gene expression of multiple cytokine and chemokine genes have revealed that RV significantly stimulates CXCL10 transcription in macrophages. To examine the onset of CXCL10 expression, RAW264 cells were stimulated with RV, and the expression of CXCL10 mRNA was monitored by RT-PCR during the course of incubation. In the RV-stimulated macrophages, the induction of CXCL10 transcription was readily observed 12 h after stimulation, and a similar transcription pattern was also observed in the cells stimulated with the UV-inactivated virions (Fig. 4A). In the experiments shown in Fig. 4B, the relative amounts of CXCL10 mRNAs were measured by densitometry. Upon RV stimulation, the CXCL10 mRNAs rapidly accumulated in the cells between 8 and 12 h, and they went on increasing gradually up to 24 h after incubation (Fig. 4B). To determine whether the enhanced expression of CXCL10 mRNAs in the RV-stimulated macrophages correlated with protein production, we measured the amounts of CXCL10 proteins in culture supernatants by enzyme-linked immunosorbent assay. As shown in Fig. 4C, the RV-stimulated macrophages exhibited a marked increase in the CXCL10 protein content in culture fluids at 12 and 24 h, while only small amounts of CXCL10 were released from the unstimulated cells, suggesting that enhanced gene expression of CXCL10 in RV-stimulated macrophages leads to the production and release of CXCL10.
FIG. 4.
RV stimulates gene expression and protein production of CXCL10 in macrophages. (A) Time course of CXCL10 transcription in macrophages. Cells were incubated with (+) or without (−) infectious and UV-inactivated (Inact.) CVS virions for 2 h. After additional incubation for the indicated times, CXCL10 mRNAs from each sample were amplified by using RT-PCR as described in the text. The data are from one of three independent experiments with similar results. (B) Measurement of the relative amounts of CXCL10 mRNA in macrophages upon RV stimulation. RAW264 cells were stimulated with RV (10 FFU/cell), washed, and further incubated. Immediately thereafter (0 h) and at the time points indicated, CXCL10 mRNAs were detected by using RT-PCR, and the relative amounts of CXCL10 mRNA were determined by image analysis of the band intensity of each PCR product as described in the text. The results are shown as n-fold increase in the expression of CXCL10 mRNA with reference to the levels of CXCL10 mRNA at 0 h after incubation. Mean values and standard deviations from the results of three independent experiments are shown. (C) Protein contents of CXCL10 in culture fluids from RV-stimulated macrophages. Cells were stimulated with RV inocula (10 FFU/ml) for 2 h or unstimulated (Mock) and then incubated for the times indicated. After incubation, the culture supernatants were removed, and the CXCL10 protein content was measured by enzyme-linked immunosorbent assay. The data are averages of three independent experiments, and the error bars represent standard deviations.
RV virions trigger ERK1/2 activation in macrophages.
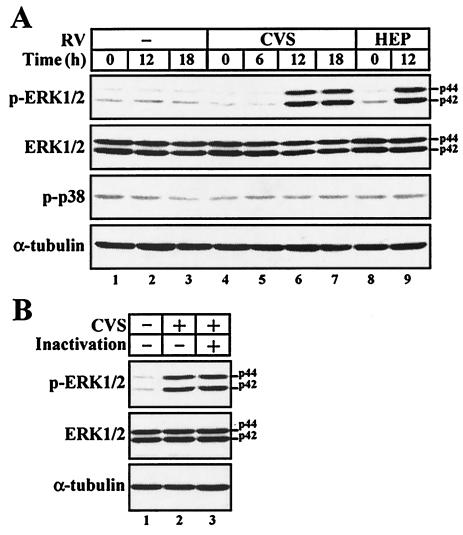
The enhanced expression of iNOS and CXCL10 genes in the RV-stimulated macrophages implies the possibility that RV virions activate the cellular signaling pathway underlying the expression of these genes. Considering the important role of the MAPK-mediated signaling pathway in host defense, we examined the activation of two types of MAPK subfamilies, ERK1/2 and p38 MAPK. To examine whether RV stimulates the MAPK pathway in macrophages, cells were mock stimulated or stimulated with RV (10 FFU/cell), and the degrees of MAPK phosphorylation were examined by Western blotting using antibodies specific for the phosphorylated forms of ERK1/2 (p-ERK1/2) and p38 MAPK (p-p38) (Fig. 5A). In order to avoid an additional effect of growth factors on ERK1/2 activation, the cells were incubated without fetal calf serum throughout the incubation periods. When macrophages were stimulated with either strain of RV, the strong signals of p-ERK1/2 were detected 12 h after incubation (Fig. 5A, lanes 6 and 9), while only low, but detectable, levels of p-ERK were seen in the mock-stimulated cells at all the time points tested (Fig. 5A, lanes 1 to 3). The increased levels of p-ERK in the RV-stimulated macrophages were not due to the enhanced production of total ERK or the difference in protein extracts loaded, as the protein levels of total ERK and α-tubulin, a major component of the cytoskeleton, in all samples were comparable. In contrast to the enhanced ERK1/2 phosphorylation, the levels of p-p38 were not changed during the course of incubation. To test whether the RV-induced phosphorylation of ERK1/2 is triggered in response to RV virions, we examined the ERK1/2 phosphorylation in macrophages stimulated with RV virions that had been inactivated by UV irradiation. As shown in Fig. 5B, the UV-inactivated virions induced the phosphorylation of ERK1/2 at a level similar to that seen with infectious RV. These results suggest that RV virions directly trigger the activation of the ERK1/2-mediated signaling pathway in macrophages and that the RV-induced activation of macrophages is independent of the p38 MAPK pathway.
FIG. 5.
RV virions activate the ERK1/2-mediated signaling pathway in macrophages. (A) Western blot analyses of ERK1/2 and p38 MAPK in RV-stimulated macrophages. RAW264 cells were stimulated with RV for 2 h or unstimulated (−), washed, and further incubated for the times indicated. Equal amounts of protein extracts from each sample (15 μg/lane) were loaded on the gel and analyzed by Western blotting using antibodies against p-ERK1/2, total ERK1/2, and p-p38. The amount of α-tubulin was also assessed to monitor the equal loading of protein extracts. (B) Cells were left unstimulated or stimulated with (+) either infectious or UV-inactivated virions of a CVS strain, and Western blot analyses of p-ERK1/2, total ERK1/2, and α-tubulin were performed as described for panel A. Similar results were obtained from three separate experiments.
Endosomal-lysosomal process is required for RV-induced activation of ERK pathway.
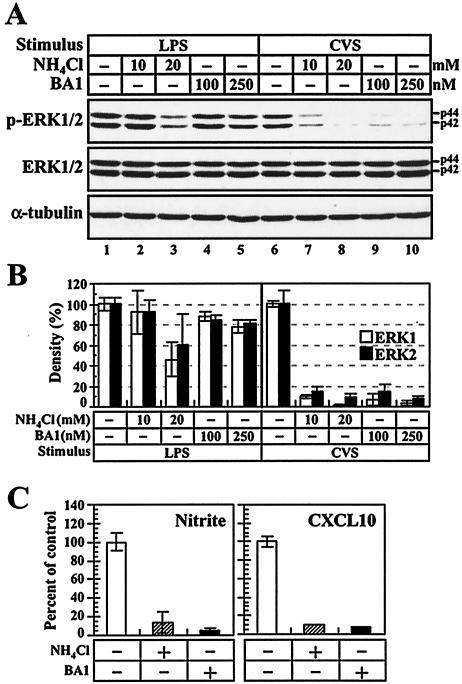
We next assessed the process by which RV virions trigger the activation of the ERK1/2-mediated signaling pathway in macrophages. Previous studies demonstrated that macrophages actively engulf RV virions in intracellular compartments, thereby destroying the exogenous particles (86). Considering the essential nature of the endosomal-lysosomal vesicles in macrophage function and activity, we hypothesized that the endosomal-lysosomal pathway might be involved in the RV-induced activation of the ERK1/2 pathway. To elucidate this possibility, we examined RV-induced ERK1/2 phosphorylation in the presence of lysosomotropic drugs, which interfere with the endosomal-lysosomal process by neutralizing the low-pH environment of endosomal-lysosomal vesicles. RAW264 macrophages were treated with a weak base, ammonium chloride (NH4Cl), and an inhibitor of vacuolar H+-ATPase, BA1 (11, 21), and then the RV-induced phosphorylation of ERK1/2 was analyzed by Western blotting. To control the nonspecific effect of these drugs on ERK1/2 phosphorylation, the LPS-induced macrophage activation, which is known to have low sensitivity to vacuolar alkalization (28, 88), was also examined. Figure 6A shows the protein levels of p-ERK1/2, total ERK1/2, and α-tubulin. In the absence of inhibitors, the band intensity of p-ERK1/2 in the RV-stimulated macrophages (lane 6) was slightly weaker than that in the LPS-stimulated cells at the concentration tested (50 ng/ml) (lane 1). In the LPS-stimulated cells, treatment with 10 mM NH4Cl (lane 2) and 100 and 250 nM BA1 (lanes 4 and 5, respectively) had little or no effect on the amount of p-ERK1/2, although phosphorylation levels of ERK1/2 were slightly reduced at 20 mM NH4Cl (lane 3). On the other hand, treatment with either drug diminished the RV-induced phosphorylation of ERK1/2 (lanes 7 to 10). In the presence of 20 mM NH4Cl or 250 nM BA1, only low, but detectable, levels of p-ERK1/2 were observed in the RV-stimulated cells (lanes 8 and 10). The decreased level of ERK1/2 phosphorylation was not due to the degradation of total ERK1/2, as the protein levels of ERK1/2 in each sample were comparable (lanes 6 to 10). As shown in Fig. 6B, the neutralization of vacuolar pH resulted in the reduction of p-ERK1/2 by >90% in the RV-stimulated macrophages compared to that in the non-drug-treated control. When the cells were treated with NH4Cl (20 mM) or BA1 (250 nM), the RV-induced production of nitrite and CXCL10 protein was significantly reduced (Fig. 6C). These results suggest that the endosomal-lysosomal process is a key event leading to ERK1/2 activation and the production of NO and CXCL10 in the RV-stimulated macrophages.
FIG. 6.
Endosomal-lysosomal process is required for RV-induced macrophage activation. (A and B) Effects of lysosomotropic agents on ERK1/2 phosphorylation in LPS- and RV-stimulated macrophages. (A) RAW264 cells, which had been treated with the indicated concentrations of NH4Cl and BA1 or untreated (−), were stimulated with either LPS (50 ng/ml) or CVS virions (10 FFU/ml) for 2 h. After additional incubation in the absence or presence of inhibitors, the cells were subjected to Western blot analyses of p-ERK1/2, total ERK1/2, and α-tubulin as described in the text. The data are from one of three individual experiments. (B) Quantitative analyses of ERK1/2 phosphorylation in LPS- and RV-stimulated macrophages. The digital images of each blot shown in panel A were prepared, and the density of each band was quantified by image analysis. The percentages of band densities of p-ERK1/2 were calculated with reference to the values for the mock-treated controls. The data are averages of three separate experiments, and the error bars indicate standard deviations. (C) Effects of lysosomotropic agents on production of nitrite and CXCL10 protein in RV-stimulated macrophages. Cells were stimulated with CVS viruses in the absence (−) or presence (+) of NH4Cl (20 mM) and BA1 (250 nM). At 24 h after stimulation, the concentrations of nitrite and CXCL10 in culture fluids were determined as described in the text. The percentages of nitrite and CXCL10 concentrations were calculated with reference to the values for the mock-treated control. The data are averages of three separate experiments, and the error bars indicate standard deviations.
RV activates iNOS and CXCL10 transcription via the MEK1/2-ERK1/2 pathway.
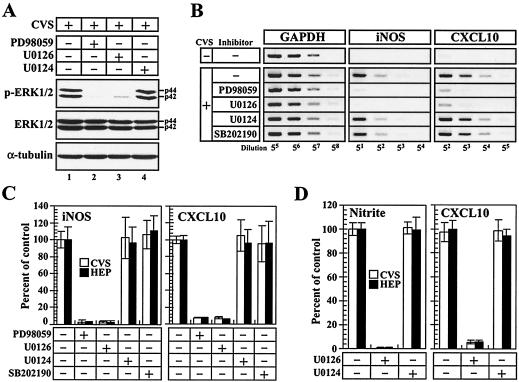
To further characterize the RV-induced activation of cellular signaling, we assessed the effects of MEK inhibitors on the phosphorylation of ERK1/2 in RV-stimulated macrophages. MEK1 and -2 are dual-specificity MAPK kinases that phosphorylate ERK1/2 (22), and their activities are suppressed by treatment with the specific inhibitors PD98059 and U0126. As shown in Fig. 7A, when macrophages were treated with PD98059 or U0126, the band densities of p-ERK1/2 were severely impaired, while the protein level of p-ERK1/2 was not affected by treatment with U0124, an inactive analogue of U0126, indicating that RV virions stimulate the ERK1/2 pathway through the activation of MEK1/2. If the RV-induced expression of the iNOS and CXCL10 genes were attributable to the activation of the MEK1/2-ERK1/2 pathway, then the inhibition of MEK activity should interfere with the expression of these genes. To assess this possibility, RAW264 cells were untreated or treated with PD98059, U0126, U0124, or SB202190 prior to RV stimulation, and the expression patterns of the iNOS and CXCL10 genes were examined by RT-PCR (Fig. 7B). When the cells were treated with the MEK1/2 inhibitors PD98059 and U0126, the RV-induced transcription of iNOS and CXCL10 was impaired, while U0124, an inactive analogue of U0126, and SB202190, a potent inhibitor of p38 MAPK, showed no effect on the RV-induced gene expression. Quantitative analyses of the gene expression revealed that the treatments with MEK inhibitors reduced the RV-induced expression of the iNOS and CXCL10 genes by ∼97 and 93%, respectively (Fig. 7C). As shown in Fig. 7D, the inhibition of MEK1/2 activities by U0126 treatment markedly reduced the RV-induced production of nitrite and the CXCL10 protein by ∼99 and 94%, respectively. These results indicate that the RV-induced transcription of iNOS and CXCL is achieved through the activation of the MEK1/2-ERK1/2 signaling pathway.
FIG. 7.
Effects of MEK1/2 inhibitors on macrophage activation in response to RV virions. (A) Phosphorylation levels of ERK1/2 in the presence of MEK1/2 inhibitors. RAW264 cells were left untreated or treated (+) with PD98059 and U0126 prior to stimulation with CVS virions (10 FFU/cell). U0124, an inactive analogue of U0126, was also used as a control to verify the specificity of U0126. At 12 h after stimulation, the cells were lysed and subjected to Western blot analyses of p-ERK1/2, total ERK1/2, and α-tubulin as described in the text. The data are from one of two individual experiments with similar results. (B and C) Effects of MEK1/2 inhibitors on expression of iNOS and CXCL10 genes. Cells were left untreated or treated with PD98059, U0126, U0124, and SB202190, a potent inhibitor of p38MAPK, prior to stimulation with RV (CVS). (B) At 12 h after stimulation, iNOS and CXCL10 mRNAs were examined by RT-PCR, and the amplified products were electrophoresed and stained. (C) The digital images of DNA patterns in panel B were prepared, the band intensities of PCR products were quantified, and the percentages of mRNA expression were calculated in comparison with the values for the untreated control. The data are averages of three independent experiments. The error bars represent standard deviations. (D) Effects of MEK1/2 inhibitor on the release of NO and CXCL10 proteins from RV-stimulated macrophages. Cells were left untreated or treated with either the MEK1/2 inhibitor U0126 or the control drug U0124 for 1 h prior to stimulation with RV (CVS; 10 FFU/cell). At 24 h after RV stimulation, the culture fluids were removed, and the concentrations of nitrite and CXCL10 were measured as described in the text. The percentages of nitrite and CXCL10 contents were calculated with reference to the values for the untreated control. Mean values and standard deviations from three individual experiments are shown.
DISCUSSION
Macrophages are resident immune effector cells within various tissues and are hence likely to encounter infectious agents at very early stages of infection, as well as at later stages, when monocyte-derived macrophages are recruited to the site of infection. The antiviral macrophage response is predominantly triggered by the replication of viruses that target macrophages (5, 9, 56). A well-known virus-induced stimulus is double-stranded (ds) RNA derived from viral RNAs containing ds structure and dsRNA formed during viral replication (34). Based on the results obtained in the present study, and on previous reports, it has been shown that macrophages have extremely low susceptibility to RV infection, and thus, we hypothesized that the mode of macrophage activation against RV may differ from that against other macrophage-tropic viruses.
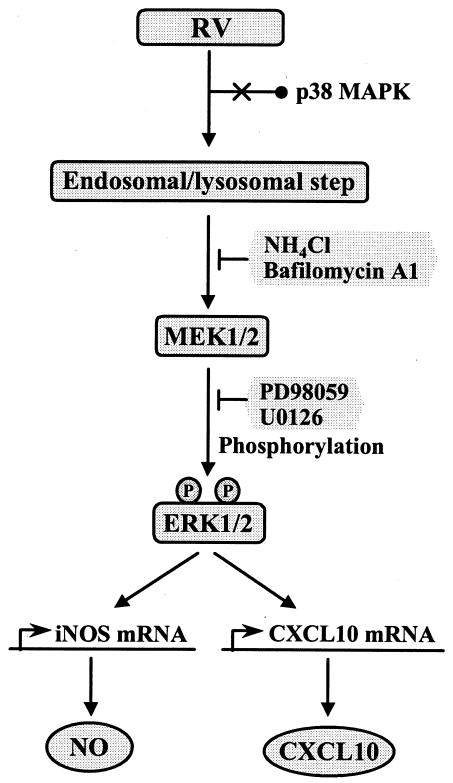
In the present study, we systemically characterized the RV-induced activation of macrophages. On the basis of our data, we provide a model for the cellular signaling events underlying macrophage activation in response to RV (Fig. 8). We suggest here that the activation of ERK1/2-dependent signaling is a key process leading to the selective induction of iNOS and CXCL10 transcription. ERK1/2 phosphorylation was triggered by stimulation with inactivated virions, as well as infectious virions, indicating that virus replication is not required for this process. Furthermore, the data obtained here demonstrate that the inhibition of endosomal-lysosomal functions results in diminished activation of the ERK1/2 pathway upon RV stimulation, implying that endocytic clearance of RV virions in the acidic environment of intracellular vesicles is required for the RV-induced macrophage response. We also observed that RV virions stimulate the proliferation of macrophages. Since ERK1/2-mediated cellular signaling plays an important role in mitogenesis (17), it is reasonable that the enhanced proliferation of the RV-stimulated macrophages might be a consequence of activated ERK1/2 activation. Indeed, the RV-induced proliferation of macrophages was severely impaired in the presence of MEK1/2 inhibitors (data not shown).
FIG. 8.
Schematic model for the induction of NO and CXCL10 production in macrophages in response to RV virions. The model is based on the data in the present study and previous publications (see the text for appropriate references). RV virions may be taken up by macrophages via endocytosis, and this process precedes the RV-induced activation of the cellular signaling mediated by the MEK1/2-ERK1/2 pathway. The acidic environment of endosomal-lysosomal compartments is essential for inducing ERK1/2 phosphorylation, which is inhibited by neutralization of cytoplasmic vesicles with lysosomotropic drugs, NH4Cl, and BA1. Phosphorylated forms of ERK1/2 promote the expression of the iNOS and CXCL10 genes, leading to the induction of NO and CXCL10 production through the activation of MEK1/2, which is inhibited by specific inhibitors, PD98059 and U0126. However, RV stimulation has little or no effect on the activity of p38 MAPK, a member of the stress-activated MAPKs.
The striking feature of the RV-induced macrophage response is the selective induction of gene expression. When macrophages were stimulated with RV virions, the transcription of iNOS and CXCL10 genes was markedly induced, whereas RV stimulation had little or no effect on the expression of other types of chemokines and cytokines, including proinflammatory (IL-1β, IL-6, and TNF-α) and antiviral (IFN-α, -β, and -γ) cytokines. We examined the expression of these cytokines at different time points, but intensive activation of transcription was not observed (data not shown). Recently, it has been reported that vesicular stomatitis virus, a rhabdovirus, productively replicates in monocytes and induces the expression of CXCL10 and CCL2 without affecting the production of proinflammatory cytokines other chemokines (12). Thus, the mode of CXCL10 expression in the RV-stimulated macrophages seems to be similar to that induced by other rhabdoviruses, although the RV-induced chemokine expression is more selective in that the activation of CCL2 transcription is much weaker than that observed for CXCL10 expression.
As a notable feature of the RV-stimulated macrophages, we found that RV virions are capable of inducing iNOS gene expression and NO production. NO, a free radical species that is predominantly produced by activated macrophages, exhibits an inhibitory effect on virus replication, as well as cytotoxicity to the infected cells (38, 78). It has also been demonstrated that mice with a disrupted iNOS gene exhibit greater mortality rates and reduced viral clearance compared to wild-type mice following infection with various kinds of viruses (78). Thus, the RV-induced production of NO in macrophages can be considered one of the important factors in innate immunity, especially in controlling the early stage of viral replication at the peripheral sites of virus infection. However, several studies indicate that NO production in the CNS during RV infection inversely correlates with the neuropathological process. It was shown that the increased activity of iNOS leads to tissue damage within the CNS in RV-infected animals, thereby increasing the severity of clinical signs (1, 31, 42, 87). Based on these data, and on evidence that the infiltrating monocyte-macrophage cell types are detected in the RV-invaded CNS (53), it is likely that the RV-induced NO production by the macrophage lineage cells might be responsible for the neuropathogenesis of RV infection.
When examining the expression profiles of multiple cytokine and chemokine genes, we found that the gene expression and protein secretion of CXCL10 were dramatically induced in the RV-stimulated macrophages. CXCL10, CXCL9, and CXCL11 are related members of the IFN-inducible CXC chemokines, which are produced in response to cytokines such as IFNs and TNF-α, and they interact with a common chemokine receptor, CXCR3 (94). Interestingly, the expression patterns of other IFN-inducible chemokines, CXCL9 and CXCL11, were not affected, or were less affected, by the RV stimulation of macrophages than was CXCL10 expression. These findings suggest that RV-induced CXCL10 expression is triggered through a mechanism that is distinct from that regulating the expression of other IFN-inducible chemokines. A CXCL10 receptor, CXCR3, is principally expressed on T cells of the Th1 phenotype and plays an essential role in the recruitment of Th1 cells to the peripheral sites of infection, where they control the cell-mediated immune response by secreting IFN-γ and lymphotoxin (10, 49). Thus, the interaction between CXCL10 and CXCR3 plays a pivotal role in Th1-dependent cellular immunity (35). From these lines of evidence, it is possible that the RV-induced expression of CXCL10 at the local sites contributes to the Th1-dependent response that leads to the strong cell-mediated immunity against attenuated RV strains and RV-based vectors (57, 89, 90).
As for the cellular mechanism responsible for the transcription of iNOS and CXCL10, our findings suggest that the ERK1/2 signaling pathway is a cardinal mediator of RV-induced gene expression. Some groups of viruses, including murine hepatitis virus strain 3, visna virus, and the D variant of encephalomyocarditis virus, as well as virion components of human immunodeficiency virus, have been shown to induce ERK1/2 activation in monocytes and macrophages (3, 5, 6, 9, 30, 56, 75). However, most of them simultaneously stimulate p38 MAPK, a stress-activated MAPK (18, 30, 56). Our results obtained in the present study are unique in that the RV-induced macrophage response is not accompanied by p38 MAPK activation. This observation suggests that the preferential stimulation of ERK1/2, but not of p38 MAPK, might contribute to the selective activation of antiviral cellular function without causing an excessive proinflammatory response.
Based on previous reports (41, 77, 86), it is likely that RV virions are engulfed in endosomal-lysosomal vesicles in macrophages, in which they are destroyed in the acidic environment, resulting in defective replication of RV in macrophages. In the present study, inhibition of the acidic environment within endosomal-lysosomal vesicles significantly reduced the phosphorylation levels of ERK1/2, suggesting that the endocytic process is required for the activation of the ERK1/2 pathway in RV-stimulated macrophages. Several authors have reported that the ERK1/2-mediated signaling cascade in macrophages is activated via endocytosis of unmethylated CpG dinucleotide (CpG-ODN), a DNA motif of bacterial DNA, and by phagocytosis of fungal pathogens and apoptotic cells (28, 32, 33, 44). We believe this to be the first report of the endocytic process being required for the virus-induced activation of ERK1/2 signaling in macrophages.
Upon activation by the MEK1/2-ERK1/2 cascade, ERK1/2 mediates various kinds of biological responses involved in cell proliferation and differentiation by phosphorylating a large number of substrates, including transcription factors (17). Previous studies demonstrated that the iNOS and IP-10 genes each contain one or more cognate binding motifs for ISGF3 (IFN-stimulated gene factor 3), which consists of STAT1 (signal transducer and activator of transcription 1), STAT2, and IRF-9 (IFN regulatory factor 9), and for some kinds of transcription factors, such as IRF-3 and NF-κB (16, 19, 50, 67, 69, 70, 91). We think that activated ERK1/2 may phosphorylate these transcription factors, thereby inducing the selective expression of the iNOS and CXCL10 genes in response to RV virions.
With respect to the MAPK-dependent regulation of NO production in macrophages, recent data suggest that the induction of iNOS expression is partly mediated by the activation of the ERK1/2-dependent pathway of macrophages in response to some stimuli, such as LPS, CpG-ODN, and group IIA sPLA2 (secretory phospholipase A2), a cellular enzyme produced by activated macrophages, but not to dsRNA (4, 20, 28, 51). However, it remains uncertain whether the virus-induced activation of ERK1/2 in macrophages leads to the induction of iNOS expression. As to CXCL10 expression, a previous report indicates that adenovirus entry into epithelial cells results in the induction of CXCL10 via simultaneous activation of ERK1/2 and p38 MAPK (83), but the ERK1/2-dependent mechanism regulating CXCL10 expression in immunocytes, including macrophages, is totally unknown. Therefore, our data constitute the evidence that the virus recognition of macrophages selectively triggers the production of NO and CXCL10 through the activation of a cellular signaling pathway mediated by ERK1/2, but not p38, thereby regulating antiviral cellular functions without causing excessive proinflammatory responses.
Acknowledgments
We are grateful to N. Inoue for helpful discussions and encouragement and to Y. Shoji for providing technical assistance.
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan and a Research on Emerging and Reemerging Infectious Diseases grant from the Ministry of Health, Labor and Welfare of Japan.
REFERENCES
- 1.Akaike, T., E. Weihe, M. Schaefer, Z. F. Fu, Y. M. Zheng, W. Vogel, H. Schmidt, H. Koprowski, and B. Dietzschold. 1995. Effect of neurotropic virus infection on neuronal and inducible nitric oxide synthase activity in rat brain. J. Neurovirol. 1:118-125. [DOI] [PubMed] [Google Scholar]
- 2.Astoul, E., M. Lafage, and M. Lafon. 1996. Rabies superantigen as a Vβ T-dependent adjuvant. J. Exp. Med. 183:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Badou, A., Y. Bennasser, M. Moreau, C. Leclerc, M. Benkirane, and E. Bahraoui. 2000. Tat protein of human immunodeficiency virus type 1 induces interleukin-10 in human peripheral blood monocytes: implication of protein kinase C-dependent pathway. J. Virol. 74:10551-10562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baek, S. H., J. H. Lim, D. W. Park, S. Y. Kim, Y. H. Lee, J. R. Kim, and J. H. Kim. 2001. Group IIA secretory phospholipase A2 stimulates inducible nitric oxide synthase expression via ERK and NF-κB in macrophages. Eur. J. Immunol. 31:2709-2717. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee, S., K. Narayanan, T. Mizutani, and S. Makino. 2002. Murine coronavirus replication-induced p38 mitogen-activated protein kinase activation promotes interleukin-6 production and virus replication in cultured cells. J. Virol. 76:5937-5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber, S. A., L. Bruett, B. R. Douglass, D. S. Herbst, M. C. Zink, and J. E. Clements. 2002. Visna virus-induced activation of MAPK is required for virus replication and correlates with virus-induced neuropathology. J. Virol. 76:817-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barchet, W., M. Cella, B. Odermatt, C. Asselin-Paturel, M. Colonna, and U. Kalinke. 2002. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 195:507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bidani, A., and T. A. Hemming. 1995. Effects of bafilomycin A1 on functional capabilities of LPS-activated macrophages. J. Leukoc. Biol. 57:275-281. [DOI] [PubMed] [Google Scholar]
- 9.Biggs, T. E., S. J. Cooke, C. H. Barton, M. P. Harris, K. Saksela, and D. A. Mann. 1999. Induction of activator protein 1 (AP-1) in macrophages by human immunodeficiency virus type-1 NEF is a cell-type-specific response that requires both hck and MAPK signaling events. J. Mol. Biol. 290:21-35. [DOI] [PubMed] [Google Scholar]
- 10.Bonecchi, R., G. Bianchi, P. P. Bordignon, D. D'Ambrosio, R. Lang, A. Borsatti, S. Sozzani, P. Allavena, P. A. Gray, A. Mantovani, and F. Sinigaglia. 1998. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 187:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchmuller-Rouiller, Y., S. B. Corradin, J. Smith, and J. Mauel. 1994. Effect of increasing intravesicular pH on nitrite production and leishmanicidal activity of activated macrophages. Biochem. J. 301:243-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussfeld, D., M. Nain, P. Hofmann, D. Gemsa, and H. Sprenger. 2000. Selective induction of the monocyte-attracting chemokines MCP-1 and IP-10 in vesicular stomatitis virus-infected human monocytes. J. Interferon Cytokine Res. 20:615-621. [DOI] [PubMed] [Google Scholar]
- 13.Caivano, M. 1998. Role of MAP kinase cascades in inducing arginine transporters and nitric oxide synthetase in RAW264 macrophages. FEBS Lett. 429:249-253. [DOI] [PubMed] [Google Scholar]
- 14.Charlton, K. M., and G. A. Casey. 1979. Experimental rabies in skunks: immunofluorescence light and electron microscopic studies. Lab. Investig. 41:36-44. [PubMed] [Google Scholar]
- 15.Claassen, I. J., A. D. Osterhaus, and E. Claassen. 1995. Antigen detection in vivo after immunization with different presentation forms of rabies virus antigen: involvement of marginal metallophilic macrophages in the uptake of immune-stimulating complexes. Eur. J. Immunol. 25:1446-1452. [DOI] [PubMed] [Google Scholar]
- 16.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis, R. J. 1993. The mitogen-activated protein kinase signal transduction pathway. J. Biol. Chem. 268:14553-14556. [PubMed] [Google Scholar]
- 18.Del Corno, M., Q. H. Liu, D. Schols, E. de Clercq, S. Gessani, B. D. Freedman, and R. G. Collman. 2001. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 98:2909-2916. [DOI] [PubMed] [Google Scholar]
- 19.Doyle, S., S. Vaidya, R. O'Connell, H. Dadgostar, P. Dempsey, T. Wu, G. Rao, R. Sun, M. Haberland, R. Modlin, and G. Cheng. 2002. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity 17:251-263. [DOI] [PubMed] [Google Scholar]
- 20.Feng, G. J., H. S. Goodridge, M. M. Harnett, X. Q. Wei, A. V. Nikolaev, A. P. Higson, and F. Y. Liew. 1999. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages: Leishmania phosphoglycans subvert macrophage IL-12 production by targeting ERK MAP kinase. J. Immunol. 163:6403-6412. [PubMed] [Google Scholar]
- 21.Gagliardi, S., M. Rees, and C. Farina. 1999. Chemistry and structure activity relationships of bafilomycin A1, a potent and selective inhibitor of the vacuolar H+-ATPase. Curr. Med. Chem. 6:1197-1212. [PubMed] [Google Scholar]
- 22.Garrington, T. P., and G. L. Johnson. 1999. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol. 11:211-218. [DOI] [PubMed] [Google Scholar]
- 23.Geisow, M. J., P. D'arcy Hart, and M. R. Young. 1981. Temporal changes of lysosome and phagosome pH during phagolysosome formation in macrophages: studies by fluorescence microscopy. J. Cell Biol. 89:645-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass, W. G., H. F. Rosenberg, and P. M. Murphy. 2003. Chemokine regulation of inflammation during acute viral infection. Curr. Opin. Allergy Clin. Immunol. 3:467-473. [DOI] [PubMed] [Google Scholar]
- 25.Green, L. C., D. A. Wagner, J. Glogowski, P. L. Skipper, J. S. Wishnok, and S. R. Tannenbaum. 1982. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 126:131-138. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto, M., and K. Yoshino. 1976. Alteration of the in vitro host range of rabies virus after serial chick embryo cell passage using alkaline maintenance medium. Jpn. J. Microbiol. 20:339-346. [DOI] [PubMed] [Google Scholar]
- 27.Hayashi, T., T. Kaneda, Y. Toyama, M. Kumegawa, and Y. Hakeda. 2002. Regulation of receptor activator of NF-kappa B ligand-induced osteoclastogenesis by endogenous interferon-beta (IFN-β) and suppressors of cytokine signaling (SOCS). The possible counteracting role of SOCSs in IFN-β-inhibited osteoclast formation. J. Biol. Chem. 277:27880-27886. [DOI] [PubMed] [Google Scholar]
- 28.He, H., and M. H. Kogut. 2003. CpG-ODN-induced nitric oxide production is mediated through clathrin-dependent endocytosis, endosomal maturation, and activation of PKC, MEK1/2 and p38 MAPK, and NF-κB pathways in avian macrophage cells (HD11). Cell Signal 15:911-917. [DOI] [PubMed] [Google Scholar]
- 29.Heitmeier, M. R., A. L. Scarim, and J. A. Corbett. 1998. Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-κB activation. J. Biol. Chem. 273:15301-15307. [DOI] [PubMed] [Google Scholar]
- 30.Hirasawa, K., H. S. Jun, H. S. Han, M. L. Zhang, M. D. Hollenberg, and J. W. Yoon. 1999. Prevention of encephalomyocarditis virus-induced diabetes in mice by inhibition of the tyrosine kinase signaling pathway and subsequent suppression of nitric oxide production in macrophages. J. Virol. 73:8541-8548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hooper, D. C., S. T. Ohnishi, R. Kean, Y. Numagami, B. Dietzschold, and H. Koprowski. 1995. Local nitric oxide production in viral and autoimmune diseases of the central nervous system. Proc. Natl. Acad. Sci. USA 92:5312-5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu, B., A. Punturieri, J. Todt, J. Sonstein, T. Polak, and J. L. Curtis. 2002. Recognition and phagocytosis of apoptotic T cells by resident murine tissue macrophages require multiple signal transduction events. J. Leukoc. Biol. 71:881-889. [PMC free article] [PubMed] [Google Scholar]
- 33.Ibata-Ombetta, S., T. Jouault, P. A. Trinel, and D. Poulain. 2001. Role of extracellular signal-regulated protein kinase cascade in macrophage killing of Candida albicans. J. Leukoc. Biol. 70:149-154. [PubMed] [Google Scholar]
- 34.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 35.Kafri, T., D. Morgan, T. Krahl, N. Sarvetnick, L. Sherman, and I. Verma. 1998. Cellular immune response to adenoviral vector infected cells does not require de novo viral gene expression: implications for gene therapy. Proc. Natl. Acad. Sci. USA 95:11377-11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaneda, T., T. Nojima, M. Nakagawa, A. Ogasawara, H. Kaneko, T. Sato, H. Mano, M. Kumegawa, and Y. Hakeda. 2000. Endogenous production of TGF-beta is essential for osteoclastogenesis induced by a combination of receptor activator of NF-kappa B ligand and macrophage-colony-stimulating factor. J. Immunol. 165:4254-4263. [DOI] [PubMed] [Google Scholar]
- 37.Karin, M. 1998. Mitogen-activated protein kinase cascades as regulators of stress responses. Ann. N. Y. Acad. Sci. 851:139-146. [DOI] [PubMed] [Google Scholar]
- 38.Karupiah, G., Q. W. Xie, R. M. Buller, C. Nathan, C. Duarte, and J. D. MacMicking. 1993. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science 261:1445-1448. [DOI] [PubMed] [Google Scholar]
- 39.Kawai, A. 1977. Transcriptase activity associated with rabies virion. J. Virol. 24:826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawakami, K. 2002. Interleukin-18 and host defense against infectious pathogens. J. Immunother. 25:S12-S19. [DOI] [PubMed] [Google Scholar]
- 41.King, A. A., J. J. Sands, and J. S. Porterfield. 1984. Antibody-mediated enhancement of rabies virus infection in a mouse macrophage cell line (P388D1). J. Gen. Virol. 65:1091-1093. [DOI] [PubMed] [Google Scholar]
- 42.Koprowski, H., Y. M. Zheng, E. Heber-Katz, N. Fraser, L. Rorke, Z. F. Fu, C. Hanlon, and B. Dietzschold. 1993. In vivo expression of inducible nitric oxide synthase in experimentally induced neurologic diseases. Proc. Natl. Acad. Sci. USA 90:3024-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristensson, K., D. K. Dastur, D. K. Manghani, H. Tsiang, and M. Bentivoglio. 1996. Rabies: interactions between neurons and viruses. A review of the history of Negri inclusion bodies. Neuropathol. Appl. Neurobiol. 22:179-187. [PubMed] [Google Scholar]
- 44.Kurosaka, K., M. Takahashi, and Y. Kobayashi. 2003. Activation of extracellular signal-regulated kinase 1/2 is involved in production of CXC-chemokine by macrophages during phagocytosis of late apoptotic cells. Biochem. Biophys. Res. Commun. 306:1070-1074. [DOI] [PubMed] [Google Scholar]
- 45.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix-Lamande, S., R. Mancassola, M. Naciri, and F. Laurent. 2002. Role of gamma interferon in chemokine expression in the ileum of mice and in a murine intestinal epithelial cell line after Cryptosporidium parvum infection. Infect. Immun. 70:2090-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lafon, M., D. Scott-Algara, P. N. Marche, P. A. Cazenave, and E. Jouvin-Marche. 1994. Neonatal deletion and selective expansion of mouse T cells by exposure to rabies virus nucleocapsid superantigen. J. Exp. Med. 180:1207-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lafon, M., M. Lafage, A. Martinez-Arends, R. Ramirez, F. Vuillier, D. Charron, V. Lotteau, and D. Scott-Algara. 1992. Evidence for a viral superantigen in humans. Nature 358:507-510. [DOI] [PubMed] [Google Scholar]
- 49.Lichtman, A. H., and A. K. Abbas. 1997. T-cell subsets: recruiting the right kind of help. Curr. Biol. 7:242-244. [DOI] [PubMed] [Google Scholar]
- 50.Lowenstein, C. J., E. W. Alley, P. Raval, A. M. Snowman, S. H. Snyder, S. W. Russell, and W. J. Murphy. 1993. Macrophage nitric oxide synthase gene: two upstream regions mediate induction by interferon gamma and lipopolysaccharide. Proc. Natl. Acad. Sci. USA 90:9730-9734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maggi, L. B., Jr., J. M. Moran, R. M. Buller, and J. A. Corbett. 2003. ERK activation is required for double-stranded RNA- and virus-induced interleukin-1 expression by macrophages. J. Biol. Chem. 278:16683-16689. [DOI] [PubMed] [Google Scholar]
- 52.Maggi, L. B., Jr., M. R. Heitmeier, D. Scheuner, R. J. Kaufman, R. M. Buller, and J. A. Corbett. 2000. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J. 19:3630-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marquette, C., A. M. Van Dam, P. E. Ceccaldi, P. Weber, F. Haour, and H. Tsiang. 1996. Induction of immunoreactive interleukin-1 beta and tumor necrosis factor-alpha in the brains of rabies virus infected rats. J. Neuroimmunol. 68:45-51. [DOI] [PubMed] [Google Scholar]
- 54.McGettigan, J. P., R. J. Pomerantz, C. A. Siler, P. M. McKenna, H. D. Foley, B. Dietzschold, and M. J. Schnell. 2003. Second-generation rabies virus-based vaccine vectors expressing human immunodeficiency virus type 1 gag have greatly reduced pathogenicity but are highly immunogenic. J. Virol. 77:237-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGettigan, J. P., S. Sarma, J. M. Orenstein, R. J. Pomerantz, and M. J. Schnell. 2001. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J. Virol. 75:8724-8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGilvray, I. D., Z. Lu, A. C. Wei, A. P. Dackiw, J. C. Marshall, A. Kapus, G. Levy, and O. D. Rotstein. 1998. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J. Biol. Chem. 273:32222-32229. [DOI] [PubMed] [Google Scholar]
- 57.McKenna, P. M., R. J. Pomerantz, B. Dietzschold, J. P. McGettigan, and M. J. Schnell. 2003. Covalently linked human immunodeficiency virus type 1 gp120/gp41 is stably anchored in rhabdovirus particles and exposes critical neutralizing epitopes. J. Virol. 77:12782-12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mellman, I., R. Fuchs, and A. Helenius. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55:663-700. [DOI] [PubMed] [Google Scholar]
- 59.Meyer, M., P. J. Hensbergen, E. M. van der Raaij-Helmer, G. Brandacher, R. Margreiter, C. Heufler, F. Koch, S. Narumi, E. R. Werner, R. Colvin, A. D. Luster, C. P. Tensen, and G. Werner-Felmayer. 2001. Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection. Eur. J. Immunol. 31:2521-2527. [DOI] [PubMed] [Google Scholar]
- 60.Moncada, S., R. M. Palmer, and E. A. Higgs. 1991. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 43:109-142. [PubMed] [Google Scholar]
- 61.Morimoto, K., D. C. Hooper, S. Spitsin, H. Koprowski, and B. Dietzschold. 1999. Pathogenicity of different rabies virus variants inversely correlates with apoptosis and rabies virus glycoprotein expression in infected primary neuron cultures. J. Virol. 73:510-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murphy, F. A., and S. P. Bauer. 1974. Early street rabies virus infection in striated muscle and later progression to the central nervous system. Intervirology 3:256-268. [DOI] [PubMed] [Google Scholar]
- 63.Nakamichi, K., D. Kuroki, Y. Matsumoto, and H. Otsuka. 2001. Bovine herpesvirus 1 glycoprotein G is required for prevention of apoptosis and efficient viral growth in rabbit kidney cells. Virology 279:488-498. [DOI] [PubMed] [Google Scholar]
- 64.Nakamichi, K., Y. Matsumoto, and H. Otsuka. 2002. Bovine herpesvirus 1 glycoprotein G is necessary for maintaining cell-to-cell junctional adherence among infected cells. Virology 294:22-30. [DOI] [PubMed] [Google Scholar]
- 65.Nakamichi, K., Y. Matsumoto, and H. Otsuka. 2002. Bovine herpesvirus 1 US ORF8 protein induces apoptosis in infected cells and facilitates virus egress. Virology 304:24-32. [DOI] [PubMed] [Google Scholar]
- 66.Nakamichi, K., Y. Matsumoto, Y. Tohya, and H. Otsuka. 2002. Induction of apoptosis in rabbit kidney cell under high-level expression of bovine herpesvirus 1 US ORF8 product. Intervirology 45:85-93. [DOI] [PubMed] [Google Scholar]
- 67.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 283:1150-1156. [DOI] [PubMed] [Google Scholar]
- 68.Neumann, B., N. Zantl, A. Veihelmann, K. Emmanuilidis, K. Pfeffer, C. D. Heidecke, and B. Holzmann. 1999. Mechanisms of acute inflammatory lung injury induced by abdominal sepsis. Int. Immunol. 11:217-227. [DOI] [PubMed] [Google Scholar]
- 69.Ohmori, Y., R. D. Schreiber, and T. A. Hamilton. 1997. Synergy between interferon-γ and tumor necrosis factor-α in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor κB. J. Biol. Chem. 272:14899-14907. [DOI] [PubMed] [Google Scholar]
- 70.Ohmori, Y., and T. A. Hamilton. 1993. Cooperative interaction between interferon (IFN) stimulus response element and κB sequence motifs controls IFN γ- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 268:6677-6688. [PubMed] [Google Scholar]
- 71.Olson, J. K., A. M. Girvin, and S. D. Miller. 2001. Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler's virus. J. Virol. 75:9780-9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ousman, S. S., and S. David. 2001. MIP-1α, MCP-1, GM-CSF, and TNF-alpha control the immune cell response that mediates rapid phagocytosis of myelin from the adult mouse spinal cord. J. Neurosci. 21:4649-4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Paludan, S. R. 2001. Requirements for the induction of interleukin-6 by herpes simplex virus-infected leukocytes. J. Virol. 75:8008-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pati, S., M. Cavrois, H. G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Popik, W., J. E. Hesselgesser, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6406-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prehaud, C., S. Lay, B. Dietzschold, and M. Lafon. 2003. Glycoprotein of nonpathogenic rabies viruses is a key determinant of human cell apoptosis. J. Virol. 77:10537-10547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ray, N. B., L. C. Ewalt, and D. L. Lodmell. 1995. Rabies virus replication in primary murine bone marrow macrophages and in human and murine macrophage-like cell lines: implications for viral persistence. 69:764-772. [DOI] [PMC free article] [PubMed]
- 78.Reiss, C. S., and T. Komatsu. 1998. Does nitric oxide play a critical role in viral infections? J. Virol. 72:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schnell, M. J., H. D. Foley, C. A. Siler, J. P. McGettigan, B. Dietzschold, and R. J. Pomerantz. 2000. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc. Natl. Acad. Sci. USA 97:3544-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shoji, Y., S. Inoue, K. Nakamichi, I. Kurane, T. Sakai, and K. Morimoto. 2004. Generation and characterization of P gene-deficient rabies virus. Virology 318:295-305. [DOI] [PubMed] [Google Scholar]
- 81.Siler, C. A., J. P. McGettigan, B. Dietzschold, S. K. Herrine, J. Dubuisson, R. J. Pomerantz, and M. J. Schnell. 2002. Live and killed rhabdovirus-based vectors as potential hepatitis C vaccines. Virology 292:24-34. [DOI] [PubMed] [Google Scholar]
- 82.Thoulouze, M. I., M. Lafage, M. Schachner, U. Hartmann, H. Cremer, and M. Lafon. 1998. The neural cell adhesion molecule is a receptor for rabies virus. J. Virol. 72:7181-7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tibbles, L. A., J. C. Spurrell, G. P. Bowen, Q. Liu, M. Lam, A. K. Zaiss, S. M. Robbins, M. D. Hollenberg, T. J. Wickham, and D. A. Muruve. 2002. Activation of p38 and ERK signaling during adenovirus vector cell entry lead to expression of the C-X-C chemokine IP-10. J. Virol. 76:1559-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsiang, H., and J. Koenig. 1987. Different behaviour of fixed and street rabies virus strains in cultured rat myotubes, p. 363-368. In B. Mahy and O. Kolafofsky (ed.), The biology of negative strand viruses. Elsevier Science, Amsterdam, The Netherlands.
- 85.Tsiang, H., S. de la Porte, D. J. Ambroise, M. Derer, and J. Koenig. 1986. Infection of cultured rat myotubes and neurons from the spinal cord by rabies virus. J. Neuropathol. Exp. Neurol. 45:28-42. [DOI] [PubMed] [Google Scholar]
- 86.Turner, G. S., and R. Ballard. 1976. Interaction of mouse peritoneal macrophages with fixed rabies virus in vivo and in vitro. J. Gen. Virol. 30:223-231. [DOI] [PubMed] [Google Scholar]
- 87.Ubol, S., C. Sukwattanapan, and Y. Maneerat. 2001. Inducible nitric oxide synthase inhibition delays death of rabies virus-infected mice. J. Med. Microbiol. 50:238-242. [DOI] [PubMed] [Google Scholar]
- 88.Weber, S. M., and S. M. Levitz. 2000. Chloroquine interferes with lipopolysaccharide-induced TNF-alpha gene expression by a nonlysosomotropic mechanism. J. Immunol. 165:1534-1540. [DOI] [PubMed] [Google Scholar]
- 89.Wiktor, T. J. 1978. Cell-mediated immunity and postexposure protection from rabies by inactivated vaccines of tissue culture origin. Dev. Biol. Stand. 40:255-264. [PubMed] [Google Scholar]
- 90.Wiktor, T. J., P. C. Doherty, and H. Koprowski. 1977. In vitro evidence of cell-mediated immunity after exposure of mice to both live and inactivated rabies virus. Proc. Natl. Acad. Sci. USA 74:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie, Q. W., Y. Kashiwabara, and C. Nathan. 1994. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J. Biol. Chem. 269:4705-4708. [PubMed] [Google Scholar]
- 92.Xu, H., H. An, Y. Yu, M. Zhang, R. Qi, and X. Cao. 2003. Ras participates in CpG oligodeoxynucleotide signaling through association with toll-like receptor 9 and promotion of interleukin-1 receptor-associated kinase/tumor necrosis factor receptor-associated factor 6 complex formation in macrophages. J. Biol. Chem. 278:36334-36340. [DOI] [PubMed] [Google Scholar]
- 93.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 94.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]