Abstract
A stable latent reservoir for human immunodeficiency virus type 1 (HIV-1) in resting memory CD4+ T cells presents a barrier to eradication of the infection even in patients on highly active antiretroviral therapy. Potential mechanisms for latency include inaccessibility of the integrated viral genome, absence of key host transcription factors, premature termination of HIV-1 RNAs, and abnormal splicing patterns. To differentiate among these mechanisms, we isolated extremely pure populations of resting CD4+ T cells from patients on highly active antiretroviral therapy. These cells did not produce virus but retained the capacity to do so if appropriately stimulated. Products of HIV-1 transcription were examined in purified resting CD4+ T cells. Although short, prematurely terminated HIV-1 transcripts have been suggested as a marker for latently infected cells, the production of short transcripts had not been previously demonstrated in purified populations of resting CD4+ T cells. By separating RNA into polyadenylated and nonpolyadenylated fractions, we showed that resting CD4+ T cells from patients on highly active antiretroviral therapy produce abortive transcripts that lack a poly(A) tail and that terminate prior to nucleotide 181. Short transcripts dominated the pool of total HIV-1 transcripts in resting CD4+ T cells. Processive, polyadenylated HIV-1 mRNAs were also present at a low level. Both unspliced and multiply spliced forms were found. Taken together, these results show that the nonproductive nature of the infection in resting CD4+ T cells from patients on highly active antiretroviral therapy is not due to absolute blocks at the level of either transcriptional initiation or elongation but rather relative inefficiencies at multiple steps.
The advent of highly active antiretroviral therapy to treat human immunodeficiency virus type 1 (HIV-1) infection has allowed the suppression of plasma virus levels in adherent patients to below the limit of detection of ultrasensitive clinical assays (<50 copies of HIV-1 RNA/ml). This degree of viral suppression has revealed much about the basic biology of the virus itself, including mechanisms of HIV-1 persistence in viral reservoirs. The most clearly defined reservoir for HIV-1 is a small pool of latently infected resting memory CD4+ T cells with integrated provirus (14, 15, 17, 24, 25, 79). Although latently infected cells harboring replication-competent virus are present only at low frequency, this reservoir assumes tremendous importance in the setting of highly active antiretroviral therapy because of its extraordinary stability (24, 71, 72), a property that reflects a fundamental aspect of memory T-cell biology.
In order to provide lifelong protection against pathogens previously encountered by the immune system, memory T cells must survive in a quiescent state for long periods of time. Because HIV-1 can establish latent infection in resting memory CD4+ T cells (8, 14, 60), it can persist through mechanisms that govern immunologic memory, a very fundamental aspect of the immune system. Although reactivation from the latent reservoir contributes only a small fraction of the plasma virus in untreated individuals, the stability of the latent reservoir makes it extremely significant in patients on highly active antiretroviral therapy in whom this reservoir represents a major barrier to virus eradication (17, 24, 25, 71, 72, 79).
Much attention has been focused recently on reactivation and subsequent targeting of the latent reservoir as a strategy for virus eradication (10, 29, 46; T.-W. Chun, D. Engel, S. Mizell, J. Metcalf, J. Hallahan, J. Kovacs, R. Davey, M. Dybul, J. Mican, C. Lane, and A. Fauci, 6th Conference on Retroviruses and Opportunistic Infections, Chicago, Ill., 1999). It is therefore important to understand the molecular mechanisms involved in maintaining HIV-1 latency. Many potential mechanisms have been proposed. These include mechanisms in which initiation of transcription at the HIV-1 long terminal repeat is blocked due to proviral integration into sites that repress transcription (40, 41, 78), DNA and histone modifications that inhibit transcription (20, 31, 32), the absence of host transcriptional activators necessary for HIV-1 gene expression in resting CD4+ T cells (7, 21, 56, 75), or the presence of transcriptional repressors (20, 31).
In addition, there are other potential mechanisms in which transcription initiates but does give rise to productive infection as a result of premature termination of HIV-1 transcripts in the absence of the viral protein Tat and Tat-associated host factors (1, 34, 36, 42). Studies in cell lines and unfractionated peripheral blood mononuclear cells (PBMCs) from infected individuals have shown that the HIV-1 long terminal repeat produces two distinct populations of transcripts, a set of short, prematurely terminated transcripts of about 60 nucleotides and a set of full-length mRNAs (1, 2, 42, 48, 58, 59, 70). In the absence of Tat, the short abortive transcripts predominate, whereas in the presence of Tat, there is a dramatic increase in the levels of long transcripts. Tat binds to a stem-loop structure formed by the first 59 nucleotides of the HIV-1 transcript as well as to a cellular protein kinase complex originally referred to as TAK (Tat-associated kinase) (36, 39, 74). TAK is a complex of cyclin T1 and cyclin-dependent kinase CDK9, which bears homology to pTEFb, a positive-acting elongation factor known to be present in early elongation complexes in Drosophila melanogaster (85). Once in close proximity to the HIV-1 transcription machinery, CDK9 can phosphorylate RNA polymerase II at serine-5 in the C-terminal domain repeat and thus allow efficient transcriptional elongation (35, 48, 52, 61, 83). The absence of Tat and components of the TAK complex in resting CD4+ T cells represents one potential mechanism for latency (1, 34, 36, 42). A final class of potential mechanisms view latency as a consequence of failure to export unspliced HIV-1 RNA in the absence of sufficient levels of the viral protein Rev (53, 55, 62, 63, 69).
Despite the impressive amount of work that has been done on mechanisms of HIV-1 latency, there remains considerable uncertainty over how latency is maintained in vivo. Some of the uncertainty stems from the model systems and experimental approaches used. Molecular studies of the status of HIV-1 DNA and RNA in infected cells do not provide an indication of whether the relevant cells can produce replication-competent virus. Many in vitro studies have utilized infected cell lines as models of HIV-1 latency. In light of the profoundly quiescent, G0 state of resting memory CD4+ T cells in vivo, continuously dividing cell lines may not provide an accurate mechanistic representation of the latent reservoir in vivo. In addition, many of the original in vivo studies were performed before the advent of highly active antiretroviral therapy, when patients had detectable viral loads and active viral replication, making the analysis of latency difficult.
It is now clear that the analysis of HIV-1 latency is best carried out in patients on highly active antiretroviral therapy, in whom active replication of the virus is almost completely suppressed and in whom latently infected cells provide the principal mechanism of viral persistence (17, 24, 25, 71, 72, 79). A further problem is that many carefully devised and sensitive studies have been performed on unfractionated PBMCs derived from patient samples. Although this approach gives valuable information about the state of circulating lymphocytes as a whole, it cannot reveal distinctions between HIV-1 activity in activated and resting CD4+ T cells. Thus, in order to truly understand the state of HIV-1 in the latent reservoir in vivo, it is important to obtain highly purified populations of resting CD4+ T cells from patients who have had prolonged suppression of viral replication on highly active antiretroviral therapy. In an effort to differentiate between the many proposed mechanisms of latency, we examined various potential products of HIV-1 transcription in this purified population of cells with the goal of determining what forms of HIV-1 RNA can be produced in infected resting CD4+ T cells in vivo.
MATERIALS AND METHODS
Purification of resting CD4+ T cells from patients on highly active antiretroviral therapy.
Peripheral blood was obtained from aviremic patients who had achieved suppression of plasma HIV-1 RNA levels to <50 copies/ml with antiretroviral drugs for at least 3 months. Informed consent was obtained from all patients. This study was approved by an institutional review board.
Resting CD4+ T cells were purified with a previously described (14, 25) two-stage process. PBMCs were negatively selected to remove CD8+ T cells, B cells, monocytes, NK cells, and activated CD4+ T cells with appropriate monoclonal antibodies and magnetic beads conjugated with antibodies to mouse immunoglobulin G. The depletion of activated CD4+ T cells was accomplished with antibodies to both early (CD69 and CD25) and late (HLA-DR) activation markers. Further purification of resting CD4+ T cells was accomplished by sorting for small lymphocytes with high CD4 and low HLA-DR surface expression. The resulting populations of resting CD4+ T cells showed <1% contamination with activated cells.
Activation of purified resting CD4+ T cells.
We resuspended 106 freshly sorted resting CD4+ T cells in 1 ml of RPMI supplemented with 10% fetal bovine serum and penicillin-streptomycin and placed in a 48-well plate. Half the wells received 1 μg of anti-CD3 monoclonal antibody and 2.5 μg of anti-CD28 monoclonal antibody (BD Biosciences) and were supplemented with medium containing interleukin-2. At days 2, 4, and 6, culture supernatants were harvested, and cell-free HIV-1 virions were quantitated with the Roche 1.5 ultrasensitive assay for HIV-1 RNA (detection limit: 50 copies/ml).
Isolation of mRNA from CD4+ T lymphocytes.
Aliquots of 106, 0.5 × 106, 0.25 × 106, and 0.1 × 106 sorted resting cells were lysed in l ml of Trizol, and total RNA was isolated according to the manufacturer's protocol (Invitrogen). Polyadenylated RNA and nonpolyadenylated RNA were separated with the Oligotex mRNA mini kit (Qiagen). RNA eluted from the oligo(dT) beads was labeled polyadenylated RNA. RNA that did not bind the oligo(dT) beads was labeled nonpolyadenylated RNA. The nonpolyadenylated fraction was incubated twice with oligo(dT) beads to ensure removal of polyadenylated mRNA. Magnesium chloride was added to the nonpolyadenylated fraction after isolation to bind excess EDTA in the binding buffer. Total RNA for splicing analysis was isolated with the RNeasy mini kit according to the manufacturer's protocol (Qiagen).
Synthesis of cDNA from CD4+ T lymphocytes.
Nonpolyadenylated, polyadenylated, and total RNAs were first DNase treated to remove any genomic DNA (DNase I, amplification grade; Invitrogen). RNA was reverse transcribed in the presence of 2 μM concentrations of the relevant gene-specific primer, either SHORT-RT, 5′-GGGTTCCCTAGTT-3′, nucleotides 40 to 54, repeat region; LONG-RT, 5′-GCTCTCGCACCCA-3′, nucleotides 337 to 349 gag; E5RT1, 5′-TCGTCGCTGTCTCCGCTTC-3′, nucleotides 5526 to 5544 tat/rev; or E7RT3, 5′-CAATCAAGAGTAAGTCTCTCAAGCGG-3′, nucleotides 8077 to 8102 env); 130 ng of random hexamers (Invitrogen); and 1 μl of RNase inhibitor (RNAguard; Amersham Pharmacia Biotech) with Superscript II according to the manufacturer's protocol (Invitrogen). One-fourth of the isolated RNA was used in each reaction. For each RNA isolation, one reaction which contained all components except for Superscript II was performed.
Single-round PCR amplification of nonpolyadenylated and polyadenylated cDNA.
PCR amplification of the resulting cDNA reverse transcribed with SHORT-RT or LONG-RT was carried out with Expand High Fidelity PCR enzyme, 1 μM gene-specific primer, 0.2 μM each of the four deoxynucleoside triphosphates, and 5 μl of cDNA in a 50-μl reaction volume. Primers used for single-round amplification were as described previously (2): HIV-START.2, 5′-GGGTCTCTCTGGTTAGA-3′, nucleotides 1 to 16, repeat region; HIV-SHORT3′, 5′-GGGTTCCCTAGTTAGCC-3′, nucleotides 42 to 58, repeat region; and HIV-LONG3′, 5′-CTGCTAGAGATTTTCCACACTGAC-3′ nucleotides 158 to 181 from the transcription start site. Single-round PCRs of 35 cycles were carried out with either the HIV-START.2 and HIV-SHORT3′ primers or the HIV-START.2 and HIV-LONG3′ primers. Cycling parameters were as follows for single-round PCR: (i) denaturation for 2 min at 94°C; (ii) 35 cycles of 94°C for 30 s and 55°C for 30 s; and (iii) a final extension at 72°C for 60 s. The specificity of PCR products was confirmed by Southern blot hybridization with an HIV-1-specific probe, TAR.3 (5′-GATCTGAGCCTGGGAGCTCT-3′, nucleotides 19 to 36 from the transcription start site).
Heminested PCR amplification of total cDNA.
PCR amplification of the resulting cDNA reverse transcribed with E5RT1 or E7RT3 was carried out with Expand High Fidelity PCR enzyme and the PCR conditions described above. PCR primers used in heminested reactions were as follows: HIV-START5′, 5′-GGGTCTCTCTGGTTAGACCAGATCTGAGCC-3′, nucleotides 1 to 29, repeat region; IN1.3 to 5′, 5′-GACATAATAGCAACAGACATAC-3′, nucleotides 4379 to 4400, pol; E2.3-5′, 5′-GCAGAGATCCACTTTGGAAAGG-3′, nucleotides 4464 to 4485, pol; E5-3′, 5′-CTCCGCTTCTTCCTGCCAT-3′, nucleotides 5516 to 5534, tat/rev; and E7.1 INNER, 5′-GTCTCTGTCTCTCTCTCCACC-3′, nucleotides 7979-7999, env. Outer PCRs were carried out with primer set E2 (E2.3-5′ and E5RT1), US (In1.3-5′ and E5RT1), E5 (HIV-START5′ and E5RT1), or FL (HIV-START5′ and E7RT3). Inner PCRs were carried out with primer set E2 (E2.3-5′ and E5-3′), US (In1.3-5′ and E5-3′), E5 (HIV-START5′ and E5-3′), or FL (HIV-START5′ and E7.1 inner). Cycling parameters for inner and outer PCRs were as follows: (i) denaturation for 2 min at 94°C; (ii) 30 cycles of 94°C for 60 s, 60°C for 60 s, and 72°C for 2 min 45 s; and (iii) a final extension at 72°C for 5 min. PCR products were cloned by TOPO TA cloning (Invitrogen) and sequenced.
Evaluation of PCR sensitivities with DNA and RNA standards.
The HIV-SHORT and HIV-LONG PCR primer sets were tested on a plasmid DNA template (pLAI) with the PCR conditions above. Both HIV-SHORT and HIV-LONG primer sets produced a signal after Southern blotting down to a single copy of pLAI. Plasmid pSP64poly(A)HIV-FL was constructed for use in in vitro transcription experiments. Plasmid pLAI was digested with BspE1, which cuts 150 bp upstream of the transcription start site in the 5′ U3 region and at nucleotide 8981 in the 3′ U3 region. The resulting 9,132-bp band was cloned into the Ava1 site of the multiple cloning site of pSP64poly(A) vector (Promega). For synthesis of in vitro transcripts, pSP64poly(A)HIV-FL was linearized with BsrB1. In vitro transcription of large quantities of RNA was performed with the RiboMAX large scale RNA production system (Promega). In vitro transcription reactions were DNase treated, and RNA was isolated with the Oligotex mRNA mini kit. RNA was quantified by absorbance.
For nonpolyadenylated HIV-1 RNA, RNA containing the first 59 nucleotides of HIV-1 transcript (TAR region) was synthesized by Dharmacon Research. Both polyadenylated and nonpolyadenylated HIV-1 RNAs of known copy number were serially diluted into 106 bead-depleted resting CD4+ T cells from HIV-1-negative donors. Standards were taken throughout the RNA isolation, reverse transcription, and PCR procedures described above. Both nonpolyadenylated and polyadenylated HIV-1 templates had sensitivities of approximately 12.5 copies/PCR.
RESULTS
Isolation of resting CD4+ T-cell populations containing latently infected cells.
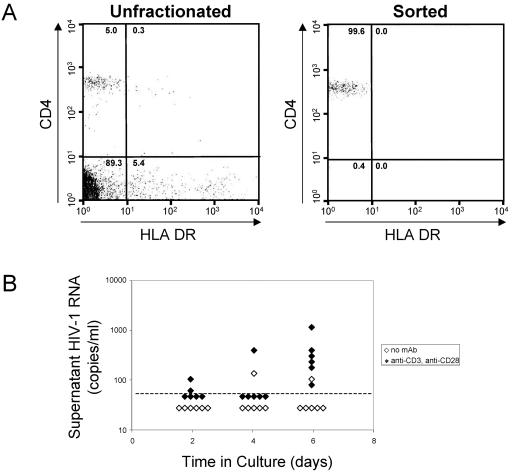
We examined HIV-1 transcriptional initiation and elongation in purified populations of resting CD4+ T cells from patients on highly active antiretroviral therapy who had stable suppression of viremia to below the limit of detection. In such patients, latent HIV-1 in resting CD4+ T cells represents a major mechanism for the persistence of replication-competent virus (8, 17, 24, 25, 79). PBMCs were obtained from patients who had achieved and maintained suppression of plasma HIV-1 RNA to below the limit of detection of the ultrasensitive clinical assay (<50 copies/ml) for at least 9 months (mean, 45.2 months; range, 9 to 66 months). Resting CD4+ T cells were purified from PBMCs with a previously described (14, 25) procedure that involves depletion of unwanted cell populations, including activated T cells, followed by sorting for small cells that express high levels of CD4 but not the activation marker HLA-DR. Resting CD4+ T cells isolated in this way were consistently >99.0% pure as measured by fluorescence-activated cell sorting analysis (Fig. 1A).
FIG. 1.
Purification and characterization of resting CD4+ T cells from aviremic patients on highly active antiretroviral therapy. (A) Flow cytometric analysis of unfractionated PBMCs (left panel) and highly purified resting CD4+, DR− T cells (right panel) stained with phycoerythrin-conjugated anti-CD4 and fluorescein isothiocyanate-conjugated anti-HLA-DR antibodies. (B) Production of virus particles by purified resting CD4+ T cells. Resting CD4+ T cells were cultured in medium alone (open diamonds) or with anti-CD3 and anti-CD28 monoclonal antibodies (solid diamonds). Virus particle concentrations in the supernatant were measured by RT-PCR at the indicated times.
To confirm that resting cell populations purified in this way contained latently infected cells but not productively infected cells, purified resting CD4+ T cells were cultured in the presence or absence of the activating combination of anti-CD3 and anti-CD28 antibodies. Culture supernatants were isolated on days 2, 4, and 6 and subjected to ultrasensitive viral load measurement with a reverse transcription-PCR assay sensitive to 50 molecules of HIV-1 RNA/ml. Only when cultured in the presence of anti-CD3 plus anti-CD28 were resting CD4+ T cells able to consistently produce and release measurable levels of virus (Fig. 1B). Although some level of viral replication can occur in resting CD4+ T cells in tissue microenvironments (22, 73, 81) or following various types of activating stimuli (18, 44, 45, 68, 76), our results show that infected cells contained within the resting CD4+ T-cell compartment from the peripheral blood of aviremic patients are in a nonproductive state of infection. Because virus production was induced with anti-CD3 plus anti-CD28, at least some of these cells are in a reversibly nonproductive or latent state of infection.
Presence of two populations of HIV-1 transcripts in resting CD4+ T cells from aviremic patients.
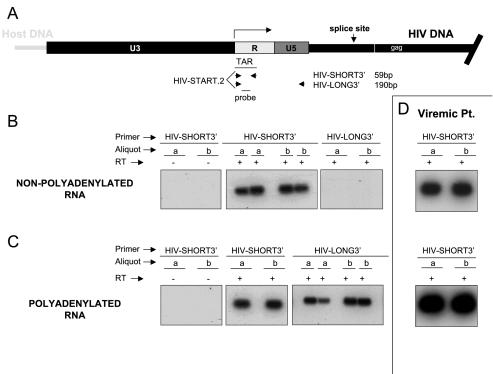
To examine HIV-1 transcription in resting CD4+ T cells, we first looked for the presence of short, abortive transcripts. Although it has been suggested that short HIV-1 transcripts may be a marker for latently infected cells (1, 2), the production of short transcripts has not been analyzed previously in purified populations of resting CD4+ T cells from infected individuals, the population of cells known to harbor latent HIV-1 in vivo. Total RNA isolated from 106 purified resting CD4+ T cells was separated into polyadenylated and nonpolyadenylated fractions. After DNase treatment, the HIV-1 RNA was reverse transcribed and amplified with one primer set (HIV-SHORT = HIV-START and HIV-SHORT3′) that amplifies all products of transcription including the short, abortive transcripts (Fig. 2A). In addition, HIV-1 RNA was amplified with another primer set (HIV-LONG = HIV-START and HIV-LONG3′) whose product extends beyond transcriptional pausing sites.
FIG. 2.
Detection of nonpolyadenylated and polyadenylated HIV-1 RNAs associated with resting CD4+ T cells from patients on highly active antiretroviral therapy. (A) Schematic organization of the HIV-1 long terminal repeat with location of PCR primers. The HIV-START.2 and HIV-SHORT3′ primer set amplifies both abortive and full-length products of transcription. The HIV-START.2 and HIV-LONG3′ primer set amplifies any full-length product of transcription. (B) Southern blots of RT-PCR products amplified from nonpolyadenylated HIV-1 RNA isolated from 106 resting CD4+ T cells with HIV-START.2 and either the HIV-SHORT3′ or HIV-LONG3′ primer. Patient samples were analyzed in duplicate with (+) and without (−) reverse transcriptase (RT). (C) Southern blots of RT-PCR products amplified from polyadenylated HIV-1 RNA isolated from 106 resting CD4+ T cells with HIV-START.2 and either the HIV-SHORT3′ or HIV-LONG3′ primer. Patient samples were analyzed in duplicate with (+) and without (−) reverse transcriptase. (D) Southern blots of RT-PCR products amplified from nonpolyadenylated (top) and polyadenylated (bottom) HIV-1 RNA isolated from 106 PBMCs isolated from a representative viremic patient. Amplification was carried out with the HIV-SHORT3′ primer pair.
When nonpolyadenylated RNA was used as a template in the RT-PCR, a robust signal was observed with the HIV-SHORT primer set, while the HIV-LONG primer set gave no consistent signal (Fig. 2B). When polyadenylated RNA was used, both the HIV-SHORT and HIV-LONG primer sets gave signals (Fig. 2C). This analysis was performed on resting CD4+ T cells from 14 aviremic patients, each yielding similar results. In all cases, signals were dependent upon inclusion of reverse transcriptase in the reaction, demonstrating that the PCR products did not result from the presence of contaminating HIV-1 DNA (Fig. 2B). These results provide direct evidence that latently infected resting CD4+ T cells produce abortive transcripts that lack a poly(A) tail and that terminate prior to nucleotide 181.
The failure to detect long transcripts in the nonpolyadenylated fraction from resting CD4+ T cells was not due to a difference in the sensitivity of the two primer pairs. Both sets of primers give equivalent signals on DNA and in vitro-transcribed RNA templates (data not shown). As a control for the success of the RNA isolation and subsequent RNA fractionation, the cellular housekeeping gene glyceraldehhyde-3-phosphate dehydrogenase was amplified by RT-PCR with primers that span a splice junction. Spliced glyceraldehhyde-3-phosphate dehydrogenase mRNA was detected in the polyadenylated fraction but not in the nonpolyadenylated fraction (data not shown).
Our results also demonstrate that HIV-1 RNAs extending beyond the transcriptional pausing site and containing a poly(A) tail are present in populations of infected resting CD4+ T cells from aviremic patients (Fig. 3C). While many of the products of transcription are abortive, prematurely terminated RNAs lacking a poly(A) tail, consistent with models originally proposed by Peterlin and colleagues (1, 2, 48), the same cell populations also contain detectable levels of polyadenylated HIV-1 mRNAs resulting from processive transcription. Importantly, levels of nonpolyadenylated and polyadenylated HIV-1 RNAs were low compared to those found in cells from patients with active viral replication.
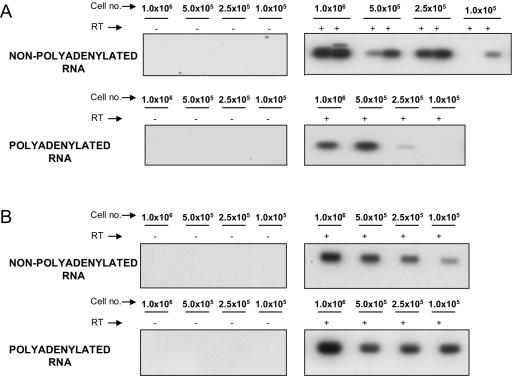
FIG. 3.
Analysis of nonpolyadenylated and polyadenylated HIV-1 RNAs at various cell dilutions. (A) Nonpolyadenylated RNA (top panels) and polyadenylated RNA (bottom panels) were isolated from aliquots of 106, 0.5 × 106, 0.25 × 106, and 105 resting CD4+ T cells. Southern blots of RT-PCR products at each cell dilution with HIV-START.2 and HIV-SHORT3′ primers are shown. (B) Southern blots of RT-PCR products amplified from HIV-1 RNA isolated from 106, 0.5 × 106, 0.25 × 106, and 105 PBMCs from viremic patients.
For purposes of comparison, Fig. 2D shows nonpolyadenylated and polyadenylated signals from 106 PBMCs isolated from a representative viremic patient. Based on standard curves performed with in vitro-transcribed HIV-1 RNA, levels of processive polyadenylated HIV-1 RNAs range only from 200 to 5,000 copies/106 resting CD4+ T cells (not shown). The method described here allows more sensitive detection of HIV-1 RNAs than previously described methods (35) because all processive products of transcription are captured by a single primer pair. Even so, the number of RNA molecules detected is remarkably low considering that the level of HIV-1 RNA in a single productively infected CD4+ T-cell is estimated to be about 3,600 copies (37). Taken together, these results show that, in resting CD4+ T cells from patients on highly active antiretroviral therapy, the HIV-1 long terminal repeat maintains the ability to initiate transcription. Our results support a model in which HIV-1 latency results not from absolute blocks at the level of either transcriptional initiation or elongation but rather from the relative inefficiencies of both steps.
Differential expression of nonpolyadenylated and polyadenylated HIV-1 RNAs in resting CD4+ T cells.
Having established the presence of two distinct types of transcripts in resting CD4+ T cells from patients on highly active antiretroviral therapy, we next examined the relative contributions of the nonpolyadenylated and polyadenylated RNAs to the total pool of HIV-1 RNAs. Relative levels of nonpolyadenylated and polyadenylated HIV-1 RNAs could be assessed in cell dilution experiments in which cells were diluted prior to cell lysis. After oligo(dT) fractionation and DNase treatment, nonpolyadenylated and polyadenylated HIV-1 RNAs were reverse transcribed with the primer SHORT-RT and PCR amplified with the HIV SHORT primer pair (Fig. 2A). These primers detect both prematurely terminated and processive transcripts. The use of the same set of primers to detect HIV-1 RNAs in the polyadenylated and nonpolyadenylated fractions facilitated the analysis of the relative amounts of these transcripts in resting CD4+ T cells by eliminating the possible effects of variability in primer binding sites or differences in PCR sensitivities. At such low levels of expression, small variations in sensitivities can produce large errors in quantitation.
PCR products were detected by Southern blotting with an internal probe (Fig. 2A). Relative HIV-1 expression levels were compared at each cell dilution. In seven of seven patients studied, HIV-1 RNA from the nonpolyadenylated RNA fraction gave stronger signals than RNA from the polyadenylated fraction of an equal number of cells. In contrast, when PBMCs from viremic patients were subjected to the same analysis, polyadenylated transcripts were abundant compared to abortive nonpolyadenylated transcripts (Fig. 3B). Thus, our results demonstrate directly that in purified resting CD4+ T cells from aviremic patients, the steady-state levels of abortive transcripts exceed the levels of processive transcripts.
Presence of low levels of spliced and unspliced HIV-1 RNA in resting CD4+ T cells.
The detection of low levels of polyadenylated transcripts in resting CD4+ T cells populations is consistent with some level of processive transcription but can also result from the presence of full-length genomic HIV-1 RNA in incoming virions that have attached to the cell surface or recently entered the cells. Therefore, the detection of spliced RNA provides more definitive evidence for the ongoing production of functional HIV-1 transcripts in infected resting CD4+ T cells. Previous studies of the levels of multiply spliced HIV-1 RNAs in latently infected cells have reached conflicting conclusions (4, 27, 33, 55, 63), although few of the studies have been done in purified resting CD4+ T cells from patients.
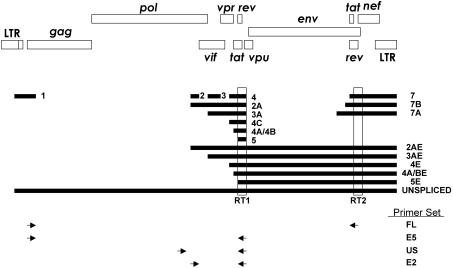
To investigate the nature of the polyadenylated HIV-1 transcripts found in resting CD4+ T cells, we designed heminested RT-PCR primers to detect all possible transcripts produced by alternative splicing. With alternative splicing of a single genomic RNA, HIV-1 can generate 47 different mRNA species (65). It was therefore necessary to design RT-PCR primers without any bias for a particular transcript type (66, 67). Three classes of these nested primers were used. The first detects multiply spliced species only, the second detects unspliced species only, and the third is able to amplify multiply spliced or partially spliced and unspliced species in the same reaction (Fig. 4). Although great care was taken to design primers in the most highly conserved regions, four different primer sets were used to overcome any patient-to-patient sequence variability in these regions (66).
FIG. 4.
Schematic of the HIV-1 genome and locations of nested HIV-1 primers. The primers used to prime cDNA synthesis are shown as E5RT1 and E7RT3. Four different primer sets were used: US is able to amplify unspliced HIV-1 only, and E2 is able to amplify unspliced HIV-1 RNA or spliced HIV-1 RNAs. With E2, HIV-1 RNAs that are unspliced or contain exon 2A, 2AE, 3A, or 3A will produce a larger band, while HIV-1 RNAs that contain exon 2 or 3 and 4, 4A, 4B, 4C, or 5 will produce a smaller band. The E5 and FL primer sets can only amplify multiply spliced HIV-1 RNAs. cDNA synthesized with E5RT1 was amplified with the E2 and US sets. cDNA synthesized with E7RT3 was amplified with E5 and FL.
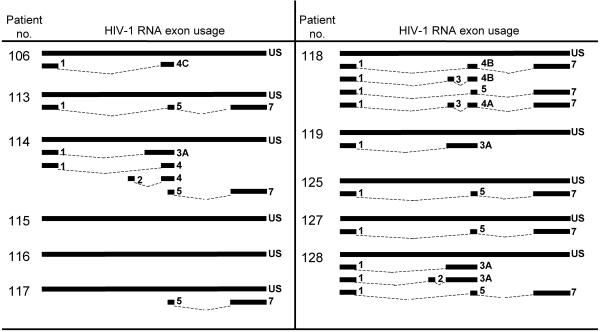
Total RNA was isolated from 106 purified CD4+ resting T cells from 11 aviremic patients, and RT-PCR was performed with the four different primer sets. Although the steady-state level of polyadenylated HIV-1 transcripts is low in resting CD4+ T cells, various species of HIV-1 RNA, including unspliced and multiply spliced forms, were detected in the patients studied (Fig. 5). The identity of the PCR products was confirmed by sequence analysis. Full-length unspliced forms of HIV-1 RNA were detected most consistently. Various spliced forms of HIV-1 RNA were also detected, but detection required a very sensitive heminested PCR approach, and patterns were different in each patient, consistent with sporadic detection of rare spliced RNA molecules near the limit of detection of the assay (Fig. 5). These results suggest that despite the defects in transcriptional elongation in resting CD4+ T cells, some full-length HIV-1 RNAs are made and some of these undergo subsequent splicing to generate a variety of different mRNAs.
FIG. 5.
HIV-1 RNA exon usage in transcripts detected from resting CD4+ T cells isolated from patients on highly active antiretroviral therapy.
DISCUSSION
Although some proposed mechanisms of HIV-1 latency envision an absence of transcriptional initiation due to the lack of key host transcription factors or restraints imposed by chromatin structure (7, 20, 21, 31, 32, 40, 41, 56, 75, 78), our data provide evidence that low levels of transcriptional initiation and elongation can occur in latently infected resting CD4+ T cells from infected individuals. Short, abortive transcripts predominated over processive transcripts, demonstrating a defect in elongation. Some processive transcripts, both spliced and full length, were also detected. However, the levels of HIV-1 RNA in resting CD4+ T cells were so low that ultrasensitive PCR methods were required for detection. Taken together, our results suggest that HIV-1 latency results not from a complete block at any single step in replication but rather from partial defects at multiple steps. It is important to note that while most studies of HIV-1 latency have been carried out in transformed cell lines, unfractionated PBMCs, or cells from viremic patients, our results were obtained in highly purified resting CD4+ T cells from aviremic patients on highly active antiretroviral therapy, the precise cell population in which latent infection has been documented in vivo (17, 24, 25, 79).
We analyzed HIV-1 transcription by separating total RNA into polyadenylated and nonpolyadenylated fractions. This provided novel information that has previously been convoluted by the presence of both populations of HIV-1 transcripts. If RT-PCR is performed on total RNA, primers designed to amplify abortive products of transcription also amplify processive products of transcription. Separation of the total RNA into polyadenylated and nonpolyadenylated fractions allows these primers to specifically detect abortive or processive transcripts in separate reactions without the need for mapping the 3′ end of the RNA. With this approach, we provide here the first direct demonstration of short, abortive products of HIV-1 transcription in purified resting CD4+ T cells from patients on highly active antiretroviral therapy.
Our results are consistent with previous findings from other groups who analyzed RNA in unfractionated PBMCs from viremic patients (1, 2). The preponderance of short or nonprocessive transcripts in resting CD4+ T cells compared to full-length transcripts supports the notion that suboptimal conditions for transcription elongation contribute to latency in resting CD4+ T cells by reducing the formation of functional mRNAs encoding viral proteins. In the setting of active viral replication, processive transcripts become dominant over short transcripts. Thus, it is not simply the presence but rather the preponderance of short transcripts that is associated with the state of HIV-1 latency. The possibility that these short products actually arise from cleavage and subsequent degradation of full-length HIV-1 mRNAs cannot be entirely excluded. However, the lack of any consistent signal in the nonpolyadenylated fraction with the longer primer set supports the notion that these short transcripts are the result of abortive transcription initiation events (Fig. 2B).
The production in resting CD4+ T cells of abortive transcripts demonstrates that initiation of HIV-1 transcription can occur in resting CD4+ T-cell populations that do not produce measurable levels of virus particles. This result is consistent with a recent study showing that the overwhelming majority of integrated HIV-1 genomes in resting CD4+ T cells are located within introns of actively expressed genes rather than in inaccessible regions of heterochromatin (30). Both studies used purified primary resting CD4+ T cells from aviremic patients on highly active antiretroviral therapy. This is the cell population that harbors latent HIV-1 in vivo. The findings that integrated viral genomes reside within the introns of active genes and that abortive HIV-1 transcripts can be produced in these cells together argue strongly that latency is not simply a matter of inaccessibility of the HIV-1 long terminal repeat. The demonstration that transcription can be initiated at the HIV-1 long terminal repeat in latently infected resting CD4+ T cells from infected individuals suggests that further attention be given to therapeutic approaches designed to drive cells out of latency by overcoming the block in elongation. For example, exogenous Tat has recently been show to upregulate HIV-1 gene expression in PBMCs from patients on highly active antiretroviral therapy (48).
Our results also show that the block to transcriptional elongation is not complete in that some full-length HIV-1 transcripts can also be detected in resting CD4+ T cells at low levels. This result is in contrast to the findings of some other groups, who were unable to detect full-length transcripts in unfractionated PBMCs from infected individuals (1). However, numerous other studies have detected HIV-1 RNAs in unfractionated PBMCs from infected individuals (4, 27, 28, 37, 47, 80, 84). Unfortunately, interpretation of gene expression studies done with unfractionated PBMCs is greatly complicated by the fact that a small number of productively infected activated cells may contain large amounts of HIV-1 RNA. With respect to latency, the critical question is whether purified resting CD4+ T cells express functional RNAs. In the small number of studies that have previously addressed this issue, unspliced transcripts have been detected in several studies, while spliced products have not been seen (16, 33). We used specially designed heminested RT-PCR assays to detect various forms of HIV-1 RNA, and we were able to detect both unspliced and spliced forms.
Although products of processive HIV-1 transcription were detected in each patient, the steady-state levels were extremely reduced compared to the levels of abortive transcripts (Fig. 3). Many aspects of the complex regulation of HIV-1 gene expression may contribute to reducing the level of functional viral transcripts in infected resting cells. The HIV-1 promoter contains binding sites for numerous host transcription factors, including factors such as NF-κB and NFAT that are sequestered in the cytoplasm in resting CD4+ T cells. Although viruses with mutations in the NF-κB binding sites can replicate in some settings (11, 38), NF-κB appears to play a particularly important role in promoting HIV-1 gene expression in primary cells and in activating latent HIV-1 (3, 5, 7, 9, 9, 21, 75).
It is likely that in infected, resting CD4+ T cells, NF-κB is sequestered in the cytoplasm through its interaction with IκB (50). HIV-1 transcription is also heavily dependent upon the stimulation of elongation by Tat (23, 39, 42, 74) and the associated cyclin T1-CDK9 complex (35, 36, 49, 52, 61, 83, 85). Thus, insufficient levels of Tat and/or cyclin T1-CDK9 likely limit HIV-1 gene expression in resting CD4+ T cells. Several studies suggest that the roles of NF-κB and Tat are more complex than originally thought. NF-κB can promote elongation (77), and Tat has been shown to have functions in addition to its role in upregulating transcriptional elongation. These include overcoming chromatin structure constraints through complex interactions with proteins containing histone acetyltransferase activity (6, 19, 43, 51, 54, 57, 64). Tat also counteracts negative elongation factors (26) and promotes cotranscriptional mRNA capping (12, 13, 82).
According to some previously proposed models of HIV-1 latency, there is an aberrant pattern of viral RNA expression in latently infected cells (53, 63). These reports cited an abundance of multiply spliced and partially spliced viral RNA species compared to unspliced viral RNA in latently infected cell lines. Our results do not support this abundance of multiply spliced products. Instead, we demonstrated the presence of unspliced and spliced variants alike. Both unspliced and spliced variants were at low levels, barely above the limit of detection. It is also apparent that the multiply spliced viral RNA species found were extremely variable in their exon usage. This may reflect the sporadic detection of rare species at the detection limit of the assay. The present study extends previous studies of latently infected cells which used a single primer set to detect multiply spliced species (10, 33). In the approach described here, a wide variety of multiply spliced variants can be detected, and the presence of multiply spliced RNAs in resting CD4+ T cells was more readily apparent. However, despite the presence of multiply spliced and unspliced HIV-1 species in resting CD4+ T cells, the levels are so low in each cell that they are insufficient to support observable virus production (Fig. 1B).
Our results support a revised view of HIV-1 latency in which the absence of virus production is not determined solely by a single factor or mechanism. It does not appear that there is an absolute block at any of the stages examined. Rather, it appears that the resting CD4+ T cell provides a nonideal transcriptional environment for the HIV-1 promoter, resulting in reduced production of full-length functional transcripts. In addition to the low level of functional transcripts, additional defects at the level of export, translation, or virus assembly may further limit virus production by infected resting CD4+ T cells. These multiple deficiencies in virus production are all related to the profound change in state which occurs as infected CD4+ T lymphoblasts revert to a resting memory state, in which they can carry latent HIV-1 for prolonged periods of time.
Acknowledgments
We thank Mike Paradise and Scott Barnett for help in recruiting patients. Thanks to Karen Chadwick and Hao Zhang for cell sorting.
This work was supported by NIH grant AI43222, the Doris Duke Charitable Foundation, and the Howard Hughes Medical Institute.
REFERENCES
- 1.Adams, M., L. Sharmeen, J. Kimpton, J. M. Romeo, J. V. Garcia, B. M. Peterlin, M. Groudine, and M. Emerman. 1994. Cellular latency in human immunodeficiency virus-infected individuals with high CD4 levels can be detected by the presence of promoter-proximal transcripts. Proc. Natl. Acad. Sci. USA 91:3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, M., C. Wong, D. Wang, and J. Romeo. 1999. Limitation of Tat-associated transcriptional processivity in HIV-infected PBMC. Virology 257:397-405. [DOI] [PubMed] [Google Scholar]
- 3.Alcami, J., D. L. Lain, L. Folgueira, M. A. Pedraza, J. M. Jacque, F. Bachelerie, A. R. Noriega, R. T. Hay, D. Harrich, and R. B. Gaynor. 1995. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 14:1552-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagnarelli, P., A. Valenza, S. Menzo, R. Sampaolesi, P. E. Varaldo, L. Butini, M. Montroni, C. F. Perno, S. Aquaro, D. Mathez, J. Leibowitch, C. Balotta, and M. Clementi. 1996. Dynamics and modulation of human immunodeficiency virus type 1 transcripts in vitro and in vivo. J. Virol. 70:7603-7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barboric, M., R. M. Nissen, S. Kanazawa, N. Jabrane-Ferrat, and B. M. Peterlin. 2001. NF-kappaB binds P-TEFb to stimulate transcriptional elongation by RNA polymerase II. Mol. Cell 8:327-337. [DOI] [PubMed] [Google Scholar]
- 6.Benkirane, M., R. F. Chun, H. Xiao, V. V. Ogryzko, B. H. Howard, Y. Nakatani, and K. T. Jeang. 1998. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J. Biol. Chem. 273:24898-24905. [DOI] [PubMed] [Google Scholar]
- 7.Bohnlein, E., J. W. Lowenthal, M. Siekevitz, D. W. Ballard, B. R. Franza, and W. C. Greene. 1988. The same inducible nuclear proteins regulates mitogen activation of both the interleukin-2 receptor-alpha gene and type 1 HIV. Cell 53:827-836. [DOI] [PubMed] [Google Scholar]
- 8.Brenchley, J. M., B. J. Hill, D. R. Ambrozak, D. A. Price, F. J. Guenaga, J. P. Casazza, J. Kuruppu, J. Yazdani, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J. Virol. 78:1160-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks, D. G., P. A. Arlen, L. Gao, C. M. Kitchen, and J. A. Zack. 2003. Identification of T cell-signaling pathways that stimulate latent HIV in primary cells. Proc. Natl. Acad. Sci. USA 100:12955-12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks, D. G., D. H. Hamer, P. A. Arlen, L. Gao, G. Bristol, C. M. Kitchen, E. A. Berger, and J. A. Zack. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413-423. [DOI] [PubMed] [Google Scholar]
- 11.Chen, B. K., M. B. Feinberg, and D. Baltimore. 1997. The kappaB sites in the human immunodeficiency virus type 1 long terminal repeat enhance virus replication yet are not absolutely required for viral growth. J. Virol. 71:5495-5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu, Y. L., E. Coronel, C. K. Ho, S. Shuman, and T. M. Rana. 2001. HIV-1 Tat protein interacts with mammalian capping enzyme and stimulates capping of TAR RNA. J. Biol. Chem. 276:12959-12966. [DOI] [PubMed] [Google Scholar]
- 13.Chiu, Y. L., C. K. Ho, N. Saha, B. Schwer, S. Shuman, and T. M. Rana. 2002. Tat stimulates cotranscriptional capping of HIV mRNA. Mol. Cell 10:585-597. [DOI] [PubMed] [Google Scholar]
- 14.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 15.Chun, T. W., D. Finzi, J. Margolick, K. Chadwick, D. Schwartz, and R. F. Siliciano. 1995. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat. Med. 1:1284-1290. [DOI] [PubMed] [Google Scholar]
- 16.Chun, T. W., J. S. Justement, R. A. Lempicki, J. Yang, G. Dennis, Jr., C. W. Hallahan, C. Sanford, P. Pandya, S. Liu, M. McLaughlin, L. A. Ehler, S. Moir, and A. S. Fauci. 2003. Gene expression and viral production in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc. Natl. Acad. Sci. USA 100:1908-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun, T. W., L. Stuyver, S. B. Mizell, L. A. Ehler, J. A. Mican, M. Baseler, A. L. Lloyd, M. A. Nowak, and A. S. Fauci. 1997. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 94:13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chun, T.-W., D. Engel, S. B. Mizell, L. A. Ehler, and A. S. Fauci. 1998. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J. Exp. Med. 188:83-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Col, E., B. Gilquin, C. Caron, and S. Khochbin. 2002. Tat-controlled protein acetylation. J. Biol. Chem. 277:37955-37960. [DOI] [PubMed] [Google Scholar]
- 20.Coull, J. J., F. Romerio, J. M. Sun, J. L. Volker, K. M. Galvin, J. R. Davie, Y. Shi, U. Hansen, and D. M. Margolis. 2000. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J. Virol. 74:6790-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duh, E. J., W. J. Maury, T. M. Folks, A. S. Fauci, and A. B. Rabson. 1989. Tumor necrosis factor alpha activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-kappa B sites in the long terminal repeat. Proc. Natl. Acad. Sci. USA 86:5974-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg, M. B., D. Baltimore, and A. D. Frankel. 1991. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc. Natl. Acad. Sci. USA 88:4045-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finzi, D., J. Blankson, J. D. Siliciano, J. B. Margolick, K. Chadwick, T. Pierson, K. Smith, J. Lisziewicz, F. Lori, C. Flexner, T. C. Quinn, R. E. Chaisson, E. Rosenberg, B. Walker, S. Gange, J. Gallant, and R. F. Siliciano. 1999. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat. Med. 5:512-517. [DOI] [PubMed] [Google Scholar]
- 25.Finzi, D., M. Hermankova, T. Pierson, L. M. Carruth, C. Buck, R. E. Chaisson, T. C. Quinn, K. Chadwick, J. Margolick, R. Brookmeyer, J. Gallant, M. Markowitz, D. D. Ho, D. D. Richman, and R. F. Siliciano. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295-1300. [DOI] [PubMed] [Google Scholar]
- 26.Fujinaga, K., D. Irwin, Y. Huang, R. Taube, T. Kurosu, and B. M. Peterlin. 2004. Dynamics of human immunodeficiency virus transcription: P-TEFb phosphorylates RD and dissociates negative effectors from the transactivation response element. Mol. Cell Biol. 24:787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furtado, M. R., D. S. Callaway, J. P. Phair, K. J. Kunstman, J. L. Stanton, C. A. Macken, A. S. Perelson, and S. M. Wolinsky. 1999. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N. Engl. J. Med. 340:1614-1622. [DOI] [PubMed] [Google Scholar]
- 28.Gunthard, H. F., D. V. Havlir, S. Fiscus, Z. Q. Zhang, J. Eron, J. Mellors, R. Gulick, S. D. Frost, A. J. Brown, W. Schleif, F. Valentine, L. Jonas, A. Meibohm, C. C. Ignacio, R. Isaacs, R. Gamagami, E. Emini, A. Haase, D. D. Richman, and J. K. Wong. 2001. Residual human immunodeficiency virus (hiv) type 1 rna and dna in lymph nodes and hiv rna in genital secretions and in cerebrospinal fluid after suppression of viremia for 2 years. J. Infect. Dis. 183:1318-1327. [DOI] [PubMed] [Google Scholar]
- 29.Hamer, D. H., S. Bocklandt, L. McHugh, T. W. Chun, P. M. Blumberg, D. M. Sigano, and V. E. Marquez. 2003. Rational design of drugs that induce human immunodeficiency virus replication. J. Virol. 77:10227-10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han, Y., K. Lassen, D. Monie, A. R. Sedaghat, S. Shimoji, X. Liu, T. C. Pierson, J. B. Margolick, R. F. Siliciano, and J. D. Siliciano. 2004. Resting CD4+ T cells from HIV-1-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J. Virol. [DOI] [PMC free article] [PubMed]
- 31.He, G., and D. M. Margolis. 2002. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol. Cell. Biol. 22:2965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He, G., L. Ylisastigui, and D. M. Margolis. 2002. The regulation of HIV-1 gene expression: the emerging role of chromatin. DNA Cell Biol. 21:697-705. [DOI] [PubMed] [Google Scholar]
- 33.Hermankova, M., J. D. Siliciano, Y. Zhou, D. Monie, K. Chadwick, J. B. Margolick, T. C. Quinn, and R. F. Siliciano. 2003. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J. Virol. 77:7383-7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herrmann, C. H., R. G. Carroll, P. Wei, K. A. Jones, and A. P. Rice. 1998. Tat-associated kinase, TAK, activity is regulated by distinct mechanisms in peripheral blood lymphocytes and promonocytic cell lines. J. Virol. 72:9881-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herrmann, C. H., M. O. Gold, and A. P. Rice. 1996. Viral transactivators specifically target distinct cellular protein kinases that phosphorylate the RNA polymerase II C-terminal domain. Nucleic Acids Res. 24:501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herrmann, C. H., and A. P. Rice. 1995. Lentivirus Tat proteins specifically associate with a cellular protein kinase, TAK, that hyperphosphorylates the carboxyl-terminal domain of the large subunit of RNA polymerase II: candidate for a Tat cofactor. J. Virol. 69:1612-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hockett, R. D., J. M. Kilby, C. A. Derdeyn, M. S. Saag, M. Sillers, K. Squires, S. Chiz, M. A. Nowak, G. M. Shaw, and R. P. Bucy. 1999. Constant mean viral copy number per infected cell in tissues regardless of high, low, or undetectable plasma HIV RNA. J. Exp. Med. 189:1545-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ilyinskii, P. O., and R. C. Desrosiers. 1996. Efficient transcription and replication of simian immunodeficiency virus in the absence of NF-kappaB and Sp1 binding elements. J. Virol. 70:3118-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones, K. A., and B. M. Peterlin. 1994. Control of RNA initiation and elongation at the HIV-1 promoter. Annu. Rev. Biochem. 63:717-743. [DOI] [PubMed] [Google Scholar]
- 40.Jordan, A., D. Bisgrove, and E. Verdin. 2003. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 22:1868-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan, A., P. Defechereux, and E. Verdin. 2001. The site of HIV-1 integration in the human genome determines basal transcriptional activity and response to Tat transactivation. EMBO J. 20:1726-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kao, S. Y., A. F. Calman, P. A. Luciw, and B. M. Peterlin. 1987. Anti-termination of transcription within the long terminal repeat of HIV-1 by tat gene product. Nature 330:489-493. [DOI] [PubMed] [Google Scholar]
- 43.Kiernan, R. E., C. Vanhulle, L. Schiltz, E. Adam, H. Xiao, F. Maudoux, C. Calomme, A. Burny, Y. Nakatani, K. T. Jeang, M. Benkirane, and C. Van Lint. 1999. HIV-1 tat transcriptional activity is regulated by acetylation. EMBO J. 18:6106-6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korin, Y. D., D. G. Brooks, S. Brown, A. Korotzer, and J. A. Zack. 2002. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J. Virol. 76:8118-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulkosky, J., D. M. Culnan, J. Roman, G. Dornadula, M. Schnell, M. R. Boyd, and R. J. Pomerantz. 2001. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood 98:3006-3015. [DOI] [PubMed] [Google Scholar]
- 46.Kulkosky, J., G. Nunnari, M. Otero, S. Calarota, G. Dornadula, H. Zhang, A. Malin, J. Sullivan, Y. Xu, J. DeSimone, T. Babinchak, J. Stern, W. Cavert, A. Haase, and R. J. Pomerantz. 2002. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J. Infect. Dis. 186:1403-1411. [DOI] [PubMed] [Google Scholar]
- 47.Lewin, S. R., M. Vesanen, L. Kostrikis, A. Hurley, A. Duran, L. Zhang, D. D. Ho, and M. Markowitz. 1999. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiviral therapy. J. Virol. 73:6099-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin, X., D. Irwin, S. Kanazawa, L. Huang, J. Romeo, T. S. Yen, and B. M. Peterlin. 2003. Transcriptional profiles of latent human immunodeficiency virus in infected individuals: effects of Tat on the host and reservoir. J. Virol. 77:8227-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin, X., R. Taube, K. Fujinaga, and B. M. Peterlin. 2002. P-TEFb containing cyclin K and Cdk9 can activate transcription via RNA. J. Biol. Chem. 277:16873-16878. [DOI] [PubMed] [Google Scholar]
- 50.Liou, H. C. 2002. Regulation of the immune system by NF-kappaB and IkappaB. J. Biochem. Mol. Biol. 35:537-546. [DOI] [PubMed] [Google Scholar]
- 51.Lusic, M., A. Marcello, A. Cereseto, and M. Giacca. 2003. Regulation of HIV-1 gene expression by histone acetylation and factor recruitment at the LTR promoter. EMBO J. 22:6550-6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majello, B., G. Napolitano, A. Giordano, and L. Lania. 1999. Transcriptional regulation by targeted recruitment of cyclin-dependent CDK9 kinase in vivo. Oncogene 18:4598-4605. [DOI] [PubMed] [Google Scholar]
- 53.Malim, M. H., and B. R. Cullen. 1991. HIV-1 structural gene expression requires the binding of multiple Rev monomers to the viral RRE: implications for HIV-1 latency. Cell 63:241-248. [DOI] [PubMed] [Google Scholar]
- 54.Marzio, G., M. Tyagi, M. I. Gutierrez, and M. Giacca. 1998. HIV-1 tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc. Natl. Acad. Sci. USA 95:13519-13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michael, N. L., P. Morrow, J. Mosca, M. Vahey, D. S. Burke, and R. R. Redfield. 1991. Induction of human immunodeficiency virus type 1 expression in chronically infected cells is associated primarily with a shift in RNA splicing patterns. J. Virol. 65:7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nabel, G., and D. Baltimore. 1987. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature 326:711-713. [DOI] [PubMed] [Google Scholar]
- 57.Ott, M., M. Schnolzer, J. Garnica, W. Fischle, S. Emiliani, H. R. Rackwitz, and E. Verdin. 1999. Acetylation of the HIV-1 Tat protein by p300 is important for its transcriptional activity. Curr. Biol. 9:1489-1492. [DOI] [PubMed] [Google Scholar]
- 58.Pendergrast, P. S., and N. Hernandez. 1997. RNA-targeted activators, but not DNA-targeted activators, repress the synthesis of short transcripts at the human immunodeficiency virus type 1 long terminal repeat. J. Virol. 71:910-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pendergrast, P. S., D. Morrison, W. P. Tansey, and N. Hernandez. 1996. Mutations in the carboxy-terminal domain of TBP affect the synthesis of human immunodeficiency virus type 1 full-length and short transcripts similarly. J. Virol. 70:5025-5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pierson, T., T. L. Hoffman, J. Blankson, D. Finzi, K. Chadwich, J. B. Margolick, C. Buck, J. D. Siliciano, R. W. Doms, and R. F. Siliciano. 2000. Characterization of chemokine receptor utilization of viruses in the latent reservoir for HIV-1. J. Virol. 74:7824-7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ping, Y. H., and T. M. Rana. 1999. Tat-associated kinase (P-TEFb): a component of transcription preinitiation and elongation complexes. J. Biol. Chem. 274:7399-7404. [DOI] [PubMed] [Google Scholar]
- 62.Pomerantz, R. J., T. Seshamma, and D. Trono. 1992. Efficient replication of human immunodeficiency virus type 1 requires a threshold level of Rev: potential implications for latency. J. Virol. 66:1809-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pomerantz, R. J., D. Trono, M. B. Feinberg, and D. Baltimore. 1990. Cells nonproductively infected with HIV-1 exhibit an aberrant pattern of viral RNA expression: a molecular model for latency. Cell 61:1271-1276. [DOI] [PubMed] [Google Scholar]
- 64.Pumfery, A., L. Deng, A. Maddukuri, F. C. de la, H. Li, J. D. Wade, P. Lambert, A. Kumar, and F. Kashanchi. 2003. Chromatin remodeling and modification during HIV-1 Tat-activated transcription. Curr. HIV Res. 1:343-362. [DOI] [PubMed] [Google Scholar]
- 65.Purcell, D. F., and M. A. Martin. 1993. Alternative splicing of human immunodeficiency virus type 1 mRNA modulates viral protein expression, replication, and infectivity. J. Virol. 67:6365-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saltarelli, M. J., E. Hadziyannis, C. E. Hart, J. V. Harrison, B. K. Felber, T. J. Spira, and G. N. Pavlakis. 1996. Analysis of human immunodeficiency virus type 1 mRNA splicing patterns during disease progression in peripheral blood mononuclear cells from infected individuals. AIDS Res. Hum. Retroviruses 12:1443-1456. [DOI] [PubMed] [Google Scholar]
- 67.Schwartz, S., B. K. Felber, D. M. Benko, E. M. Fenyo, and G. N. Pavlakis. 1990. Cloning and functional analysis of multiply spliced mRNA species of human immunodeficiency virus type 1. J. Virol. 64:2519-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scripture-Adams, D. D., D. G. Brooks, Y. D. Korin, and J. A. Zack. 2002. Interleukin-7 induces expression of latent human immunodeficiency virus type 1 with minimal effects on T-cell phenotype. J. Virol. 76:13077-13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seshamma, T., O. Bagasra, D. Trono, D. Baltimore, and R. J. Pomerantz. 1992. Blocked early-stage latency in the peripheral blood cells of certain individuals infected with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 89:10663-10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheldon, M., R. Ratnasabapathy, and N. Hernandez. 1993. Characterization of the inducer of short transcripts, a human immunodeficiency virus type 1 transcriptional element that activates the synthesis of short RNAs. Mol. Cell. Biol. 13:1251-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Siliciano, J. D., J. Kajdas, D. Finzi, T. C. Quinn, K. Chadwick, J. B. Margolick, C. Kovacs, S. J. Gange, and R. F. Siliciano. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat. Med. 9:727-728. [DOI] [PubMed] [Google Scholar]
- 72.Strain, M. C., H. F. Gunthard, D. V. Havlir, C. C. Ignacio, D. M. Smith, A. J. Leigh-Brown, T. R. Macaranas, R. Y. Lam, O. A. Daly, M. Fischer, M. Opravil, H. Levine, L. Bacheler, C. A. Spina, D. D. Richman, and J. K. Wong. 2003. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc. Natl. Acad. Sci. USA 100:4819-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Swingler, S., B. Brichacek, J. M. Jacque, C. Ulich, J. Zhou, and M. Stevenson. 2003. HIV-1 Nef intersects the macrophage CD40L signalling pathway to promote resting-cell infection. Nature 424:213-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taube, R., K. Fujinaga, J. Wimmer, M. Barboric, and B. M. Peterlin. 1999. Tat transactivation: a model for the regulation of eukaryotic transcriptional elongation. Virology 264:245-253. [DOI] [PubMed] [Google Scholar]
- 75.Tong-Starksen, S. E., P. A. Luciw, and B. M. Peterlin. 1987. Human immunodeficiency virus long terminal repeat responds to T-cell activation signals. Proc. Natl. Acad. Sci. USA 84:6845-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Unutmaz, D., V. N. KewalRamani, S. Marmon, and D. R. Littman. 1999. Cytokine signals are sufficient for HIV-1 infection of resting human T lymphocytes. J. Exp. Med. 189:1735-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.West, M. J., A. D. Lowe, and J. Karn. 2001. Activation of human immunodeficiency virus transcription in T cells revisited: NF-κB p65 stimulates transcriptional elongation. J. Virol. 75:8524-8537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winslow, B. J., R. J. Pomerantz, O. Bagasra, and D. Trono. 1993. HIV-1 latency due to the site of proviral integration. Virology 196:849-854. [DOI] [PubMed] [Google Scholar]
- 79.Wong, J. K., M. Hezareh, H. F. Gunthard, D. V. Havlir, C. C. Ignacio, C. A. Spina, and D. D. Richman. 1997. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science 278:1291-1295. [DOI] [PubMed] [Google Scholar]
- 80.Zhang, J., and C. S. Crumpacker. 2001. Human immunodeficiency virus type 1 RNA in peripheral blood mononuclear cells of patients receiving prolonged highly active antiretroviral therapy. J. Infect. Dis. 184:1341-1344. [DOI] [PubMed] [Google Scholar]
- 81.Zhang, Z., T. Schuler, M. Zupancic, S. Wietgrefe, K. A. Staskus, K. A. Reimann, T. A. Reinhart, M. Rogan, W. Cavert, C. J. Miller, R. S. Veazey, D. Notermans, S. Little, S. A. Danner, D. D. Richman, D. Havlir, J. Wong, H. L. Jordan, T. W. Schacker, P. Racz, K. Tenner-Racz, N. L. Letvin, S. Wolinsky, and A. T. Haase. 1999. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science 286:1353-1357. (Erratum, 286:2273.) [DOI] [PubMed] [Google Scholar]
- 82.Zhou, M., L. Deng, F. Kashanchi, J. N. Brady, A. J. Shatkin, and A. Kumar. 2003. The Tat/TAR-dependent phosphorylation of RNA polymerase II C-terminal domain stimulates cotranscriptional capping of HIV-1 mRNA. Proc. Natl. Acad. Sci. USA 100:12666-12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou, M., M. A. Halanski, M. F. Radonovich, F. Kashanchi, J. Peng, D. H. Price, and J. N. Brady. 2000. Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription. Mol. Cell. Biol. 20:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu, T., D. Muthui, S. Holte, D. Nickle, F. Feng, S. Brodie, Y. Hwangbo, J. I. Mullins, and L. Corey. 2002. Evidence for human immunodeficiency virus type 1 replication in vivo in CD14+ monocytes and its potential role as a source of virus in patients on highly active antiretroviral therapy. J. Virol. 76:707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhu, Y., T. Pe'ery, J. Peng, Y. Ramanathan, N. Marshall, T. Marshall, B. Amendt, M. B. Mathews, and D. H. Price. 1997. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 11:2622-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]