Abstract
We investigated the functional trait responses to 5 nm metallic silver nanoparticle (AgNPs) exposure in the Lessepsian-entry bivalve B. pharaonis. Respiration rate (oxygen consumption), heartbeat rate, and absorption efficiency were evaluated across an 8-day exposure period in mesocosmal conditions. Basal reference values from not-exposed specimens were statistically compared with those obtained from animals treated with three sublethal nanoparticle concentrations (2 μg L−1, 20 μg L−1, and 40 μg L−1). Our data showed statistically significant effects on the average respiration rate of B. pharaonis. Moreover, complex nonlinear dynamics were observed as a function of the concentration level and time. Heartbeat rates largely increased with no acclimation in animals exposed to the two highest levels with similar temporal dynamics. Eventually, a decreasing trend for absorption efficiency might indicate energetic constraints. In general, these data support the possible impact of engineered nanomaterials in marine environments and support the relevance of functional trait assessment in present and future ecotoxicological studies.
1. Introduction
The quick development of nanotechnology is posing concerns on the environmental impact of engineered nanomaterials (ENMs) into delicate environments such as marine coastal areas. The production and commercial use of ENMs have been linearly increased during the last decade and involve the following: personal care, cosmetics, fabrics and textile, household appliances, electronics and computers, health and medicine, renewable energies, and environmental remediation [1]. According to the Nanotechnology Consumer Products Inventory [2], currently, there are 1841 ENM-containing products in the consumer marketplace, of which 438 (24%) contain nanosilver. Silver is a xenobiotic metal widely used in industrial applications (more than 60% of the metal demand) such as chemical catalysis, batteries, photography, electronics, and brazing and soldering [3]. Due to its antimicrobial properties it has been largely employed as additive in personal care products, coatings, paints, fabrics, food, and beverage. Nowadays, most of the latter applications as well as those targeted to health, fitness, and household use are based on silver ENMs [2]. Indeed, silver at the nanoscale level shows distinctive physicochemical properties and biological activities like excellent conductivity, chemical stability, and increased catalytic activity [4]. These distinctive properties differ significantly from those of larger particles and have made silver nanoparticles (AgNPs) extremely attractive for the production of consumer materials and, therefore, the investigation of their environmental fate and toxicological properties [4, 5]. Nanosilver predicted environmental concentrations (PECs) are yet affected by uncertainty since different approaches in modeling and measurement methods complicate validation. Gottschalk and coworkers [6] reviewed the outcome from different predictive models. For superficial waters they indicated a wide silver range spanning from 10−4 to 100 ppm with a median value around 10−2 ppm. Waste water treatment plants are able to capture most (nano)silver into an insoluble sulfide form [7]; however, ENMs may enter into the environment through different routes such as outdoor urban sources [8] that are not necessarily intercepted by treatment plants. Air transport and in general the hydrogeological cycle have a primary role in ENM run-off and transport, thus, making marine ecosystems the terminal sinks [9]. PECs for seawater environments are practically unknown.
The antimicrobial as well as toxic effects of silver are attributed to the Ag+ ion that is a strong electrophile reacting with most macromolecules containing S, O, and N [5, 10, 11]. The toxicity of AgNPs in the environment is certainly a function of the silver metal speciation that highly depends on the environment itself but also intrinsic characteristics such as particle sintering, coating, surface potential, size, and Ag+ dissolution rate [12–14]. It is widely recognized that Ag-ENMs can exert toxic effects on aquatic organisms such as algae, mollusks, crustaceans, and fish for which classical ecotoxicological endpoints have been evaluated [1, 15]; however, very little is known about marine species [16].
Marine bivalves are model species to study ENM effects. Since they are filter-feeder organisms, they bioaccumulate toxicants either in dissolved or in particle-adsorbed forms in the water column [17, 18]. However, the main routes of NPs uptake and the level at which they can penetrate into the organism are not completely known [5]. In the bivalve Mytilus edulis Moore [17] showed that the principal process for the translocation of polystyrene NPs across the membrane is endocytosis. Regarding AgNP data on toxicological effects on bivalves, this information is sparse. According to Zuykov et al. [19] AgNPs can be accumulated in the extrapallial fluid of the blue mussel Mytilus edulis and concentrate mostly in ganglia. Gomes et al. [20] reported an oxidative stress syndrome in soft tissues of Mytilus spp. with induction of heavy metal binding proteins in gills. In the oyster C. virginica there were embryo toxicity and effects on lysosomal integrity of hepatopancreas cells [21]. In this context, functional traits such as food assimilation, respiration, and heartbeat rates [22] may represent a straightforward and useful approach to investigate AgNPs metabolic and bioenergetic effects. Here we report the effects of AgNPs on main functional traits of a new proposed model species, the Lessepsian-entry bivalve Brachidontes pharaonis [23, 24]. Indeed, we tested the sensitivity of B. pharaonis to submicromolar amounts (0–40 μg L−1) of a 5 nm commercial AgNP in an 8-day exposure experiment carried out in laboratory under mesocosmal conditions. The response variables observed were respiration rate (RR), heartbeat rate (HBR), and absorption efficiency (AE).
2. Material and Methods
2.1. Animals and Treatments with Silver Nanoparticles
Specimens of Brachidontes pharaonis were collected from the Ettore Pond of Stagnone di Marsala (Trapani, Western Sicily, 37° 52′ north; 12° 28′ east). Once collected, mussels were brought back to the laboratory in controlled conditions of temperature (16°C) and humidity (100%). They were cleaned from epibionts and moved into tanks at 0.5 L per animal in filtered recirculating seawater at 20°C. Organisms were acclimated in laboratory conditions for 15 days at 20°C, 36‰ salinity, and pH 8.2 ± 0.1. The total number of animals used in this experiment was 360, with a size ranging from 22 to 27 mm. Both during the acclimation and during experimental periods, organisms were fed three times per day (ad libitum) with fresh cultures of Isochrysis galbana at an initial titer of 15,000 cells mL−1. Animals were divided into 4 experimental groups of 90 specimens, with 3 independent tanks per group. Each group corresponded to a different exposure level to silver nanoparticles: 0 (not-exposed, reference control), 2, 20, and 40 μg L−1. This concentration range is compatible with previous studies on AgNPs [20, 25].
Silver nanoparticles (5 nm average diameter) with an alkane coating were supplied by AMEPOX (Lodz, Poland) in a stable ultrapure-water solution at 1 g L−1. This material has been already used in a large ENM toxicity-testing framework within the NanoFATE project (https://wiki.ceh.ac.uk/display/nanofate/Home) [26–29].
2.2. Respiration Rate
Metabolic respiration rates were evaluated as oxygen consumption according to a reliable procedure already tested in companion papers [30, 31]. Measurements were carried out every 2 days on six randomly selected specimens per treatment (0, 2, 20, and 40 μg L−1). Briefly, single B. pharaonis specimens were placed in glass respirometric chambers (0.5 L) containing filtered air-saturated seawater. Magnetic stirring ensured water mixing within the chamber, while oxygen reduction was measured by means of computer-assisted optical oxygen-meter probes (FireSting O2, PyroScience GmbH, Aachen, Germany) in four different chambers (one per condition) simultaneously. The rate of oxygen consumption was then calculated according to [32]: RR (O2 μmoles/h) = [C(t 0) − C(t 1)]·(Vr) · 60/(t 1 − t 0), where t 0 and t 1 represent start and finish times (min) of the measurement period; C(t) is concentration of oxygen in the water (μmoles O2 L−1) at time t; and Vr is volume of respirometer minus the animal. Respiration rate correction for body mass was carried out using dry weight [32].
2.3. Heartbeat Rate
Heartbeat rate (HBR, beats/min) was evaluated by means of a noninvasive cardioplethysmographic technique [22, 33]. To record the mussel heartbeat, infrared sensors were glued on the left side of the mussel shell, just below the umbone. To avoid stress to the organism, sensors were positioned the day before the measurement. The heartbeat signals obtained were amplified, filtered, and then detected by means of a portable oscilloscope PicoScope 236 (Pico Technology Ltd., UK) connected to a laptop computer equipped with PicoScope 6.0 software. During the experimental session, HBR was recorded at intervals of 10 minutes per mussel. HBR values for each animal were obtained as the average of 3 randomly selected views of the 10-min measurement. Measurements were made every 2 days on six randomly selected individuals per treatment.
2.4. Absorption Efficiency
Food absorption efficiency was measured by comparing the proportions of organic matter in the algal cells and mussels feces according to the equation of Conover [34]: AE = (F − E)/[(1 − E)F], where F is a relationship between dry weight and ash-free dry weight of algal food while E is a relationship between dry weight and ash-free dry weight of fecal pellets. Fecal pellets from each treatment were collected daily and placed in separate vials. Collected feces were filtered on preweighted glass fiber filters (Whatman GF/C, 0.45 μm) and washed with 0.5 M ammonium formate to remove salts [35]. Then, samples were dried at 100°C for 48 h and dry weights were recorded immediately after cooling in a desiccator. Eventually, samples were ashed in a furnace at 450°C for 2 h and then reweighted to obtain the ash-free dry weight. The results are the average of six different measurements per condition.
2.5. Statistical Analysis
An analysis of variance (ANOVA) was performed using R (software version 2.15.1) to test the dependence of RR, HBR, and AE on AgNP concentration (CONC, fixed, 4 levels) and time (TIME, fixed, 5 levels). The assumption of homoscedasticity was tested using Cochran's C test. Post hoc comparisons were made using the Student-Newman-Keuls test (SNK-test). The alpha values are reported on each table or figure.
3. Results and Discussion
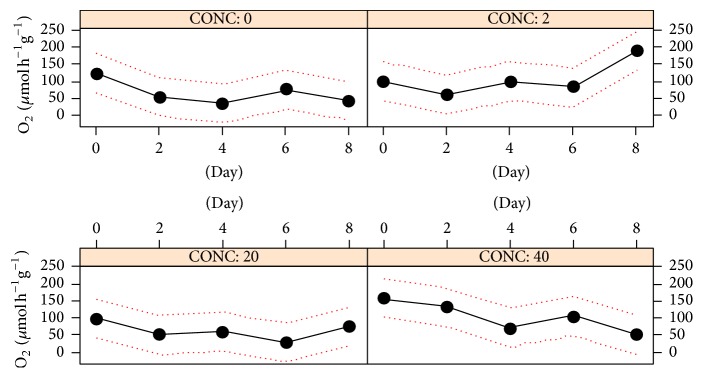
The exposure to 5 nm AgNPs significantly influenced the overall B. pharaonis respiration rate (RR) (Table 1, Figure 1). This is the result of complex dynamics especially occurring in specimens treated with 2 and 40 μg L−1 AgNP during the 8-day exposure (Figure 2). These samples showed opposite temporal trends and seem to reflect different compensation strategies to discrete nanoparticle amounts. The lowest concentration, in fact, determined a progressive increase of oxygen consumption in time and by contrast the highest Ag level caused an increase of respiration during the first part of the exposure, then, followed by a constant decrease to an average value of about 49 μmol h−1 g−1 similar to that of control (Figure 2). The intermediate Ag level displayed negligible effects with respect to control. The average individual respiration rate of control specimens observed in this study was around 10 μM mol O2 h−1. This measure was compatible with previous values reported in the same species [24].
Table 1.
ANOVA details for the effects of 5 AgNP exposure on B. pharaonis normalized respiration rate (RR).
| Source | DF | MS | F | P |
|---|---|---|---|---|
| CONC | 3 | 8570.4 | 3.62 | ∗ |
| TIME | 4 | 5490.6 | 2.32 | ns |
| CONC × TIME | 12 | 4336.8 | 1.83 | ns |
| Residuals | 40 | 2365.7 |
Factors tested for dependence were concentration (CONC) and exposure time (TIME). Shown are DF, degree of freedom; MS, mean square; F, F-test result; and P, P value [∗ = P ≤ 0.05; and ns = no significant difference (P > 0.05)].
Figure 1.
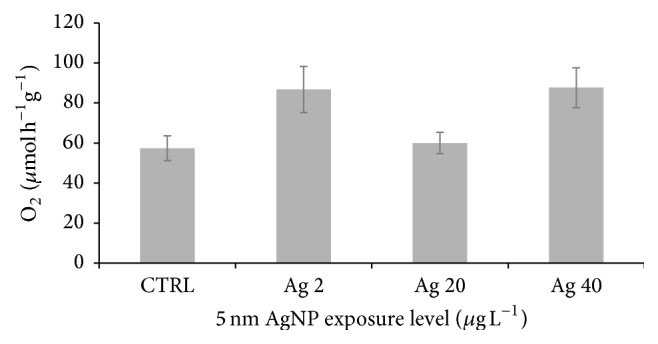
Effects of 5 nm AgNP on average B. pharaonis respiration rates (RR). Shown is the normalized average (±SEM) respiration rate (μmol h−1 g−1) across the 8-day exposure period. The ANOVA post hoc test did not show statistically significant differences between the control not-exposed samples and the AgNP treated ones (TNK-test P > 0.05).
Figure 2.
B. pharaonis respiration rates (RR) dynamics. The plot shows respiration rates (RR, μmol h−1 g−1) versus AgNP concentrations (μg L−1). Continuous line: average RR; dotted-line, 1-SD confidence interval.
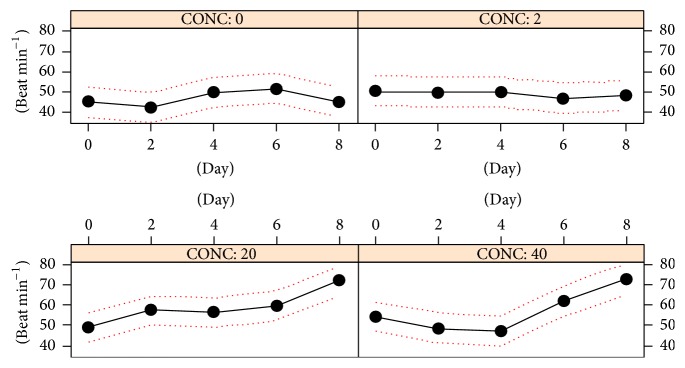
5 nm AgNPs had statistically significant effects on the heartbeat rate (HBR) (Table 2). Figure 3 shows the temporal trends obtained across the 8-day exposure period. These are consistent with an increase of HBR in samples exposed to the highest silver levels, that is, 20 and 40 μg L−1. The ANOVA output (Table 3) clearly indicates that HBRs are higher in the latter samples than in control animals as well as those exposed to 2 μg L−1 AgNPs (Table 3).
Table 2.
ANOVA details for the effects of AgNP exposure on B. pharaonis heart beat rate (HBR).
| Source | DF | MS | F | P |
|---|---|---|---|---|
| CONC | 4 | 215.2 | 5.342 | ∗∗ |
| TIME | 3 | 510.8 | 12.679 | ∗∗∗ |
| CONC × TIME | 12 | 124.8 | 3.098 | ∗∗ |
| Residuals | 40 | 40.3 |
Factors tested for dependence were concentration (CONC) and exposure time (TIME). Shown are DF, degree of freedom; MS, mean square; F, F-test result; and P, P value [∗∗ = P ≤ 0.01; ∗∗∗ = P ≤ 0.001; and ns = no significant difference (P > 0.05)].
Figure 3.
B. pharaonis heartbeat rates (HBRs) dynamics. The plot shows heartbeat rates (HBRs, beat min−1) versus AgNP concentrations (μg L−1). Continuous line: average HBR; dotted-line, 1-SD confidence interval.
Table 3.
ANOVA post hoc comparison for HBR.
| CONC | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 2 | 20 | 20 | 20 | 20 | 20 | 40 | 40 | 40 | 40 | 40 | |
| CONC × | TIME | 0 | 2 | 4 | 6 | 8 | 0 | 2 | 4 | 6 | 8 | 0 | 2 | 4 | 6 | 8 | 0 | 2 | 4 | 6 | 8 |
|
| |||||||||||||||||||||
| 0 | 0 | — | |||||||||||||||||||
| 0 | 2 | ns | — | ||||||||||||||||||
| 0 | 4 | ns | ns | — | |||||||||||||||||
| 0 | 6 | ns | ns | ns | — | ||||||||||||||||
| 0 | 8 | ns | ns | ns | ns | — | |||||||||||||||
| 2 | 0 | ns | ns | ns | ns | ns | — | ||||||||||||||
| 2 | 2 | ns | ns | ns | ns | ns | ns | — | |||||||||||||
| 2 | 4 | ns | ns | ns | ns | ns | ns | ns | — | ||||||||||||
| 2 | 6 | ns | ns | ns | ns | ns | ns | ns | ns | — | |||||||||||
| 2 | 8 | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | ||||||||||
| 20 | 0 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | |||||||||
| 20 | 2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | ||||||||
| 20 | 4 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | |||||||
| 20 | 6 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | ||||||
| 20 | 8 | ∗∗ | ∗∗∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | ∗∗ | ∗∗ | ∗∗ | ns | ns | ns | — | |||||
| 40 | 0 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | ||||
| 40 | 2 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ∗∗ | ns | — | |||
| 40 | 4 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ∗∗ | ns | ns | — | ||
| 40 | 6 | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | — | |
| 40 | 8 | ∗∗∗ | ∗∗∗ | ∗∗ | ∗ | ∗∗∗ | ∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ns | ns | ns | ns | ns | ∗∗ | ∗∗ | ns | — |
SNK-test and P value [∗ = P ≤ 0.05; ∗∗ = P ≤ 0.01; ∗∗∗ = P ≤ 0.001; and ns = no significant difference (P > 0.05)].
Basal HBRs were similar to values previously reported [33].
Finally, no statistically significant effects were found in absorption efficiency (AE) of mussels exposed to AgNPs (P > 0.05). However, Figure 4 depicts a decreasing AE trend across the silver concentration gradient.
Figure 4.
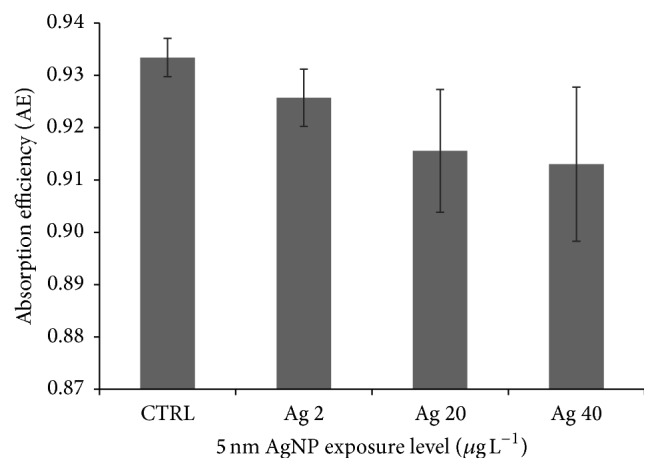
B. pharaonis absorption efficiency (AE). AE average (±SEM) values for the effects of 5 nm AgNP exposure are depicted. The decreasing trend observed was not statistically significant (1-way ANOVA, P = 0.18).
Respiration and heartbeat rates are two important physiological traits in ectothermic organisms, such as bivalves. They may reflect the energetic budget availability thus informing on higher organizational levels, that is, growth and fecundity [36–38]. For this reason RR and HBR have been used to predict physical and chemical stress effects for a long time. Numerous researchers have reported a dose dependent decrease of HBR for the chronic effects of heavy metal exposure, such as copper, however, starting from concentrations higher than 50 μg L−1 [39–41]. It should be pointed out that, in the present study, silver was continuously presented to mussel in a nonreactive form, that is, the elemental state. Preliminary data obtained by our research group suggest a sudden precipitation of AgNP in seawater and a relatively low dissolution rate of silver to Ag+ ions (unpublished observations). This means that the actual bioavailable as well as active silver fraction in the water column was certainly below the nominal concentration used, shifting the actual tested range within a genuine ppb scale. The results presented in this work indicate that AgNPs can affect two important physiological processes of the marine bivalve B. pharaonis, that is, metabolic respiration rate and heartbeat rate. HBRs showed a consistent increase in samples exposed to the two highest AgNP levels (20 and 40 μg L−1) (Figure 3). This finding is only in apparent contrast with the aforementioned previous studies, since our data can be reconciled by hormesis. Hormesis represents a relatively new paradigm in toxicology for which responses to different kinds of toxic agents (or drugs) behave nonlinearly within a threshold level, usually very low and thus disregarded in common toxicological frameworks [42, 43]. Indeed, in specimens of B. pharaonis and Mytilaster minimus subjected to relatively high and recurrent levels of disturbance—such as hypersalinity or hyperthermia—a sharp and continuous increase of HBR has been described before occurring of severe bradycardia [33, 44]. We, therefore, can conclude that the observed HBR changes are likely to represent a compensation strategy to counteract the disturbance level escalation, for example, increasing silver burdens accumulating into mussel soft tissues across the exposure.
The outcome of this study showed a more complex response pattern to 5 nm AgNPs concerning the respiration rate (RR) (Figures 1 and 2). ANOVA pointed out significant effects for the variable concentration (CONC, Table 1) but nonlinear responses were observed (Figure 1). In 40 μg L−1 exposed animals RR dynamics displayed a fair increase followed by acclimation to control values. This is consistent with the acclimation properties of RR, as reported for moderate temperature shift by Widdows [45]. Instead, no response was observed at the intermediate dose and a delayed RR intensification was seen at 2 μg L−1 AgNPs. Similar results were seen in the bivalve Mytilus galloprovincialis Lam. exposed to ZnO NPs for 12 weeks, that is, low-to-intermediate doses did not render a perfect dose response pattern on some physiological and behavioral traits such as RR and survival [46]. These findings confirm that, in general, ENM toxicity should be a function of factors other than the mere nominal tested concentration since the evolution and fate of NPs in the tested environment can eventually influence the internal dose. To this end, it is worth noting that Mullera et al. [47], as part of the same toxicological framework studied by Hanna et al. [46], reported a good correlation between the internal ZnO level and RR. Furthermore, as previously discussed for HBR, nonlinearity (sensu hormesis) represents an intrinsic item of the toxicological response; therefore, RR fluctuations across increasing AgNP doses should be considered a genuine feature of the physiological adaptation process.
Energy flows through the food chain in ecosystems and ecosystems themselves rely on that energy [48]. Our data on AgNP exposure indicated a decreasing trend for food absorption efficiency, although this effect was not statistically significant (Figure 4). This result is not trivial since, as recently demonstrated by our research group [24], even a small reduction in the efficiency to assimilate food may give rise to important impacts in terms of life history trait's magnitude (fecundity and growth rate) or energy allocated to byssus quality and quantity [36], eventually increasing the likelihood of subpopulation dislodgment and/or extinction.
While we are aware that mesocosmal conditions can be highly controlled to generate reliable outcomes [24, 49–51], present results, despite their relevance in an ecotoxicological framework of ENM toxicity assessment, showed that a relatively short exposure period (8 days) is probably not enough to delineate the entire dynamics that characterize Brachidontes sp. response to stress. Therefore, longer exposure period should be envisaged in further studies.
4. Conclusions
This work demonstrates that in marine bivalves basic physiological functions are impacted by relatively low concentrations of AgNP, an emerging contaminant, and supports the use of functional bioenergetics into mechanistic frameworks able to inform on ecological niche changes starting from simple individual responses [52].
Acknowledgments
This study was supported by a grant to Francesco Dondero (CP-FP 247739 NanoFATE) under the European Commission FP7-Program. Authors are grateful to Valeria Palmeri, Valeria Montalto, and Alessandro Rinaldi for their technical contribution and assistance in laboratory.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Massarsky A., Trudeau V. L., Moon T. W. Predicting the environmental impact of nanosilver. Environmental Toxicology and Pharmacology. 2014;38(3):861–873. doi: 10.1016/j.etap.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Vance M. E., Kuiken T., Vejerano E. P., McGinnis S. P., Hochella M. F., Hull D. R. Nanotechnology in the real world: redeveloping the nanomaterial consumer products inventory. Beilstein Journal of Nanotechnology. 2015;6(1):1769–1780. doi: 10.3762/bjnano.6.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Silver Institute and Thomson Reuters. World Silver Survey 2015. A Summary. 2015. [Google Scholar]
- 4.Wijnhoven S. W. P., Peijnenburg W. J. G. M., Herberts C. A., et al. Nano-silver—a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3(2):109–138. doi: 10.1080/17435390902725914. [DOI] [Google Scholar]
- 5.Fabrega J., Luoma S. N., Tyler C. R., Galloway T. S., Lead J. R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environment International. 2011;37(2):517–531. doi: 10.1016/j.envint.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Gottschalk F., Sun T., Nowack B. Environmental concentrations of engineered nanomaterials: review of modeling and analytical studies. Environmental Pollution. 2013;181:287–300. doi: 10.1016/j.envpol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Nowack B. Nanosilver revisited downstream. Science. 2010;330(6007):1054–1055. doi: 10.1126/science.1198074. [DOI] [PubMed] [Google Scholar]
- 8.Baalousha M., Yang Y., Vance M. E., et al. Outdoor urban nanomaterials: the emergence of a new, integrated, and critical field of study. Science of The Total Environment. 2016;557-558:740–753. doi: 10.1016/j.scitotenv.2016.03.132. [DOI] [PubMed] [Google Scholar]
- 9.Wiesner M. R., Lowry G. V., Casman E., et al. Meditations on the ubiquity and mutability of nano-sized materials in the environment. ACS Nano. 2011;5(11):8466–8470. doi: 10.1021/nn204118p. [DOI] [PubMed] [Google Scholar]
- 10.Morones J. R., Elechiguerra J. L., Camacho A., et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 11.Ratte H. T. Bioaccumulation and toxicity of silver compounds: a review. Environmental Toxicology and Chemistry. 1999;18(1):89–108. doi: 10.1897/1551-5028(1999)01860;0089:batosc62;2.3.co;2. [DOI] [Google Scholar]
- 12.Bondarenko O., Juganson K., Ivask A., Kasemets K., Mortimer M., Kahru A. Toxicity of Ag, CuO and ZnO nanoparticles to selected environmentally relevant test organisms and mammalian cells in vitro: a critical review. Archives of Toxicology. 2013;87(7):1181–1200. doi: 10.1007/s00204-013-1079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunningham S., Brennan-Fournet M. E., Ledwith D., Byrnes L., Joshi L. Effect of nanoparticle stabilization and physicochemical properties on exposure outcome: acute toxicity of silver nanoparticle preparations in zebrafish (Danio rerio) Environmental Science and Technology. 2013;47(8):3883–3892. doi: 10.1021/es303695f. [DOI] [PubMed] [Google Scholar]
- 14.Shin S. W., Song I. H., Um S. H. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials. 2015;5(3):1351–1365. doi: 10.3390/nano5031351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walters C. R., Pool E. J., Somerset V. S. Ecotoxicity of silver nanomaterials in the aquatic environment: a review of literature and gaps in nano-toxicological research. Journal of Environmental Science and Health—Part A Toxic/Hazardous Substances and Environmental Engineering. 2014;49(13):1588–1601. doi: 10.1080/10934529.2014.938536. [DOI] [PubMed] [Google Scholar]
- 16.Corsi I., Cherr G. N., Lenihan H. S., et al. Common strategies and technologies for the ecosafety assessment and design of nanomaterials entering the marine environment. ACS Nano. 2014;8(10):9694–9709. doi: 10.1021/nn504684k. [DOI] [PubMed] [Google Scholar]
- 17.Moore M. N. Do nanoparticles present ecotoxicological risks for the health of the aquatic environment? Environment International. 2006;32(8):967–976. doi: 10.1016/j.envint.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Canesi L., Ciacci C., Fabbri R., Marcomini A., Pojana G., Gallo G. Bivalve molluscs as a unique target group for nanoparticle toxicity. Marine Environmental Research. 2012;76:16–21. doi: 10.1016/j.marenvres.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Zuykov M., Pelletier E., Demers S. Colloidal complexed silver and silver nanoparticles in extrapallial fluid of Mytilus edulis . Marine Environmental Research. 2011;71(1):17–21. doi: 10.1016/j.marenvres.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Gomes T., Pereira C. G., Cardoso Ć., et al. Effects of silver nanoparticles exposure in the mussel Mytilus galloprovincialis. Marine Environmental Research. 2014;101(1):208–214. doi: 10.1016/j.marenvres.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy M. P., Carroll D. L., Ringwood A. H. Tissue specific responses of oysters, Crassostrea virginica, to silver nanoparticles. Aquatic Toxicology. 2013;138:123–128. doi: 10.1016/j.aquatox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Burnett L. E., Holman J. D., Jorgensen D. D., Ikerd J. L., Burnett K. G. Immune defense reduces respiratory fitness in Callinectes sapidus, the Atlantic blue crab. The Biological Bulletin. 2006;211(1):50–57. doi: 10.2307/4134577. [DOI] [PubMed] [Google Scholar]
- 23.Sarà G., Vizzini S., Mazzola A. Sources of carbon and dietary habits of new Lessepsian entry Brachidontes pharaonis (Bivalvia, Mytilidae) in the western Mediterranean. Marine Biology. 2003;143(4):713–722. doi: 10.1007/s00227-003-1118-4. [DOI] [Google Scholar]
- 24.Montalto V., Palmeri V., Rinaldi A., Kooijman S. A. L. M., Sarà G. Dynamic energy budget parameterisation of Brachidontes pharaonis, a Lessepsian bivalve in the Mediterranean Sea. Journal of Sea Research. 2014;94:47–51. doi: 10.1016/j.seares.2014.05.007. [DOI] [Google Scholar]
- 25.Chan C. Y. S., Chiu J. M. Y. Chronic effects of coated silver nanoparticles on marine invertebrate larvae: A Proof of Concept Study. PLoS ONE. 2015;10(7, article e0132457) doi: 10.1371/journal.pone.0132457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro F., Gallego-Urrea J. A., Jurkschat K., et al. Silver nanoparticles and silver nitrate induce high toxicity to Pseudokirchneriella subcapitata, Daphnia magna and Danio rerio . Science of the Total Environment. 2014;466-467:232–241. doi: 10.1016/j.scitotenv.2013.06.101. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro F., Gallego-Urrea J. A., Goodhead R. M., et al. Uptake and elimination kinetics of silver nanoparticles and silver nitrate by Raphidocelis subcapitata: the influence of silver behaviour in solution. Nanotoxicology. 2015;9(6):686–695. doi: 10.3109/17435390.2014.963724. [DOI] [PubMed] [Google Scholar]
- 28.Tourinho P. S., Van Gestel C. A. M., Jurkschat K., Soares A. M. V. M., Loureiro S. Effects of soil and dietary exposures to Ag nanoparticles and AgNO3 in the terrestrial isopod Porcellionides pruinosus . Environmental Pollution. 2015;205:170–177. doi: 10.1016/j.envpol.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 29.Tourinho P. S., van Gestel C. A. M., Morgan A. J., et al. Toxicokinetics of Ag in the terrestrial isopod Porcellionides pruinosus exposed to Ag NPs and AgNO3 via soil and food. Ecotoxicology. 2016;25(2):267–278. doi: 10.1007/s10646-015-1585-7. [DOI] [PubMed] [Google Scholar]
- 30.Sarà G., Palmeri V., Rinaldi A., Montalto V., Helmuth B. Predicting biological invasions in marine habitats through eco-physiological mechanistic models: a case study with the bivalve Brachidontes pharaonis . Diversity and Distributions. 2013;19(10):1235–1247. doi: 10.1111/ddi.12074. [DOI] [Google Scholar]
- 31.Prusina I., Sarà G., De Pirro M., et al. Variations in physiological responses to thermal stress in congeneric limpets in the Mediterranean Sea. Journal of Experimental Marine Biology and Ecology. 2014;456:34–40. doi: 10.1016/j.jembe.2014.03.011. [DOI] [Google Scholar]
- 32.Widdows J., Staff F. ICES Techniques in Marine Environmental Sciences. 40. Copenhagen, Denmark: International Council for the Exploration of the Sea; 2006. Biological effects of contaminants: measurement of scope for growth in mussels. [Google Scholar]
- 33.Sarà G., de Pirro M. Heart beat rate adaptations to varying salinity of two intertidal Mediterranean bivalves: the invasive Brachidontes pharaonis and the native Mytilaster minimus . Italian Journal of Zoology. 2011;78(2):193–197. doi: 10.1080/11250001003657360. [DOI] [Google Scholar]
- 34.Conover R. J. Assimilation of organic matter by zooplankton. Limnology and Oceanography. 1966;11(3):338–345. doi: 10.4319/lo.1966.11.3.0338. [DOI] [Google Scholar]
- 35.Zhu C. J., Lee Y. K. Determination of biomass dry weight of marine microalgae. Journal of Applied Phycology. 1997;9(2):189–194. doi: 10.1023/A:1007914806640. [DOI] [Google Scholar]
- 36.Carrington E., Waite J. H., Sarà G., Sebens K. P. Mussels as a model system for integrative ecomechanics. Annual Review of Marine Science. 2015;7:443–469. doi: 10.1146/annurev-marine-010213-135049. [DOI] [PubMed] [Google Scholar]
- 37.Kooijman S. A. L. M. Energy budgets can explain body size relations. Journal of Theoretical Biology. 1986;121(3):269–282. doi: 10.1016/S0022-5193(86)80107-2. [DOI] [Google Scholar]
- 38.Sarà G., Rinaldi A., Montalto V. Thinking beyond organism energy use: a trait-based bioenergetic mechanistic approach for predictions of life history traits in marine organisms. Marine Ecology. 2014;35(4):506–515. doi: 10.1111/maec.12106. [DOI] [Google Scholar]
- 39.Curtis T. M., Williamson R., Depledge M. H. Simultaneous, long-term monitoring of valve and cardiac activity in the blue mussel Mytilus edulis exposed to copper. Marine Biology. 2000;136(5):837–846. doi: 10.1007/s002270000297. [DOI] [Google Scholar]
- 40.Scott D. M., Major C. W. The Effect of Copper (II) on Survival, Respiration, and Heart Rate in the Common Blue Mussel, Mytilus edulis. Biological Bulletin. 1972;143(3):679–688. doi: 10.2307/1540190. [DOI] [PubMed] [Google Scholar]
- 41.Grace A. L., Gainey L. F., Jr. The effects of copper on the heart rate and filtration rate of Mytilus edulis . Marine Pollution Bulletin. 1987;18(2):87–91. doi: 10.1016/0025-326x(87)90574-1. [DOI] [Google Scholar]
- 42.Calabrese E. J. Hormesis: a revolution in toxicology, risk assessment and medicine. EMBO Reports. 2004;5(supplement 1):S37–S40. doi: 10.1038/sj.embor.7400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calabrese E. J., Baldwin L. A. The frequency of U-shaped dose responses in the toxicological literature. Toxicological Sciences. 2001;62(2):330–338. doi: 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- 44.Braby C. E., Somero G. N. Following the heart: temperature and salinity effects on heart rate in native and invasive species of blue mussels (genus Mytilus) Journal of Experimental Biology. 2006;209(13):2554–2566. doi: 10.1242/jeb.02259. [DOI] [PubMed] [Google Scholar]
- 45.Widdows J. Effect of temperature and food on the heart beat, ventilation rate and oxygen uptake of Mytilus edulis . Marine Biology. 1973;20(4):269–276. doi: 10.1007/bf00354270. [DOI] [Google Scholar]
- 46.Hanna S. K., Miller R. J., Muller E. B., Nisbet R. M., Lenihan H. S. Impact of engineered zinc oxide nanoparticles on the individual performance of Mytilus galloprovincialis . PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0061800.e61800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mullera E. B., Hannab S. K., Lenihanb H. S., Millera R. J., Nisbetc R. M. Impact of engineered zinc oxide nanoparticles on the energy budgets of Mytilus galloprovincialis . Journal of Sea Research. 2014;94:29–36. doi: 10.1016/j.seares.2013.12.013. [DOI] [Google Scholar]
- 48.Odum E. P. Energy flow in ecosystems: a historical review. American Zoologist. 1968;8(1):11–18. doi: 10.1093/icb/8.1.11. [DOI] [Google Scholar]
- 49.Manachini B., Arizza V., Rinaldi A., Montalto V., Sarà G. Effect of the commercial BT-based pesticide on eco-physiological response of two marine bivalves. Marine Environmental Research. 2013;83:29–37. doi: 10.1016/j.marenvres.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Gianguzza P., Visconti G., Gianguzza F., Vizzini S., Sarà G., Dupont S. Temperature modulates the response of the thermophilous sea urchin Arbacia lixula early life stages to CO2-driven acidification. Marine Environmental Research. 2014;93:70–77. doi: 10.1016/j.marenvres.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Rinaldi A., Montalto V., Lika K., Sanfilippo M., Manganaro A., Sarà G. Estimation of dynamic energy budget parameters for the Mediterranean toothcarp (Aphanius fasciatus) Journal of Sea Research. 2014;94:65–70. doi: 10.1016/j.seares.2014.05.006. [DOI] [Google Scholar]
- 52.Kearney M., Simpson S. J., Raubenheimer D., Helmuth B. Modelling the ecological niche from functional traits. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1557):3469–3483. doi: 10.1098/rstb.2010.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]