Abstract
Experimental vaccines based on recombinant vesicular stomatitis viruses (VSV) expressing foreign viral proteins are protective in several animal disease models. Although these attenuated viruses are nonpathogenic in nonhuman primates when given by nasal, oral, or intramuscular routes, they are pathogenic in mice when given intranasally, and further vector attenuation may be required before human trials with VSV-based vectors can begin. Mutations truncating the VSV glycoprotein (G) cytoplasmic domain from 29 to 9 or 1 amino acid (designated CT9 or CT1, respectively) were shown previously to attenuate VSV growth in cell culture and pathogenesis in mice. Here we show that VSV recombinants carrying either the CT1 or CT9 deletion and expressing the human immunodeficiency virus (HIV) Env protein are nonpathogenic in mice, even when given by the intranasal route. We then carried out a detailed analysis of the CD8+ T-cell responses, including in vivo cytotoxic T-cell activity, induced by these vectors. When given by either the intranasal or intraperitoneal route, the VSV-CT9 vector expressing HIV Env elicited primary and memory CD8+ T-cell responses to Env equivalent to those elicited by recombinant wild-type VSV expressing Env. The VSV-CT1 vector also induced potent CD8+ T-cell responses after intraperitoneal vaccination, but was less effective when given by the intranasal route. The VSV-CT1 vector was also substantially less effective than the VSV-CT9 or wild-type vector at inducing antibody to Env. The VSV-CT9 vector appears ideal because of its lack of pathogenesis, propagation to high titers in vitro, and stimulation of strong cellular and humoral immune responses.
Vesicular stomatitis virus (VSV) is a membrane-bound enveloped virus and the prototypic member of the Rhabdoviridae family. VSV has a single negative-stranded RNA genome that encodes five structural proteins: the nucleocapsid (N), the phosphoprotein (P), the matrix protein (M), the glycoprotein (G), and the RNA-dependent RNA polymerase (L). VSV causes a self-limiting disease in livestock, resulting in lesions on the gums, mouth, and teats. Infection of humans with VSV is rare and often asymptomatic, but can result in mild flu-like symptoms (reviewed in reference 24).
Our laboratory established a system for recovery of recombinant VSVs (rVSVs) from plasmid DNA (14) and developed VSV as a vector for high-level expression of foreign genes (29). We have also explored the use of VSV recombinants expressing foreign genes as experimental vaccines (12, 20, 22, 23, 25, 27). For example, VSV recombinants expressing human immunodeficiency virus (HIV) 89.6 Env and simian immunodeficiency virus (SIV) mac239 Gag have protected macaques from progression to AIDS for over 3 years following challenge with SHIV 89.6P (25).
In addition to being effective, human vaccines must be safe for use in both healthy and immunocompromised people. Vaccines based on live viruses are typically highly effective but are normally attenuated by multiple mutations before they are considered safe for human use. The recombinant wild-type VSV (rVSV) we have used as an experimental vaccine vector is attenuated for pathogenesis in mice compared to wild-type VSV (23), although the specific mutation(s) responsible for the attenuated phenotype has not been identified. This recombinant also does not cause vesicular stomatitis in cattle (L. Rodriguez, personal communication). Despite the lack of pathogenesis of this attenuated rVSV in nonhuman primates when given by the oral, nasal, or intramuscular route (25), additional specific attenuating mutations are likely to be required before human trials with VSV-based vaccine vectors can be conducted.
Previous studies have shown that deletion mutations resulting in truncations of the VSV G cytoplasmic tail from 29 amino acids to 9 or 1 amino acid reduce or eliminate all vector-associated pathogenesis (weight loss) after intranasal (i.n.) immunization of 6-week-old mice (23). In contrast, i.n. inoculation of mice with rVSV lacking these mutations typically causes a weight loss of 15 to 17% at day 4 postinoculation, followed by complete recovery (23). The highly attenuated vectors, designated VSV-CT9 and VSV-CT1, grow to lower titers than rVSV in vitro but retain their ability to replicate in vivo and induce immune responses in mice. Our laboratory reported previously that immunization of mice with VSV-CT1 expressing influenza virus hemagglutinin (HA) induced humoral immune responses to HA (22). The antibody response to HA encoded by the VSV-CT1 vector was reduced 16-fold compared with that induced by the rVSV expressing HA, but the reduced response was still sufficient to protect mice from influenza virus challenge (22). Induction of cellular immune responses to foreign proteins by highly attenuated VSV vectors has not been evaluated previously, nor has the effect of route of administration been examined.
In the present study we demonstrated that highly attenuated vectors expressing HIV Env are nonpathogenic in mice. In addition, we have carried out a detailed analysis of the immune responses generated to HIV Env by these vectors. Emphasis was placed on evaluation of the cellular immune responses by using state-of-the art techniques, including in vivo cytotoxic T-lymphocyte (CTL) assays, as well as the effect of the route of vaccination on the responses to the attenuated vectors.
MATERIALS AND METHODS
Construction of plasmids and recovery of recombinant viruses.
The plasmids used to recover the rVSVs diagrammed in Fig. 1 were constructed as follows. Plasmid pVSVCT1-HA (22) and an analogous pVSVCT9-HA were digested with XmaI and NotI to release the 1.7-kb insert encoding HA, and the vector was purified. A 2.2-kb DNA fragment encoding HIV Env with the cytoplasmic tail of VSV G was amplified from pBSEnvG709 (19) using the forward primer 5′-AGCTAGCTCCCGGGATTATGAGAGTGAAGGAGAAATATC-3′ and the reverse primer 5′-GGCCTTAAGGGCGGCCGCTTACTTTCCAAGTCGGTTCATCTC-3′. These primers introduced the underlined XmaI and NotI sites upstream and downstream of the EnvG coding sequence. The PCR product was digested with XmaI and NotI, purified, and ligated into the pVSVCT1 or pVSVCT9 vector that had been digested with the same enzymes. Plasmids were recovered after transformation of Escherichia coli and purified using a Maxi kit (QIAGEN). The insert sequences were verified (Yale Keck Sequencing Facility).
FIG. 1.
Viral genomes and protein expression. (A) Gene orders in VSV recombinants, diagrammed 3′ to 5′ on the negative-strand RNA genome. The 29-amino-acid cytoplasmic tail of VSV G has been truncated to 9 amino acids (CT9) or 1 amino acid (CT1) in the attenuated vectors. (B) SDS-PAGE of lysates of BHK cells infected with the indicated viruses and labeled with [35S]methionine. The gel image was collected on a PhosphorImager. Positions of VSV and EnvG proteins are indicated.
Recombinant viruses were recovered from these plasmids as described previously (14). Briefly, BHK-21 cells were grown to 50% confluency and infected at a multiplicity of infection (MOI) of 10 with vTF7-3, vaccinia virus expressing T7 polymerase (7). One hour after infection, cells were transfected with 10 μg of pVSVCT9-EnvG or pVSVCT1-EnvG along with 3 μg of pBS-N, 5 μg of pBS-P, 1 μg of pBS-L, and 4 μg of pBS-G. Medium containing virus was collected about 24 h after cytopathic effect was seen. Stocks were grown on BHK-21 cells from individual plaques and stored at −80°C. Stocks of VSV-EnvG described previously (10) and rVSV (14) (described in Fig. 1) were grown and titers were determined on BHK-21 cells.
Indirect immunofluorescence staining and microscopy were performed initially to confirm VSV G and HIV Env expression in cells infected with the recombinants. BHK cells on coverslips were infected with virus at an MOI of 10. After 8 h cells were fixed with 3% formaldehyde and incubated with mouse monoclonal antibodies I1 and I14 to VSV G followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody to confirm VSV G expression. Separate coverslips were incubated with polyclonal sheep anti-gp120 (1:100; AIDS Research and Reference Reagent Program) followed by FITC-conjugated donkey anti-sheep antibody (1:50).
Metabolic labeling and SDS-PAGE.
To confirm expression of proteins of the appropriate size, BHK cells (106) were infected at an MOI of 20 with VSV recombinants. After 5 h, medium was removed and cells were washed twice with methionine-free Dulbecco's modified Eagle's medium (DMEM). Methionine-free DMEM (1 ml) containing 100 μCi of [35S]methionine was added to each plate for two additional hours. Medium was removed, cells were washed with phosphate-buffered saline (PBS), lysed with 500 μl of detergent solution (1% Nonidet P-40, 0.4% deoxycholate, 50 mM Tris-HCl [pH 8], 62.5 mM EDTA) on ice for 5 min, and collected into 1.5-ml Eppendorf tubes. The protein extracts were centrifuged for 2 min at 16,000 × g to remove the nuclei and stored at −20°C. Protein extracts were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide),and proteins were visualized on a PhosphorImager (Molecular Dynamics).
Inoculation of mice.
Six-week-old female BALB/c mice were obtained from Charles River Laboratories and kept for at least 1 week before experiments were initiated. Mice were housed in microisolator cages in a biosafety level 2-equipped animal facility. Viral stocks were diluted to appropriate titers in serum-free DMEM. For intraperitoneal (i.p.) vaccination, mice were injected with 5 × 106 PFU of virus in 125 μl. For i.n. vaccination, mice were lightly anesthetized with methoxyflurane (Metafane; Medical Developments Australia Pty. Ltd.) and administered 106 to 107 PFU of virus in a 25-μl volume. The Institutional Animal Care and Use Committee of Yale University approved all animal experiments done in this study.
Tetramer assay.
Splenocytes were obtained by disrupting spleens between the frosted ends of two microscope slides. Red blood cells were removed using red blood cell lysing buffer (Sigma). Staining was performed on freshly isolated splenocytes as previously described (9). Briefly, approximately 5 × 106 cells were added to the wells of a 96-well V-bottom plate and were blocked with unconjugated streptavidin (Molecular Probes) and Fc block (Pharmingen) for 15 min at room temperature (RT). Following a 5-min centrifugation at 500 × g, splenocytes were labeled with a FITC-conjugated anti-CD62L antibody or FITC-conjugated anti-CD44 (Pharmingen), an allophycocyanin-conjugated anti-CD8 antibody (Pharmingen), and tetramer for 30 min at RT. The tetramer was a phycoerythrin-conjugated major histocompatibility complex (MHC) class I Dd tetramer (provided by the NIAID Tetramer Facility) containing the Env peptide p18-I10 (N-RGPGRAFVTI-C; Research Genetics). CD8+ T cells which were tetramer+ and activated (CD62Llo or CD44hi) were identified. Animals vaccinated with rVSV were used to determine background levels of tetramer binding. Background was <0.5% for mice infected i.p. and <0.2% for mice infected i.n. and was subtracted from all reported percentages.
Intracellular cytokine assay.
The assay for intracellular cytokine production was performed essentially as previously described (9). Briefly, splenocytes were obtained as described above. Stimulated and unstimulated cell populations were made by adding 5 × 106 splenocytes to each well of a 24-well plate. To the stimulated well, 1 μg of Env peptide p18-I10 (N-RGPGRAFVTI-C; Research Genetics)/ml was added, and the cells were incubated for 2 h at 37°C with 5% CO2. After 2 h, 1 μg of brefeldin A (Golgi Plug kit; Pharmingen) was added to each well and cells were incubated for an additional 4 h. The cells were transferred to a V-bottom 96-well plate and labeled with tetramer for 15 min at RT. After 15 min, 1 μl of allophycocyanin-conjugated anti-CD8 antibody was added, and incubation continued for an additional 15 min. The cells were washed and resuspended in 100 μl of fix solution (2% formaldehyde in PBS) for 10 min at RT. The cells were washed and permeabilized for 5 min at RT in 100 μl of 0.1% Saponin (Sigma, St. Louis, Mo.). The cells were pelleted and stained for 15 min at RT with FITC-conjugated anti-gamma-interferon (anti-IFN-γ) antibody diluted in Saponin. The percentage of IFN-γ-producing tetramer+ CD8+ T cells was identified.
CTL assay in vivo.
This assay was performed as described previously (5) using Env peptide p18-I10 (N-RGPGRAFVTI-C; Research Genetics). On day 7 postinoculation, splenocytes were obtained as described above from an uninfected mouse and resuspended in 1 ml of 5% fetal bovine serum-DMEM. The donor (target) cells were split into two populations. Env p18-I10 peptide was added to one population (+peptide) to a final concentration of 10−6 M, and to the other population no peptide was added (−peptide). Cells were incubated at 37°C in 5% CO2 for 45 min with occasional mixing. Cells were washed and resuspended in 1 ml of PBS. One milliliter of 10 μM 5,6-carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, Oreg.) was added to +peptide cells (final concentration, 5 μM) to generate a CFSEhi group, and 1 ml of 1 μM CFSE was added to −peptide cells (final concentration, 0.5 μM) to generate the CFSElo group. Cells were vortexed as the CFSE was added and then incubated for 5 min at RT. Cells were then washed three times in PBS and resuspended in PBS at a concentration of 108 cells/ml. Ten million cells (100 μl) were injected intravenously (i.v.) into vaccinated or control (immunized with rVSV) mice. After 4 h, the recipient mice were euthanized and spleens were obtained and prepared as above. CFSEhi and CFSElo populations were identified by flow cytometry. Percent specific lysis was calculated by using the following formula: percent specific lysis = [1 − (ratio for vaccinated mice/ratio for control mice)] × 100, where “ratio” = (percent CFSElo/percent CFSEhi).
ELISA.
Blood was obtained from mice at 4 days prior to vaccination and at 56 and 84 days after vaccination. Serum was collected and heat inactivated as previously described (26). Preparation of the antigen, gp140 from recombinant vaccinia virus vBD1, was previously described, along with the enzyme-linked immunosorbent assay (ELISA) procedure (26). Twenty microliters of gp140 was used in each well, and the serum was diluted in PBS from 1:25 to 1:1,600. Plates were read in a Bio-Rad ELISA plate reader at an absorbance of 415 nm. Backgrounds obtained from preimmune serum were subtracted. An absorbance of 0.1 above background was used to determine endpoint titers.
Statistical analysis.
Statistical analysis was done using the two-tailed Mann-Whitney test (Prism software). Results were considered significant when P was <0.05.
RESULTS
Construction of attenuated recombinants expressing HIV Env.
Truncations of the VSV G cytoplasmic domain are known to reduce VSV growth in culture (28) and pathogenicity in mice (23). To determine the extent to which the attenuation affects immune responses to foreign proteins encoded by these vectors, we constructed two VSV vectors expressing an HIV envelope protein, designated EnvG, with its cytoplasmic domain replaced with that from VSV G. This modification of Env enhances its incorporation into VSV particles (10) and was used initially because it enhanced antibody responses to Env (unpublished results). The vectors expressing this EnvG protein were designated VSV-CT9EnvG and VSV-CT1EnvG. The numbers indicate the number of cytoplasmic amino acids remaining from the original 29-amino-acid cytoplasmic domain on the VSV G protein (Fig. 1A). Note that these mutations are present only in the VSV G protein and not in the EnvG proteins.
To confirm appropriate protein expression by these recombinant viruses, we labeled infected BHK cells with [35S]methionine. When lysates of infected cells were analyzed by SDS-PAGE, a protein of approximately 145 kDa corresponding to HIV EnvG appeared in lysates of cells infected with VSV EnvG, VSV-CT9EnvG, and VSV-CT1EnvG (Fig. 1B). The mobilities of the CT9 and CT1 G proteins were also consistent with truncations of 20 or 28 amino acids from the cytoplasmic domain (Fig. 1B).
Recombinant viruses are attenuated for pathogenesis in vivo.
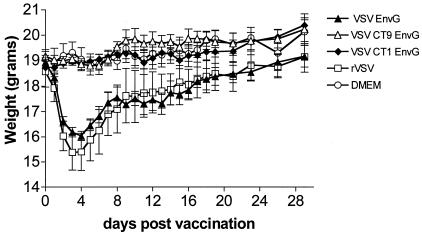
Mice infected i.n. with rVSV typically lose weight for 3 to 7 days after infection. The weight loss is transient, with animals recovering to their preinfection weight by about 2 weeks postinfection (23). We used weight loss as a measure of the degree of attenuation of the recombinant vectors expressing HIV EnvG.
We infected groups of 7-week-old BALB/c mice i.n. with rVSV, VSV-EnvG, VSV-CT9EnvG, or VSV-CT1EnvG virus and weighed them daily. Mice infected with rVSV lost an average of 17.6% of preinfection body weight by day 4 postvaccination and then began to recover (Fig. 2). Mice infected with VSV-EnvG lost slightly less weight (15.4% by day 4) than those infected with rVSV, although the difference between these groups was not statistically significant. In contrast, mice infected with the highly attenuated VSV-CT9EnvG or VSV-CT1EnvG did not lose weight, and the differences in average weights (day 4) between these animals and those receiving VSV-EnvG were highly significant (P = 0.0001). Mice infected with VSV-CT9EnvG and VSV-CT1EnvG also appeared healthy, while those infected with rVSV or VSV-EnvG had ruffled fur and were less active during the first week after vaccination.
FIG. 2.
Pathogenesis measured by weight loss after vaccination. Seven-week-old female BALB/c mice were inoculated i.n. with VSV-EnvG (solid triangles), VSV-CT9EnvG (open triangles), VSV-CT1EnvG (solid diamonds), rVSV (open squares), or DMEM (open circles). Mice were weighed (minimum of three mice per group) each day after vaccination at the same time of day. Error bars are the standard errors of the means.
Analysis of CD8+ T-cell response with MHC I tetramers and intracellular cytokine staining after i.p. vaccination.
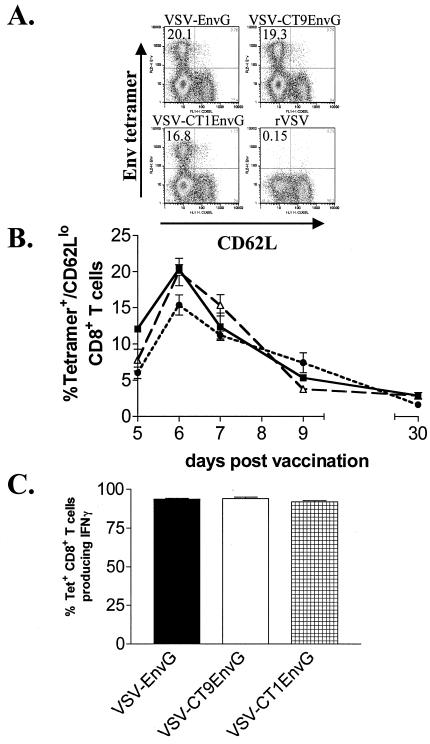
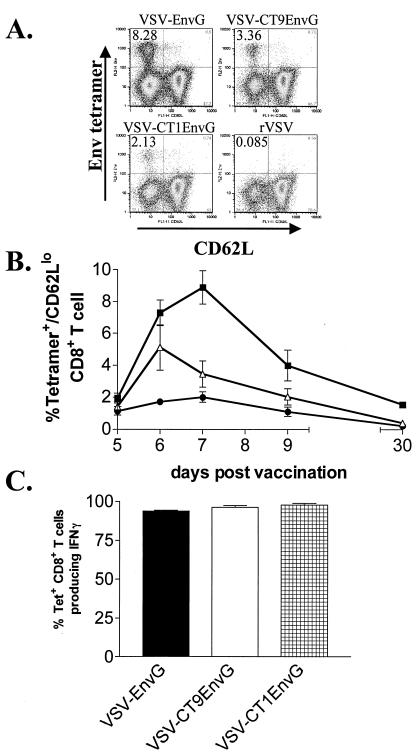
Previously, our laboratory reported that VSV vectors expressing HIV Env elicit strong CD8+ T-cell responses in mice (9). In order to determine the degree to which attenuation of VSV affected the development of immune responses to HIV Env, we compared CD8+ T-cell responses elicited by VSV-EnvG, VSV-CT9EnvG, and VSV-CT1EnvG. Accordingly, we vaccinated mice i.p. with each virus and initially measured the appearance of Env-specific CD8+ T cells using an MHC I tetramer (H-2Dd) bound to the immunodominant Env peptide p18-I10 (N-RGPGRAFVTI-C) (30). As a measure of activation, we identified cells which expressed low amounts of CD62L, a lymph node-homing receptor which is down regulated upon recent activation (1, 2, 11, 17). Activated (CD62Llo), tetramer+, CD8+ splenocytes were enumerated using flow cytometry at days 5, 6, 7, 9, and 30 (Fig. 3B).
FIG. 3.
Detection of antigen-specific CD8+ T cells after i.p. vaccination. Mice were vaccinated i.p. with VSV-EnvG, VSV-CT9EnvG, or VSV-CT1EnvG. Splenocytes were isolated and stained with anti-CD8, Env tetramer, and anti-CD62L. (A) Representative fluorescence-activated cell sorter (FACS) plots (pregated on CD8+ T cells) at 6 days postvaccination for the indicated viruses. Numbers in the upper left corner of each plot indicate the percentage of CD8+ T cells that were Env tetramer+ and CD62Llo. (B) Graph of the percentages of Env tetramer+ CD62Llo CD8+ T cells at the indicated days postvaccination with VSV-EnvG (filled squares), VSV-CT9EnvG (open triangles), or VSV-CT1EnvG (filled circles). Error bars are the standard error of the mean (three to five mice per point). Background from mice vaccinated with rVSV (≥0.40%) was subtracted in each case. (C) Analysis of IFN-γ production by intracellular cytokine staining. Seven days postvaccination, splenocytes were isolated and analyzed for intracellular IFN-γ production. Bars represent the percentage of tetramer-specific CD8+ T cells producing IFN-γ upon stimulation ± the standard error of the mean. At least three animals were analyzed for each time point.
For independent confirmation of the activation, we separately identified Env-specific CD8+ T cells which expressed high levels of CD44, a surface protein required for lymphocyte extravasation to inflammatory sites (4, 6) (data not shown).
The peak of activated (CD62Llo), tetramer+, CD8+ T cells elicited by all viruses was on day 6 postvaccination (Fig. 3B). Consistent with the idea that less-attenuated viruses might produce a stronger immune response, mice infected with VSV-EnvG and VSV-CT9EnvG had the greatest number of Env-specific T cells (20.4% ± 0.6% and 19.9% ± 1.9%, respectively [means ± standard errors of the means]). Mice infected with VSV-CT1EnvG produced only slightly fewer tetramer+ cells (15.3% ± 1.4%), but the difference between VSV-CT1EnvG and the less-attenuated vectors was statistically significant (P = 0.0167). After day 6, the percentage of antigen-specific T cells declined in all animals until day 30, at which time a stable population was present in mice vaccinated with VSV-EnvG (2.9% ± 0.45%), VSV-CT9EnvG (2.9% ± 0.28%), and VSV-CT1EnvG (1.7% ± 0.34%) (Fig. 3B).
While all three viruses elicited high numbers of antigen-specific T cells, it was important to determine whether these cells were functional. We used intracellular cytokine staining to measure the ability of the tetramer+ CD8+ T cells to produce IFN-γ as an initial measure of CD8+ T-cell function. At 7 days postvaccination, nearly all (92 to 94%) of the tetramer-specific CD8+ T cells from all three EnvG groups produced IFN-γ in response to peptide stimulation (Fig. 3C).
Direct measurement of CTL killing in vivo after i.p. vaccination.
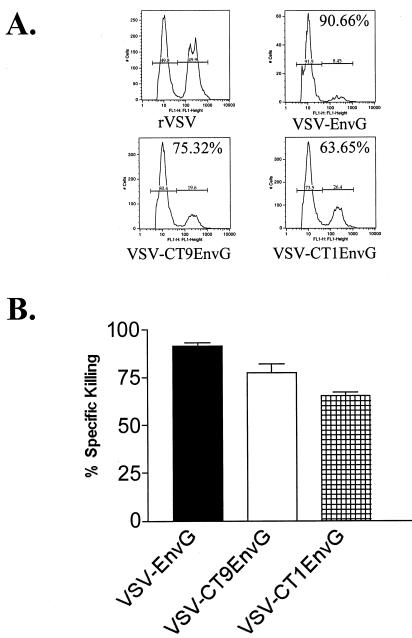
To determine if the antigen-specific T cells generated by vaccination were cytotoxic, we performed CTL assays in vivo using an assay described previously (5). The assay measures CTL activity by quantifying loss of target cells loaded with Env peptide in vaccinated or control animals. Splenocytes from a naïve mouse were used to create two populations of cells, one labeled with a high concentration of CFSE and loaded with p18-I10 Env peptide and the other labeled with a low concentration of CFSE and lacking peptide. Vaccinated mice were then injected i.v. with equal numbers of these cells, and killing of the target population by CTL was measured by flow cytometry. Mice vaccinated i.p. with VSV-EnvG or VSV-CT9EnvG were able to kill 83% of target cells (Fig. 4B). Killing of target cells in mice vaccinated with VSV-CT1EnvG was slightly less efficient (74%) (Fig. 4B).
FIG. 4.
Quantitation of cytotoxic T-cell killing in vivo after i.p. vaccination. Mice were inoculated i.p. with rVSV (n = 3), VSV-EnvG (n = 3), VSV-CT9EnvG (n = 3), and VSV-CT1EnvG (n = 3). At 7 days postvaccination, mice were injected i.v. with two populations of splenocytes from naïve mice. One population was loaded with p18-I10 Env peptide and labeled with a high concentration of CFSE, and the other population was not loaded with peptide and was labeled with a low concentration of CFSE. (A) Representative FACS histograms for each group. (B) Percentages of CFSEhi and CFSElo seen in mice that had been infected with rVSV (53.5 and 45.7%), VSV-EnvG (15 and 83.6%), VSV-CT9 EnvG (14.5 and 84.2%), and VSV-CT1EnvG (21.2 and 77.7%) were used to calculate percent specific killing. Percent specific killing of peptide-loaded cells in vaccinated mice was obtained as described in Materials and Methods. Mice vaccinated with rVSV were used as a control. The bars show the average percent specific killing for each group. Error bars indicate standard errors of the means.
Antibody response post-i.p. vaccination.
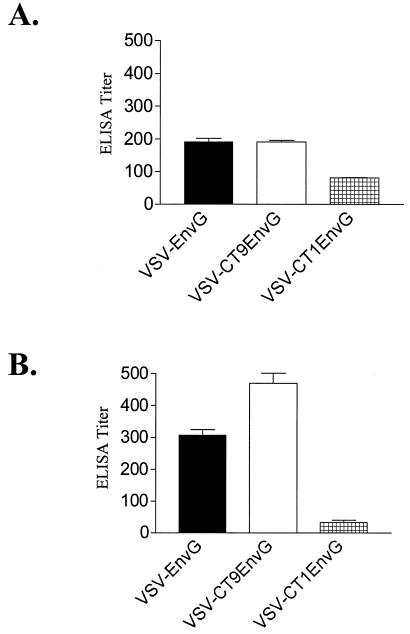
Postinfection CTL responses to HIV or SIV infection are important in controlling viral load (3, 13). However, vaccine-induced CTL responses are unlikely to be able to prevent infection (reviewed in reference 15). In contrast, high levels of neutralizing antibodies are able to prevent infection by SIV or SHIV in nonhuman primate models (16). We therefore wanted to determine to what extent the antibody responses elicited by our attenuated vectors were affected. At 3 months after i.p. vaccination, we measured the serum antibody response to HIV Env using an ELISA that detects immunoglobulin G and immunoglobulin M antibody to oligomeric gp140 (21). Mice vaccinated with VSV-EnvG had a serum ELISA titer of 190 ± 11.5 to gp140, and mice vaccinated with VSV-CT9EnvG had an equivalent titer of 190 ± 5.77 (Fig. 5A). However, mice vaccinated with VSV-CT1EnvG generated a lower titer of 81.0 ± 1.00, indicating some attenuation with respect to antibody induction (Fig. 5A). We did not attempt to measure neutralizing antibody directed to HIV Env, because previous studies have shown that it does not appear until after boosting of responses with VSV envelope exchange vectors expressing HIV Env (26).
FIG. 5.
ELISA of antibody response after i.p. and i.n. vaccination. Mice were vaccinated either i.p. (A) or i.n. (B) with VSV-EnvG (solid bars), VSV-CT9EnvG (open bars), or VSV-CT1EnvG (hatched bars). Eighty-four days postvaccination, serum antibody titers to gp140 were analyzed using ELISA. Bars indicate the reciprocal dilution at which absorbance at 415 nm was 0.1 over the background. All bars indicate a pooled serum from at least three mice (± the standard error of the mean of triplicate wells). Background was obtained with control serum from unvaccinated mice.
CD8+ T-cell response to HIV Env following i.n. vaccination.
Intranasal immunization with VSV vectors is an excellent route for generation of antigen-specific T cells in mice (9) and also would be a convenient route for mass immunization of humans with a VSV-based AIDS vaccine. We therefore determined how effective the highly attenuated vectors were when given by this route. We vaccinated mice and determined the percentage of activated, Env tetramer-specific CD8+ T cells elicited 5, 6, 7, 9, and 30 days postvaccination (Fig. 6B).
FIG. 6.
Detection of antigen-specific CD8+ T cells after i.n. vaccination. Mice were vaccinated i.n. with VSV-EnvG, VSV-CT9EnvG, or VSV-CT1EnvG. Splenocytes were isolated and stained with anti-CD8, Env tetramer, and anti-CD62L. (A) Representative FACS plots (pregated on CD8+ T cells) at 7 days postvaccination for the indicated viruses. Numbers in the upper left corner of each plot indicate the percentage of CD8+ T cells that were Env tetramer+ and CD62Llo. (B) Graph of the percentages of Env tetramer+, CD62Llo CD8+ T cells at the indicated days postvaccination with VSV-EnvG (filled squares), VSV-CT9EnvG (open triangles), or VSV-CT1EnvG (filled circles). Error bars are standard errors of the means (three to five mice per point). Background from mice vaccinated with rVSV (≥0.16%) was subtracted in each case. (C) Analysis of IFN-γ production by intracellular cytokine staining. Seven days postvaccination, splenocytes were isolated and analyzed for intracellular IFN-γ production. Bars represent percentage of tetramer-specific CD8+ T cells producing IFN-γ after stimulation with peptide in animals vaccinated with the indicated viruses (error bars are standard errors of the means). At least three animals were analyzed for each time point.
In all cases, vaccination by the i.n. route resulted in production of two- to sevenfold-fewer antigen-specific CTL than seen after i.p. vaccination (P = 0.001). Interestingly, there was a strong correlation between the degree of attenuation and the decrease in immune response with i.n. immunization. For example, the peak response to VSV-EnvG declined only twofold when the i.p. and i.n. routes were compared, while the peak response to the more-attenuated VSV-CT9EnvG declined fourfold and that to the most-attenuated VSV-CT1EnvG decreased sevenfold.
The peak of 8.90% ± 1.0% activated, Env-specific CD8+ T cells elicited by VSV-EnvG was on day 7 postvaccination. The VSV-CT9EnvG peak of 5.1% ± 1.4% appeared on day 6. The peak of the Env-specific CD8+ T-cell response to VSV-CT1EnvG occurred 7 days postvaccination and equaled 2.0% ± 0.3% (Fig. 6B). In all vaccinated animals, a distinct population of Env tetramer-specific, activated CD8+ T cells remained on day 30 postvaccination (Fig. 6B). The average percentage of tetramer-specific CD8+ T cells elicited in mice that received VSV-EnvG was 1.5% ± 0.1%, for VSV-CT9EnvG it was 0.4% ± 0.07%, and for VSV-CT1EnvG it was 0.23% ± 0.07%. A background of 0.08% (determined in mice receiving rVSV) was subtracted from these values.
We used intracellular cytokine staining to measure function of the Env-specific CD8+ T cells produced and found that 93 to 97.5% of Env-specific CD8+ T cells elicited on day 7 produced IFN-γ upon stimulation with Env peptide. (Fig. 6C).
CTL function in vivo after i.n. vaccination.
While i.n. immunization induced fewer numbers of antigen-specific T cells, an important question is whether this reduction significantly affects the ability of the host to kill cells presenting HIV Env. To determine cytotoxic capabilities of the CD8+ T cells produced by i.n. vaccination, we used an in vivo CTL assay. VSV-EnvG showed a specific killing of 91.6% ± 1.8% of Env-loaded splenocytes 7 days postvaccination. The percent specific killing of the attenuated vectors decreased as they became more attenuated but still showed high percentages, with 77.5% ± 4.9% for VSV-CT9EnvG and 65.5% ± 1.8% for VSV-CT1EnvG (Fig. 7). These high percentages suggest that while fewer antigen-specific CTL are produced after vaccination with the VSV-CT9 and VSV-CT1 vectors, the CTL that are present are functional and capable of detecting and killing peptide-loaded cells.
FIG. 7.
Quantitation of cytotoxic T-cell killing in vivo after i.n. vaccination. Mice were inoculated i.n. with rVSV (n = 3), VSV-EnvG (n = 3), VSV-CT9EnvG (n = 3), or VSV-CT1EnvG (n = 3). At 7 days postvaccination, mice were injected i.v. with two populations of splenocytes from naïve mice. One population was loaded with p18-I10 Env peptide and labeled with a high concentration of CFSE, and the other population was not loaded with peptide and was labeled with a low concentration of CFSE. (A) Representative FACS histograms for each group. (B) Percentages of CFSEhi and CFSElo seen in mice that had been infected with rVSV (49.9 and 49.8%), VSV-EnvG (8.45 and 91.5%), VSV-CT9 EnvG (19.6 and 80.4%), and VSV-CT1EnvG (26.4 and 73.5%) were used to calculate percent specific killing. Percent specific killing of peptide-loaded cells in vaccinated mice was obtained as described in Materials and Methods. Mice vaccinated with rVSV were used as a control. Bars show average percent specific killing for each group. Error bars indicate the standard error of the mean.
Antibody response post-i.n. vaccination.
We obtained serum from vaccinated mice 3 months postvaccination to measure the ELISA titer to oligomeric gp140 after i.n. immunization with these vectors (Fig. 5B). Vaccination with VSV-EnvG produced an antibody titer of 306.7 ± 17.6, while vaccination with VSV-CT9EnvG elicited a somewhat higher titer of 470.0 ± 32.1. In contrast, vaccination with VSV-CT1EnvG produced a much lower antibody titer of 33.3 ± 6.7 (Fig. 5B).
DISCUSSION
Vaccines based on live-attenuated, replicating viruses typically activate multiple components of the immune system and elicit a balanced immune response, including antibody- and cell-mediated responses. There is, however, a critical balance between the degree of attenuation of viral replication and pathogenesis and maintenance of immunogenicity (18). Here we investigated the degree of attenuation of pathogenesis and its effect on generation of cellular immune responses for two highly attenuated VSV-based vectors with truncations in the cytoplasmic domain of the single viral glycoprotein G. Both of these vectors expressed an HIV Env protein, allowing us to carry out a detailed evaluation of the effects of attenuation on the cellular immune responses to a foreign protein.
The most important finding of our study is that a VSV vector with the cytoplasmic tail of VSV G truncated from 29 to 9 amino acids (and expressing HIV Env) was completely attenuated for pathogenesis in mice when delivered via the nasal route, yet still induced cellular immune responses to Env nearly comparable to those induced by the rVSV vector. This VSV-CT9 vector was highly effective when given by either the i.n. or i.p. routes and also induced levels of antibody to Env similar to those induced by the rVSV vector. VSV-CT9 grows to titers that are about twofold reduced compared to rVSV (28), suggesting that it could be easily produced in the quantities that would be required for mass vaccination.
Further attenuation of the rVSV vector by truncation of its G cytoplasmic tail to 1 amino acid generates a VSV-CT1 vector that grows to approximately 10-fold-lower titers than rVSV (28) and induces 4- to 5-fold-lower cellular immune responses compared to rVSV when given by the nasal route. Despite the clear reduction in cellular immune responses as measured by MHC class I tetramer staining, the highly attenuated VSV-CT1 vector still induced 65% killing in in vivo CTL assays, compared to 78% for CT9 and 91% for rVSV. The lower level of CTL activity induced by the CT1 vector may be sufficient to limit HIV replication, but further experimentation in nonhuman primates is required to test the efficacy of both the VSV-CT1 and VSV-CT9 vectors in vaccination.
Interestingly, when given by the i.p. route, the VSV-CT1 vector induced cellular immune responses to Env (tetramer assay) that were almost as strong as those induced by rVSV. However, after i.n. delivery there was a greatly reduced response to Env generated by VSV-CT1 compared to the rVSV vector. This difference may result from the greatly reduced virus production by VSV-CT1 compared to rVSV. We suggest that induction of strong immune responses after i.n. vaccination is highly dependent on the level of virus replication and the extent of spread from the site of inoculation. A requirement for multicycle virus replication to induce strong immune responses could magnify the differences in responses generated by viruses that produce low titers (VSV-CT1) and those that produce higher titers (VSV-CT9 and rVSV). In contrast, after i.p. inoculation, there may be rapid control of virus spread for all vectors and more limited requirement for spread to induce strong immune responses. In fact, i.p. inoculation with rVSV induces no measurable pathogenesis, consistent with minimal virus replication. In a situation where virus replication is controlled rapidly, the magnitude of the immune response generated would depend on the number of cells infected initially and the amount of Env protein produced per cell (which is the same for all vectors), and not on the number of virus particles produced per cell.
A previous study from our laboratory addressed the pathogenesis induced by the VSV-CT1 and VSV-CT9 vectors given i.n. This study reported that VSV-CT1 was completely attenuated with respect to causing measurable weight loss or any other signs of pathogenesis, while VSV-CT9 still caused a small but significant weight loss (23). In the present study the VSV-CT9EnvG recombinant did not cause detectable weight loss. This difference could be due to the use of slightly younger mice in the previous study or to the addition of the EnvG gene in the VSV-CT9 recombinant in the present study. In fact, a small reduction in weight loss caused by inclusion of EnvG in the rVSV background was observed (Fig. 2), and we have previously noted that HIV Env expression in VSV vectors has a significant effect on reduction of VSV titers in vitro (8).
The nonpathogenic and highly effective VSV-based vectors described here clearly give us important new options that should be explored further in nonhuman primate models for AIDS and other vaccines.
Acknowledgments
We thank Karl Haglund for his generous help in the initial stages of this project, and we thank Nina Rose and Michael Hendry for helpful suggestions on the manuscript.
This work was supported by NIH grant R37-AI40357 and by an HIV-1 Vaccine Design and Development Team contract (NIH NO1-AI-25458).
REFERENCES
- 1.Andersson, E. C., J. P. Christensen, O. Marker, and A. R. Thomsen. 1994. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J. Immunol. 152:1237-1245. [PubMed] [Google Scholar]
- 2.Andersson, E. C., J. P. Christensen, A. Scheynius, O. Marker, and A. R. Thomsen. 1995. Lymphocytic choriomeningitis virus infection is associated with long-standing perturbation of LFA-1 expression on CD8+ T cells. Scand. J. Immunol. 42:110-118. [DOI] [PubMed] [Google Scholar]
- 3.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budd, R. C., J. C. Cerottini, C. Horvath, C. Bron, T. Pedrazzini, R. C. Howe, and H. R. MacDonald. 1987. Distinction of virgin and memory T lymphocytes. Stable acquisition of the Pgp-1 glycoprotein concomitant with antigenic stimulation. J. Immunol. 138:3120-3129. [PubMed] [Google Scholar]
- 5.Coles, R. M., S. N. Mueller, W. R. Heath, F. R. Carbone, and A. G. Brooks. 2002. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J. Immunol. 168:834-838. [DOI] [PubMed] [Google Scholar]
- 6.DeGrendele, H. C., P. Estess, and M. H. Siegelman. 1997. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science 278:672-675. [DOI] [PubMed] [Google Scholar]
- 7.Fuerst, T. R., E. G. Niles, F. W. Studier, and B. Moss. 1986. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc. Natl. Acad. Sci. USA 83:8122-8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haglund, K., J. Forman, H. G. Krausslich, and J. K. Rose. 2000. Expression of human immunodeficiency virus type 1 Gag protein precursor and envelope proteins from a vesicular stomatitis virus recombinant: high-level production of virus-like particles containing HIV envelope. Virology 268:112-121. [DOI] [PubMed] [Google Scholar]
- 9.Haglund, K., I. Leiner, K. Kerksiek, L. Buonocore, E. Pamer, and J. K. Rose. 2002. High-level primary CD8+ T-cell response to human immunodeficiency virus type 1 Gag and Env generated by vaccination with recombinant vesicular stomatitis viruses. J. Virol. 76:2730-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, J. E., M. J. Schnell, L. Buonocore, and J. K. Rose. 1997. Specific targeting to CD4+ cells of recombinant vesicular stomatitis viruses encoding human immunodeficiency virus envelope proteins. J. Virol. 71:5060-5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung, T. M., W. M. Gallatin, I. L. Weissman, and M. O. Dailey. 1988. Down-regulation of homing receptors after T cell activation. J. Immunol. 141:4110-4117. [PubMed] [Google Scholar]
- 12.Kahn, J. S., A. Roberts, C. Weibel, L. Buonocore, and J. K. Rose. 2001. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 75:11079-11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawson, N. D., E. A. Stillman, M. A. Whitt, and J. K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA 92:4477-4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letvin, N. L., and B. D. Walker. 2003. Immunopathogenesis and immunotherapy in AIDS virus infections. Nat. Med. 9:861-866. [DOI] [PubMed] [Google Scholar]
- 16.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mueller, C., H. K. Gershenfeld, C. G. Lobe, C. Y. Okada, R. C. Bleackley, and I. L. Weissman. 1988. A high proportion of T lymphocytes that infiltrate H-2-incompatible heart allografts in vivo express genes encoding cytotoxic cell-specific serine proteases, but do not express the MEL-14-defined lymph node homing receptor. J. Exp. Med. 167:1124-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy, B. R., and R. M. Chanock. 2001. Immunization against viral diseases, p. 435-468. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 19.Owens, R. J., and J. K. Rose. 1993. Cytoplasmic domain requirement for incorporation of a foreign envelope protein into vesicular stomatitis virus. J. Virol. 67:360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter, J. D., B. E. Vivas-Gonzalez, D. Gomez, J. H. Wilson, J. L. Brandsma, H. L. Greenstone, J. K. Rose, and A. Roberts. 2002. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J. Virol. 76:8900-8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richardson, T. M., Jr., B. L. Stryjewski, C. C. Broder, J. A. Hoxie, J. R. Mascola, P. L. Earl, and R. W. Doms. 1996. Humoral response to oligomeric human immunodeficiency virus type 1 envelope protein. J. Virol. 70:753-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts, A., L. Buonocore, R. Price, J. Forman, and J. K. Rose. 1999. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 73:3723-3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, A., E. Kretzschmar, A. S. Perkins, J. Forman, R. Price, L. Buonocore, Y. Kawaoka, and J. K. Rose. 1998. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 72:4704-4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose, J. K., and M. A. Whitt. 2001. Rhabdoviridae: the viruses and their replication, p. 1221-1244. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 25.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 26.Rose, N. F., A. Roberts, L. Buonocore, and J. K. Rose. 2000. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 74:10903-10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlereth, B., J. K. Rose, L. Buonocore, V. ter Meulen, and S. Niewiesk. 2000. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 74:4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnell, M. J., L. Buonocore, E. Boritz, H. P. Ghosh, R. Chernish, and J. K. Rose. 1998. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 17:1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnell, M. J., L. Buonocore, M. A. Whitt, and J. K. Rose. 1996. The minimal conserved transcription stop-start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 70:2318-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirai, M., C. D. Pendleton, and J. A. Berzofsky. 1992. Broad recognition of cytotoxic T cell epitopes from the HIV-1 envelope protein with multiple class I histocompatibility molecules. J. Immunol. 148:1657-1667. [PubMed] [Google Scholar]