Abstract
Objectives
The aims of this study are (1) to evaluate the safety and feasibility of using optical frequency domain imaging (OFDI) during balloon pulmonary angioplasty (BPA) procedures, (2) to assess the correlations between the vessel area (VA) and luminal area (LA) obtained by OFDI and intravascular ultrasound (IVUS), and (3) to compare inter‐ and intra‐observer variability among measurements taken from OFDI and IVUS images.
Background
The BPA in patients with chronic thromboembolic pulmonary hypertension (CTEPH) is an evolving procedure.
Methods
Twenty‐three consecutive attempts of pair of OFDI and IVUS during BPA were evaluated. All complications that occurred during‐BPA and up to 48 hr post‐BPA were recorded. Using side branches as landmarks, 48 pairs of regions were chosen to compare measurements of VA and LA.
Results
OFDI images can be obtained without any procedurally related complications. Although the VA and LA measurements obtained by OFDI were smaller than those obtained by IVUS, high correlations were found (VA: r = 0.78, P < 0.0001 and LA: r = 0.75, P < 0.0001). Less inter‐ and intra‐observer variability was found when using measurements taken from OFDI versus IVUS images.
Conclusions
OFDI during BPA was safe and feasible. The reproducibility of OFDI imaging was excellent and offered a favorable addition to the BPA procedures. © 2016 The Authors Catheterization and Cardiovascular Interventions Published by Wiley Periodicals, Inc.
Keywords: intravascular ultrasonography, pulmonary hypertension, pulmonary embolism
INTRODUCTION
The balloon pulmonary angioplasty (BPA) in patients with chronic thromboembolic pulmonary hypertension (CTEPH) is an evolving procedure 1, 2, 3, 4, 5. The effectiveness of BPA in improving pulmonary circulation, symptoms, and life expectancy has been reported 6, 7, 8, 9, 10, 11. In 2001, Feinstein et al. reported the first results of using pulmonary balloon angioplasty in a limited number of patients. In this report, the hemodynamic efficacy of BPA was demonstrated; however, 11 of the 18 patients who were included developed reperfusion pulmonary edema as a post‐BPA complication 1. Several previous reports have elucidated inventive methods of predicting and preventing procedure‐related complications 2, 3, 12. For example, high‐resolution imaging is a useful way to visualize the complex structures that form within the pulmonary arteries of patients affected by CTEPH.
The use of intravascular ultrasound (IVUS) imaging during BPA has been shown to reduce complication frequency 2. Optical frequency domain imaging (OFDI) (Terumo Corp., Tokyo, Japan) has been suggested to be a promising alternative to IVUS for the visualization of coronary arteries 13. The spatial resolution of OFDI is ∼10–20 μm; this resolution is significantly (about tenfold) higher than what can be obtained with IVUS imaging. Although the safety and feasibility of this procedure has been established for use in the coronary artery, no previous reports have examined the adaptability of OFDI to BPA procedures.
The aims of this study included the following: (1) to evaluate the safety and feasibility of using OFDI in the pulmonary artery during BPA procedures, (2) to assess the correlations between the vessel and luminal areas obtained by OFDI and IVUS, and (3) to compare inter‐ and intra‐observer variability among measurements taken from OFDI and IVUS images.
MATERIALS AND METHODS
OFDI and IVUS were used in tandem on a total of consecutive 23 lesions undergoing BPA for CTEPH at the National Centre for Global Health and Medicine (Tokyo, Japan). In these patients, CTEPH was diagnosed based on the demonstration of organized pulmonary thromboembolism using contrast‐enhanced lung computed tomography, perfusion lung scintigraphy, pulmonary function tests, blood tests, echocardiography, and pulmonary angiography; other causes of pulmonary hypertension were ruled out.
This study complied with the Declaration of Helsinki, and written informed consent was obtained from each patient. The local ethics committee approved the use of clinical data for this study.
Imaging Procedures
Heparin was administered to maintain an active clotting time of 250–300 sec throughout the procedure. A 9‐Fr sheath was inserted into the right internal jugular vein. From the 9‐Fr sheath, a 6‐Fr 80‐cm long sheath was inserted into the main pulmonary artery tract. All BPA procedures were performed using 6‐Fr guiding catheters that were inserted through the 6‐Fr long sheath. After orienting the guiding catheter to target the pulmonary artery, contrast media (Iopamidol, OYPALOMIN®300, KONICA MINOLTA Inc., Tokyo, Japan) was injected to visualize the angiographical features. Under fluoroscopic guidance, a 0.014 inch wire (Chevalier 14 Universal 190®, Cordis, Tokyo, Japan) was then advanced into the distal region of the target pulmonary artery. Both OFDI and IVUS imaging were performed without pre‐dilatation. All balloon sizes were determined based on OFDI measurements.
OFDI Imaging
The OFDI catheter (Fast ViewTM, Terumo Corp., Tokyo, Japan) was advanced into the target vessel under fluoroscopic guidance, and the optical lens was placed more than 5 mm distal of the target lesion. The imaging procedure was calibrated using the outer border of the circumference of the catheter. Lens pullbacks were performed under continuous injection of contrast media (diluted by 50% with saline) via hand injection by experienced interventionalists. OFDI images were obtained at a rate of 160 frames/sec, and the pullback speed was 20 mm/sec.
IVUS Imaging
IVUS imaging was performed using a commercially available 20‐MHz ultrasound VOLCANO system (Volcano Japan Corp., Tokyo, Japan). The IVUS catheter (Eagle Eye® Platinum, Volcano Japan Corp., Tokyo, Japan) was advanced into the target vessel under fluoroscopic guidance, and the transducer was placed more than 5 mm distal of the target lesion. Catheter pullbacks were performed manually. To distinguish lumen and vessel structures in the IVUS images, the advanced functionality options of a color Doppler (ChromaFlo®, Volcano Japan Corp., Tokyo, Japan) were used.
Risk Assessment
Two experienced cardiologists assessed the outcomes of each of the BPA procedures. All complications that occurred during BPA and up to 48 hr post BPA were recorded. All adverse events that were found by angiography and non‐contrasted computerized tomography (CT) following the procedures were recorded. A worsening of respiratory status after BPA was defined as the need for non‐invasive positive pressure ventilation using either high‐concentration oxygen inhalation or an artificial respirator. The homodynamic parameters before and after the sessions were compared.
Analysis
Two experienced cardiologists evaluated the OFDI and IVUS images. The images were classified as being “fair,” “possible,” and “poor.” To evaluate the measurements of vessel area (VA) and lumen area (LA) that were obtained using OFDI and IVUS, selected regions of the images were identified using side branches as landmarks. The VA and LA of these regions were then analyzed by two independent observers using the software that is included in the OFDI and VOLCANO equipment.
To assess the inter‐observer variability among the VA and LA measurements that were obtained from OFDI and IVUS imaging, two independent observers repeated each measurement. To assess the intra‐observer variability among the VA and LA measurements that were obtained from OFDI and IVUS imaging, each image was re‐evaluated by the same observer at least 4 weeks after the initial analysis.
Statistical Analysis
Continuous variables are presented as the mean values ± SDs or median [Interquartile range (IQR)]. ANOVA analysis was used to compare the VA and LA measurements that were obtained using OFDI and IVUS. Correlations between variables were calculated using Pearson's r coefficient. Intra‐class correlation coefficients (ICC) were used to evaluate inter‐ and intra‐observer variability. The homodynamic parameters were compared and significant differences were determined using Wilcoxon matched‐pairs signed rank test. All of the tests were two‐sided, and P‐values <0.05 were considered significant. The SPSS (IBM Japan, Tokyo) software package (ver. 23) was used for the analyses.
RESULTS
Target Lesion Characteristics and Acquisition of OFDI and IVUS Images
The lesion characteristics are summarized in Table 1. Because 23 consecutive lesions were evaluated, 2 complete obstruction lesions were included. OFDI was performed directly after wire crossing and was followed by IVUS. In both cases, the catheters that were used for the procedures could advance distally to the target lesions in all attempts. Among the 23 pullbacks, 14 fair, 2 possible, and 7 poor OFDI images were obtained without using pre‐dilatation. Incomplete blood clearance led to a reduction in the quality of the OFDI images. The mean contrast volume used per pullback was 7.4 mL (range 5–10 mL). Due to machine trouble that arose when using the Eagle Eye® Platinum catheter, one IVUS image could not be recorded. In the remaining 22 attempts, fair IVUS images were obtained. In the end, 18 out of 23 (78%) OFDI pullbacks could be matched to IVUS pullbacks. Using side branches as landmarks, 48 pairs of regions were chosen to compare measurements of VA and LA.
Table 1.
Lesion Characteristics
| Lesion type | Total n = 23 (100%) |
|---|---|
| Band | 8 (35%) |
| Web | 8 (35%) |
| Abrupt narrowing | 2 (9%) |
| Complete obstruction | 2 (9%) |
| Others | 3 (13%) |
Safety of OFDI Procedure
In each attempt, the OFDI catheter was successfully positioned at the target lesion without causing any adverse angiographical events. Although two micro pulmonary hemorrhages were found during post BPA CT scanning, no causal link between the hemorrhages and the OFDI procedure could be confirmed. None of the patients required non‐invasive positive pressure ventilation with either high‐concentration oxygen inhalation or artificial respiration after BPA.
Comparison of OFDI and IVUS Images
The VA and LA that were measured by OFDI were smaller than those measured by IVUS (VA: 10.0 ± 6.6 mm2 vs. 14.9 ± 9.4 mm2, P < 0.0001 and LA: 5.9 ± 4.0 mm2 vs. 9.2 ± 5.9 mm2, P < 0.0001, respectively); however, the correlation between the two measurements was highly significant (VA: r = 0.78, P < 0.0001 and LA: r = 0.75, P < 0.0001) (Fig. 1). Additionally, the high resolution images that were obtained using OFDI‐enabled visualization of the complex pulmonary artery structures associated with CTEPH (Fig. 2).
Figure 1.
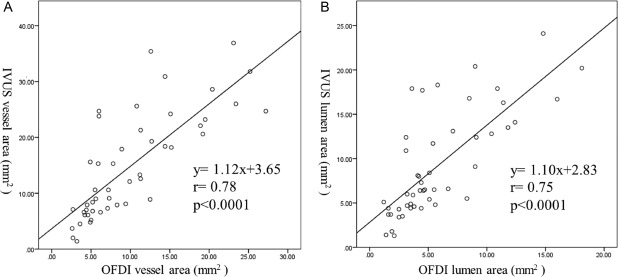
(a) Correlation between the vessel areas measured by OFDI and IVUS. Correlation coefficient (r) = 0.78, P < 0.0001. (b) Correlation between the lumen areas measured by OFDI and IVUS. Correlation coefficient (r) = 0.75, P < 0.0001.
Figure 2.
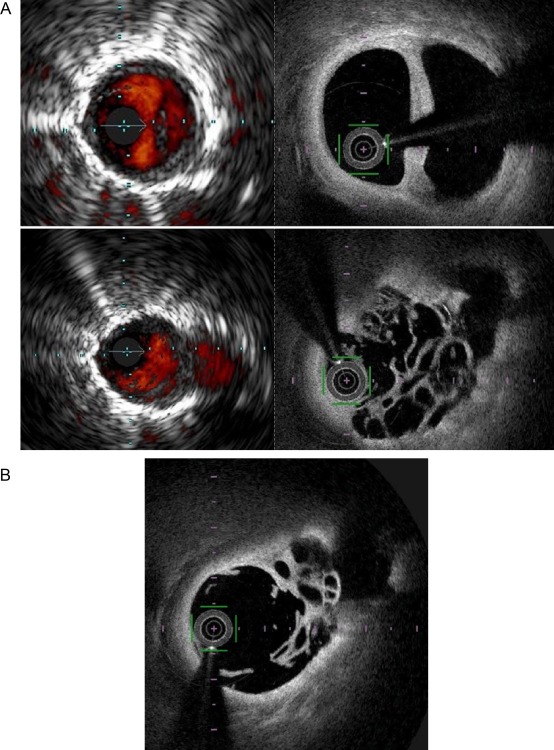
(a) Corresponding IVUS and OFDI images. Above: Double chamber structure is shown. Below: Web and a side branch are shown. The OFDI system provided high resolution images of a double chamber structure (above) and web and a side branch (below). (b) OFDI image after ballooning. The same region with Fig. 2a (below). Compressed web structure was clearly revealed by OFDI system.
Inter‐ and Intra‐Observer Variability
The inter‐observer variability among VA measurements that were obtained using the OFDI system was excellent. The intra‐class correlation coefficient (ICC) was 0.967. The inter‐observer variability among LA measurements that were obtained by OFDI was also excellent (ICC 0.996). The inter‐observer variability among these measurements when taken using the IVUS system was also excellent (VA: ICC 0.865, LA: ICC 0.962). However, the ICCs that were measured for OFDI were higher than those measured for IVUS (Table 2).
Table 2.
Inter‐Observer Variability Evaluated by Intraclass Correlation Coefficients (ICC) Values
| ICC of vessel area | ICC of lumen area | |
|---|---|---|
| OFDI | 0.967 | 0.996 |
| IVUS | 0.865 | 0.962 |
OFDI measurements presented better inter‐observer variability than IVUS measurements.
There was virtually no intra‐observer variability among VA and LA measurements obtained by either OFDI or IVUS (Table 3).
Table 3.
Intra‐Observer Variability Evaluated by Intraclass Correlation Coefficients (ICC) Values
| ICC of vessel area | ICC of lumen area | |
|---|---|---|
| OFDI | 0.993 | 0.999 |
| IVUS | 0.970 | 0.993 |
OFDI measurements presented better intra‐observer variability than IVUS measurements.
Hemodynamic Changes
Comparisons of the hemodynamic examinations pre‐ and post BPA are presented in Fig. 3. Significant improvements were observed in the mean pulmonary arterial pressure (Pre 28 [25–39] mm Hg vs. Post 23 [21–30] mm Hg, P = 0.018) and the pulmonary vascular resistance (Pre 5.3 [4.3–6.6] Wood U vs. 4.1 [3.8–4.3] Wood U, P = 0.018). In contrast, a significant difference was not observed in the cardiac index (Pre 2.73 [2.58–3.33] L/min/m2 vs. 2.68 [2.52–2.94] L/min/m2, ns).
Figure 3.
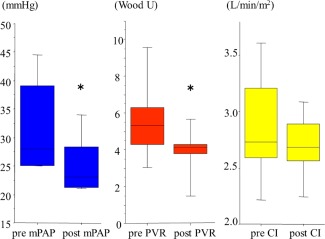
Therapeutic efficacy of BPA in all the patients enrolled. Hemodynamic changes in the mean pulmonary arterial pressure (mPAP), the pulmonary vascular resistance (PVR), and the cardiac index (CI). mPAP and PVR were significantly improved post procedures. *P < 0.05.
DISCUSSION
There are some reports which showed the usefulness of optical coherence tomography (OCT) as a diagnostic tool for CTEPH 14, 15. However, no previously published studies have compared the use of OFDI versus IVUS during BPA in CTEPH patients. The principal findings of our study include the following: (1) OFDI images of CTEPH patients’ pulmonary arteries can be obtained without incurring any procedurally related life‐threatening adverse events, (2) the VA and LA measurements obtained by OFDI are smaller than those obtained by IVUS, (3) a high correlation was found between the measurements of VA and LA that were obtained by the two different imaging methods that were employed, and (4) less inter‐ and intra‐observer variability was found when using measurements taken from OFDI versus IVUS images.
Feasibility and Safety
In all 23 attempts, the OFDI catheter (Fast ViewTM, Terumo Corp., Tokyo, Japan) could be successfully positioned distal to the target lesions. In 14 of the 23 attempts (61%), fair OFDI images were obtained without pre‐dilatation. CTEPH patients typically present with pulmonary arteries that are filled with web (fibrous organized thrombus). The observable depth that is attained using OFDI is not attenuated in web and is only slightly attenuated in fibrin. Therefore, the OFDI system may be a more suitable choice for visualizing the pulmonary artery in patients presenting with CTEPH (versus using this technique in lipid‐rich coronary arteries). Even in cases in which possible or poor images could be obtained without pre‐dilatation, fair images could still be obtained after pre‐dilatation.
It should be noted that the IVUS catheter (Eagle Eye® Platinum, Volcano Japan Corp., Tokyo, Japan) was also successfully positioned distal to the target lesions. Fair IVUS images were obtained in 22 out of 23 attempts; one attempt failed due to mechanical problems. The VOLCANO system enabled more distal imaging than OFDI. This was due to the different positioning that is required when using the Eagle Eye® Platinum transducer compared to the Fast ViewTM lens. The Eagle Eye® Platinum transducer was positioned at 11 mm proximal to the tip, and the lens of Fast ViewTM was positioned at 24 mm proximal to the tip. For cases in which precise information of extremely distal pulmonary arteries is needed, the VOLCANO system may be favorable. However, this scenario is rare in most clinical settings because the majority of operators will only push deflated balloons into very distal pulmonary arteries (with diameters estimated to be less than 1.5 mm by angiogram) for the sake of the “Dotter effect.” The mean contrast volume that was used per OFDI pullback was 7.4 mL. This volume would be acceptable for use in patients without renal dysfunction. No life‐threatening events developed in relation to the imaging procedures. Thus, OFDI can be considered safe for use during BPA.
Assessment of OFDI and IVUS Measurements
In our study, VA and LA measurements that were made using OFDI were significantly smaller than those produced by IVUS. These results are in agreement with those of a previous study that was performed in coronary arteries 16. In this study, the mean diameters of the vessels and lumina were compared between OFDI and IVUS images (vessel diameters: 3.6 mm vs. 4.4 mm, lumen diameters 2.7 mm vs. 3.4 mm), and the difference was found to be −22% in the mean vessel diameter and −25% in the mean lumen diameter. These differences are greater than what has been found previously 13, 16, 17.
One possible explanation for this discrepancy is the much higher resolution that can be obtained when using OFDI to visualize the intima interface of the lumen compared to that found when using IVUS. Therefore, ODFI measurements more precisely evaluated the true luminal area, whereas IVUS measurements overestimated this area. Previous research has reported that IVUS measurements can be influenced by eccentric catheter placement, blood flow velocity, blood temperature, and the incidence angle of the echo signal. These factors caused a 16 ± 6% increase in a phantom model and a 14 ± 9% increase in human arteries in vitro 18. In CTEPH patients in particular, the lumen of the pulmonary artery, which typically contains the target lesions, is usually filled with web (fibrous organized thrombus). Therefore, it is difficult to advance wires into the center of this artery. Another possible explanation for the discrepancy described above is the order of imaging procedures that was used. In our study, IVUS procedures were always performed after OFDI procedures. Therefore, any organized, fibrous thrombus associated with a lesion would have been compressed by the OFDI catheter, leading to a potential improvement in perfusion pressure distal to the lesion. A Dotter effect caused by the OFDI catheter might therefore reduce the incidence of vessel collapse. It is not uncommon that pulmonary arteries of CTEPH dramatically dilate after some devices advance distal to target lesions. Therefore, it is difficult to compare VA or LA measured by OFDI and IVUS in same condition. The diameter of the Fast ViewTM lens is 0.034 inch, and the diameter of the Eagle Eye® Platinum transducer is 0.053 inch. A central aim of this study was to evaluate the feasibility and safety of the OFDI imaging procedure. If the IVUS procedure (using a greater diameter than that used for OFDI) had been performed prior to OFDI, the feasibility of ODFI could not have been properly assessed.
The information obtained by OFDI may facilitate the selection of smaller balloon sizes than are required by IVUS. However, unlike in coronary stenting, just size balloon selection is not needed in BPA procedure. In BPA, the selection of a small balloon size is acceptable for preventing pulmonary artery rupture or the development of post BPA reperfusion pulmonary oedema.
Assessment of Reproducibility
The high resolution images obtained using OFDI led to excellent inter‐ and intra‐observer variability rates in both VA and LA measurements. Indeed, there was virtually no intra‐observer variability when using this method. OFDI also produced more highly reproducible images than IVUS, which may contribute to stability in and standardization of BPA procedures. The high reproducibility afforded by OFDI imaging is especially favorable in the case of inexperienced operators.
Study Limitations
This study employed a relatively small number of CTEPH patients to evaluate the feasibility and safety of using OFDI during the perioperative period; therefore, long‐term follow‐up of patient outcomes was not performed. A larger study population and longer follow‐up time will be necessary to assess such outcomes. In each of the BPA procedures evaluated in this study, IVUS images were not obtained until after OFDI was performed. Therefore, the target lesions were already dilated by the passage of the Fast ViewTM catheter, which could have affected our results. Some hemodynamic changes were statistically significant but they were not so big. Because the changes were caused by each one session.
CONCLUSIONS
In this study, the use of OFDI during BPA in patients with CTEPH was found to be safe and demonstrated the feasibility of using this technique. The reproducibility of OFDI imaging was excellent and offered a favorable addition to the BPA procedures.
Conflicts of interest: Nothing to report.
REFERENCES
- 1. Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001;103:10–13. [DOI] [PubMed] [Google Scholar]
- 2. Mizoguchi H, Ogawa A, Munemasa M, Mikouchi H, Ito H, Matsubara H. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:748–755. [DOI] [PubMed] [Google Scholar]
- 3. Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Fukuda K, Yoshino H, Satoh T. Pressure‐wire‐guided percutaneous transluminal pulmonary angioplasty: A breakthrough in catheter‐interventional therapy for chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2014;7:1297–1306. [DOI] [PubMed] [Google Scholar]
- 4. Yanagisawa R, Kataoka M, Inami T, Shimura N, Ishiguro H, Fukuda K, Yoshino H, Satoh T. Efficacy of 360‐degree three‐dimensional rotational pulmonary angiography to guide percutaneous transluminal pulmonary angioplasty. EuroIntervention 2014;9:1483. [DOI] [PubMed] [Google Scholar]
- 5. Ishiguro H, Kataoka M, Inami T, Yanagisawa R, Shimura N, Taguchi H, Kohshoh H, Yoshino H, Satoh T. Percutaneous transluminal pulmonary angioplasty for central‐type chronic thromboembolic pulmonary hypertension. JACC Cardiovasc Interv 2013;6:1212–1213. [DOI] [PubMed] [Google Scholar]
- 6. Sugimura K, Fukumoto Y, Satoh K, Nochioka K, Miura Y, Aoki T, Tatebe S, Miyamichi‐Yamamoto S, Shimokawa H. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long‐term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J 2012;76:485–488. [DOI] [PubMed] [Google Scholar]
- 7. Inami T, Kataoka M, Ando M, Fukuda K, Yoshino H, Satoh T. A new era of therapeutic strategies for chronic thromboembolic pulmonary hypertension by two different interventional therapies; pulmonary endarterectomy and percutaneous transluminal pulmonary angioplasty. PLoS One 2014;9:e94587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inami T, Kataoka M, Ishiguro H, Yanagisawa R, Shimura N, Yoshino H, Satoh T. Percutaneous transluminal pulmonary angioplasty for chronic thromboembolic pulmonary hypertension with severe right heart failure. Am J Respir Crit Care Med 2014;189:1437–1439. [DOI] [PubMed] [Google Scholar]
- 9. Yanagisawa R, Kataoka M, Inami T, Shimura N, Ishiguro H, Fukuda K, Yoshino H, Satoh T. Safety and efficacy of percutaneous transluminal pulmonary angioplasty in elderly patients. Int J Cardiol 2014;175:285–289. [DOI] [PubMed] [Google Scholar]
- 10. Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, Tamura Y, Ando M, Fukuda K, Yoshino H, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012;5:756–762. [DOI] [PubMed] [Google Scholar]
- 11. Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013;99:1415–1420. [DOI] [PubMed] [Google Scholar]
- 12. Inami T, Kataoka M, Shimura N, Ishiguro H, Yanagisawa R, Taguchi H, Fukuda K, Yoshino H, Satoh T. Pulmonary edema predictive scoring index (PEPSI), a new index to predict risk of reperfusion pulmonary edema and improvement of hemodynamics in percutaneous transluminal pulmonary angioplasty. JACC Cardiovasc Interv 2013;6:725–736. [DOI] [PubMed] [Google Scholar]
- 13. Okamura T, Onuma Y, Garcia‐Garcia HM, van Geuns RJ, Wykrzykowska JJ, Schultz C, van der Giessen WJ, Ligthart J, Regar E, Serruys PW. First‐in‐man evaluation of intravascular optical frequency domain imaging (OFDI) of Terumo: A comparison with intravascular ultrasound and quantitative coronary angiography. EuroIntervention 2011;6:1037–1045. [DOI] [PubMed] [Google Scholar]
- 14. Tatebe S, Fukumoto Y, Sugimura K, Nakano M, Miyamichi S, Satoh K, Oikawa M, Shimokawa H. Optical coherence tomography as a novel diagnostic tool for distal type chronic thromboembolic pulmonary hypertension. Circ J 2010;74:1742–1744. [DOI] [PubMed] [Google Scholar]
- 15. Tatebe S, Fukumoto Y, Sugimura K, Miura Y, Nochioka K, Aoki T, Miura M, Yamamoto S, Yaoita N, Satoh K, et al. Optical coherence tomography is superior to intravascular ultrasound for diagnosis of distal‐type chronic thromboembolic pulmonary hypertension. Circ J 2013;77:1081–1083. [DOI] [PubMed] [Google Scholar]
- 16. Kubo T, Akasaka T, Shite J, Suzuki T, Uemura S, Yu B, Kozuma K, Kitabata H, Shinke T, Habara M, et al. OCT compared with IVUS in a coronary lesion assessment: The OPUS‐CLASS study. JACC Cardiovasc Imaging 2013;6:1095–1104. [DOI] [PubMed] [Google Scholar]
- 17. Capodanno D, Prati F, Pawlowsky T, Cera M, La Manna A, Albertucci M, Tamburino C. Comparison of optical coherence tomography and intravascular ultrasound for the assessment of in‐stent tissue coverage after stent implantation. EuroIntervention 2009;5:538–543. [DOI] [PubMed] [Google Scholar]
- 18. Chae JS, Brisken AF, Maurer G, Siegel RJ. Geometric accuracy of intravascular ultrasound imaging. J Am Soc Echocardiogr 1992;5:577–587. [DOI] [PubMed] [Google Scholar]


