Abstract
Human herpesvirus 8 (HHV-8), also called Kaposi's sarcoma (KS) herpesvirus, can cause KS but is inefficient. Untreated human immunodeficiency virus type 1 (HIV-1) coinfection is a powerful risk factor. The HHV-8 chemokine receptor, vGPCR (ORF74), activates NF-κB and NF-AT, and their levels of activation are synergistically increased by HIV-1 Tat. Transgenic vGPCR mice develop KS-like tumors. A cell line derived from one such tumor expresses vGPCR and forms tumors in nude mice. Here we show that transfection of DNA encoding HIV-1 tat (but not a transactivation-defective mutant) into these tumor cells increases NF-κB and NF-AT activation levels and accelerates tumor formation. Tumorigenesis was also accelerated when Tat DNA was transfected into normal cells and the transfected cells were mixed with the tumor cells and injected into a single site. Tumorigenesis was also increased when the two cell types were injected at separate sites, suggesting that tumorigenesis is accelerated by Tat through soluble factors.
Kaposi's sarcoma (KS) is a tumor-like lesion characterized by angiogenesis and inflammation (20). It is unclear whether KS lesions are true tumors or are, instead, areas of inflammatory hyperplastic proliferation. The multifocal appearance of KS, its lack of aneuploidy, and its spontaneous regression in some settings argue against a tumorigenic nature. KS lesions from some patients are clonal in origin, indicative of a true neoplasm, but this is not the case with other patients (13, 23, 36). Taken together, the data suggest that KS originates as a benign hyperproliferation that under some circumstances progresses to a malignancy.
KS is caused by infection with human herpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (10). Antibodies and viral DNA are universally present in KS patients, and seroconversion predicts the appearance of KS (22, 28, 31, 37, 44). HHV-8 infection is not necessarily sufficient to cause KS, however, as many more people are infected than develop the disease. Infection with human immunodeficiency virus type 1 (HIV-1) is a powerful risk factor, elevating KS incidence in HHV-8 infected people by four orders of magnitude (6, 24). Although immune dysfunction likely accounts for some of the increased incidence of KS in double infections, it may not be the sole contributing factor; the incidence of KS in HIV-2-infected people with AIDS is much lower than in HIV-1 AIDS (2). Interestingly, the HIV-1 Tat protein acts as a growth factor for KS-derived endothelial cells (15, 16), possibly in part through synergy with or dysregulation of the expression of pro-inflammatory cytokines (5, 7, 17, 18, 21). Although HIV-1 and HHV-8 generally do not infect the same cell, cells infected by the two viruses are often in close proximity. Tat is released by HIV-1-infected cells and can be taken up by uninfected cells (9, 16, 19), suggesting a mechanism by which Tat could act on HHV-8-infected cells in vivo.
Although the mechanisms of KS pathogenesis by HHV-8 are not known, an increasing body of evidence suggests that the virally encoded chemokine receptor or G protein-coupled receptor (vGPCR), the product of ORF74, plays an important role. It activates multiple signaling pathways and transcription factors in the absence of added ligands (3, 12, 30, 32-34, 39, 41, 42), resulting in the induction of synthesis of various proinflammatory cytokines, growth factors, and cell surface adhesion proteins. More strikingly, mice transgenic (Tg) for vGPCR develop lesions that closely resemble KS (25, 26, 46).
The vGPCR clearly does not function as a classic oncogene. It is expressed during the lytic phase of viral gene expression (11, 25, 26, 35, 38, 46), and cells expressing it likely die. However, factors released by these cells could influence the behavior of surrounding cells that are not expressing vGPCR; indeed, factors released by cells expressing vGPCR elicit chemotaxis of T lymphoid and monocytic cells and induce NF-κB activation in cells not expressing vGPCR (33). Consistent with this, tumors of vGPCR Tg mice express detectable transgene product in only a minority of cells in the tumor (25, 46). Indeed, mice with a vGPCR transgene regulated by a CD2 promoter express the transgene only in T cells that infiltrate the tumor and not in the endothelial spindle cells that make up the bulk of the tumor and are thought to be the abnormal cells in KS (46).
Recently, it has been shown that Tat synergistically increases the levels of NF-AT and NF-κB activation by vGPCR (34, 46), suggesting a possible pathogenic mechanism for Tat in KS. We have derived a cell line from a KS-like tumor of a vGPCR Tg mouse that causes tumors when transplanted into nude mice (25). Here we show that Tat accelerates tumorigenesis by this KS tumor-derived cell line.
MATERIALS AND METHODS
Mice.
The generation of vGPCR Tg mice has been described previously (25). Briefly, a plasmid expressing the HHV-8 vGPCR under the regulation of an early simian virus 40 promoter was microinjected into fertilized one-cell C57BL/6J eggs, and the resultant eggs were transplanted into a pseudopregnant female. A Tg line was developed from the offspring in which approximately 30% of the mice develop KS-like lesions by 1 year of age. Tumor induction studies were performed with a cell line developed from one of the tumors and CR:NIH-Bg-Nu-XID immunodeficient mice.
Cells and plasmids.
The GR3 cell line was developed by two serial passages through nude mice of cells from a KS-like tumor of a vGPCR Tg mouse. GR3 cells express vGPCR and cause tumors in 100% of nude mice and with lesser efficiency in immunocompetent mice. The GR5 cell line was derived from the tail tissue of a non-Tg littermate by explantation as previously described (25) and is not tumorigenic in nude mice. A plasmid containing a codon-optimized synthetic open reading frame encoding the full-length Tat of HIV-1(MN) has been described previously (1). A codon-optimized deletion mutant of Tat, TatΔ30-51, was also synthesized and has been previously described (1). TatΔ30-51 lacks the cysteine-rich region of Tat and is defective for transactivation of the HIV-1 long terminal repeat. Plasmids were transfected into GR3 and GR5 cells by the Ca3(PO4)2 method.
Signal transduction assays and Western blot analyses.
The activation of the transcription factors NF-κB and NF-AT was assayed by DNA binding assays by using nuclear extracts prepared from transfected cells or tumor tissues. Binding assays were performed on nuclear extracts by using oligonucleotides containing NF-κB or NF-AT consensus binding sites and TransAM enzyme-linked immunosorbent assay (ELISA)-based kits from Active Motif (Carlsbad, Calif.) under conditions recommended by the manufacturer. Binding by the appropriate antibody was detected with a horseradish peroxidase-coupled second antibody, and absorbance at 450 nm was determined. In some cases, the activation levels of NF-κB and NF-AT were measured by cotransfection with a luciferase gene driven by a basal promoter containing multiple consensus binding sites for the appropriate factor under conditions specified by the manufacturer (PathDetect; Stratagene, La Jolla, Calif.).
Western blotting for vGPCR was performed by using a previously described antibody against a synthetic peptide based on the vGPCR sequence (11). The phosphorylation of Akt and selected substrates of Akt was analyzed by Western blotting by using phospho-specific antibodies and by reprobing the blots with antibodies to several of the appropriate total proteins and comparing the signals. Antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.) or Cell Signaling Technology (Beverly, Mass.).
Tumor induction studies.
For tumorigenesis studies, GR3 cells (generally, 0.5 × 106 cells except where otherwise noted) were injected intramuscularly into the flanks of nude mice. Transfected cells were injected 72 h following transfection. Tumor formation was followed by visual inspection every 2 to 3 days. When tumors were visible, they were measured in two dimensions. When animals were euthanized, tumors were excised, weighed, and sectioned for histology, immunohistochemistry, and signal transduction assays.
RT-PCR.
Reverse transcription-PCR (RT-PCR) and real-time RT-PCR for vGPCR, vascular endothelial growth factor A (VEGF-A) and VEGF-C mRNA, and 18S rRNA were performed as described previously (25). Real-time RT-PCR for macrophage inflammatory protein 2 (MIP-2) was performed by using 5′-CCACCAACCACCAGGCTACA-3′ and 5′-GCGAGGCACATCAGGTACGA-3′ as the sense and antisense primers, respectively, to generate a 351-bp product.
RESULTS
Tumor formation by vGPCR Tg GR3 tumor cells.
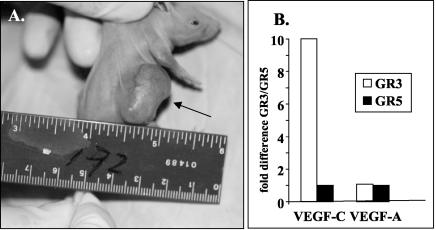
It was previously reported that approximately 30% of mice with a transgene encoding the HHV-8 vGPCR develop KS-like tumors (25). When injected into mice, a cell line developed from a tumor of one of the F2 generation of Tg mice caused tumors within 3 weeks in 100% of nude mice and in one of four immunocompetent mice. In contrast, cells derived from normal tail tissue of a non-Tg littermate did not cause tumors in any of 10 nude mice. Cells from one of the nude mouse tumors were cultured and injected into nude mice and again gave tumors in all recipients within 3 weeks, and cells cultured from these tumors retained their tumorigenic potential. Figure 1A shows a typical tumor. A cell line (GR3) developed from one of these tumors was used for subsequent studies.
FIG. 1.
Tumor induction by GR3 cell line. (A) GR3 Tg tumor cells were injected into the side of a nude mouse. The arrow indicates the tumor formed at the site 3 weeks after injection. (B) GR3 cell lines overproduce VEGF-C mRNA. RNA levels were measured by real-time RT-PCR as described in Materials and Methods. Shown on the y axis are the ratios of the expression levels in GR3 cells to levels in GR5 cells, with the levels of 18S rRNA used as an internal standard.
The GR3 cell line expresses vGPCR RNA and protein, as determined by RT-PCR, immunohistochemistry, and Western blotting. The GR3 cell line also expresses high levels of mRNA for VEGF-C, but not VEGF-A, compared to levels in cells cultured from normal mouse tail tissue, as determined by real-time RT-PCR (Fig. 1B). However, the expression of VEGF-C RNA does not appear to be directly induced by vGPCR, as judged by transfection of vGPCR into NIH 3T3 cells (data not shown).
Enhancement of NF-κB and NF-AT activation by Tat.
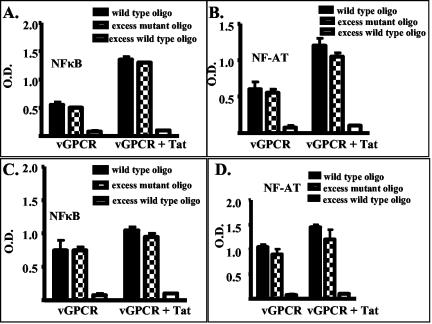
It has previously been shown that HIV-1 Tat protein, either provided as an extracellular protein or encoded by transfected DNA, synergistically increases the activation of NF-κB and NF-AT by vGPCR (34). We wondered whether Tat would also increase levels of NF-κB and NF-AT activation in the GR3 tumor cell line, which constitutively expresses vGPCR. GR3 cells were transfected with either a Tat expression plasmid or an empty control vector, and nuclear extracts were prepared from the transfected cells. Transfected Tat increased the levels of activation of both NF-κB (Fig. 2A) and NF-AT (Fig. 2B) in GR3 cells two- to threefold, similar to its effects in normal cells transiently expressing vGPCR. Tat alone had little effect on NF-κB and NF-AT signaling. Transfection of a Tat expression construct into GR5 cells did not greatly increase the activation level of either factor unless an expression construct for vGPCR was cotransfected (Fig. 3). The expression levels of vGPCR protein were similar in GR3 cells with and without transfected Tat (Fig. 4A), indicating that the signal increases are not due to upregulation of vGPCR expression.
FIG. 2.
Tat increases vGPCR-mediated NF-κB and NF-AT activation. Shown are results of ELISA-based DNA binding assays for NF-κB (A and C) and NF-AT (B and D) carried out as described in Materials and Methods on transfected GR3 cells (A and B) or on lysates of nude mouse tumors (C and D). The black bars show binding to deoxyoligonucleotides containing consensus binding sites immobilized on plastic and analyzed as described in Materials and Methods. The binding was competed with excess wild-type deoxyoligonucleotides (open bars) or deoxyoligonucleotides containing mutated binding sites (checkered bars). OD, optical density.
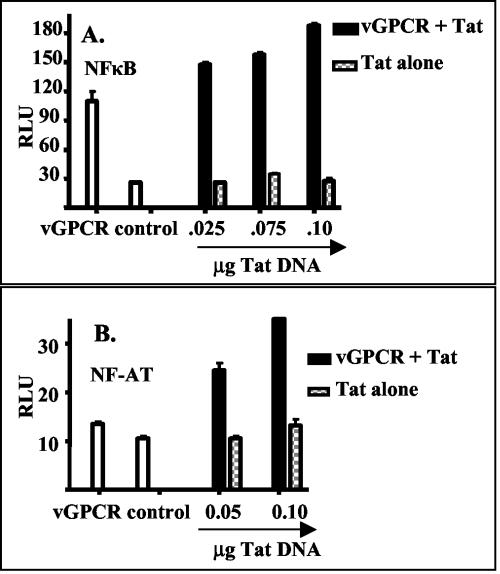
FIG. 3.
Tat alone has little effect on NF-κB and NF-AT activation. GR5 cells were transfected with an expression construct for vGPCR or an empty control vector and with different amounts of an expression vector for Tat. The amount of total DNA was held constant for each transfection by the addition of the appropriate amounts of control vector. Activation levels were measured by cotransfection of a luciferase reporter construct, described in Materials and Methods, and measurement of luciferase activity with a luminometer. Activity is expressed as relative luciferase units (RLU), an arbitrary unit. Experiments were performed twice in triplicate, and the results were normalized for constant activity with the control vector alone. Transfection efficiency was analyzed by using a cotransfected expression vector for fluorescent green protein and was approximately 15% for both sets of experiments. Shown are the results measuring the levels of activation of NF-κB (A) and NF-AT (B). Open bars, cells transfected with vGPCR DNA alone or control vector; solid bars, cells transfected with vGPCR DNA plus the indicated amount of Tat DNA; checkered bars, cells transfected with the indicated amount of Tat DNA plus control vector.
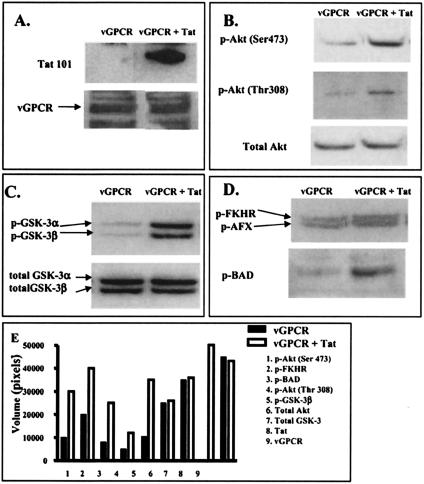
FIG. 4.
Tat increases vGPCR-mediated phosphorylation of Akt and its downstream targets. GR3 cells were transfected with Tat101 DNA or a control vector, and cell lysates were analyzed by Western blotting as described in Materials and Methods. Blots were probed as follows: with antibodies against Tat or vGPCR (A), with antibodies against phosphorylated Akt or total Akt (B), with antibodies against phosphorylated GSK-3 or total GSK-3 (C), or with antibodies to phosphorylated FKHR, AFX, or BAD (D). Panel E shows the quantification of the blots performed with a PhosphorImager and measured in arbitrary pixel units. The solid bars show results from control cells, and the open bars show results from cells transfected with Tat101 DNA.
Tat synergistically increases NF-κB and NF-AT activation by vGPCR in human cells through the phosphatidylinositol 3-kinase (PI3-K)/Akt/protein kinase B pathway (34). We asked whether Tat would have a similar effect on the PI3-K/Akt pathway in GR3 cells. We therefore measured the phosphorylation levels of Akt as well as that of BAD and glycogen synthetase kinase 3β (GSK-3β), two of the downstream targets of Akt phosphorylation. Phosphorylation of both Akt (Fig. 4B) and its downstream targets (Fig. 4C and D) was clearly increased by Tat, while the levels of total protein remained constant. Figure 4E shows the quantification of the results by PhosphorImager analysis of selected bands, expressed in arbitrary pixel units. Thus, it is likely that Tat is acting by similar mechanisms on vGPCR signaling in GR3 cells and vGPCR-transfected human cells.
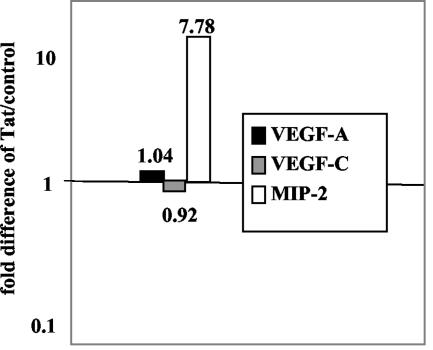
The murine chemokine MIP-2, a homologue of human Groα, is an agonist for vGPCR (26), and vGPCR mutants that retain constitutive signal transduction but fail to respond to MIP-2 are not highly tumorigenic when expressed as transgenes (26). Since MIP-2 is regulated in part by NF-κB (45), we asked whether Tat increased the level of MIP-2 mRNA expression in GR3 cells. As judged by real-time RT-PCR, this was indeed the case. In numerous experiments upregulation at 24 and 48 h posttransfection ranged from approximately twofold (data not shown) to eightfold (Fig. 5). ELISAs of media collected 48 h posttransfection showed an approximately 50% increase in secreted MIP-2 (data not shown). Neither VEGF-A nor VEGF-C mRNA levels were affected by Tat, although VEGF-C is already upregulated in GR3 cells (Fig. 1B).
FIG. 5.
Expression of Tat in GR3 cells increases the expression level of MIP-2. GR3 cells were transfected with Tat101 DNA or a control vector as described in Materials and Methods. After 48 h, RNA was purified from the transfected cells, and the concentrations of MIP-2, VEGF-A, and VEGF-C mRNA were determined by real-time RT-PCR by using 18S rRNA as an internal reference. The y axis shows the ratios of the expression levels in Tat-transfected cells to levels in controls, with the levels of 18S rRNA used as an internal standard.
Enhancement of tumorigenesis by Tat.
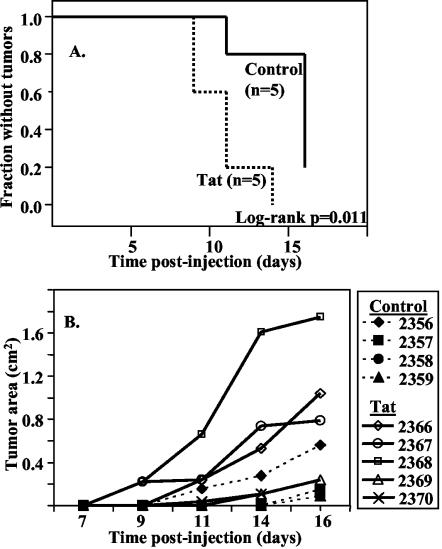
Since Tat increased NF-κB and NF-AT activation levels in GR3 cells, an obvious question was whether Tat would also increase or accelerate tumorigenesis by GR3 cells. GR3 cells were transfected with an expression construct for Tat or a control plasmid (pSG5) as described in Materials and Methods. Cells were cultured for 72 h and then injected into groups of five nude mice for each construct. Mice were observed every 2 to 3 days for the appearance of tumors at the site of injection, and the tumors were measured in two dimensions after they first became visible. As shown in Fig. 6A, tumors in two of the mice receiving cells transfected with Tat were detectable by day 9 postinoculation. The earliest appearance of a tumor in the control group was at day 11, by which time four out of five mice from the Tat group had detectable tumors. As judged from the surface area of the tumor, the tumor growth rate was strikingly higher with the Tat group compared to the rate for the controls. The data are summarized in Fig. 6B. Tumors were removed at day 16 and sectioned for further analysis, including weight measurement. Only one of the four tumors in mice injected with GR3 cells transfected with the control vector was large enough to excise clearly; it weighed 0.36 g. The five tumors in the Tat group had an average weight of 0.6 ± 0.2 g.
FIG. 6.
Transfection of GR3 cells with Tat101 increases their tumorigenicity. Cells were transfected with Tat101 DNA or a control vector and injected into nude mice as described in Materials and Methods. (A) Kaplan-Meier plot of the time of appearance of palpable tumors at the site of injection. The log-rank P value was 0.011, indicating that the difference between the two groups was highly significant. (B) Plot of the surface area of the tumors.
Nuclear extracts were prepared from tumor slices, and the levels of activation of NF-κB and NF-AT were measured by using ELISA-based DNA binding assays. Activation levels of both factors were slightly but reproducibly higher in tumors containing the Tat-transfected cells (Fig. 2C and D). The relatively small differences between cells treated with Tat and the control vector may reflect a gradual decrease in the expression level of the transiently transfected Tat. Indeed, although we could readily detect by Western blotting Tat expression in recently transfected cells (Fig. 4), we could not detect Tat expression in the tumors (data not shown). Thus, it is likely that the effects of Tat occur relatively early in tumor formation.
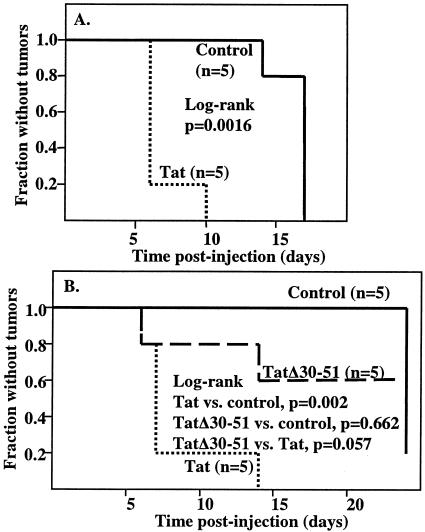
Although HIV-1 and HHV-8 do not generally infect the same cells, cells infected with each virus likely exist in close proximity. Therefore, Tat and vGPCR would mostly be expressed in different cells, although Tat can be released from HIV-1-infected cells and enter or bind to HHV-8-infected cells. To model this relationship, we transfected normal GR5 cells with a Tat expression vector or a control vector, mixed them with GR3 cells, and injected the mixture into nude mice. We again observed the mice every 2 to 3 days and determined the time of appearance of tumors. As shown in Fig. 7A, when the GR5 normal cells were transfected with the Tat plasmid, tumors appeared more rapidly than when the GR5 cells were transfected with the control vector. We also carried out a series of experiments in which the two cell types were not mixed but were injected on opposite sides of nude mice. Interestingly, under these conditions Tat again accelerated tumor formation, indicating that the effects of Tat on tumorigenesis can occur at a distance (Fig. 7B). In no instance did we observe tumors on the sides of mice that were injected with Tat-transfected GR5 cells, suggesting that Tat itself is not tumorigenic.
FIG. 7.
Expression of Tat in normal cells increases tumorigenesis by GR3 cells. (A) Normal GR5 cells were transfected with Tat101 DNA or a control vector. Transfected normal cells were mixed with GR3 cells and injected into mice as described in Materials and Methods. A Kaplan-Meier plot for the time of appearance of palpable tumors is shown. A log-rank analysis, performed by using StatXact version 6 (Cytel Software Corp., Cambridge, Mass.), gave a P value of 0.0016. (B) Normal GR5 cells were transfected with Tat101, the deletion mutant TatΔ30-51, or empty vector. Mice were injected with transfected normal cells on one side and GR3 cells on the opposite side. A Kaplan-Meier plot for the time of appearance of palpable tumors is shown. A log-rank analysis gave a P value of 0.002 for the comparison of Tat with the control vector and of 0.057 for the comparison of Tat with the TatΔ30-51 mutant. There was no significant difference (P = 0.662) between the values of the Tat mutant and the control.
DISCUSSION
We and others have previously shown that the HHV-8 vGPCR activates multiple parallel signaling pathways, including NF-κB, NF-AT, and AP-1, in the absence of added ligands (3, 8, 12, 30, 32-34, 39-42). NF-κB and NF-AT signaling by vGPCR are synergistically increased by HIV-1 Tat protein (34). The effects of both vGPCR and Tat on signaling are dependent on the PI3-K/Akt pathway. In this regard, Tat has been shown to prevent down-regulation of Akt in vincristine-treated KS cells (14). The expression of vGPCR from a transgene or from a transfected expression vector causes the formation of KS-like tumors in mice (4, 25, 26, 46). The transduction of endothelial cells with a vGPCR retroviral vector also induces KS-like lesions in mice (29).
In the present studies we evaluated whether Tat can increase vGPCR-mediated tumorigenesis. For this purpose, we used GR3, a cell line derived from a KS-like tumor of a vGPCR Tg mouse and passaged several times as a tumor through nude mice. GR3 cells constitutively express vGPCR and elevated levels of VEGF-C mRNA and form tumors when injected into nude mice. We have looked for stimulation of tumorigenesis by Tat in this model system by two criteria, tumor size as measured by weight or surface area and the time until appearance of discernible tumors. By both criteria, transfection of a Tat expression vector into the tumor cells greatly increased tumorigenesis. Activation of NF-κB and NF-AT in the GR3 cells was increased by the transfection of Tat, consistent with previous results in human cells (34). Tat did not increase the level of expression of vGPCR, suggesting that its primary effect on tumorigenesis is to increase the strength of vGPCR signaling.
Although these data suggest that Tat could contribute to KS pathogenesis by cooperating with vGPCR, infection of the same cells by the two viruses is likely to be infrequent. Hence, expression of both proteins in the same cell is not likely. However, Tat is released by HIV-1 infected cells and can be taken up by other cells (9, 16, 19). We therefore sought to more closely mimic the in vivo situation by expressing Tat and vGPCR in separate cells and looking for a paracrine effect on tumorigenesis. We transfected the Tat expression vector into normal mouse tail GR5 cells and either mixed them with GR3 tumor cells and injected them together into nude mice or injected the Tat-transfected and tumor cells separately into opposite sides of nude mice. In both cases, the results were similar to those obtained with Tat-transfected tumor cells. The formation of tumors was clearly enhanced, indicating that Tat and vGPCR need not be expressed in the same cell in order for Tat to cooperate in vGPCR-mediated tumorigenesis and that effects from Tat may occur at a distance.
The precise mechanism by which Tat increases vGPCR-mediated signaling is not clear. Interestingly, however, expression of Tat in GR3 cells increased the level of expression of MIP-2, the murine homologue of Groα. HHV-8 infection induces the expression of Groα from human endothelial cells (27). Both Groα and MIP-2 are agonists for vGPCR, and the interaction of MIP-2 and vGPCR appears to be important for tumorigenesis in Tg mice (26). Tat may therefore upregulate the activities of vGPCR through its induction of Groα, although this then begs the question of how Tat induces Groα.
The data indicate that Tat may indeed play a role in KS pathogenesis by increasing vGPCR-mediated signaling in people dually infected with HIV-1 and HHV-8, although the degree to which this interaction plays a role is not clear. Tat concentrations of up to 1 ng/ml in serum have been reported (43). Since Tat is readily taken up by cells or bound on their surfaces, however, it would probably act locally, and local concentrations are likely to be considerably higher than the concentration in serum. It is also possible, however, that the enhancement of tumorigenesis is not mediated by extracellular Tat but, rather, by cytokines or other soluble factors induced from cells expressing Tat. In either case, the present data provide a potential mechanism by which HIV-1 could contribute to KS pathogenesis by HHV-8.
Acknowledgments
This work was supported by NIH grant R01 CA99905.
We thank Gary Hayward (Johns Hopkins, Baltimore, Md.) for providing us with antisera to vGPCR.
REFERENCES
- 1.Agwale, S. M., M. T. Shata, M. S. Reitz, Jr., V. S. Kalyanaraman, R. C. Gallo, M. Popovic, and D. M. Hone. 2002. A Tat subunit vaccine confers protective immunity against the immune-modulating activity of the human immunodeficiency virus type-1 Tat protein in mice. Proc. Natl. Acad. Sci. USA 99:10037-10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariyoshi, K., M. S. van der Loeff, P. Cook, D. Whitby, T. Corrah, S. Jaffar, F. Cham, S. Sabally, D. O'Donovan, R. A. Weiss, T. F. Schulz, and H. Whittle. 1998. Kaposi's sarcoma in the Gambia, West Africa is less frequent in human immunodeficiency virus type 2 than in human immunodeficiency virus type 1 infection despite a high prevalence of human herpesvirus 8. J. Hum. Virol. 1:193-199. [PubMed] [Google Scholar]
- 3.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 4.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gershengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 5.Barillari, G., C. Sgadari, C. Palladino, R. Gendelman, A. Caputo, C. B. Morris, B. C. Nair, P. Markham, A. Nel, M. Sturzl, and B. Ensoli. 1999. Inflammatory cytokines synergize with the HIV-1 Tat protein to promote angiogenesis and Kaposi's sarcoma via induction of basic fibroblast growth factor and the αvβ3 integrin. J. Immunol. 163:1929-1935. [PubMed] [Google Scholar]
- 6.Biggar, R. J., P. S. Rosenberg, T. Cote, et al. 1996. Kaposi's sarcoma and non-Hodgkin's following the diagnosis of AIDS. Int. J. Cancer 68:754-758. [DOI] [PubMed] [Google Scholar]
- 7.Buonaguro, L., G. Barillari, H. K. Chang, C. A. Bohan, V. Kao, R. Morgan, R. C. Gallo, and B. Ensoli. 1992. Effects of the human immunodeficiency virus type 1 Tat protein on the expression of inflammatory cytokines. J. Virol. 66:7159-7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannon, M., N. J. Philpott, and E. Cesarman. 2003. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor has broad signaling effects in primary effusion lymphoma cells. J. Virol. 77:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang, H. C., F. Samaniego, B. C. Nair, L. Buonaguro, and B. Ensoli. 1997. HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. AIDS 11:1421-1431. [DOI] [PubMed] [Google Scholar]
- 10.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 11.Chiou, C.-J., L. J. Poole, P. S. Kim, D. M. Ciufo, J. S. Cannon, C. M. ap Rhys, D. J. Alcendor, J.-C. Zong, R. F. Ambinder, and G. S. Hayward. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3421-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Couty, J. P., E. Geras-Raaka, B. B. Weksler, and M. C. Gershengorn. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor signals through multiple pathways in endothelial cells. J. Biol. Chem. 276:33805-33811. [DOI] [PubMed] [Google Scholar]
- 13.Delabesse, E., E. Oksenhendler, C. Lebbe, O. Verola, B. Varet, and A. G. Turhan. 1997. Molecular analysis of clonality in Kaposi's sarcoma. J. Clin. Pathol. 50:664-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deregibus, M. C., V. Cantaluppi, S. Doublier, M. F. Brizzi, I. Deambrosis, A. Albini, and G. Camussi. 2002. HIV-1-Tat protein activates phosphatidylinositol 3-kinase/Akt-dependent survival pathways in Kaposi's sarcoma cells. J. Biol. Chem. 277:25195-25202. [DOI] [PubMed] [Google Scholar]
- 15.Ensoli, B., G. Barillari, S. Z. Salahuddin, R. C. Gallo, and F. Wong-Staal. 1990. Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345:84-86. [DOI] [PubMed] [Google Scholar]
- 16.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ensoli, B., R. Gendelman, P. Markham, V. Fiorelli, S. Colombini, M. Raffeld, A. Cafaro, H. K. Chang, J. N. Brady, and R. C. Gallo. 1994. Synergy between basic fibroblast growth factor and HIV-1 Tat protein in induction of Kaposi's sarcoma. Nature 371:674-680. [DOI] [PubMed] [Google Scholar]
- 18.Fiorelli, V., R. Gendelman, F. Samaniego, P. D. Markham, and B. Ensoli. 1995. Cytokines from activated T cells induce normal endothelial cells to acquire the phenotypic and functional features of AIDS-Kaposi's sarcoma spindle cells. J. Clin. Investig. 95:1723-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frankel, A. D., and C. O. Pabo. 1988. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189-1193. [DOI] [PubMed] [Google Scholar]
- 20.Gallo, R. C. 1998. The enigmas of Kaposi's sarcoma. Science 282:1837-1839. [DOI] [PubMed] [Google Scholar]
- 21.Ganju, R. K., N. Munshi, B. C. Nair, Z.-Y. Liu, P. Gill, and J. E. Groopman. 1998. Human immunodeficiency virus Tat modulates the Flk-1/KDR receptor, mitogen-activated protein kinases, and components of focal adhesion in Kaposi's sarcoma cells. J. Virol. 72:6131-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, S. J., L. Kingsley, D. R. Hoover, T. J. Spira, C. R. Rinaldo, A. Saah, J. Phair, R. Detels, P. Parry, Y. Chang, and P. S. Moore. 1996. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N. Engl. J. Med. 335:233-241. [DOI] [PubMed] [Google Scholar]
- 23.Gill, P. S., Y. C. Tsai, A. P. Rao, C. H. Spruck III, T. Zheng, W. A. Harrington, Jr., T. Cheung, B. Nathwani, and P. A. Jones. 1998. Evidence for multiclonality in multicentric Kaposi's sarcoma. Proc. Natl. Acad. Sci. USA 95:8257-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goedert, J. J. 2000. The epidemiology of acquired immunodeficiency syndrome malignancies. Semin. Oncol. 27:390-401. [PubMed] [Google Scholar]
- 25.Guo, H.-G., M. Sadowska, W. Reid, E. Tschachler, G. S. Hayward, and M. S. Reitz. 2003. Kaposi's sarcoma-like tumors in a human herpesvirus 8 ORF74 transgenic mouse. J. Virol. 77:2631-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holst, P. J., M. M. Rosenkilde, D. Manfra, S. C. Chen, M. T. Wiekowski, B. Holst, F. Cifire, M. Lipp, T. W. Schwartz, and S. A. Lira. 2001. Tumorigenesis induced by the HHV8-encoded chemokine receptor requires ligand modulation of high constitutive activity. J. Clin. Investig. 108:1789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane, B. R., J. Liu, P. J. Bock, D. Schols, M. J. Coffey, R. M. Strieter, P. J. Polverini, and D. M. Markovitz. 2002. Interleukin-8 and growth-regulated oncogene alpha mediate angiogenesis in Kaposi's sarcoma. J. Virol. 76:11570-11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefrere, J. J., M. C. Meyohas, M. Mariotti, J. L. Meynard, M. Thauvin, and J. Frottier. 1996. Detection of human herpesvirus 8 DNA sequences before the appearance of Kaposi's sarcoma in human immunodeficiency virus (HIV)-positive subjects with a known date of HIV seroconversion. J. Infect. Dis. 174:283-287. [DOI] [PubMed] [Google Scholar]
- 29.Montaner, S., A. Sodhi, A. Molinolo, T. H. Bugge, E. T. Sawai, Y. He, Y. Li, P. E. Ray, and J. S. Gutkind. 2003. Endothelial infection with KSHV genes in vivo reveals that vGPCR initiates Kaposi's sarcomagenesis and can promote the tumorigenic potential of viral latent genes. Cancer Cell 3:23-36. [DOI] [PubMed] [Google Scholar]
- 30.Montaner, S., A. Sodhi, S. Pece, E. A. Mesri, and J. S. Gutkind. 2001. The Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor promotes endothelial cell survival through the activation of Akt/protein kinase B. Cancer Res. 61:2641-2648. [PubMed] [Google Scholar]
- 31.Moore, P. S., L. A. Kingsley, S. D. Holmberg, T. J. Spira, P. Gupta, D. R. Hoover, J. P. Parry, L. J. Conley, H. W. Jaffe, and Y. Chang. 1996. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 10:175-180. [DOI] [PubMed] [Google Scholar]
- 32.Munshi, N., R. K. Ganju, S. Avraham, E. A. Mesri, and J. E. Groopman. 1999. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor activation of c-jun amino-terminal kinase/stress-activated protein kinase and lyn kinase is mediated by related adhesion focal tyrosine kinase/proline-rich tyrosine kinase 2. J. Biol. Chem. 274:31863-31867. [DOI] [PubMed] [Google Scholar]
- 33.Pati, S., M. Cavrois, H.-G. Guo, J. S. Foulke, Jr., J. Kim, R. A. Feldman, and M. Reitz. 2001. Activation of NF-κB by the human herpesvirus 8 chemokine receptor ORF74: evidence for a paracrine model of Kaposi's sarcoma pathogenesis. J. Virol. 75:8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pati, S., J. S. Foulke, Jr., O. Barabitskaya, J. Kim, B. C. Nair, D. Hone, J. Smart, R. A. Feldman, and M. Reitz. 2003. Human herpesvirus 8-encoded vGPCR activates nuclear factor of activated T cells and collaborates with human immunodeficiency virus type 1 Tat. J. Virol. 77:5759-5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulose-Murphy, M., N.-K. Ha, C. Xiang, Y. Chen, L. Gillim, R. Yarchoan, P. Meltzer, M. Bittner, J. Trent, and S. Zeichner. 2001. Transcription program of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus). J. Virol. 75:4843-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rabkin, C. S., S. Janz, A. Lash, A. E. Coleman, E. Musaba, L. Liotta, R. J. Biggar, and Z. Zhuang. 1997. Monoclonal origin of multicentric Kaposi's sarcoma lesions. N. Engl. J. Med. 336:988-993. [DOI] [PubMed] [Google Scholar]
- 37.Renwick, N., T. Halaby, G. J. Weverling, N. H. Dukers, G. R. Simpson, R. A. Coutinho, J. M. Lange, T. F. Schulz, and J. Goudsmit. 1998. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi's sarcoma. AIDS 12:2481-2488. [DOI] [PubMed] [Google Scholar]
- 38.Sarid, R., T. Sato, R. A. Bohenzky, J. J. Russo, and Y. Chang. 1997. Kaposi's sarcoma-associated herpesvirus encodes a functional bcl-2 homologue. Nat. Med. 3:293-298. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz, M., and P. M. Murphy. 2001. Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor constitutively activates NF-kappa B and induces proinflammatory cytokine and chemokine production via a C-terminal signaling determinant. J. Immunol. 167:505-513. [DOI] [PubMed] [Google Scholar]
- 40.Shepard, L. W., M. Yang, P. Xie, D. D. Browning, T. Voyno-Yasenetskaya, T. Kozasa, and R. D. Ye. 2001. Constitutive activation of NF-kappa B and secretion of interleukin-8 induced by the G protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus involve G alpha(13) and RhoA. J. Biol. Chem. 276:45979-45987. [DOI] [PubMed] [Google Scholar]
- 41.Smit, M. J., D. Verzijl, P. Casarosa, M. Navis, H. Timmerman, and R. Leurs. 2002. Kaposi's sarcoma-associated herpesvirus-encoded G protein-coupled receptor ORF74 constitutively activates p44/p42 MAPK and Akt via Gi and phospholipase C-dependent signaling pathways. J. Virol. 76:1744-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 43.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 44.Whitby, D., M. R. Howard, M. Tenant-Flowers, N. S. Brink, A. Copas, C. Boshoff, T. Hatzioannou, F. E. Suggett, D. M. Aldam, and A. S. Denton. 1995. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet 346:799-802. [DOI] [PubMed] [Google Scholar]
- 45.Widmer, U., K. R. Manogue, A. Cerami, and B. Sherry. 1993. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J. Immunol. 150:4996-5012. [PubMed] [Google Scholar]
- 46.Yang, B. T., S. C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]